Abstract
Mitogen-activated protein kinase kinase kinase 7 (MAP3K7), known as TAK1, is a central mediator of intracellular host defense signaling promoting inflammatory gene expression. Hence, TAK1 is a prime target of intracellular bacterial effectors in blocking inflammatory responses. However, when TAK1 is inhibited, host cells alternatively activate multiple cell death pathways, namely caspase 8-dependent apoptosis and pyroptosis, and receptor interacting protein kinase 3 (RIPK3)-dependent necroptosis. While these pathways ultimately lead to cell death, we found that they also modulate mitochondria to produce mitochondrial reactive oxygen species (ROS). Although as cell death executors, mixed lineage kinase-like (MLKL) and gasdermins are known to form pores in the plasma membrane, we found that TAK1 inhibition translocates them to mitochondria resulting in elevated mitochondrial ROS. Ablation of both MLKL and gasdermins diminished TAK1 inhibition-induced elevation of ROS and exacerbated intracellular bacterial colonization. Our results reveal that these cell death pathways have an alternative host defense role to prevent intracellular pathogen colonization.
Similar content being viewed by others
Introduction
Bacterial invasion initiates host inflammatory signaling pathways, which include pathways leading to canonical inflammatory responses and several different types of cell death, apoptosis, pyroptosis, and necroptosis [1]. An initiator caspase, caspase 8, mediates apoptosis and pyroptosis, while activation of receptor interacting protein kinase 3 (RIPK3) leads to necroptosis. Mitogen-activated protein kinase (MAPK) kinase kinase 7, known as TAK1, is one of the central signaling intermediates in the canonical inflammatory pathway [2]. TAK1 transmits the Toll like receptors and TNF signals to transcription factors, NF-κB and AP-1, through activating IKKs and MAPKs, respectively. In characterizing murine tissues and cultured cells harboring Tak1 gene deletion, we have found that Tak1 ablation abolishes the NF-κB and MAPK-driven inflammatory pathway but activates cell death pathways, which causes severe inflammatory conditions [3, 4]. Thus, both activation and inhibition of TAK1 results in inflammation, which had long been puzzling to us. Pathogens express a variety of effector molecules to block host inflammatory signaling pathways [5] including TAK1 [6]. Such efforts are supposed to benefit pathogens. However, we have recently found that the TAK1 inhibition-induced caspase 8 and RIPK3 cell death pathways elevate mitochondrial reactive oxygen species (ROS), which ultimately block intracellular pathogen growth before killing host cells [7]. We proposed that TAK1 inhibition-induced cell death pathways are alternative host defenses that have evolved to counteract the effects of pathogens’ effectors [7]. The current study aimed to define TAK1 inhibition-induced alternative host defense mechanism. Specifically, we determined the mechanism by which caspase 8 and RIPK3 induce mitochondrial ROS upon TAK1 inhibition.
Results
Compound deletion of caspase 8 and Mlkl blocks Tak1 deficiency-induced ROS and cell death
Tak1 gene deletion or pharmacological inhibition of TAK1’s protein kinase activity elevates ROS, which ultimately kill the cells [2]. This ROS-cell killing requires inflammatory cytokine TNF stimulation in many cell lines and primary keratinocytes [8,9,10]. However, in bone marrow derived macrophages (BMDMs), Tak1 gene deletion or TAK1 inhibition alone can elevate ROS and cell death without additional stimuli [7, 11], because BMDMs produce TNF. We previously reported that double deletion of caspase 8 and Ripk3 but no single deletion blocked Tak1 deficiency-induced mitochondrial ROS in BMDMs [7]. Thus, both caspase 8 and RIPK3 pathways are involved in elicitation of mitochondrial ROS. Many studies have determined the effector molecules downstream of caspase 8 and RIPK3 [12]. Caspase 8 initiates the caspase cascade leading to apoptosis, but also cleaves and activates gasdermin D (GSDMD) [13]. RIPK3 phosphorylates and activates mixed lineage kinase-like (MLKL) [14]. Both GSDMD and MLKL are known to be oligomerized and form pores in the cytoplasmic membrane, which execute pyroptosis and necroptosis, respectively. While they preferentially bind to phospholipids particularly phosphatidylinositol in the cytoplasmic membrane, they are also known to bind to cardiolipin [15,16,17], which is abundant in the mitochondrial inner membranes. Several earlier studies reported that gasdermins and MLKL modulate mitochondria [18,19,20,21]. We hypothesized that GSDMD and MLKL participate in elicitation of mitochondrial ROS in TAK1 ablation (Fig. 1A). We began with investigating involvement of MLKL by using Mlkl deficient mice [22]. We have previously reported that TAK1 inhibition with two different pharmacological inhibitors, 5Z-7oxozeaenol (5ZOZ) [23] and Takinib [24] as well as Tak1 gene deletion-induced ROS [7]. These ROS can be detected with two different mitochondrial specific ROS dyes, MitoSOX and MitoPY1, but ineffectively with general ROS dye CM-H2DCFDA. The ROS signals colocalize with mitochondria [7]. This ROS elevation begins around 5–6 h post TAK1 inhibition and is observed at a high level after 18 h when BMDMs with TAK1 inhibition are still similarly viable compared with no treatment [7]. Thus, in the current study, we examined the MitoSOX levels at 18 h post TAK1 inhibition, when TAK1 inhibition did not reduce cell viability compared with non-treated BMDMs (Supplementary Fig. S1). Consistent with our previous results [7], TAK1 inhibition alone with the TAK1 inhibitor 5Z-7oxozeaenol (5ZOZ) elevated mitochondrial ROS in BMDMs, which were abolished by double gene deletion of caspase 8 and Ripk3 (Fig. 1B, C). Mlkl deficiency did not block TAK1 inhibition-induced mitochondrial ROS (Fig. 1B, C). However, macrophages harboring double deletions of caspase 8 and Mlkl exhibited diminished TAK1 inhibition-induced mitochondrial ROS. Double deletions of caspase 8 and Mlkl’s effectiveness in diminishing ROS was slightly less or comparable to caspase 8 and Ripk3 double deficiency (Fig. 1B, C). When floxed Tak1 gene was deleted by inducible Cre recombinase (CreERT) expression, TAK1 protein was decreased, mitochondrial ROS increased, and BMDMs started dying at 5 days (Supplementary Fig. S1B and Fig. 1D). We observed that caspase 8 and RIPK3 protein levels also decreased compared with the loading control as cells started dying (Supplementary Fig. S1B, lane 2), which may be partially due to caspase 8 activation (self-cleavage) and RIPK3 degradation [25]. At 8 days post-Tak1 gene deletion, BMDMs were almost completely killed (Supplementary Fig. S1C, D). Compound inducible deletion of Caspase 8 and germline Ripk3 deletion but not any single deletion reduced Tak1 deficiency-induced mitochondrial ROS and cell death (Fig. 1D and Supplementary Fig. S1D). We examined whether caspase 8 and Mlkl double deletion rescued Tak1 gene deletion-induced mitochondrial ROS and cell death, The rescues were highly noticeabe but not complete (Fig. 1D). Overall, caspase 8 and Mlkl double deletions was similarly effective compared with caspase 8 and Ripk3 double deletion. These results demonstrate that MLKL is the major mediator of RIPK3-dependent mitochondrial ROS and subsequent cell death.
A Working hypothesis. We hypothesize that TAK1 inhibition-induced pathways block intracellular bacteria colonization through pore-forming proteins, MLKL and gasdermins. B, C Caspase 8-deficient and Caspase 8 and Mlkl double-deficient BMDMs were generated by treating BMDMs isolated from mice harboring inducible Casp8 deletion (Rosa26CreERT Casp8flox/flox) and inducible Casp8 and whole body Mlkl deletions (Rosa26CreERT Casp8flox/flox, Mlkl−/−) with the Cre inducer, 4OHT, for 5 days. Other BMDMs were isolated from the mice harboring indicated genotypes. All BMDMs were treated with vehicle (DMSO) or 300 nM 5z-7oxozeanol (5ZOZ) for 18 h. SytoxGreen-negative (live) BMDMs were gated and analyzed. Representative MitoSOX analyses were shown in (B) and quantification of replicate experiments is shown in (C). D BMDMs were isolated from mice harboring inducible Tak1 gene deletion (Rosa26CreERT Tak1flox/flox) alone or together with other gene deletions, and were incubated with 4OHT for 5 days. MitoSOX analysis and crystal violet cell viability were performed. Median fluorescence intensity (MFI) in live BMDMs and crystal violet absorbance relative to vehicle treated BMDMs are shown. One-way ANOVA, multiple comparisons, Tukey test; ***, p < 0.001; **, p < 0.01; *, p < 0.05; NS not significant.
Gasdermins participate in TAK1 inhibition-induced mitochondrial ROS
We next asked whether gasdermin D (GSDMD), which is known to play a major role downstream of caspase 8 [26, 27], participates in elicitation of mitochondrial ROS by using BMDMs with Gsdmd and Ripk3 gene deletion. These BMDMs were still viable by TAK1 inhibition at 18 h at a similar level to non-treated BMDMs (Supplementary Fig. S2A). Gene deletion of Gsdmd [28] alone did not block TAK1 inhibition-induced mitochondrial ROS (Fig. 2A, B). We generated mice harboring Ripk3 and Gsdmd double deletion and tested the TAK1 inhibition in BMDMs. However, unlike Caspase8 Mlkl double deletion, Ripk3 and Gsdmd double deletion did not effectively block TAK1 inhibition-induced mitochondrial ROS (Fig. 2A, B). Gasdermins are a protein family [13, 29], and multiple members are known to be expressed in macrophages including gasdermin E (GSDME) [18]. To test whether gasdermin E is involved in TAK1 inhibition-induced mitochondrial ROS, we used BMDMs from Ripk3, Gsdmd and Gsdme triple deletion mice. Ripk3, Gsdmd and Gsdme triple deletion inhibited TAK1 inhibition-induced mitochondrial ROS (Fig. 2A, B), suggesting that both GSDMD and GSDME participate in mitochondrial ROS elevation. Likewise, gasdermin inhibitor disulfiram [30], which can inhibit not only GSDMD but also GSDME [31], inhibited TAK1 inhibition-induced mitochondrial ROS in Ripk3-deficient BMDMs (Supplementary Fig. S2B). These results demonstrate that two types of pore forming proteins, MLKL and gasdermins, mediate TAK1 inhibition-induced mitochondrial ROS.
A, B BMDMs isolated from the indicated genotypes were treated with 300 nM 5ZOZ for 18 h. Representative MitoSox analysis in live BMDMs is shown (A). Median fluorescence intensity (MFI) in live BMDMs relative to vehicle treated BMDMs is shown (B). One-way ANOVA, multiple comparisons, Tukey test; *, p < 0.05.
Intracellular Salmonella growth suppression is dependent on MLKL and gasdermins
We reported that TAK1 inhibition-induced mitochondrial ROS is associated with blockade of intracellular bacterial colonization [7]. We asked whether MLKL and gasdermins mediate this bactericidal activity. We used Salmonella enterica serovar Typhimurium LT-2, hereafter referred to as Salmonella, as a model intracellular bacterial strain. While certain intracellular bacteria, such as Yersinia genus, express an effector directly inhibiting TAK1 enzymatic activity [6], Salmonella does not inhibit TAK1 [32]. Thus, we can examine the effect of TAK1 inhibition in intracellular bacterial growth with and without TAK1 inhibitor. We incubated BMDMs with Salmonella (multiplicity of infection (MOI), 10), and at 30 min post-infection, extracellular Salmonella were eliminated by gentamicin, and TAK1 inhibitor 5ZOZ were added. As Salmonella LT-2 strain is non-virulent in mice [33], it does not cause cell lysis in BMDMs. At 18 h post-infection, BMDMs are similarly viable with and without TAK1 inhibition (Supplementary Fig. S3A). We measured the mitochondrial ROS levels in live BMDMs and the intracellular Salmonella numbers (Fig. 3). Salmonella infection alone did not affect mitochondrial ROS, which is consistent with the notion that Salmonella does not inhibit TAK1 (Supplementary Fig. S3B). Mlkl or Gsdmd single or Ripk3 Gsdmd double deletion did not effectively block TAK1 inhibitor-induced mitochondrial ROS, whereas double deletion of caspase 8 and Mlkl or triple deletion of Ripk3, Gsdmd and Gsdme was effective on blocking it (Fig. 3A, left). We previously reported that hydrogen sulfide, cysteine, and N-acetyl cysteine (NAC), all of which can provide electrons to the mitochondrial electron transport chain [34], completely block bacteria growth suppression by TAK1 inhibition, whereas a general ROS scavenger, tert-butylhydroquinone, is ineffective [7]. Thus, ROS by themselves are not sufficient, but other events associated with disruption mitochondrial respiration are also important to block intracellular bacteria growth. We also reported that NAC exacerbates intracellular colonization of Yersinia that expresses a TAK1 inhibiting effector YopJ [7]. Therefore, in this study, we used NAC treatment as a control for restoration of TAK1 inhibitor-induced events. NAC abolished TAK1 inhibition-induced mitochondrial ROS in BMDMs with any gene deletion (Fig. 3A, right). The intracellular bacteria numbers were reduced by TAK1 inhibition, which were restored by caspase 8 and Mlkl double deletion or Ripk3, Gsdmd, and Gsdme triple deletion but not by Mlkl or Gsdmd single deletion or Ripk3 Gsdmd double deletion (Fig. 3B). NAC restored intracellular Salmonella numbers in all genotypes (Fig. 3B and Supplementary Fig. S3C). These results demonstrate that MLKL and gasdermins (GSDMD and GSDME) cooperatively promote blockade of intracellular Salmonella colonization through mitochondrial modulation.
A BMDMs isolated from the indicated genotypes were infected with Salmonella (MOI, 10) for 30 min, and extracellular Salmonella was eliminated by gentamicin treatment. BMDMs treated with 300 nM 5ZOZ alone or together with 3 mM N-acetyl cysteine (NAC) for 18 h. MitoSOX MFIs in live BMDMs relative to samples with Salmonella infection alone are shown. Salmonella infection alone or with NAC did not elevate mitochondrial ROS (Supplementary Fig. S3B). B Intracellular Salmonella colonization was assessed at 18 h post infection with and without 300 nM 5ZOZ and/or 3 mM NAC. Representative Salmonella colony photos are shown (left). Each column of colony photos are from one agar plate. Colony forming units (CFUs) relative to vehicle treatment are shown (right). One-way ANOVA, multiple comparisons, Tukey test; ***, p < 0.001; **, p < 0.01; *, p < 0.05; NS not significant.
Mitochondrial localization of MLKL and gasdermins upon TAK1 inhibition
We next examined whether MLKL and gasdermins are physically associated with mitochondria upon TAK1 inhibition. If MLKL and GSDMD modulate mitochondria to produce ROS, they should translocate to mitochondria prior to mitochondrial ROS elevation. We isolated cytosolic (C), including the plasma membrane, as well as mitochondrial (M) fractions at 5 h post-TAK1 inhibition. Without TAK1 inhibition, GSDMD and MLKL were found to reside in the cytosol (Fig. 4A, top and 2nd panels) [35]. N-terminus GSDMD (GSDMD-N), which forms a pore [13, 29], was observed both in the cytosolic and mitochondrial fractions with TAK1 inhibition in wild type BMDMs (Fig. 4A, top panel, lanes 3, 4). MLKL was not detectable in the mitochondrial fraction (2nd panel, lane 4). These suggest that GSDMD-N physically translocated to the mitochondria upon TAK1 inhibition. Ripk3 deletion did not affect GSDMD-N translocation to the mitochondrial fraction (top panel, lane 8). In caspase 8-deficient BMDMs, we observed increased MLKL in the mitochondria with TAK1 inhibition (2nd panel, lane 12), whereas GSDMD cleavage was marginal (top panel, lanes 11 and 12). These are consistent with the previous notion that caspase 8 is the direct mediator of GSDMD cleavage upon bacterial TAK1 inhibition [26, 27]. Single deletion of Gsdmd seemed to exacerbate mitochondrial translocation of MLKL (2nd panel, lane 16). We also found that mitochondrial MLKL was detectable with S345 phospho-specific MLKL antibody in wild type BMDMs with pan-caspase inhibition (see Fig. 5B, lane 12), indicating that MLKL is activated for pore formation. Single deletion of Mlkl induced mitochondrial translocation of GSDMD (top panel, lane 20) at the level comparable to that in wild type (lane 4). Double deletion of Ripk3 and Gsdmd or caspase 8 abolished both GSDMD and MLKL translocation to the mitochondria (lanes 21–28). We also found that another TAK1 inhibitor Takinib induces GSDMD translocation to the mitochondria (Supplementary Fig. S4A). These results demonstrate that either or both GSDMD-N or/and phosphorylated MLKL are translocated to mitochondria in response to TAK1 inhibition depending on availability of caspase 8-GSDMD and RIPK3-MLKL pathways. If one pathway is disrupted, the other pore forming proteins translocate to mitochondria upon TAK1 inhibition. Given barely detectable MLKL translocation in wild type and its exacerbation in Gsdmd-deficient BMDMs, GSDMD may be a primary mediator of TAK1 inhibition-induced mitochondrial modulation. Our results in Figs. 2 and 3 indicate that double deletion of Ripk3 and Gsdmd is insufficient, but that triple deletion of Ripk3, Gsdmd, and Gsdme can block TAK1 inhibition-induced mitochondrial ROS (Fig. 2B). Like GSDMD, GSDME is also cleaved by caspases and the resulting N-terminal GSDME (GSDME-N) forms a pore [13, 29]. We examined GSDME cleavage and its localization in wild type, Gsdmd single- and Ripk3 and Gsdmd double-deficient BMDMs (Fig. 4B and Supplementary Fig. S4B). GSDME-N was found to be in the mitochondrial fraction in Gsdmd- and Ripk3 and Gsdmd double-deficient BMDMs when TAK1 was inhibited, whereas the GSDME-N was only marginally observed in wild type BMDMs (Fig. 4B, lanes 4, 10, Supplementary Fig. S4B, lanes 4, 13). Consistent with a previous study [31], disulfiram blocked cleavage of GSDME as well as GSDMD (Supplementary Fig. S3B, lanes, 5,6,10,14). These results suggest that GSDME plays a compensatory role when GSDMD is absent.
A BMDMs were treated with vehicle or 300 nM 5ZOX for 5 h. Cell lysates were fractionated into the cytosol including plasma membrane (C) and the mitochondrial (M) fractions, and GSDMD and MLKL were analyzed by Western blotting. Full length GSDMD (GSDMD-F) and N-terminal GSDMD (GSDMD-N) are indicated. B GSDME and GSDMD in the whole cell lysate (W), the cytosol including plasma membrane (C) and the mitochondrial (M) fractions in wild type (left panels) and Gsdmd-deficient BMDMs with vehicle or 300 nM 5ZOZ (5 h) were analyzed by Western blotting. Full length GSDME (GSDME-F) and N-terminal GSDME (GSDME-N) are also indicated. *, non-specific band. Mitochondrial marker TOM20 and cytosolic marker GAPDH are shown as loading controls.
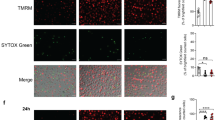
A, B Wild type BMDMs were treated with vehicle or 300 nM 5ZOZ for 5 h. The cytosolic including plasma membrane (C) and the mitochondrial (M) proteins were resolved by SDS-PAGE with and without a reducing agent (2-mercaptoehtanol), and analyzed by Western blotting. Oligomerized bands of GSDMD and MLKL and N-terminal GSDME are indicated. Mitochondrial marker TOM20 and cytosolic marker GAPDH are shown as loading controls. B 20 µM pan-caspase inhibitor, emricasan, was also treated for 5 h. Smaller amounts of proteins were loaded to avoid anti-P-MLKL background bands. C HeLa-RIPK3 cells were transfected with vectors expressing GFP alone or GFP-GSDMD. At 24 h post-transfection, cells were treated with vehicle or 50 ng/ml TNF and 1 μM 5ZOZ (TZ) for 5 h. Cytosol and mitochondrial fractions were analyzed in reducing and non-reducing conditions by Western blotting. TOM20 and GAPDH are shown as loading controls for mitochondria and cytosol, respectively. The estimated molecular weights of GFP-GSDMD and cleaved GFP-GSDMD-N are about 75 and 50 kDa, respectively. D HeLa-RIPK3 with GFP-GSDMD were treated with vehicle (top and 2nd panels) or with 50 ng/ml TNF and 1 µM 5ZOZ (3rd and bottom panels) for 5 h. Immunofluorescence staining of anti-TOM20 (red) and GFP-GSDMD (green) was analyzed by confocal microscopy. 2nd and bottom panels show enlarged images of top and 3rd panels, respectively. Scale bars, 10 µm. Control GFP expressing cells are shown in Supplementary Fig. S5A. Volumetric (3D) image of the TNF and 5ZOZ treated cell are shown in Supplementary Video 1. E Wild type BMDMs were treated with vehicle or 300 nM 5ZOZ for 4 h. Mitochondrial respiration was determined by Seahorse assay (Oligo, oligomycin; FCCP, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone; Rote/Ant, rotenone + antimycin A). A representative analysis is shown (upper panel). Oxygen consumption rate (OCR) was normalized with protein concentration per well (lower graph). Bars represent mean values of basal respiration and maximal respiration from three independent Seahorse assays. Students’ t-test, ***, p < 0.001; **, p < 0.01; *, p < 0.05; NS not significant.
Both MLKL and N-terminal gasdermins form pores by oligomerization [13, 29]. We examined whether TAK1 inhibition induces MLKL and gasdermin oligomerization in the mitochondria. In non-reducing SDS-PAGEs, GSDMD were observed as high molecular weight smear bands with TAK1 inhibition in the both cytosolic and mitochondrial fractions of wild type BMDMs (Fig. 5A top panel, lanes 3, 4). A higher molecular weight MLKL was observed when Western blotting was overexposed (Fig. 5A 2nd panel, lanes 3, 4). RIPK3 phosphorylates MLKL at serine 352, which promotes MLKL oligomerization [22]. Phosphorylated MLKL was clearly detected in the mitochondrial fraction as smear bands at high molecular weights with TAK1 inhibition when pan-caspase inhibitor, emricasan, was treated (Fig. 5B top panel, lane 6). These suggest that TAK1 inhibition primarily induces N-terminal GSDMD oligomerization in mitochondria, and phosphorylation and oligomerization of MLKL occurs when caspases are inhibited. To visualize physical location of GSDMD, we used GFP-tagged GSDMD. GFP-tagged GSDMD was expressed in RIPK3 expressing HeLa (HeLa-RIPK3) cells, in which we previously reported that co-treatment of TNF and TAK1 inhibitor mimics pathogen infection and induces mitochondrial ROS [7]. Although N-terminal tag is known to disrupt functional pore formation [36], we were able to observe cleaved GFP-GSDMD and oligomerization in the mitochondrial fraction at 5 h post-TNF and TAK1 inhibitor treatment (Fig. 5C). Analysis via confocal microscopy indicated that the GFP-GSDMD fusion protein was dispersed within the cell (Fig. 5D, upper panes), and GFP-GSDMD puncta were formed in the cytoplasm following TNF and TAK1 inhibitor treatment, which were overlapped with mitochondria (Fig. 5D, lower panels, Supplementary Fig. S5B and Supplementary Video 1). Control cells expressing only GFP lacked specific colocalization with mitochondria (Supplementary Fig. S5A, B and Supplementary Video 2). Live cell time lapse images also demonstrate co-localization of GFP-GSDMD with mitochondria starting around 5 h post stimulation (Supplementary Fig. S5C). This co-localization was also observed in cells with Salmonella (Supplementary Fig. S5D). Thus, GFP-GSDMD is likely to be cleaved and localized to mitochondria under pathogen infection. Finally, we found that mitochondrial respiration was disrupted with TAK1 inhibition (Fig. 5E). Mitochondrial impairment was also observed as a lower level of the mitochondrial membrane potential with TAK1 inhibition (Supplementary Fig. S5E). Mitochondrial respiration and membrane potential were partially restored by Ripk3, Gsdmd, and Gsdme triple deletion (Supplementary Fig. S5E, F). Collectively, these results demonstrate that gasdermins, GSDMD and GSDME, and/or MLKL are translocated to mitochondria upon TAK1 inhibition, which disrupt mitochondrial respiration resulting in elevation of mitochondrial ROS and blocking intracellular Salmonella colonization (Graphic summary, Supplementary Fig. S6).
Discussion
In the current study, we investigated how host cells prevent intracellular bacteria growth through mitochondrial modulation when TAK1 is inhibited. We found that, as initially hypothesized (Fig. 1A), both caspase 8-GSDMD and RIPK3-MLKL pathways cooperatively modulate mitochondria. We found that their cell death executor pore forming proteins, MLKL and GSDMD, are translocated to mitochondria, and that GSDME plays a compensatory role when GSDMD is absent. We previously reported that hydrogen sulfide and its precursors, NAC, but not general antioxidants effectively block TAK1 inhibition-induced mitochondrial ROS [7]. As hydrogen sulfide facilitates mitochondrial respiration by donating electrons to complex III in the respiratory chain, TAK1 inhibition is likely to promote ROS production by disrupting the electron flux in the respiratory chain. Our current study identified that TAK1 inhibition indeed impairs mitochondrial respiration, and that RIPK3-MLKL and gasdermins are the executors of this disruption. How do these proteins disrupt mitochondrial respiration? Both MLKL and gasdermins are known to bind particular membrane phospholipids such as phosphatidylinositol to form pores, but also exhibit a high affinity to cardiolipin [15,16,17]. Cardiolipin is abundant in the mitochondrial inner membrane where the respiratory chains reside. Thus, it may be reasonable to assume that MLKL and gasdermins form pores in the mitochondrial inner membrane, which results in disrupting the membrane potential leading to blocking the electron flux. However, how these proteins reach the mitochondrial inner membrane is still elusive as the outer mitochondrial membrane is impermeable to them under normal conditions. Rogers et al. reported that N-terminus GSDME has an ability to permeabilize the outer mitochondrial membrane [18]. Caspase 8 cleaves and activates BH3-only protein Bid, and cleaved Bid (tBid) might promote the canonical apoptotic outer membrane pores of BAX and BAK [37], which could permeabilize outer mitochondrial membrane to MLKL and gasdermins. Alternatively, cardiolipin translocation to outer mitochondrial membrane is known to occur under apoptotic conditions [38] and pyroptosis [39], which might recruit MLKL and gasdermins and permeabilize outer mitochondria membrane. These mechanisms may be cooperatively accountable for disrupting the mitochondrial outer membrane in BMDMs with TAK1 inhibition.
Tak1 gene deletion causes cell death in many types of cells and tissues [2]. We initially thought that TAK1 is required for cell survival. However, it turns out that artificial deletion of Tak1 gene mimics pathogen effectors that target host inflammatory responses. Specifically, Yersinia species including Y. pseudotuberculosis, Y. enterocolitica, and Y. pestis, express an effector YopJ, an acetyl transferase, which directly modifies the amino acid residue (Thr-187) of TAK1’s activation loop disrupting the protein kinase catalytic activity [6]. Once TAK1 is inhibited or ablated, host cells initiate the alternative defense mechanisms involving cell death pathways. Our current results indicate that such inhibition initiates activation of MLKL and gasdermins to block pathogen colonization. Besides TAK1, many other protein kinases in the inflammatory signaling pathways are the targets of bacterial and viral effectors [5], which may be covalently modified or degraded [40]. For example, IκB kinases (IKKs), activators of NF-κB, and TANK binding kinase 1 (TBK1), an activator of the IRF-interferon pathway, can be inhibited upon pathogen invasion. Interestingly, similar to Tak1 ablation [2], mice harboring Ikk or Tbk1 gene deletion exhibit tissue damage associated with cell death [41, 42]. The severity of tissue damage varies dependent on tissues and genes. Nevertheless, both caspase 8 and RIPK3 pathways appear to be involved in Tak1-, Ikk-, and Tbk1-deficient cell death. This might suggest that a common mechanism mediates the alternative host defense when inflammatory protein kinases are inhibited by pathogen effectors.
The limitation of our current results lies in the absence of identification of the mechanism by which TAK1 inhibition initiates the caspase 8 and RIPK3 cell death pathways. Receptor interacting protein kinase 1 (RIPK1) has been reported as a substrate of TAK1, IKKs, and TBK1 [43,44,45,46]. Their phosphorylation limits RIPK1 activity resulting in blockade of caspase 8 and RIPK3. Thus, RIPK1 may serve as a common sensor for pathogen effectors’ inhibition of inflammatory protein kinases. However, RIPK1 alone cannot be accountable for TAK1 ablation-induced cell death [47]. Another limitation of this study is that the triple deletion of Ripk3, Gsdmd, and Gsdme largely, but not completely, blocks mitochondrial ROS and bacterial growth suppression by TAK1 inhibition (Fig. 3). This indicates that additional mechanisms are involved in TAK1 inhibition-induced mitochondrial ROS elevation. Other gasdermins may be involved, although their expression levels are much lower than those of GSDMD and GSDME in macrophages [29]. Apoptotic mitochondrial pore-forming proteins BAX/BAK are activated by caspase 8 through caspase 3. With BAX/BAK pores, it is reported that caspase 3 translocates into the mitochondria and disrupts the mitochondrial electron transport chain [48]. These possibilities need to be evaluated.
MLKL and gasdermins were originally described as cell death executors that form pores in the plasma membranes [13, 29]. However, emerging studies have been revealing that they also play other roles independently of executing cell death. MLKL binds to intracellular Listeria and blocks their colonization without inducing host cell death [49]. Gasdermin pores release interleukin 1 without largely disrupting the plasma membrane when the formation of gasdermin pores are limited [50]. Gasdermins are also reported to attack gram negative bacteria [51]. Our results demonstrate that MLKL and gasdermins target and disrupt mitochondria to produce ROS when TAK1 is inhibited by intracellular bacteria. All these functional roles of MLKL and gasdermins are associated with the host defense system. When MLKL and gasdermins are activated in response to invading microorganisms, the pore forming proteins may initially attempt to kill intracellular microorganisms by directly attacking them and/or activating inflammation by releasing IL-1. When microorganisms evade the initial host defense by inhibiting the canonical inflammatory signaling pathways, the pore forming proteins are translocated to and modulate mitochondria to initiate the bacteria-killing process. Finally, if the level of activated MLKL and gasdermins exceeds a certain threshold, they form pores in the host cell’s plasma membrane, resulting in host cell killing. Dead cells release danger associated molecular patterns, which promote inflammation in surrounding cells. MLKL and gasdermins may be the central mediators of this multi-layered host defense system.
Materials and methods
Study design
No power analysis for the sample size calculation was used. All experiments were conducted in BMDMs isolated from two-five different mice for each genotype. All independently acquired data points are shown in the results. The results are confirmed by at least three separately performed experiments. All methods were performed in accordance with the relevant guidelines and regulations.
Mice, BMDMs, HeLa cells, and bacteria
As mice harboring germline Tak1 or caspase 8 gene deletion are lethal during embryogenesis [2], we used an inducible gene deletion system. For other gene deletions, mice with germline gene deletions were used. Specifically, wild type [no-Cre Tak1flox/flox [52]], inducible caspase 8-deficient [Rosa26-CreERT, JAX ® Mice, B6;129-Gt(ROSA)26Sortm1(cre/ERT)Nat/J [53] Casp8flox/flox [54]], Ripk3-deficient [Ripk3−/− [55]], caspase 8 and Ripk3 double deficient [Casp8−/− (spontaneously acquired from Casp8flox/flox) Ripk3−/−], Mlkl-deficient [Mlkl−/− [22]], Gsdmd-deficient [Gsdmd−/−, JAX ® Mice C57BL/6N-Gsdmdem4Fcw/J #032410 [28]], Gsdme-deficient [Gsdmde−/−, JAX ® Mice C57BL/6N-Gsdmeem1Fsha/J #032411 [56]], Casp8 Mlkl double-deficient (Rosa26.CreERT Casp8flox/flox Mlkl−/−), Gsdmd Ripk3 double-deficient (Ripk3−/− Gsdmd−/−), Ripk3, Gsdmd, Gsdme triple-deficient (Ripk3−/− Gsdmd−/− Gsdme−/−), inducible Tak1-deficient (Rosa26-CreERT Tak1flox/flox), Tak1 Casp8 double-deficient (Rosa26.CreERT Tak1flox/flox, Casp8flox/flox) Tak1 Ripk3 double-deficient (Rosa26.CreERT Tak1flox/flox, Ripk3−/−), Tak1, Casp8 and Ripk3 triple-deficient (Rosa26.CreERT Tak1flox/flox, Casp8flox/flox Ripk3−/−) and littermate or age-matched no-Cre mice were used. The genotypes were determined by PCR and were confirmed by Western blotting. The mouse care and the procedures using mice were conducted with the approval of the North Carolina State University Institutional Animal Care and Use Committee.
Bone marrow cells were isolated with a standard method and were cultured in macrophage media; Dulbecco’s modified Eagle’s medium supplemented with 10% bovine growth serum (HyClone), 50 I.U./mL penicillin–streptomycin, and 30% L929 conditioned media at 37 °C with 5% CO2. For Cre-dependent gene deletion, BMDMs were treated with 0.3 μM 4-hydroxytamoxifen (4OHT) or vehicle (ethanol) alone for 5 days to achieve complete floxed gene deletion. To determine cell viability after gene deletions, BMDMs were fixed using 10% formalin in PBS, and stained with 0.1% crystal violet solution. The dye was eluted and analyzed at 595 nm. Salmonella enterica Typhimurium LT-2 were culture in Luria–Bertani (LB) broth at 30 °C.
Reagents
TAK1 protein kinase inhibitor, 300 nM 5Z-7-oxozeaenol (5ZOZ) [23] and 10 µM Takinib [24] were used. 3 mM N-acetyl-L-cysteine (NAC) (Sigma-Aldrich), 50 μM disulfiram (Sigma-Aldrich) and pan-caspase inhibitor, emricasan, (MedKoo Biosciences, Inc.) were also used. To measure the level of ROS, 1.25 μM MitoSOX (Thermo Fisher Scientific), was used. To monitor cell viability, 30 nM Sytox Green (Thermo Fisher Scientific) was used. Antibodies against TAK1 [57], caspase 8 (CASP8) (Cell Signaling #4927), RIPK3 (Sigma-Aldrich, R4277 and MABC1595 clone 8G7), MLKL (Abcam, ab184718), phospho-MLKL (phospho S345) (Abcam, ab196436), gasdermin D (GSDMD) (Abcam, ab209845), gasdermin E (GSDME) (Abcam, ab215191), GFP (Abcam, ab1218), TOM20 (Santa Cruz, FL-145, Sigma-Aldrich, HPA011562), GAPDH (Chemicon, MAB374, Sigma-Aldrich, G9545), β-actin (Sigma-Aldrich, AC15) were used. Human TNF (Peprotech, 300-01 A), gentamicin sulfate (VWR), and 4OHT (Sigma-Aldrich) were also used.
ROS analysis
BMDMs were washed with phosphate-buffered saline (PBS) and were detached from culture plates with gentle scraping. Cells were precipitated with centrifugation at 5000 × g for 1 min were incubated with mixtures of 1.25 μM MitoSOX and 30 nM Sytox Green in PBS for 30 min at room temperature. Live (Sytox green-negative) cells were gated, and the median fluorescence intensities (MFIs) were determined with a flow cytometer (Accuri C6 Plus, BD Bioscience) and FlowJo software (BC Bioscience).
Salmonella infection
Salmonella were pre-cultured over-night, and were inoculated into fresh culture broth at 1:10 dilution. After 1.5 h-incubation at 30 °C, bacterial numbers were enumerated by OD, and bacteria were suspended in PBS after centrifugation at 5000 × g for 10 min. BMDMs were seeded on 6-well plates 1–3 days before infection. When cells reached to the confluency level at 80–100%, the culture medium was changed to one without any antibiotics, and the cell numbers per wells were counted. The cells were incubated with the indicated MOI of Salmonella for 30 min at 37 °C in 5% CO2. The cells were then washed with sterile PBS and incubated in a medium supplemented with gentamicin (100 μg/ml) for 18 h with the indicated chemicals. To determine the intracellular Salmonella growth, cells were washed with sterile PBS and lysed in PBS containing 0.2% Triton X-100 at 18 h-post infection. Serial dilutions of the lysates were spotted (10 μl/spot) on LB agar plates for enumeration of intracellular bacteria. For imaging, mCherry-Salmonella [7] were infected with the same procedure.
Immunofluorescence Staining
To determine GSDMD localization, GFP-tagged GSDMD (pAcGFPC3-GSDMD) was generated using pAcGFPC3 (Takara) and mouse GSDMD [Addgene, Plasmid #80950 [51]]. HeLa cells stably expressing receptor interacting protein kinase 3 (HeLa-RIPK3) [49] were cultured with Dulbecco’s modified Eagle’s medium supplemented with 10% bovine growth serum (Hyclone) and were co-transfected with an empty GFP expressing vector or pAcGFPC3-GSDMD using TransIT-X2® (Mirus Bio). At 24 h post-transfection, cells were treated with 50 ng/ml TNF and 1 µM 5ZOZ. Some cells were used for live imaging with Mitotracker deep red (30 nM, Thermo Fisher) and Hoechst 33342 (1 µg/ml, Thermo Fisher) for 4–5 h by a confocal microscope (FV3000, Olympus) with a 40× oil objective (UPLXAPO40XO). Other cells were fixed with 4% paraformaldehyde for 10 min at room temperature, permeabilized with 0.5% Triton X100 for 10 min, and incubated with a blocking buffer, PBS containing 5% goat serum for 1 h at room temperature. Antibodies, anti-TOM20 (1:1000), and secondary antibodies, anti-rabbit IgG conjugated with Alexa-647 (1:1000, Thermo Fisher Scientific) were used. Cells were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich). The coverslips were mounted with ProLong Diamond Antifade Mountan (Thermo Fisher) and were observed and Z-stack images were acquired by a confocal microscope (FV3000, Olympus) with a 40× and 60× oil objective (UPLXAPO40XO, UPLXAPO60XOHR).
Western Blotting
BMDMs and HeLa-RIPK3 cells were lysed in extraction buffer (20 mM HEPES [pH 7.4], 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM PMSF, 20 µg/ml aprotinin, 0.5% Triton X-100). For fractionation of cell proteins, BMDMs were processed with the Qproteome Mitochondrial Isolation Kit (QIAGEN), and the cytosol and the mitochondrial fractions were subjected to Western blotting. Proteins with and without a reducing agent 2-mercaptoethanol were resolved using sodium dodecyl sulphate (SDS)–polyacrylamide gel electrophoresis, and were transferred to polyvinylidene difluoride membranes (Thermo Fisher Scientific). Anti-TAK1 (1:1000), anti-CASP8 (1:1000), anti-MLKL (1:1000), anti-P-MLKL (1:1000), anti-GSDMD (1:1000), anti-GSDME (1:1000), anti-TOM20 (1:1000), anti-GAPDH (1:10,000), and anti-β-actin (1:10,000) were used, and the bound antibodies were visualized with horseradish peroxidase–conjugated antibodies by Clarity and Clarity Max chemiluminescence assay (Bio-Rad).
Bioenergetics measurements and mitochondrial membrane potential
BMDMs (1 × 105 cells/well) were seeded on a Seahorse cell culture plate. The assay medium was Agilent Seahorse XF Base Medium supplemented with 10 mM glucose, 2 mM glutamine and 1 mM sodium pyruvate and manually adjusted to pH 7.4 with NaOH 0.1 N. To measure oxygen consumption rate (OCR), 0.5 µM Oligomycin A (to block ATP synthesis), 1 µM FCCP (to uncouple mitochondria proton pumping) and 1 µM antimycin A + rotenone (to completely block the electron transport chain) were injected sequentially in a Seahorse XFe96 Analyzer (Agilent Technologies). For measuring mitochondrial membrane potential, tetramethylrhodamine ethyl ester (TMRE) (100 nM) at 30 min prior to harvesting cells, and harvested cells suspension was divided into two tubes followed by incubation with 50 µM carbonyl cyanide m-chlorophenyl hydrazone (CCCP) or vehicle for 15 min. After washing cells with PBS, cells were incubated with 30 nM Sytox Green for 30 min. Live (Sytox green-negative) cells were gated, and the mean fluorescence intensities (MFIs) were determined with a flow cytometer (Accuri C6 Plus, BD Bioscience). The MFIs, subtracted by those with CCCP treated cells, are used as relative membrane potential values.
Statistical analysis
The box and whisker graphs represent the mean (middle line), the 25th and 75th percentiles (box), the minimum and maximal date points (whisker), and all data points acquired from separately processed samples. Differences between experimental groups were assessed for significance by using the one-way ANOVA with Tukey multiple comparisons test or the unpaired Student’s t test (two-tailed) with equal distributions as indicated in the figure legends. Each experiments were repeated three times or more.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Samir P, Malireddi RKS, Kanneganti TD. The PANoptosome: a deadly protein complex driving pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol 2020;10:238.
Mihaly SR, Ninomiya-Tsuji J, Morioka S. TAK1 control of cell death. Cell Death Differ. 2014;21:1667–76.
Kajino-Sakamoto R, Inagaki M, Lippert E, Akira S, Robine S, Matsumoto K, et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol 2008;181:1143–52.
Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, et al. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem 2006;281:19610–7.
Krachler AM, Woolery AR, Orth K. Manipulation of kinase signaling by bacterial pathogens. J Cell Biol 2011;195:1083–92.
Paquette N, Conlon J, Sweet C, Rus F, Wilson L, Pereira A, et al. Serine/threonine acetylation of TGFbeta-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc Natl Acad Sci USA 2012;109:12710–5.
Lopez-Perez W, Sai K, Sakamachi Y, Parsons C, Kathariou S, Ninomiya-Tsuji J. TAK1 inhibition elicits mitochondrial ROS to block intracellular bacterial colonization. Proc Natl Acad Sci USA 2021;25:e2023647118.
Omori E, Matsumoto K, Zhu S, Smart RC, Ninomiya-Tsuji J. Ablation of TAK1 upregulates reactive oxygen species and selectively kills tumor cells. Cancer Res. 2010;70:8417–25.
Morioka S, Omori E, Kajino T, Kajino-Sakamoto R, Matsumoto K, Ninomiya-Tsuji J. TAK1 kinase determines TRAIL sensitivity by modulating reactive oxygen species and cIAP. Oncogene. 2009;28:2257–65.
Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem 2008;283:26161–8.
Sakamachi Y, Morioka S, Mihaly SR, Takaesu G, Foley JF, Fessler MB, et al. TAK1 regulates resident macrophages by protecting lysosomal integrity. Cell Death Dis. 2017;8:e2598.
Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024;187:235–56.
Liu X, Xia S, Zhang Z, Wu H, Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat Rev Drug Discov. 2021;20:384–405.
Murphy JM. The killer pseudokinase mixed lineage kinase domain-like protein (MLKL). Cold Spring Harb Perspect Biol 2020;12;8:a036376.
Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–6.
Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–46.
Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–81.
Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10:1689.
Huang LS, Hong Z, Wu W, Xiong S, Zhong M, Gao X, et al. mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity. 2020;52:475–86.e475.
Weindel CG, Martinez EL, Zhao X, Mabry CJ, Bell SL, Vail KJ, et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell. 2022;185:3214–31.e3223.
Valenti M, Molina M, Cid VJ. Human gasdermin D and MLKL disrupt mitochondria, endocytic traffic and TORC1 signalling in budding yeast. Open Biol. 2023;13:220366.
Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–53.
Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 2003;278:18485–90.
Totzke J, Gurbani D, Raphemot R, Hughes PF, Bodoor K, Carlson DA, et al. Takinib, a selective TAK1 inhibitor, broadens the therapeutic efficacy of TNF-alpha inhibition for cancer and autoimmune disease. Cell Chem Biol 2017;24:1029–39.e1027.
Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 2007;19:2056–67.
Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci USA 2018;115:E10888–97.
Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–9.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5.
Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143–57.
Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol 2020;21:736–45.
Wang C, Yang T, Xiao J, Xu C, Alippe Y, Sun K, et al. NLRP3 inflammasome activation triggers gasdermin D-independent inflammation. Sci Immunol 2021;6:eabj3859.
Jones RM, Wu H, Wentworth C, Luo L, Collier-Hyams L, Neish AS. Salmonella AvrA Coordinates Suppression of Host Immune and Apoptotic Defenses via JNK Pathway Blockade. Cell Host Microbe. 2008;3:233–44.
Swords WE, Cannon BM, Benjamin WH Jr. Avirulence of LT2 strains of Salmonella typhimurium results from a defective rpoS gene. Infect Immun 1997;65:2451–3.
Ezerina D, Takano Y, Hanaoka K, Urano Y, Dick TP. N-Acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem Biol 2018;25:447–59.e444.
Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27:673–84.
He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–98.
Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501.
Pizzuto M, Pelegrin P. Cardiolipin in immune signaling and cell death. Trends Cell Biol. 2020;30:892–903.
Miao R, Jiang C, Chang WY, Zhang H, An J, Ho F, et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity. 2023;56:2523–41.e2528.
Galan JE. Common themes in the design and function of bacterial effectors. Cell Host Microbe. 2009;5:571–9.
Pasparakis M, Luedde T, Schmidt-Supprian M. Dissection of the NF-kappaB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006;13:861–72.
Bonnard M, Mirtsos C, Suzuki S, Graham K, Huang J, Ng M, et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 2000;19:4976–85.
Geng J, Ito Y, Shi L, Amin P, Chu J, Ouchida AT, et al. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun. 2017;8:359.
Menon MB, Gropengiesser J, Fischer J, Novikova L, Deuretzbacher A, Lafera J, et al. p38(MAPK)/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection. Nat Cell Biol 2017;19:1248–59.
Lafont E, Draber P, Rieser E, Reichert M, Kupka S, de Miguel D, et al. TBK1 and IKKepsilon prevent TNF-induced cell death by RIPK1 phosphorylation. Nat Cell Biol 2018;20:1389–99.
Xiao Y, Zou Q, Xie X, Liu T, Li HS, Jie Z, et al. The kinase TBK1 functions in dendritic cells to regulate T cell homeostasis, autoimmunity, and antitumor immunity. J Exp Med 2017;214:1493–507.
Malireddi RKS, Gurung P, Kesavardhana S, Samir P, Burton A, Mummareddy H, et al. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med 2020;217;3:e20191644.
Ricci JE, Munoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–86.
Sai K, Parsons C, House JS, Kathariou S, Ninomiya-Tsuji J. Necroptosis mediators RIPK3 and MLKL suppress intracellular Listeria replication independently of host cell killing. J Cell Biol 2019;218:1994–2005.
Zhou B, Abbott DW. Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Rep. 2021;35:108998.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8.
Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 2005;6:1087–95.
Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci 2003;23:2314–22.
Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–95.
Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol 2004;24:1464–9.
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103.
Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–6.
Acknowledgements
We thank Dr. Akira for Tak1-floxed, Dr. Dixit for Ripk3−/−, Drs. Hakem and Green for Casp8-floxed, Dr. Murphy for Mlkl−/− mice. This work was supported by National Institutes of Health (NIH) grant R01GM112986 (JN-T), R35GM139601 (JN-T), the National Institute of Environmental Health Sciences Intramural Grant Z01 ES102005 (MF) and The United States Department of Agriculture- National Institute of Food and Agriculture award 2018-07464 (GRANT12687985) (SK). WL-P was supported NIH fellowship F31GM135975. YS, RG-C, and WL-P were also partly supported by NIH training grant T32 ES007046.
Author information
Authors and Affiliations
Contributions
Conceptualization: WLP, KS, JNT. Methodology/Materials: CP, SK, MF. Investigation: WLP, RGC, KS, YS. PR, JMM, JNT. Funding acquisition: KS, MF, JNT. Writing–original draft: WLP, KS, JNT. Writing–review & editing: WLP, KS, SK, MF, JNT.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
No human subjects are included in this study. Bone marrow derived macrophages were isolated from mice. The mouse care and the procedures using mice were conducted with the approval of the North Carolina State University Institutional Animal Care and Use Committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Stephen Tait
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López-Pérez, W., González-Calderón, R.E., Sai, K. et al. TAK1 inhibition activates pore-forming proteins to block intracellular bacterial growth through modulating mitochondria. Cell Death Dis 16, 456 (2025). https://doi.org/10.1038/s41419-025-07760-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41419-025-07760-4