Abstract
Efferocytosis, the clearance of apoptotic cells, is a critical process that maintains tissue homeostasis and immune regulation. Defective efferocytosis is linked to the development of chronic inflammatory conditions, including atherosclerosis, neurological disorders, and autoimmune diseases. Moreover, the interplay between autophagy and efferocytosis is crucial for inflammation control, as autophagy enhances the ability of phagocytic cells. Efficient efferocytosis, in turn, regulates autophagic pathways, fostering a balanced cellular environment. Dysregulation of this balance can contribute to the pathogenesis of various disorders. Phytochemicals, bioactive compounds found in plants, have emerged as promising therapeutic agents owing to their diverse pharmacological properties, including antioxidant, anti-inflammatory, and immunomodulatory effects. This review aims to highlight the pivotal role of phytochemicals in enhancing efferocytosis and autophagy and explore their potential in the prevention and treatment of related disorders. This study examines how phytochemicals influence key aspects of efferocytosis, including phagocytic cell activation, macrophage polarization, and autophagy induction. The therapeutic potential of phytochemicals in atherosclerosis and neurological diseases is highlighted, emphasizing their ability to enhance efferocytosis and autophagy and reduce inflammation. This review also discusses innovative approaches, such as nanoformulations and combination therapies to improve the targeting and bioavailability of phytochemicals. Ultimately, this study inspires further research and clinical applications in phytochemical-mediated efferocytosis enhancement for managing chronic inflammatory and autoimmune conditions.

Similar content being viewed by others
Facts
-
Dysregulation of balance between autophagy and efferocytosis can contribute to the pathogenesis of various disorders.
-
Phytochemicals derived from natural sources can enhance efferocytosis and potentially treat the related conditions.
-
Different phytochemicals, such as flavones, flavonoids, and anthocyanidins have anti-inflammatory effects and can prevent various disorders.
-
EGCG, Baicalin, 6-Gingerol, Apigenin, and Berberine promote efferocytosis and inhibit inflammation.
-
The synergistic effects of combining multiple phytochemicals could lead to enhanced anti-inflammatory and efferocytosis-promoting effects, warranting systematic studies on optimal combinations.
Open Questions
-
1.
What are the specific molecular mechanisms by which different phytochemicals enhance efferocytosis and autophagy in various cell types, and how do these mechanisms differ between phytochemicals?
-
2.
How can the findings regarding phytochemical effects on efferocytosis and autophagy be translated into clinical practice for treating autoimmune and inflammatory disorders, and what specific clinical trials are needed to evaluate their efficacy?
-
3.
What are the potential synergistic effects of combining different phytochemicals on efferocytosis and autophagy, and how might these combinations be optimized for therapeutic use?
-
4.
What are the long-term effects of phytochemical supplementation on efferocytosis and autophagy in chronic inflammatory conditions, and how do these effects impact overall disease progression and patient outcomes?
Introduction
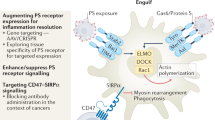
Every day, approximately 0.4% of an adult human’s estimated 37.2 trillion cells die [1]. Maintaining homeostasis in multicellular organisms is a crucial process that relies on cell death, and efficient removal of dying cells. In almost all physiological and pathological situations, a controlled series of signaling events (regulated cell death) triggers cells to participate in their demise [2]. The clearance of dead cells is crucial for maintaining normal bodily functions as well as preventing diseases. Efferocytosis, the process of removing apoptotic cells (ACs) and non-inflammatory phagocytic activity by both professional and non-professional phagocytes, is required for tissue homeostasis during physiological function and restoration of homeostasis after disorders [3, 4] (Fig. 1). Efferocytosis becomes defective in several non-resolving, chronic inflammatory diseases, leading to the accumulation of ACs [5, 6]. In this case, the release of cellular and inflammatory mediators can trigger autoimmune and inflammatory disorders, such as atherosclerosis, autoimmune diseases, and neurodegenerative conditions. The failure to efficiently clear dead cells disrupts tissue homeostasis and promotes pathological inflammation [7, 8]. Abnormal efferocytosis is linked to different autoimmune and inflammatory disorders, such as acute lung injury (ALI, a clinical syndrome characterized by widespread inflammation and increased permeability of the alveolar-capillary barrier) rheumatoid arthritis (RA), asthma, autoimmunity lymphoproliferative syndrome (ALPS), infection, diabetes, systemic lupus erythematosus (SLE), multiple sclerosis (MS), and other inflammatory conditions [8,9,10,11,12,13]. During inflammation, the ability of phagocytes to engulf ACs is inhibited by the generation of reactive oxygen species (ROS) by neutrophils. ROS triggers the activation of RhoA, a GTPase that acts as a negative regulator of efferocytosis, leading to a decrease in the engulfment of ACs by neighboring cells [14,15,16]. Rho GTPases regulate efferocytosis by controlling the actin cytoskeleton, which is essential for phagocyte motility and AC engulfment. In addition, Rac1, a member of the Rho GTPase family, plays a central role in regulating the actin cytoskeleton and signaling pathways involved in efferocytosis [17, 18] (Table 1).
Antioxidants derived from natural sources have significant bioactivities that can be used to prevent or cure diseases related to oxidative stress. These antioxidants work by reducing or scavenging ROS, inhibiting lipid peroxidation, and chelating free metal ions. Moreover, phytochemicals with antioxidant properties increase efferocytosis and have the potential to treat efferocytosis-related conditions [19,20,21]. Numerous studies published over the recent have indicated the antioxidant and anti-inflammatory effects of various phytochemical nutrients, such as flavones, flavonoids, flavanols, isoflavones, and anthocyanidins. Phytochemicals possess bioactive properties that can prevent or treat an extremely wide array of diseases [22,23,24,25,26,27,28].
Phytochemicals are natural compounds found in plants that have been studied for their health benefits and possible therapeutic uses. There are several advantages to using phytochemicals compared with other treatments. These compounds come from natural sources, such as fruits, vegetables, herbs, and spices, which many people prefer because they fit well with a holistic view of health and wellness. Phytochemicals offer a wide range of health benefits due to their different chemical structures. They can act as antioxidants, reduce inflammation, fight microbes, and even help prevent cancer. Additionally, phytochemical treatments have fewer side effects than conventional medications. Foods rich in phytochemicals also provide important nutrients, vitamins, minerals, and dietary fiber, all of which contribute to overall health. However, it’s important to note that the effectiveness of phytochemical treatments are can depend on factors, such as the specific compound, the amount taken, how it’s prepared, individual differences, and the health issue being addressed [29, 30]. The aim of this review was to comprehensively examine the role of phytochemical antioxidants in modulating efferocytosis and their potential therapeutic applications for autoimmune and inflammatory disorders. Furthermore, this review explored the mechanisms by which phytochemicals modulate efferocytosis, including their effects on ROS generation, RhoA activation, and phagocytic clearance. The association between abnormal efferocytosis and various autoimmune and inflammatory conditions. Ultimately, the review consolidated the existing knowledge, identified research gaps, and offered insights into the therapeutic potential of phytochemicals against efferocytosis-related disorders.
Efferocytosis: a key process in immune regulation and tissue homeostasis
The immune response differs significantly between the two primary types of cell death, apoptosis (planned cell death) and necrosis (random cell death). Phagocytosis, the process by which cells engulf and digest cellular debris, is initiated by efferocytosis (the clearance of ACs) without inducing an inflammatory or immune response. Efferocytosis is believed to be anti-inflammatory and promote immune tolerance, meaning that the immune system does not exhibit a strong reaction. Necrosis, on the other hand, happens when cells die prematurely as a result of external causes, resulting in a pro-inflammatory and immunostimulatory response [31,32,33]. The failure to clear ACs can lead to necrotic features in ACs, causing autoimmune disorders over time through the stimulation of pro-inflammatory pathways [11, 34, 35].
Apoptosis occurs daily in several cells, and is part of normal development and tissue maintenance. To prevent the release of cytotoxic materials into the environment, it is important to remove these ACs quickly. Efferocytosis is the process by which cellular corpses are removed [36] (Fig. 1). Since the 1990s, this process has been of interest and involves complicated molecular relationships and a variety of phagocytosis and efferocytosis pathways [37,38,39]. AC clearance is an important process that helps maintain and heal homeostasis after injury. When ACs are not efficiently cleaned, their membrane integrity is compromised, leading to intracellular content leakage and secondary necrosis. Efferocytosis is the rapid removal of ACs from tissues by efferocytes to prevent subsequent necrosis [40].
Efferocytosis involves five steps: attraction of phagocytes to ACs, identification of ACs by phagocytes, internalization of ACs into phagocytes, degradation of ACs, and anti-inflammatory responses [41] (Table 1). The process of efferocytosis requires coordination between three signals: “Find-Me”, “Eat-Me”, and “Don’t Eat-Me”, in order to maintain equilibrium. Various cell types can participate in efferocytosis, including professional phagocytes, such as immature dendritic cells and macrophages, as well as nonprofessional cells, such as epithelial cells, fibroblasts, and endothelial cells [42,43,44] (Fig. 1). Macrophages, as professional phagocytes, play an important role in enabling successful efferocytosis. Generally, the signals recognized as valuable surface markers are found on ACs that facilitate the proper performance of phagocytic processes [39].
Efferocytosis plays a crucial role in maintaining homeostasis in biological systems (Table 1). It is essential for human health because it helps to avoid the negative consequences of cell death, guarantees the integrity of tissues and organs and fosters a healthy immunological response. Efferocytosis is a critical process that maintains tissue homeostasis by clearing ACs, suppressing inflammation, promoting self-tolerance, and activating resolution pathways. The regulation of macrophage polarization plays a key role in the successful execution of efferocytosis.
The failure to effectively clear dead cells results in the buildup of uncleare dead cells. These cells then release pro-inflammatory molecules, which in turn leads to an excessive inflammatory response. This highlights the crucial role of clearing ACs in maintaining homeostasis. Consequently, any changes in the ability of phagocytes, whether professional or non-professional, to engulf ACs can have an impact on tissue homeostasis. This condition can potentially trigger inflammation and/or developmental abnormalities, depending on the specific tissue [3, 13, 45]. Efferocytosis has three critical effects in addition to avoiding additional cell death: it inhibits inflammatory responses, promotes self-tolerance, and activates pathways that help resolve the problem. Efferocytosis promotes the generation of anti-inflammatory and tissue-repair chemicals, whereas deficient efferocytosis can result in excessive inflammation and various diseases [46]. The clearance of ACs necessitates the presence of receptors on the phagocytes that can identify the ligands associated with ACs. Additionally, phagocytes must undergo cytoskeletal reorganization to engulf ACs bound to other cells [41]. The elimination of ACs by macrophages is achieved through the generation of specific molecules produced during the degradation of ACs within the phagolysosome. Furthermore, the fusion of phagosomes with lysosomes is required to degrade ACs contents. When macrophages ingest ACs, they regulate the production of pro-inflammatory cytokines, suppressing their release while increasing the production of molecules that mitigate inflammation and facilitate resolution and repair [47, 48]. These processes are carried out through pathways that are both integrated and mechanistically distinct. AC interaction with the efferocyte receptor T cell immunoglobulin mucin receptor 1 (TIM1), for example, reduces the generation of TNF, CC-chemokine ligand 5 (CCL5), and interleukin (IL)-6 by blocking the activation of NF-κB. AC binding to the efferocyte receptor stabilin 2, on the other hand, causes the synthesis of transforming growth factor beta (TGFβ) [49, 50]. ACs block Toll-like receptor (TLR) as well as type 1 interferon (IFN)-mediated pro-inflammatory signaling pathways by interacting with the MertK and AXL [51]. ACs inhibit the function of IκB kinase (IKK), thereby hindering the activation of TLR4-induced, NF-κB-dependent TNF expression. On the other hand, AXL activation leads to an increase in the levels of Twist-related proteins, which act as transcriptional repressors that suppress TLR4-induced TNF promoter activity. Additionally, these receptors on efferocytes upregulate the expression of the E3 ubiquitin ligase suppressors of cytokine signaling 1 (SOCS1) and SOCS3. The upregulation of SOCS1 and SOCS3 inhibits the signaling of signal transducer and activator of transcription 1 (STAT1) mediated by IFNα, consequently blocking pro-inflammatory gene expression [52,53,54]. Sterols are transported to efferocytes after the degradation of phagolysosomal AC. Once delivered, these sterols activate nuclear sterol receptors (e.g., liver X receptor-alpha (LXRα), and peroxisome proliferator-activated receptor (PPAR)γ, PPARδ). When these receptors are activated, anti-inflammatory cytokines, such as IL-10 and TGFβ are produced. Furthermore, it induces the differentiation of Tregulatory cells and Thelper 2 cells, thereby aiding in the resolution of immune responses [55,56,57]. Moreover, the nuclear receptor corepressor is bound by PPARγ and LXRs, thereby preventing its removal from the promoter sequences of genes encoding specific pro-inflammatory cytokines (e.g. IL-1β and TNF) [58, 59]. Furthermore, the interaction between efferocytes and ACs promotes the generation of specific pro-resolving mediators while reducing the formation of pro-inflammatory leukotrienes. The activation of the AC receptor MertK causes the translocation of lipoxygenase 5 from the nucleus to the cytoplasm, thereby increasing the production of lipoxin A4. These combined activities effectively suppress inflammation and enhance the resolution of inflammation [48, 60].
Any deficiency in this process is linked to several conditions, including inflammatory, autoimmune, and atherosclerosis conditions [5, 11, 61]. When phagocytes, such as macrophages, fail to destroy ACs quickly owing to cell membrane breakdown, they change into secondary necrotic cells (NCs). As a result, the release of cellular and DNA from these NCs into the environment might trigger an inflammatory reaction. The precise control of macrophage polarization is crucial for efficient efferocytosis in both pathological and physiological contexts. Therefore, understanding and regulating this polarization are of significant importance [45]. The activation state and functional characteristics of macrophages, depending on the condition, determine the emergence of two distinct phenotypes: M1 (classically activated) and M2 (alternatively activated) [62, 63]. The polarization of macrophages is influenced by inflammatory parameters. Pro-inflammatory cytokines, such as IL-12, pathogen-associated molecular patterns (PAMPs), TNF-α, and IFN-ϒ, such as lipopolysaccharide (LPS), polarize M1 macrophages. In macrophages, these stimuli efficiently promote the M1 phenotype. The notable actions of M1 macrophages (pro-inflammatory) include the secretion of TNF-α, IL-6, and IL-1, which contribute to inflammatory responses and elevation of type-1 Th1 cell responses. Furthermore, M1 macrophages have tumoricidal properties [63] (Table 3).
Overall, macrophage polarization favors the M1 phenotype for the attack of intracellular infections and enhancement of anticancer effects. Furthermore, M2 macrophages are known anti-inflammatory cells that express high levels of IL-10 and low levels of pro-inflammatory cytokines. These qualities are important in a variety of physiological processes, including wound healing and inflammation control. Inflammatory M1 macrophages are present in the early stages of wound healing. In this respect, M1 macrophages and neutrophils work together to eliminate pathogens and debris. Subsequently, apoptotic neutrophils are absorbed by inflammatory M1 macrophages during the efferocytosis process [63]. M1 macrophages produce nitric oxide (NO), ROS via inducible nitric oxide synthase (iNOS), and pro-inflammatory cytokines, such as IL-1, IL-12, IL-23, and TNF when activated. M2 macrophages (anti-inflammatory), on the other hand, release anti-inflammatory cytokines and growth factors, such as IL-10, IL-4, and TGFβ, as well as angiogenic and pro-fibrotic characteristics [13, 64].
Autophagy and efferocytosis
The interplay between autophagy and efferocytosis is crucial for maintaining cellular homeostasis and preventing inflammation. Autophagy is a highly conserved cellular process that serves as a crucial pathway for the clearance of damaged organelles and misfolded proteins. Importantly, autophagy has been shown to play a key role in the efficient clearance of ACs by activated inflammatory cells, such as neutrophils and macrophages [65, 66]. Autophagy is integral to macrophage function and efferocytosis. By regulating polarization, cytokine secretion, metabolism, and phagocytosis, autophagy not only supports macrophage survival and function but also promotes the resolution of inflammation and maintenance of tissue homeostasis through the effective clearance of ACs [67] (Fig. 2).
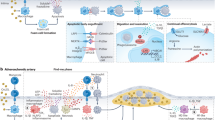
A The entry of monocytes into the intima. Monocytes migrate from the bloodstream into the innermost layer of the artery wall, called the intima. Once in the intima, the monocytes differentiate into macrophages. B illustrates the formation of foam cells. In intima, macrophages start to accumulate modified lipoproteins, such as oxidized low-density lipoprotein (Ox-LDL), leading to the formation of foam cells. The accumulation of these foam cells, along with other cellular debris and extracellular matrix, leads to the formation of an atherosclerotic plaque within the intima of the artery wall. In this case, this cell can modulate by normal autophagy via phytochemicals. C, D shows the effect of drug-eluting stents containing phytochemicals, leading to an increase in autophagy. E This illustrates that improved autophagy induced by phytochemicals can promote efferocytosis, and conversely, increased efferocytosis can enhance autophagy. Ultimately, this balance fosters an anti-inflammatory environment, reduces atherosclerotic plaque development, and improves plaque stability. Thus, by incorporating phytochemicals, the drug-eluting stents could potentially provide added therapeutic benefits beyond the currently used pharmaceutical drugs, leading to improved long-term outcomes for patients with atherosclerotic cardiovascular disease. Additional details about the intricate factors and pathways connecting autophagy and efferocytosis, along with specific phytochemicals that can regulate this balance, can be found in Table 2.
Autophagy significantly influences the polarization of macrophages into various functional states, including M1 and M2 types [68, 69]. M2 macrophages exhibit greater efficiency in efferocytosis, facilitating tissue repair and the resolution of inflammation. Through the regulation of polarization, autophagy enhances the capacity of macrophages to recognize and clear ACs. Additionally, it affects the secretion of various cytokines that regulate immune responses. By promoting the release of anti-inflammatory cytokines, autophagy fosters a favorable environment for efferocytosis, thereby enhancing the ability of macrophages to engulf and digest ACs [69, 70].
IL-10 inhibits the mechanistic target of rapamycin (mTOR) activation through the signal transducer and activator of transcription 3- DNA damage-inducible transcript 4 (STAT3-DDIT4) pathway, which promotes mitophagy and oxidative phosphorylation while suppressing aerobic glycolysis. In contrast, the mTOR signaling pathway facilitates the shift toward glycolysis necessary for M1 macrophages by increasing HIF-1 expression [70]. mTOR is a key regulator of autophagy, that suppresses the autophagy process. When mTOR is active, it inhibits autophagy, thereby preventing the degradation of cellular components. This regulation helps maintain cellular homeostasis under nutrient-rich conditions, but it can hinder autophagy during periods of stress or nutrient deprivation [71, 72]. Adenosine Monophosphate-activated protein kinase (AMPK) also plays a role in regulating M2 polarization of macrophages. By activating autophagy and promoting metabolic reprogramming, AMPK supports the transition of macrophages toward the M2 phenotype, which is associated with anti-inflammatory responses and tissue repair. This regulation enhances the ability of macrophages to clear ACs and secrete anti-inflammatory cytokines, contributing to the resolution of inflammation and overall tissue homeostasis [73]. Thus, autophagy plays a vital role in cellular metabolism by providing energy and recycling cellular components [70]. This metabolic flexibility allows macrophages to adapt to different functional states, thereby supporting their efficiency in performing efferocytosis, particularly under stress conditions. Furthermore, autophagy enhances the machinery responsible for recognizing and engulfing ACs, facilitating the phagocytosis process and ensuring effective clearance of dead cells while preventing secondary necrosis [67].
Numerous studies have highlighted the close association between impaired autophagy and decreased efferocytosis, or the phagocytic clearance of ACs, in the context of various inflammatory disorders, such as atherosclerosis, inflammatory bowel disease (IBD), and neurodegenerative diseases [65, 66, 74,75,76,77,78,79] (Table 2).
Enhancing autophagy may significantly improve AC recognition and clearance [66, 78]. Inhibiting autophagy by silencing autophagy-related gene 5 (ATG5) or other autophagy-related proteins increases apoptosis and oxidative stress mediated by NADPH oxidase. ATG5 is involved in the formation of autophagosome, cellular structures that encapsulate and degrade damaged or unnecessary cellular components. Additionally, this inhibition reduces the identification of ACs in efferocytes in macrophages lacking ATG5 [78]. In the context of microglia, the primary phagocytes in the brain, autophagy facilitates the efficient removal of dead cells, thereby preventing necrosis and subsequent inflammatory damage to healthy tissue [77].
When autophagy is disrupted, either in laboratory settings or in living organisms, there is a concomitant increase in apoptosis and a decreased ability of efferocytic phagocytes to recognize and engulf deceased cells [67, 76, 78]. This indicates that autophagy can trigger two distinct protective responses: promoting cell survival under normal conditions and facilitating efficient removal of cells by neighboring phagocytes when repair is not possible.
During the onset of atherosclerosis, when macrophages consume oxidized LDL (Ox-LDL) or other modified lipoproteins, they transform into foam cells. The buildup of these foam cells leads to the development of atherosclerotic plaques. Autophagy in macrophages helps prevent foam cell formation by decreasing the ingestion of Ox-LDL and enhancing both efferocytosis and cholesterol efflux. Efficient cholesterol efflux is important for maintaining macrophage function and their ability to effectively perform efferocytosis. [79, 80]. Moreover, defects in autophagy can lead to impaired efferocytosis, resulting in the accumulation of ACs and increased inflammation, as seen in conditions, such as stroke and neurodegeneration [74, 75, 77]. In therapeutic contexts, strategies aimed at boosting autophagy may enhance the ability of immune cells, like macrophages, to clear ACs effectively. This approach could be particularly beneficial in diseases characterized by defective efferocytosis, such as atherosclerosis and IBD, where improved clearance of dead cells can mitigate inflammation and promote tissue repair [66, 78]. Therefore, the interplay between autophagy and efferocytosis has emerged as an important area of research for understanding the regulation of inflammation and tissue homeostasis.
Phytochemicals: nature’s toolbox for efferocytosis enhancement
Phytochemical diversity and sources
Phytochemicals, naturally occurring compounds found in plants, have shown great potential for enhancing efferocytosis, the process of clearing ACs. These diverse plant-derived molecules possess various bioactive properties, including antioxidant, anti-inflammatory, and antimicrobial activities [29, 30]. Phytochemical treatments may offer advantages over conventional medications, such as a more natural and holistic approach, broad health benefits, potential synergistic effects, and fewer side effects [29, 30]. The mechanisms by which various phytochemicals modulate the key factors and pathways related to efferocytosis in various disease conditions are summarized in the Table 2. This table provides a comprehensive overview of the potential of phytochemicals to enhance efferocytosis and its therapeutic implications. By consolidating the existing knowledge on phytochemical-mediated modulation of efferocytosis, this chapter aims to offer valuable insights for future research and clinical applications, particularly in the context of chronic inflammatory and autoimmune disorders.
Conventional treatments for efferocytosis-related disorders often have limited efficacy and are associated with adverse side effects. For example, in the context of atherosclerosis, standard therapies, such as statins and anti-inflammatory drugs have had a modest impact on disease progression due to their inability to fully restore defective efferocytosis [81]. In this line, phytochemicals (alone or in combination with other treatments) have demonstrated the ability to modulate key pathways and cellular processes involved in efferocytosis, such as regulating phagocytic activity, macrophage polarization, and autophagy. This multifaceted approach to enhancing efferocytosis and resolving inflammation is an area of growing interest [30, 82,83,84,85,86,87,88,89,90].
Mechanisms of phytochemical-mediated enhancement of efferocytosis and autophagy
The different mechanisms through which various phytochemicals mediate the factors and pathways related to efferocytosis in different conditions/disorders are summarized in Table 2. As shown in Table, there is great potential for enhancing the efferocytosis properties of phytochemicals in disorders.
Efferocytosis and autophagy dysregulation under pathophysiological conditions and its implications
Modulation of efferocytosis and autophagy by phytochemicals in atherosclerosis
Atherosclerosis, the main cause of cardiovascular and cerebrovascular illnesses, is also a major contributor to other ailments, such as cerebral infarction and chronic cerebral insufficiency. It is commonly regarded as a disease characterized by cholesterol accumulation and inflammation caused by lipids [4]. This accumulation is believed to release pro-inflammatory cytokines and formation of cholesterol microcrystals within cells, activating the inflammasome [91]. Macrophages filled with cholesterol, known as “foam cells,” are prone to death, releasing their contents and potentially impairing efferocytosis, which can worsen inflammation [92] (Fig. 2).
Efferocytosis, the clearance of ACs, is crucial for resolving inflammation in atherosclerosis. This process helps prevent the buildup of ACs and the release of inflammatory substances [93,94,95]. Failing efferocytosis can lead to necrosis and the release of pro-inflammatory factors, creating a detrimental cycle that exacerbates atherosclerosis. Factors like TNF-α play a significant role in this process by promoting inflammation and inhibiting pathways that facilitate AC clearance [96, 97].
The ingestion of extracellular components including lipids, carbohydrates, proteins, and nucleic acids occurs as a result of AC engulfment. To manage the increased metabolic burden, macrophages must engage in degradation and efflux pathways, which are critical for reducing inflammation and promoting tissue repair [98,99,100]. Experiments in animals lacking TIM-4, MertK, milk fat globule-EGF factor 8 protein (MFGE8), or protein S (ProS) revealed reduced AC clearance, increased inflammation, and worsened atherosclerosis. As defective efferocytosis accelerates macrophage apoptosis, the necrotic core enriched with lipids expands as atherosclerotic plaque progression occurs [101,102,103,104].
Several variables affect vulnerable plaques and acute coronary artery syndrome, including decreased fibrous cap formation, the presence of high inflammatory cytokine levels, intimal cell death, and the growth of the lipid-laden necrotic core. Furthermore, the lack of efferocytosis signals hinders the following pathways involved in the reverse transfer of intracellular cholesterol, allowing foam cells to form and the initiation of atherosclerosis to begin (Fig. 2). Notably, C1q protects against atherosclerosis by enhancing macrophage survival and improving the activity of foam cells in the early stages [105,106,107]. The interplay between autophagy and efferocytosis is particularly important in macrophages within atherosclerotic plaques. Autophagy enhances efferocytosis efficiency by providing the necessary resources for phagocytes to effectively engulf and process ACs. For instance, autophagy-related proteins can promote the formation of phagophores that facilitate the engulfment of apoptotic debris. Additionally, autophagy can regulate the expression of surface receptors involved in efferocytosis, thereby improving AC recognition and clearance. Conversely, impaired autophagy can lead to defective efferocytosis, resulting in the accumulation of ACs and a heightened inflammatory response. This dysregulation contributes to the progression of atherosclerosis and increases the risk of cardiovascular events [65, 76, 78, 79].
Experimental findings have provided evidence for the regulatory function of extracellular signal-regulated kinase 5 (ERK5) during macrophage efferocytosis. ERK5, a member of the mitogen-activated protein kinase (MAPK) family, is essential for maintaining macrophage phagocytosis and slowing the development of atherosclerosis [94]. In LDLR−/− mice (a genetically modified mouse model that is deficient in the LDL receptor gene and is commonly used in atherosclerosis research), the elimination of the ERK5 gene intensifies atherosclerosis and inhibits the expression of proteins associated with efferocytosis. Moreover, the use of an ERK5 inhibitor reduces the phagocytic activity of RAW264.7 cells (a macrophage cell line) in laboratory settings. Consequently, it can be inferred that the regulation of macrophage efferocytosis via ERK5 can be beneficial for combating atherosclerosis [108]. According to the data, there is a rising emphasis on treatment techniques that directly target the atherosclerotic macrophage phenotype in order to enhance disease regression. Various compounds originating from natural sources may provide a great possibility in this area (Table 2). These molecules possess multiple functions, including antioxidant properties, the ability to lower lipid levels, and the capacity to modulate cell signaling. As a result, they are likely to effectively counteract the development of lesions and, more importantly, in regulating the inflammatory response of macrophages.
Anti-atherogenic compounds found in nature, such as phytochemicals, have the ability to modify the pharmacological targets of trained immunity. Epigallocatechin gallate (EGCG) has been shown in studies to have antibacterial, antiviral, antioxidant, anti-vascular proliferation, anti-arteriosclerosis, anti-thrombosis, anti-inflammatory, and anti-tumor properties [109] (Table 2). EGCG also demonstrated inhibitory effects on the overexpression of type A scavenger receptor (SR-A) induced by Ox-LDL in the identical cell line, resulting in a reduction in the absorption of Ox-LDL and the formation of foam cells [110]. Curcumin has been demonstrated to regulate inflammation in both in vitro and in vivo studies. This characteristic renders curcumin a highly efficient remedy for inflammatory conditions, such as rheumatoid arthritis [111, 112]. The inhibitory effect of curcumin on the activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome in macrophages has been established through numerous scientific investigations. This inhibitory effect is critical for preventing HFD-induced insulin resistance and the activation of the LPS-priming and NLRP3 inflammasome pathways in macrophages [113]. When it comes to controlling M1/M2 macrophages, curcumin can increase the release of M2 markers (e.g., macrophage mannose receptor (MMR), arginase 1, PPAR-, IL-4, and/or IL-13). Curcumin causes a shift in M1 macrophages towards an M2 phenotype in autoimmune myocarditis (EAM) and hyaline membrane disease in vivo [114]. A prior investigation revealed that curcumin significantly decreased the accumulation of lipids induced by Ox-LDL in J774.A1 macrophages. This reduction occurred by diminishing Ox-LDL uptake through SR-A and by enhancing cholesterol efflux via ATP binding cassette transporter A1 (ABCA1). Notably, curcumin did significantly affect other cholesterol transporters [115]. Among its multiple mechanisms, resveratrol plays a crucial role in protecting against atherosclerosis by regulating the differentiation of monocytes and macrophages. Furthermore, it inhibits the oxidation of LDL, enhances the protection of endothelial cells, reduces the levels of trimethylamine N-oxide (TMAO) through the modulation of gut flora, and hinders the proliferation and migration of vascular smooth muscle cells (VSMCs) [116]. Resveratrol effectively inhibited the formation of “foam cells” in RAW264.7 cells stimulated by LPS. This inhibition was achieved by reducing ROS production and MCP1 expression through the activation of the Akt/Forkhead Box O3a (Foxo3a) and AMPK/sirtuin 1 (Sirt1) pathways. Notably, the involvement of NADPH oxidase 1 (Nox1) was crucial for the aforementioned pathways to exert their effects [117, 118]. Furthermore, quercetin, a naturally occurring compound, has the ability to mitigate the development of atherosclerosis by modulating the occurrence of endothelial cellular senescence induced by Ox-LDL [119].
The potential of phytochemical-rich compounds in treating atherosclerosis by focusing on their ability to induce effective autophagy (not excessive) and efferocytosis. Specifically, the neuroprotective effects of berberine, 6-gingerol, and celosins through autophagy induction are evident in important disorders [74, 75, 120, 121]. Berberine activates autophagy through AMPK signaling, reducing inflammation induced by Ox-LDL, and improving the bacterial killing ability of macrophages [122, 123]. 6-gingerol exhibits neuroprotective effects by inhibiting inflammation, apoptosis, and oxidative stress through autophagy activation. It protects against brain ischemia/reperfusion damage and apoptosis caused by 6-hydroxydopamine [74]. Celosin inhibits atherosclerosis and promotes autophagy flow. In atherosclerosis models, celosins reduced the plaque prevalence and increased the number of autophagy bodies. In-vitro experiments demonstrated reduced phagocytosis and foam cell formation, along with increased of autophagy-specific protein expression [120].
Additionally, Sirt1 can enhance the clearance of Ox-LDL-induced ACs by macrophages through autophagy activation. Stimulating Sirt1 with a low concentration of resveratrol increased the expression of Sirt1 and autophagy markers, leading to improved efferocytosis. Conversely, inhibiting Sirt1 with nicotinamide decreased efferocytosis [76]. Further research in this area could provide valuable insights into developing more effective treatments for atherosclerosis by improving efferocytosis through autophagy modulation.
Furthermore, curcumin, ginger, and magnolol were the three natural medications studied. To modulating the medication release, a biodegradable polymer with varying molecular weights was utilized as a carrier for the medicines. In-vitro experiments revealed that all three medicines exhibited a burst release followed by continuous release lasting up to 38 days. Ginger exhibited the best compatibility compared with magnolol and curcumin, which had less ideal compatibility and fell into the mild-to-severe blood toxicity category. Thus, ginger has potential as a drug-coating material for drug-eluting stents, but more in-vitro testing is needed to validate its suitability [124].
The modulation of efferocytosis and autophagy by phytochemicals in brain disorders
Proper elimination of ACs is critical for maintaining homeostasis of the central nervous system (CNS) and allowing its recovery after damage. Efferocytosis is critical for avoiding inflammation and other pathological problems in cases of physiological AC mortality. When ACs are not cleaned properly, the integrity of the AC membrane is compromised, leading to the release of intracellular contents and eventual secondary necrosis. Microglia, which are principally responsible for efferocytosis, are critical for the removal of ACs from the CNS [40, 125, 126]. A variety of cell types, including neural crest cells, neural stem cells, and astrocytes, can function as efferocytes. However, these cells are often less effective than microglia. When the blood-brain or blood-spinal cord barrier is disrupted during trauma, monocytes originating from the blood penetrate the CNS. Under normal conditions, seeing astrocytes in the CNS is difficult since they are rapidly consumed by microglia due to the very effective process of efferocytosis [13, 127]. Nonetheless, because efferocytosis is controlled by a mix of age, inflammation, and certain genetic risk factors, it can contribute to the development of CNS disorders. Several studies have highlighted the importance of dysregulated microglial/macrophage activity in the etiology of numerous clinical disorders, such as Alzheimer’s disease (AD), stroke, amyotrophic lateral sclerosis (ALS), and Parkinson’s disease (PD). Poor efferocytosis results in inadequate AC elimination [128, 129]. As a result, the excessive buildup of ACs overwhelms the efferocytosis ability of microglia/macrophages, generating a negative feedback loop. Secondary necrosis of uncleared ACs aggravates this situation by causing the production of pro-inflammatory factors, intracellular molecules, and danger-related molecular patterns (DAMPs), which increase the level of damage [130, 131]. Hence, efferocytosis is significantly important for promoting neuronal survival and facilitating axonal regeneration. By inhibiting the pro-inflammatory release as well as antigenic autoimmune constituents, efferocytosis contributes to the clearance of apoptotic debris. The remarkable therapeutic potential of this process in the context of neurodegenerative diseases necessitates additional preclinical development to fully exploit its benefits [46].
Dietary phytochemicals can influence numerous aspects of neuroinflammation. The neuroprotective effects of these compounds have been observed to depend on both the dosage and duration of exposure, as indicated by several studies. The neuroprotective effects of phytochemicals are believed to be mediated through various mechanisms. While the antioxidant properties of dietary phytochemicals have been extensively studied, it has been demonstrated that these compounds cannot only scavenge free radicals in the brain but also specifically target stress-activated signaling pathways that play crucial roles in neuroprotection. Numerous studies conducted in laboratory settings as well as in living organisms have provided significant evidence indicating that a wide range of phytochemicals have the ability to influence the expression of various genes that encode proteins promoting cell survival. These proteins include antioxidant enzymes, neurotrophic factors, and anti-apoptotic factors [132,133,134].
Curcumin at a concentration of 20 μM has a significant effect on the microglial transcriptome. This effect results in the development of an anti-inflammatory and neuroprotective phenotype in LPS-activated microglia [135]. Curcumin, when present at concentrations ranging from 5 to 25 μM, exerts its neuroprotective effects through the activation of the Akt/nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Nrf2 is a transcription factor that plays a crucial role in the cellular response to oxidative stress and the regulation of genes involved in antioxidant and cytoprotective processes. Thus, Nrf2 plays a critical role in mediating the neuroprotective effects of curcumin against oxidative damage [136, 137]. The neuroprotective properties of curcumin are also attributed to its ability to modulate Sirt1. Recent findings suggest that Sirt1 signaling activation is linked to the neuroprotective effects of curcumin. Moreover, administering curcumin as a pre-treatment (at a dosage of 50 mg/kg) has been shown to reduce apoptosis, inflammation, and mitochondrial dysfunction in ischemic brain injury in vivo [138]. Curcumin, at concentrations of 5–10 μM, has demonstrated efficacy in stimulating FoxO3a activity within monocytes/macrophages. Hence, curcumin may have an anti-oxidative effect on the inflammatory cells of the vascular system [139].
6-gingerol derived from Zingiber officinale (ginger) has demonstrates significant effects on related disorders through modulation of autophagy and efferocytosis. In the context of cerebral ischemia/reperfusion injury, studies on adult male Sprague-Dawley rats have shown that the protective effects of 6-gingerol are mediated through its ability to enhance autophagy and facilitate the clearance of apoptotic neuronal cells [74, 75]. 6-gingerol inhibits the NLRP3 inflammasome and apoptosis by promoting the dissociation of transient receptor potential vanilloid 1 (TRPV1) from Fas-associated factor 1 (FAF1), which leads to the activation of autophagy. This process not only reduces cerebral infarct volume but also improves brain edema and neurological scores, effectively reversing brain histomorphological damage, 6-gingerol upregulates autophagy by reducing inflammation derived from the NLRP3 inflammasome and neuronal apoptosis, thereby enhancing cellular survival and function [74, 75].
The modulation of efferocytosis by phytochemicals in respiratory disorders (e.g., COPD and cystic fibrosis)
In lung inflammatory diseases, the rate of programmed cell death (apoptosis) is elevated, or the removal of these dead cells is impaired, resulting in cell accumulation [140]. This pathological process is primarily caused by the ineffective clearance of ACs by airway macrophages, a process known as efferocytosis [9]. Millions of people worldwide suffer from the degenerative lung disease known as chronic obstructive pulmonary disease (COPD). Oxidative stress and inflammation resulting from exposure to cigarette smoke or other environmental dangers play a significant role in the development of COPD [141]. Increased apoptosis and impaired clearance of ACs through a process called efferocytosis, contribute to chronic inflammation and tissue damage in patients with COPD [142].
Cystic fibrosis (CF) is caused by a mutation in the CF transmembrane conductance regulator (CFTR) gene. This genetic defect is characterized by significant airway inflammation and the accumulation of apoptotic (dying) cells [143]. Efferocytosis, the process of phagocytosis (engulfment) of ACs, is a crucial regulator of inflammation. Efferocytosis prevents the progression of apoptosis to necrosis (cell death) and actively suppresses the release of various pro-inflammatory mediators, such as IL-8 [144]. CFTR deficiency disrupts efferocytosis in airway epithelial cells, primarily due to increased expression of RhoA, a negative regulator, rather than altered binding or receptor expression [143]. Inhibiting RhoA or its downstream Rho kinase can restore efferocytosis in these cells. Additionally, an amiloride-sensitive ion channel is involved, as amiloride can improve phagocytic function in CFTR-deficient cells. This impaired efferocytosis leads to pro-inflammatory effects, with ACs boosting IL-8 release in CFTR-deficient cells but not in those with normal CFTR [145].
The modulation of efferocytosis by phytochemicals in autoimmune-related disorders
Defective efferocytosis has emerged as a common pathogenic mechanism contributing to the chronic inflammation and tissue damage observed in autoimmune disorders, such as MS, T2D, and Crohn’s disease [146]. In an autoimmune encephalomyelitis (EAE) model of MS, inhibition of 12/15-lipoxygenase (12/5-LOX) by baicalein increased expression of PPARβ/δ in microglia. This was associated with reduced microglia activation, suppressed phagocytosis, and decreased production of pro-inflammatory cytokines and chemokines in the CNS. The modulation of efferocytosis and inflammatory pathways in microglia by 12/15-lipoxygenase inhibition attenuated the clinical severity of EAE, suggesting therapeutic potential in MS [147]. Moreover, defective efferocytosis has been linked to key pathological processes in T2DM, including pancreatic islet β cell destruction, skeletal muscle dysfunction, adipose tissue inflammation, and liver metabolism abnormalities [148]. The failure to effectively clear ACs leads to their accumulation and secondary necrosis, releasing of pro-inflammatory factors that contribute to the glucose homeostasis imbalance. Impaired efferocytosis is considered an initial node in development and progression of T2DM and its complications and is therefore a potential therapeutic target [148]. In a chronic mouse model of intestinal inflammation (SAMP/Yit), mesenchymal stem cell (MSC) therapy promoted mucosal healing and immunomodulation. The long-term therapeutic effects of MSCs were partially mediated by macrophage efferocytosis of apoptotic MSCs, leading to anti-inflammatory reprogramming of macrophages. This highlights the importance of efferocytosis in regulating the inflammatory response and promoting tissue repair in the context of chronic intestinal inflammation, as seen in Crohn’s disease [147]. Thus, modulating efferocytosis pathways represents a promising therapeutic approach for these autoimmune-related disorders by several phytochemicals, such as curcumin, crocin, PCA, and baicalein [146, 147, 149,150,151] (for more details see Table 3).
Clinical trials targeting efferocytosis-related factors/pathways
Clinical studies investigating phytochemicals in efferocytosis-related pathologies
In 2020, a randomized clinical trial was conducted to examine the safety of quercetin supplementation in patients with COPD. The researchers enrolled individuals with varying degrees of lung disease. The results confirmed that there were no severe adverse events associated with the administration of the study drug, as determined by blood tests. However, it is worth noting that one patient reported experiencing mild gastro-oesophageal reflux disease (GERD) in both the quercetin and placebo groups. Overall, the study showed significant changes in blood profile, lung function, and the COPD assessment questionnaire indicated that quercetin was well-tolerated up to a dose of 2000 mg/day (NCT01708278) [152] (Table 3).
Furthermore, another randomized and double-blind clinical trial was conducted to evaluate the effectiveness of quercetin in the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-COV2) patients. Although the trial recognizes the need for further understanding of quercetin, its antioxidant, anti-inflammatory, and antihistamine characteristics have been observed in numerous in vitro and animal investigations (NCT04377789) (Table 3).
Defects in the gene that encodes the CFTR have been identified as the cause of CF, predominantly affecting the pulmonary and digestive systems and resulting in early mortality owing to gradual deterioration in pulmonary function [144, 145]. In vitro studies have shown that quercetin can activate CFTR. The Nasal Potential Difference (NPD) test, which measures voltage across the nasal membrane, is an important biomarker of CFTR function in vivo [153]. In vitro and in vivo studies have revealed that quercetin activates CFTR in addition to the effects of existing NPD reagents [154, 155]. Furthermore, it has been discovered to activate rescued mutant CFTR in vitro, namely the ∆F508 CFTR mutation, which is the most prevalent cause of CF. The preliminary data suggests that the administration of quercetin may improve defective activation of the ΔF508 CFTR mutation, which is a common CF-causing mutation in which the CFTR protein is present on the cell surface. Therefore, the use of quercetin in the NPD protocol is likely to enhance the detection of the ΔF508 CFTR present at the cell surface.
This potential improvement in the detection of surface-localized ΔF508 CFTR represents a possible means to identify new candidates that may benefit from systemic CFTR potentiator therapy. Potentiator therapies aim to enhance the activity of CFTR channels, which can be beneficial for individuals with CF who have CFTR mutations that result in protein localization on the cell surface(NCT01348204) (Table 3).
Sarcoidosis, an inflammatory disease, is believed to be caused by oxidative stress and inadequate antioxidant levels. Oxidative stress, defined as an imbalance between ROS generation and the body’s ability to protect against them, plays an important role in the development of sarcoidosis. In individuals with sarcoidosis, the levels of antioxidants, which are crucial for defending against ROS, are lower. Consequently, antioxidant therapy aimed at strengthening the diminished antioxidant defense against sarcoidosis could be beneficial. Additionally, since ROS can initiate and mediate inflammation, antioxidant therapy may also alleviate the heightened inflammation observed in sarcoidosis. A clinical trial was conducted to evaluate the effects of a double-blind intervention on two groups of non-smoking, untreated sarcoidosis patients, according to this information. Malondialdehyde levels in plasma were examined as a sign of oxidative damage, while tumor necrosis factor (TNF)/IL-10 and IL-8/IL-10 ratios in plasma were assessed as indicators of inflammation. Quercetin supplementation was shown to be beneficial in sarcoidosis because it is a flavonoid with anti-inflammatory and anti-oxidative effects, making it a viable antioxidant treatment (NCT00402623) [156] (Table 3).
Another group of scientists investigated the benefits of a combination of dietary supplements, specifically quercetin, curcumin, and vitamin D3, as an extra remedy for the initial mild indications of COVID-19 infection in patients who are not hospitalized. The results revealed that these dietary supplements possessed potent antioxidant, anti-inflammatory/immunomodulatory, and extensive antiviral properties (NCT05130671) [157] (Table 3).
A clinical trial was conducted to examine the effects of curcumin in Crohn’s disease. This goal of the study was to determine if the combination of curcumin and thiopurine could effectively prevent the recurrence of Crohn’s disease following surgery (NCT02255370). This study revealed that oral curcumin was not more effective than placebo in preventing he of Crohn’s disease recurrence after surgery [149] (Table 3).
The efficacy of curcumin, a biologically active phytochemical present in turmeric, has been evaluated for the maintenance of remission in patients with ulcerative colitis (UC) [158, 159]. A randomized, double-blind, multicenter trial was conducted to specifically assess the potential benefits of curcumin as a maintenance therapy for patients with quiescent UC [158]. The trial enrolled 89 patients with quiescent UC, with 45 receiving curcumin (2 g/day) in addition to their standard treatment of sulfasalazine or mesalamine, and 44 receiving placebo plus the standard medications [158]. The study found that significantly fewer patients relapsed in the curcumin group than in the placebo group at the end of the 6-month treatment period [158]. Additionally, the use of curcumin was associated with improvements in the clinical activity index (CAI) and endoscopic index (EI), indicating a suppression of the morbidity associated with UC [158]. These findings were supported by a systematic review [159]. The review confirmed the beneficial effects of curcumin, reporting that 4% of patients in the curcumin group experienced relapse after 6 months, compared with 18% in the placebo group [159]. At 12-month follow-up, the relapse rates were 22% and 32% in the curcumin and placebo groups, respectively [159]. Consistent with the original trial, the systematic review also found that curcumin significantly improved the CAI and EI at 6 months compared with placebo [159].
Future perspectives: expanding the frontiers of modulating efferocytosis by phytochemicals
Emerging technologies and approaches for targeted efferocytosis modulation
Synergistic interactions of phytochemical combinations
The potential to understand how phytochemical combination interact holds great promise for maximizing health benefits and preventing chronic diseases. Understanding these pathways is important for maximizing synergistic effects while avoiding antagonistic effects in daily diets and phytochemical-based therapies for chronic disorders [160]. In a specific study, the combination of curcumin and piperine exerted synergistic effects on pain-like behaviors without any potential side effects on the CNS in mouse models. These findings suggest that this combination could be a safe and effective strategy for managing pain [161]. Other studies have also highlighted the immunomodulatory effects of phytochemical combinations, such as the synergistic enhancement of macrophage-mediated immune responses through the combination of vegetable soup and beta-glucan [162]. Another study showed increased phagocytic activity of macrophages through the synergistic actions of Caulerpa racemosa and Eleutherine americana extracts [163]. These findings offer valuable insights into the development of functional foods and phytochemical-based treatments for chronic diseases.
Nanotechnology-enabled delivery systems for phytochemicals in efferocytosis modulation
Using nano-sized phytochemicals and spray technology for sublingual absorption can potentially improve the uptake and bioavailability of phytochemicals [164,165,166]. Nano-sized particles can enhance the surface area and penetration of the compounds, allowing for more efficient absorption through the sublingual mucosa. Sublingual administration involves placing the spray under the tongue, where it is directly absorbed into the bloodstream, bypassing the digestive system. This can accelerate the onset of action and avoid potential degradation or loss of efficacy in the gastrointestinal tract. However, it is important to note that the effectiveness and safety of nano-sized phytochemical sprays can vary depending on various factors, such as the specific formulation, dosage, and individual response [164, 165].
The combination of phytochemicals and nanotechnology has shown enormous potential for the long-term development and manufacture of nanotechnology-enabled goods. In one study, an aqueous extract of Olax nana Wall. ex Benth. was used to create biogenic silver and gold nanoparticles with biocompatibility and potential biomedical applications, such as anticancer, antibacterial, antileishmanial, antinociceptive, enzyme inhibition, and anti-inflammatory properties [167]. Similarly, ginseng-derived exosome-like nanoparticles (GENs) have been demonstrated to be effective in the treatment of colitis via modulating the intestinal microbiota and immune surrounding environment [168]. Curcumin-silver nanoparticles (Cur-AgNPs) produced from the phytochemical curcumin are efficient against Gram-positive and Gram-negative bacteria, while being less harmful to human keratinocytes [169]. Finally, liquid crystalline nanoparticles (LCNs) encapsulating berberine, a phytochemical with potent anti-inflammatory and antioxidant properties, showed potent anti-inflammatory and antioxidant activities in vitro in LPS-induced mouse RAW264.7 macrophages [170]. Elaeagnus angustifolia leaf extracts were used as a non-toxic source of reducing and stabilizing chemicals to create zinc oxide nanoparticles (ZnONPs). The ZnONPs demonstrated a variety of biological uses and were proven to be biocompatible when tested with human erythrocytes and macrophages [171].
A new treatment method for osteoarthritis that involves repolarizing synovial macrophages from the M1 to M2 phenotype employs apoptotic chondrocyte membrane-coated, quercetin-loaded metalorganic framework nanoparticles. These nanoparticles mimic efferocytosis, are easily phagocytosed by synovial macrophages, promoting M2 polarization and inhibiting chondrocyte apoptosis [172, 173]. These studies demonstrate the potential of combining nanotechnology with phytochemicals for various biomedical applications.
Conclusion
This comprehensive review explored the potential of phytochemicals in modulating efferocytosis, the critical process of clearing ACs, and its implications for the prevention and treatment of inflammatory and autoimmune disorders. This review highlighted the central role of efferocytosis in maintaining immune regulation and tissue homeostasis. Dysregulation of this process is closely linked to the pathogenesis of various chronic inflammatory conditions, such as atherosclerosis, neurodegenerative diseases, and autoimmune disorders. By consolidating the current understanding of the mechanisms by which phytochemicals enhance efferocytosis, this review offers valuable insights for future research and clinical applications. Exploring phytochemicals as enhancers of efferocytosis, inflammation resolution, and wound healing presents new strategies for immune regulation and tissue balance. Defective efferocytosis is linked to chronic inflammatory and autoimmune disorders, emphasizing the need for therapeutic interventions. Phytochemical antioxidants from dietary sources show promise for increasing efferocytosis and autophagy, and may be effective against related conditions. They possess diverse bioactive properties and can reduce ROS, promote efficient removal of ACs, reduce inflammation, and induce autophagy. Understanding the potential of phytochemicals in efferocytosis-related disorders provides valuable insights for future research and clinical applications. Particularly for chronic inflammatory diseases like atherosclerosis, phytochemical-based interventions may offer new treatment avenues. Moreover, phytochemicals have neuroprotective properties and can influence gene expression, promoting cell survival. However, it is important to note that the effectiveness of phytochemical treatments can vary, and further research, including clinical trials and preclinical studies, is needed to optimize their use in therapeutic interventions. Further studies are recommended to fully elucidate the precise mechanisms of action and optimize the use of phytochemicals in therapeutic interventions, paving the way for their effective integration into clinical practice.
In conclusion, this review underscores the significant potential of phytochemicals as enhancers of efferocytosis and autophagy and modulators of inflammation resolution, offering new avenues for the management of chronic inflammatory and autoimmune disorders. Continued research in this field is promising for the development of effective, natural therapies that improve tissue homeostasis and overall patient outcomes.
References
Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, et al. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40:463–71.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541.
Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015;16:907–17.
Back M, Yurdagul A Jr, Tabas I, Oorni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019;16:389–406. https://doi.org/10.1038/s41569-019-0169-2
Yurdagul JrA, Doran AC, Cai B, Fredman G, Tabas IA. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front. Cardiovascular Med. 2018;4:86.
Han M, Ryu G, Shin SA, An J, Kim H, Park D, et al. Physiological Roles of Apoptotic Cell Clearance: Beyond Immune Functions. Life (Basel, Switzerland). 2021;11. https://doi.org/10.3390/life11111141.
Kawano M, Nagata S. Efferocytosis and autoimmune disease. Int. Immunol. 2018;30:551–8.
Szondy Z, Garabuczi É, Joós G, Tsay GJ, Sarang Z. Impaired clearance of apoptotic cells in chronic inflammatory diseases: therapeutic implications. Front. Immunol. 2014;5:354.
McCubbrey AL, Curtis JL. Efferocytosis and lung disease. Chest. 2013;143:1750–7.
Karaji N, Sattentau QJ. Efferocytosis of pathogen-infected cells. Front. Immunol. 2017;8:1863.
Abdolmaleki F, Farahani N, Gheibi Hayat SM, Pirro M, Bianconi V, Barreto GE, et al. The role of efferocytosis in autoimmune diseases. Front. Immunol. 2018;9:1645.
Horst AK, Tiegs G, Diehl L. Contribution of macrophage efferocytosis to liver homeostasis and disease. Front. Immunol. 2019;10:2670.
Doran AC, Yurdagul JrA, Tabas I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020;20:254–67.
McPhillips K, Janssen WJ, Ghosh M, Byrne A, Gardai S, Remigio L, et al. TNF-α inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J. Immunol. 2007;178:8117–26.
Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J. Biol. Chem. 2006;281:8836–42.
Tosello-Trampont A–C, Nakada-Tsukui K, Ravichandran KS. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J. Biol. Chem. 2003;278:49911–9.
Kim SY, Kim S, Bae DJ, Park SY, Lee GY, Park GM, et al. Coordinated balance of Rac1 and RhoA plays key roles in determining phagocytic appetite. PLoS One. 2017;12:e0174603 https://doi.org/10.1371/journal.pone.0174603. Epub 20170404
Patel JC, Hall A, Caron E Rho GTPases and macrophage phagocytosis. In: Balch WE, Der CJ, Hall A, editors. Methods in Enzymology. 325. p. 462–73. Academic Press; San Diego, CA, 2000.
Moon C, Lee Y-J, Park H-J, Chong YH, Kang JL. N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. Am. J. respiratory Crit. care Med. 2010;181:374–87.
Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–8006.
Elias RJ, Kellerby SS, Decker EA. Antioxidant activity of proteins and peptides. Crit. Rev. food Sci. Nutr. 2008;48:430–41.
Bland JS. Application of Phytochemicals in Immune Disorders: Their Roles Beyond Antioxidants. Integr. Med. (Encinitas, Calif.). 2021;20:16–21.
Lai YS, Putra R, Aui SP, Chang KT. M2(C) Polarization by Baicalin Enhances Efferocytosis via Upregulation of MERTK Receptor. Am. J. Chin. Med. 2018;46:1899–914. https://doi.org/10.1142/s0192415x18500957.
Nascimento L, Moraes A, Costa K, Galúcio J, Taube P, Costa C, et al. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: new findings and potential applications. Biomolecules. 2020;10:988.
Guan R, Van Le Q, Yang H, Zhang D, Gu H, Yang Y, et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere. 2021;271:129499.
Rahman MA, Hannan MA, Dash R, Rahman MH, Islam R, Uddin MJ, et al. Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front. Pharmacol. 2021;12:639628 https://doi.org/10.3389/fphar.2021.639628.
Wen T, Song L, Hua S. Perspectives and controversies regarding the use of natural products for the treatment of lung cancer. Cancer Med. 2021;10:2396–422.
Ghoreishi PS, Shams M, Nimrouzi M, Zarshenas MM, Lankarani KB, Fallahzadeh Abarghooei E, et al. The Effects of Ginger (Zingiber Officinale Roscoe) on Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blinded Placebo-Controlled Clinical Trial. J Diet Suppl. 2024;21:294–312.
Serafini M, Peluso I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016;22:6701–15. https://doi.org/10.2174/1381612823666161123094235
Leitzmann C. Characteristics and Health Benefits of Phytochemicals. Forsch. Komplementmed. 2016;23:69–74. https://doi.org/10.1159/000444063.
Cvetanovic M, Ucker DS. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J. Immunol. 2004;172:880–9.
Shimamura H, Sunamura M, Tsuchihara K, Egawa S, Takeda K, Matsuno S. Irradiated pancreatic cancer cells undergo both apoptosis and necrosis, and could be phagocytized by dendritic cells. Eur. surgical Res. 2005;37:228–34.
Moon B, Yang S, Moon H, Lee J, Park D. After cell death: the molecular machinery of efferocytosis. Exp Mol Med. 2023:1-8.
Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–9.
Tajbakhsh A, Gheibihayat SM, Taheri RA, Fasihi-Ramandi M, Bajestani AN, Taheri A. Potential diagnostic and prognostic of efferocytosis-related unwanted soluble receptors/ligands as new non-invasive biomarkers in disorders: a review. Mol. Biol. Rep. 2022;49:5133–52.
Kumar S, Birge RB. Efferocytosis. Curr. Biol. 2016;26:R558–R9.
Hart S, Haslett C, Dransfield I. Recognition of apoptotic cells by phagocytes. Experientia. 1996;52:950–6.
Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol. today. 1993;14:131–6.
Gheibi Hayat SM, Bianconi V, Pirro M, Sahebkar A. Efferocytosis: molecular mechanisms and pathophysiological perspectives. Immunol. cell Biol. 2019;97:124–33.
Zhao J, Zhang W, Wu T, Wang H, Mao J, Liu J, et al. Efferocytosis in the central nervous system. Front. Cell Developmental Biol. 2021;9:773344.
Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 2013;5:a008748.
Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–55.
Kelley SM, Ravichandran KS. Putting the brakes on phagocytosis:“don’t‐eat‐me” signaling in physiology and disease. EMBO Rep. 2021;22:e52564.
Boada-Romero E, Martinez J, Heckmann BL, Green DR. The clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol. 2020;21:398–414.
Razi S, Yaghmoorian Khojini J, Kargarijam F, Panahi S, Tahershamsi Z, Tajbakhsh A, et al. Macrophage efferocytosis in health and disease. Cell Biochem. Funct. 2023;41:152–65.
Zhang Y, Wang Y, Ding J, Liu P. Efferocytosis in multisystem diseases. Mol. Med. Rep. 2022;25:1–15.
Kasikara C, Doran AC, Cai B, Tabas I. The role of non-resolving inflammation in atherosclerosis. J. Clin. Investig. 2018;128:2713–23.
Fredman G, Tabas I. Boosting inflammation resolution in atherosclerosis: the next frontier for therapy. Am. J. Pathol. 2017;187:1211–21.
Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule–1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008;118:1657–68.
Park S, Jung M, Kim H, Lee S, Kim S, Lee B, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201.
Elliott MR, Koster KM, Murphy PS. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J. Immunol. 2017;198:1387–94.
Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, et al. Apoptotic cells induce Mer tyrosine kinase–dependent blockade of NF-κB activation in dendritic cells. Blood. 2007;109:653–60.
Sharif MN, Šošić DE, Rothlin CV, Kelly E, Lemke G, Olson EN, et al. Twist mediates suppression of inflammation by type I IFNs and Axl. J. Exp. Med. 2006;203:1891–901.
Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–36.
Proto JD, Doran AC, Gusarova G, Yurdagul A, Sozen E, Subramanian M, et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity. 2018;49:666–77. e6.
Oh SA, Li MO. TGF-β: guardian of T cell function. J. Immunol. 2013;191:3973–9.
Noelia A, Castrillo A. Liver X receptors as regulators of macrophage inflammatory and metabolic pathways. Biochimica et. Biophysica Acta (BBA)-Mol. Basis Dis. 2011;1812:982–94.
Jennewein C, Kuhn A-M, Schmidt MV, Meilladec-Jullig V, von Knethen A, Gonzalez FJ, et al. Sumoylation of peroxisome proliferator-activated receptor γ by apoptotic cells prevents lipopolysaccharide-induced NCoR removal from κB binding sites mediating transrepression of proinflammatory cytokines. J. Immunol. 2008;181:5646–52.
Ghisletti S, Huang W, Ogawa S, Pascual G, Lin M-E, Willson TM, et al. Parallel SUMOylation-dependent pathways mediate gene-and signal-specific transrepression by LXRs and PPARγ. Mol. cell. 2007;25:57–70.
Cai B, Kasikara C, Doran AC, Ramakrishnan R, Birge RB, Tabas I. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci. Signal. 2018;11:eaar3721.
Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Front. Immunol. 2011;2:57.
Martin CJ, Peters KN, Behar SM. Macrophages clean up: efferocytosis and microbial control. Curr. Opin. Microbiol. 2014;17:17–23.
Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018;19:1801.
Morioka S, Maueröder C, Ravichandran KS. Living on the Edge: Efferocytosis at the Interface of Homeostasis and Pathology. Immunity. 2019;50:1149–62. https://doi.org/10.1016/j.immuni.2019.04.018.
Rochette L, Dogon G, Rigal E, Zeller M, Cottin Y, Vergely C. Interplay between efferocytosis and atherosclerosis. Arch. Cardiovasc Dis. 2023;116:474–84. https://doi.org/10.1016/j.acvd.2023.07.007.
Wang EJ, Wu MY, Ren ZY, Zheng Y, Ye RD, Tan CSH, et al. Targeting macrophage autophagy for inflammation resolution and tissue repair in inflammatory bowel disease. Burns Trauma. 2023;11:tkad004 https://doi.org/10.1093/burnst/tkad004.
Pan W, Zhang J, Zhang L, Zhang Y, Song Y, Han L, et al. Comprehensive view of macrophage autophagy and its application in cardiovascular diseases. Cell Prolif. 2024;57:e13525 https://doi.org/10.1111/cpr.13525.
Zhang X, Qin Y, Wan X, Liu H, Lv C, Ruan W, et al. Rosuvastatin exerts anti-atherosclerotic effects by improving macrophage-related foam cell formation and polarization conversion via mediating autophagic activities. J. Transl. Med. 2021;19:62 https://doi.org/10.1186/s12967-021-02727-3.
Luo Y, Lu S, Gao Y, Yang K, Wu D, Xu X, et al. Araloside C attenuates atherosclerosis by modulating macrophage polarization via Sirt1-mediated autophagy. Aging (Albany NY). 2020;12:1704–24. https://doi.org/10.18632/aging.102708.
Bories GFP, Leitinger N. Macrophage metabolism in atherosclerosis. FEBS Lett. 2017;591:3042–60. https://doi.org/10.1002/1873-3468.12786.
Wu MY, Lu JH Autophagy and Macrophage Functions: Inflammatory Response and Phagocytosis. Cells. 2019;9. https://doi.org/10.3390/cells9010070.
Deretic V. Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr. Opin. Immunol. 2012;24:21–31. https://doi.org/10.1016/j.coi.2011.10.006.
Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–48. https://doi.org/10.1042/BJ20061520
Rezazadeh-Shojaee FS, Ramazani E, Kasaian J, Tayarani-Najaran Z. Protective effects of 6-gingerol on 6-hydroxydopamine-induced apoptosis in PC12 cells through modulation of SAPK/JNK and survivin activation. J. Biochem Mol. Toxicol. 2022;36:e22956 https://doi.org/10.1002/jbt.22956.
Luo J, Chen J, Yang C, Tan J, Zhao J, Jiang N, et al. 6-Gingerol protects against cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome and apoptosis via TRPV1 / FAF1 complex dissociation-mediated autophagy. Int Immunopharmacol. 2021;100:108146 https://doi.org/10.1016/j.intimp.2021.108146.
Liu B, Zhang B, Guo R, Li S, Xu Y. Enhancement in efferocytosis of oxidized low-density lipoprotein-induced apoptotic RAW264.7 cells through Sirt1-mediated autophagy. Int J. Mol. Med. 2014;33:523–33. https://doi.org/10.3892/ijmm.2013.1609.
Singh S. Beyond LC3-associated phagocytosis: cross-talk between autophagy and efferocytosis during microglial corpse clearance. Australia: University of Adelaide; 2023.
Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–53. https://doi.org/10.1016/j.cmet.2012.01.022.
Shao BZ, Han BZ, Zeng YX, Su DF, Liu C. The roles of macrophage autophagy in atherosclerosis. Acta Pharm. Sin. 2016;37:150–6. https://doi.org/10.1038/aps.2015.87.
Das M, Das DK. Resveratrol and cardiovascular health. Mol Asp Med. 2010;31:503–12. https://doi.org/10.1016/j.mam.2010.09.001.
Shahraki MN, Jouabadi SM, Bos D, Stricker BH, Ahmadizar F. Statin Use and Coronary Artery Calcification: a Systematic Review and Meta-analysis of Observational Studies and Randomized Controlled Trials. Curr Atheroscler Rep. 2023;25:769–84. https://doi.org/10.1007/s11883-023-01151-w.
Hedayati S, Tarahi M, Azizi R, Baghbali V, Ansarifar E, Hashempur MH Encapsulation of mint essential oil: Techniques and applications. Adv. Coll. Interf. Sci. 2023:321:103023. https://doi.org/10.1016/j.cis.2023.103023.
Kazemi A, Iraji A, Esmaealzadeh N, Salehi M, Hashempur MH. Peppermint and menthol: a review on their biochemistry, pharmacological activities, clinical applications, and safety considerations. Critic Rev Food Sci Nutr. 2024;1–26. https://doi.org/10.1080/10408398.2023.2296991. Online ahead of print.
Hajimonfarednejad M, Ostovar M, Hasheminasab FS, Shariati MA, Thiruvengadam M, Raee MJ, et al. Medicinal Plants for Viral Respiratory Diseases: A Systematic Review on Persian Medicine. Evi Based Complement Alter Med. 2023;2023:1928310.
Morand C, Tomás-Barberán FA. Contribution of plant food bioactives in promoting health effects of plant foods: why look at interindividual variability? Eur. J. Nutr. 2019;58:13–9. https://doi.org/10.1007/s00394-019-02096-0. Epub 2019/10/23
Ramawat K, Dass S, Mathur M. The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants. In: Ramawat, K. (eds) Herbal Drugs: Ethnomedicine to Modern Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-79116-4_2.
Hahn NI. Are phytoestrogens nature’s cure for what ails us? A look at the research. J. Am. Dietetic Assoc. 1998;98:974–7.
Zhang L, Virgous C, Si H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019;69:19–30. https://doi.org/10.1016/j.jnutbio.2019.03.009.
Townsend JR, Kirby TO, Sapp PA, Gonzalez AM, Marshall TM, Esposito R. Nutrient synergy: definition, evidence, and future directions. Front Nutr. 2023;10:1279925 https://doi.org/10.3389/fnut.2023.1279925.
Farooqui AA. Phytochemicals, signal transduction, and neurological disorders: New York, NY: Springer Science & Business Media; 2012. https://doi.org/10.1007/978-1-4614-3804-5.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61.
Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circulation Res. 2016;118:653–67.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377:1119–31.
Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010;10:36–46.
Kojima Y, Volkmer J-P, McKenna K, Civelek M, Lusis AJ, Miller CL, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90.
Martinet W, Schrijvers DM, De Meyer GR. Necrotic cell death in atherosclerosis. Basic Res. Cardiol. 2011;106:749–60.
Yurdagul A, Subramanian M, Wang X, Crown SB, Ilkayeva OR, Darville L, et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. 2020;31:518–33. e10.
Tabas I. 2016 Russell Ross Memorial Lecture in Vascular Biology: Molecular–Cellular Mechanisms in the Progression of Atherosclerosis. Arteriosclerosis, Thrombosis, Vasc. Biol. 2017;37:183–9.
Han CZ, Ravichandran KS. Metabolic connections during apoptotic cell engulfment. Cell. 2011;147:1442–5.
Tajbakhsh A, Rezaee M, Kovanen PT, Sahebkar A. Efferocytosis in atherosclerotic lesions: malfunctioning regulatory pathways and control mechanisms. Pharmacol. Therapeutics. 2018;188:12–25.
Kimani SG, Geng K, Kasikara C, Kumar S, Sriram G, Wu Y, et al. Contribution of defective PS recognition and efferocytosis to chronic inflammation and autoimmunity. Front. Immunol. 2014;5:566.
Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, et al. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J. Clin. Investig. 2017;127:564–8.
Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, et al. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–77.
Schrijvers DM, De MeyerGR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovascular Res. 2007;73:470–80.
Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J. Interventional Cardiol. 2002;15:439–46.
Pulanco MC, Cosman J, Ho M-M, Huynh J, Fing K, Turcu J, et al. Complement protein C1q enhances macrophage foam cell survival and efferocytosis. J. Immunol. 2017;198:472–80.
Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, et al. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J. Clin. Investig. 2019;124:1083–97.
Heo K-S, Cushman HJ, Akaike M, Woo C-H, Wang X, Qiu X, et al. ERK5 activation in macrophages promotes efferocytosis and inhibits atherosclerosis. Circulation. 2014;130:180–91.
Liu B, Yan W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019;272:663–9.
Chen S-J, Kao Y-H, Jing L, Chuang Y-P, Wu W-L, Liu S-T, et al. Epigallocatechin-3-gallate reduces scavenger receptor A expression and foam cell formation in human macrophages. J. Agric. food Chem. 2017;65:3141–50.
Chen B, Li H, Ou G, Ren L, Yang X, Zeng M. Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IκBα and blocking mitochondrial damage. Arthritis Res. Ther. 2019;21:1–15.
Ahmad RS, Hussain MB, Sultan MT, Arshad MS, Waheed M, Shariati MA, et al. Biochemistry, safety, pharmacological activities, and clinical applications of turmeric: a mechanistic review. Evi Based Complement Alter Medicine. 2020;2020.
Yin H, Guo Q, Li X, Tang T, Li C, Wang H, et al. Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J. Immunol. 2018;200:2835–46.
Saqib U, Sarkar S, Suk K, Mohammad O, Baig MS, Savai R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget. 2018;9:17937.
Zhao JF, Ching LC, Huang YC, Chen CY, Chiang AN, Kou YR, et al. Molecular mechanism of curcumin on the suppression of cholesterol accumulation in macrophage foam cells and atherosclerosis. Mol. Nutr. food Res. 2012;56:691–701.
Vasamsetti SB, Karnewar S, Gopoju R, Gollavilli PN, Narra SR, Kumar JM, et al. Resveratrol attenuates monocyte-to-macrophage differentiation and associated inflammation via modulation of intracellular GSH homeostasis: Relevance in atherosclerosis. Free Radic. Biol. Med. 2016;96:392–405.
Park D-W, Baek K, Kim J-R, Lee J-J, Ryu S-H, Chin B-R, et al. Resveratrol inhibits foam cell formation via NADPH oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp. Mol. Med. 2009;41:171–9.
Dong W, Wang X, Bi S, Pan Z, Liu S, Yu H, et al. Inhibitory effects of resveratrol on foam cell formation are mediated through monocyte chemotactic protein-1 and lipid metabolism-related proteins. Int. J. Mol. Med. 2014;33:1161–8.
Jiang Y-H, Jiang L-Y, Wang Y-C, Ma D-F, Li X. Quercetin attenuates atherosclerosis via modulating oxidized LDL-induced endothelial cellular senescence. Front. Pharmacol. 2020;11:512.
Tang Y, Wu H, Shao B, Wang Y, Liu C, Guo M. Celosins inhibit atherosclerosis in ApoE(-/-) mice and promote autophagy flow. J. Ethnopharmacol. 2018;215:74–82. https://doi.org/10.1016/j.jep.2017.12.031.
Limanaqi F, Biagioni F, Busceti CL, Ryskalin L, Polzella M, Frati A, et al. Phytochemicals Bridging Autophagy Induction and Alpha-Synuclein Degradation in Parkinsonism. Int J. Mol. Sci. 2019;20:3274 https://doi.org/10.3390/ijms20133274.
Li CG, Yan L, Jing YY, Xu LH, Liang YD, Wei HX, et al. Berberine augments ATP-induced inflammasome activation in macrophages by enhancing AMPK signaling. Oncotarget. 2017;8:95–109. https://doi.org/10.18632/oncotarget.13921
Fan X, Wang J, Hou J, Lin C, Bensoussan A, Chang D, et al. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J. Transl. Med. 2015;13:92 https://doi.org/10.1186/s12967-015-0450-z.
Ghafoor B, Ali MN, Riaz Z. Synthesis and Appraisal of Natural Drug-Polymer-Based Matrices Relevant to the Application of Drug-Eluting Coronary Stent Coatings. Cardiol. Res Pr. 2020;2020:4073091 https://doi.org/10.1155/2020/4073091.
Wolf SA, Boddeke H, Kettenmann H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017;79:619–43.
Tajbakhsh A, Read M, Barreto GE, Avila-Rodriguez M, Gheibi-Hayat SM, Sahebkar A. Apoptotic neurons and amyloid-beta clearance by phagocytosis in Alzheimer’s disease: Pathological mechanisms and therapeutic outlooks. Eur. J. Pharm. 2021;895:173873 https://doi.org/10.1016/j.ejphar.2021.173873.
Borggrewe M, Kooistra SM, Noelle RJ, Eggen BJ, Laman JD. Exploring the VISTA of microglia: immune checkpoints in CNS inflammation. J. Mol. Med. 2020;98:1415–30.
Márquez-Ropero M, Benito E, Plaza-Zabala A, Sierra A. Microglial corpse clearance: lessons from macrophages. Front. Immunol. 2020;11:506.
Hall-Roberts H, Di Daniel E, James WS, Davis JB, Cowley SA. In vitro quantitative imaging assay for phagocytosis of dead neuroblastoma cells by iPSC-macrophages. JoVE. 2021:e62217.
Flannagan RS, Jaumouillé V, Grinstein S. The cell biology of phagocytosis. Annu. Rev. Pathol.: Mechanisms Dis. 2012;7:61–98.
Mike JK, Ferriero DM. Efferocytosis mediated modulation of injury after neonatal brain hypoxia-ischemia. Cells. 2021;10:1025.
Lee J, Jo D-G, Park D, Chung HY, Mattson MP. Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system. Pharmacol. Rev. 2014;66:815–68.
Faria A, Pestana D, Teixeira D, Couraud P-O, Romero I, Weksler B, et al. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011;2:39–44.
Davinelli S, Maes M, Corbi G, Zarrelli A, Willcox DC, Scapagnini G. Dietary phytochemicals and neuro-inflammaging: from mechanistic insights to translational challenges. Immun. Ageing. 2016;13:1–17.
Karlstetter M, Lippe E, Walczak Y, Moehle C, Aslanidis A, Mirza M, et al. Curcumin is a potent modulator of microglial gene expression and migration. J. neuroinflammation. 2011;8:1–12.
Wu J, Li Q, Wang X, Yu S, Li L, Wu X, et al. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PloS one. 2013;8:e59843.
Wu J-X, Zhang L-Y, Chen Y-L, Yu S-S, Zhao Y, Zhao J. Curcumin pretreatment and post-treatment both improve the antioxidative ability of neurons with oxygen-glucose deprivation. Neural Regeneration Res. 2015;10:481.
Miao Y, Zhao S, Gao Y, Wang R, Wu Q, Wu H, et al. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res. Bull. 2016;121:9–15.
Zingg JM, Hasan ST, Cowan D, Ricciarelli R, Azzi A, Meydani M. Regulatory effects of curcumin on lipid accumulation in monocytes/macrophages. J. Cell. Biochem. 2012;113:833–40.
Krysko O, Vandenabeele P, Krysko DV, Bachert C. Impairment of phagocytosis of apoptotic cells and its role in chronic airway diseases. Apoptosis. 2010;15:1137–46. https://doi.org/10.1007/s10495-010-0504-x
Tajbakhsh A, Gheibihayat SM, Mortazavi D, Medhati P, Rostami B, Savardashtaki A, et al. The Effect of Cigarette Smoke Exposure on Efferocytosis in Chronic Obstructive Pulmonary Disease; Molecular Mechanisms and Treatment Opportunities. COPD. 2021;18:723–36. https://doi.org/10.1080/15412555.2021.1978419.
Asare PF, Tran HB, Hurtado PR, Perkins GB, Nguyen P, Jersmann H, et al. Inhibition of LC3-associated phagocytosis in COPD and in response to cigarette smoke. Ther. Adv. Respir. Dis. 2021;15:17534666211039769 https://doi.org/10.1177/17534666211039769
Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, et al. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Invest. 2002;109:661–70. https://doi.org/10.1172/JCI13572
Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 2004;10:487–93. https://doi.org/10.1038/nm1028.
Vandivier RW, Richens TR, Horstmann SA, deCathelineau AM, Ghosh M, Reynolds SD, et al. Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297:L677–86. https://doi.org/10.1152/ajplung.00030.2009.
Li Q, Liu H, Yin G, Xie Q. Efferocytosis: Current status and future prospects in the treatment of autoimmune diseases. Heliyon. 2024;10:e28399 https://doi.org/10.1016/j.heliyon.2024.e28399.
Xu J, Zhang Y, Xiao Y, Ma S, Liu Q, Dang S, et al. Inhibition of 12/15-lipoxygenase by baicalein induces microglia PPARbeta/delta: a potential therapeutic role for CNS autoimmune disease. Cell Death Dis. 2013;4:e569 https://doi.org/10.1038/cddis.2013.86.
Liu X, Liu H, Deng Y. Efferocytosis: An Emerging Therapeutic Strategy for Type 2 Diabetes Mellitus and Diabetes Complications. J. Inflamm. Res. 2023;16:2801–15. https://doi.org/10.2147/JIR.S418334.
Bommelaer G, Laharie D, Nancey S, Hebuterne X, Roblin X, Nachury M, et al. Oral Curcumin No More Effective Than Placebo in Preventing Recurrence of Crohn’s Disease After Surgery in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2020;18:1553–60 e1. https://doi.org/10.1016/j.cgh.2019.08.041.
Behrouz V, Dastkhosh A, Hedayati M, Sedaghat M, Sharafkhah M, Sohrab G. The effect of crocin supplementation on glycemic control, insulin resistance and active AMPK levels in patients with type 2 diabetes: a pilot study. Diabetol. Metab. Syndr. 2020;12:59 https://doi.org/10.1186/s13098-020-00568-6.
Cosaro E, Bonafini S, Montagnana M, Danese E, Trettene MS, Minuz P, et al. Effects of magnesium supplements on blood pressure, endothelial function and metabolic parameters in healthy young men with a family history of metabolic syndrome. Nutr. Metab. Cardiovasc Dis. 2014;24:1213–20. https://doi.org/10.1016/j.numecd.2014.05.010.
Han MK, Barreto TA, Martinez FJ, Comstock AT, Sajjan US. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020;7:e000392 https://doi.org/10.1136/bmjresp-2018-000392
Rowe SM, Clancy JP, Wilschanski M. Nasal potential difference measurements to assess CFTR ion channel activity. Methods Mol. Biol. 2011;741:69–86. https://doi.org/10.1007/978-1-61779-117-8_6
Pyle LC, Fulton JC, Sloane PA, Backer K, Mazur M, Prasain J, et al. Activation of the cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of DeltaF508 cystic fibrosis transmembrane conductance regulator rescue. Am. J. Respir. Cell Mol. Biol. 2010;43:607–16. https://doi.org/10.1165/rcmb.2009-0281OC.
Zhang S, Smith N, Schuster D, Azbell C, Sorscher EJ, Rowe SM, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: therapeutic implications for chronic rhinosinusitis. Am. J. Rhinol. Allergy. 2011;25:307–12. https://doi.org/10.2500/ajra.2011.25.3643
Boots AW, Drent M, de Boer VC, Bast A, Haenen GR. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011;30:506–12. https://doi.org/10.1016/j.clnu.2011.01.010.
Khan A, Iqtadar S, Mumtaz SU, Heinrich M, Pascual-Figal DA, Livingstone S, et al. Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results From a Pilot Open-Label, Randomized Controlled Trial. Front Pharm. 2022;13:898062 https://doi.org/10.3389/fphar.2022.898062.
Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006;4:1502–6. https://doi.org/10.1016/j.cgh.2006.08.008.
Kumar S, Ahuja V, Sankar MJ, Kumar A, Moss AC. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2012;10:CD008424 https://doi.org/10.1002/14651858.CD008424.pub2.
Chen X, Li H, Zhang B, Deng Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022;62:5658–77. https://doi.org/10.1080/10408398.2021.1888693.
Boonrueng P, Wasana PWD, Hasriadi, Vajragupta O, Rojsitthisak P, Towiwat P. Combination of curcumin and piperine synergistically improves pain-like behaviors in mouse models of pain with no potential CNS side effects. Chin. Med. 2022;17:119 https://doi.org/10.1186/s13020-022-00660-1.
Lee HN, Choi JH, Park JY, Ahn JH, Jang DE, Shim JG, et al. Combination of vegetable soup and glucan demonstrates synergistic effects on macrophage-mediated immune responses. Food Sci. Biotechnol. 2021;30:583–8. https://doi.org/10.1007/s10068-021-00888-x.
Pakki E, Tayeb R, Usmar U, Ridwan IA, Muslimin L. Effect of orally administered combination of Caulerpa racemosa and Eleutherine americana (Aubl) Merr extracts on phagocytic activity of macrophage. Res Pharm. Sci. 2020;15:401–9. https://doi.org/10.4103/1735-5362.293518.
Kumar S, Shen J, Burgess DJ. Nano-amorphous spray dried powder to improve oral bioavailability of itraconazole. J. Control Release. 2014;192:95–102. https://doi.org/10.1016/j.jconrel.2014.06.059.
Hua S. Advances in Nanoparticulate Drug Delivery Approaches for Sublingual and Buccal Administration. Front Pharm. 2019;10:1328 https://doi.org/10.3389/fphar.2019.01328.
Yadav N, Parveen S, Banerjee M. Potential of nano-phytochemicals in cervical cancer therapy. Clin. Chim. Acta. 2020;505:60–72. https://doi.org/10.1016/j.cca.2020.01.035.
Ovais M, Khalil AT, Raza A, Islam NU, Ayaz M, Saravanan M, et al. Multifunctional theranostic applications of biocompatible green-synthesized colloidal nanoparticles. Appl Microbiol Biotechnol. 2018;102:4393–408. https://doi.org/10.1007/s00253-018-8928-2.
Kim J, Zhang S, Zhu Y, Wang R, Wang J. Amelioration of colitis progression by ginseng-derived exosome-like nanoparticles through suppression of inflammatory cytokines. J. Ginseng Res. 2023;47:627–37. https://doi.org/10.1016/j.jgr.2023.01.004.
Jaiswal S, Mishra P. Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med Microbiol Immunol. 2018;207:39–53. https://doi.org/10.1007/s00430-017-0525-y.
Alnuqaydan AM, Almutary AG, Azam M, Manandhar B, De Rubis G, Madheswaran T, et al. Phytantriol-Based Berberine-Loaded Liquid Crystalline Nanoparticles Attenuate Inflammation and Oxidative Stress in Lipopolysaccharide-Induced RAW264.7 Macrophages. Nanomaterials (Basel). 2022;12:4312 https://doi.org/10.3390/nano12234312.
Iqbal J, Abbasi BA, Yaseen T, Zahra SA, Shahbaz A, Shah SA, et al. Green synthesis of zinc oxide nanoparticles using Elaeagnus angustifolia L. leaf extracts and their multiple in vitro biological applications. Sci. Rep. 2021;11:20988 https://doi.org/10.1038/s41598-021-99839-z.
Yang H, Yu Z, Ji S, Yan J, kong Y, Huo Q, et al. Regulation of Synovial Macrophages Polarization by Mimicking Efferocytosis for Therapy of Osteoarthritis. Adv Funct Mater. 2022;32. https://doi.org/10.1002/adfm.202207637.
Huwait EA, Saddeek SY, Al-Massabi RF, Almowallad SJ, Pushparaj PN, Kalamegam G. Antiatherogenic Effects of Quercetin in the THP-1 Macrophage Model In Vitro, With Insights Into Its Signaling Mechanisms Using In Silico Analysis. Front Pharm. 2021;12:698138 https://doi.org/10.3389/fphar.2021.698138.
Medina C, Ravichandran K. Do not let death do us part:‘find-me’signals in communication between dying cells and the phagocytes. Cell Death Differ. 2016;23:979–89.
Lauber K, Bohn E, Kröber SM, Xiao Y-j, Blumenthal SG, Lindemann RK, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–30.
Horino K, Nishiura H, Ohsako T, Shibuya Y, Hiraoka T, Kitamura N, et al. A monocyte chemotactic factor, S19 ribosomal protein dimer, in phagocytic clearance of apoptotic cells. Lab. Investig.; a J. Tech. methods Pathol. 1998;78:603–17.
Hou Y, Plett PA, Ingram DA, Rajashekhar G, Orschell CM, Yoder MC, et al. Endothelial-monocyte–activating polypeptide II induces migration of endothelial progenitor cells via the chemokine receptor CXCR3. Exp. Hematol. 2006;34:1125–32.
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. (Baltim., Md: 1950). 1992;148:2207–16.
Fadok VA, De Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001;276:1071–7.
Kourtzelis I, Hajishengallis G, Chavakis T. Phagocytosis of apoptotic cells in resolution of inflammation. Front. Immunol. 2020;11:553.
Gardai SJ, Bratton DL, Ogden CA, Henson PM. Recognition ligands on apoptotic cells: a perspective. J. Leukoc. Biol. 2006;79:896–903.
Vafadar A, Vosough P, Jahromi HK, Tajbakhsh A, Savardshtaki A, Butler AE, et al. The role of efferocytosis and transplant rejection: Strategies in promoting transplantation tolerance using apoptotic cell therapy and/or synthetic particles. Cell Biochem Funct. 2023;41:959–77.
Castellano F, Montcourrier P, Chavrier P. Membrane recruitment of Rac1 triggers phagocytosis. J cell Sci. 2000;113:2955–61. https://doi.org/10.1002/cbf.3852.
Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 2007;7:964–74.
Das S, Owen KA, Ly KT, Park D, Black SG, Wilson JM, et al. Brain angiogenesis inhibitor 1 (BAI1) is a pattern recognition receptor that mediates macrophage binding and engulfment of Gram-negative bacteria. Proc. Natl Acad. Sci. 2011;108:2136–41.
Park S-Y, Kang K-B, Thapa N, Kim S-Y, Lee S-J, Kim I-S. Requirement of adaptor protein GULP during stabilin-2-mediated cell corpse engulfment. J. Biol. Chem. 2008;283:10593–600.
Park D, Hochreiter-Hufford A, Ravichandran KS. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr. Biol. 2009;19:346–51.
Toda S, Hanayama R, Nagata S. Two-step engulfment of apoptotic cells. Mol Cell Biol. 2012;32:118–25.
Flannagan RS, Canton J, Furuya W, Glogauer M, Grinstein S. The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis. Mol. Biol. cell. 2014;25:1511–22.
Park B, Lee J, Moon H, Lee G, Lee DH, Cho JH, et al. Co-receptors are dispensable for tethering receptor-mediated phagocytosis of apoptotic cells. Cell Death Dis. 2015;6:e1772. e
Nishi C, Toda S, Segawa K, Nagata S. Tim4-and MerTK-mediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Mol. Cell. Biol. 2014;34:1512–20.
Moon B, Lee J, Lee S-A, Min C, Moon H, Kim D, et al. Mertk interacts with Tim-4 to enhance Tim-4-mediated efferocytosis. Cells. 2020;9:1625.
Beizavi Z, Gheibihayat SM, Moghadasian H, Zare H, Yeganeh BS, Askari H, et al. The regulation of CD47-SIRPalpha signaling axis by microRNAs in combination with conventional cytotoxic drugs together with the help of nano-delivery: a choice for therapy? Mol. Biol. Rep. 2021;48:5707–22. https://doi.org/10.1007/s11033-021-06547-y.
Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nat. Rev. Mol. cell Biol. 2008;9:781–95.
Kinchen JM, Doukoumetzidis K, Almendinger J, Stergiou L, Tosello-Trampont A, Sifri CD, et al. A pathway for phagosome maturation during engulfment of apoptotic cells. Nat. cell Biol. 2008;10:556–66.
Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl Acad. Sci. 2011;108:17396–401.
Fond AM, Lee CS, Schulman IG, Kiss RS, Ravichandran KS. Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. J. Clin. Investig. 2015;125:2748–58.
Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, et al. PPAR-δ senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 2009;15:1266–72.
Park D, Han CZ, Elliott MR, Kinchen JM, Trampont PC, Das S, et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011;477:220–4.
Ampomah PB, Cai B, Sukka SR, Gerlach BD, Yurdagul JrA, Wang X, et al. Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution. Nat. Metab. 2022;4:444–57.
Zhang S, Weinberg S, DeBerge M, Gainullina A, Schipma M, Kinchen JM, et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 2019;29:443–56. e5.
Morioka S, Perry JS, Raymond MH, Medina CB, Zhu Y, Zhao L, et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature. 2018;563:714–8.
Lin D, Kang X, Shen L, Tu S, Lenahan C, Chen Y, et al. Efferocytosis and Its Associated Cytokines: A Light on Non-tumor and Tumor Diseases? Mol. Ther. Oncolytics. 2020;17:394–407. https://doi.org/10.1016/j.omto.2020.04.010.
Mota AC, Dominguez M, Weigert A, Snodgrass RG, Namgaladze D, Brune B. Lysosome-Dependent LXR and PPARdelta Activation Upon Efferocytosis in Human Macrophages. Front Immunol. 2021;12:637778 https://doi.org/10.3389/fimmu.2021.637778.
Wang T, Xiang Z, Wang Y, Li X, Fang C, Song S, et al. (-)-Epigallocatechin Gallate Targets Notch to Attenuate the Inflammatory Response in the Immediate Early Stage in Human Macrophages. Front Immunol. 2017;8:433 https://doi.org/10.3389/fimmu.2017.00433.
Zeng P, Liu B, Wang Q, Fan Q, Diao JX, Tang J, et al. Apigenin Attenuates Atherogenesis through Inducing Macrophage Apoptosis via Inhibition of AKT Ser473 Phosphorylation and Downregulation of Plasminogen Activator Inhibitor-2. Oxid. Med Cell Longev. 2015;2015:379538 https://doi.org/10.1155/2015/379538.
Zhu W, Jin Z, Yu J, Liang J, Yang Q, Li F, et al. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int Immunopharmacol. 2016;35:119–26. https://doi.org/10.1016/j.intimp.2016.03.030.
Kim ME, Kim HK, Park HY, Kim DH, Chung HY, Lee JS. Baicalin from Scutellaria baicalensis impairs Th1 polarization through inhibition of dendritic cell maturation. J. Pharm. Sci. 2013;121:148–56. https://doi.org/10.1254/jphs.12200fp
Ding XM, Pan L, Wang Y, Xu QZ. Baicalin exerts protective effects against lipopolysaccharide-induced acute lung injury by regulating the crosstalk between the CX3CL1-CX3CR1 axis and NF-kappaB pathway in CX3CL1-knockout mice. Int J. Mol. Med. 2016;37:703–15. https://doi.org/10.3892/ijmm.2016.2456.
Abdi H, Roshanravan M, Mirzavi F, Hosseinzadeh H, Mosaffa F. Crocin’s effect on phenotype switching of J774A.1 macrophages depends on their polarization state during exposure. Iran. J. Basic Med Sci. 2023;26:1431–7. https://doi.org/10.22038/IJBMS.2023.70859.15392
Benvenutti L, Nunes R, Venturi I, Ramos SA, Broering MF, Goldoni FC, et al. Anti-Inflammatory and Healing Activity of the Hydroalcoholic Fruit Extract of Solanum diploconos (Mart.) Bohs. J. Immunol. Res. 2021;2021:9957451 https://doi.org/10.1155/2021/9957451.
Manickam V, Dhawan UK, Singh D, Gupta M, Subramanian M. Pomegranate Peel Extract Decreases Plaque Necrosis and Advanced Atherosclerosis Progression in Apoe (-/-) Mice. Front Pharm. 2022;13:888300 https://doi.org/10.3389/fphar.2022.888300.
Eisvand F, Tajbakhsh A, Seidel V, Zirak MR, Tabeshpour J, Shakeri A. Quercetin and its role in modulating endoplasmic reticulum stress: A review. Phytother. Res. 2022;36:73–84. https://doi.org/10.1002/ptr.7283.
Tonin TD, Thiesen LC, de Oliveira Nunes ML, Broering MF, Donato MP, Goss MJ, et al. Rubus imperialis (Rosaceae) extract and pure compound niga-ichigoside F1: wound healing and anti-inflammatory effects. Naunyn Schmiedebergs Arch. Pharm. 2016;389:1235–44. https://doi.org/10.1007/s00210-016-1285-8.
Bonay M, Roux AL, Floquet J, Retory Y, Herrmann JL, Lofaso F, et al. Caspase-independent apoptosis in infected macrophages triggered by sulforaphane via Nrf2/p38 signaling pathways. Cell Death Discov. 2015;1:15022 https://doi.org/10.1038/cddiscovery.2015.22.
Li Q, Liu X, Du Y, Zhang X, Xiang P, Chen G, et al. Protocatechuic acid boosts continual efferocytosis in macrophages by derepressing KLF4 to transcriptionally activate MerTK. Sci. Signal. 2023;16:eabn1372 https://doi.org/10.1126/scisignal.abn1372.
Xia W, Zhu Z, Xiang S, Yang Y. Ginsenoside Rg5 promotes wound healing in diabetes by reducing the negative regulation of SLC7A11 on the efferocytosis of dendritic cells. J. Ginseng Res. 2023;47:784–94. https://doi.org/10.1016/j.jgr.2023.06.006.
Veena SM, Muddapur U, Sajjanar S, Mirajkar KK, Shivaram AK, et al. Inhibition of LDL Oxidation and Foam Cell Development of Tannin Methanol Extract from Citrus limon and Honey Formulation on Cell lines. Pharmacogn. Res. 2022;14:158–65. https://doi.org/10.5530/pres.14.2.23
Nambiar SS, Shetty NP, Bhatt P, Neelwarne B. Inhibition of LDL oxidation and oxidized LDL-induced foam cell formation in RAW 264.7 cells show anti-atherogenic properties of a foliar methanol extract of Scoparia dulcis. Pharmacogn. Mag. 2014;10:S240–8. https://doi.org/10.4103/0973-1296.133241
Liu F, Shan S, Li H, Shi J, Yang R, Li Z. Millet shell polyphenols ameliorate atherosclerosis development by suppressing foam cell formation. J. Nutr. Biochem. 2023;115:109271 https://doi.org/10.1016/j.jnutbio.2023.109271.
Liu Z, Wang J, Huang E, Gao S, Li H, Lu J, et al. Tanshinone IIA suppresses cholesterol accumulation in human macrophages: role of heme oxygenase-1. J. Lipid Res. 2014;55:201–13. https://doi.org/10.1194/jlr.M040394.
Onal H, Arslan B, Ucuncu Ergun N, Topuz S, Yilmaz Semerci S, Kurnaz ME, et al. Treatment of COVID-19 patients with quercetin: a prospective, single center, randomized, controlled trial. Turk. J. Biol. 2021;45:518–29. https://doi.org/10.3906/biy-2104-16.
Ujjan ID, Khan S, Nigar R, Ahmed H, Ahmad S, Khan A. The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19-Results from a pragmatic randomized clinical trial. Front Nutr. 2022;9:1023997 https://doi.org/10.3389/fnut.2022.1023997.
Pirro M, Francisci D, Bianconi V, Schiaroli E, Mannarino MR, Barsotti F, et al. NUtraceutical TReatment for hYpercholesterolemia in HIV-infected patients: The NU-TRY(HIV) randomized cross-over trial. Atherosclerosis. 2019;280:51–7. https://doi.org/10.1016/j.atherosclerosis.2018.11.026.
Noah TL, Zhang H, Zhou H, Glista-Baker E, Muller L, Bauer RN, et al. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: a randomized, double-blind study. PLoS One. 2014;9:e98671 https://doi.org/10.1371/journal.pone.0098671.
Muller L, Meyer M, Bauer RN, Zhou H, Zhang H, Jones S, et al. Effect of Broccoli Sprouts and Live Attenuated Influenza Virus on Peripheral Blood Natural Killer Cells: A Randomized, Double-Blind Study. PLoS One. 2016;11:e0147742 https://doi.org/10.1371/journal.pone.0147742.
He X, Yang F, Wu Y, Lu J, Gao X, Zhu X, et al. Identification of tanshinone I as cap-dependent endonuclease inhibitor with broad-spectrum antiviral effect. J. Virol. 2023;97:e0079623 https://doi.org/10.1128/jvi.00796-23.
Author information
Authors and Affiliations
Contributions
Asma Vafadar was responsible for writing and editing the article, while Amir Tajbakhsh handled the article idea, writing, editing, evaluating, and managing the project. Fatemeh Hosseinpour-Soleimani drew the illustrations and provided writing assistance. Amir Tajbakhsh, Fatemeh Hosseinpour-Soleimani, Asma Vafadar, and Mohammad Hashem Hashempur contributed to the comprehensive data analysis and interpretation of the results. Amir Savardshtaki and Mohammad Hashem Hashempur supervised and coordinated the team members, in addition to writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vafadar, A., Tajbakhsh, A., Hosseinpour-Soleimani, F. et al. Phytochemical-mediated efferocytosis and autophagy in inflammation control. Cell Death Discov. 10, 493 (2024). https://doi.org/10.1038/s41420-024-02254-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-024-02254-2