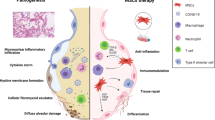
Abstract
In recent years, the morbidity and mortality caused by acute and chronic lung diseases have gradually increased, becoming a global public health burden. However, modern medicine has yet to determine the exact treatment for lung diseases associated with inflammation. Alleviating lung diseases and repairing injured lung tissue are urgent issues that need to be resolved. Mesenchymal stem cells (MSCs) have been used to treat various inflammatory diseases owing to their powerful anti-inflammatory, anti-apoptotic, and tissue-regenerative properties. MSCs show great promise and have been shown to play a role in relieving lung diseases experimentally. The immune regulatory role of MSCs is thought to be a key mechanism underlying their multiple potential therapeutic effects. Immune cells and secreted factors contribute to tissue repair following lung injury. However, the overactivation of immune cells can aggravate lung injury. Here, we review evidence that MSCs act on immune cells to relieve lung diseases. Based on the immunomodulatory properties of MSCs, the specific mechanisms by which MSCs in alleviate lung diseases are reviewed, with a focus on innate and adaptive immunity. In addition, we discuss current challenges in the treatment of lung diseases using MSCs.
Similar content being viewed by others
Facts
-
MSCs have a good application prospect in the treatment of lung diseases.
-
MSCs can act on innate immune cells (neutrophils, macrophages, eosinophils) and adaptive
-
immune cells (T cells, B cells) to play a repair role.
-
The clinical application of MSCs still faces great challenges.
Open Questions
-
What is the specific mechanism by which MSCs regulate immune cells?
-
Whether immune cells can affect the effect of MSCs?
-
Can we develop strategies to enhance the activity of mesenchymal stem cells and overcome the challenges of clinical application?
Introduction
Lung diseases, mainly caused by trauma, viral infections, air pollution, and aging populations, are the leading causes of death worldwide [1, 2]. In recent years, morbidity and mortality caused by acute and chronic lung diseases have gradually increased, becoming a global public health burden [3]. Chronic lung diseases, including chronic obstructive pulmonary disease (COPD), asthma, and idiopathic pulmonary fibrosis (IPF), affect more than 500 million people worldwide [4]. Acute lung injury (ALI) encompasses a wide range of pathological processes that can lead to severe acute respiratory distress syndrome (ARDS) with a mortality rate of up to 40% [5]. Both chronic and acute lung diseases are associated with inflammatory cell infiltration, pro-inflammatory cytokine secretion, alveolar epithelial and endothelial cell injury, and decreased alveolar fluid clearance [6, 7]. In the process of ALI, inflammatory cells such as monocytes and macrophages are activated to release pro-inflammatory factors (IL-6 and IFN-γ) to cause excessive pro-inflammatory reaction, while anti-inflammatory factors (IL-4 and IL-10) are secreted to jointly act on immune cells. The imbalance between pro-inflammatory and anti-inflammatory factors leads to the occurrence of ALI, which further develops into ARDS [8]. Neutrophil activation is an important part of the COPD process, which causes chronic mucous hypersecretion and destruction of lung substance through the release of neutrophil elastase and other bioactive substances [9]. Chronic airway inflammation is the main mechanism of asthma, and neutrophils, eosinophils and T lymphocytes are the main inflammatory cells involved [10]. Activated Th2 cells produce interleukin to activate B lymphocytes and synthesize specific IgE, which binds to IgE receptors on the surface of eosinophils. When the allergen re-enters the body, it binds to the IgE on the cell surface and produces a series of reactions. In addition, activated Th2 cell components of cytokines can also directly activate eosinophils and macrophages, causing them to gather in the airway [11]. In IPF patients, macrophages, neutrophils, and T cells increased significantly in alveolar lavage fluid, and the chemokines and cytokines released by them (such as TGF-β, IL-10, IL-4, IL-13) can promote fibrosis [12].
Modern medicine has thus far failed to pinpoint definitive therapies for inflammatory lung conditions, typically relying on anti-inflammatory agents to alleviate symptoms and reduce lung injury [13]. Presently employed medications, including non-steroidal anti-inflammatory drugs, corticosteroids, and bronchodilators, fail to impede the progression of the disease, while their associated side effects pose an additional challenge [14]. Mesenchymal stem cells (MSCs) have been used to treat various inflammatory diseases owing to their powerful anti-inflammatory, anti-apoptotic and tissue-regenerative properties. MSC-based therapies have become popular in regenerative medicine [15]. Thus, MSCs may be an ideal therapeutic agent for treating lung diseases. In animal models of lung injury, MSCs have been shown to have significant tissue repair effects [16]. These beneficial effects are mediated by multiple mechanisms, including reduced inflammation and permeability of alveolar epithelial and endothelial cells, enhanced alveolar fluid clearance, and reduced oxidative stress responses [15]. One of the reasons for the widespread study of MSCs is their powerful immunomodulatory properties, which play a role by suppressing innate and adaptive immune responses involving multiple immune cells [17]. A growing number of studies have found that MSCs can relieve lung diseases by regulating immune cells (such as neutrophils, macrophages, T cells, and B cells) [18,19,20,21].
This study aimed to summarize the specific mechanisms through which MSCs alleviate lung diseases by regulating immune cells based on their immunomodulatory properties. Therefore, we conducted a comprehensive literature search. In addition, we discuss the current challenges of MSC-based treatment of lung diseases, which will increase the possibility of applying this novel approach in the clinical treatment of lung diseases.
MSCs on the innate immune responses during lung diseases
Neutrophils
The activation and recruitment of neutrophils play important pathological roles in ALI. During acute inflammatory responses, neutrophils rapidly recruit inflamed tissues from the bloodstream via a tightly controlled multi-step recruitment cascade, and are the first white blood cells to reach a site of infection or injury [22]. Activated neutrophils control injured lesions and remove cell debris [23]. Although neutrophil activation is critical for host defense, overactivation releases a variety of toxic substances, including reactive oxygen species (ROS), pro-inflammatory cytokines (such as nuclear factor-κB (NF-κB), Interleukin (IL)-1β, and IL-17), and proteases [24]. Toxic substances released by neutrophils trigger various chemotactic signals that enhance inflammatory responses [23]. Considering the important role of neutrophils in the pathogenesis of ALI, neutrophils targeting is a new approach for ALI treatment. Several studies have shown that MSCs and extracellular vesicles (EVs) inhibit neutrophil migration and infiltration, reduce neutrophil mediated oxidative stress, and release inflammatory factors that may have protective effects on lung injury [25] (Table 1, Fig. 1A).
A MSCs regulate neutrophil. i) MSCs enhance phagocytosis activity; ii) MSCs convert activated neutrophils to senescent neutrophil phenotypes by upregating CD24 expression; iii) MSCs reduce neutrophil infiltration, decrease the expression of TNF-α, IL-6 and IL-1β, and increase IL-10; iv) MSCs derived exosomes can prevent excessive NETs formation by transferring miR-17-5p to target the TLR-4/ROS/MAPK pathway. B MSCs regulate macrophage. i) MSCs inhibit the activation of NLRP3 and NLRC4 inflammasome in macrophages and improved phagocytosis function, thereby inhibiting inflammation; ii) MSCs inhibit macrophage autophagy through miR-384-5p/Beclin-1 and HO-1 signaling pathways; iii) MSC-Exo miR-150-3p inhibits M1 polarization by down-regulating IL-6, IL-1β, iNOS, and promotes M2 polarization by up-regulating IL-10 in LPS-stimulated macrophages; iv) MSC-Exos can transfer stem cell-derived mitochondrial components to alveolar macrophages; v) MSC-Exo miR-451 regulates the MIF-PI3K-AKT signaling pathway to promote the polarization of macrophages M1 to M2; vi) PD-1 expressed on apoptotic bodies interacts with PD-L1 expressed by macrophages to shift macrophage metabolism from glycolysis to oxidative phosphorylation.
Once neutrophils are over-activated, ROS may exceed the cell’s clearance capacity and are released into the extracellular environment in large quantities, causing harm to the lung tissue [26]. ROS can act as both a messenger of tumor necrosis factor (TNF)-induced cell death and a regulator of inflammation-related signaling pathways, such as c-Jun N-terminal kinase (JNK) and NF-κB [27]. A series of studies have demonstrated the anti-oxidative stress effect of MSCs. MSC therapy converts activated neutrophils into senescent neutrophils by upregulating CD24 expression, thereby inhibiting inflammation by reducing ROS production, and nicotinamide adenine dinucleotide phosphate oxidase [28]. Moreover, in a bleomycin-induced PF model, gingival-derived MSCs intervention significantly down-regulated MDA and MPO levels, up-regulated GSH and SOD levels, and alleviated oxidative stress in lung tissue [29]. ILs released by overactivated neutrophils have a variety of functions in inflammation, are associated with the progression of ALI [30]. MSCs and EVs have been shown to reduce the infiltration of neutrophils and proinflammatory cytokines (such as IL-1β, IL-17, TNF-α, and IL-6), while increasing the expression of anti-inflammatory cytokines (such as IL-10) in injured lung tissue [31, 32]. Furthermore, when neutrophils are exposed to large numbers of bacteria and fungi, extracellular DNA and histone, as well as cytoplasmic proteases, antimicrobial peptides and oxidant molecules form neutrophil extracellular traps (NETs). NETs can intensify the inflammatory response during lung injury and promote macrophage polarization to the M1 phenotype [33]. MSCs is a promising NET targeted therapy. Soluble factors secreted by MSCs effectively inhibit NET production, thereby alleviating inflammation [34]. In addition, Chu et al. found that hypoxic-pretreated MSC-derived exosomes could prevent excessive NETs formation by transferring miR-17-5p to target the TLR-4/ROS/MAPK pathway, thereby speeding up wound healing [35]. It can be seen that MSCs affect neutrophils in multiple ways, thereby alleviating various lung diseases.
Macrophages
Macrophages in the lung tissues play a central role in inflammatory responses. Several preclinical studies have shown that MSCs and their secretory factors can repair lung tissue damage by targeting macrophages (Table 1, Fig. 1B). MSCs and their EVs can reduce the infiltration of macrophages, lower the levels of pro-inflammatory cytokines in macrophages, increase the levels of anti-inflammatory factors, as well as improve their phagocytic function, ultimately improve the lung tissue damage [36, 37]. Additionally, macrophage autophagy is closely associated with various lung diseases. Moderate autophagy is thought to protect cells from hypoxia and starvation, whereas overactivated autophagy can lead to apoptosis or necrosis [38]. However, Bone marrow-derived MSCs (BMSCs) and exosomes regulate autophagy in macrophages through phosphoinositide 3 kinase (PI3K)/ Protein Kinase B (Akt)/ heme oxygenase 1 (HO-1) pathway and by delivering miR-384-5p [39].
Under diverse environmental conditions, macrophages can polarize into distinct phenotypes, including classically activated M1 and selectively activated M2 macrophages [40]. When stimulated by LPS or Th1-associated cytokines, such as IFN-γ and TNF-α, macrophages can be polarized into an M1 phenotype. M1 macrophages exhibit heightened production of proinflammatory cytokines, leading to tissue injury, while concurrently facilitating host immune clearance of pathogens. M2 macrophages are usually induced by IL-4, IL-13, TGF-β, and M-CSF, which mainly secrete anti-inflammatory cytokines that promote wound healing and tissue damage repair [41]. Through the maintenance of immune homeostasis within the lung microenvironment, both M1 and M2 macrophages demonstrate the capacity to avert excessive inflammatory responses that precipitate tissue injury [42]. Thus, maintaining the balance between M1 and M2 macrophages is a promising strategy for treating lung injury. MSCs can regulate M1/M2 polarization of macrophages through a variety of specific mechanisms and play an important role in lung injury. Lv et al. found that MSCs mediate macrophage polarization by regulating Stanniocalcin-2, a stress- response protein with antioxidant properties, thereby alleviating lung inflammation and oxidative stress in ALI mice [31]. In addition, MSC-derived exosomes (MSC-Exos) regulate the downstream MIF-PI3K-AKT signaling pathway and inflammatory mediators (down-regulate IL-6, IL-1β; up-regulate IL-10) by delivering different non-coding RNAs (miR-451 and miR-150-3p), thus promoting the polarization of M1 to M2 macrophages [43, 44]. Mitochondria produce energy to support cellular activities, such as cell proliferation, apoptosis, and metabolism. Dysfunctional mitochondria can disrupt the metabolic health of alveolar epithelial cells and macrophages, leading to various lung diseases [45]. It is worth noting that adipose derived mesenchymal stem cell (AdMSC)-Exos can transfer stem cell-derived mitochondrial components to alveolar macrophages, improve the mitochondrial integrity of macrophages, transform macrophages into anti-inflammatory phenotypes, restore immune homeostasis, and thus relieve lung inflammation [46]. Due to the presence of human microenvironment, injected MSCs undergo programmed apoptosis and release apoptotic vesicles. Apoptotic MSCs exhibit distinctive anti-inflammatory effects and exert immunomodulatory effects [47]. Compared to normal human umbilical cord MSCs (hUC-MSCs), apoptotic hUC-MSCs can more effectively reduce inflammatory exudates and vascular permeability in the lungs of ALI rats [48]. Using a mouse model of ALI, Jiang et al. demonstrated that apoptotic bodies released by transplanted hUC-MSCs transformed macrophages from a pro-inflammatory to an anti-inflammatory state. The specific mechanism is that PD-L1 expressed by apoptotic bodies interacts with PD-1 on macrophages, which changes the metabolism of macrophages from glycolysis to oxidative phosphorylation [49]. Recently, nanotechnology utilizing the complete natural cell membrane coating of MSCs has been an emerging platform for targeted therapies. Lu et al. successfully constructed a novel nanoparticle drug carrier system for sepsis management by modifying nanoparticles with LPS-treated BMSC membranes and delivering them to the infectious microenvironment with a silver metal-organic framework as the nanocore, which exerts both anti-inflammatory and antibacterial effects, alleviates cytokine storms, and protects vital organ functions [50].
Eosinophils
Asthma is a chronic inflammatory airway ailment in which eosinophils play a significant role. Eosinophils are end-effector cells involved in allergic diseases. Following the receipt of stimulus signals, eosinophils perform immunomodulatory and proinflammatory functions by releasing various immunomodulatory factors, such as cytokines, chemokines, growth factors, and cytotoxic proteins [51]. Multiple studies have shown that MSCs and their EVs can reduce the number of eosinophils in the lung tissue of asthmatic mice, thereby reducing allergic airway inflammation and remodeling [52,53,54,55] (Table 1). Moreover, Group 2 innate lymphoid cells (ILC2) mediate the activation of eosinophils in the airway during asthma, and ILC2 is associated with persistent pulmonary eosinophilia. Small EVs derived from human mesenchymal cells inhibit ILC2 levels, inflammatory cell infiltration and airway hyperreactivity in asthmatic mice by delivering miR-146a-5p [56]. Therefore, MSCs can relieve asthma by regulating eosinophils and ILC2.
MSCs on the adaptive immune responses during lung diseases
T cells
CD4 + T cells are the main cells involved in the adaptive immune response and play an important role in body development and homeostasis. Under the synergistic effect of T cell receptor stimulation and cytokines, naïve CD4 + T cells differentiate into distinct subpopulations, including Th1, Th2, Th17, and Regulatory T (Tregs) cells [57]. Tregs secrete anti-inflammatory factors (such as TGF-β), promote neutrophil apoptosis, diminish neutrophil numbers, and foster a conducive environment for tissue repair [58]. In addition, Tregs regenerate the alveolar epithelium and induce the proliferation and differentiation of type II alveolar cells into type I cells [59]. Th17 cells produce IL -17, which induces the secretion of pro-inflammatory factors. IL-17 exerts a direct influence on monocytes by facilitating their maturation and extravasation, thereby inducing the recruitment of macrophages [60]. IL-17 also contributes to the generation of oxidizing free radicals, which can exacerbate damage to alveolar epithelial and microvascular endothelial cells [61]. Therefore, the Th17/Treg balance is critical for the progression of lung injury. A ratio of Th17 cells to Tregs greater than 0.79 was an independent predictor of poor prognosis patients [62]. Lung-resident MSCs prevent the differentiation of naïve CD4 + T cells into Th17 cells in vitro, inhibit the production of IL-17 and IL-22 by fully differentiated Th17 cells, and induce a Treg phenotype [63]. Similarly, MSCs transplantation in animals can significantly increase the levels of IL-10, Foxp3 and Tregs in peripheral blood and lung tissue samples, down-regulate the levels of IL-17 and Th17, regulate the balance of Treg/Th17, and ultimately alleviate lung diseases [64, 65].
Following lung injury, immune cells and secreted cytokines form an inflammatory microenvironment that promotes fibrosis [66]. Tu’s team have found that human CD8 + T cells are essential for the induction of PF in mice. MSCs can alleviate PF and improve lung function by inhibiting bleomycin-induced CD8 + T cell invasion and proinflammatory cytokine production, which are related to the regulation of programmed death-1/programmed death-ligand 1 pathway [20]. After MSC treatment, CD8 + T cells in the lungs expressed low levels of CXCR3. MSCs may reduce lung injury caused by neutrophil infiltration by inhibiting CD8 + T cell chemotaxis [67]. In addition to affecting CD8 + T cells, MSCs also reduce inflammatory CD3 + T cell infiltration, expression of inflammatory cytokines TNF-α, IL-6, TGF-β1, and lactate level to improve PF induced by paraquat [68] (Table 2, Fig. 2).
i) MSCs inhibit the production of IL-17 and IL-22 by fully differentiated Th17 cells, and increase IL-10 by induced the Tregs phenotype; MSCs promote proresolving mediators protectin D1, resolvin E1 and regulating Treg/Th17 balance; ii) MSCs alter the CCR2-CCL2 and PD-1/PD-L1 axis, reducing T cell infiltration; MSCs inhibit the proliferation of T cells, which is related to the reduction of iNOS expression in MSCs and the phosphorylation of STAT5 in T cells; iii) MSCs inhibit the proliferation and differentiation of B cells into plasma cells and the production of IgM and IgG; iv) MSCs influence the dynamics of B-cell recruitment by inhibiting the chemokine CXCL13; v) MSCs reduce the expression of CD205, TLR9, and CD14 and IRAK-4 expression.
B cells
In conjunction with T cells, B cells are vital components of the adaptive immune response. They function as proficient antigen-presenting cells capable of producing a spectrum of proinflammatory and anti-inflammatory cytokines. Furthermore, B cells can generate terminally differentiated antibody-secreting plasma cells [69]. In respiratory diseases, the infiltration of B cells and production of immunoglobulins (such as IgM, IgG, and IgE) are correlated with disease severity [70]. Extensive studies have shown that MSCs inhibit the proliferation and differentiation of B cells into plasma cells and the production of immunoglobulins (IgM and IgG) [71,72,73]. CCL3 and CCL4 promote local inflow of neutrophils in the body, while some reports suggest that B cells express and secrete CCL3/4 [74]. Feng et al. found that MSC treatment inhibited the expression of the lung B-cell chemokine CCL4 in ALI mice, thereby reducing the entry of neutrophils into the injured lung tissue. In addition, the expression of the immunoglobulin-related genes Iglc2, Iglc3, and Ighd was reduced [75]. HAMSCs also influence the dynamics of B-cell recruitment and homing in bleomycin-induced PF by inhibiting the chemokine CXCL13 [21]. CpG ODN-2006, a synthetic oligonucleotide, induces B-cell proliferation and differentiation [76]. Parolini et al. explored the mechanism of action of hAMSCs on B cells. They found that hAMSCs could influence early CpG-induced B cell stimulation by reducing the expression of three major CpG sensors (CD205, TLR9, and CD14), thereby inhibiting downstream inflammatory signaling pathways [77] (Table 2, Fig. 2).
Challenges for MSC therapy in lung diseases
Although the transplantation of MSCs has the potential to relieve lung diseases, the largescale application of MSCs in clinical settings still faces great challenges. Currently, the clinical applications of MSCs in treating lung diseases are mainly focused on severe coronavirus disease 2019 and acute respiratory distress syndrome, all of which are in phase I/II, with no largescale phase III clinical trials conducted.
Firstly, MSCs are heterogeneous cell populations, and MSCs from different donors and tissues exhibit different characteristics [78]. Researchers have compared MSCs derived from the umbilical cord, amniotic membrane, and bone marrow and found that although the three expressed similar surface markers, they had certain differences in paracrine factors, immunomodulatory ability, and regenerative support ability [79]. Tai et al. demonstrated that MSCs derived from different types of placental tissues exhibit distinct biological characteristics. Furthermore, heterogeneity was observed among MSCs of the same type sourced from different individuals [80]. With an increase in donor age, the multi-lineage differentiation, homing, immune regulation, and oxidative stress regulation of MSCs gradually decline and disappear [81]. In addition, the in vitro inoculation density of MSCs, different components in the growth medium (serum and growth factor combination), and oxygen concentration may affect the gene profile, epigenomic status, and phenotype of the cells [82]. As the number of cell passages increases, the expression profile of MSCs also changes; therefore, the most commonly used therapeutic passages are three to seven [83]. The result of the heterogeneity is a complexity that leads to challenges in their identification. Because the MSC subpopulation is not clearly defined and is difficult to distinguish, different combinations of markers are needed to confirm MSCs of different origins. The most commonly used markers are CD29, CD44, CD73, CD90, and CD105, whereas among the negative markers, CD34, CD45, and HLA-DR are the most commonly used [84]. Therefore, ensuring the quality, purity, potency, and stability of MSCs is one of the urgent challenges.
Secondly, the safety of MSC transplantation in vivo is a major challenge for its clinical application, including concerns about immunocompatibility, tumorigenicity, and microvascular occlusion [85]. Currently, most administrations are intravenous, and the limited number of MSCs reaching the injury site, along with a low survival rate, have restricted their therapeutic effectiveness owing to the presence of the immune microenvironment [86]. Apoptosis or autophagy occurs rapidly in MSCs following systemic or intratracheal administration [87]. Appropriate pretreatment of the cell culture medium can potentially modify the properties and therapeutic efficacy of MSCs. Preconditioning includes strategies, such as transgenics, exposure to hypoxia, administration of inflammatory factors, use of bioactive compounds, cultivation in 3D cultures, co-culture with disease-associated cells, or supplementation with patient serum. Furthermore, the clinical translation of stem cells requires ethical and safety considerations. MSCs’ ability to self-renew may pose a risk of tumor formation after entering the body [88]. The pro-tumor effects of MSCs manifest through various mechanisms within the tumor microenvironment, including differentiation into stromal components of the tumor microenvironment, suppression of immune responses, promotion of angiogenesis, enhancement of tumor cell survival, and facilitation of metastasis [89]. Moreover, there is a risk of thrombus formation when MSCs are transfused in vivo. In a Phase I/IIa study using Wharton’s jelly-derived MSCs for compression fractures, one patient in the stem cell treatment group developed pulmonary embolism [90]. Compared to observations of MSCs, MSC-EVs have higher biocompatibility and do not show evidence of carcinogenic capacity or a negative immune response [91, 92]. Additionally, MSC-EVs are easier to store and exhibit extremely high stability. They also demonstrate good biobarrier penetration and reduce the risk of microvascular embolization [93]. However, the lack of largescale production and extraction technologies, recognized standardized EV separation and characterization technologies, and handling and storage technologies have limited the clinical application of MSC-EVs [94]. In addition, determining the optimal route of administration and therapeutic dose required for different diseases using both MSCs and MSC-EVs is challenging [95, 96]. These issues have limited the clinical application of MSCs, and further research is needed to overcome these challenges.
Conclusions
Effective treatments for lung diseases are still lacking, and researchers are striving to find new and effective drugs. MSCs and their derived secretomes exhibit protective effects against lung diseases, suggesting a potential therapeutic approach. Immune cells play crucial roles in the progression of lung diseases. MSCs have immunomodulatory properties; they can act on neutrophils, macrophages, T cells, and B cells; and play a role in both innate and adaptive immune responses during lung diseases. However, MSCs and their secretomes face challenges in clinical applications, such as heterogeneity, unsafe transformation in vivo, low survival rate, and lack of standardized methods for the isolation, extraction, and storage of EVs. In the future, more comprehensive basic research and larger clinical trials are required to address these issues.
Data availability
The information supporting this study’s findings is available in this article.
References
Murray CJL. COVID-19 will continue but the end of the pandemic is near. Lancet. 2022;399:417–9.
Meng M, Zhang WW, Chen SF, Wang DR, Zhou CH. Therapeutic utility of human umbilical cord-derived mesenchymal stem cells-based approaches in pulmonary diseases: Recent advancements and prospects. World J Stem Cells. 2024;16:70–88.
Mattila T, Jormanainen V, Erhola M, Vasankari T, Toppila-Salmi S, Herse F, et al. Real-world drug use in asthma, chronic obstructive pulmonary disease, rhinitis, cough, and cold in Finland from 1990 to 2021: Association with reduced disease burden. Clin Transl Allergy. 2024;14:e12340.
GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8:585–96.
Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18.
Melo MM, Cruz FF, Rocco PRM. Mesenchymal stromal cell therapy for chronic lung diseases: experimental and clinical evidence. Expert Rev Respir Med. 2023;17:223–35.
Zhu W, Zhang Y, Wang Y. Immunotherapy strategies and prospects for acute lung injury: Focus on immune cells and cytokines. Front Pharmacol. 2022;13:1103309.
Yang SC, Tsai YF, Pan YL, Hwang TL. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed J. 2021;44:439–46.
Chen Z, Li W, Tang Y, Zhou P, He Q, Deng Z. The neutrophil-lymphocyte ratio predicts all-cause and cardiovascular mortality among United States adults with COPD: results from NHANES 1999-2018. Front Med (Lausanne). 2024;11:1443749.
Kermani NZ, Li CX, Versi A, Badi Y, Sun K, Abdel-Aziz MI, et al. Endotypes of severe neutrophilic and eosinophilic asthma from multi-omics integration of U-BIOPRED sputum samples. Clin Transl Med. 2024;14:e1771.
Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J Allergy Clin Immunol. 2019;144:1–12.
Jia C, Yang M, Xiao G, Zeng Z, Li L, Li Y, et al. ESL attenuates BLM-induced IPF in mice: Dual mediation of the TLR4/NF-κB and TGF-β1/PI3K/Akt/FOXO3a pathways. Phytomedicine. 2024;132:155545.
Loo CY, Lee WH. Nanotechnology-based therapeutics for targeting inflammatory lung diseases. Nanomedicine (Lond). 2022;17:865–79.
Li J, Chen W, Liu H, Liu H, Xiang S, You F, et al. Pharmacologic effects approach of essential oils and their components on respiratory diseases. J Ethnopharmacol. 2023;304:115962.
Zhuang X, Jiang Y, Yang X, Fu L, Luo L, Dong Z, et al. Advances of mesenchymal stem cells and their derived extracellular vesicles as a promising therapy for acute respiratory distress syndrome: from bench to clinic. Front Immunol. 2023;14:1244930.
Hu Q, Zhang S, Yang Y, Yao JQ, Tang WF, Lyon CJ, et al. Extracellular vesicles in the pathogenesis and treatment of acute lung injury. Mil Med Res. 2022;9:61.
Xu W, Yang Y, Li N, Hua J. Interaction between Mesenchymal Stem Cells and Immune Cells during Bone Injury Repair. Int J Mol Sci. 2023;24:14484
Liu C, Xiao K, Xie L. Advances in the Regulation of Macrophage Polarization by Mesenchymal Stem Cells and Implications for ALI/ARDS Treatment. Front Immunol. 2022;13:928134.
van Dalen SCM, Blom AB, Walgreen B, Slöetjes AW, Helsen MMA, Geven EJW, et al. IL-1β-Mediated Activation of Adipose-Derived Mesenchymal Stromal Cells Results in PMN Reallocation and Enhanced Phagocytosis: A Possible Mechanism for the Reduction of Osteoarthritis Pathology. Front Immunol. 2019;10:1075.
Ni K, Liu M, Zheng J, Wen L, Chen Q, Xiang Z, et al. PD-1/PD-L1 Pathway Mediates the Alleviation of Pulmonary Fibrosis by Human Mesenchymal Stem Cells in Humanized Mice. Am J Respir Cell Mol Biol. 2018;58:684–95.
Cargnoni A, Romele P, Bonassi Signoroni P, Farigu S, Magatti M, Vertua E, et al. Amniotic MSCs reduce pulmonary fibrosis by hampering lung B-cell recruitment, retention, and maturation. Stem Cells Transl Med. 2020;9:1023–35.
Petri B, Sanz MJ. Neutrophil chemotaxis. Cell Tissue Res. 2018;371:425–36.
Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307.
Wang SW, Zhang Q, Lu D, Fang YC, Yan XC, Chen J, et al. GPR84 regulates pulmonary inflammation by modulating neutrophil functions. Acta Pharmacol Sin. 2023;44:1665–75.
Yang T, Xiang CG, Wang XH, Li QQ, Lei SY, Zhang KR, et al. RIPK1 inhibitor ameliorates pulmonary injury by modulating the function of neutrophils and vascular endothelial cells. Cell Death Discov. 2024;10:152.
Xue Y, Zhang Y, Chen L, Wang Y, Lv Z, Yang LQ, et al. Citrulline protects against LPS‑induced acute lung injury by inhibiting ROS/NLRP3‑dependent pyroptosis and apoptosis via the Nrf2 signaling pathway. Exp Ther Med. 2022;24:632.
Morgan MJ, Liu ZG. Reactive oxygen species in TNFalpha-induced signaling and cell death. Mol Cells. 2010;30:1–12.
Feng B, Feng X, Yu Y, Xu H, Ye Q, Hu R, et al. Mesenchymal stem cells shift the pro-inflammatory phenotype of neutrophils to ameliorate acute lung injury. Stem Cell Res Ther. 2023;14:197.
Wang X, Zhao S, Lai J, Guan W. Gao Y. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Gingival-Derived MSCs on Bleomycin-Induced Pulmonary Fibrosis in Mice. Int J Mol Sci. 2021;23:99
De Alessandris S, Ferguson GJ, Dodd AJ, Juss JK, Devaprasad A, Piper S, et al. Neutrophil GM-CSF receptor dynamics in acute lung injury. J Leukoc Biol. 2019;105:1183–94.
Lv H, Liu Q, Sun Y, Yi X, Wei X, Liu W, et al. Mesenchymal stromal cells ameliorate acute lung injury induced by LPS mainly through stanniocalcin-2 mediating macrophage polarization. Ann Transl Med. 2020;8:334.
Hezam K, Wang C, Fu E, Zhou M, Liu Y, Wang H, et al. Superior protective effects of PGE2 priming mesenchymal stem cells against LPS-induced acute lung injury (ALI) through macrophage immunomodulation. Stem Cell Res Ther. 2023;14:48.
Song C, Li H, Li Y, Dai M, Zhang L, Liu S, et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization. Exp Cell Res. 2019;382:111486.
Navas A, Magaña-Guerrero FS, Domínguez-López A, Chávez-García C, Partido G, Graue-Hernández EO, et al. Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair. Stem Cells Transl Med. 2018;7:906–17.
Chu Z, Huang Q, Ma K, Liu X, Zhang W, Cui S, et al. Novel neutrophil extracellular trap-related mechanisms in diabetic wounds inspire a promising treatment strategy with hypoxia-challenged small extracellular vesicles. Bioact Mater. 2023;27:257–70.
Tu C, Wang Z, Xiang E, Zhang Q, Zhang Y, Wu P, et al. Human Umbilical Cord Mesenchymal Stem Cells Promote Macrophage PD-L1 Expression and Attenuate Acute Lung Injury in Mice. Curr Stem Cell Res Ther. 2022;17:564–75.
Xia TT, Hu R, Shao CJ, Feng Y, Yang XL, Xie YP, et al. Stanniocalcin-1 secreted by human umbilical mesenchymal stem cells regulates interleukin-10 expression via the PI3K/AKT/mTOR pathway in alveolar macrophages. Cytokine. 2023;162:156114.
Zeki AA, Yeganeh B, Kenyon NJ, Post M, Ghavami S. Autophagy in airway diseases: a new frontier in human asthma? Allergy. 2016;71:5–14.
Wang NF, Bai CX. Bone marrow-derived mesenchymal stem cells modulate autophagy in RAW264.7 macrophages via the phosphoinositide 3-kinase/protein kinase B/heme oxygenase-1 signaling pathway under oxygen-glucose deprivation/restoration conditions. Chin Med J (Engl). 2021;134:699–707.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95.
Aggarwal NR, King LS. D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–25.
Liu J, Xing F, Fu Q, He B, Jia Z, Du J, et al. hUC-MSCs exosomal miR-451 alleviated acute lung injury by modulating macrophage M2 polarization via regulating MIF-PI3K-AKT signaling pathway. Environ Toxicol. 2022;37:2819–31.
Liang G, Feng Y, Tang W, Yao L, Huang C, Chen Y. Proinflammatory Bone Marrow Mesenchymal Stem Cell-Derived Exosomal miR-150-3p Suppresses Proinflammatory Polarization of Alveolar Macrophages in Sepsis by Targeting Inhibin Subunit Beta A. J Interferon Cytokine Res. 2023;43:518–30.
Riou M, Alfatni A, Charles AL, Andrès E, Pistea C, Charloux A. New Insights into the Implication of Mitochondrial Dysfunction in Tissue, Peripheral Blood Mononuclear Cells, and Platelets during Lung Diseases. J Clin Med. 2020;9:1253
Xia L, Zhang C, Lv N, Liang Z, Ma T, Cheng H, et al. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. 2022;12:2928–47.
Thum T, Bauersachs J, Poole-Wilson PA, Volk HD, Anker SD. The dying stem cell hypothesis: immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle. J Am Coll Cardiol. 2005;46:1799–802.
Liu FB, Lin Q, Liu ZW. A study on the role of apoptotic human umbilical cord mesenchymal stem cells in bleomycin-induced acute lung injury in rat models. Eur Rev Med Pharmacol Sci. 2016;20:969–82.
Jiang T, Xia Y, Wang W, Zhao J, Liu W, Liu S, et al. Apoptotic bodies inhibit inflammation by PDL1-PD1-mediated macrophage metabolic reprogramming. Cell Prolif. 2024;57:e13531.
Lu L, Quan L, Li J, Yuan J, Nie X, Huang X, et al. Bioengineered stem cell membrane functionalized nanoparticles combine anti-inflammatory and antimicrobial properties for sepsis treatment. J Nanobiotechnology. 2023;21:170.
Shen K, Zhang M, Zhao R, Li Y, Li C, Hou X, et al. Eosinophil extracellular traps in asthma: implications for pathogenesis and therapy. Respir Res. 2023;24:231.
Mo Y, Kang H, Bang JY, Shin JW, Kim HY, Cho SH, et al. Intratracheal administration of mesenchymal stem cells modulates lung macrophage polarization and exerts anti-asthmatic effects. Sci Rep. 2022;12:11728.
Abreu SC, Xisto DG, de Oliveira TB, Blanco NG, de Castro LL, Kitoko JZ, et al. Serum from Asthmatic Mice Potentiates the Therapeutic Effects of Mesenchymal Stromal Cells in Experimental Allergic Asthma. Stem Cells Transl Med. 2019;8:301–12.
Fang SB, Zhang HY, Meng XC, Wang C, He BX, Peng YQ, et al. Small extracellular vesicles derived from human MSCs prevent allergic airway inflammation via immunomodulation on pulmonary macrophages. Cell Death Dis. 2020;11:409.
Ren J, Liu Y, Yao Y, Feng L, Zhao X, Li Z, et al. Intranasal delivery of MSC-derived exosomes attenuates allergic asthma via expanding IL-10 producing lung interstitial macrophages in mice. Int Immunopharmacol. 2021;91:107288.
Fang SB, Zhang HY, Wang C, He BX, Liu XQ, Meng XC, et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J Extracell Vesicles. 2020;9:1723260.
Guan T, Zhou X, Zhou W, Lin H. Regulatory T cell and macrophage crosstalk in acute lung injury: future perspectives. Cell Death Discov. 2023;9:9.
Lewkowicz P, Lewkowicz N, Sasiak A, Tchórzewski H. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J Immunol. 2006;177:7155–63.
Mock JR, Dial CF, Tune MK, Gilmore RC, O’Neal WK, Dang H, et al. Impact of Regulatory T Cells on Type 2 Alveolar Epithelial Cell Transcriptomes during Resolution of Acute Lung Injury and Contributions of IFN-γ. Am J Respir Cell Mol Biol. 2020;63:464–77.
Raucci F, Saviano A, Casillo GM, Guerra-Rodriguez M, Mansour AA, Piccolo M, et al. IL-17-induced inflammation modulates the mPGES-1/PPAR-γ pathway in monocytes/macrophages. Br J Pharmacol. 2022;179:1857–73.
Ge S, Hu J, Gao S, Ren J, Zhu G. LncRNA NEAT1: A novel regulator associated with the inflammatory response in acute respiratory distress syndrome. Gene. 2023;878:147582.
Yu ZX, Ji MS, Yan J, Cai Y, Liu J, Yang HF, et al. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit Care. 2015;19:82.
Wang L, Shi M, Tong L, Wang J, Ji S, Bi J, et al. Lung-Resident Mesenchymal Stem Cells Promote Repair of LPS-Induced Acute Lung Injury via Regulating the Balance of Regulatory T cells and Th17 cells. Inflammation. 2019;42:199–210.
Qian HB, Zou G, Li C, He QF, Liu J. Bone marrow mesenchymal stem cells attenuate LPS-induced acute lung injury in mice by promoting RvE1/ProD1 and modulating Treg/Th17 balance. Turk J Biol. 2022;46:173–85.
Li Y, Li H, Cao Y, Wu F, Ma W, Wang Y, et al. Placenta‑derived mesenchymal stem cells improve airway hyperresponsiveness and inflammation in asthmatic rats by modulating the Th17/Treg balance. Mol Med Rep. 2017;16:8137–45.
Liu M, Ren D, Wu D, Zheng J, Tu W. Stem Cell and Idiopathic Pulmonary Fibrosis: Mechanisms and Treatment. Curr Stem Cell Res Ther. 2015;10:466–76.
Zhu J, Feng B, Xu Y, Chen W, Sheng X, Feng X, et al. Mesenchymal stem cells alleviate LPS-induced acute lung injury by inhibiting the proinflammatory function of Ly6C(+) CD8(+) T cells. Cell Death Dis. 2020;11:829.
He F, Wang Y, Li Y, Yu L. Human amniotic mesenchymal stem cells alleviate paraquat-induced pulmonary fibrosis in rats by inhibiting the inflammatory response. Life Sci. 2020;243:117290.
Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27.
Cheng G, Zhang N, Wang Y, Rui J, Yin X, Cui T. Antibodies of IgG, IgA and IgM against Human Bronchial Epithelial Cell in Patients with Chronic Obstructive Pulmonary Disease. Clin Lab. 2016;62:1101–8.
Feng X, Feng B, Zhou J, Yang J, Pan Q, Yu J, et al. Mesenchymal stem cells alleviate mouse liver fibrosis by inhibiting pathogenic function of intrahepatic B cells. Hepatology. 2024.
Kim DK, Lee HJ, Lee IH, Lee JJ. Immunomodulatory Effects of Primed Tonsil-Derived Mesenchymal Stem Cells on Atopic Dermatitis via B Cell Regulation. Cells. 2023;13:80
Luk F, Carreras-Planella L, Korevaar SS, de Witte SFH, Borràs FE, Betjes MGH, et al. Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front Immunol. 2017;8:1042.
Benet ZL, Marthi M, Ke F, Wu R, Turner JS, Gabayre JB, et al. CCL3 Promotes Germinal Center B Cells Sampling by Follicular Regulatory T Cells in Murine Lymph Nodes. Front Immunol. 2018;9:2044.
Feng B, Zhu J, Xu Y, Chen W, Sheng X, Feng X, et al. Immunosuppressive effects of mesenchymal stem cells on lung B cell gene expression in LPS-induced acute lung injury. Stem Cell Res Ther. 2020;11:418.
Huggins J, Pellegrin T, Felgar RE, Wei C, Brown M, Zheng B, et al. CpG DNA activation and plasma-cell differentiation of CD27- naive human B cells. Blood. 2007;109:1611–9.
Magatti M, Masserdotti A, Bonassi Signoroni P, Vertua E, Stefani FR, Silini AR, et al. B Lymphocytes as Targets of the Immunomodulatory Properties of Human Amniotic Mesenchymal Stromal Cells. Front Immunol. 2020;11:1156.
Costa LA, Eiro N, Fraile M, Gonzalez LO, Saá J, Garcia-Portabella P, et al. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci. 2021;78:447–67.
Wegmeyer H, Bröske AM, Leddin M, Kuentzer K, Nisslbeck AK, Hupfeld J, et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013;22:2606–18.
Tai C, Wang L, Xie Y, Gao T, Huang F, Wang B. Analysis of Key Distinct Biological Characteristics of Human Placenta-Derived Mesenchymal Stromal Cells and Individual Heterogeneity Attributing to Donors. Cells Tissues Organs. 2021;210:45–57.
Wang Z, Chai C, Wang R, Feng Y, Huang L, Zhang Y, et al. Single-cell transcriptome atlas of human mesenchymal stem cells exploring cellular heterogeneity. Clin Transl Med. 2021;11:e650.
Rennerfeldt DA, Van Vliet KJ. Concise Review: When Colonies Are Not Clones: Evidence and Implications of Intracolony Heterogeneity in Mesenchymal Stem Cells. Stem Cells. 2016;34:1135–41.
Selich A, Daudert J, Hass R, Philipp F, von Kaisenberg C, Paul G, et al. Massive Clonal Selection and Transiently Contributing Clones During Expansion of Mesenchymal Stem Cell Cultures Revealed by Lentiviral RGB-Barcode Technology. Stem Cells Transl Med. 2016;5:591–601.
Maličev E, Jazbec K. An Overview of Mesenchymal Stem Cell Heterogeneity and Concentration. Pharmaceuticals (Basel). 2024;17:350
Cao C, Zhang L, Liu F, Shen J. Therapeutic Benefits of Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome: Potential Mechanisms and Challenges. J Inflamm Res. 2022;15:5235–46.
Li M, Jiang Y, Hou Q, Zhao Y, Zhong L, Fu X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: current status and future prospects. Stem Cell Res Ther. 2022;13:146.
Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824–33.
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, et al. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36–45.
Afkhami H, Mahmoudvand G, Fakouri A, Shadab A, Mahjoor M, Komeili Movahhed T. New insights in application of mesenchymal stem cells therapy in tumor microenvironment: pros and cons. Front Cell Dev Biol. 2023;11:1255697.
Shim J, Kim KT, Kim KG, Choi UY, Kyung JW, Sohn S, et al. Safety and efficacy of Wharton’s jelly-derived mesenchymal stem cells with teriparatide for osteoporotic vertebral fractures: A phase I/IIa study. Stem Cells Transl Med. 2021;10:554–67.
Yoshida T, Ishidome T, Hanayama R. High Purity Isolation and Sensitive Quantification of Extracellular Vesicles Using Affinity to TIM4. Curr Protoc Cell Biol. 2017;77:3.45.1-3..18.
Hosseini NF, Dalirfardouei R, Aliramaei MR, Najafi R. Stem cells or their exosomes: which is preferred in COVID-19 treatment? Biotechnol Lett. 2022;44:159–77.
Cheng L, Yu P, Li F, Jiang X, Jiao X, Shen Y, et al. Human umbilical cord-derived mesenchymal stem cell-exosomal miR-627-5p ameliorates non-alcoholic fatty liver disease by repressing FTO expression. Hum Cell. 2021;34:1697–708.
Guo G, Tan Z, Liu Y, Shi F, She J. The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer. Stem Cell Res Ther. 2022;13:138.
Ahmed L, Al-Massri K. New Approaches for Enhancement of the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cardiovascular Diseases. Tissue Eng Regen Med. 2022;19:1129–46.
Didamoony MA, Soubh AA, Atwa AM, Ahmed LA. Innovative preconditioning strategies for improving the therapeutic efficacy of extracellular vesicles derived from mesenchymal stem cells in gastrointestinal diseases. Inflammopharmacology. 2023;31:2973–93.
Li LL, Zhu YG, Jia XM, Liu D, Qu JM. Adipose-Derived Mesenchymal Stem Cells Ameliorating Pseudomonas aeruginosa-induced Acute Lung Infection via Inhibition of NLRC4 Inflammasome. Front Cell Infect Microbiol. 2020;10:581535.
Li R, Li Y, Dong X. Preconditioning mesenchymal stromal cells with flagellin enhances the anti‑inflammatory ability of their secretome against lipopolysaccharide‑induced acute lung injury. Mol Med Rep. 2020;22:2753–66.
Sadeghi S, Mosaffa N, Hashemi SM, Mehdi Naghizadeh M, Ghazanfari T. The immunomodulatory effects of mesenchymal stem cells on long term pulmonary complications in an animal model exposed to a sulfur mustard analog. Int Immunopharmacol. 2020;80:105879.
Yuan W, Song HY, Xiong J, Jiang WL, Kang GJ, Huang J, et al. Placenta‑derived mesenchymal stem cells ameliorate lipopolysaccharide‑induced inflammation in RAW264.7 cells and acute lung injury in rats. Mol Med Rep. 2020;22:1458–66.
Liu X, Gao C, Wang Y, Niu L, Jiang S, Pan S. BMSC-Derived Exosomes Ameliorate LPS-Induced Acute Lung Injury by miR-384-5p-Controlled Alveolar Macrophage Autophagy. Oxid Med Cell Longev. 2021;2021:9973457.
Lv H, Yuan X, Zhang J, Lu T, Yao J, Zheng J, et al. Heat shock preconditioning mesenchymal stem cells attenuate acute lung injury via reducing NLRP3 inflammasome activation in macrophages. Stem Cell Res Ther. 2021;12:290.
Deng H, Zhu L, Zhang Y, Zheng L, Hu S, Zhou W, et al. Differential Lung Protective Capacity of Exosomes Derived from Human Adipose Tissue, Bone Marrow, and Umbilical Cord Mesenchymal Stem Cells in Sepsis-Induced Acute Lung Injury. Oxid Med Cell Longev. 2022;2022:7837837.
Wang G, Zhang Y, Hu N, Liu Q, Ma F, Xie J. Mesenchymal Stem Cells Attenuate Acute Lung Injury in Mice Partly by Suppressing Alveolar Macrophage Activation in a PGE2-Dependent Manner. Inflammation. 2022;45:2000–15.
Wang X, Liu D, Zhang X, Yang L, Xia Z, Zhang Q. Exosomes from adipose-derived mesenchymal stem cells alleviate sepsis-induced lung injury in mice by inhibiting the secretion of IL-27 in macrophages. Cell Death Discov. 2022;8:18.
Xu L, Zhu Y, Li C, Wang Q, Ma L, Wang J, et al. Small extracellular vesicles derived from Nrf2-overexpressing human amniotic mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting NLRP3. Biol Direct. 2022;17:35.
Xu Z, Lin L, Fan Y, Huselstein C, De Isla N, He X. Secretome of Mesenchymal Stem Cells from Consecutive Hypoxic Cultures Promotes Resolution of Lung Inflammation by Reprogramming Anti-Inflammatory Macrophages. Int J Mol Sci. 2022;23:4333
Zhao R, Wang L, Wang T, Xian P, Wang H, Long Q. Inhalation of MSC-EVs is a noninvasive strategy for ameliorating acute lung injury. J Control Release. 2022;345:214–30.
Chen Y, Wang L, Liu M, Zhao J, Xu X, Wei D, et al. Mechanism of exosomes from adipose-derived mesenchymal stem cells on sepsis-induced acute lung injury by promoting TGF-β secretion in macrophages. Surgery. 2023;174:1208–19.
Jiang T, Xia Y, Wang W, Zhao J, Liu W, Liu S. Apoptotic bodies inhibit inflammation by PDL1-PD1-mediated macrophage metabolic reprogramming. Cell Prolif. 2023;57:e13531
Kim YE, Sung DK, Bang Y, Sung SI, Yang M, Ahn SY. SOCS3 Protein Mediates the Therapeutic Efficacy of Mesenchymal Stem Cells against Acute Lung Injury. Int J Mol Sci. 2023;24:8256
Zhang TY, Zhang H, Deng JY, Gong HR, Yan Y, Zhang Z, et al. BMMSC-derived Exosomes Attenuate Cardiopulmonary Bypass-related Acute Lung Injury by Reducing Inflammatory Response and Oxidative Stress. Curr Stem Cell Res Ther. 2023;18:720–8.
Deng H, Wu L, Liu M, Zhu L, Chen Y, Zhou H, et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Attenuate LPS-Induced ARDS by Modulating Macrophage Polarization Through Inhibiting Glycolysis in Macrophages. Shock. 2020;54:828–43.
Xu H, Nie G, Yin T, Shao C, Ding D, Zou M. Umbilical Cord-derived Mesenchymal Stem Cells with Surfactant Protein B Alleviates Inflammatory Response in Acute Respiratory Distress Syndrome by Regulating Macrophage Polarization. Balkan Med J. 2022;39:130–9.
Wang LT, Yen BL, Wang HH, Chao YY, Lee W, Huang LY, et al. Placental mesenchymal stem cells boost M2 alveolar over M1 bone marrow macrophages via IL-1β in Klebsiella-mediated acute respiratory distress syndrome. Thorax. 2023;78:504–14.
Dong B, Wang C, Zhang J, Zhang J, Gu Y, Guo X, et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res Ther. 2021;12:204.
Kim RL, Bang JY, Kim J, Mo Y, Kim Y, Lee CG, et al. Mesenchymal stem cells exert their anti-asthmatic effects through macrophage modulation in a murine chronic asthma model. Sci Rep. 2022;12:9811.
Mo Y, Kim Y, Bang JY, Jung J, Lee CG, Elias JA, et al. Mesenchymal Stem Cells Attenuate Asthmatic Inflammation and Airway Remodeling by Modulating Macrophages/Monocytes in the IL-13-Overexpressing Mouse Model. Immune Netw. 2022;22:e40.
Braza F, Dirou S, Forest V, Sauzeau V, Hassoun D, Chesné J, et al. Mesenchymal Stem Cells Induce Suppressive Macrophages Through Phagocytosis in a Mouse Model of Asthma. Stem Cells. 2016;34:1836–45.
Abreu SC, Lopes-Pacheco M, da Silva AL, Xisto DG, de Oliveira TB, Kitoko JZ, et al. Eicosapentaenoic Acid Enhances the Effects of Mesenchymal Stromal Cell Therapy in Experimental Allergic Asthma. Front Immunol. 2018;9:1147.
Choi SM, Mo Y, Bang JY, Ko YG, Ahn YH, Kim HY, et al. Classical monocyte-derived macrophages as therapeutic targets of umbilical cord mesenchymal stem cells: comparison of intratracheal and intravenous administration in a mouse model of pulmonary fibrosis. Respir Res. 2023;24:68.
Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med. 2018;197:104–16.
Porzionato A, Zaramella P, Dedja A, Guidolin D, Bonadies L, Macchi V, et al. Intratracheal administration of mesenchymal stem cell-derived extracellular vesicles reduces lung injuries in a chronic rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2021;320:L688–l704.
Kim M, Kwon JH, Bae YK, Kim GH, Um S, Ha J, et al. Soluble PTX3 of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Attenuates Hyperoxic Lung Injury by Activating Macrophage Polarization in Neonatal Rat Model. Stem Cells Int. 2020;2020:1802976.
Kim YE, Ahn SY, Sung DK, Chang YS, Park WS. Mesenchymal Stem Cells and Formyl Peptide Receptor 2 Activity in Hyperoxia-Induced Lung Injury in Newborn Mice. Int J Mol Sci. 2022;23:10604
Su W, Nong Q, Wu J, Fan R, Liang Y, Hu A, et al. Anti-inflammatory protein TSG-6 secreted by BMSCs attenuates silica-induced acute pulmonary inflammation by inhibiting NLRP3 inflammasome signaling in macrophages. Int J Biol Macromol. 2023;253:126651.
Klinger JR, Pereira M, Del Tatto M, Brodsky AS, Wu KQ, Dooner MS, et al. Mesenchymal Stem Cell Extracellular Vesicles Reverse Sugen/Hypoxia Pulmonary Hypertension in Rats. Am J Respir Cell Mol Biol. 2020;62:577–87.
Chen X, Wei Q, Sun H, Zhang X, Yang C, Tao Y, et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Regulate Macrophage Polarization to Attenuate Systemic Lupus Erythematosus-Associated Diffuse Alveolar Hemorrhage in Mice. Int J Stem Cells. 2021;14:331–40.
Bao P, Zhao W, Mou M, Liu X. MicroRNA-21 mediates bone marrow mesenchymal stem cells protection of radiation-induced lung injury during the acute phase by regulating polarization of alveolar macrophages. Transl Cancer Res. 2020;9:231–9.
Takao S, Nakashima T, Masuda T, Namba M, Sakamoto S, Yamaguchi K, et al. Human bone marrow-derived mesenchymal stromal cells cultured in serum-free media demonstrate enhanced antifibrotic abilities via prolonged survival and robust regulatory T cell induction in murine bleomycin-induced pulmonary fibrosis. Stem Cell Res Ther. 2021;12:506.
Sun J, Han ZB, Liao W, Yang SG, Yang Z, Yu J, et al. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem. 2011;27:587–96.
Ge X, Bai C, Yang J, Lou G, Li Q, Chen R. Intratracheal transplantation of bone marrow-derived mesenchymal stem cells reduced airway inflammation and up-regulated CD4+CD25+ regulatory T cells in asthmatic mouse. Cell Biol Int. 2013;37:675–86.
Cahill EF, Tobin LM, Carty F, Mahon BP, English K. Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther. 2015;6:19.
Cho KS, Park MK, Kang SA, Park HY, Hong SL, Park HK, et al. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediators Inflamm. 2014;2014:436476.
Kang SY, Park DE, Song WJ, Bae BR, Lee JW, Sohn KH, et al. Immunologic regulatory effects of human umbilical cord blood-derived mesenchymal stem cells in a murine ovalbumin asthma model. Clin Exp Allergy. 2017;47:937–45.
Nozari P, Mokhtari P, Nemati M, Zainodini N, Taghipour Z, Asadi F, et al. Investigation of the effect of IFN-γ/TNF-α-treated mesenchymal stem cells on Th9- and Treg cell-related parameters in a mouse model of ovalbumin-induced allergic asthma. Immunopharmacol Immunotoxicol. 2022;44:773–85.
Lathrop MJ, Brooks EM, Bonenfant NR, Sokocevic D, Borg ZD, Goodwin M, et al. Mesenchymal stromal cells mediate Aspergillus hyphal extract-induced allergic airway inflammation by inhibition of the Th17 signaling pathway. Stem Cells Transl Med. 2014;3:194–205.
Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner DE, Coffey A, et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem Cells Transl Med. 2015;4:1302–16.
Duong KM, Arikkatt J, Ullah MA, Lynch JP, Zhang V, Atkinson K, et al. Immunomodulation of airway epithelium cell activation by mesenchymal stromal cells ameliorates house dust mite-induced airway inflammation in mice. Am J Respir Cell Mol Biol. 2015;53:615–24.
Chan CK, Lin TC, Huang YA, Chen YS, Wu CL, Lo HY, et al. The modulation of Th2 immune pathway in the immunosuppressive effect of human umbilical cord mesenchymal stem cells in a murine asthmatic model. Inflamm Res. 2016;65:795–801.
Keyhanmanesh R, Rahbarghazi R, Aslani MR, Hassanpour M, Ahmadi M. Systemic delivery of mesenchymal stem cells condition media in repeated doses acts as magic bullets in restoring IFN-γ/IL-4 balance in asthmatic rats. Life Sci. 2018;212:30–6.
Shin JW, Ryu S, Ham J, Jung K, Lee S, Chung DH, et al. Mesenchymal Stem Cells Suppress Severe Asthma by Directly Regulating Th2 Cells and Type 2 Innate Lymphoid Cells. Mol Cells. 2021;44:580–90.
Li X, Wang J, Cao J, Ma L, Xu J. Immunoregulation of Bone Marrow-Derived Mesenchymal Stem Cells on the Chronic Cigarette Smoking-Induced Lung Inflammation in Rats. Biomed Res Int. 2015;2015:932923.
Li X, Xu J, Li P. Rat bone mesenchymal stem cells exert antiproliferative effects on nicotine‑exposed T cells via iNOS production. Mol Med Rep. 2020;21:2267–75.
Gore AV, Bible LE, Song K, Livingston DH, Mohr AM, Sifri ZC. Mesenchymal stem cells increase T-regulatory cells and improve healing following trauma and hemorrhagic shock. J Trauma Acute Care Surg. 2015;79:48–52. discussion
Liu D, Kong F, Yuan Y, Seth P, Xu W, Wang H, et al. Decorin-Modified Umbilical Cord Mesenchymal Stem Cells (MSCs) Attenuate Radiation-Induced Lung Injuries via Regulating Inflammation, Fibrotic Factors, and Immune Responses. Int J Radiat Oncol Biol Phys. 2018;101:945–56.
Cao M, Liu H, Dong Y, Liu W, Yu Z, Wang Q, et al. Mesenchymal stem cells alleviate idiopathic pneumonia syndrome by modulating T cell function through CCR2-CCL2 axis. Stem Cell Res Ther. 2021;12:378.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing. The figures were created with BioRender.com.
Funding
This study was financially supported by the Research Project of Affiliated Hospital of Zhejiang Chinese Medical University in 2023 (2023FSYYZY02); Natural Science Foundation of Zhejiang Province (LY24H290001); Zhejiang Lin Shengyou Famous Traditional Chinese Medicine Expert Inheritance Studio Project (GZS202002); National Natural Science Foundation of China (8240140866); Natural Science Foundation of Zhejiang Province (ZCLQ24H2901); Medical and Health Technology Program of Zhejiang Province (2024KY1328); Traditional Chinese Medicine Science and Technology Program of Zhejiang Province (2024ZL712).
Author information
Authors and Affiliations
Contributions
Yuqian Feng: conceptualization, writing-original draft preparation. Jiamin Lu and Jing Jiang: writing-original draft preparation. Kezhan Shen: writing-reviewing and editing. Kaibo Guo and Yazhen Zhong: writing-reviewing and editing, supervision, conceptualization. Shengyou Lin: project administration, supervision, conceptualization, writing-reviewing and editing. All authors contributed to the paper and approved the submitted version. Figures were created with biorender.com.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All of the authors are aware of and agree to the content of the paper and their being listed as a co-author of the paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, Y., Lu, J., Jiang, J. et al. Mesenchymal stem cells for lung diseases: focus on immunomodulatory action. Cell Death Discov. 11, 52 (2025). https://doi.org/10.1038/s41420-025-02303-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-025-02303-4