Abstract
Background/Objectives
The effect of mild dehydration on plasma and serum volume has not been well established. Furthermore, the ability of urinary and blood biomarkers to monitor small hydration changes have not been solidified. There were two objectives of this research: 1. Determine if mild dehydration affects plasma and serum volume; 2. Determine if mild dehydration can be detected better by urinary or blood biomarkers.
Subjects/Methods
47 subjects were recruited; 10 subjects were removed from the study and 37 subjects (27% male) completed the study. This was a crossover study design such that each subject underwent all protocols in a counterbalanced order. Protocols consisted of 12-h dehydration, 12-h hydration, and control.
Results
Neither plasma volume (p = 0.914), plasma volume status (p = 0.649), nor serum volume (p = 0.273) were different among protocols. Body mass (p < 0.001) was lower following the dehydration protocol. Urine color (p < 0.001), urine osmolality (p < 0.001), urine specific gravity (p < 0.001), serum osmolality (p < 0.001), and plasma osmolality (p < 0.001) were all lower following the hydration protocol. Hematocrit (p = 0.842) and hemoglobin concentration (p = 0.558) were not different among protocols.
Conclusions
Dehydration did not affect plasma or serum volume. Therefore, a 12-h fast from food and water as done in this study will not likely affect laboratory test results of biomarker concentration. All 3 urinary measures were able to detect changes in hydration status, whereas only 2 blood measures were able to detect changes in hydration status. This may indicate that urinary measures are best at detecting small changes in hydration status.
Similar content being viewed by others
Introduction
Hydration status has been shown to affect exercise performance [1,2,3], cognitive performance [4, 5], and laboratory test results [6]. It is likely the effect of hydration on these factors is a result of changes in plasma volume (PV). The effect hydration status and exercise have on PV is well understood [2, 7,8,9,10]. However, less research has investigated how mild dehydration, in the absence of exercise affects both PV and serum volume (SV). Understanding the effects of hydration status on PV and SV may be important clinically, as the concentration of plasma [11,12,13,14] and serum [15,16,17] biomarkers are used to diagnose disease. Concentration of these biomarkers may vary based on hydration status, ultimately affecting diagnoses. For example, body mass index as an estimate of PV has been shown to effect biomarkers used to assess Alzheimer’s disease [6]. Hydration status may also affect the bioavailability of water-soluble drugs [18]. Previous research has found changes in PV due to hydration status [19,20,21] which is likely to affect SV, as serum is simply plasma without clotting factors [22]. Yet, we were unable to find research specifically assessing the effect of hydration status on SV.
While many studies use body weight as a means of assessing hydration status [23, 24], dehydration may occur with no significant changes in body weight [25]. Additionally, it may be difficult to determine changes in body weight as a result of hydration status in the clinic, where patients are wearing different clothing from previous visits or may have experienced weight losses or gains due to changes in lean and fat mass [26]. Therefore, urinary or blood measurements may be better alternatives for assessing hydration status clinically.
This study was conducted for two primary purposes: 1. To determine if dehydration causes PV or SV changes at rest; and 2. To determine which urinary and blood markers of hydration are able to detect dehydration in subjects adhering to protocols causing modest changes in hydration status.
Materials/subjects and methods
Study design
This was a crossover study design. A formal sample size calculator was used to determine that at least 30 subjects were needed to reach a power of 90% with an alpha level 0.01 [27]. Forty-seven subjects were recruited for this study and all were Caucasian. None of the subjects had comorbid heart disease, nor were any of them using diuretics. Informed consent was obtained from all subjects, and the Appalachian State University Institutional Review Board for the protection of human subjects (Boone, NC, USA) approved the study (IRB#18-0083). All subjects participated in the control, hydration, and dehydration protocols in a counterbalanced order.
The hydration and dehydration protocol required a 12-h fast from food. The hydration protocol required subjects to drink water during the 12-h fast (at least 6, 8-ounce (237 mL) glasses for males and at least 4, 8-ounce (237 mL) glasses for females), while the dehydration protocol required a 12-h fast from fluid ingestion. Participants were reminded via email the day before their lab visit to begin the protocol exactly 12-h before coming to lab. Twelve hours was chosen to mimic the clinical relevance of fasting 12-h prior to common blood tests. For the control protocol, subjects maintained their normal dietary routine prior to measurements. Subjects reported to lab the same time of day for all protocols to control for diurnal rhythm. Each subject was required to complete all three testing protocols within a period of 7days to minimize effects from weight changes, with a washout period of at least 24 h between protocols. Of the 47 subjects, 1 did not follow the protocol on their hydration day and 5 had issues with blood draw. Data from these 6 subjects were removed from analysis leaving a total of 41 subjects who completed the study (Fig. 1).
Variables
To determine hydration status, the following measurements were analyzed: spot urine osmolality (UOSM), spot urine specific gravity (USG), spot urine color (UC), body mass (BM), percent total body water (%TBW), hematocrit (Hct), hemoglobin concentration ([Hb]), plasma osmolality (POSM), serum osmolality (SOSM), plasma volume (PV), plasma volume status (PVS), and serum volume (SV). When a subject came to the laboratory, blood was drawn from a prominent vein in the antecubital space while seated [28] using a 21-gauge butterfly needle with a 7-inch luer lock extension connected to a vacutainer adapter (Becton-Dickinson, Franklin Lakes, NJ USA). Blood was collected into a 4 ml heparin and a 4 ml serum separation vacutainer (Becton-Dickinson, Franklin Lakes, NJ USA).
After blood collection, the subject entered a private restroom where he/she provided a urine sample, emptied his/her bladder completely, and then performed a naked body weight and %TBW measurement using the Tanita BC-533 bioelectrical impedance (BIA) scale (Tanita Corporation of America, Inc., Arlington Heights, IL USA). This scale was used due to its ability to give repeatable weight and %TBW measurements, as tested in-house prior to commencement of the study. This type of scale does not distinguish between intracellular water and extracellular water. The urine sample was not a first morning sample as first morning urine may not strongly correlate with fluid intake [29].
The urine sample was visually analyzed for color using the sample-over-chart method [30] in ambient fluorescent laboratory lighting by the same non-blinded investigator [31]. Urine Osmolality was measured using a single sample osmometer (Advanced Instruments Model 3250, Norwood, MA USA). Specific gravity was measured using an analog handheld refractometer (ATAGO U.S.A., Inc., Bellevue, WA USA).
Blood from the heparin vacutainer was used to determine Hct in duplicate (1.32% intra-assay coefficient of variation) by filling two microhematocrit tubes with blood from the heparinized vacutainer, which were then centrifuged at 13,700 × g for two minutes using a microhematocrit centrifuge (StatSpin CritSpin, HemoCue America, Brea, CA USA). Hematocrit was determined by measuring the height of blood and plasma divided by the height of red blood cells (RBCs) and multiplied by 100. A 10-fold dilution was performed by placing 1 ml of blood from the heparinized vacutainer into 9 ml of deionized water (dH2O) for 30 min to allow lysing of RBCs. Following this incubation period a 10-fold series dilution was performed two more times using dH2O to obtain a final 1000-fold dilution. This 1000-fold dilution was used to determine [Hb] via the Harboe method with an Allen correction factor [32]. Briefly, the 1000-fold diluted sample was poured into a cuvette and underwent spectrophotometry at the wavelengths of 380, 415, and 450 nanometers (nm) using an Eon spectrophotometer (BioTek Instruments, Inc., Winooski, VT USA). The absorbance at each wavelength, after subtraction from a dH2O blank, were used to determine [Hb] with a modified version of the Harboe equation to give hemoglobin results in g/dl instead of mg/dl. Thus, the following equation was used: Hb (g/dl) = ((0.01672 x A415) – (0.00836 x A380) – (0.00836 x A450)) * 1,000; where 0.01672 and 0.00836 are constants, A415 represents absorbance at 415 nm, A380 represents absorbance at 380 nm, A450 represents absorbance at 450 nm, and multiplying by 1,000 accounts for the 1,000-fold dilution.
Hematocrit and [Hb] were then used to determine plasma volume using the following equations developed by Dill and Costill, 1974 [7]:
where BV = blood volume, Hb = hemoglobin, CV = red blood cell volume, Hct = hematocrit, PV = Plasma Volume, A = after dehydration, B = before dehydration, BVB was considered to be 100.
Plasma volume was calculated with control values for [Hb] and Hct entered in the “before” part of the formulas and [Hb] and Hct values for dehydration or hydration put in the “after” part of the formulas to provide consistency of the PV calculations.
Plasma volume status has also been shown to be an accurate measure to assess plasma volume [33, 34]. Therefore, PVS was calculated according to Hoshika et al. [33]:
where PV = plasma volume, a = 1530 in males and 864 in females, b = 41 in males and 47.9 in females, kg = kilograms, c = 39 in males and 40 in females, PVS = plasma volume status.
The remaining 3 mL of blood in the heparin vacutainer underwent centrifugation at 2630 × g for 10 min at 4 °C (ThermoScientific Sorvall Legend RT+ refrigerated centrifuge, Thermo Fisher Scientific, Inc., Walthum, MA USA) to separate the plasma. Following centrifugation, POSM was determined via the single-sample osmometer.
3 mL of blood were removed from the serum separation vacutainer and placed into a serum transfer tube. This was done to ensure the same amount of blood was used to determine SV for each sample, as no formula exists for calculating SV. The 3 mL of blood was then allowed to coagulate at room temperature for greater than 30 min, but no longer than 60 min. Following coagulation, the blood underwent centrifugation at 2630 × g for 10 min at 4 °C. The separated serum was then poured into a graduated cylinder standing on a chemical scale (Mettler Toledo XS104, Mettler-Toledo, LLC, Columbus, OH USA) to determine SV produced per 3 mL of blood both by visual measurement using the graduated cylinder and by weighing the serum sample with the assumption that 1 µl of serum has a mass of 1 mg. Once the measurement of SV was complete, SOSM was measured via freezing point depression using the single sample osmometer. All measurements were performed immediately following blood and urine collection to avoid any fluid changes due to storage [35].
Statistical analysis
One subject forgot to measure %TBW on their control day creating one missing data point. To account for this, a multiple imputation approach using 5 imputations and taking the mean was used. The original data set of %TBW was then compared to the %TBW data following multiple imputation using a two-tailed paired T-test. Outliers for all variables were identified using the Z score method with cutoff points of 3.0 and −3.0. This decision was made a priori in order to remove subjects who likely did not comply with one of the protocols.
A repeated measures analysis of variance (RMANOVA) was used to compare all variables when multivariate normality was met. When Mauchley’s test of sphericity was not met, a Greenhouse–Geisser Correction factor was used. For variables in which multivariate normality was not met, a two-way Friedman’s non-parametric test was used. Bonferroni post-hoc analyses were used when significance was found to determine differences among variables. Partial eta squared (η2) was used to calculate effect size when RMANOVA was used, and Kendall’s W was used for a Friedman test.
To determine sex differences between age, a two-tailed independent samples T-test was conducted when the assumption of normality and homogeneity of variance for mean was met. A two-tailed Mann–Whitney U test was conducted when normality or homogeneity of variance for mean was not met. Cohen’s D (d) was used to calculate effect size when a two-tailed independent samples T-test was conducted and η2 was used to calculate effect size when a Mann–Whitney U test was conducted.
Sex differences for all other variables were compared using a multivariate ANOVA (MANOVA) with Pillai’s trace even if normality was violated, as long as homogeneity of variance was met, as the MANOVA has been found to outperform the nonparametric test when only the assumption of normal distribution is violated [36]. If the assumption of homogeneity of variance was violated, a nonparametric multivariate Kruskal–Wallis test was used.
Normality was tested using a Shapiro–Wilk Normality test. Homogeneity of variance was tested using a Levene statistic. Alpha was set at 0.05 to determine statistical significance. All statistical analyses were generated using the Statistical Package for the Social Sciences (SPSS) version 28 (SPSS Inc., Chicago, IL USA).
Results
The paired T-test between the original %TBW data set and the %TBW data set following multiple imputation indicated a standard deviation of zero. Therefore, we used the data set derived from multiple imputation for all statistical analyses. The Z score method showed 4 subjects with outliers. These 4 subjects were removed giving a total of 37 subjects (10 males and 27 females) for statistical analysis (Fig. 1).
Normality was met in all protocols for %TBW and SOSM (p > 0.05) but not for BM, age, %TBW, UC, USG, UOSM, Hct, [Hb], PV, PVS, POSM, SV in grams, or SV in milliliters (p < 0.05). Homogeneity of variance based on mean for all protocols was met for each variable (p > 0.05) except USG and UOSM (p < 0.05). Normality based on sex was met for PV, PVS, and SOSM (p > 0.05) but not for any other variable (p < 0.05). Homogeneity of variance based on mean for sex was met in all variables (p > 0.05).
There were no differences among protocols for %TBW or age (p = 1.000). Serum osmolality was lower in the hydration group compared to the dehydration (p < 0.001) and control groups (p < 0.001). There were no significant differences among protocols for Hct (p = 0.842), [Hb] (p = 0.558), PV (p = 0.914), PVS (p = 0.649), SV in grams (p = 0.273), or SV in milliliters (p = 0.334).
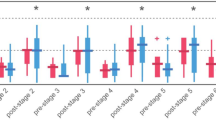
Body mass was 0.14% lower following dehydration compared to control (p = 0.001) and 0.86% lower than the hydration protocol (p < 0.001). Urine color was lower following the hydration protocol compared to both the dehydration (p < 0.001) and control (p = 0.011) protocols. Urine osmolality, USG, and POSM, were all lower following the hydration protocol compared to the dehydration (p < 0.001) and control (p < 0.001) protocols. See Fig. 2 and Supplementary Data 1 for comparisons among protocols.
A Body mass (B), urine color (C), urine specific gravity (D), urine osmolality (E), hematocrit (F), percent body water (G), hemoglobin concentration (H), plasma volume (I), plasma volume status (J), plasma osmolality (K), serum volume in milliliters (L), serum volume in grams (M), and serum osmolality (N) among the control, hydration, and dehydration protocols. Box and whisker plots are used to represent nonparametric data where the ‘+’ represents the mean value and the line through the box represents the median value. Interquartile ranges were calculated using inclusive median. Bar graphs are used to represent parametric data with standard deviation. * = significantly different compared to the hydration protocol (p < 0.05). + = significantly different compared to the dehydration protocol (p < 0.05).
Sex differences
Body mass, %TBW, Hct, and [Hb], were greater in males compared to females for all protocols (p < 0.001). Plasma osmolality was also greater in males for the control (p < 0.001), hydration (p = 0.025), and dehydration (p = 0.003) protocols. Males had higher SOSM but only at the control protocol (p = 0.026). On the other hand, females had a greater PV and PVS in all protocols (p < 0.001). Serum volume in grams was greater in females for the control (p = 0.005), hydration (p < 0.001), and dehydration (p = 0.001) protocols. Females also had a higher SV in milliliters but only at the hydration protocol (p = 0.034). There were no differences between sexes for age, UC, USG, or UOSM (p > 0.05). See Fig. 3 and supplementary Data 2 for comparison between sexes.
A, body mass (B), urine color (C), urine specific gravity (D), urine osmolality (E), hematocrit (F), percent body water (G), hemoglobin concentration (H), plasma volume (I), plasma volume status (J), plasma osmolality (K), serum volume in milliliters (L), serum volume in grams (M), and serum osmolality (N) among the control, hydration, and dehydration protocols. Box and whisker plots are used to represent nonparametric data where the ‘+’ represents the mean value and the line through the box represents the median value. Interquartile ranges were calculated using inclusive median. Bar graphs are used to represent parametric data with standard deviation. * = significantly different compared to males (p < 0.05).
Discussion
There were 2 purposes of this study: 1. To determine if a 12-h fast affects PV or SV; 2. To analyze the performance of blood and urinary markers in detecting hydration changes. In regard to the first purpose, we found that neither PV nor SV were affected by the dehydration and hydration protocols. For the second purpose, we found that BM, SOSM, POSM, UC, UOSM, and USG did show differences among protocols.
The finding that PV and SV did not change supports the practice of not considering hydration status when analyzing clinical blood tests. Hematocrit and [Hb] also did not change, which is expected given the calculation used to determine PV is dependent upon Hct and [Hb] [7]. A lack of change in Hct, [Hb], and PV among our protocols is in agreement with other studies [37,38,39,40,41] and supports the hypothesis that PV and SV are maintained despite small changes in hydration status [39].
Plasma osmolality and SOSM were all lower after the hydration protocol compared to the dehydration and control protocols. Our results agree with those of others who have found changes in POSM and SOSM with changes in hydration status [20, 37, 40, 42,43,44]. However, none of the protocols in our study caused POSM or SOSM to go outside of the normal range (≥290 mOsm) [1] for a hydrated individual. This is to be expected with our modest protocol, as POSM and SOSM have been reported to be well controlled in light of hydration status [35, 42].
Urine osmolality, USG, and UC were all lower after the hydration protocol compared to the dehydration and control protocols. All 3 urinary measures were in the dehydration category of UOSM≥700 mOsmol, USG≥1.020, and UC≥4 [4, 31, 45] following both the control and dehydration protocols. This shows that solute concentration in urine is sensitive to modest changes in fluid intake. Our finding of changes in UOSM and USG with only a 0.86% reduction in BM disagree with those of Popowski et al. who found that UOSM was not changed significantly until a 5% decrease in BM; and that USG was not changed significantly until a 3% decrease in BM [43]. This distinction is likely due to differences in protocols, as Popowski et al. was investigating acute dehydration caused by exercise, whereas our study employed a 12-h period of dehydration. Therefore, it is possible the urinary values did not have time to change in the Popowski study. Other research agrees with our study, supporting the use of urine measurements as an indicator of hydration status even in cases of mild hypohydration [38, 39]. Our findings of urinary measures changing with hydration status despite no changes in SV or PV agree with the findings of Tucker et al. who found that neither UOSM, USG, nor UC is predictive of plasma volume [46].
Although not the primary purpose of this study, we compared all data between sexes. BM, %TBW, Hct, and [Hb] were all significantly higher in males, which has been shown previously [38, 47,48,49]. A unique result was that PV and SV was greater in females for all protocols. This finding was unexpected since males tend to have greater blood volume than females [50], and would therefore be expected to have higher PV and SV. However, it is possible the menstrual cycle may have caused this difference, as women have reported retaining water at different times throughout the menstrual cycle [51]. In expectation from our findings on PV and SV, POSM and SOSM was found to be greater in males for all protocols. Urinary markers were not different between sexes.
There are limitations to be considered when interpreting data from this study. One is that water ingestion was not normalized by BM in the hydration protocol. This could cause less hydration in larger individuals and greater hydration in smaller individuals. Another limitation is that we did not require subjects to report the amount of fluid they ingested during the hydration protocol which would have allowed us to normalize data based on the amount of water each subject ingested. Although our data was within the normal ranges expected, we used single measures for UOSM, SOSM, POSM, USG, SV, PV, and [Hb]. While we did use duplicate measures for Hct, we should have used duplicate measures for all variables tested. A final limitation to consider is that all subjects in this study were young (18–22 yrs.) and apparently healthy. Since hydration status may be affected by age and disease, the results of this study should not be extrapolated to older or diseased populations.
In conclusion, no differences were found in PV or SV. Since PV and SV were not affected by these protocols, it stands to reason the concentration of clinical biomarkers would also not be affected by a 12-h fasting protocol as typically prescribed prior to common blood tests. Of the variables used to assess hydration status, the urinary variables appeared to be better at detecting small changes in hydration status compared to blood variables. Sex differences existed between BM, Hct, and [Hb] as expected. Unexpectedly, females had higher PV and SV than males. Future research may consider investigating an older population who more commonly undergo a 12-h fast prior to blood tests, normalize fluid based on BM, take multiple blood and urinary measures during the 12-hour period, and control for the menstrual cycle in women.
Data availability
Data analyzed during this study can be found in the Supplementary Data 3 excel spreadsheet.
References
Sawka MN, Burke LM, Eichner R, Maughan RJ, Montain SJ, Stachenfeld NS. Exercise and fluid replacement. Med Sci Sports Exerc. 2007;39:377–90.
James LJ, Moss J, Henry J, Papadopoulou C, Mears SA. Hypohydration impairs endurance performance: a blinded study. Physiol Rep. 2017;5:1–10.
Barley OR, Iredale F, Chapman DW, Hopper A, Abbiss CR. Repeat effort performance is reduced 24 h after acute dehydration in mixed martial arts athletes. J Strength Cond Res. 2018;32:2555–61.
Thomas DT, Erdman KA, Buke LM. Nutrition and Athletic Performance. Med Sci Sports Exerc. 2016;48:543–68.
Cian C, Koulmann N, Barraud PA, Raphel C, Jimenez C, Melin B. Influence of variations in body hydration on cognitive function: effect of hyperhydration, heat stress, and exercise-induced dehydration. 2000;4:29–36.
Tristão‐Pereira C, Fuster V, Sanchez‐Gonzalez J, Ibañez B, Zetterberg H, Blennow K, et al. Physiological confounders of blood‐based Alzheimer’s disease biomarkers in middle‐aged asymptomatic individuals. Alzheimers Dement. 2023;19:e078219.
Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–8.
Jimenez C, Melin B, Koulmann N, Allevard AM, Launay JC, Savourey G. Plasma volume changes during and after acute variations of body hydration level in humans. Eur J Appl Physiol. 1999;80:1–8.
Graham MR, Pates J, Davies B, Cooper SM, Bhattacharya K, Evans PJ, et al. Should an increase in cerebral neurochemicals following head kicks in full contact karate influence return to play? Int J Immunopathol Pharm. 2015;28:539–46.
Graham MR, Myers T, Evans P, Davies B, Cooper SM, Bhattacharya K, et al. Direct hits to the head during amateur boxing is associated with a rise in serum biomarkers for brain injury. Int J Immunopathol Pharm. 2011;24:119–25.
Aaron RE, Tian T, Fleming GA, Sacks DB, Januzzi JL. Emerging biomarkers in the laboratory and in practice: a novel approach to diagnosing heart failure in diabetes. J Diabetes Sci Technol. 2024;18:733–40.
Yu X, Sun Y, Zhao Y, Zhang W, Yang Z, Gao Y, et al. Prognostic value of plasma galectin-3 levels in patients with coronary heart disease and chronic heart failure. Int Heart J. 2015;56:314–8.
Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–9.
Feola M, Testa M, Leto L, Cardone M, Sola M, Rosso GL. Role of galectin-3 and plasma B type-natriuretic peptide in predicting prognosis in discharged chronic heart failure patients. Medicine (Baltimore). 2016;95:e4014.
Williams‐Gray CH, Wijeyekoon R, Yarnall AJ, Lawson RA, Breen DP, Evans JR, et al. Serum immune markers and disease progression in an incident P arkinson’s disease cohort (ICICLE‐PD). Mov Disord. 2016;31:995–1003.
Sahab ZJ, Semaan SM, Sang Q-XA. Methodology and applications of disease biomarker identification in human serum. Biomark Insights. 2007;2:117727190700200.
Ayrignac X, Le Bars E, Duflos C, Hirtz C, Maleska Maceski A, Carra-Dallière C, et al. Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep. 2020;10:10923.
Rastmanesh R. Inadequate hydration status of test subjects can affect bioavailability studies. ACS Pharm Transl Sci. 2023;6:1104–6.
Patterson SM, VanderKaay MM, Shanholtzer BA, Patterson CA. Influence of acute fluid loading on stress-induced hemoconcentration and cardiovascular reactivity. J Behav Med. 2008;31:319–30.
Endo Y, Torii R, Yamazaki F, Sagawa S, Yamauchi K, Tsutsui Y, et al. Water drinking causes a biphasic change in blood composition in humans. Pflüg Arch. 2001;442:362–8.
Nose H, Mack GW, Shi XR, Nadel ER. Role of osmolality and plasma volume during rehydration in humans. J Appl Physiol. 1988;65:325–31.
Hrubec TC, Whichard JM, Larsen CT, Pierson FW. Plasma versus serum: specific differences in biochemical analyte values. J Avian Med Surg. 2002;16:101–5.
Armstrong LE. Assessing hydration status: the elusive gold standard. J Am Coll Nutr. 2007;26:575S–84S.
Kavouras SA. Assessing hydration status. Curr Opin Clin Nutr Metab Care. 2002;5:519–24.
Hahn RG. Effects of diet, habitual water intake and increased hydration on body fluid volumes and urinary analysis of renal fluid retention in healthy volunteers. Eur J Nutr. 2021;60:691–702.
Kavouras SA. Hydration, dehydration, underhydration, optimal hydration: are we barking up the wrong tree? Eur J Nutr. 2019;58:471–3.
Faul F, Erdfelder E, Lang A-G, Buchner AG. Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Harrison MH. Effects on thermal stress and exercise on blood volume in humans. Physiol Rev. 1985;65:149–209.
Perrier, Rondeau E, Poupin P, Le Bellego M, Armstrong LE L, Lang F, et al. Relation between urinary hydration biomarkers and total fluid intake in healthy adults. Eur J Clin Nutr. 2013;67:939–43.
Wardenaar F, Armistead S, Boeckman K, Butterick B, Youssefi D, Thompsett D, et al. Validity of urine color scoring using different light conditions and scoring techniques to assess urine concentration. J Athl Train. 2022;57:191–8.
Fink HH, Mikesky AE. Practical applications in sports nutrition. 5th ed. Jones & Bartlett Learning; 2018.
Han V, Serrano K, Devine DV. A comparative study of common techniques used to measure haemolysis in stored red cell concentrates. Vox Sang. 2010;98:116–23.
Hoshika Y, Kubota Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, et al. Effect of empagliflozin versus placebo on plasma volume status in patients with acute myocardial infarction and type 2 diabetes mellitus. Diabetes Ther. 2021;12:2241–8.
Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail. 2015;17:35–43.
Bohnen N, Terwel D, Markerink M, Ten Haaf JA, Jolles J. Pitfalls in the measurement of plasma osmolality pertinent to research in vasopressin and water metabolism. Clin Chem. 1992;38:2278–80.
Finch H. Comparison of the performance of nonparametric and parametric MANOVA test statistics when assumptions are violated. Methodology. 2005;1:27–38.
Stookey JD, Hamer J, Killilea DW. Change in hydration indices associated with an increase in total water intake of more than 0.5 L/day, sustained over 4 weeks, in healthy young men with initial total water intake below 2 L/day. Physiol Rep. 2017;5:e13356.
Armstrong LE, Maresh CM, Castellani JW, Bergeron MF, Kenefick RW, LaGasse KE, et al. Urinary indices of hydration status. Int J Sport Nutr. 1994;4:265–79.
Francesconi RP, Hubbard RW, Szlyk PC, Schnakenberg D, Carlson D, Leva N, et al. Urinary and hematologic indexes of hypohydration. J Appl Physiol. 1987;62:1271–6.
Williams TD, Seckl JR, Lightman SL. Dependent effect of drinking volume on vasopressin but not atrial peptide in humans. Am J Physiol-Regul Integr Comp Physiol. 1989;257:R762–4.
Geelen G, Keil LC, Kravik SE, Wade CE, Thrasher TN, Barnes PR, et al. Inhibition of plasma vasopressin after drinking in dehydrated humans. Am J Physiol-Regul Integr Comp Physiol. 1984;247:R968–71.
Armstrong LE. Hydration assessment techniques. Nutr Rev. 2005;63:S40–54.
Popowski LA, Oppliger RA, Patrick Lambert G, Johnson RF, Kim Johnson A, Gisolfi CV. Blood and urinary measures of hydration status during progressive acute dehydration. Med Sci Sports Exerc. 2001;33:747–53.
Moran DS, Heled Y, Margaliot M, Shani Y, Laor A, Margaliot S, et al. Hydration status measurement by radio frequency absorptiometry in young athletes—a new method and preliminary results. Physiol Meas. 2004;25:51–9.
Kavouras SA, Johnson EC, Bougatsas D, Arnaoutis G, Panagiotakos DB, Perrier E, et al. Validation of a urine color scale for assessment of urine osmolality in healthy children. Eur J Nutr. 2016;55:907–15.
Tucker MA, Butts CL, Satterfield AZ, Six A, Johnson EC, Ganio MS. Spot sample urine specific gravity does not accurately represent small decreases in plasma volume in resting healthy males. J Am Coll Nutr. 2018;37:17–23.
Malisova O, Athanasatou A, Pepa A, Husemann M, Domnik K, Braun H, et al. Water intake and hydration indices in healthy European adults: the European hydration research study (EHRS). Nutrients. 2016;8:1–12.
Weitkunat T, Knechtle B, Knechtle P, Rüst CA, Rosemann T. Body composition and hydration status changes in male and female open-water swimmers during an ultra-endurance event. J Sports Sci. 2012;30:1003–13.
Ritz P, Vol S, Berrut G, Tack I, Arnaud MJ, Tichet J. Influence of gender and body composition on hydration and body water spaces. Clin Nutr. 2008;27:740–6.
Sharma R, Sharma S. Physiology, Blood Volume. Treasure Island, FL: StatPearls Publishing; 2023.
White CP, Hitchcock CL, Vigna YM, Prior JC. Fluid retention over the menstrual cycle: 1-year data from the prospective ovulation cohort. Obstet Gynecol Int. 2011;2011:1–7.
Funding
Open access funding provided by the Carolinas Consortium.
Author information
Authors and Affiliations
Contributions
JM assisted in data collection, statistical analysis, and manuscript writing. OD assisted in experimental design, data collection, and provided feedback on the manuscript. NT assisted in experimental design, data collection, and provided feedback on the manuscript. EM assisted in experimental design, data collection, and provided feedback on the manuscript. AY assisted in statistical analysis, figure design, table design, and manuscript writing. MR assisted in experimental design, data collection, statistical analysis, and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research was conducted in accordance with the Declaration of Helsinki and was approved by the Appalachian State University Institutional Review Board for the protection of human subjects (IRB#18-0083). Written informed consent was obtained from all subjects prior to participating in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morgan, J.E., Dunning, O.N., Tocci, N.D. et al. Plasma and serum volume remain unchanged following a 12-h fast from food and drink despite changes in blood and urinary hydration markers. Eur J Clin Nutr 79, 161–167 (2025). https://doi.org/10.1038/s41430-024-01526-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-024-01526-5