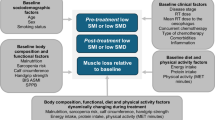
Abstract
Background
Low skeletal muscle mass and impaired muscle quality (myosteatosis) have been associated with poor outcomes in cancer patients. This study aimed to evaluate the impact of pre-therapeutic low muscle mass and myosteatosis on chemoradiotherapy (CRT)-induced toxicity and survival outcomes in patients with non-resectable pancreatic cancer (PC).
Methods
In this retrospective study, pre-therapeutic CT scans were used to measure muscle mass/density. Low muscle mass was defined as a skeletal muscle index <38.5 cm²/m² (women) and <52.4 cm²/m² (men), and myosteatosis as a mean psoas density <41 HU if BMI < 25 kg/m² or <33 HU if BMI > 25 kg/m². Adverse effects were collected per week (W) of treatment. Dose-limiting toxicity (DLT) was defined as any toxicity leading to dose reduction, treatment delays or permanent discontinuation.
Results
Among the 85 included patients, 75 (88.2%) and 18 (22.2%) had pre-therapeutic low muscle mass and myosteatosis, respectively. Only 12 patients (14.1%) experienced DLT. Patients with low muscle mass developed significantly more toxicities at W2 (p = 0.013) and W5 (p = 0.026), notably more nausea (p = 0.037) and anemia (p = 0.004). Low muscle mass was associated with poorer overall survival (HR 4.41 [1.50–12.94], p = 0.007) in multivariate Cox analysis, while myosteatosis was not associated with CRT toxicities, DLT and overall survival (p = 0.408).
Conclusion
Patients with low muscle mass experienced more toxicities and poorer outcomes during CRT for non-resectable PC.
Similar content being viewed by others
Introduction
Pancreatic cancer (PC) is a major public health issue. Its incidence is steadily increasing in France, Europe, and the United States of America while its prognosis remains poor [1,2,3]. The 5-year overall survival (OS) rate, all stages combined, is estimated to be around 10%, and PC is the seventh leading cause of cancer death in both sexes [4]. Despite the few progresses in therapeutics, PC remains highly fatal, and treatment options are limited due to late diagnosis.
Locally advanced PC (LAPC), representing 30% of cases, is defined as a pancreatic tumor having a tumor-to-artery interface ≥180°, or an unreconstructable portal vein or superior mesenteric vein, with no distant metastasis [5]. As upfront surgical resection cannot guarantee high rates of R0 resection due to extensive vascular involvement [6], gemcitabine-based induction chemotherapy is the recommended standard of care in this setting [7,8,9]. Moreover, FOLFIRINOX chemotherapy regimen is a new alternative, based on the favorable results of the NEOPAN phase 3 study [10].
The role of chemoradiotherapy (CRT) remains controversial in non-resectable PC. The concept of CRT is based on the potentiation of radiotherapy by a radiosensitizing molecule, most often capecitabine [11]. It can be proposed as a consolidation treatment after successful induction chemotherapy in selected patients [12,13,14]. The LAP07 study secondary analyses [15, 16] and the CONKO-007 trial [17] reported potential benefits in terms of local tumor control, without compromising patient’s safety. Nevertheless, CRT could impair quality of life through chemotherapy- or radiotherapy-induced adverse effects, such as abdominal pain, nausea, vomiting or diarrhea [18].
Recently, body composition has emerged as a major prognostic factor in oncology [19,20,21]. Sarcopenia is defined as the association of low muscle strength, with low muscle quantity or quality, and/or low physical performance [22]. Cancer patients with sarcopenia have higher treatment-induced toxicity, more postoperative complications, impaired quality of life and poorer OS [23, 24], especially in patients with resected PC and non-resectable PC [25,26,27]. Despite similar muscle mass, the presence of myosteatosis, defined by the excess of adiposity deposits in muscle tissue, has been associated with increased chemotherapy-induced toxicity, shorter time to tumor progression and poorer OS in cancer patients [19, 28]. Body composition analysis from computed tomography (CT) scans, commonly used to screen for sarcopenia, could also be used to detect myosteatosis through the attenuation of mean skeletal muscle Hounsfield units (HU) [29]. However, the impact of myosteatosis on survival and the treatment-related toxicities has not yet been studied in patients treated with CRT for non-resectable PC.
Therefore, this study aimed to assess the impact of low muscle mass and myosteatosis on treatment-related toxicity and survival outcomes in non-resectable PC patients treated with CRT.
Materials and methods
Study design and patients
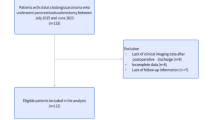
We retrospectively reviewed all medical records of patients with pancreatic adenocarcinoma treated by CRT between January 1, 2011 and May 1, 2022 in two French tertiary centers (Godinot Cancer Institute, Reims and Antoine Lacassagne Center, Nice). Patients were included if diagnosed with a histologically-confirmed non-metastatic pancreatic adenocarcinoma (or adenosquamous carcinoma), borderline resectable but non-resectable after induction chemotherapy or locally advanced according to the National Comprehensive Cancer Network (NCCN) criteria [5] and treated with concomitant definitive CRT. However, patients with an oligometastatic PC achieving a complete metastatic response after the first-line chemotherapy and then receiving CRT were also included, as the main concern was local control. A pre-therapeutic CT scan (performed before the initiation of CRT) covering from the apex of lungs to the sacrum with exploitable morphologic data was mandatory. The CT scan acquisition protocol was identical for both centers and is described in the Supplementary Materials.
Patients with other histological types of PC, and/or without an available or exploitable pre-therapeutic CT scan, and/or who received desynchronized CRT, radiotherapy alone, hemostatic, antalgic, or stereotaxic pancreatic radiotherapy, and/or who had radiotherapy performed for local PC recurrence, were not included. Patients were excluded in case of prescribed but unperformed radiotherapy, or if they were diagnosed with multiple distant metastases at the beginning of treatment.
Data collection
Baseline demographic characteristics at the initiation of CRT were retrospectively collected, including gender, age, weight (kilograms), height (centimeters), body mass index (BMI) (kg/m²) and Eastern Cooperative Oncology Group (ECOG) performance status (PS). PCs were classified according to histological subtypes and surgical resectability criteria defined by the NCCN consensus [5], based on the diagnostic CT scan and the review of the multidisciplinary board tumor meeting. The date of initial diagnosis of PC was collected. Biological data included serum albumin, C-reactive protein (CRP) and CA19.9 levels at CRT initiation, and the phenotype of dihydropyrimidine dehydrogenase (DPD) using plasma uracil rate determination. To improve accuracy, we prioritized collecting demographic and biological data as close as possible to the first day of treatment (during the month preceding the diagnosis, chemotherapy induction or CRT initiation). Previous induction treatments before CRT, such as chemotherapy (drugs and number of cycles received) or surgery, and the duration of CRT, the number of administered cycles of chemotherapy and number of days of radiotherapy effectively carried out were described.
Date of disease progression according to RECIST1.1 criteria [30] and date of death were also collected.
Chemoradiotherapy protocol
Radiotherapy was delivered with photons in three-dimensional conformal radiation therapy (3DCRT) or in intensity modulation radiation therapy (IMRT) using conventional fractionation. Radiotherapy was combined with daily oral chemotherapy (capecitabine 850 mg/m² twice daily, Monday to Friday) or with intravenous (IV) LV5FU2 simplified chemotherapy regimen every two weeks (L-leucovorin 200 mg/m² IV infusion over 2 h and 5-fluorouracile (5FU) 400 mg/m² IV bolus injection then 5FU 2400 mg/m² continuous IV infusion over 46 h). The total dose of radiotherapy could vary from 50,4 Gy in 28 sessions to 54 Gy in 27 sessions, five days a week, as recommended by the French national guidelines [8].
Toxicity
Overall and weekly treatment-related acute toxicities (nausea, vomiting, diarrhea, hand-foot syndrome, mucositis, heart problem, anemia, thrombocytopenia, neutropenia, febrile neutropenia) from the first week (W) of treatment (W1) to W7 are graded weekly (according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0) [31] during CRT in our institution. These data were collected from the medical file. Treatment-related dose-limiting toxicity (DLT) was defined as any toxicity leading to dose reduction, treatment delays or permanent discontinuation.
CT scan analyses
For each patient, the pre-therapeutic CT scan performed before the initiation of CRT and obtained from the hospital imaging database (Synapse PACS V5 software, Fujifilm Medical Systems, U.S.A., Inc) was used to measure skeletal muscle mass and density. As commonly used in the literature, measurements were performed on a single CT slice at the third lumbar vertebra (L3) at the level of the transverse processes [21, 22, 29, 32]. The CT scan image analysis was anonymized and selected in Digital Imaging and Communications in Medicine (DICOM) format with data on injection time and slice thickness. All morphologic analyses were performed by a single reader after training with an experienced radiologist, avoiding interobserver variability. Muscle area and density were measured using the AsanJ-Morphometry™ software (Asan Image Metrics, Seoul, Korea, created in 2019 and available at http://datasharing.aim-aicro.com/morphometry). Right and left psoas areas were delineated semi-automatically by predetermined thresholds for the Hounsfield Units (HU) on CT scans (−190 to −30 HU for fat and 0–100 for muscle) and manually corrected if necessary [32].
Muscle mass was studied through the total muscle area (TMA), which includes that of the psoas muscles, the lumbar muscles, the erector spinae, the transversus abdominis muscles, the internal and external oblique muscles, and the rectus abdominis. TMA was calculated in cm² and normalized to the square of the height (m²) to obtain the skeletal muscle index (SMI) (cm²/m²). Low muscle mass was defined as SMI < 38.5 cm²/m² for women and <52.4 cm²/m² for men [22, 33], according to the 2019 revised European consensus.
Myosteatosis was assessed by psoas muscle density (PMD). The mean attenuation of the left and right psoas was obtained by freehand drawing the region of interest (ROI) outlining the psoas muscles at the selected CT slice, and the average density of the two psoas muscles was calculated and used to define myosteatosis. Heterogeneous thresholds are used in the literature to define myosteatosis [19, 34]. As used in many recent studies, myosteatosis was defined as a mean PMD < 41 HU if BMI < 25 kg/m² and <33 HU if BMI > 25 kg/m² [29]. Another threshold (PMD ≤ 44.5 HU) described by Herrod et al. was also assessed in an exploratory analysis [35].
To account for a possible heterogeneity in CT injection time, we also adjusted the mean PMD on the contrast aorta density. A circular ROI on the same CT slice was drawn in the center of aorta avoiding the periphery of the vessel, particularly the atheromatous calcifications. The indexed psoas density (IPD) was then calculated using the following equation: average PMD/aorta density (HU).
Additional measurements performed for muscle mass and density are described in the Supplementary Materials.
Aims and endpoints
The primary objective was to assess the impact of pre-therapeutic low muscle mass and myosteatosis on treatment-related toxicities during CRT. Primary endpoints were the rate of overall treatment-related toxicities (any grade), weekly toxicities (severe grades 3–5) and DLT.
The secondary objective was to describe survival outcomes of patients treated by CRT according to the presence of low muscle mass or myosteatosis. Secondary endpoints included OS and progression-free survival (PFS). OS was defined as the time from CRT initiation to death from any cause; alive patients were censored at the date of their last follow-up. PFS was defined as the time from CRT initiation to the date of the RECIST-based progression or death; alive patients without progression were censored at the date of their last follow-up.
Statistical analysis
Quantitative variables were expressed as median and interquartile range (IQR). Values were compared using the non-parametric Kruskal–Wallis test. Qualitative data were described as frequencies and compared with the Chi-square test or Student’s test when appropriate.
A univariate analysis was performed for key demographic and tumoral factors, as well as for overall and weekly treatment-induced toxicities and DLT, depending on the presence of low muscle mass or myosteatosis, using the appropriate test.
Survival outcomes were described using Kaplan–Meier survival curves. The survival regression analysis was performed using the Cox proportional hazard model. A univariate analysis, followed by a multivariate analysis, was done using preselected clinically relevant covariates (sex, PS, presence of low muscle mass, presence of myosteatosis). The proportional hazard assumption was checked using a Schoenfeld plot. All p-values were two-sided, and a p-value ≤ 0.05 was considered significant.
Due to the heterogeneous population (including patients with initially borderline resectable, locally advanced or oligometastatic PC), an a posteriori sensitivity analysis was performed including only patients with LAPC.
All data were collected using EpiInfo 7.2.5.0 and analyzed using R Studio (R Core Team, version 4.2.0, 2022).
Results
Description of the population
A total of 85 patients were included. Baseline characteristics of the overall population and grouped by the presence of low muscle mass and myosteatosis are described in Table 1. The included patients were mostly women (n = 52, 61.2%) and aged over 65 (median: 68.9 years, IQR: 60.2–73.7). At CRT initiation, all pancreatic tumors were non-resectable, and initially locally advanced (n = 53, 62.4%), borderline resectable (n = 29, 34.1%) and three patients (3.5%) had an oligometastatic PC achieving a complete response after induction chemotherapy. Nearly all patients received an induction chemotherapy (n = 82, 98.8%), mostly FOLFIRINOX combination regimen (n = 68, 81.9%). The most common concomitant chemotherapy was capecitabine (n = 78, 91.8%). The median dose received during RT was 54 Gray (IQR: 50.4–54). Most patients received RT with IMRT technique (n = 74, 87.1%) whereas eleven patients (12.9%) received 3DCRT.
Among the overall population, 75 patients (88.2%) had low muscle mass whereas 18 (22.2%) had myosteatosis. Measurements of SMI and PMD did not differ between patients of the two radiotherapy centers (p = 0.477 and p = 0.08, respectively). All patients with myosteatosis also had low muscle mass (n = 18, 100%). Ten patients (11.8%) had neither myosteatosis nor low muscle mass. Patients with low muscle mass were significantly older (p = 0.005) and had significant lower BMI (p = 0.009) in univariate analysis. Patients with myosteatosis had significantly lower SMI (p = 0.038) and lower IPD (p = 0.001) in univariate analysis. CT contrast agent injection did not influence the diagnosis of myosteatosis (p = 0.183).
Description of overall and weekly toxicities
A total of 531 overall toxicities were observed during CRT. Patients mainly experienced nausea (n = 32, 50% in W3), diarrhea (n = 21, 33.3% in W5), anemia (n = 21, 33.3% in W5), and thrombocytopenia (n = 22, 34.4% in W3) (Fig. 1, Supplementary Table A1).
Cumulated weekly toxicities were mostly grade 1 (n = 455, 75.3%), and a few cases of grade 3 toxicity were observed for nausea (n = 2), vomiting (n = 1) and diarrhea (n = 1) (Supplementary Table A2). Six patients reported hand-foot syndrome during CRT. No patient experienced grade 4 or 5 toxicity, febrile neutropenia or any cardiac disorder.
Description of dose-limiting toxicities (DLT)
Eleven patients (12.9%) experienced a chemotherapy-related DLT (Table 2).
Chemotherapy was delayed for eight patients (9.4%) and stopped for a median of 8.5 days (4.75–14.25). Six patients (7.1%) underwent chemotherapy dose reductions of 20–30% of the initial dose. The most frequent chemotherapy-DLTs were thrombocytopenia (54.5%, n = 6), nausea (36.4%, n = 4), vomiting (36.4%, n = 4) and hand-foot syndrome (30.0%, n = 3). DLT was mainly managed in ambulatory care (81.8%, n = 9).
Only one patient (1.2%) experienced a radiotherapy-related DLT and had to stop radiotherapy for 11 days because of nausea and vomiting.
No patient died due to treatment-induced toxicity.
Analysis of DLT, overall and weekly toxicities according to low muscle mass and myosteatosis
In the univariate analysis, low muscle mass was significantly associated with more weekly toxicities during the second (p = 0.013) and the fifth (p = 0.026) weeks of treatment (Table 3).
Patients with low muscle mass developed more nausea (p = 0.037) and anemia (p = 0.004) (Fig. 2). The presence of myosteatosis was not associated with more weekly toxicities (Table 3) or more DLT (p = 1.000).
Survival outcomes
The median OS of the whole population was 17.1 months (IQR: 7.6–37.2) from CRT initiation (Fig. 3). The median OS from diagnosis of PC was 20.6 months (IQR: 14.9–32.7). Seventy-one patients died during the follow-up including 82.3% (n = 57) related to PC.
In the univariate analysis, factors associated with OS were the presence of low muscle mass (HR 3.63 [1.29–10.25], p = 0.015) and ECOG PS 2 (versus ECOG PS 0, HR 6.33 [2.04–19.66], p = 0.001). Myosteatosis was not associated with a poorer outcome (p = 0.513). In the multivariate analysis, ECOG PS 2 (HR 6.98 [2.07–23.54], p = 0.002) and low muscle mass (HR 4.41 [1.50–12.94], p = 0.007) were significantly associated with shorter OS, whereas myosteatosis was not (p = 0.408) (Table 4).
To support our results, a sensitivity analysis was conducted a posteriori, including only patients with LAPC. This confirmed the prognostic impact of low muscle mass on OS, while an ECOG PS 2 was no longer significant given the small number of patients in this subgroup.
PFS was not statistically different between patients according to muscle mass or density (Fig. 4).
Discussion
To the best of our knowledge, this study was the first to assess the respective impacts of pre-therapeutic low muscle mass and myosteatosis on CRT-induced toxicity in patients with non resectable PC. In this population, low muscle mass was highly prevalent (n = 75, 88.2%) and was significantly associated with more weekly toxicities (any grade) at W2 (p = 0.013) and W5 (p = 0.026) during CRT, notably more nausea (p = 0.037) and anemia (p = 0.004). Low muscle mass was also independently associated with poorer OS (HR 4.41 [1.50–12.94], p = 0.007) in multivariate analysis, while myosteatosis had no impact on the occurrence of weekly toxicities and OS (p = 0.408).
In this cohort, the prevalence of sarcopenia, estimated by low muscle mass (88.2%), was higher than that previously described in the literature. Prevalence of pre-therapeutic sarcopenia has been previously reported to be approximately 38% in patients with all-type of cancers [20, 24] and 67% in patients with LAPC treated with CRT [25]. However, most patients (85.8%) in the study of Naumann et al. had not received prior chemotherapy. In our study, almost all patients received induction chemotherapy (98.8%), including multi-drug chemotherapy in 81.9% of patients. The use of prior chemotherapy may have induced low muscle mass in our patients and may therefore have influenced the prevalence of sarcopenia before the initiation of CRT [36]. Additionally, pancreatic exocrine insufficiency has been reported to be highly prevalent in patients with PC and could have influenced the development of sarcopenia in these patients [37, 38]. A recent review [37]. has indeed reported that untreated pancreatic exocrine insufficiency can lead to maldigestion, and consequently malabsorption, malnutrition, and increased risk of osteoporosis, sarcopenia, and/or cardiovascular events. Untreated pancreatic exocrine insufficiency has also been associated with more postoperative complications after pancreatic resection and poorer response to chemotherapy through the gut microbiome dysbiosis and the modulation of the immune response. Furthermore, higher rates of sarcopenia could also be explained by the systemic catabolic syndrome in patients with PC, inducing both fat and muscle mass loss. Cancer-associated cachexia is mediated by the main cytokine and immune cell-driven pathways, especially in patients with PC [39]. PC cachexia is a multifactorial complex syndrome [38] involving both tumor-related systemic factors (pro-inflammatory cytokines, increased lipolysis and glycolysis, KRAS-dependent metabolic changes, adipose tissue browning), impaired pancreatic exocrine and endocrine functions (leading to increased gluconeogenesis, insulin resistance, malabsorption, gut microbiome dysbiosis, increased intestinal permeability and systemic inflammation) and alterations of the intestinal tract (gastric outlet obstruction, gut microbiome dysbiosis with excess bacterial growth). Another recent study has assessed low muscle mass and density in patients with PC using preoperative CT scans, with a prevalence of sarcopenia of 74.0% and myosteatosis of 67.7% [40]. This study showed the differences in fat distribution, with sarcopenic patients having lower subcutaneous adipose tissue while those with myosteatosis having greater visceral adipose tissue. Of note, transcriptomic analyses in muscles revealed increased inflammation and decreased growth in patients with sarcopenia, and a disrupted oxidative phosphorylation and lipid accumulation in patients with myosteatosis [40]. Finally, the thresholds used in the literature to define sarcopenia in cancer patients are very heterogenous. This study used gold-standard thresholds as recommended by the European consensus [33] to define low muscle mass, but the definition of sarcopenia also includes muscle strength measurement [22, 41], which was not performed routinely in our unit and therefore not available in this retrospective study. Thus, low muscle mass alone did not allow a conclusion on the presence of sarcopenia in our study, since loss of muscle mass may be independent of the presence of sarcopenia [41].
The most frequent adverse effects during CRT were digestive and hematological toxicities. Similar results have been reported in two recent studies including patients with LPAC undergoing CRT [16, 17]. In the LAP07 trial [16], six patients in the CRT group had grade 3 or 4 nausea versus none in the chemotherapy group (p = 0.008). In the CONKO-007 trial [17], hematological toxicities were increased in the CRT arm, whereas other toxicities were comparable. Several parameters can influence the radiotherapy-induced toxicity, notably the treatment technique performed and the use of concomitant chemotherapy. For example, the concomitant chemotherapy used in the CONKO-007 trial [17] was gemcitabine, which is more radio-sensitizing than capecitabine. In the LAP07 study [16], patients were treated between 2008 and 2011 with 3DCRT without IMRT, even though IMRT has been developed to spare healthy tissue and prevent radiation toxicity [42, 43]. In the present study, most patients received the IMRT technique and a concomitant chemotherapy with capecitabine.
A significant association between low muscle mass and the occurrence of weekly toxicities during CRT was shown in this study, particularly during the second (p = 0.013) and fifth (p = 0.026) weeks of CRT. A statistical trend was also observed for the other weeks of treatment, though this difference was not statistically significant, likely due to small sample sizes. The number of adverse events did not increase with the duration of treatment, and only a few DLT (14.1%) and grade 3 toxicities (4.7%) were reported, probably due to the tight weekly monitoring in our units with early management of adverse events. Consistent with our results, sarcopenia has been reported to be predictive of treatment-induced toxicity [27, 28, 44, 45], including CRT-related toxicity [25], in digestive cancer patients.
The prognostic impact of sarcopenia on survival outcomes has previously been studied in patients with LAPC treated with CRT. Naumann et al. showed that a persistent loss of weight and lean muscle was negatively associated with OS [25], supporting the importance of a longitudinal evaluation of these parameters during CRT. In our study, muscle mass and SMI were measured only once before CRT initiation on the pre-therapeutic CT scan. A potential association between the evolution of body composition during CRT and treatment-induced toxicities or survival outcomes is yet to be studied in this setting, especially since CRT is known to reduce muscle mass [25, 36]. A simple tool, the handgrip test, could be used to monitor the evolution of sarcopenia parameters during treatment could be the measurement of muscle strength, which is the key parameter in the consensus definition of sarcopenia [22]. This test has already shown its feasibility in routine and its association with muscle mass (measured with SMI) and nutritional parameters in digestive cancer patients [46,47,48]. Finally, in multivariate analysis, ECOG PS 2 was associated with the worst prognostic impact on OS, but due to its low frequency in the whole cohort (n = 4), this factor seemed less relevant to use than low muscle mass.
Myosteatosis had no impact on the occurrence of weekly toxicities and survival outcomes in the present study. The literature has used various thresholds to define myosteatosis in cancer patients [19, 34]. We chose to define myosteatosis as a cut-point for muscle attenuation of <41 HU if BMI < 25 kg/m² or <33 HU if BMI > 25 kg/m², which is currently the most used in the literature [29]. Since this definition is not yet consensual in cancer patients, another threshold for myosteatosis (PMD ≤ 44.5 HU) described by Herrod et al. [35] was also assessed in an exploratory analysis (Supplementary Tables A3 and A4), with similar results. Myosteatosis had no impact on the occurrence of weekly toxicities or survival, whatever the threshold used to define it. Although CT scans were not systematically performed with injection of contrast products, and the density of the structures may vary depending on the injection, the diagnosis of myosteatosis was not associated with contrast enhancer injection in our study (p = 0.183), which supports a potentially limited effect of this bias on our results. Another point of interest for the evaluation of myosteatosis was the precise delineation of the psoas muscles, which is meticulous work. Density may vary by a few HU depending on the delimited area and may interfere with this calculation. To limit this bias and maximize reproducibility, all morphologic analyses were performed by a single reader after a training with an experienced radiologist. Despite these biases and considering the large proportion of patients with LAPC presenting with loss of skeletal muscle mass, often of multifactorial origin, the assessment of muscle quality through myosteatosis is of increasing interest in patients with PC. Indeed, both baseline muscle mass and quality (myosteatosis) have been reported to be independent risk factors for early chemotherapy-related toxicity in metastatic PC, which would help stratifying patients for the most suitable choice of treatment [28]. Additionally, baseline muscle attenuation was reported to be associated with poorer prognosis and with the incompletion rate of adjuvant chemotherapy in PC patients treated with neoadjuvant chemoradiotherapy [49]. Ongoing and future studies should continue to explore muscle parameters for prognosis in patients with cancer.
Our study had several other limitations. First, the retrospective design may have limited the exhaustive collection of clinical toxicities. However, we emphasize the fact that all CT scans were performed before the outcome of interest. Second, a low number of events was observed for some covariates, including myosteatosis. This limited the statistical power and hampered the robustness of our conclusions. Finally, the studied population was heterogenous, including borderline resectable tumor and oligometastatic PC achieving a complete metastatic response after the first-line chemotherapy. All these patients received CRT for the same indication, i.e. definitive CRT. To support our results, a sensitivity analysis including only patients with LAPC was performed a posteriori, confirming the prognostic impact of sarcopenia. Nevertheless, the studied population remains heterogeneous, and the inclusion of these uncommon profiles could bias the results. However, we should underline that only few of them were included (n = 3, 3.5% of patients with oligometastatic PC).
An additional sensitivity analysis was carried out by calculating gender-based sarcopenia and myosteatosis using tertiles and comparing the lowest with the highest tertile. We observed similar results by comparing the first and third tertile but lost significance in the process (sarcopenia: logrank p = 0.16; multivariate Cox proportional hazard model, p = 0.16; myosteatosis: logrank, p = 1; multivariate Cox proportional hazard model, p = 0.94). Although we lost significance, we should note that we over stratified the population resulting in a considerable loss of statistical power.
Despite these limitations, this study highlights the need to optimize the management of patients with PC in current practice [27]. The prevalence of low muscle mass was high in patients with non-resectable PC initiating a definitive CRT after induction chemotherapy. Since low muscle mass was associated with CRT-related toxicities, targeted therapeutic interventions could be considered to prevent DLT in this frail population. Patients may also benefit from early and longitudinal nutritional monitoring and adapted physical activity throughout cancer care in order to reduce treatment-related toxicity and improve quality of life and survival [27, 50,51,52]. Due to limited resources, there is a need to target adapted physical activity programs to patients who need them most: patients with PC seem to be a population of choice in this indication, since the prevalence of sarcopenia and cachexia remains high. But in current practice, access to these programs is still limited and should be developed.
Conclusions
In conclusion, patients with low muscle mass experienced more weekly treatment-induced toxicities and poorer outcomes during CRT for non resectable PC. Conversely, the presence of myosteatosis did not influence CRT-related toxicity and survival. Prospective studies are necessary to evaluate the potential impact of myosteatosis and the longitudinal evolution of sarcopenia during treatment. Early detection of muscle mass reduction could optimize the management of patients with non-resectable PC, through nutritional monitoring and adapted physical activity as key points in cancer care.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Cabasag CJ, Arnold M, Rutherford M, Bardot A, Ferlay J, Morgan E, et al. Pancreatic cancer survival by stage and age in seven high-income countries (ICBP SURVMARK-2): a population-based study. Br J Cancer. 2022;126:1774–82.
Dyba T, Randi G, Bray F, Martos C, Giusti F, Nicholson N, et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 2021;157:308–47.
Gaddam S, Abboud Y, Oh J, Samaan JS, Nissen NN, Lu SC, et al. Incidence of Pancreatic Cancer by Age and Sex in the US, 2000-2018. JAMA. 2021;326:2075–7.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1028–61.
van Veldhuisen E, van den Oord C, Brada LJ, Walma MS, Vogel JA, Wilmink JW, et al. Locally advanced pancreatic cancer: work-up, staging, and local intervention strategies. Cancers. 2019;11:976.
Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v56–68.
Neuzillet C, Gaujoux S, Williet N, Bachet JB, Bauguion L, Colson Durand L, et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig Liver Dis. 2018;50:1257–71.
Palta M, Godfrey D, Goodman KA, Hoffe S, Dawson LA, Dessert D, et al. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2019;9:322–32.
Ducreux M, Desgrippes R, Rinaldi Y, Di Fiore F, Guimbaud R, Follana P, et al. PRODIGE 29-UCGI 26(NEOPAN): A phase III randomised trial comparing chemotherapy with folfirinox or gemcitabine in locally advanced pancreatic carcinoma (LAPC). Ann Oncol. 2022;33:S592–S598.
Hennequin C, Quero L, Favaudon V. Biological basis of chemo-radiotherapy associations. Bull Cancer. 2009;96:329–36.
Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of Chemoradiotherapy After Disease Control With Chemotherapy in Locally Advanced Pancreatic Adenocarcinoma in GERCOR Phase II and III Studies. JCO. 2007;25:326–31.
Krishnan S, Rana V, Janjan NA, Varadhachary GR, Abbruzzese JL, Das P, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55.
Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14:317–26.
Huguet F, Hammel P, Vernerey D, Goldstein D, Van Laethem JL, Glimelius B, et al. Impact of chemoradiotherapy (CRT) on local control and time without treatment in patients with locally advanced pancreatic cancer (LAPC) included in the international phase III LAP 07 study. JCO. 2014;32:4001–4001.
Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844–53.
Fietkau R, Ghadimi M, Grützmann R, Wittel UA, Jacobasch L, Uhl W, et al. Randomized phase III trial of induction chemotherapy followed by chemoradiotherapy or chemotherapy alone for nonresectable locally advanced pancreatic cancer: first results of the CONKO-007 trial. JCO. 2022;40:4008–4008.
Breen WG, Jethwa KR, Yu NY, Spears GM, Harmsen WS, Miller RC, et al. Patient-Reported Quality of Life Before and After Chemoradiation for Intact Pancreas Cancer: A Prospective Registry Study. Pract Radiat Oncol. 2021;11:e63–9.
Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;145:102839.
Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clinical Nutrition. 2018;37:1101–13.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31.
Rier HN, Jager A, Sleijfer S, Maier AB, Levin MD. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. The Oncologist. 2016;21:1396–409.
Couderc AL, Liuu E, Boudou-Rouquette P, Poisson J, Frelaut M, Montégut C, et al. Pre-Therapeutic Sarcopenia among Cancer Patients: An Up-to-Date Meta-Analysis of Prevalence and Predictive Value during Cancer Treatment. Nutrients. 2023;15:1193.
Naumann P, Eberlein J, Farnia B, Hackert T, Debus J, Combs SE. Continued Weight Loss and Sarcopenia Predict Poor Outcomes in Locally Advanced Pancreatic Cancer Treated with Chemoradiation. Cancers. 2019;11:709.
Mintziras I, Miligkos M, Wächter S, Manoharan J, Maurer E, Bartsch DK. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int J Surg. 2018;59:19–26.
Chan MY, Chok KSH. Sarcopenia in pancreatic cancer – effects on surgical outcomes and chemotherapy. World J Gastrointest Oncol. 2019;11:527–37.
Hong S, Kim KW, Park HJ, Ko Y, Yoo C, Park SY, et al. Impact of baseline muscle mass and myosteatosis on the development of early toxicity during first-line chemotherapy in patients with initially metastatic pancreatic cancer. Front Oncol. 2022;12:878472.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
U.S. Department of Human and Health Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol Ser A. 2019;74:1671–8.
Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35.
MacCormick A, Streeter A, Puckett M, Aroori S. The impact of myosteatosis on outcomes following surgery for gastrointestinal malignancy: a meta-analysis. Ann R Coll Surg Engl. 2023;105:203–11.
Herrod PJJ, Boyd-Carson H, Doleman B, Trotter J, Schlichtemeier S, Sathanapally G, et al. Quick and simple; psoas density measurement is an independent predictor of anastomotic leak and other complications after colorectal resection. Tech Coloproctol. 2019;23:129–34.
Bozzetti F. Chemotherapy-Induced Sarcopenia. Curr Treat Options Oncol. 2020;21:7.
Halle-Smith JM, Hall LA, Powell-Brett SF, Merali N, Frampton AE, Beggs AD, et al. Pancreatic Exocrine Insufficiency and the Gut Microbiome in Pancreatic Cancer: A Target for Future Diagnostic Tests and Therapies? Cancers. 2023;15:5140.
Kordes M, Larsson L, Engstrand L, Löhr JM. Pancreatic cancer cachexia: three dimensions of a complex syndrome. Br J Cancer. 2021;124:1623–36.
Baazim H, Antonio-Herrera L, Bergthaler A. The interplay of immunology and cachexia in infection and cancer. Nat Rev Immunol. 2022;22:309–21.
Stretch C, Aubin JM, Mickiewicz B, Leugner D, Al-Manasra T, Tobola E, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS One. 2018;13:e0196235.
Cruz-Jentoft AJ, Gonzalez MC, Prado CM. Sarcopenia≠low muscle mass. Eur Geriatr Med. 2023. https://doi.org/10.1007/s41999-023-00760-7.
Bittner MI, Grosu AL, Brunner TB. Comparison of toxicity after IMRT and 3D-conformal radiotherapy for patients with pancreatic cancer - a systematic review. Radiother Oncol. 2015;114:117–21.
Prasad S, Cambridge L, Huguet F, Chou JF, Zhang Z, Wu AJ, et al. Intensity modulated radiation therapy reduces gastrointestinal toxicity in locally advanced pancreas cancer. Pract Radiat Oncol. 2016;6:78–85.
Botsen D, Ordan MA, Barbe C, Mazza C, Perrier M, Moreau J, et al. Dynapenia could predict chemotherapy-induced dose-limiting neurotoxicity in digestive cancer patients. BMC Cancer. 2018;18:955.
Martin P, Botsen D, Brugel M, Bertin E, Carlier C, Mahmoudi R, et al. Association of Low Handgrip Strength with Chemotherapy Toxicity in Digestive Cancer Patients: A Comprehensive Observational Cohort Study (FIGHTDIGOTOX). Nutrients. 2022;14:4448.
Ordan MA, Mazza C, Barbe C, Perrier M, Botsen D, Renard Y, et al. Feasibility of systematic handgrip strength testing in digestive cancer patients treated with chemotherapy: The FIGHTDIGO study. Cancer. 2018;124:1501–6.
Moreau J, Ordan MA, Barbe C, Mazza C, Perrier M, Botsen D, et al. Correlation between muscle mass and handgrip strength in digestive cancer patients undergoing chemotherapy. Cancer Med. 2019:8:3677–84.
Perrier M, Ordan MA, Barbe C, Mazza C, Botsen D, Moreau J, et al. Dynapenia in digestive cancer outpatients: association with markers of functional and nutritional status (the FIGHTDIGO study). Support Care Cancer. 2022;30:207–15.
Akahori T, Sho M, Kinoshita S, Nagai M, Nishiwada S, Tanaka T, et al. Prognostic Significance of Muscle Attenuation in Pancreatic Cancer Patients Treated with Neoadjuvant Chemoradiotherapy. World J Surg. 2015;39:2975–82.
Neuzillet C, Bouché O, Tournigand C, Chibaudel B, Bouguion L, Bengrine-Lefevre L, et al. Adapted physical activity in patients (Pts) with advanced pancreatic cancer (APACaP): Results from a prospective national randomized GERCOR trial. JCO. 2022;40:4007–4007.
Lemoine A, Perrier M, Mazza C, Quinquenel A, Brasseur M, Delmer A, et al. Feasibility and Impact of Adapted Physical Activity (APA) in Cancer Outpatients Beginning Medical Anti-Tumoral Treatment: The UMA-CHAPA Study. Cancers. 2022;14:1993.
Vashi P, Popiel B, Lammersfeld C, Gupta D. Outcomes of systematic nutritional assessment and medical nutrition therapy in pancreatic cancer. Pancreas. 2015;44:750–5.
Acknowledgements
We would like to thank Aditya Ramani, a native English speaker who proofread and improved the quality of the manuscript. We also thank the patients and their families.
Author information
Authors and Affiliations
Contributions
Conceptualization: MP, EB, MB and OB; Methodology: MP, MD, MB and OB; Software: MB; Validation: MP, MF, EB, CC, FS, MB and OB; Formal analysis: MF, MB and OB; Investigation: MF, DB, PG, PT, AS, EF, and OB; Resources: MF, DB, PG, PT, AS, EF, and OB; Data curation: MP, MB and OB; Writing-original draft preparation: MP, MF, CC, MB, and OB; Writing-review and editing: MP, MF, MB, and OB; Visualization: MP, MF, CC, MB, and OB; Supervision: MP, CC, FS, MB, and OB; Project administration: MB and OB. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
CC reports personal fees as a speaker from Brystol Myers Squibb, outside the submitted work. DB reports personal fees as a speaker and/or in an advisory role from Accord Healthcare, Amgen, Sanofi, Servier, and Pierre Fabre, outside the submitted work. FS reports personal fees as a speaker and/or in an advisory role from Astra Zeneca, outside the submitted work. OB reports personal fees as a speaker and/or in an advisory role from Merck KGaA, Apmomia Therapeutics, Bayer, MSD, Amgen, Servier, and Pierre Fabre, outside the submitted work. All other authors have no conflict of interest.
Ethical approval
The database of this retrospective study was constituted in accordance with the reference methodology MR004 of the French National Commission on Informatics and Liberty (CNIL) and the Helsinki Declaration. According to current French regulations, no informed consent or additional ethical committee review was required for retrospective studies using data from medical records collected in daily practice by medical and nursing staff caring for the patient and without human involvement. Data were anonymized prior to statistical analysis. Based on the non-opposition principle, information on the purpose of the study and the type of data collected was given to each patient included in the study. Patients were free to decline to participate, but none refused. The present study was declared to the Health Data Hub under the number F20220624135714 and is available at https://www.health-data-hub.fr.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Perrier, M., Fontaine, M., Bertin, E. et al. Impact of low muscle mass and myosteatosis on treatment toxicity and survival outcomes in non-resectable pancreatic cancer patients treated with chemoradiotherapy. Eur J Clin Nutr 79, 576–586 (2025). https://doi.org/10.1038/s41430-025-01566-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41430-025-01566-5