Abstract
This study aimed to evaluate the impact of various dietary interventions on managing osteoarthritis (OA), a condition significantly affecting global health due to joint alterations driven by inflammatory mediators. A systematic review and meta-analysis, adhering to PRISMA guidelines, examined Randomized Controlled Trials (RCTs) investigating dietary interventions in OA. Two reviewers independently conducted study selection, data extraction, and quality assessment. Random effects models calculated standardized mean differences (SMD) and mean differences (MD). Risk of bias was evaluated with the Cochrane Risk of Bias tool (RoB2), and heterogeneity was assessed using I² values. Nine RCTs (898 participants) were identified, assessing various diets: reduced energy (n = 4), Mediterranean (n = 2), low-fat (n = 2), anti-inflammatory (n = 1), low-carbohydrate (n = 1), and plant-based (n = 1). Dietary interventions significantly improved pain (SMD: –0.67; 95% CI: [–1.01, –0.34]; p < 0.0001), and physical function (SMD: –0.62; 95% CI: [–0.94, –0.30]; p = 0.0001) and body weight (MD: –3.18; 95% CI: [–3.52, –2.83], p < 0.0001). Subgroup analyses revealed reduced energy diets improved pain (SMD: –0.85; 95% CI: [–1.15, –0.55], p < 0.0001), physical function (SMD: –0.95; 95% CI: [–1.33, –0.58], p < 0.0001) and body weight (MD: –3.13; 95% CI: [–3.77, –2.49], p < 0.0001). The Mediterranean diet did not significantly impact pain (SMD: –0.27; 95% CI: [–1.14, 0.60], P = 0.54), or physical function (SMD = –0.28; 95% CI: [–0.79, 0.24], p = 0.29). This study emphasizes the significant impact of dietary interventions on pain, physical function, and weight management in people with OA, with reduced energy diets showing the most effectiveness. Specific dietary patterns show promise but require further investigation.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a prevalent and persistent joint condition that significantly impacts the overall wellbeing and quality of life of individuals worldwide [1]. As of 2020, around 595 million people were diagnosed with OA globally, and if the present patterns continue, it is estimated that close to one billion individuals will experience OA by the year 2050 [2]. In the initiation and progression of the OA process, inflammatory mediators play a vital role. These mediators are produced locally by joint cells and, systemically, by tissues like adipose tissue, which releases them into the bloodstream [3]. Eventually, they reach the joint through the vasculature in the subchondral bone. Consequently, these mediators induce diverse structural and functional alterations in joint tissues, including the breakdown of cartilage, remodelling of bone, and changes in the synovium [3]. These alterations lead to noticeable symptoms such as pain, stiffness, swelling, and limitations in joint mobility. OA, being one of the most widespread chronic health concerns, goes beyond physical ramifications. It influences mental well-being, disrupts sleep patterns, hinders work engagement, and, notably, can even impact mortality [4].
With the incidence of OA on the rise due to the aging population and the obesity epidemic, there is a growing need to prioritize effective prevention and management of this chronic condition. Recent research highlights the potential role of dietary factors in preventing the onset or slowing the progression of OA [5, 6]. One noteworthy avenue of investigation involves the adoption weight management strategies, which have shown promising outcomes. Studies reveal that a reduction of at least 5–10% of body weight correlates with significant improvements in OA-related symptoms and functional outcomes [7, 8]. This suggests a tangible link between weight management and the amelioration of OA, presenting a compelling case for dietary interventions [8].
An expanding body of research is delving into the potential efficacy of anti-inflammatory diets, such as the Mediterranean Diet (Med Diet) and plant-based diets, as interventions for OA. These dietary patterns are characterized by an emphasis on fruits, vegetables, whole grains, and healthy fats, have garnered attention for their potential to influence the inflammatory processes underlying OA [9, 10]. Empirical evidence indicates notable efficacy of anti-inflammatory diets in reducing inflammation, irrespective of concurrent weight loss, as substantiated in academic literature [11, 12], suggesting this might be promising for OA.
Drawing insights from a comprehensive systematic review and meta-analyses, it becomes evident that a synergistic approach involving reduced energy diets and regular exercise yields a moderate alleviation of pain [7]. Another meta-analysis has highlighted the positive impact of incorporating meal replacements into a dietary regimen, revealing improvements in physical function [13]. Additionally, a separate systematic review reveals that dietary modifications can effectively reduce pain and enhance function in adults with osteoarthritis [14]. However, it is noteworthy that the existing body of evidence from systematic reviews and meta-analyses primarily focuses on the combination of a reduced energy diet and exercise, and specifically on interventions involving meal replacements. There is a notable gap in the literature regarding the efficacy of different dietary patterns such as the Med diet, anti-inflammatory diets, and distinct reduced energy dietary strategies without meal replacement on OA management. As such, there is a compelling need for further investigation to systematically assess and compare the outcomes associated with these diverse dietary approaches in the context of OA management. This study represents a significant advancement in the field by being the first meta-analysis to systematically compare the effectiveness of diverse dietary patterns in OA management, extending beyond the focus on energy restriction and exercise. By evaluating the impact of anti-inflammatory, low-carbohydrate, low-fat, and Mediterranean diets, this work fills a critical gap in the literature by focusing solely on the effects of diet without the combined influence of exercise or meal replacement formulas. Also, it can provide evidence-based insights that have the potential to shape future research directions. The primary aim of this systematic review and meta-analysis was to examine the effects of various dietary interventions, sourced from Randomized Controlled Trials (RCTs), on pain relief, weight management, and physical function in individuals with OA, offering a more comprehensive understanding of dietary strategies in OA management.
Methods
The systematic review and meta-analysis protocol were pre-registered on the Open Science Framework (Registration https://doi.org/10.17605/OSF.IO/CYHWP). There were minor deviations from the pre-registered protocol. Specifically, the quality assessment tool. These were addressed by using the Cochrane Risk of Bias tool (RoB 2), the updated tool for quality assessment in RCTs. This revised tool RoB2 was developed to address limitations identified in the other tools, incorporate advances in the assessment of risk of bias used in other recently developed tools, and integrate recent developments in the estimation of intervention effects from randomized trials [15, 16]. Adherence to reporting standards was ensured by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
Search strategy
In conducting this systematic review and meta-analysis, we identify relevant studies by systematically searched the following electronic databases from their inception until January 2024: PubMed (Medline), EMBASE, Web of Science, CINAHL, PsycINFO, Scopus, Central Register of Controlled Trials, and grey literature. Additionally, we set up automated updates in PubMed to track newly published papers, with the most recent update received on January 29, 2025. To ensure comprehensive coverage, we also conducted manual searches in other databases to capture any newly available studies. The search strategy was developed with input from an information librarian, ensuring the comprehensiveness and accuracy of the search process. This ensured that the strategy was comprehensive and aligned with the study’s objectives. The detailed search strategy for all databases is provided in Supplementary Appendix 1. We did not impose any language restrictions on the search and provided a translator when required. Further, we screened reference lists of the included studies, and systematic reviews on the topic.
Study selection
We included RCTs studies that recruited male and female adults (over 18 years of age) who were diagnosed with OA. OA is a chronic joint condition characterized by the breakdown and gradual loss of cartilage in the joints. It typically has the potential to affect any joint within the body, although it typically has a stronger presence in joints located in the hands, knees, hips, and spine [18]. This systematic review did not impose restrictions on the joints affected.
Studies that report any diet strategies including any type of diet intervention like weight loss diet, low fat diet, Med diet, low carbohydrate diet, anti-inflammatory diet, and plant-based diet for the management of pain, physical function, quality of life or other health outcomes in patients with OA were included. Diet strategies were classified based on the diet reported in each study. Health outcomes included body weight, and inflammatory markers such as C-reactive protein (CRP), interleukin-1(IL-1), interleukin-6 (IL-6), and tumour necrosis factor-alpha (TNF-α).
Studies carried out in healthcare settings, communities, and homes were included. There were no limitations based on geographic location or cultural context. Inclusion criteria for RCTs involved direct comparisons between different diets or a usual care control group. If an exercise component was provided in both the intervention and control groups, and the effect of the dietary intervention could be isolated, then these studies were included.
Studies combining diet and meal replacement formulas, longitudinal study designs, analytical observational studies, such as prospective and retrospective cohort studies, case-control studies, analytical cross-sectional studies, and qualitative literature, surveys, case series, studies without full text and case reports were excluded.
Screening strategy
The authors followed the strict screening procedures reported by the Cochrane Handbook for Systematic Reviews. Articles were exported from databases into COVIDENCE software (Australia, ABN 41 600 366 274). Screening of titles and abstracts to remove irrelevant reports was undertaken by one author (SA), as permitted by the Cochrane Handbook, which states that while duplicate screening is ideal, initial screening by a single reviewer is acceptable [19]. Articles or documents that met the inclusion criteria without meeting any exclusion criteria were retrieved for full text review. The full texts of these selected articles and documents were then evaluated by two authors (SA & BP). Each author operated independently and remained blind to each other’s decision throughout the screening process. Regarding reviewer blinding, while researchers have historically recommended blinding reviewers to reduce potential bias, there is currently no empirical evidence to demonstrate that blinding significantly reduces or prevents bias [20]. Consequently, reviewers in this study were not blinded to author identities, institutional affiliations, or other details, which aligns with common practice in systematic reviews. Instead, we minimized bias through clearly defined inclusion and exclusion criteria, adherence to systematic procedures, and independent assessment by multiple reviewers [20]. Discrepancies during the full-text screening phase were addressed through discussion, consistent with Cochrane’s recommendation [19] that disagreements are often resolved through dialogue to identify simple oversights or differences in interpretation. When necessary, a third reviewer (SG) provided arbitration to reach a final decision. Multiple publications arising from the same study were linked together, and the publication most relevant to the research question was selected as the primary source, with secondary papers used for data resources if needed.
Data extraction
Two authors (SA & CF) worked independently to extract data from the included studies. Disagreements were resolved by a discussion. If consensus was not reached, a third author (SG) was consulted. The following information was decided upon a-priori and extracted from included papers: study characteristics including first author, year of publication, country, study design, sample size, study duration, adherence, participants’ characteristics such as body mass index (BMI), sex, race, study population characteristics (e.g., overweight, pre-diabetic state, or type 2 diabetes), intervention and control conditions such as type of diets, details of the diet intervention and control group, changes in outcomes such as changes in weight, BMI, pain, physical function, quality of life (QoL), mental health, and inflammatory markers including CRP, IL-1, IL-6, and TNF-α.
If the mean and standard deviation (SD) of the differences were unavailable, our initial step was to attempt to communicate with the study authors. In the event of no response, we proceeded by extracting the mean and SD of both pre-intervention and post-intervention values for the control and intervention groups. Authors were contacted twice for missing data. If no response was received within one month of initiating contact, and both the mean and SD values for pre-intervention and post-intervention were unavailable, the paper was considered to meet the inclusion criteria but lacked sufficient data for the meta-analysis.
Quality assessment
The studies included were assessed for bias using the Rob2 tailored for RCTs [16]. Two independent reviewers evaluated each study to ascertain whether it exhibited low, moderate, or high risk of bias. Evaluation criteria encompassed potential biases stemming from subject recruitment, randomization procedures, intervention deviations, missing data handling, outcome measurement, and reported result selection. The two reviewers resolved any disagreements about bias and quality assessments by discussing them. If consensus was not reached, a third author was consulted.
Data synthesis and statistical analysis
The statistical analysis was conducted using R softwareTM (Indianapolis, Indiana, USA) where the mean difference, SD, and sample size were entered. When a study did not report SD, we used the estimation methods recommended by the Cochrane Handbook [21]. If the difference values were not directly available, the differences were computed using below equation:
assuming a correlation coefficient (R value) of 0.8 [22].
If the standard error (SE) was provided in the article, SD was calculated using below equation:
with n representing the sample size [21].
We analysed continuous data by calculating the mean difference (MD) (meta-analysis of the same scale) or standardized mean difference (SMD) (meta-analysis of different scales), and 95% confidence intervals.
Forest plots were utilized to visually represent the point estimate and the 95% CI of each individual effect size. Effect sizes were interpreted according to Cohen’s interpretation ‘trivial’ (<0.20), ‘small’ ( ≥ 0.20 to <0.50), ‘moderate’ ( ≥0.50 to <0.80) and ‘large’ ( ≥ 0.80) [23]. The heterogeneity of data between studies was evaluated and quantified using Cochrane’s Q test, with a p-value of <0.1 indicating statistical significance, and I2 [24]. The Cochrane Handbook provides general guidelines for interpreting heterogeneity levels and a random-effects meta-analysis was used to incorporate heterogeneity among studies. Heterogeneity between 0% and 40% might not be deemed important, while between 30% and 60% may suggest moderate heterogeneity. Heterogeneity between 50% and 90% may indicate substantial heterogeneity, and between 75% and 100% may represent considerable heterogeneity [25]. In cases where a study’s I2 value fell into two intervals, we opted for the higher, more conservative interval. We conducted meta-regression analyses to investigate how continuous factors, such as the length of follow-up periods, could impact the results. The p-value of regression coefficient was used to indicate whether this difference was statistically significant [26]. Sensitivity analyses were performed using leave-one-out meta-analyses to evaluate the impact of each individual study on the overall estimate of the treatment effect. Additionally, we conducted a sensitivity analysis by removing the single study with ‘low risk’ of bias to assess whether its inclusion was disproportionately affecting the results. The meta-regression and sensitivity were conducted using R software.
Results
Search Results
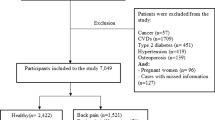
Figure 1 shows the PRISMA flow diagram of the study selection process. The search identified 13,239 records. Of these, 8293 were screened after removal of duplicates, and 8237 were excluded based on titles and abstracts. Of 56 potentially relevant records screened in full text for eligibility (Supplementary Appendix 2), nine RCTs (n = 898) met our inclusion criteria [7, 8, 27,28,29,30,31,32,33]. Additionally, Supplementary Appendix 3 provides a detailed summary of how each PRISMA 2020 checklist item is addressed within the manuscript, ensuring transparency and adherence to reporting guidelines.
Study characteristics
Participant and intervention characteristics are outlined in Tables 1, 2. All nine RCTs encompass a diverse geographic distribution, with contributions from the United States (n = 4), Iran (n = 2), Tunisia (n = 1), Taiwan (n = 1), and the United Kingdom (n = 1). The studies encompassed a total of 898 participants, with sample sizes across the studies varying from 21 to 316 individuals. The age of participants included in the studies ranged from 41 ± 11.7 to 72 ± 1.97 years old. The study population comprised individuals with a BMI range from 26.9 ± 3.02 to 36 ± 7 kg/m2. Additionally, four studies included patients with OA who also had other comorbidities such as diabetes mellitus, cardiovascular diseases, hyperlipidaemia, liver disease, and kidney failure. Regarding gender diversity, seven of the studies included participants of all genders, one study specifically focused on female participants, and one study did not specify gender.
The diets encompassed in the study varied, including reduced energy (n = 4) [7, 8, 30], Med Diet (n = 2) [29, 31], low-fat (defined as 20% fat) (n = 2) [31, 32], anti-inflammatory (n = 1) [28], low-carbohydrate (defined as 20 g/d for first three weeks then 40 g thereafter) (n = 1) [32], and whole-foods, plant-based diet (n = 1) [27]. Messier et al. described reduced energy diet as achieving a 5% reduction in body weight through calorie reduction [7]. Another study defined it as adhering to a balanced low-energy diet of 1200 kcal/day (1 kcal = 4.186 kJ) [8]. Ghroubi et al. characterized it as reducing caloric intake by 25–30% for individuals whose usual consumption ranges from 1500 to 3000 calories [30]. Wolf et al. referred to it as weight control through modified eating habits [33]. Therefore, we propose renaming all these approaches collectively as a “reduced energy diet.” The length of time the dietary interventions ran varied significantly across the trials, with a mean duration of 29.55 ± 34.46 weeks, range 6 to 104. Control/comparator groups across the studies received different interventions, including eat-as-usual (n = 6), a low-calorie diet (n = 1), and health education sessions covering topics related to OA, obesity, and exercise (n = 1). Compliance with the intervention was evaluated using various dietary assessment methods, such as weekly food journals, 24-h recalls, and 7-day food diaries, in seven of the papers [7, 8, 27,28,29, 31, 32]. However, two studies did not specify the method used to assess compliance [30, 33]. The RCTs investigated several outcome measures, including pain (n = 9), weight reduction (n = 8), physical function (n = 7), QoL (n = 3), and inflammatory markers (n = 2). However, we could not perform a meta-analysis on QoL and inflammatory outcomes due to the insufficient number of studies and data. All the studies, with the exception of Wolf et al. [33], reported findings on pain. All studies reported on body weight change, with the exception of Dyer et al. [29]. Two studies did not report on physical function [27, 32].
Risk of bias (Rob)
Figure 2 presents an overview of the risk of bias within the studies, which were evaluated based on the pre-established criteria of the Cochrane Rob2 tool for randomized control [16].
Within Domain 1: Randomization Process, seven studies were identified as having a low risk of bias [7, 8, 27,28,29,30,31], while the other three studies were classified as having some concerns of bias [32, 33]. In Domain 2: Deviations from Intended Interventions, every study was assessed to have a low risk of bias except Ghroubi et al. [30]. One study had some concerns of bias for Domain 3: Missing outcome data [27], and the remainder had a low risk of bias. In Domain 4: Measurement of the outcome, six studies had low risk of bias [7, 8, 28,29,30, 33] and three had some concerns of bias [27, 31, 32]. Five studies [7, 27, 30, 32, 33] had low risk of bias for Domain 5: Selection of the reported result, with the remainder having some concerns of bias [8, 28, 29, 31]. Overall, analysis showed some concerns with risk of bias for the eight studies included in this meta-analysis.
Changes in pain
Across the seven studies [7, 8, 28,29,30,31,32] that measured subjective pain via questionnaire, there was a significant improvement in subjective pain levels, with the experimental groups demonstrating moderate and significant improvements compared to the control groups (SMD -0.67; 95% CI: [–1.01, –0.34]; p < 0.0001). However, there was notable heterogeneity (I2 = 63%, p < 0.01) among the studies [Fig. 3].
When analysing by sub-groups, a reduced energy diet led to a large, significant improvement in pain (SMD: –0.85; 95% CI: [–1.15, –0.55], p < 0.0001), with zero heterogeneity (I2 = 0%; p = 0.76). However, no significant improvement was observed for the low-fat diet (SMD: –0.36; 95% CI: [–0.76, 0.04], p = 0.08) and Med Diet (SMD: –0.27; 95% CI: [–1.14, 0.60], p = 0.54) diets. Heterogeneity for the low-fat diet (I2 = 0%, p = 0.87) was low, but high and significant for the Med Diet (I2 = 85%, p = 0.01). Due to a limited number of studies, we were unable to complete a sub-group analysis for the anti-inflammatory or low carbohydrate diets, however the anti-inflammatory diet showed promising results with a large and significant reduction in pain (SMD –1.41; 95% CI: [–1.98, –0.85], P < 0.0001).
Changes in physical function
In the seven studies [7, 8, 28,29,30,31, 33] that assessed subjective physical function using questionnaires, there was a significant moderate improvement in physical function overall, with groups undergoing experimental treatments showing better outcomes than control groups (SMD: –0.62; 95% CI: [–0.94, –0.30]; p = 0.0001). However, significant heterogeneity (I2 = 68%, p < 0.01) was observed across the studies [Fig. 4].
Sub-group analysis revealed that a reduced energy diet led to a large and significant improvement in physical function (SMD: –0.95; 95% CI: [–1.33, –0.58], p < 0.0001) with moderate heterogeneity (I2 = 37%; p = 0.19). In contrast, the Med diet had a small, non-significant effect (SMD: –0.28; 95% CI: [–0.79, 0.24], p = 0.29) with notable, but non-significant heterogeneity (I2 = 58%, p = 0.12). We were unable to conduct a subgroup analysis for the anti-inflammatory diet due to limited study numbers. However, this diet type displayed promising results, indicating a significant and noteworthy improvement in physical function (SMD: –0.72; 95% CI: [–1.24, –0.20], p < 0.0001).
Changes in body weight
Overall, there was a statistically significant body weight loss across six studies [8, 28, 30,31,32,33], with those in the intervention groups showing better outcomes (MD: –3.18; 95% CI: [–3.52, –2.83], p < 0.0001) with a low heterogeneity (I2 = 0%, p = 0.98) across the studies [Fig. 5].
Sub-group analysis showed statistical differences in outcomes for those on a reduced energy diet (MD: –3.13; 95% CI: [–3.77, –2.49], p < 0.0001) and low-fat diet (MD: –3.09; 95% CI: [–3.62, –2.55], p < 0.0001). There was no heterogeneity among studies for the reduced energy diet (I2 = 0%, p = 0.97) and low-fat diet interventions (I2 = 0%, p = 0.71). Due to the scarcity of research, we could not carry out a subgroup analysis for studies using the anti-inflammatory diet, Med Diet, or low-carbohydrate diet. Nonetheless, both the Med Diet (SMD: –3.29; 95% CI: [–3.98, –2.60], p < 0.0001), and anti-inflammatory diet (SMD: –4.13; 95% CI: [–6.90, –1.36], p < 0.0001), showed promising results.
Sensitivity analyses
To assess the influence of individual studies on the overall pooled efficacy estimate for the primary outcome, we conducted leave-one-out meta-analyses. In this approach, each study was sequentially removed, and the meta-analysis was recalculated to determine its impact on the pooled effect size.
The leave-one-out analyses demonstrated a consistent pooled effect size, indicating the robustness of the findings. The sensitivity analyses for pain, weight reduction, and physical function demonstrated that no single study significantly influenced the overall results. These findings underscore the robustness of the intervention’s impact across all three outcomes. Detailed results of the leave-one-out analyses can be found in Supplementary Appendix 4.
We also conducted a sensitivity analysis by excluding the study with ‘low risk’ of bias to assess its impact on the results. After removal, the pooled effect sizes remained consistent for both primary outcomes. For physical function, the SMD was –0.52 (95% CI: [–0.83, –0.21]; I² = 54%), and for pain, the SMD was –0.65 (95% CI: [–1.04, –0.26]; I² = 66%). These results further confirm the robustness of the intervention’s effect.
Meta-regression
To explore how continuous variables, such as the duration of follow-up periods, might influence the outcomes, we conducted meta-regression analyses. We found no significant impact on pain (β = −0.0043, SE = 0.0056, p = 0.442), body weight (β = −0.0098, SE = 0.0301, p = 0.74), or physical function (β = −0.0071, SE = 0.0047, p = 0.126). We were unable to calculate meta regression on other variables due to a limited number of studies.
Discussion
This systematic review and meta-analysis revealed that dietary interventions have a noteworthy impact on improving pain levels, enhancing physical function, reducing body weight in patients with OA. Specifically, we found that reduced energy diets resulted in significant improvements in pain, physical function, and body weight. However, no significant enhancements in pain and physical function were observed with the Med Diet. To our knowledge, this is the first meta-analysis examining the efficacy of various dietary interventions in managing OA.
Our research indicates that dietary interventions are effective in alleviating pain among patients with OA, with reduced energy diets showing particularly promising results. Consistent with our findings, a meta-analysis revealed that a combination of diet and exercise treatments moderately reduced pain. However, diet-only treatments did not yield pain reduction [34]. It is important to highlight that while our study focused on dietary interventions without the inclusion of meal replacement formulas, the meta-analysis encompassed trials involving a combination of reduced energy diet and formula. However, when subgroup analyses were conducted, the same level of efficacy was not observed with low-fat diets or the Med Diet. A systematic review of three studies (comprising two cohort studies and one trial) concluded a possible association between OA and adherence to the Med Diet [9]. However, our meta-analysis included only two studies on the Med diet, one of which lacked sufficient details about the intervention. Additionally, a significant limitation across the included studies was the method used to assess dietary adherence, which may have affected the reliability of the findings [29]. The high heterogeneity observed in some subgroups, such as the Mediterranean diet, suggests significant variability in study outcomes, which may reflect differences in study design, population characteristics, or intervention protocols. Additionally, the wide confidence intervals for some standardized mean differences (e.g., [-1.42; -0.16] for the reduced-energy diet) indicate uncertainty in the effect estimates. These findings, coupled with the significant subgroup differences (p = 0.03), highlight the need for caution in interpreting the results and underscore the importance of further research to clarify the effectiveness of specific dietary interventions in OA. Anti-inflammatory and low carbohydrate diets showed promising results for improving pain, however with only one study available to analyse for each diet type, the literature would benefit from further research utilising these diets.
Furthermore, our research highlights the potential of dietary interventions to enhance physical function among individuals with OA, with particularly positive outcomes associated with reduced energy diets. Aligned with our findings, a systematic review and meta-analysis revealed that incorporating dietary changes alongside meal replacements, and very low-energy diets using meal replacements exclusively, led to improvements in physical function [13]. In contrast, the Med Diet did not demonstrate a notable improvement in physical function. However, due to the inclusion of only two studies on the Med Diet in this meta-analysis, it was challenging to thoroughly examine this relationship. Despite the apparent effectiveness of anti-inflammatory and low-fat diets in alleviating physical function-related issues, the scarcity of studies focusing on these dietary interventions underscores the necessity for additional research in this area.
In relation to weight, the collective findings from seven studies indicated a statistically significant body weight reduction. Subgroup analysis emphasized notable differences in outcomes, especially among individuals following reduced energy or low-fat diets. Across the studies analyzed, treatment durations varied widely, spanning from 6 to 104 weeks. Our investigation involved conducting a meta-regression analysis to probe the influence of treatment duration and our findings revealed no notable impact on pain levels, body weight, or physical function. The evidence suggests that weight reduction may offer a plausible explanation for the decrease in pain. Specifically, in overweight individuals, a reduction of 5% in body weight can alleviate certain joint discomfort, whereas a loss of at least 10% in body weight is associated with substantial clinical improvement in joint pain [34, 35]. Obesity is considered a chronic inflammatory condition, with IL-1 contributing to the susceptibility to knee OA. Consequently, obesity and increased body fat play a significant role in the development of OA, even in non-mechanical contexts. Therefore, weight loss not only reduces mechanical stress on the joints but also mitigates the inflammatory processes associated with obesity, further contributing to the reduction of OA symptoms [36]. In the initiation and progression of OA, inflammatory mediators are pivotal [37, 38]. Managing weight and reducing the consumption of proinflammatory food components can potentially decrease the incidence of chronic inflammation [39]. Various food components may possess anti-inflammatory effects; for instance, polyunsaturated fatty acids (PUFAs) can hinder the production of nitric oxide and TNF-α, while vitamins C and E may mitigate systemic inflammation by reducing oxidative stress and the expression levels of proinflammatory cytokines [40]. Enhancing the intake of food components with a lower Dietary Inflammatory Index (DII), such as those advocated in the Med Diet, can help maintain the body in a more anti-inflammatory state [39].
The current study possesses both strengths and limitations. Notably, it is the first meta-analysis of randomised controlled trials investigating the impact of various dietary strategies on OA management. A thorough search of six databases employing MeSH terms, broad scope, and comprehensive keyword strategy was conducted to encompass the entirety of existing literature and the study refrained from imposing restrictions based on language or time frame. Also, the adoption of RoB 2 ensured a more robust and systematic framework for evaluating potential biases, aligning with contemporary standards in systematic reviews and meta-analyses. The modification provided a clearer and more precise categorization of bias levels while maintaining consistency with the overall trends and conclusions derived from the included studies. This consistency in findings supports the validity of our results and mitigates concerns about potential bias introduced by this change [15, 16]. Additionally, there were certain constraints to consider. A known limitation of diet trials is the issues in blinding participants undergoing interventions. Another limitation arises from the significant heterogeneity observed across the included studies. To address this, we utilized random-effects models to account for variability between studies. Subgroup analyses based on dietary intervention type indicated consistent results with low heterogeneity (I² = 0%) for reduced energy and low-fat diets. Additionally, meta-regression was conducted to evaluate the impact of study duration, but it did not identify duration as a significant source of heterogeneity. The limited number of studies per subgroup restricted further exploration of other potential sources of heterogeneity. According to the Cochrane Handbook, meaningful regression analyses typically require at least ten studies for each characteristic being modelled. This becomes even more critical when covariates are unevenly distributed across studies. Given the small number of included studies in our review, findings from subgroup analyses or meta-regression should be interpreted with caution. Future research with larger datasets is necessary to provide more definitive insights into the factors contributing to heterogeneity.
Furthermore, it is important to note that several included studies did not provide comprehensive data on dietary adherence, complicating the interpretation of the results. Specifically, while seven studies monitored compliance, two studies lacked adherence data entirely. This gap introduces uncertainty about the extent to which participants adhered to the prescribed interventions, which may have affected the reliability of the findings. We also emphasize the need for future research to prioritize standardized and transparent reporting of compliance to ensure more robust and reliable conclusions. Another significant limitation is the variability in the types of reduced energy diet interventions used across different studies. These variations can lead to differing outcomes in terms of weight loss and its impact on OA management. For instance, the effects of a 5% weight reduction might not be directly comparable to those achieved through a fixed 1200 kcal/day diet or a 25–30% caloric reduction. Each of these dietary strategies may influence OA outcomes differently due to variations in the degree of calorie restriction, nutritional balance, and participant compliance. To improve the quality and comparability of future research, it is essential to standardize the definitions and reporting of dietary interventions. Also, we now note that the differences in baseline characteristics, such as age, BMI, and comorbidities, could potentially influence how different populations respond to dietary interventions, thus affecting the generalizability of our results. While this variability adds breadth to the analysis, it also suggests that the findings may not be equally applicable to all subgroups. Future research should aim to investigate the impact of these factors more thoroughly to help tailor dietary recommendations to specific populations. While this study focused solely on the effects of dietary interventions without the inclusion of exercise or meal replacement formulas, we recognize the potential for synergistic effects when diet and exercise are combined. Future research should explore the cumulative impact of these combined interventions on OA outcomes, as there is evidence that integrated approaches involving both dietary modifications and exercise may provide more substantial benefits in alleviating pain and improving physical function [7, 13].
In conclusion, our systematic review and meta-analysis points to a significant impact of dietary interventions on pain relief, enhanced physical function among OA patients. Specifically, reduced energy diets emerged as particularly effective in improving pain levels, physical function, and body weight. Although the Med Diet did not exhibit significant benefits in pain relief or physical function improvement, highlighting the need for further research in this area. Additionally, the scarcity of studies on anti-inflammatory and low-carbohydrate diets calls for more investigation to fully understand their potential in OA management.
Data availability
The data from this study are available upon request to the corresponding author.
References
Maly M, Marriott K, Chopp-Hurley J. Osteoarthritis year in review 2019: rehabilitation and outcomes. Osteoarthr Cartil. 2020;28:249–66.
Steinmetz JD, Culbreth GT, Haile LM, Rafferty Q, Lo J, Fukutaki KG, et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e508–e22.
Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil. 2013;21:16–21.
Allen K, Thoma L, Golightly Y. Epidemiology of osteoarthritis. Osteoarthr Cartil. 2022;30:184–95.
Green JA, Hirst-Jones KL, Davidson RK, Jupp O, Bao Y, MacGregor AJ, et al. The potential for dietary factors to prevent or treat osteoarthritis. Proc Nutr Soc. 2014;73:278–88.
Thomas S, Browne H, Mobasheri A, Rayman MP. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology. 2018;57:iv61–iv74.
Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the arthritis, diet, and activity promotion trial. Arthritis Rheum. 2004;50:1501–10.
Hsu Y-I, Chen Y-C, Lee C-L, Chang N-J. Effects of diet control and telemedicine-based resistance exercise intervention on patients with obesity and knee osteoarthritis: a randomized control trial. Int J Environ Res Public Health. 2021;18:7744.
Morales-Ivorra I, Romera-Baures M, Roman-Viñas B, Serra-Majem L. Osteoarthritis and the Mediterranean diet: a systematic review. Nutrients. 2018;10:1030.
Asadi S, Aminianfar A, Shiva F, Asadi S, Yarizadeh H, Qorbani M, et al. Association between dietary inflammatory index scores and diabetes sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: a case‐control study. Evid‐Based Complementary Alternat Med. 2022;2022:2661649.
Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79.
Cooper I, Brukner P, Devlin BL, Reddy AJ, Fulton M, Kemp JL, et al. An anti-inflammatory diet intervention for knee osteoarthritis: a feasibility study. BMC Musculoskelet Disord. 2022;23:1–13.
Webb EJ, Osmotherly PG, Baines SK. Physical function after dietary weight loss in overweight and obese adults with osteoarthritis: a systematic review and meta-analysis. Public Health Nutr. 2021;24:338–53.
Stanfar K, Hawes C, Ghajar M, Byham-Gray L, Radler DR. Diet modification reduces pain and improves function in adults with osteoarthritis: a systematic review. J Human Nutr Dietet. 2024;37(4):847–84.
Minozzi S, Cinquini M, Gianola S, Gonzalez-Lorenzo M, Banzi R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J Clin Epidemiol. 2020;126:37–44.
Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. bmj. 2019;366.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam physician. 2012;85:49–56.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.5 (updated August 2024). Cochrane, 2024. Available from www.training.cochrane.org/handbook.
Berlin JA. Does blinding of readers affect the results of meta-analyses? Lancet. 1997;350:185–6.
Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. Cochrane handbook for systematic reviews of interventions. 2019:143–76.
Anzures‐Cabrera J, Sarpatwari A, Higgins JP. Expressing findings from meta‐analyses of continuous outcomes in terms of risks. Stat Med. 2011;30:2967–85.
Cohen J. Statistical power analysis for the behavioral sciences: Routledge; 2013.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–58.
Deeks JJ, Higgins JP, Altman DG, Group CSM. Analysing data and undertaking meta‐analyses. Cochrane handbook for systematic reviews of interventions. 2019:241–84.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP. et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;2019:ED000142.
Clinton CM, O'Brien S, Law J, Renier CM, Wendt MR. Whole-foods, plant-based diet alleviates the symptoms of osteoarthritis. Arthritis.2015;2015:708152
Dolatkhah N, Toopchizadeh V, Barmaki S, Salekzamani Y, Najjari A, Farshbaf-Khalili A, et al. The effect of an anti-inflammatory in comparison with a low caloric diet on physical and mental health in overweight and obese women with knee osteoarthritis: a randomized clinical trial. Eur J Nutr. 2023;62:659–72.
Dyer J, Davison G, Marcora SM, Mauger AR. Effect of a Mediterranean type diet on inflammatory and cartilage degradation biomarkers in patients with osteoarthritis. The journal of nutrition. Health Aging. 2017;21:562–6.
Ghroubi S, Elleuch H, Kaffel N, Echikh T, Abid M, Elleuch M, editors. Apport de l’exercice physique et du régime dans la prise en charge de la gonarthrose chez l’obèse. Annales de réadaptation et de médecine physique: Elsevier 2008.
Sadeghi A, Zarrinjooiee G, Mousavi SN, Abdollahi Sabet S, Jalili N. Effects of a Mediterranean diet compared with the low-fat diet on patients with knee osteoarthritis: a randomized feeding trial. Int J Clin Pract. 2022;2022:7275192
Strath LJ, Jones CD, Philip George A, Lukens SL, Morrison SA, Soleymani T, et al. The effect of low-carbohydrate and low-fat diets on pain in individuals with knee osteoarthritis. Pain Med. 2020;21:150–60.
Wolf S, Foley S, Budiman-Mak E, Moritz T, O’Connell S, Jelinek C, et al. Predictors of weight loss in overweight veterans with knee osteoarthritis who participated in a clinical trial. J Rehabil Res Dev. 2010;47:171–81.
Hall M, Castelein B, Wittoek R, Calders P, Van Ginckel A, editors. Diet-induced weight loss alone or combined with exercise in overweight or obese people with knee osteoarthritis: a systematic review and meta-analysis. Seminars in arthritis and rheumatism. Elsevier (2019).
Vincent HK, Heywood K, Connelly J, Hurley RW. Obesity and weight loss in the treatment and prevention of osteoarthritis. PMR 2012;4:S59–S67.
Lementowski PW, Zelicof SB. Obesity and osteoarthritis. Am J Orthopedics-Belle Mead. 2008;37:148.
Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–8.
Goldring MB, Culley KL, Otero M. Pathogenesis of osteoarthritis in General. Cartilage: Volume 2: Pathophysiology. 2017:1-25.
Chen Q-J, Ou L, Li K, Ou F-R. Meta-analysis of the relationship between Dietary Inflammatory Index and esophageal cancer risk. Medicine. 2020;99:e23539.
Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134:1806–11.
Acknowledgements
We extend our gratitude to Professor David Simar for translating the French paper. We also thank the librarians and statisticians at UNSW for their invaluable assistance with the search strategy and data analysis.
Funding
This study was funded by the Estate of the Late Faye Williams. Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
SA contributed to the following aspects of the study: design and coordination, screening abstracts and full texts, data extraction, risk of bias assessment, data analysis and interpretation of the results, writing the initial draft of the manuscript. SG contributed to the following aspects of the study: design and coordination, screening the full texts as the third reviewer, risk of bias assessment, reviewing the draft manuscript. CB contributed to the design and coordination of the study and reviewed the draft manuscript. CF contributed to data extraction. PH helped with the analysis. All authors read and approved the final manuscript. BP contributed to the following aspects of the study: supervising, designing, and coordinating the study, screening the full texts, data analysis and interpretation of the results, reviewing all drafts of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asadi, S., Grafenauer, S., Burley, C.V. et al. The effectiveness of dietary intervention in osteoarthritis management: a systematic review and meta-analysis of randomized clinical trials. Eur J Clin Nutr 79, 959–971 (2025). https://doi.org/10.1038/s41430-025-01622-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-025-01622-0
This article is cited by
-
Does metabolically healthy obesity increase the risk of knee and hand osteoarthritis? A population-based cohort study
BMC Musculoskeletal Disorders (2026)