Abstract
Background
Genetically modified (GMO) high-amylose barley lowers postprandial glucose. Since certain EU countries do not allow GMO barley, we therefore assessed if high-amylose barley made from traditional breeding (Lean Baking Barley, LBB) lowers postprandial glucose compared to bread made from regular barley (RB) or wheat (WF) in individuals with or without type 2 diabetes (T2D).
Methods
In a randomised crossover design, 38 participants (18 T2D and 20 non-T2D) consumed 160 g of bread made from 100% LBB, RB, or WF. Postprandial metabolic responses, appetite and bread perception were measured. A mixed model ANOVA was used for analysis.
Results
LBB bread reduced 4 h postprandial glucose measured as incremental area under the curve (iAUC) by 41% and 39% vs. WF and RB bread in T2D and by 28% and 32% in non-T2D (all, P < 0.05). In T2D, LBB reduced postprandial insulin (iAUC) by 52% and 38% vs. WF and RB, and by 60% vs. WF in non-T2D (all, P < 0.05). Postprandial GIP (iAUC) was lower after LBB in both groups vs. RB and WF (P < 0.05). GLP-1 (iAUC) and FFA (tAUC) were lower after LBB vs. WF in non-T2D (P < 0.05), but not in T2D. Appetite scores were similar for all breads. Overall liking was higher for WF but did not differ between barley types.
Conclusion
LBB breads reduce postprandial glucose and insulin compared to RB and WF bread in individuals irrespective of T2D. LBB may have potential as a functional food in prevention and management of T2D.
ClinicalTrails.gov registration: NCT04702672.
Similar content being viewed by others

Introduction
Diabetes management, including the prevention and remission of type 2 diabetes (T2D), relies on effective evidence-based studies. Well-designed dietary recommendations and nutrition therapy are essential to improve both life expectancy and quality [1, 2]. Among the key factors implicated in the development and progression of diabetes is increased postprandial glucose levels following the consumption of high-glycemic index foods, e.g. wheat flour bread [3]. Dietary strategies aimed at modulating postprandial glucose responses have therefore emerged as promising interventions to mitigate the risk and progression of T2D.
Both high-amylose flour and barley flour have garnered considerable attention in this context due to their unique nutritional properties. High-amylose flour, characterised by a higher proportion of resistant starch, has been shown to elicit a blunted postprandial glucose response compared to traditional flours high in rapidly digestible starches [4, 5]. Similarly, barley flour, rich in dietary fibre, especially beta-glucans, exhibits a slower and more sustained release of glucose into the bloodstream, thus exerting favourable effects on glycemic control and appetite [6,7,8].
We have previously demonstrated that by replacing 50% of wheat flour with 50% of genetically modified high-amylose barley flour [9] postprandial glucose responses were reduced by 34% compared with 100% wheat flour bread [10]. However, genetically modified flour is not a commercially viable option in many European countries. Consequently, we have developed a high-amylose barley variety, termed Lean Baking Barley (LBB), using publicly accepted mutation-based breeding protocols [11].
The present study aims to investigate the postprandial glycemic responses of bread baked with LBB flour compared to bread baked with either regular barley flour (RB) or wheat flour (WF). Additionally, we measured acute postprandial changes in satiety, incretins, lipids and glucagon. Furthermore, evaluation of test breads in regard to look, taste, texture, and overall liking were performed.
We hypothesised that bread made from LBB has the potential to improve postprandial glucose responses, measured as incremental area under the curve (iAUC), compared to both RB and WF bread in subjects with or without T2D, respectively.
Methods
Study design
This study was performed as an acute, single blind, randomised, controlled, crossover trial with three test meals consisting of bread made with either 100% LBB, 100% RB, or 100% WF. Randomisation was done using RedCap®.
The primary outcome was changes in postprandial glucose (given as 4 h iAUC) in subjects with or without T2D. The secondary outcomes were postprandial changes in insulin, glucagon, triglyceride (TG), FFA, gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) calculated as either iAUC or total AUC (tAUC). Furthermore, questionnaires were completed to evaluate e.g. satiety and fullness, as well as perception scores of the test meals.
The study was registered at ClinicalTrails.gov as NCT04702672.
Participants
Adults with and without T2D were recruited via local newspapers and online ads. The study took place at Steno Diabetes Centre Aarhus, Aarhus University Hospital, between February and June 2024. All participants provided written informed consent after receiving oral and written information. Eligibility was determined through physical exams, medical history, and blood tests.
Inclusion criteria without T2D: Adults ( ≥ 18 years) without any form of diabetes. With T2D: Adults ( ≥ 18 years) diagnosed according to IDF criteria, with HbA1c between 6 and 9.3% (42–78 mmol/mol).
Exclusion criteria (both groups): Insulin use, once-weekly GLP-1 agonists, acarbose, significant cardiovascular, kidney, liver, psychiatric, or endocrine conditions, steroid treatment, substance abuse, pregnancy, breastfeeding, or legal incompetence. Stable treatment for hypertension or high cholesterol was permitted.
Experimental protocol
After a standardised evening meal and an overnight fast (from midnight), the study participants arrived at the clinic at 07.30 AM on all three study days.
Smoking was not allowed during the overnight fast or the study visits. Alcohol consumption was not permitted the day before the study days. Anti-hypertensive, cholesterol-lowering and anti-diabetic drugs were paused 24 h before every study day. The three intervention days were separated by a six-day minimum washout.
Antihypertensive medications were temporarily discontinued due to their potential confounding effects on blood glucose regulation, although blood pressure was not an outcome measure in this study. The half-lives of metformin and simvastatin - used by the majority of study participants - are approximately 2–4 h, while those of SGLT-2 inhibitors, DPP-4 inhibitors, and atorvastatin range from 12 to 13 h. We acknowledge that a one-day discontinuation may not allow for complete washout of all drugs across there therapeutic classes; however, due to practical considerations, all medications were paused for the same duration.
During the study days, a catheter was placed in a cubital vein for blood sampling. Baseline questionnaires were completed, and blood samples were drawn. At 0 min the test bread was consumed within the next 10 min along with 250 ml of tap water. A bread perception questionnaire was completed within the first 10 min.
During the following 4 h, blood samples were drawn as following: glucose, insulin, and glucagon at –10, 0, 10, 20, 30, 45, 60, 90, 120, 150, 180, 210, and 240 min; TG, FFA, GLP-1 and GIP at –10, 0, 30, 60, 120, 180, and 240 min. All blood samples were immediately centrifuged at 3000 g for 10 min at 4 °C; thereafter, plasma samples were frozen at –20 °C and the next day stored at –80 °C, except from plasma for glucose measurements, since this was analysed on the study day.
Questionnaires regarding satiety were completed at: 0, 30, 60, 120, 180, and 240 min. At 120 min, an additional 250 ml of tap-water was served.
Study breads
Regular nude barley (RB, H. vulgare var. nudum PS3) and high-amylose nude barley (LBB, H. vulgare var. nudum LBB) were grown in 2023 at Aarhus University, Flakkebjerg. RB was developed by Agrologica (Mariager, Denmark). Grains from both varieties were milled using a Komo Fidibus 21 (KOMO GmbH, Germany). The LBB variety was bred to lack Starch Branching Enzyme IIa (SBEIIa), resulting in 46.5% amylose content. Its yield, grain weight, and starch granule shape were similar to the original line, as described in detail previously [12]. Wheat bread was made with commercial Manitoba flour (HavneMøllen, Denmark). All three bread types followed similar recipes and were produced by P.A. Andersen Bakery (Vejle, Denmark). Breads were portioned (160 g), sealed, frozen at −20 °C, defrosted overnight before study days, and served unheated.
Participants consumed a standard commercial spaghetti bolognese meal (1750 kJ; 15.4 g fat, 49 g carbs, 18.2 g protein) the night before each study day (Salling Group A/S, Denmark). Extra foods were allowed if intake was measured and replicated across all test days.
Bread component analysis
Bread analysis were performed on breads prepared similarly to the study breads.
The moisture content was determined by the weight loss after drying in a vacufuge vacuum concentrator from Eppendorf overnight. The total carbohydrate content was measured as the sum of the dietary fibre and starch content. The Megazyme total starch assay kit (K-TSTA-100A, Wicklow, Ireland) was used to determine the total starch content of samples containing resistant starch following the manufacturer’s instructions. This method variant uses dimethyl sulfoxide and a boiling bath, and dissolution in dimethyl sulfoxide at 100 °C is effective for solubilizing all starches in the bread. The dietary fibre content was determined using the Megazyme total fibre assay kit (K-TDFR-200A, Wicklow, Ireland) in accordance with the manufacturer’s instructions. This method variant uses 1 h incubation with heat-stable α-amylase, which is a critical enzymatic digestion step to remove digestible starch components. The resistant starch content was determined using the Megazyme resistant starch assay kit (K-RAPRS, Ireland).
Blood analyses
Plasma glucose was measured by enzyme sensor technology Xylem Brand on YSI 2500 or 2900 (YSI Incorporated, Ohio, USA). EDTA-plasma insulin and glucagon were measured with ELISA (insulin no. 10- 1113-01 and glucagon no. 10-1271-01; Mercodia AB, Sweden). Plasma FFA concentrations were measured with enzymatic colorimetric assays by using commercial kits (code 270–7700, Wako Chemicals GmbH, Germany) on the NOVI apparatus (Perkin Elmer, Connecticut, USA). Triglycerides were measured on an Indiko apparatus with quantitative enzymatic methods using commercial kits (REF 981786, Thermo Fisher Scientific, Roskilde, Denmark). GLP-1 and GIP were measured with NL-ELISA techniques (GLP-1 no. 10-1278-01 and GIP no. 10-1258-01; Mercodia AB, Sweden) on the Multimode Plate Reader EnVision (Perkin Elmer, Connecticut, USA).
Questionnaires
When consuming the test meal (time 0–10 min), the participants evaluated the looks, texture, taste, and overall liking of the bread. The evaluation consisted of seven boxes rating from the most negative “1; do not like”, over “3; neither/nor” to the most positive “7; like very much”.
Visual analogue scale (VAS) was used to assess hunger, satiety, fullness, desire to eat and prospective consumption of the test breads. VAS consists of a 150 mm line scale with words anchored at each end, expressing the most negative and the most positive rating. The questionnaires were made on paper at time 0, 30, 60, 120, 180 and 240 min a new paper was used for every time point [13, 14]. Results were converted from mm to percentage when results were analysed.
Statistical analysis
The power calculation was made to detect a difference in our primary outcome (i.e., postprandial glucose response, given as iAUC) of 20% between diets [10]. The number of participants needed to complete the study and achieve a statistical power of 80% was calculated to be 18 subjects with T2D and 20 subjects without T2D (a < 0.05, b = 0.80). A mixed model ANOVA was used to examine the difference between bread types. P < 0.05 was considered statistically significant. Results are given as mean ± 95% confidence interval (CI) in tables and as mean ± SEM in graphs, unless otherwise stated. All statistical calculations were performed with STATA version 18 (StataCorp LP, Texas, USA) and graphical elements were generated using GraphPad Prism 10 (GraphPad Software, Boston, USA).
Results
Baseline clinical characteristics
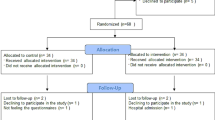
Thirty-eight participants were randomised and 36 completed the study. The two dropouts were due to personal reasons; there was one dropout in each group.
Table 1 presents baseline characteristics of the 36 completing participants. The group with T2D consisted of significantly less women, had higher HbA1c, lower cholesterols and blood pressure, and more subjects were treated with statins and antihypertensive drugs than the non-T2D group. These findings are expected due to international guidelines for diabetes care striving towards lower levels of lipids and blood pressure in subjects with, than without, T2D.
Bread assessment
Table 2 presents the moisture content, total carbohydrate, total fibre, total starch and resistant starch content in g per 100 g of each bread sample. Total carbohydrate content is the sum of total starch and dietary fibre.
Both barley bread contained more dietary fibre and resistant starch than WF, with LBB containing even more than RB. The amount of carbohydrate did not differ between LBB and RB but was higher than in the WF bread.
Glucose, insulin and glucagon
Fasting concentrations of glucose, insulin and glucagon are shown in Table 3, alongside 4 h postprandial responses such as iAUC (glucose and insulin) or tAUC (glucagon) for the T2D and the non-T2D group. The postprandial changes for glucose and insulin as well as their corresponding iAUC for the T2D and the non-T2D group (Fig. 1) demonstrated that, in the T2D group, postprandial glucose was reduced by 41% (P < 0.001) after LBB compared to WF and was reduced by 39% (P < 0.001) compared to RB.
To the left, curves represent the 17 completing participants with type 2 diabetes (T2D) in response to test meals of either 100% wheat flour bread (WF), 100% regular barley flour bread (RB), or 100% high-amylose barley (Lean Baking Barley, LBB). To the right, curves show postprandial glucose responses in the 19 completing participants without T2D. The bottom row displays changes in insulin alongside the corresponding iAUC for the T2D group (left) and the group without T2D (right). *Significantly different from each other (P < 0.05). SEMS are indicated.
In the non-T2D group, reductions in postprandial glucose for LBB were 28% (P = 0.009) and 32% (P = 0.001) compared to WF and RB, respectively.
Surprisingly no significant differences in postprandial glucose responses (iAUC) were observed between RB and WF in the T2D nor in the non-T2D group.
In the T2D group, postprandial insulin responses (iAUC) for LBB were reduced by 52% (P < 0.001) and 38% (P < 0.001) compared to WF and RB, respectively. RB reduced insulin responses by 23% (P = 0.001) compared to WF in the T2D group.
In the non-T2D group, postprandial insulin responses (iAUC) for LBB were reduced by 60% (P = 0.002) compared to WF. There was a trend towards lower postprandial insulin after LBB compared to RB, however, not significant (P = 0.053).
In the T2D group, postprandial glucagon responses did not differ between groups (P > 0.05).
In the non-T2D group, fasting glucagon levels were lower on the LBB intervention day compared to the RB intervention day. In the non-T2D population postprandial glucagon responses (tAUC) were reduced after RB compared to LBB (P = 0.002).
FFA and TG
Fasting and 4 h postprandial changes (tAUC) in plasma concentrations of FFA and TG are shown in Table 3.
In the T2D group, postprandial FFA responses (tAUC) did not differ between groups. In the non-T2D group, postprandial FFA (tAUC) was reduced by 23% (P = 0.027) after LBB compared to WF, but did not significantly differ from RB (P = 0.055).
Postprandial TG (tAUC) did not differ between interventions regardless of T2D status.
GIP and GLP-1
Fasting and 4 h postprandial changes (iAUC) in GIP and GLP-1 are shown in Table 3.
In the T2D group, postprandial GIP responses (iAUC) for LBB were reduced by 65% (P < 0.001) and 47% (P < 0.001) compared to WF and RB, respectively. GIP was reduced after RB by 33% (P = 0.001) compared to WF.
In the non-T2D group the same pattern was found. Postprandial GIP after LBB was reduced by 52% (P < 0.001) and 32% (P = 0.012) compared with after WF and RB, respectively. Postprandial GIP response after RB was reduced by 30% (P = 0.002) compared to WF.
In the T2D group, no differences were found between interventions on postprandial GLP-1 responses.
In the non-T2D, postprandial GLP-1 was reduced after LBB compared to WB (P = 0.004 and P = 0.006, respectively). In the non-T2D group no differences were seen between LBB and RB, nor between RB and WF, regarding postprandial GLP-1.
Bread perceptions and satiety
The participants’ evaluation of the looks, texture, taste and overall liking of the different breads showed that the WF bread performed better than both LBB and RB on all parameters (P < 0.05). In terms of appearance, RB bread scored better than LBB bread; however, on the other parameters, we found no differences between the barley breads (Table 4).
Fullness and hunger
The participants’ self-evaluated hunger, satiety, fullness, desire to eat and expected prospective consumption was assessed after each of the three test meals. After 1 h we found a small reduction in hunger after LBB by 5.3%, 95% CI: 0.5, 10.2; (P = 0.033), compared with WF. However, no other differences were found after 1 h on the other parameters (P > 0.05). We found no difference after 2 h or 4 h on any of the parameters (P < 0.05, all).
Discussion
This cross-over study examined 4-h postprandial glucose responses to high-amylose barley bread (LBB) versus regular barley (RB) and wheat bread (WF) in participants with and without T2D. In the T2D group, LBB reduced glucose 4 h iAUC by 41% vs. WF and 39% vs. RB. In non-T2D participants, reductions were 28% and 32%, respectively. These findings align with prior meta-analyses and highlight the potential of high-amylose starch as part of a healthy diet [15].
There is consensus that diets low in glycemic load are important for the prevention and management of diabetes and coronary heart disease, and probably obesity, particularly of relevance for individuals with insulin resistance [1, 2, 16]. Furthermore, it has been found that postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in T2D mellitus, particularly in women [17].
The reduction in glucose after LBB was simultaneously with a lowering of postprandial insulin. This lowering of postprandial insulin is closely associated with the reduction in glucose [18]. This is of great importance since hyperinsulinemia can precede and cause obesity and insulin resistance [19]. Furthermore, it has been underlined that interventions that normalise/reduce plasma insulin concentrations might play a key role in the prevention and treatment of age-related decline, obesity, T2D, CVD and cancer [2, 20, 21].
The relatively short interruption of antidiabetic treatment may have contributed to higher postprandial insulin levels in non-T2D participants compared to those with T2D. Nonetheless, we consider that the crossover study design sufficiently mitigates this potential bias and that it did not affect the observed differences in the effect of the bread types.
In addition to reduced glucose and insulin, we found a reduction in postprandial GIP after LBB compared to both RB and WF regardless of having T2D or not, and a reduction in GLP-1 after LBB compared to RB and WF in the T2D group. These findings were consistent with our previous findings with genetically modified high-amylose barley [10]. This is in line with our glucose results since higher plasma glucose concentrations are associated with greater relative insulin stimulation by GIP and GLP-1 [22]. Furthermore, the contribution of GIP to mediating the incretin effect after oral glucose in healthy human subjects is greater than that of GLP-1 [22]. This might explain why we found reduction in postprandial GIP but not in GLP-1 in the non-T2D group. Previous studies have rather consistently shown that glucose tolerance and insulin sensitivity are improved with reduced or absent GIP receptor signalling [22]. Typically, fasting GLP-1 levels are higher in individuals without T2D compared to those with T2D [23]. In our study, we found the opposite. Both DPP4 inhibitors and metformin may increase GLP-1 concentrations, and we cannot rule out that a one-day pause in antidiabetic medication may have been too short.
Nevertheless, other studies also reported higher GLP-1 levels in individuals with T2D compared to those without [24]. It has been suggested that an increase may reflect a compensatory adaptive response to elevated insulin resistance. In this study we did not measure insulin resistance.
We found no overall differences in postprandial satiety evaluations between bread types. This in in line with previous meta-analyses evaluating the effects of amylose content on postprandial subjective satiety score in healthy subjects, where no association was found between satiety and amylose content [15].
Flour from LBB has an amylose content at 47.5%, whereas flour from RB has an amylose content at 30.6% [12]. The relations between amylose content and starch digestibility is complex [25]. However, earlier studies covering the full range of amylose content in barley (0% to 100%) generally show that higher amylose content is correlated with a greater fraction of in vitro undigestible starch [26]. In a previous study using a GM method to increase amylose content in barley, the amount of resistant starch increased significantly [9, 10].
Our perception analyses of the breads showed that wheat bread was preferred over both barley breads. However, the evaluation of taste did not differ between barley breads in relation to amylose content. Taste preferences influencing food choice vary among individuals, depending on many factors such as culture, learning experiences, and genetics [27]. Given that barley is not commonly used for bread, this might to some extent explain why the taste of wheat was preferred. However, for future commercial potential, it is of high priority to work on improving the taste perception of LBB breads.
In general, it is of great importance to focus on development strategies for transformation of the agricultural sector. Modern agricultural practices, focusing on high-yielding and input-dependent monoculture cash crops, have been linked to both greenhouse gas emissions and loss of biodiversity which is of significant concern for governing unions [28, 29]. In this development process, we find it highly relevant to take the effects of crops on human metabolism into evaluation in addition to biodiversity and greenhouse gas emission. Therefore, it is of great interest to study the long-term effects of high-amylose barley in future studies.
Our findings with significantly reduced postprandial glucose following consumption of breads based on high-amylose barley (LBB), compared to wheat and regular barley, in participants with T2D as well as in participants without T2D, lead us to conclude, that that traditionally bred high-amylose barley bread may have future potential as a functional food in prevention and management of T2D.
Data availability
The data are available from the corresponding author upon reasonable request.
References
Anne-Marie A, Mette A, Chaitong C, Kjeld H, Cyril W C K, Hana K. et al. Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023;66:965–85.
Uusitupa M, Khan TA, Viguiliouk E, Kahleova H, Rivellese AA, Hermansen K. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis. Nutrients. 2019;11:2611.
Miller V, Jenkins DA, Dehghan M, Srichaikul K, Rangarajan S, Mente A, et al. Associations of the glycaemic index and the glycaemic load with risk of type 2 diabetes in 127 594 people from 20 countries (PURE): a prospective cohort study. Lancet Diabetes Endocrinol. 2024;12:330–8.
Corrado M, Ahn-Jarvis JH, Fahy B, Savva GM, Edwards CH, Hazard BA. Effect of high-amylose starch branching enzyme II wheat mutants on starch digestibility in bread, product quality, postprandial satiety and glycaemic response. Food Funct. 2022;13:1617–27.
Hallström E, Sestili F, Lafiandra D, Björck I, Ostman E. A novel wheat variety with elevated content of amylose increases resistant starch formation and may beneficially influence glycaemia in healthy subjects. Food Nutr Res. 2011;55:7074.
Fuse Y, Higa M, Miyashita N, Fujitani A, Yamashita K, Ichijo T, et al. Effect of High β-glucan Barley on Postprandial Blood Glucose and Insulin Levels in Type 2 Diabetic Patients. Clin Nutr Res. 2020;9:43–51.
Kim IS, Park SY, Park MJ, Kim KJ, Kim JY. Effect of barley on postprandial blood glucose response and appetite in healthy individuals: a randomized, double-blind, placebo-controlled trial. Nutrients. 2024;16:3899.
Matsuoka T, Tsuchida A, Yamaji A, Kurosawa C, Shinohara M, Takayama I, et al. Consumption of a meal containing refined barley flour bread is associated with a lower postprandial blood glucose concentration after a second meal compared with one containing refined wheat flour bread in healthy Japanese: A randomized control trial. Nutrition. 2020;72:110637.
Carciofi M, Blennow A, Jensen SL, Shaik SS, Henriksen A, Buléon A, et al. Concerted suppression of all starch branching enzyme genes in barley produces amylose-only starch granules. BMC Plant Biol. 2012;12:223.
Bohl M, Gregersen S, Zhong Y, Hebelstrup KH, Hermansen K. Beneficial glycaemic effects of high-amylose barley bread compared to wheat bread in type 2 diabetes. Eur J Clin Nutr. 2024;78:243–50.
Jost M, Szurman-Zubrzycka M, Gajek K, Szarejko I, Stein N. TILLING in Barley. Methods Mol Biol. 2019;1900:73–94.
Dagmara P-s, Andreas B, Kim H, Jan M, inventors barley plant having increased amylose content. https://patents.google.com/patent/WO2024246182A1 2024.
Chaput J-P, Gilbert J-A, Gregersen NT, Pedersen SD, Sjödin AM. Comparison of 150-mm versus 100-mm visual analogue scales in free living adult subjects. Appetite. 2010;54:583–6.
Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes. 2000;24:38–48.
Cai M, Dou B, Pugh JE, Lett AM, Frost GS. The impact of starchy food structure on postprandial glycemic response and appetite: a systematic review with meta-analysis of randomized crossover trials. Am J Clin Nutr. 2021;114:472–87.
Augustin LSA, Kendall CWC, Jenkins DJA, Willett WC, Astrup A, Barclay AW, et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovascul Dis. 2015;25:795–815.
Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813–9.
Dimitriadis GD, Maratou E, Kountouri A, Board M, Lambadiari V. Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: an integrative approach. Nutrients. 2021;13:159.
Zhang AMY, Wellberg EA, Kopp JL, Johnson JD. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab J. 2021;45:285–311.
Janssen J. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int J Mol Sci. 2021;22:7797.
Xing J, Chen C. Hyperinsulinemia: beneficial or harmful or both on glucose homeostasis. Am J Physiol Endocrinol Metab. 2022;323:E2–7.
Nauck MA, Quast DR, Wefers J, Pfeiffer AFH. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes Metab. 2021;23:5–29.
Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down?. Diabetologia. 2011;54:10–8.
Chong SC, Sukor N, Robert SA, Ng KF, Kamaruddin NA. Fasting and stimulated glucagon-like peptide-1 exhibit a compensatory adaptive response in diabetes and pre-diabetes states: A multi-ethnic comparative study. Front Endocrinol. 2022;13:961432.
Tian Y, Petersen BL, Liu X, Li H, Kirkensgaard JJK, Enemark-Rasmussen K, et al. Characterization of different high amylose starch granules. Part II: Structure evolution during digestion and distinct digestion mechanisms. Food Hydrocoll. 2024;149:109593.
Liang W, Ding L, Guo K, Liu Y, Wen X, Kirkensgaard JJK, et al. The relationship between starch structure and digestibility by time-course digestion of amylopectin-only and amylose-only barley starches. Food Hydrocoll. 2023;139:108491.
Galindo MM, Schneider NY, Stähler F, Töle J, Meyerhof W. 2012108:383–426. Academic Press.
Jiang C, Kan J, Gao G, Dockter C, Li C, Wu W, et al. Barley2035: A decadal vision for barley research and breeding. Molecular Plant. 2025;18:195–218.
McCouch S, Baute GJ, Bradeen J, Bramel P, Bretting PK, Buckler E, et al. Agriculture: Feeding the future. Nature. 2013;499:23–4.
Acknowledgements
Personal thanks: We thank the study participants for their contribution and time. We thank Lene Trudsø, Lisa Buus, Lone Kvist and Susanne Sørensen for their excellent laboratory technical assistance. Thanks to the medical students Philippa V. Nielsen and Signe Ringgard for their valuable assistance on the participants’ study days. We are grateful for the production of study bread by chief baker P.A. Andersen and his staff (P.A. Andersen Bakery, Vejle, Denmark). We thank Ulla Kidmose for valuable sparring on testing of satiety and bread perceptions.
Funding
Funding was provided by Innovation Fund Denmark (9067-00004A). Open access funding provided by Aarhus University Hospital.
Author information
Authors and Affiliations
Contributions
and Guarantor Statement: M.B., S.G., K.H.H. and K.H. designed research; M.B. conducted research; M.B., Z.L. and A.B. analysed data; M.B., S.G., A.B., K.H.H. and K.H. wrote the paper. M.B. is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
K.H.H. is the co-owner of Plantcarb Aps, which is a company that specialises in the production and application of high-amylose starches. The other authors declare no conflicts of interest.
Ethical approval
The study protocol was carried out in accordance with the Helsinki Declaration of 1975 as revised in 1983 and was approved by the Central Denmark Region Committees on Health Research Ethics (1-10-72-299-20).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bohl, M., Gregersen, S., Li, Z. et al. High-amylose barley bread improves postprandial glycemia compared to regular barley and wheat bread in subjects with or without type 2 diabetes. Eur J Clin Nutr 79, 1000–1006 (2025). https://doi.org/10.1038/s41430-025-01646-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41430-025-01646-6