Abstract
WD40 and SOCS box protein-2 (WSB2), a member of the large family of suppressor of cytokine signaling (SOCS)-box proteins, has recently been identified as a substrate receptor of cullin 5 E3 ligase that plays an important role in proteomic regulation through substrate ubiquitination and proteasomal degradation. Here we report five patients from four unrelated families presenting with neurodevelopmental delay, dysmorphic features, brain structural abnormalities with or without growth restriction, hypotonia, and microcephaly, all of whom are homozygous for extremely rare and predicted loss-of-function (pLoF) or missense variants in WSB2, inherited from consanguineous parents. The Wsb2-mutant mice exhibited several neurological findings that included hyperactivity, altered exploration, and hyper alertness. They also weighed less, had a lower heart rate, and presented an abnormal retinal blood vessel morphology and vasculature pattern along with decreased total thickness of the retina. Our findings suggest that homozygous LoF WSB2 variants cause a novel neurodevelopmental disorder in humans with similar neurologic and developmental findings seen in Wsb2-mutant mouse models.
Similar content being viewed by others
Introduction
Neurodevelopmental disorders (NDDs) represent a large group of disorders arising from alterations of tightly coordinated processes that regulate development and function of the brain, including intellectual disability (ID), autism spectrum disorders, and epilepsy [1]. With genomic advances, novel genes and related pathways are being identified at a rapid pace, but a significant gap continues to exist in our molecular understanding of NDDs.
Ubiquitination is one of the key regulatory mechanisms that control protein stability in a myriad of cellular processes, including neural development [2,3,4], and its disruption has been linked to many neurodevelopmental and neurodegenerative disorders [5,6,7]. The process of protein ubiquitination requires an enzymatic cascade that consists of a ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and an E3 ubiquitin ligase (E3). Although there are only 2 E1 and 30–50 E2 genes, the human genome encodes for >600 E3 ubiquitin ligases, which act post-translationally to regulate the activity and stability of the entire proteome [8]. The majority of E3s belong to the ‘really interesting new gene’ (RING)-type gene family. Among the RING-type ligases, Cullin-RING-type ligases (CRL) are the multi-subunit ligases whose major component is a specific cullin (CUL) molecule which binds to a RING-box protein (Rbx1 or Rbx2) and a substrate receptor (SR; via an adapter subunit in some cases) at its C- and N-terminus, respectively [9]. Since the original reports associating UBE3A with Angelman syndrome in 1997 [10], approximately fifty-five genes coding for either E3 ubiquitin ligases or CRL SRs have been identified as causative genes for fifty-eight different forms of NDDs [6, 11, 12]
The human WSB2 (hg38; chr12:118,032,687-118,061,179) encodes for a CRL SR protein that contains seven WD-repeats (WD40) spanning most of the protein and a suppressor of cytokine signaling (SOCS)-box in the C-terminus. WSB2 belongs to a large family of SOCS-box proteins which shares 65% similarity with a related protein WSB1[13]. Northern blotting of different mouse tissues has shown that high levels of Wsb2 transcript are present in all tissues examined, including brain, heart, and skeletal muscle [13]. It is postulated that WSB2 may act as an SR component of Cullin 5-RBX2-Elongin B/C (CRL5) E3 ubiquitin ligase complex [14], which mediates the ubiquitination and subsequent proteasomal degradation of target proteins. Only a few WSB2-targeted substrates have been identified, including the granulocyte colony-stimulating factor (G-CSF) receptor [15], interleukin-21 (IL-21) receptor [16], cyclin D1 [17], p53 [18], and lysine-methylated RelA [19]. Differential expressions of WSB2 have been reported in drug-resistant multiple myeloma cell lines [20] and human melanoma and lung cancer tissue samples [21, 22]. However, this gene has not been linked with any human disease and its role in neurodevelopment remains unexplored.
Here, we describe five patients from four unrelated families affected by developmental delays, brain anomalies, and dysmorphic features with or without intrauterine growth restriction (IUGR) and hypotonia, who were found to have homozygous, ultra-rare, predicted loss-of-function (pLoF) or missense variants in WSB2. We report the findings from a comprehensive phenotypic screening of the Wsb2-mutant (mut) mice which suggests overlapping findings between human disease and mouse model.
Subjects and methods
Participant identification and recruitment
This study was approved by the Institutional Review Board (IRB) at Boston Children’s Hospital (BCH at Boston, MA, USA) under the protocol 10-02-0253 and University of Miami (IRB protocol 20230140). All patients or their guardians provided written informed consent under BCH protocol 10-02-0253, collaborator protocol (HMO-0306-10), or through GeneDx protocol Research to Expand the Understanding of Genetic Variants: Clinical and Genetic Correlations, Western Institutional Review Board (protocol# 20171030). Participants were identified through the Manton Center for Orphan Disease Research, GeneDx, and GeneMatcher [23,24,25]. Informed consent has been obtained from all subjects or their legal guardians, and all clinical investigations adhered to the principles of the Declaration of Helsinki. All patients were examined by a clinical geneticist and/or neurologist. Pedigrees and deep phenotypic data for each patient were collected from collaborating clinicians using a standardized template. Brain magnetic resonance imaging (MRI) was collected whenever possible and reviewed by a board-certified neuroradiologist.
Exome and genome sequencing
For the GeneDx exome sequencing cases, using genomic DNA from the patient and parents, the exonic regions and flanking splice junctions of the genome were captured using the Clinical Research Exome kit (Agilent Technologies, Santa Clara, CA) (Patient 1) or the IDT xGen Exome Research Panel v1.0 (Integrated DNA Technologies, Coralville, IA) (Patient 5). Massively parallel (NextGen) sequencing was done on an illumina system with 2x150bp paired-end reads. For exome sequencing of family 3 (Patient 3 and 4), genome capture was done using the IDT xGen Exome Research Panel v2.0 combined with xGen Human mtDNA Research Panel v1.0 (Integrated DNA Technologies), sequencing done on illumina NOVA-X. For the GeneDx genome sequencing case (Patient 2), using genomic DNA from the patient and parents, PCR-free whole genome sequencing libraries were prepared using illumina® DNA PCR-Free Library Prep following the manufacturer’s protocol (illumina, San Diego, CA). Massively parallel (NextGen) sequencing was performed on an illumina NovaSeq6000 with 2x150bp paired-end reads. Reads were aligned to human genome build GRCh37/UCSC hg19 and analyzed for sequence variants using a custom-developed analysis tool or Geneyx analysis software for secondary pipeline using DRAGEN. Reported variants were confirmed, if necessary, by an appropriate orthogonal method in the patient and, if submitted, in selected relatives. For both exome and genome cases, additional sequencing technology and variant interpretation protocol have been previously described [26]. The general assertion criteria for variant classification are publicly available on the GeneDx ClinVar submission page (http://www.ncbi.nlm.nih.gov/clinvar/submitters/26957/).
Molecular modeling
The WSB2 protein structure model was built on the predicted 3D conformation via AlphaFold [27]. The protein structure illustration and amino acid mutations were generated by PyMOL (The PyMOL Molecular Graphics System, version 2.0 Schrödinger).
Mouse strain and phenotyping
The Wsb2-mutant (mut) (C57BL/6N Charles River-Wsb2tm1b(EUCOMM)Hmgu/Ieg; EM:08073) mice were derived from the International Knockout Mouse Consortium (Knockout Mouse Project (KOMP) Repository, IKMC project 22842; https://www.mousephenotype.org/data/alleles/MGI:2144041/tm1a (EUCOMM)Hmgu), which was constructed using the IMPC ‘knockout first’ targeting strategy at Helmholtz Zentrum München, Germany.
From the age of 8–16 weeks, the Wsb2-mut mice were phenotyped systematically in the German Mouse Clinic (GMC) at Helmholtz Munich (Ingolstaedter Landstrasse 1, 85764, Neuherberg, Germany; www.mouseclinic.de) as described previously [28,29,30] and in accordance with the standardized phenotyping pipeline of the IMPC (IMPReSS: https://www.mousephenotype.org/impress/index). In brief, a cohort of 14 Wsb2-mut mice (7 males and 7 females in two batches) with corresponding wildtype (WT) controls (7 males and 8 females) were compared. The sample size was determined in the context of the general guidelines of the IMPC (https://www.mousephenotype.org/about-impc/animal-welfare/arrive-guidelines/) [31]. Animal numbers may vary depending on the test performed, as indicated in the text or respective figure/table legend. Body weight was measured weekly. Further phenotyping measures included growth/size/body composition, morphology, behavior, cardiovascular, craniofacial, homeostasis, muscle, reproductive, skeleton, and vision/eye. Phenotyping protocols are in agreement with the IMPC standard procedures (https://www.mousephenotype.org/impress/PipelineInfo?id=14). The method description of the tests presented in the manuscript can be found in the Supplementary Material.
Mice were housed in individually ventilated cages (IVC) available ad libitum according to the European Union directive 2010/63/EU, German laws, and German Mouse Clinic (GMC) housing conditions (www.mouseclinic.de). All animal care and use in this study met approval by, and complied with, the rules of the district government of Upper Bavaria (Regierung von Oberbayern) Germany and were conducted according to the rules outlined by the Helmholtz Zentrum München ethical committee.
Statistics
Sample size was chosen according to our previous experience and common standards. If not stated otherwise, phenotypic data that were generated by the German Mouse Clinic were analyzed using automated R-scripts (version 3.2.3). Depending on parameter distribution and the questions addressed to the data, tests for genotype effects were made by using Wilcoxon rank sum test, Fisher’s exact test, analysis of variance (ANOVA) with post-hoc Tukey honestly significant difference (HSD) test and/or linear models. Where necessary, body weight was included as confounder. For categorical data, a Fisher’s exact test was used. For each normally distributed parameter, mean and standard deviation, whereas for non-normally distributed data, median, 25th percentiles and 75th percentiles were calculated. For all tests, a p-value < 0.05 has been used as level of significance in the comparison of phenotypic observations between mut and WT mice; a correction for multiple testing has not been performed. All data are publicly and freely available on the GMC (www.mouseclinic.de/results/phenomap-and-results/index.html) and on the IMPC portal (https://www.mousephenotype.org/data/search?term=&type=gene).
Results
Identification of WSB2 variants in five patients from four families with a syndromic NDD
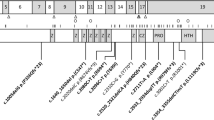
An international collaboration through Matchmaker Exchange [24] facilitated the identification of five patients from four unrelated families carrying homozygous predicted loss-of-function (pLoF) or missense variants in WSB2 (Fig. 1A and Table 1), inherited from asymptomatic consanguineous parents. The variants were identified through exome or genome sequencing and confirmed by Sanger sequencing. Patient (P) 1 (P:1) harbored a nonsense variant in the exon 2 of WSB2 (NM_018639.5: c.128G>A, p.Trp43Ter); P:2 had a single nucleotide deletion in exon 3 (NM_018639.5: c.399delG, p.Gln134ArgfsTer14), resulting in frameshift and premature stop gain; P:3 and P:4 (siblings) had the same homozygous missense variant in exon 9 (NM_018639.5: c.1121G>A, p.Arg374Gln); and P:5 had a 2-bp deletion at the end of last exon 9 (NM_018639.5: c.1187_1188delAA, p.Lys396ArgfsTer19). The variants in P:1 and P:2 were absent from the Genome Aggregation Database (gnomAD v.4.1.0), while those from P:3/P:4 and P:5 were seen only as a heterozygous variant with an extremely low frequency of 3.098 ×10−6 (5/1,614,188) and 1.239 ×10−6 (2/1,614,190), respectively. Furthermore, P:3 and P:4, siblings carrying the homozygous missense variant, had three unaffected siblings who were either heterozygous for the variant or carried the normal allele (pedigree, Supplementary Fig. S1). The WSB2 gene is highly constrained for variants with a high probability of pLoF (pLI = 1, LOEUF = 0.363) as well as missense intolerance (missense Z score = 3.4). Molecular modeling was performed for the only missense variant in the cohort (p.Arg374Gln, Fig. 1B), rest being pLoF. The substitution of Gln for Arg at position 374 likely decreases the strength of the side-chain interactions between Arg374 (red) and Phe398/Phe404 (blue), which could lead to greater conformational dynamics in the SOCS box region and potentially affect WSB2’s function in E3 ubiquitin ligase complex formation.
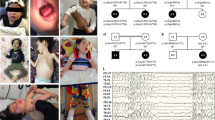
A Representative schematic of WSB2 gene (NM_018639.5) containing seven WD-repeats and a suppressor of cytokine signaling (SOCS) box in the C-terminus. The WSB2 variants are positioned in WD1, WD2, and SOCS box domain, respectively. B Molecular modeling of WSB2 missense variant (NM_018639.5: c.1121G>A, p.Arg374Gln). As shown in the top panel, the wildtype residue’s (Arg374, red) positively charged side chain interacts with the aromatic rings of Phe398 and Phe404 (shown in blue), helping stabilize this region. In contrast, the side chain of Gln374 (red, bottom panel) forms weaker hydrophobic contacts with these phenylalanine, thereby potentially increasing local flexibility of the SOCS box domain. C Representative magnetic resonance imaging (MRI) of patient 2 with homozygous c.399del p.(Q134Rfs*14) at 12 months of age. (i) Sagittal T1-weighted MRI showing microcephaly, callosal hypogenesis (white arrow), tectal dysplasia (yellow arrow), and severe cerebellar hypoplasia/atrophy (dotted oval). (ii and iii) Axial T1- and T2-weighted MRI show undersulcation and white matter hypomyelination for age. (iv-vi) Coronal T2-weighted with fat suppression show small olfactory bulbs (black arrows), optic nerves (white arrows), and hippocampi (yellow arrows).
The patients carrying homozygous nonsense or frameshifting variants had a more severe phenotype including global developmental delay (GDD), generalized hypotonia and muscle weakness, dysmorphic features, and microcephaly along with abnormal brain morphology on magnetic resonance imaging (MRI) (Fig. 1C; Table 2 and Supplementary Table S1). Their other clinical findings included intrauterine growth restriction (IUGR), low birth weight, and apnea. All three patients had feeding difficulties with dependency on enteral nutrition, and two patients had gastrointestinal (GI) dysmotility, expressed as dysphagia or constipation. In comparison, the two siblings (P:3 and P:4) from family 3 carrying the homozygous missense variant had a milder phenotype. They did have GDD, hypotonia, mild dysmorphic features, but no IUGR or microcephaly, and MRI performed at an early age were reported as normal (the older affected sibling’s MRI at a 11-year age was abnormal with hypoplasia of the vermis and possible heterotopia in the left frontal region). History of seizures was present in P:1, P:3 and P:5.
Various dysmorphic features included microcephaly, micrognathia, high-arched palate, foot deformity with high arch, overlapping toes, and clinodactyly of the 5th finger. Vision problems were noted in 4 out of 5 patients but they were heterogeneous, including hyperopia, ptosis, bilateral abnormal pupil morphology, and hypoplastic optic nerves. No hearing issue was reported.
Wsb2-mutant mice exhibit an overlapping phenotype with human patients
The Wsb2-mutant (mut; Wsb2tm1b(EUCOMM)Hmgu, Supplementary Fig. S2) mouse line was generated and analyzed at the German Mouse Clinic [32]. Systemic phenotyping of the Wsb2-mut mice revealed several overlapping phenotypes with human patients affecting multiple organ systems. Compared to control (con) mice, both male (M) and female (F) mutant (mut) mice showed reduced body weight through all the phenotyping pipeline (final body weight M con vs M mut: 28.2 g vs 21.8 g; p = 0.001; final body weight F con vs F mut: 23.9 g vs 17.8 g; p < 0.001; T-test), (Fig. 2A). This finding correlated with lower food intake (M con vs M mut: 1.6 g vs 0.5 g and F con vs F mut: 2.1 g vs 0.2 g; p < 0.001, ANCOVA), decrease in respiratory exchange ratio (con vs mut, p < 0.001), and decrease in metabolic rate (con vs mut, p < 0.001) performed using indirect calorimetry (Supplementary Fig. S3). Dual-energy X-ray absorptiometry (DXA) analysis revealed a significantly decreased bone mineral content (BMC) and fat mass in male Wsb2-mut mice. Body length was slightly reduced in 14-week-old mutants, when compared to controls (M: 9.71 ± 0.30 cm vs 9.34 ± 0.45 cm; F: 9.51 ± 0.25 cm vs 9.07 ± 0.15 cm; p < 0.01). Upon morphological examination, 5/7 female and 4/7 male mutant mice showed abnormal upper teeth; X-ray analyses also showed a decreased size of maxillary incisors in mutant mice (3/5 female and 4/5 male mutants). Ophthalmologic examination revealed an abnormal retinal blood vessel morphology and vasculature pattern (Fig. 2B, D; p < 0.001) with a reduction in total retinal thickness in mutant mice compared to controls (Fig. 2C, E; mean ±SD, left eye: con 231.7 ±14.4 μm vs mut 177.6 ±22.1 μm; right eye: con 235.4 ±9.8 μm vs mut 180.5 ±22.0 μm; p < 0.001 Wilcoxon rank-sum test).
A Shows the body weight at different ages (4–15 week) between control and Wsb2-mut mice. n = 7–8 mice per group and per genotype, median and interquartile range shown. B–E Show the retinal abnormalities in Wsb2-mut mice, including irregular retinal blood vessel morphology (B, D) and reduced total retinal thickness (C, E) as shown in the left eye. For control group: n = 15, 8 females and 7 males; and for Wsb2-mut mice: n = 11, 6 females and 5 males. The right eye showed a similar phenotype. Red boxes in (C) represent an arbitrary evaluation of total retinal thickness, using equally sized rectangles over the retinal layers in control and Wsb2-mut mice to illustrate the reduction in thickness across all retinal layers.
There were behavioral abnormalities in the Wsb2-mut mice such as decreased vertical exploration, as indexed by reduced rearing activity (Fig. 3A) and decreased resting time (Fig. 3B), in response to a novel mildly stressful environment (open field). Additionally, they exhibited decreased acoustic reactivity to lower sound pressure levels (Fig. 3C) in the Prepulse Inhibition (PPI)/Acoustic Startle Response (ASR) test. Further observations indicated increased locomotor activity (SHIRPA p = 0.03 ANOVA) and more tail elevation than control mice (92.9%, 13/14 vs 0%, 0/14, p < 0.00001, Fisher’s Exact test), hinting towards heightened alertness. Transthoracic echocardiography (TTE, Supplementary Fig. S4) revealed a significantly reduced heart rate in mut mice after correcting for lower body weight, confirmed by electrocardiogram. Additionally, both male and female homozygous mutant mice were found to be infertile. In the histological examination of testes, changes in testicular architecture and cell composition with severe testicular tubular atrophy and Leydig cell hyperplasia (Supplementary Fig. S5) were evident in male Wsb2-mut mice.
In the novel open field test, the mutant mice showed decreased rearing activity (A) and decreased resting time (B) when compared to control mice. C Prepulse Inhibition (PPI)/Acoustic Startle Response (ASR) testing showed decreased acoustic reactivity in the mutant mice at lower non-startling sound pressure levels. Con control, mut mutant, NS no stimulus.
Discussion
Cullin-RING-type ligases (CRL), the largest family of E3 ubiquitin ligases, promote the ubiquitination of approximately 20% of cellular proteins destined for degradation via ubiquitin-proteasome system [33]. Mutations in these ubiquitin ligases are associated with neurodevelopmental phenotype in humans, for instance cullin3 (CUL3) [34,35,36] and CUL4B [37, 38], underscoring the importance of regulated proteolysis in neurons. In this study, we present five patients with neurodevelopmental disorders (NDDs) associated with extremely rare variants in WSB2, encoding a CRL substrate receptor (SR) not previously linked to human disease.
The WSB2 protein has been identified as an SR of the Cullin 5-RBX2-Elongin B/C (CRL5) E3 ubiquitin ligase complex [14] characterized by the presence of two conserved domains, the N-terminal 7 WD-40 repeat and the C-terminal suppressor of cytokine signaling (SOCS)-box domain. A (SOCS)-box consists of a BC box for Elongin B/C binding [39] and a CUL5 box for CUL5 binding via its amino acid sequence LPΦP (Φ represents a hydrophobic residue) [14], whereas the WD-40 domain serves as a rigid scaffold for protein-protein interactions [40]. It is suspected that the stop-gain (in P:1) and frameshift (in P:2) variants will cause nonsense-mediated mRNA decay (NMD) and loss of function of WSB2, whereas the missense (in P:3 and P:4) and frameshift variant (in P:5) in SOCS-box domain may disrupt its protein interaction with the CRL5 complex, thus affecting their ability to ubiquitinate and degrade its protein targets.
All patients in this study exhibited global developmental delay and four of them showed abnormal brain morphology on MRI. Single-cell RNA sequencing data from human samples indicate that the major isoform of human WSB2 gene (NM_018639.5) is ubiquitously expressed across various brain regions, with a notable enrichment in the neocortex after late fetal period (Supplementary Fig. S6). Consistently, Wsb2-mutant mice exhibited behavioral abnormalities, including hyperactivity, altered exploration, and hyper alertness, although potential structural brain changes remain unknown. Similarly, the low birth weight and failure to thrive phenotype seen in three of the five patients (P:1, P:2 and P:5) carrying homozygous pLoF variants was also seen in both male and female Wsb2-mutant mice. The visual findings in human patients are relatively mild and heterogeneous (Supplementary Table S1) but the retinal abnormalities seen in Wsb2-mutant mutant, characterized by decreased retinal thickness and abnormal retinal blood vessel morphology and vasculature patterns suggests the need for future ophthalmology evaluation. Similarly, future cardiac evaluation should be considered as patient P:5 has been diagnosed with Wenckebach second-degree heart block at 8 year of age and lower heart rates have been noted in Wsb2-mut mice.
WSB2 may regulate various developmental and physiological processes given its role in ubiquitination and its widespread expression. The phenotypic findings from human patients and mutant mice suggest that it may be involved in growth regulation, neurodevelopment, retinal vascular development, and autonomic regulation, though the precise molecular mechanisms remain to be elucidated. Previous studies on WSB2 have primarily concentrated on its expression in cancer tissue and cells, as well as its roles in carcinogenesis [20,21,22]. From these studies, a limited number of WSB2 substrates have been characterized, including the G-CSF receptor and IL-21 receptor in immune cells [15, 16], cyclin D1 (Ccnd1) [17], p53 [18], and chromatin-bound methylated RelA [19], summarized in Supplementary Table S2. Among these substrates, Ccnd1, along with its catalytic counterpart cyclin-dependent kinase 4 (Cdk4), plays a crucial role in regulating G1 phase length and influences the decision of neural stem cells to proliferate or differentiate [41,42,43,44]. Overexpression of Ccnd1-Cdk4 shortens G1 phase, delays neurogenesis, and promotes the generation and expansion of basal progenitors [43]. Similarly, the transcription factor p53, a well-known tumor suppressor [45, 46], is broadly expressed in the brain and its excessive activation can lead to developmental defects and increased neuronal cell death in various human diseases, correlating with a range of phenotypes, including craniofacial, cardiovascular, and neuronal defects [47,48,49,50,51,52,53,54,55]. Beyond these substrates, other potentially critical proteins that may be regulated by WSB2 are yet to be determined.
In summary, our study provides evidence that recessive WSB2 variants result in a new neurodevelopmental disorder characterized by global developmental delays, hypotonia, seizures, abnormal brain morphology, dysmorphic features, and failure to thrive. Future in vitro studies and mouse models of human WSB2 mutations are needed to further elucidate WSB2 function, its downstream targets, and the precise mechanisms by which WSB2 mutations contribute to disease pathogenesis.
Data availability
Raw data were generated at GeneDx, LLC and the German Mouse Clinic. Derived data supporting the findings of this study are available from the corresponding author [PBA and MHdA] on request. The mouse phenotyping data are available at the International Mouse Phenotyping Consortium platform (IMPC) (https://www.mousephenotype.org/data/genes/MGI:2144041).
References
Niemi MEK, Martin HC, Rice DL, Gallone G, Gordon S, Kelemen M, et al. Common genetic variants contribute to risk of rare severe neurodevelopmental disorders. Nature. 2018;562:268–71.
Ding M, Shen K. The role of the ubiquitin proteasome system in synapse remodeling and neurodegenerative diseases. Bioessays. 2008;30:1075–83.
Upadhyay A, Joshi V, Amanullah A, Mishra R, Arora N, Prasad A, et al. E3 ubiquitin ligases neurobiological mechanisms: development to degeneration. Front Mol Neurosci. 2017;10:151.
Rape M. Ubiquitylation at the crossroads of development and disease. Nat Rev Mol Cell Biol. 2018;19:59–70.
Schmidt MF, Gan ZY, Komander D, Dewson G. Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ. 2021;28:570–90.
Ebstein F, Kury S, Papendorf JJ, Kruger E. Neurodevelopmental disorders (NDD) caused by genomic alterations of the ubiquitin-proteasome system (UPS): the possible contribution of immune dysregulation to disease pathogenesis. Front Mol Neurosci. 2021;14:733012.
Le Guerroue F, Youle RJ. Ubiquitin signaling in neurodegenerative diseases: an autophagy and proteasome perspective. Cell Death Differ. 2021;28:439–54.
Clague MJ, Heride C, Urbe S. The demographics of the ubiquitin system. Trends Cell Biol. 2015;25:417–26.
Harper JW, Schulman BA. Cullin-RING ubiquitin ligase regulatory circuits: a quarter century beyond the F-box hypothesis. Annu Rev Biochem. 2021;90:403–29.
Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15:70–3.
Weng PL, Majmundar AJ, Khan K, Lim TY, Shril S, Jin G, et al. De novo TRIM8 variants impair its protein localization to nuclear bodies and cause developmental delay, epilepsy, and focal segmental glomerulosclerosis. Am J Hum Genet. 2021;108:357–67.
White SM, Bhoj E, Nellaker C, Lachmeijer AMA, Marshall AE, Boycott KM, et al. A DNA repair disorder caused by de novo monoallelic DDB1 variants is associated with a neurodevelopmental syndrome. Am J Hum Genet. 2021;108:749–56.
Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS, et al. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–9.
Mahrour N, Redwine WB, Florens L, Swanson SK, Martin-Brown S, Bradford WD, et al. Characterization of Cullin-box sequences that direct recruitment of Cul2-Rbx1 and Cul5-Rbx2 modules to Elongin BC-based ubiquitin ligases. J Biol Chem. 2008;283:8005–13.
Erkeland SJ, Aarts LH, Irandoust M, Roovers O, Klomp A, Valkhof M, et al. Novel role of WD40 and SOCS box protein-2 in steady-state distribution of granulocyte colony-stimulating factor receptor and G-CSF-controlled proliferation and differentiation signaling. Oncogene. 2007;26:1985–94.
Nara H, Onoda T, Rahman M, Araki A, Juliana FM, Tanaka N, et al. Regulation of interleukin-21 receptor expression and its signal transduction by WSB-2. Biochem Biophys Res Commun. 2010;392:171–7.
Lu K, Zhang M, Wei G, Xiao G, Tong L, Chen D. Multiple cullin-associated E3 ligases regulate cyclin D1 protein stability. Elife. 2023;12:e80327.
Li X, Zhang CC, Lin XT, Zhang J, Zhang YJ, Yu HQ, et al. Elevated expression of WSB2 degrades p53 and activates the IGFBP3-AKT-mTOR-dependent pathway to drive hepatocellular carcinoma. Exp Mol Med. 2024;56:177–91.
Zhang J, Yu Y, Zou X, Du Y, Liang Q, Gong M, et al. WSB1/2 target chromatin-bound lysine-methylated RelA for proteasomal degradation and NF-kappaB termination. Nucleic Acids Res. 2024;52:4969–84.
Mutlu P, Ural AU, Gunduz U. Differential oncogene-related gene expressions in myeloma cells resistant to prednisone and vincristine. Biomed Pharmacother. 2012;66:506–11.
Zhang Y, Li Z, Zhao W, Hu H, Zhao L, Zhu Y, et al. WD repeat and SOCS box containing protein 2 in the proliferation, cycle progression, and migration of melanoma cells. Biomed Pharmacother. 2019;116:108974.
Wei X, Liao J, Lei Y, Li M, Zhao G, Zhou Y, et al. WSB2 as a target of Hedgehog signaling promoted the malignant biological behavior of Xuanwei lung cancer through regulating Wnt/beta-catenin signaling. Transl Cancer Res. 2020;9:7394–404.
Wohler E, Martin R, Griffith S, Rodrigues EDS, Antonescu C, Posey JE, et al. PhenoDB, GeneMatcher and VariantMatcher, tools for analysis and sharing of sequence data. Orphanet J Rare Dis. 2021;16:365.
Sobreira N, Schiettecatte F, Valle D, Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat. 2015;36:928–30.
Arachchi H, Wojcik MH, Weisburd B, Jacobsen JOB, Valkanas E, Baxter S, et al. matchbox: an open-source tool for patient matching via the Matchmaker Exchange. Hum Mutat. 2018;39:1827–34.
Retterer K, Juusola J, Cho MT, Vitazka P, Millan F, Gibellini F, et al. Clinical application of whole-exome sequencing across clinical indications. Genet Med. 2016;18:696–704.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9.
Fuchs H, Aguilar-Pimentel JA, Amarie OV, Becker L, Calzada-Wack J, Cho YL, et al. Understanding gene functions and disease mechanisms: Phenotyping pipelines in the German Mouse Clinic. Behav Brain Res. 2018;352:187–96.
Fuchs H, Gailus-Durner V, Adler T, Aguilar-Pimentel JA, Becker L, Calzada-Wack J, et al. Mouse phenotyping. Methods. 2011;53:120–35.
Gailus-Durner V, Fuchs H, Becker L, Bolle I, Brielmeier M, Calzada-Wack J, et al. Introducing the German Mouse Clinic: open access platform for standardized phenotyping. Nat Methods. 2005;2:403–4.
Karp NA, Meehan TF, Morgan H, Mason JC, Blake A, Kurbatova N, et al. Applying the ARRIVE guidelines to an in vivo database. PLoS Biol. 2015;13:e1002151.
da Silva-Buttkus P, Spielmann N, Klein-Rodewald T, Schutt C, Aguilar-Pimentel A, Amarie OV, et al. Knockout mouse models as a resource for the study of rare diseases. Mamm Genome. 2023;34:244–61.
Zhao Y, Xiong X, Sun Y. Cullin-RING ligase 5: functional characterization and its role in human cancers. Semin Cancer Biol. 2020;67:61–79.
Blackburn PR, Ebstein F, Hsieh TC, Motta M, Radio FC, Herkert JC, et al. Loss-of-function variants in CUL3 cause a syndromic neurodevelopmental disorder. medRxiv. 2023. https://www.medrxiv.org/content/10.1101/2023.06.13.23290941v1.
Dong Z, Chen W, Chen C, Wang H, Cui W, Tan Z, et al. CUL3 deficiency causes social deficits and anxiety-like behaviors by impairing excitation-inhibition balance through the promotion of cap-dependent translation. Neuron. 2020;105:475–90.e6.
Thiffault I, Cadieux-Dion M, Farrow E, Caylor R, Miller N, Soden S, et al. On the verge of diagnosis: Detection, reporting, and investigation of de novo variants in novel genes identified by clinical sequencing. Hum Mutat. 2018;39:1505–16.
Shim T, Kim JY, Kim W, Lee YI, Cho B, Moon C. Cullin-RING E3 ubiquitin ligase 4 regulates neurite morphogenesis during neurodevelopment. iScience. 2024;27:108933.
Tarpey PS, Raymond FL, O’Meara S, Edkins S, Teague J, Butler A, et al. Mutations in CUL4B, which encodes a ubiquitin E3 ligase subunit, cause an X-linked mental retardation syndrome associated with aggressive outbursts, seizures, relative macrocephaly, central obesity, hypogonadism, pes cavus, and tremor. Am J Hum Genet. 2007;80:345–52.
Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–4.
Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300.
Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711.
Lukaszewicz AI, Anderson DJ. Cyclin D1 promotes neurogenesis in the developing spinal cord in a cell cycle-independent manner. Proc Natl Acad Sci USA. 2011;108:11632–7.
Lange C, Huttner WB, Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5:320–31.
Miyashita S, Owa T, Seto Y, Yamashita M, Aida S, Sone M, et al. Cyclin D1 controls development of cerebellar granule cell progenitors through phosphorylation and stabilization of ATOH1. EMBO J. 2021;40:e105712.
Jain AK, Barton MC. p53: emerging roles in stem cells, development and beyond. Development. 2018;145:dev158360.
Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–58.
Li H, Zhang Z, Li H, Pan X, Wang Y. New insights into the roles of p53 in central nervous system diseases. Int J Neuropsychopharmacol. 2023;26:465–73.
Szybinska A, Lesniak W. P53 dysfunction in neurodegenerative diseases - the cause or effect of pathological changes? Aging Dis. 2017;8:506–18.
Lessel D, Wu D, Trujillo C, Ramezani T, Lessel I, Alwasiyah MK, et al. Dysfunction of the MDM2/p53 axis is linked to premature aging. J Clin Invest. 2017;127:3598–608.
Van Nostrand JL, Brady CA, Jung H, Fuentes DR, Kozak MM, Johnson TM, et al. Inappropriate p53 activation during development induces features of CHARGE syndrome. Nature. 2014;514:228–32.
Bowen ME, Attardi LD. The role of p53 in developmental syndromes. J Mol Cell Biol. 2019;11:200–11.
Tsai YY, Su CH, Tarn WY. p53 activation in genetic disorders: different routes to the same destination. Int J Mol Sci. 2021;22:9307.
Williams SE, Garcia I, Crowther AJ, Li S, Stewart A, Liu H, et al. Aspm sustains postnatal cerebellar neurogenesis and medulloblastoma growth in mice. Development. 2015;142:3921–32.
Marjanovic M, Sanchez-Huertas C, Terre B, Gomez R, Scheel JF, Pacheco S, et al. CEP63 deficiency promotes p53-dependent microcephaly and reveals a role for the centrosome in meiotic recombination. Nat Commun. 2015;6:7676.
Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, et al. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med. 2008;14:125–33.
Acknowledgements
The authors would like to thank the patients and their parents for their commitment to this research and to finding a cause for this disorder. We sincerely appreciate Prof. Alan Beggs for providing resources through the Manton Center for Orphan Disease Research, which greatly supported this study. We acknowledge the expert technical support of our GMC and ICE Unit technicians and of the Core Facility Laboratory Animal Services at Helmholtz Munich.
Funding
This work was supported by the “Because of Bella” foundation (PBA), the Batchelor foundation at the University of Miami (PBA and SL), the German Federal Ministry of Education and Research (Infrafrontier grant 01KX1012 to MHdA) and German Center for Diabetes Research (DZD) (MHdA).
Author information
Authors and Affiliations
Consortia
Contributions
PBA designed the experiments and project administration. All authors participated in performing the experiments, data analyses, manuscript drafting and/or figure generation. MHdA, HF and VGD conceptualized and supervised the mouse phenotyping experiments. MHdA lead the research activity and acquired the funding necessary to conduct the research. GMC consortium members conducted the mouse research and analyzed and interpreted the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
BM and EAE are employees of and may own stock in GeneDx, LLC.
Ethics approval
This study was approved by the Institutional Review Board (IRB) at Boston Children’s Hospital (under the protocol#10-02-0253) and University of Miami (IRB protocol# 20230140). All patients or their guardians provided written informed consent under BCH protocol 10-02-0253, collaborator protocol (HMO-0306-10), or through GeneDx protocol Research to Expand the Understanding of Genetic Variants: Clinical and Genetic Correlations, Western Institutional Review Board (protocol# 20171030). Mice were housed in individually ventilated cages (IVC) available ad libitum according to the European Union directive 2010/63/EU, German laws, and German Mouse Clinic (GMC) housing conditions (www.mouseclinic.de). All animal care and use in this study met approval by, and complied with, the rules of the district government of Upper Bavaria (Regierung von Oberbayern) Germany and were conducted according to the rules outlined by the Helmholtz Zentrum München ethical committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, S., Gailus-Durner, V., McGivern, B. et al. Recessive variants in WSB2 encoding a substrate receptor of E3 ubiquitin ligase underlie a neurodevelopmental syndrome. Eur J Hum Genet (2025). https://doi.org/10.1038/s41431-025-01863-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41431-025-01863-4