Abstract
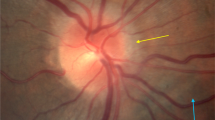
Ophthalmic abnormalities including unilateral and bilateral optic disc edema, optic nerve sheath distention, globe flattening, choroidal folds, and hyperopic shifts have been observed in astronauts during and after long-duration spaceflight. An increased understanding of factors contributing to this syndrome, termed spaceflight-associated neuro-ocular syndrome, is currently a top priority for the ESA and NASA, especially since this medical obstacle could impact the visual health of astronauts as well as the success of future missions, including continued trips to the International Space Station, a return to the moon, or a future human mission to Mars. Currently, the exact mechanisms causing this neuro-ocular syndrome are not fully understood. In the present paper, we propose a hypothetical framework by which optic disc edema in astronauts may result, at least partly, from the forcing of perioptic cerebrospinal fluid into the optic nerve and optic disc along perivascular spaces surrounding the central retinal vessels, related to long-standing microgravity fluid shifts and variations in optic nerve sheath anatomy and compliance. Although this hypothesis remains speculative at the present time, future research in this area of investigation could not only provide exciting new insights into the mechanisms underlying microgravity-induced optic disc swelling but also offer opportunities to develop countermeasure strategies.
摘要
宇航员在历经长期太空飞行和重返地球后的眼部异常改变, 包括单侧或双侧视盘水肿、视神经鞘扩张、眼球扁平化、脉络膜皱褶、远视偏移。对于这种被称为航天相关的神经-眼综合征的发病机制研究, 是目前欧洲航天局和美国国家航空航天局的首要任务, 尤其是此医学难题会影响宇航员的视觉健康以及未来航天任务的顺利完成, 包括继续前往国际空间站、重返月球和人类未来的火星计划。目前, 引起这种神经-眼综合症的发病机制还未完全明确。本文提出了一种关于宇航员视盘水肿发病机制的假说, 其中可能的因素是眼球后的脑脊液沿着视网膜中央血管的周围间隙流入了视神经和视盘, 这与长期微重力环境下的流体移动和视神经鞘的解剖结构及顺应性的变化有关。虽然这项假说目前尚未被证实, 但今后关于此方面的研究不仅可为微重力环境下视盘水肿的发病机制提供令人振奋新的观点, 而且为制定及应对的决策提供机会。
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118:2058–69.
Lee AG, Mader TH, Gibson CR, Tarver W. Space flight-associated neuro-ocular syndrome. JAMA Ophthalmol. 2017;135:992–4.
Lawley JS, Petersen LG, Howden EJ, Sarma S, Cornwell WK, Zhang R, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol. 2017;595:2115–27.
Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain. 2007;130:514–20.
Pircher A, Montali M, Pircher J, Berberat J, Remonda L, Killer HE. Perioptic cerebrospinal fluid dynamics in idiopathic intracranial hypertension. Front Neurol. 2018;9:506.
Killer HE, Jaggi GP, Miller NR, Huber AR, Landolt H, Mironov A, et al. Cerebrospinal fluid dynamics between the basal cisterns and the subarachnoid space of the optic nerve in patients with papilloedema. Br J Ophthalmol. 2011;95:822–7.
Mader TH, Gibson CR, Otto CA, Sargsyan AE, Miller NR, Subramanian PS, et al. Persistent asymmetric optic disc swelling after long-duration space flight: implications for pathogenesis. J Neuroophthalmol. 2017;37:133–9.
Xin X, Fan B, Flammer J, Miller NR, Jaggi GP, Killer HE, et al. Meningothelial cells react to elevated pressure and oxidative stress. PLoS ONE. 2011;6:e20142.
Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure. Prog Retin Eye Res. 2016;50:108–44.
Vaiman M, Gottlieb P, Bekerman I. Quantitative relations between the eyeball, the optic nerve, and the optic canal important for intracranial pressure monitoring. Head Face Med. 2014;10:32.
Wostyn P, De Winne F, Stern C, De Deyn PP. Dilated prelaminar paravascular spaces as a possible mechanism for optic disc edema in astronauts. Aerosp Med Hum Perform. 2018;89:1089–91.
Berdahl J. The eye in space. US Ophthalmic Rev. 2016;9:76–7.
Alperin N, Bagci AM. Spaceflight-induced visual impairment and globe deformations in astronauts are linked to orbital cerebrospinal fluid volume increase. Acta Neurochir Suppl. 2018;126:215–9.
Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR. The optic nerve: a new window into cerebrospinal fluid composition? Brain. 2006;129:1027–30.
Jaggi GP, Harlev M, Ziegler U, Dotan S, Miller NR, Killer HE. Cerebrospinal fluid segregation optic neuropathy: an experimental model and a hypothesis. Br J Ophthalmol. 2010;94:1088–93.
Killer HE. Production and circulation of cerebrospinal fluid with respect to the subarachnoid space of the optic nerve. J Glaucoma. 2013;22:S8–10.
Shanthaveerappa TR, Bourne GH. Arachnoid villi in the optic nerve of man and monkey. Exp Eye Res. 1964;3:31–5.
Killer HE, Laeng HR, Groscurth P. Lymphatic capillaries in the meninges of the human optic nerve. J Neuroophthalmol. 1999;19:222–8.
Mader TH, Gibson CR, Pass AF, Lee AG, Killer HE, Hansen HC, et al. Optic disc edema in an astronaut after repeat long-duration space flight. J Neuroophthalmol. 2013;33:249–55.
Wostyn P, De Deyn PP. Intracranial pressure-induced optic nerve sheath response as a predictive biomarker for optic disc edema in astronauts. Biomark Med. 2017;11:1003–8.
Wostyn P, De Deyn PP. Optic nerve sheath distention as a protective mechanism against the visual impairment and intracranial pressure syndrome in astronauts [letter]. Invest Ophthalmol Vis Sci. 2017;58:4601–2.
Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87:34–40.
Singh S, Dass R. The central artery of the retina. I. Origin and course. Br J Ophthalmol. 1960;44:193–212.
Hayreh SS. The sheath of the optic nerve. Ophthalmologica. 1984;189:54–63.
Wostyn P, Killer HE, De Deyn PP. Glymphatic stasis at the site of the lamina cribrosa as a potential mechanism underlying open-angle glaucoma. Clin Exp Ophthalmol. 2017;45:539–47.
Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10.
Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:6.
Killer HE, Laeng HR, Flammer J, Groscurth P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol. 2003;87:777–81.
Saboori P, Sadegh A. Histology and morphology of the brain subarachnoid trabeculae. Anat Res Int. 2015;2015:279814.
Zeleny TNC, Kohler C, Neutzner A, Killer HE, Meyer P. Cell-cell interaction proteins (gap junctions, tight junctions, and desmosomes) and water transporter aquaporin 4 in meningothelial cells of the human optic nerve. Front Neurol. 2017;8:308.
Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40:2583–99.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111.
Kramer LA, Sargsyan AE, Hasan KM, Polk JD, Hamilton DR. Orbital and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology. 2012;263:819–27.
Riascos R, Heymann JC, Hakimelahi R, Hasan K, Sargsyan A, Barr YR, et al. Novel finding of optic nerve central T2 hypointensity utilizing 3 Tesla MR imaging. Neuroradiol J. 2015;28:133–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PW is the inventor of a pending patent application pertaining biomarkers for spaceflight-associated neuro-ocular syndrome. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wostyn, P., Mader, T.H., Gibson, C. et al. The escape of retrobulbar cerebrospinal fluid in the astronaut’s eye: mission impossible?. Eye 33, 1519–1524 (2019). https://doi.org/10.1038/s41433-019-0453-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41433-019-0453-8
This article is cited by
-
A multifactorial, evidence-based analysis of pathophysiology in Spaceflight Associated Neuro-Ocular Syndrome (SANS)
Eye (2025)
-
Spaceflight associated neuro-ocular syndrome (SANS): an update on potential microgravity-based pathophysiology and mitigation development
Eye (2023)
-
The ex vivo human translaminar autonomous system to study spaceflight associated neuro-ocular syndrome pathogenesis
npj Microgravity (2022)
-
The odyssey of the ocular and cerebrospinal fluids during a mission to Mars: the “ocular glymphatic system” under pressure
Eye (2022)
-
The glymphatic pathway in the optic nerve: did astronauts already reveal signs of its existence?
npj Microgravity (2021)