Abstract
Background/Objectives
This systematic literature review examines the quality of early clinical evaluation of artificial intelligence (AI) decision support systems (DSS) reported in glaucoma care. Artificial Intelligence applications within glaucoma care are increasing within the literature. For such DSS, there needs to be standardised reporting to enable faster clinical adaptation. In May 2022, a checklist to facilitate reporting of early AI studies (DECIDE-AI) was published and adopted by the EQUATOR network.
Methods
The Cochrane Library, Embase, Ovid MEDLINE, PubMed, SCOPUS, and Web of Science Core Collection were searched for studies published between January 2020 and May 2023 that reported clinical evaluation of DSS for the diagnosis of glaucoma or for identifying the progression of glaucoma driven by AI. PRISMA guidelines were followed (PROSPERO registration: CRD42023431343). Study details were extracted and were reviewed against the DECIDE-AI checklist. The AI-Specific Score, Generic-Item Score, and DECIDE-AI Score were generated.
Results
A total of 1,552 records were screened, with 19 studies included within the review. All studies discussed an early clinical evaluation of AI use within glaucoma care, as defined by the a priori study protocol. Overall, the DECIDE-AI adherence score was low, with authors under reporting the AI specific items (30.3%), whilst adhering well to the generic reporting items (84.7%).
Conclusion
Overall, reporting of important aspects of AI studies was suboptimal. Encouraging editors and authors to incorporate the checklist will enhance standardised reporting, bolstering the evidence base for integrating AI DSS into glaucoma care workflows, thus help improving patient care and outcomes.
Similar content being viewed by others
Introduction
Glaucoma is an optic neuropathy that manifests with peripheral vision loss. It is a progressive and irreversible condition with a poorly understood aetiology. Clinically, glaucoma can pose a challenge to diagnose [1], therefore, clinical examination by senior ophthalmologists, visual field (VF) measurements, optical coherence tomography (OCT), along with other clinical tests are all used to determine if a patient has glaucoma [2]. Glaucoma comprises of a heterogeneous group of disorders, that are broadly categorised as open angle glaucoma (OAG) and angle closure glaucoma (ACG), defined by the irideocorneal angle [3]. The current prevalence estimate of glaucoma worldwide is 76 million, and this statistic is estimated to increase to 111.8 million by 2040 [4]. With 11% of world blindness in adults over 50 years being attributed to glaucoma in 2020, glaucoma is a leading public health concern and burden for healthcare systems worldwide [5]. Within the United Kingdom (UK), glaucoma care accounts for 20% of out-patient hospital eye services workload [6], and represents a significant financial burden to health care providers.
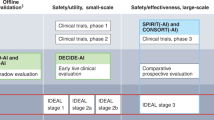
Within many disciplines of medicine, there has been a stark increase within the body of literature citing artificial intelligence (AI) applications to assist medical practitioners [7]. These applications show promise that could lead to physicians having more time to focus on high-yield activities such as surgical waiting lists, though despite the promise of these decision support systems (DSS) they must undergo the same scrutiny as any drug, medical device, or surgical innovation. The development pathways for such interventions are unclear as DSS lack compelling definitions of study stage that exist for drug trials and surgical innovations [8]. In order for the body of literature to be put into effect within the practice of medicine, DSS using AI must be supported with systematic reporting in order to build a sound, robust and comprehensive body of evidence. The DECIDE-AI guideline was published in May 2022 to give authors a standardised template to use when reporting early-stage clinical evaluation of such systems [8].
The DECIDE-AI guideline is a “stage specific reporting guideline for the early, small scale and live clinical evaluation of DSS based on AI” [8]. It was developed under a consensus process involving 20 stakeholder groups across 18 countries with 151 experts involved in the process. The checklist provides a framework of minimum reporting standards and is comprised of 27 items. The DECIDE-AI checklist is made of two parts: 17 AI specific reporting items, and 10 generic reporting items. Such checklists aid the synthesis of standardised evidence, to improve appraisal and replicability of the study findings.
In this systematic review we aim to evaluate the current reporting of AI DSS in glaucoma care. The review specifically examines the body of literature for AI DSS used to diagnose glaucoma and/or its progression to assess how well authors report their findings in the early clinical evaluation.
Methods
Literature search strategy
The study protocol was registered on PROSPERO (ID: CRD42023431343) and the systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9]. A list of the search terms are provided within the supplementary materials (Fig. S1), which were used to systematically search: The Cochrane Library, Embase, Ovid MEDLINE, PubMed, SCOPUS, and Web of Science Core Collection. Databases were searched between 01/01/2020 – 25/05/2023. The search strategy used key terms and subject headings relating to the three themes of this review: (1) AI, (2) Glaucoma, and (3) Detection/Progression. Identified records were exported into Rayyan where automatic duplication of records were reviewed [10]. EndNote 20 was used to manage selected articles [11].
Study selection
The articles identified from the bibliographic search were de-duplicated using Rayyan software and subsequently underwent screening by title and abstract, completed independently by two authors (BLH and BEH), following the predefined criteria. To be eligible for inclusion, studies had to be: (1) published in the English language; (2) dated from January 2020 to current; (3) include glaucoma patients; and (4) include use of AI, machine learning, or deep learning to diagnosis or predict progression. Studies were excluded if they were review articles, letters to the editor, published protocols, conference abstracts, or non-human studies. Papers that were unclear on these criteria were brought to full-text screening.
Articles that passed screening by title and abstract were brought to full text review. Two authors independently screened the articles by full text to determine their eligibility according to the following criteria: (1) use of any AI to diagnosis or predict glaucoma, (2) early clinical evaluation (informed consent received), and (3) full text published in English. Conflicts were discussed and resolved through consensus. If a consensus could not be agreed the article was passed to a third author (AAB) for arbitration.
Data collection and synthesis
The articles identified for inclusion underwent data extraction by one investigator (BLH) and data was inputted into a data synthesis table. Information extracted from each article included: Title, Publication Date, Author, Journal, Input data, Aim, Method (Deep Learning/Machine Learning), and review against the DECIDE-AI Checklist. A meta-analysis was not appropriate given the diversity of the publications and aim to review adherence to the DECIDE-AI checklist.
DECIDE-AI checklist
The DECIDE-AI guideline was produced under an international consensus process to aid better reporting of early clinical evaluation of DSS using AI [8]. The review against the DECIDE-AI included both the 17 AI specific reporting items and the 10 generic reporting items. The AI specific reporting items include a further 11 sub-sections, thus the highest score for the purposes of this review was 28.
The DECIDE-AI checklist was used in this review to assess how well authors adhered to the guidance; using a binary system in which reported was denoted by a ‘1’ and ‘0’ represented not reported. Though the checklist provides 27 reporting items, it also includes recommendations. For example, AI specific item two has two subparts, making an enquiry into both the targeted medical condition and the intended user. These subparts were also assessed using the same binary scoring described above and therefore, if an author reports all items on the checklist the paper could ‘score’ a maximum of 38; 28 points for the AI and 10 for the Generic items.
Reporting item 13, safety and errors has two parts but has added complexity. Part a assesses the reporting of safety and errors and gives four further domains to consider, for this systematic review, a paper would receive a point for the subpart if it reported any of the further domains. Part b assesses risk to patient safety and does not give domains to consider, therefore a paper would receive ‘1’ if it considered aspects of patient safety.
The overall score, the AI specific score and generic score were collated and recorded within a data synthesis table. In addition to the raw score, means of the score are reported to convey average adherence to the DECIDE-AI checklist, with median reported to account for potential skewness or outliers, providing a more robust measure of central tendency. The DECIDE-AI checklist is provided below in Table 1 [8].
Results
Search results
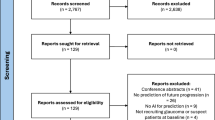
The study selection process is outlined in the PRISMA flow diagram (Fig. 1). The predefined bibliographic search strategy yielded 3,607 articles, of which 2,055 were removed after duplicate detection using Rayyan. Title and abstract review removed a further 1,219 papers, leaving 333 papers to be reviewed by full text. Only one article, by Touahri et al., was not accessible via our institution or network [12]. The primary reason for exclusion during secondary screening was articles reporting within the in silico phase. From full text review, 19 papers met the study criteria and were included for data extraction.
Study characteristics
Data from the selected studies has been tabulated and presented in Table 2. All studies selected for data extraction made use of AI and had received informed consent from the patient.
Generally from the selected articles, authors aimed to diagnose glaucoma using AI [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30], while one study aimed to identify subtypes of glaucoma [31]. No papers attempted to predict the progression of glaucoma. The selected studies primarily came from the medical literature (78.9%) [30, 31], while 10.5% came from engineering [18, 19], 5.3% from mathematical [29], and 5.3% from cross-disciplinary [13] journals. On average, papers within the medical literature performed better overall, but papers within the engineering literature performed better for the AI-specific items, while mathematical journals adhered best to the generic reporting items, see Table 3.
Authors predominantly used deep learning (73.7%) [13, 23, 30, 31] to diagnose glaucoma though 21.1% of the papers used machine learning [14, 15, 24, 29] to identify glaucoma and one paper(5.3%) [22] used a mixture of both deep learning and machine learning to achieve the aim, Supplementary Materials S2: Distribution of AI Method used.
Authors used many different inputs, such as ultra-wide fundus photograph, OCT, fundus image, eye movement tracking, VR headset, visual field (VF) and electroretinography. The most commonly used imaging modality used was OCT with 31.6% of selected studies using it, the distributions of the other inputs can be seen in Supplementary Materials S3: Distribution of Data inputs.
DECIDE-AI checklist
No author referenced the DECIDE-AI guidance within their publication. Generally, adherence to the checklist was low, with an overall mean adherence of 44.6%, the median score was 17. The maximum score possible was 38 points. Figure 2 shows the total DECIDE-AI score for each paper out of a possible 38 points. The highest scoring paper was by Li et al. [30]. achieving 23 points out of 38 and the lowest score of 11 for Sunija et al. [13].
Generic reporting items
Overall, authors reported most of the generic reporting items, with a mean adherence of 84.7%. The highest possible score for this section was 10, the median score was 9. Of the 19 papers included within this review, 14 papers [14, 16, 26,27,28,29,30,31] scored 90%, 2 papers [15, 17] scored 80%, 2 papers [18, 25] scored 70%, and 1 paper [13] scored 50%, individual scores can be reviewed in Fig. 3.
More broadly, across the 19 included studies, all studies adhered to generic reporting items I (abstract), IV (outcomes), VII (main results) and VIII (subgroup analysis). Generic reporting item VI, patient involvement was least reported with only one paper referencing it. Figure 4 shows the respective overall score for each reporting item.
AI-specific reporting items
Across the 19 included papers, the mean adherence to the AI specific reporting items was low at 30.3%. The highest possible score for this section was 28, the median score was 8 and the highest score was 14, published by Li et al [30]. The lowest, and more prevalent score was 5, with three papers [15, 25, 27] attaining this result. Figure 5 shows the individual study score.
All 19 studies adhered to the AI specific reporting items 2a (targeted medical condition), 4a (describe AI system) and 4b (describe data input), while items 3c (participants), 6a, 6b (safety and errors), 7 (human factors), 10a, 10b (implementation), 13a (safety and errors), 14a and 14b (human factors) were not reported at all. Figure 6 shows how many papers reported each of the AI specific items.
Discussion
This systematic review aimed to determine how well the current literature citing early clinical evaluation of AI DSS to detect glaucoma and/or predict its progression is reported. The DECIDE-AI reporting guideline was used to generate a score of adherence and therefore assess how well each of the included papers reported according to the guideline. The guideline aims to standardise the reporting of early clinical evaluation of DSS and help provide clarity on this stage of evaluation. The definition of ‘early live clinical evaluation’ for AI studies is ambiguous and less well defined than the stages of drug trials and surgical innovations. The DECIDE-AI reporting guideline was first published in May 2022 [8]. Many of the studies included within this review were published in 2021, the year preceding the DECIDE-AI publication.
Artificial intelligence is a popular topic across all areas of the literature, with applications spanning from agriculture to medicine. By nature, AI is a cross-disciplinary field based within computer science, which can give rise to complexities when reporting AI methods within fields where there are already well-established research and reporting methods. Papers within this review were predominantly from the medical literature (15), with two from the discipline of engineering, and one from mathematics. One of the included papers were published within a cross-disciplinary journal. Those included from the medical literature scored the highest with an overall DECIDE-AI score of 17.5/38 (median = 18.0) with the cross-disciplinary paper scoring the lowest mean score of 11/38. For the AI specific reporting items, papers from medical journals scored the highest with a mean score of 8.7/28 (median = 9.0), while the paper published in the mathematical journal scored the highest (9/10) for the generic reporting items. A potential reason for the overall score being highest within the medical literature could be due to the DECIDE-AI checklist being designed for early clinical evaluations, and authors outside this field may not be accustomed to how evidence is reported in the medical field. Publications within other disciplines are reported in a different manner to that seen within medical journals.
Overall, adherence to the DECIDE-AI checklist (maximum =38) was low, brought around by low reporting of the AI specific items (maximum = 28), with a mean score of 30.3% (median = 8.0). The generic reporting items (maximum = 10) were reported well with an 84.7% mean adherence to the checklist (median = 9). The DECIDE-AI checklist was published in 2022 to assist the reporting of the growing number of AI based clinical DSS to ensure safety and evaluate the human factors surrounding the use of such systems. The guideline was developed under a consensus based agreement by a multi-stakeholder group. As the publication was produced in 2022, many authors would be unaware of its presence, which could account for the low adherence, particularly in papers published before 2022.
Of the generic reporting items (maximum = 10), only VI (patient involvement) showed low adherence, while the AI specific reporting items (maximum = 28) 3c (steps to familiarise user), 6a (identifying malfunctions), 6b (how patient risk was delt with), 7 (human factors), 10a (user exposure), 10b (changes to clinical work flow), 13a (safety and error), 14a (usability evaluation) and 14b (user learning curves) were not reported at all. These reporting items predominately represent participation, safety, and human factors, all key elements of a decision support system. Without usability, a DSS could easily become unused or cause adverse events owing to the user not being able to easily understand and use the system.
Despite the low adherence to the DECIDE-AI guidance, it would be prudent to reassess compliance in the future to determine if adherence to this novel reporting guideline has increased as authors become aware of its presence. By abiding by these minimum reporting standards authors can systematically report AI driven DSS consistently and duly consider the “proof of clinical utility at small scale, safety, human factors evaluation and preparation for larger scale summative trials” [8]. It would also be useful for other disciplines of medicine to repeat this study to see how they adhere to the DECIDE-AI checklist, allowing for comparison and knowledge transfer between specialities. Although different specialities have their own technical needs, such knowledge transfer could assist with usability and integration of systems into current workflows to optimise patient care and outcomes.
This study has several strengths presenting the adherence to a novel guideline designed by a consensus process. Adherence to these guidelines standardised the reporting and allows for comparability, an important factor with the rising number of papers citing AI to aid healthcare. The presented review assesses a narrow time-frame of papers that represent the current literature of AI in glaucoma care and the exponential growth of AI applications in medicine, thus providing a timely narrative of the importance of standardised reporting when building the evidence base for including AI DSS into the current workflow of healthcare systems.
Despite the many strengths of this study we also acknowledge some limitations, such as the use of informed consent as a proxy for early clinical evaluation. As noted within the DECIDE-AI explanation and elaboration, the stage specific terminology is ambiguous. Therefore, the authors felt that use of informed consent would be a good discriminator between in silico and live evaluations of this novel technology. Another factor to consider is the novelty of both the body of literature surrounding AI applications in glaucoma care and the checklist, therefore it would be interesting to review in the future if there is a significant difference in the reporting of early clinical evaluation of these DSS in glaucoma care owing to the publication of the DECIDE-AI guideline.
This systematic review highlights the current under-use of the DECIDE-AI checklist when authors are reporting the early stage clinical evaluation of DSS driven by AI for identifying glaucoma or its progression. Generally, authors adhered well to the generic reporting items, while falling short on the AI specific reporting items. In particular, this review found that authors underreported the human factors and those of patient and public involvement associated with the reporting guideline. As the DECIDE-AI guidance was only published in 2022, it is hoped that journal editors and authors will soon adopt citing it in their work to help improve the standardisation of reporting and robustness of this specific stage of the evaluation of AI driven DSS to allow systematic comparisons between model evaluations.
Data availability
NA
References
Scott A. Diagnosis and investigations in glaucoma. In: Gillmann K, Mansouri K, eds. The Science of Glaucoma Management. Academic Press; 2023:35-39:chap 3.
European Glaucoma Society. Terminology and Guidelines for Glaucoma, 5th Edition. Br J Ophthalmol. 2021;105:1-169. https://doi.org/10.1136/bjophthalmol-2021-egsguidelines.
Stein JD, Khawaja AP, Weizer JS. Glaucoma in Adults—Screening, Diagnosis, and Management. Jama. 2021;325:164. https://doi.org/10.1001/jama.2020.21899.
Allison K, Patel D, Alabi O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus. 2020. https://doi.org/10.7759/cureus.11686.
Sun Y, Chen A, Zou M, Zhang Y, Jin L, Li Y, et al. Time trends, associations and prevalence of blindness and vision loss due to glaucoma: an analysis of observational data from the Global Burden of Disease Study 2017. BMJ Open. 2022;12:e053805. https://doi.org/10.1136/bmjopen-2021-053805.
Harper RA, Gunn PJG, Spry PGD, Fenerty CH, Lawrenson JG. Care pathways for glaucoma detection and monitoring in the UK. Eye. 2020;34:89–102. https://doi.org/10.1038/s41433-019-0667-9.
Meskó B, Görög M. A short guide for medical professionals in the era of artificial intelligence. npj Digital Med. 2020;3 https://doi.org/10.1038/s41746-020-00333-z.
Vasey B, Nagendran M, Campbell B, Clifton DA, Collins GS, Denaxas S, et al. Reporting guideline for the early stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. BMJ. 2022;(Supplementary appendix 2: DECIDE-AI reporting item checklist):e070904. https://doi.org/10.1136/bmj-2022-070904.
Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:n71. https://doi.org/10.1136/bmj.n71.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5 https://doi.org/10.1186/s13643-016-0384-4.
EndNote. Version EndNote 20. Clarivate; 2013.
Touahri R, Azizi N, Hammami NE, Aldwairi M, Benzebouchi NE, Moumene O. Multi source retinal fundus image classification using convolution neural networks fusion and Gabor-based texture representation. Int J Comput Vis Robot. 2021;11:401–28. https://doi.org/10.1504/ijcvr.2021.116557.
Sunija AP, Gopi VP, Palanisamy P. Redundancy reduced depthwise separable convolution for glaucoma classification using OCT images. Biomed Signal Process Control. 2022;71:103192. https://doi.org/10.1016/j.bspc.2021.103192. 2022PT - Article
Wu C-W, Shen H-L, Lu C-J, Chen S-H, Chen H-Y. Comparison of different machine learning classifiers for glaucoma diagnosis based on spectralis oct. Diagnostics. 2021;11:1718. https://doi.org/10.3390/diagnostics11091718. 2021PT - Article
Wu C-W, Chen H-Y, Chen J-Y, Lee C-H. Glaucoma Detection Using Support Vector Machine Method Based on Spectralis OCT. Diagnostics. 2022;12:391. https://doi.org/10.3390/diagnostics12020391. 2022PT - Article
Cao J, You K, Zhou J, Xu M, Xu P, Wen L, et al. A cascade eye diseases screening system with interpretability and expandability in ultra-wide field fundus images: A multicentre diagnostic accuracy study. EClinicalMedicine. 2022;2022:53. https://doi.org/10.1016/j.eclinm.2022.101633. 11EA SEP 2022
Hong J, Liu X, Guo Y, Gu H, Gu L, Xu J, et al. A Novel Hierarchical Deep Learning Framework for Diagnosing Multiple Visual Impairment Diseases in the Clinical Environment. Front Med. 2021;8:654696. https://doi.org/10.3389/fmed.2021.654696. 2021PT - Article
Krishnan S, Amudha J, Tejwani S. Gaze Exploration Index (GE i)-Explainable Detection Model for Glaucoma. IEEE Access. 2022;10:74334–50.
Kunumpol P, Lerthirunvibul N, Phienphanich P, Munthuli A, Temahivong K, Tantisevi V, et al. GlauCUTU: Time Until Perceived Virtual Reality Perimetry With Humphrey Field Analyzer Prediction-Based Artificial Intelligence. IEEE Access. 2022;10:36949–62.
Dong L, He W, Zhang R, Ge Z, Wang YX, Zhou J, et al. Artificial Intelligence for Screening of Multiple Retinal and Optic Nerve Diseases. JAMA Network Open. 2022;5:e229960. https://doi.org/10.1001/jamanetworkopen.2022.9960.
Li F, Pan J, Yang D, Wu J, Ou Y, Li H, et al. A Multicenter Clinical Study of the Automated Fundus Screening Algorithm. Transl. 2022;11:22. https://doi.org/10.1167/tvst.11.7.22. 2022PT - Journal Article, Multicenter Study, Research Support, Non-U.S. Gov’t
Lim WS, Ho H-Y, Ho H-C, Chen Y-W, Lee C-K, Chen P-J, et al. Use of multimodal dataset in AI for detecting glaucoma based on fundus photographs assessed with OCT: focus group study on high prevalence of myopia. BMC Med 2022;22:206. https://doi.org/10.1186/s12880-022-00933-z. 2022PT - Journal Article, Research Support, Non-U.S. Gov’t
Lin TPH, Hui HYH, Ling A, Chan PP, Shen R, Wong MOM, et al. Risk of Normal Tension Glaucoma Progression From Automated Baseline Retinal-Vessel Caliber Analysis: A Prospective Cohort Study. Am J Ophthalmol. 2022;08 https://doi.org/10.1016/j.ajo.2022.09.015.
Sarossy M, Crowston J, Kumar D, Weymouth A, Wu Z. Time-Frequency Analysis of ERG With Discrete Wavelet Transform and Matching Pursuits for Glaucoma. Transl Vis Sci Technol. 2022;11:19. https://doi.org/10.1167/tvst.11.10.19. 2022PT - Article
Panda SK, Cheong H, Tun TA, Devella SK, Senthil V, Krishnadas R, et al. Describing the Structural Phenotype of the Glaucomatous Optic Nerve Head Using Artificial Intelligence. Am J Ophthalmol. 2022;236:172–82. https://doi.org/10.1016/j.ajo.2021.06.010. 2022PT - Journal Article, Research Support, Non-U.S. Gov’t
Shroff S, Rao DP, Savoy FM, Shruthi S, Hsu CK, Pradhan ZS, et al. Agreement of a Novel Artificial Intelligence Software With Optical Coherence Tomography and Manual Grading of the Optic Disc in Glaucoma. J Glaucoma. 2023;32:280–6. https://doi.org/10.1097/ijg.0000000000002147.
Thiery AH, Braeu F, Tun TA, Aung T, Girard MJA. Medical Application of Geometric Deep Learning for the Diagnosis of Glaucoma. Transl. 2023;12:23. 2023 https://doi.org/10.1167/tvst.12.2.23 PT - Journal Article, Research Support, Non-U.S. Gov’t
Xiong J, Li F, Song D, Tang G, He J, Gao K, et al. Multimodal Machine Learning Using Visual Fields and Peripapillary Circular OCT Scans in Detection of Glaucomatous Optic Neuropathy. Ophthalmology. 2022;129:171–80. 2 2022 https://doi.org/10.1016/j.ophtha.2021.07.032.
Xu Y, Yang Y, Huo Y, Hu M. A group of novel indexes of optical coherence tomography for computer-aided diagnosis of glaucoma. J Nonlinear Convex Anal. 2022;23:2437–47.
Li F, Song D, Chen H, Xiong J, Li X, Zhong H, et al. Development and clinical deployment of a smartphone-based visual field deep learning system for glaucoma detection. npj Digital Med. 2020;3 https://doi.org/10.1038/s41746-020-00329-9.
Eslami Y, Mousavi Kouzahkanan Z, Farzinvash Z, Safizadeh M, Zarei R, Fakhraie G, et al. Deep Learning-Based Classification of Subtypes of Primary Angle-Closure Disease With Anterior Segment Optical Coherence Tomography. J Glaucoma. 2023;32:540–7. https://doi.org/10.1097/ijg.0000000000002194.
Acknowledgments
NA
Author information
Authors and Affiliations
Contributions
Conceptualisation, BLH & AAB; methodology BLH, BEH, DW & AAB; data acquisition, BLH & BEH; analysis, BLH; writing—original draft preparation, BLH; writing—review and editing, BLH, BEH, DW & AAB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leonard-Hawkhead, B., Higgins, B.E., Wright, D. et al. AI for glaucoma, Are we reporting well? a systematic literature review of DECIDE-AI checklist adherence. Eye 39, 1070–1080 (2025). https://doi.org/10.1038/s41433-025-03678-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03678-5