Abstract
Diabetic retinopathy (DR) is a leading cause of acquired blindness. Retinal non-perfusion (RNP) is associated with DR worsening and vision loss. There are no treatments available that specifically address RNP in DR. The semaphorin 3A (Sema3A)/neuropilin 1 (Nrp1) pathway may be involved in RNP progression in DR. In DR, capillary dropout leads to RNP, subsequent hypoxia and ischaemia. Upon chronic hypoxia, retinal cells produce various factors, including vascular endothelial growth factor (VEGF) and Sema3A. While VEGF promotes the growth of new vessels, elevated Sema3A forms a chemical barrier in the retina that directs new blood vessels away from the ischaemic retina. The imbalance of VEGF and Sema3A in DR is believed to dysregulate physiological revascularisation in the retina and may guide blood vessels away from ischaemic regions into the vitreous cavity, causing the pathological neovascularisation typically found in advanced DR. Approved treatments can improve DR severity, but do not appear to improve the underlying RNP. This may lead to a high treatment burden over time and a risk for disease worsening once therapy is stopped, as the underlying disease may progress despite treatment. Therapeutic agents targeting the Sema3A/Nrp1 pathway may have the potential to improve RNP as a core pathophysiologic aspect of DR. This potential disease-modifying effect may sustainably improve DR and preserve the patient’s visual function and quality of life. This review summarises Sema3A/Nrp1 pathway involvement in DR and RNP and its role as a potential target to treat DR in the context of current treatment options.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is a common complication of diabetes mellitus and a leading cause of acquired blindness in working-age adults with diabetes; DR is estimated to affect approximately a third of patients with diabetes worldwide [1]. As non-proliferative DR (NPDR) advances to proliferative DR (PDR), retinal neovascularisation develops, and serious complications may arise, including retinal and vitreous haemorrhages and tractional retinal detachment, which can lead to substantial vision impairment [2]. Currently approved treatments for DR generally target late‑stage disease, at which point irreversible damage to retinal tissue has often occurred [3].
Many of the cellular and clinical alterations associated with DR result in a breakdown of the blood-retinal barrier and loss of normal retinal vasculature [4]. This creates areas of non-perfusion in the retina known as retinal non-perfusion (RNP) [4]. RNP manifests early in DR, even in eyes that do not show any evidence of clinical DR [4]. Although it is most frequently found in the retinal periphery [5, 6], RNP can also affect the central retina (macula), and in the latter case, the term diabetic macular ischaemia (DMI) is also used [7]. RNP is an independent risk factor for DR progression [6], but it can also directly affect the function of retinal cells, which can be of particular importance if RNP is located centrally in the macula [7]. Indeed, RNP has been associated with reduced retinal sensitivity on microperimetry, deterioration of best corrected visual acuity and a risk of developing vision-threatening complications [8]. RNP can substantially affect an individual patient’s vision, although the exact association between RNP, ischaemia and loss of function remains poorly understood [9].
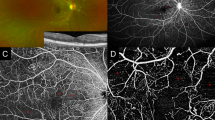
Areas of RNP usually cannot be easily seen on fundoscopic or colour fundus imaging, and the visualisation of RNP requires dedicated imaging techniques [4]. Fluorescein angiography remains the diagnostic standard for the identification of RNP, and advancements in ultra-widefield angiographic techniques enable more comprehensive examination of peripheral retinal areas [10]. Angiography only allows for two-dimensional imaging of the retinal vasculature, requires skilled technicians for good imaging quality, and the intravenous injection of the dye may cause allergic reactions in some patients [10]. Areas of RNP are usually defined as areas with capillary loss leading to reduced fluorescence in the affected areas [11]. The analysis of RNP requires well-trained personnel because various factors can impact the visualisation of areas of RNP, including image quality, timing of the dye injection and eccentricity changes [12,13,14].
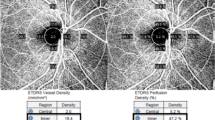
Optical coherence tomography angiography (OCTA), a technique that allows three-dimensional assessment of the retinal vasculature, is an alternative to fluorescein angiography [10]. Advantages of OCTA include its non-invasiveness, reproducibility and the better contrast of areas of RNP compared with the surrounding areas [10, 14]. However, the availability of OCTA is limited, and visualisation is focused on the central retina, because the imaging of peripheral retinal areas using OCTA is difficult [10]. Visualisation of non-perfusion in the peripheral retina can be achieved using swept-source OCTA, which allows a wider field of vision, but experience with this new technique remains limited.
In general, there is a lack of consensus on the optimal imaging biomarkers for RNP, although fluorescein angiography and OCTA are well-established techniques. The most common methods of assessing RNP on fundus fluorescein angiography involve measuring the total area of RNP or calculating the non-perfusion index (total area of RNP divided by gradable retinal area) [6, 10]. However, there is no published consensus on the exact definition of RNP and whether to use specific overlayed grids to assess the topography of measurements. Metrics derived from OCTA focusing on the central retina include vessel density, size of the foveal avascular zone and fractal dimension, and these may function as surrogates for the measurement of peripheral RNP [15]. Overall, there is a need for a consensus on imaging techniques and biomarkers for RNP. This may also include the application of automated algorithms to standardise the analysis of RNP from retinal images.
Preclinical data have identified semaphorin (Sema) proteins and their co-receptors (neuropilin [Nrp] and plexins) as key regulators of morphology and motility in many different cell types, including in the nervous, cardiovascular, immune, endocrine, hepatic, renal, reproductive, respiratory and musculoskeletal systems [16,17,18,19,20,21]. Semaphorins were initially characterised by their role in axonal guidance and subsequent development of the nervous system [22]. However, Sema3 proteins (Sema3) are now gaining increasing attention for their key role in vascular guidance [22]. Sema3 proteins inhibit physiological neovascularisation and promote pathological neovascularisation via vascular endothelial cells and macrophages, respectively (Fig. 1) [16,17,18,19,20, 23]. In particular, Sema3A has been shown to exhibit vasorepulsive effects, predominantly via plexin A‑mediated cytoskeletal collapse, leading to impaired migration and proliferation of vascular endothelial cells [22]. Therefore, Sema3A is important in diseases characterised by angiogenesis, such as cancer.
A negative relationship between Sema3A and matrix metalloproteinase (MMP) enzymes, which facilitate tumour invasion and metastasis, has been demonstrated in cancer [24, 25]. Specifically, research has highlighted that the degradation of perlecan-Sema3A-PlexinA1-Nrp1 receptor complexes on prostate cancer cells by MMP7 regulates tumour cell migration by destabilising cell junctions [24, 25].
The role of Sema3A in directing angiogenesis is also important in the context of DR. In individuals with DR, Sema3A is secreted by hypoxic neurons in the avascular retina in response to proinflammatory cytokine interleukin 1 beta [17]. Sema3A promotes vascular decay, inhibiting normal physiological revascularisation and forming a chemical barrier that forces angiogenesis towards the vitreous, where neovascularisation is pathological [17]. Therefore, silencing retinal Sema3A/Nrp1 signalling represents a potentially important therapeutic target for patients with RNP, because doing so could redirect angiogenesis towards the retina, ultimately leading to repair of the ischaemic tissue and reduced pathophysiological neovascularisation in the vitreous [17]. As such, Sema3A may be of major clinical relevance to the management of DR in the future.
This review describes the Sema3A/Nrp1 pathway and its relation to ischaemia, summarises the impact and limitations of current therapies for DR and diabetic macular oedema (DMO) on RNP, and discusses potential therapeutic strategies targeting the Sema3A/Nrp1 pathway, which may address the unmet need of improving RNP.
Structural changes in DR and RNP
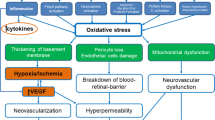
Chronic hyperglycaemia triggers the initial clinical features of DR through changes to the vascular wall and microvascular damage; microvascular changes in DR include capillary blockages (which may involve leucostasis), increased vessel leakage and irregularities in blood flow (Fig. 2) [2, 17, 26,27,28,29,30]. Microvascular changes can result in inadequate blood flow to the metabolically active retina, leading to RNP and subsequent ischaemia [4]. Ischaemia-related damage to the retina and surrounding vasculature can initiate a cycle that perpetuates the progression of ischaemia by releasing various mediators (e.g. Sema3A) and can lead to complications [17, 31]. One such complication is DMI secondary to RNP, which can cause irreversible vision loss [4, 9]. Irreversible vision loss in DMI occurs due to extensive damage to both the retinal microvasculature and the neurosensory layer of the retina, which is an interconnected process [31]. The likelihood of DMI increases with the severity and duration of DR [31].
Adapted from Ansari et al. [26]. Additional links shown in red are based on publications by Kang et al. [27], Cerani et al. [28], Joyal et al. [17], Lechner et al. [2], Khanh Vu et al. [29] and Zhou et al. [30]. Adapted under the open-access Creative Commons Attribution license. Ang angiopoietin, DME diabetic macular edema, DR diabetic retinopathy, IGF-1 insulin-like growth factor 1, PDR proliferative DR, PKC protein kinase C, Sema3A semaphorin 3A, VEGF vascular endothelial growth factor.
The Sema3a/Nrp1 pathway in DR and RNP
In RNP secondary to DR, physiological angiogenesis is disrupted [17]. Initial damage to the retinal microvasculature and hypoxia are followed by the release of various factors by retinal glial cells (e.g. vascular endothelial growth factor [VEGF]) to drive physiological angiogenesis and aim to restore the metabolic equilibrium [17]. This mechanism was demonstrated in a murine model in which mouse pups were exposed to 75% oxygen from P7 to P12 to develop oxygen-induced retinopathy (OIR), followed by room air until P17 [23]. Relative to wild-type mice, mice with OIR had a peak in Sema3A messenger RNA levels at P12 (~7-fold increase) followed by a peak in VEGF messenger RNA levels at P14 (~6-fold increase) [23]. Increased Sema3A levels form a chemical barrier in the retina that directs new blood vessels away from the ischaemic retina, locally preventing physiological revascularisation [17] and contributing to retinal neurosensory and capillary damage that results in progressive vision loss [31]. This imbalance between VEGF and Sema3A is believed to dysregulate angiogenesis, guiding blood vessels away from ischaemic regions of the retina and into the vitreous cavity, leading to pathological retinal neovascularisation [17].
Sema3A is a guidance molecule that directs vessel growth by vasorepulsion [17]. It induces cytoskeletal collapse in the filopodia of endothelial tip cells, thus locally repelling them from the ischaemic retina [17]. Nrp1 has two extracellular ligand-binding domains, A and B, that respectively bind Sema3A and VEGF-A proteins [18]. Dysregulation of Sema3A and VEGF-A signalling can promote misguided pathological angiogenesis and hyperpermeability in the eye (Fig. 1) [17,18,19,20].
The role of the Sema3A/Nrp1 pathway in regulation of angiogenesis and vascular permeability has been indicated in preclinical models. In the murine OIR model, mice with Nrp1 hypomorphism (partial loss of function) have reduced pathological angiogenesis compared with wild-type mice [32]. An antibody against Nrp1 increased physiological revascularisation of avascular areas in the OIR model [33]. This shows redirection of angiogenesis towards the ischaemic retina and away from the vitreous cavity [33]. In addition, OIR mice lacking endothelial Nrp1 have reduced neovascularisation in the retina [34], and an antibody targeting Nrp1 reduced VEGF-induced retinal permeability in an Evans blue rat model [33]. In patients with PDR, Nrp1 was expressed in fibrovascular proliferative tissue surgically excised at vitrectomy, and Nrp1 co-expression with VEGF receptor 2 correlated with vascular density of the tissue, suggesting a potential role for Nrp1 in angiogenic activity in humans [35].
Elevated levels of Sema3A have been found in the plasma of patients with DR [36] and in the vitreous of patients with late-stage proliferative DR [23] and DMO [28]. Animal studies have shown that, under hypoxic conditions, retinal ganglion cells secrete Sema3A, and Sema3A blocks physiological revascularisation in the ischaemic retina [17]. In a murine model of OIR, silencing Sema3A gene expression enhanced physiological vascular regeneration in the ischaemic retina, thus inhibiting destructive vitreal neovascularisation and preserving neuroretinal function [17], suggesting that Sema3A may be a suitable therapeutic target for the treatment of DR.
Impact of currently available therapies on RNP
In this section, the available evidence for the impact of current therapies for DR and DMO on RNP is discussed, including laser treatment, intravitreal (IVT) anti-VEGF agents and IVT corticosteroids.
Currently, there is no consensus on whether pan-retinal photocoagulation (PRP) impacts RNP apart from a known destructive effect on ischaemic cells, with older studies suggesting that PRP results in decreased retinal blood flow and more recent studies suggesting either no effect or increased perfusion when measured centrally [37]. In a prospective, observational, consecutive case series of 20 eyes from 15 patients with PDR treated with a single session of PRP and assessed with widefield OCTA, no change in RNP was observed immediately after PRP treatment, and RNP appeared to remain stable for up to 1 year [37]. However, differentiation of RNP from secondary effects of PRP, such as inflammation, is challenging. As such, further research is needed to confirm the treatment effects of PRP on RNP.
Ranibizumab and aflibercept are currently the only anti-VEGF agents approved for the treatment of DR with and without DMO [38,39,40]. Additionally, two other anti-VEGF agents, brolucizumab and faricimab, are approved for the treatment of DMO [38, 41, 42]. The impact of approved anti-VEGF agents on underlying RNP and ischaemia in patients with DR with or without DMO has been reviewed previously, with authors concluding that the results are conflicting and differ depending on the imaging modality used to assess RNP and the specific location of RNP [43, 44]. Generally, studies of anti-VEGF agents report improvements in Diabetic Retinopathy Severity Scale (DRSS) score and, if at all, only a limited impact on perfusion and disease progression [43, 44]. These studies include several post hoc and retrospective analyses of Phase III fluorescein angiography studies in participants with DMO, such as the RISE, RIDE, RESTORE and VISTA trials [45,46,47].
The major challenge is the lack of robust natural history studies about progression of RNP, and accordingly, reported potential treatment effects are difficult to differentiate from the natural history of RNP in DR. For example, some studies in PDR reported increases in RNP area after treatment with anti-VEGF agents; however, these changes are difficult to differentiate from the expected progression of RNP in this population [48,49,50]. In a prospective, randomised single-centre substudy of the larger CLARITY trial, 40 participants (40 eyes) with PDR were treated with either aflibercept or PRP and underwent mechanistic evaluation. Mean total area of RNP increased after 52 weeks in both the aflibercept (131.2–158.4 disc areas) and PRP groups (125.1–156.1 disc areas), with no statistically significant difference between groups [48]. Moreover, in the prospective, randomised RECOVERY trial, 40 participants with PDR and substantial RNP were randomised 1:1 to monthly or quarterly IVT aflibercept 2 mg [49, 50]. At 1 year of follow-up, the mean total RNP area on ultra-widefield fluorescein angiography imaging remained stable at 264 mm2 (p = 0.70) with monthly treatment but increased from 207 mm2 at baseline to 268 mm2 (p = 0.01) with quarterly treatment (p = 0.05, monthly vs quarterly) [50]. After 1 year of follow-up, patients switched to the alternative aflibercept schedule (i.e. from monthly to quarterly treatment or vice versa) [49]. The corresponding values at 2 years of follow-up were 386 mm2 with monthly treatment (p < 0.0001 vs baseline) and 421 mm2 with quarterly treatment (p < 0.0001 vs baseline; p = 0.023, monthly vs quarterly) [49].
In a recent meta-analysis of randomised controlled trials of 1296 eyes with 1 year of follow-up and 1131 eyes with 2 years of follow-up in patients with DR, RNP progression at both time points was slower among patients who received anti-VEGF therapy compared with macular laser therapy/PRP or sham [51]. Nonetheless, according to the Grading of Recommendations Assessment, Development and Evaluation guidelines, evidence was classified as ‘low’ due to indirectness and imprecision [51]. Taken together, these data suggest that anti-VEGF therapies may at best slow but not prevent RNP progression, with more frequent injections resulting in a stronger effect. However, it is important to highlight that the variable outcomes from these studies were likely influenced by the differences in the definition of RNP and the imaging modality used to assess it across studies [44, 51]. Differences in study design and patient populations may have also impacted these outcomes [48, 51]. For further evidence from larger randomised sham-controlled trials, development of standardised methodology for the assessment of RNP and a better understanding of the natural history of RNP are needed to fully understand the impact of anti-VEGF therapy on RNP at different stages of DR and to explore additional metrics to measure disease severity and impact.
The use of IVT corticosteroids in DMO, which is well established in the second line and in selected cases as first-line therapy [52], was reviewed by Rittiphairoj et al., who concluded that these agents effectively improved vision compared with sham or control in patients with DMO [53]. However, IVT corticosteroids are associated with an increased risk of cataract progression, an increased need for intraocular pressure‐lowering medications and, although rare, an increased need for glaucoma surgery, all of which can limit treatment benefits [53].
In general, the impact of IVT corticosteroids on DR has been less well investigated than that of anti-VEGF agents, although various IVT corticosteroids seem to be effective in preventing progression to vision-threatening complications of DR [54, 55]. Evidence on potential effects of corticosteroids on RNP is even more limited, as it is mainly based on few uncontrolled case series of the potential efficacy of IVT corticosteroids in patients with RNP secondary to DMO [56, 57]. As baseline DMO as well as the resolution of DMO in response to treatment is expected to have a substantial effect on measurement of RNP, data should be interpreted cautiously [58]. Although small-sample studies have evaluated perfusion status before and after administration of IVT corticosteroids, these studies lacked comparator arms and may have been subject to reporting bias [57, 59]. Some studies have reported reduced perfusion on OCTA with IVT corticosteroids [60, 61]. Therefore, while a slowdown of DR progression upon IVT corticosteroid treatment may be possible, it is difficult to draw robust conclusions on the effects of IVT corticosteroids on RNP owing to limited evidence from small studies and the lack of natural history data.
Limitations of currently available therapies
Current standards of care for DR and its complications are invasive and include PRP and IVT anti‑VEGF agents [62].
PRP achieves regression of neovascularisation through the creation of thermal burns in the peripheral retina, ablating ischaemic retinal cells, enhancing retinal oxygenation and reducing VEGF release [38, 63]. PRP was established as the standard of care for PDR more than 40 years ago and has reduced the risk of severe vision loss by half [64, 65]. However, patients treated with PRP can experience loss of visual field [66] and dark adaptation [67], resulting in loss of ability to drive [68], and new-onset DMO [69]. In rare cases, PRP can burn other structures in the eye, including the lens and fovea [70, 71]. Worsened vision following PRP treatment, due to cystoid macular oedema or vitreous haemorrhage, can also occur [72]. Furthermore, many patients treated with PRP (>70%) experience moderate or high levels of pain [73], which are significantly higher than the pain levels experienced by patients treated with IVT injections [74]. As such, PRP can lead to the deterioration of patients’ perceived functional status, quality of life and treatment satisfaction [75].
Although IVT anti-VEGF agents are widely used to treat DMO [62], they are also becoming more popular for treating PDR without DMO in selected cases, and they have shown favourable outcomes on the DRSS and prevention of vision-threatening complications if administered at a sufficient frequency [76]. However, these agents have a high treatment burden, as intensive injection regimens may be needed due to a relatively short duration of action and limited impact on the underlying DR [39,40,41]. In addition, frequent, long-term treatment may be needed to maintain any initial improvements, and there is a risk of disease rebound if the injection interval is too long or injections are missed [77]. In the second year of the PANORAMA study, the proportion of patients who transitioned from aflibercept 2 mg every 8 weeks to as-needed dosing achieving a two-step or greater improvement in DRSS scores decreased from 79.9% of patients at Week 52 to 50.0% at Week 100, suggesting that a reduction in treatment frequency may increase the risk of disease progression [76]. In a post hoc analysis of the RIDE and RISE trials and their open-label extensions, more than 30% of eyes whose scores improved to mild-to-moderate NPDR (DRSS score ≤ 43) with ranibizumab treatment during RIDE/RISE experienced a one- to two-step worsening in DRSS scores during the open-label extension with less controlled and less frequent treatment regimens [78]. Patients with improved (mild-to-moderate) NPDR showed significant worsening in DRSS scores from open-label extension baseline to Month 48 compared with the native group between RIDE/RISE baseline and Month 12 (mean increase in DRSS score of 1.0 [95% CI 0.7–1.4] vs 0.1 [95% CI −0.1 to 0.4]; p < 0.0001) [78].
This is of particular concern, as missed visits are a relatively frequent finding among patients with DR [77]. Reasons for missed IVT injection appointments include the presence of comorbidities, personal and family reasons, or problems with a clinic, insurance or change of physician [79]. In a real-world study in patients with DR and DMO, more than one quarter of patients were lost to follow-up within the first year of treatment [80]. In another study, treatment breaks of >100 days have been reported in almost half of patients with DR and DMO, with the number of missed appointments correlating with a rising number of scheduled treatment visits [79].
Early treatment of severe or moderately severe NPDR with anti-VEGF agents may prevent progression to vision-threatening PDR [81]. Although early treatment reduces the risk of progression to DMO or PDR and improves the anatomical appearance of NPDR, it does not appear to benefit visual acuity [82]. As such, although it is sometimes difficult to justify the burden of regular IVT injections to patients, frequent long-term treatment may be required to attain optimal outcomes [82].
Due to the high treatment burden, compliance can also be low among patients with PDR receiving anti-VEGF treatment [66, 83]. In a small retrospective study, eyes with PDR lost to follow-up during IVT anti-VEGF monotherapy exhibited worse anatomic and functional outcomes than eyes receiving PRP [77]. In long-term trials, loss to follow-up is common (22–39% of patients) [66, 83] and has been associated with younger age, lower income, and race [83]. Adherence to follow-up for anti-VEGF IVT injections is critical for the effective management of DR and for maintaining visual outcomes in the long term; cessation of regular injections may result in disease worsening and, ultimately, irreversible vision loss [77]. Among patients lost to follow-up, mean visual acuity is significantly worse at the return and final visits compared with the visit before loss to follow-up, and there is a high incidence of tractional retinal detachment (10 of 30 patients) and neovascularisation of the iris (4 of 30 patients) [77].
Adverse events and complications of IVT anti-VEGF therapies have been extensively reviewed [38, 84]. Adverse events associated with anti-VEGF therapy are mainly injection-related and include cataracts, vitreous haemorrhage, uveitis and ocular inflammation, floaters, retinal vessel changes, retinal detachment, endophthalmitis and elevated intraocular pressure [38, 84]. Anti-VEGF therapy may also affect systemic VEGF levels, which may explain the suggested association of anti-VEGF treatments with systemic adverse events such as cardiovascular events [38, 84]; however, more research is needed to confirm these findings.
IVT anti-VEGF therapies have a negative effect on patients’ quality of life through the intensive injection regimen, effects on patients’ ability to work and absenteeism, and anxiety and discomfort [85]. Injection appointments, including travel time, have been estimated to take an average of 4.5 h, and the total injection appointment burden over 6 months has been estimated at 20 h per patient [85]. More than half of working patients (53%) need to use at least 1 day of holiday per appointment, and 71% of patients need a carer’s assistance at the time of the injection appointment [85]. Moreover, 75% of patients experience anxiety about their upcoming injection, with 54% reporting feelings of anxiety for at least 2 days before the injection [85]. These feelings of anxiety affect the ability to sleep well in 30% of patients, reduce concentration in 17% of patients, and can cause physical adverse events such as exhaustion [85].
Novel agents targeting the Sema3a/Nrp1 pathway in ophthalmology
The Sema3A/Nrp1 pathway is considered a key driver of vascular guidance during physiological revascularisation in the ischaemic retina [16, 17, 86]. Thus, modulation of Sema3A and/or Nrp1 activity (i.e. by neutralising antibodies) may shift the balance between pro-angiogenic mediators, such as VEGF, and vasorepulsive mediators, such as Sema3A. This would redirect angiogenesis towards physiological revascularisation within the retina, thereby revascularising areas of RNP, and might modify one of the underlying pathophysiological causes of DR, leading to sustained improvement of DR and preservation of vision.
One Phase I/IIa trial has investigated a Sema3A antibody (BI 764524; NCT04424290, HORNBILL; Fig. 3) in participants with PRP-treated DMI secondary to DR [87].
Adapted from Chong et al. [87]. Adapted under the open-access Creative Commons Attribution license. AE adverse event, BCVA best corrected visual acuity, CRT central retinal thickness, D day, FAZ foveal avascular zone, IVT intravitreal, MD multiple dose, SRD single rising dose, W week.
The trial comprised a non-randomised, open-label, single rising dose part and a randomised, masked, sham-controlled multiple-dose part to investigate the safety, tolerability and early biological responses to the IVT administration of Sema3A antibody in adults ≥18 years of age with DMI secondary to DR [87]. The primary endpoint of the single rising dose part was the number of patients with dose-limiting events until Day 8, and the primary endpoint of the multiple-dose part was the number of patients with drug-related adverse events from baseline to study end [87]. Secondary endpoints for assessment of early, preliminary efficacy included changes from baseline in size of the foveal avascular zone, best corrected visual acuity and central retinal thickness [87]. Results from this study will provide insight into the potential for targeting the Sema3A pathway for the treatment of ischaemia and, more broadly, RNP in DR.
There are several Phase II trials of other compounds underway or recruiting, which, based on preclinical data, have the potential to improve RNP in DR. SPECTRA (NCT05393284) is investigating the efficacy and safety of OPL-0401, an oral Rho kinase 1/2 inhibitor, in patients with NPDR or mild PDR [88, 89]. PER-001, a first-in-class, small-molecule endothelin receptor agonist [90], is being assessed in DR (NCT06003751) [91] and glaucoma (NCT05822245) [92]. Additionally, a novel frizzled class receptor 4 agonist, SZN-413, has shown promise in preclinical studies [93]. In murine models, UBX1325, a small-molecule B-cell lymphoma-extra large (Bcl-xL) inhibitor, was shown to reduce retinal vascular permeability, decrease the size of avascular areas and preserve retinal cell function [94, 95]. Phase II trials of IVT UBX1325 include BEHOLD (NCT04857996) in participants with DMO [96] and ENVISION (NCT05275205) in participants with neovascular age‑related macular degeneration [97]. At 48 weeks in both trials, treatment with UBX1325 resulted in vision maintenance or improvement and was well tolerated, with no cases of significant intraocular inflammation, retinal artery occlusion, endophthalmitis or vasculitis [96, 97]. MAGIC (NCT05681884) is evaluating the safety and efficacy in NPDR of faricimab, a humanised bispecific antibody binding to human angiopoietin 2 and VEGF, with a focus on assessing effects on RNP [98].
Another therapeutic approach under consideration for retinal ischaemia in age-related macular degeneration is the growth factors NVB001 and NVB002, which have been shown to promote the growth of new, non-leaking retinal blood vessels in murine models of ischaemic eye disease [99]. Further assessment of NVB001 is planned in toxicokinetic studies and early clinical trials in humans [99].
Conclusions
Future efforts in DR should focus on developing treatments targeting the underlying causes rather than downstream complications of DR to achieve sustainable improvements, preserved retinal function and reduced treatment burden. Treatments addressing RNP hold some promise of achieving these goals. In DR, an imbalance between VEGF and Sema3A signalling may be an important driver of the progression of RNP and the dysregulation of angiogenesis, which guides blood vessels away from ischaemic retinal regions and into the vitreous cavity. Despite improving the DRSS score, current therapies for DR do not appear to substantially improve underlying RNP or ischaemia, are limited by invasiveness, are associated with a high treatment burden and/or have negative effects on patients’ quality of life. Improving retinal perfusion in DR through modulation of angiogenesis by targeting the Sema3A/Nrp1 pathway may improve areas of ischaemia and break the cycle of retinal damage. In addition, several other pathways, such as the Wnt signalling pathway [93, 100], Rho kinase signalling pathway [88], and Bcl-xL pathway [94, 95] have been targeted by emerging agents with potential to improve ischaemia and RNP. Reductions in RNP area may correspond with more sustainable improvements in DR severity and better preservation of function, and these agents may be associated with a reduced treatment burden compared with current treatment options. A review of the studies investigating the impact of current treatments on RNP, as well as of emerging therapies for RNP in DR, highlights the need for the standardisation of imaging biomarkers related to RNP. This may include consensus-building initiatives using expert input to define the parameters of biomarkers for RNP measurement [101], the validation of automated image analysis tools to reduce variability of measurements [102] and the development of established morphological endpoints for use in clinical trials. Furthermore, there is a need for larger natural history studies to assess the natural progression of RNP and its impact on DR progression and other patient-relevant endpoints. Beyond this, further interventional studies are needed to investigate the translation of promising preclinical and clinical data into tangible benefits for patients with DR.
References
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17.
Lechner J, O’Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vision Res. 2017;139:7–14.
Dulull N, Kwa F, Osman N, Rai U, Shaikh B, Thrimawithana TR. Recent advances in the management of diabetic retinopathy. Drug Discov Today. 2019;24:1499–509.
Wykoff CC, Yu HJ, Avery RL, Ehlers JP, Tadayoni R, Sadda SR. Retinal non-perfusion in diabetic retinopathy. Eye. 2022;36:249–56.
Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88:601–12.
Silva PS, Liu D, Glassman AR, Aiello LP, Grover S, Kingsley RM, et al. Assessment of fluorescein angiography nonperfusion in eyes with diabetic retinopathy using ultrawide field retinal imaging. Retina. 2022;42:1302–10.
Datlinger F, Wassermann L, Reumueller A, Hajdu D, Steiner I, Salas M, et al. Assessment of detailed photoreceptor structure and retinal sensitivity in diabetic macular ischemia using adaptive optics-OCT and microperimetry. Investig Ophthalmol Vis Sci. 2021;62:1.
Tsai ASH, Jordan-Yu JM, Gan ATL, Teo KYC, Tan GSW, Lee SY, et al. Diabetic macular ischemia: influence of optical coherence tomography angiography parameters on changes in functional outcomes over one year. Investig Ophthalmol Vis Sci. 2021;62:9.
Mohite AA, Perais JA, McCullough P, Lois N. Retinal ischaemia in diabetic retinopathy: understanding and overcoming a therapeutic challenge. J Clin Med. 2023;12:2406.
Antropoli A, Arrigo A, La Franca L, Bianco L, Barlocci E, Fusi E, et al. Peripheral and central capillary non-perfusion in diabetic retinopathy: an updated overview. Front Med. 2023;10:1125062.
Battaglia Parodi M, Arrigo A, Antropoli A, Bianco L, Saladino A, Bandello F, et al. Deep capillary plexus as biomarker of peripheral capillary nonperfusion in central retinal vein occlusion. Ophthalmol Sci. 2023;3:100267.
Krawitz BD, Phillips E, Bavier RD, Mo S, Carroll J, Rosen RB, et al. Parafoveal nonperfusion analysis in diabetic retinopathy using optical coherence tomography angiography. Transl Vis Sci Technol. 2018;7:4.
Ehlers JP. The OCT angiography revolution: 5 emerging themes. Ophthalmol Retina. 2017;1:457–60.
Or C, Sabrosa AS, Sorour O, Arya M, Waheed N. Use of OCTA, FA, and ultra-widefield imaging in quantifying retinal ischemia: a review. Asia Pac J Ophthalmol. 2018;7:46–51.
Vujosevic S, Fantaguzzi F, Silva PS, Salongcay R, Brambilla M, Torti E, et al. Macula vs periphery in diabetic retinopathy: OCT-angiography and ultrawide field fluorescein angiography imaging of retinal non perfusion. Eye. 2024;38:1668–73.
Iragavarapu-Charyulu V, Wojcikiewicz E, Urdaneta A. Semaphorins in angiogenesis and autoimmune diseases: therapeutic targets? Front Immunol. 2020;11:346.
Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, et al. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011;117:6024–35.
Raimondi C, Brash JT, Fantin A, Ruhrberg C. NRP1 function and targeting in neurovascular development and eye disease. Prog Retin Eye Res. 2016;52:64–83.
Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–42.
Ochsenbein AM, Karaman S, Proulx ST, Berchtold M, Jurisic G, Stoeckli ET, et al. Endothelial cell-derived semaphorin 3A inhibits filopodia formation by blood vascular tip cells. Development. 2016;143:589–94.
Alto LT, Terman JR. Semaphorins and their signaling mechanisms. Methods Mol Biol. 2017;1493:1–25.
Jiao B, Liu S, Tan X, Lu P, Wang D, Xu H. Class-3 semaphorins: potent multifunctional modulators for angiogenesis-associated diseases. Biomed Pharmacother. 2021;137:111329.
Dejda A, Mawambo G, Cerani A, Miloudi K, Shao Z, Daudelin JF, et al. Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. J Clin Investig. 2014;124:4807–22.
Zhou H, Wu A, Fu W, Lv Z, Zhang Z. Significance of semaphorin-3A and MMP-14 protein expression in non-small cell lung cancer. Oncol Lett. 2014;7:1395–400.
Tellman TV, Cruz LA, Grindel BJ, Chung LWK, Farach-Carson MC. MMP-7 cleavage of the perlecan-Sema3A-plexin(A1)-neuropilin-1 complex promotes the progression of metastatic prostate cancer. Cancer Res. 2019;79:66.
Ansari P, Tabasumma N, Snigdha NN, Siam NH, Panduru RVNRS, Azam S, et al. Diabetic retinopathy: an overview on mechanisms, pathophysiology and pharmacotherapy. Diabetology. 2022;3:159–75.
Kang Q, Yang C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799.
Cerani A, Tetreault N, Menard C, Lapalme E, Patel C, Sitaras N, et al. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab. 2013;18:505–18.
Khanh Vu TH, Chen H, Pan L, Cho KS, Doesburg D, Thee EF, et al. CD4(+) T-cell responses mediate progressive neurodegeneration in experimental ischemic retinopathy. Am J Pathol. 2020;190:1723–34.
Zhou L, Xu Z, Lu H, Cho H, Xie Y, Lee G, et al. Suppression of inner blood-retinal barrier breakdown and pathogenic Müller glia activation in ischemia retinopathy by myeloid cell depletion. J Neuroinflamm. 2024;21:210.
Usman M. An overview of our current understanding of diabetic macular ischemia (DMI). Cureus. 2018;10:e3064.
Fantin A, Herzog B, Mahmoud M, Yamaji M, Plein A, Denti L, et al. Neuropilin 1 (NRP1) hypomorphism combined with defective VEGF-A binding reveals novel roles for NRP1 in developmental and pathological angiogenesis. Development. 2014;141:556–62.
Thomas L, Low S, Hansen G, Bakker RA, Zippel N. BI-Y, an neuropilin-1 antagonist, enhances revascularization and prevents vascular endothelial growth factor-A induced retinal hyperpermeability in rodent models of retinopathies. J Pharmacol Exp Ther. 2023;385:214–21.
Fernández-Robredo P, Selvam S, Powner MB, Sim DA, Fruttiger M. Neuropilin 1 involvement in choroidal and retinal neovascularisation. PLoS ONE. 2017;12:e0169865.
Ishida S, Shinoda K, Kawashima S, Oguchi Y, Okada Y, Ikeda E. Coexpression of VEGF receptors VEGF-R2 and neuropilin-1 in proliferative diabetic retinopathy. Investig Ophthalmol Vis Sci. 2000;41:1649–56.
Kwon SH, Shin JP, Kim IT, Park DH. Association of plasma semaphorin 3A with phenotypes of diabetic retinopathy and nephropathy. Investig Ophthalmol Vis Sci. 2016;57:2983–9.
Russell JF, Al-Khersan H, Shi Y, Scott NL, Hinkle JW, Fan KC, et al. Retinal nonperfusion in proliferative diabetic retinopathy before and after panretinal photocoagulation assessed by widefield OCT angiography. Am J Ophthalmol. 2020;213:177–85.
Bahr TA, Bakri SJ. Update on the management of diabetic retinopathy: anti-VEGF agents for the prevention of complications and progression of nonproliferative and proliferative retinopathy. Life. 2023;13:1098.
Genentech Inc. LUCENTIS® (ranibizumab) for intravitreal injection. Prescribing information. 2024; https://www.gene.com/download/pdf/lucentis_prescribing.pdf.
Regeneron Pharmaceuticals Inc. EYLEA® (aflibercept) injection, for intravitreal use. Prescribing information. 2023; https://www.regeneron.com/downloads/eylea_fpi.pdf.
Genentech Inc. VABYSMO® (faricimab-svoa) injection, for intravitreal use. Prescribing information. 2024; https://www.gene.com/download/pdf/vabysmo_prescribing.pdf.
Novartis Pharmaceuticals Corporation. BEOVU® (brolucizumab-dbll) injection, for intravitreal use. Prescribing information. 2024; https://www.novartis.com/us-en/sites/novartis_us/files/beovu.pdf.
Elnahry AG, Abdel-Kader AA, Habib AE, Elnahry GA, Raafat KA, Elrakhawy K. Review on recent trials evaluating the effect of intravitreal injections of anti-VEGF agents on the macular perfusion of diabetic patients with diabetic macular edema. Rev Recent Clin Trials. 2020;15:188–98.
Chatziralli I, Touhami S, Cicinelli MV, Agapitou C, Dimitriou E, Theodossiadis G, et al. Disentangling the association between retinal non-perfusion and anti-VEGF agents in diabetic retinopathy. Eye. 2022;36:692–703.
Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121:1783–9.
Karst SG, Deak GG, Gerendas BS, Waldstein SM, Lammer J, Simader C, et al. Association of changes in macular perfusion with ranibizumab treatment for diabetic macular edema: a subanalysis of the RESTORE (extension) study. JAMA Ophthalmol. 2018;136:315–21.
Wykoff CC, Shah C, Dhoot D, Coleman HR, Thompson D, Du W, et al. Longitudinal retinal perfusion status in eyes with diabetic macular edema receiving intravitreal aflibercept or laser in VISTA study. Ophthalmology. 2019;126:1171–80.
Nicholson L, Crosby-Nwaobi R, Vasconcelos JC, Prevost AT, Ramu J, Riddell A, et al. Mechanistic evaluation of panretinal photocoagulation versus aflibercept in proliferative diabetic retinopathy: CLARITY substudy. Investig Ophthalmol Vis Sci. 2018;59:4277–84.
Wykoff CC, Nittala MG, Villanueva Boone C, Yu HJ, Fan W, Velaga SB, et al. Final outcomes from the randomized RECOVERY trial of aflibercept for retinal nonperfusion in proliferative diabetic retinopathy. Ophthalmol Retina. 2022;6:557–66.
Wykoff CC, Nittala MG, Zhou B, Fan W, Velaga SB, Lampen SIR, et al. Intravitreal aflibercept for retinal nonperfusion in proliferative diabetic retinopathy: outcomes from the randomized RECOVERY trial. Ophthalmol Retina. 2019;3:1076–86.
Nanji K, Sarohia GS, Xie J, Patil NS, Phillips M, Zeraatkar D, et al. Anti-vascular endothelial growth factor therapy and retinal non-perfusion in diabetic retinopathy: a meta-analysis of randomised trials. Acta Ophthalmol. 2024;102:e31–41.
Downey L, Acharya N, Devonport H, Gale R, Habib M, Manjunath V, et al. Treatment choices for diabetic macular oedema: a guideline for when to consider an intravitreal corticosteroid, including adaptations for the COVID-19 era. BMJ Open Ophthalmol. 2021;6:e000696.
Rittiphairoj T, Mir TA, Li T, Virgili G. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. 2020;11:Cd005656.
Wykoff CC, Chakravarthy U, Campochiaro PA, Bailey C, Green K, Cunha-Vaz J. Long-term effects of intravitreal 0.19 mg fluocinolone acetonide implant on progression and regression of diabetic retinopathy. Ophthalmology. 2017;124:440–9.
Iglicki M, Zur D, Busch C, Okada M, Loewenstein A. Progression of diabetic retinopathy severity after treatment with dexamethasone implant: a 24-month cohort study the ‘DR-Pro-DEX Study’. Acta Diabetol. 2018;55:541–7.
Capelanes NC, Malerbi FK, Novais EA, Regatieri CVS. Optical coherence tomography angiographic evaluation of macular vessel density in diabetic macular edema after intravitreal dexamethasone implants: a prospective interventional trial. Ophthalmic Surg Lasers Imaging Retina. 2023;54:174–82.
Brambati M, Borrelli E, Capone L, Querques L, Sacconi R, Battista M, et al. Changes in macular perfusion after ILUVIEN® intravitreal implant for diabetic macular edema: an OCTA study. Ophthalmol Ther. 2022;11:653–60.
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55.
Borrelli E, Parravano M, Querques L, Sacconi R, Giorno P, De Geronimo D, et al. One-year follow-up of ischemic index changes after intravitreal dexamethasone implant for diabetic macular edema: an ultra-widefield fluorescein angiography study. Acta Diabetol. 2020;57:543–48.
Carnota-Méndez P, Méndez-Vázquez C, Pérez-Gavela C. OCT-angiography changes in patients with diabetic macular edema treated with intravitreal dexamethasone implant. Clin Ophthalmol. 2022;16:247–63.
Vujosevic S, Toma C, Villani E, Muraca A, Torti E, Florimbi G, et al. Diabetic macular edema with neuroretinal detachment: OCT and OCT-angiography biomarkers of treatment response to anti-VEGF and steroids. Acta Diabetol. 2020;57:287–96.
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on diabetic eye care: The International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125:1608–22.
Reddy SV, Husain D. Panretinal photocoagulation: a review of complications. Semin Ophthalmol. 2018;33:83–8.
The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88:583–600.
Gonzalez VH, Wang PW, Ruiz CQ. Panretinal photocoagulation for diabetic retinopathy in the RIDE and RISE trials: not “1 and done”. Ophthalmology. 2021;128:1448–57.
Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136:1138–48.
Boynton GE, Stem MS, Kwark L, Jackson GR, Farsiu S, Gardner TW. Multimodal characterization of proliferative diabetic retinopathy reveals alterations in outer retinal function and structure. Ophthalmology. 2015;122:957–67.
Bro T, Andersson J. The effects of visual-field loss from panretinal photocoagulation of proliferative diabetic retinopathy on performance in a driving simulator. Eye. 2023;37:103–8.
Brucker AJ, Qin H, Antoszyk AN, Beck RW, Bressler NM, Browning DJ, et al. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol. 2009;127:132–40.
Abdi F, Daneshtalab A, Gordiz A, Zand A. Complete visual recovery after an inadvertent foveal burn. Lat Am J Ophthalmol. 2023;6:8.
Kumar K, Ganguly A, Sinha TK, Bhattacharya D. Lenticular burns following PASCAL photocoagulation. Indian J Ophthalmol. 2020;68:908–9.
Soman M, Ganekal S, Nair U, Nair K. Effect of panretinal photocoagulation on macular morphology and thickness in eyes with proliferative diabetic retinopathy without clinically significant macular edema. Clin Ophthalmol. 2012;6:2013–7.
Wu WC, Hsu KH, Chen TL, Hwang YS, Lin KK, Li LM, et al. Interventions for relieving pain associated with panretinal photocoagulation: a prospective randomized trial. Eye. 2006;20:712–9.
Lucena CR, Ramos Filho JA, Messias AM, Silva JA, Almeida FP, Scott IU, et al. Panretinal photocoagulation versus intravitreal injection retreatment pain in high-risk proliferative diabetic retinopathy. Arq Bras Oftalmol. 2013;76:18–20.
Vasilijević JB, Kovačević IM, Bukumirić ZM, Marić GD, Slijepčević NA, Pekmezović TD. Vision-related quality of life and treatment satisfaction following panretinal photocoagulation in diabetic retinopathy—a panel study. Medicina. 2022;58:1741.
Brown DM, Wykoff CC, Boyer D, Heier JS, Clark WL, Emanuelli A, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139:946–55.
Obeid A, Su D, Patel SN, Uhr JH, Borkar D, Gao X, et al. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation versus intravitreal anti-vascular endothelial growth factor. Ophthalmology. 2019;126:407–13.
Goldberg RA, Hill L, Davis T, Stoilov I. Effect of less aggressive treatment on diabetic retinopathy severity scale scores: analyses of the RIDE and RISE open-label extension. BMJ Open Ophthalmol. 2022;7:e001007.
Weiss M, Sim DA, Herold T, Schumann RG, Liegl R, Kern C, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti-vascular endothelial growth factor therapy in daily practice. Retina. 2018;38:2293–300.
Best AL, Fajnkuchen F, Nghiem-Buffet S, Grenet T, Quentel G, Delahaye-Mazza C, et al. Treatment efficacy and compliance in patients with diabetic macular edema treated with ranibizumab in a real-life setting. J Ophthalmol. 2018;2018:4610129.
Nguyen QD, Andrew AM, Jennifer IL, Ekaterina P, Ankita C, Rohini R, et al. Simulation of long-term impact of intravitreal anti-VEGF therapy on patients with severe non-proliferative diabetic retinopathy. BMJ Open Ophthalmol. 2023;8:e001190.
Maturi RK, Glassman AR, Josic K, Baker CW, Gerstenblith AT, Jampol LM, et al. Four-year visual outcomes in the protocol W randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy. JAMA. 2023;329:376–85.
Obeid A, Gao X, Ali FS, Talcott KE, Aderman CM, Hyman L, et al. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF injections. Ophthalmology. 2018;125:1386–92.
Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye. 2013;27:787–94.
Sivaprasad S, Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin Ophthalmol. 2016;10:939–46.
Cerani A, Tetreault N, Binet F, Rezende FA, Sitaras N, Lapalme E, et al. The guidance protein semaphorin3A provokes vascular leakage in diabetic retinopathy. Can J Diabetes. 2012;36:S52.
Chong V, Nguyen QD, Sepah Y, Giani A, Pearce E. HORNBILL: a phase I/IIa trial examining the safety, tolerability and early response of BI 764524 in patients with diabetic retinopathy and diabetic macular ischaemia-rationale, study design and protocol. Trials. 2022;23:669.
ClinicalTrials.gov. Phase 2 Spectra study to evaluate the safety and efficacy of OPL-0401 in patients with diabetic retinopathy. 2024; https://clinicaltrials.gov/study/NCT05393284.
Valo Health. Valo Health completes enrollment of OPL-0401 phase 2 study for the treatment of diabetic retinopathy. 2024; https://www.valohealth.com/press/valo-health-completes-enrollment-of-opl-0401-phase-2-study-for-the-treatment-of-diabetic-retinopathy.
Perfuse Therapeutics. Perfuse Therapeutics announces initiation of enrollment to the phase 2a clinical trial of PER001 intravitreal implant in diabetic retinopathy. 2023; https://perfusetherapeutics.com/perfuse-therapeutics-announces-initiation-of-enrollment-to-the-phase-2a-clinical-trial-of-per001-intravitreal-implant-in-diabetic-retinopathy/.
ClinicalTrials.gov. A study of PER-001 in participants with diabetic retinopathy. 2023; https://clinicaltrials.gov/study/NCT06003751.
ClinicalTrials.gov. A study of PER-001 in participants with open-angle glaucoma. 2023; https://clinicaltrials.gov/study/NCT05822245.
Nguyen H, Chen H, Vuppalapaty M, Whisler E, Logas KR, Sampathkumar P, et al. SZN-413, a FZD4 agonist, as a potential novel therapeutic for the treatment of diabetic retinopathy. Transl Vis Sci Technol. 2022;11:19.
Tsuruda P, Chaney S, Dejda A, Dasgupta S, Crespo-Garcia S, Rao S, et al. UBX1325, a small molecule inhibitor of Bcl-xL, attenuates vascular dysfunction in two animal models of retinopathy. Investig Ophthalmol Vis Sci. 2021;62:1163.
Crespo-Garcia S, Fournier F, Diaz-Marin R, Klier S, Ragusa D, Masaki L, et al. Therapeutic targeting of cellular senescence in diabetic macular edema: preclinical and phase 1 trial results. Nat Med. 2024;30:443–54.
UNITY Biotechnology. UNITY Biotechnology announces positive 48-week results from phase 2 BEHOLD study of UBX1325 in patients with diabetic macular edema. 2023; https://ir.unitybiotechnology.com/news-releases/news-release-details/unity-biotechnology-announces-positive-48-week-results-phase-2.
UNITY Biotechnology. UNITY Biotechnology announces 48-week results from phase 2 ENVISION study of UBX1325 in patients with wet age-related macular degeneration. 2023; https://ir.unitybiotechnology.com/news-releases/news-release-details/unity-biotechnology-announces-48-week-results-phase-2-envision.
ClinicalTrials.gov. Safety and efficacy of faricimab in patients with NPDR (MAGIC). 2024; https://clinicaltrials.gov/study/NCT05681884.
Retinal Degeneration Fund. The Retinal Degeneration Fund announces an investment in NVasc. 2023; https://retinaldegenerationfund.org/news/news-posts/the-retinal-degeneration-fund-announces-an-investment-in-nvasc.
ClinicalTrials.gov. A 2-part study consisting of multiple ascending dose (MAD) safety study, and a dose-finding masked study to assess the safety and efficacy of intravitreal (IVT) EYE103 in patients with diabetic macular edema (DME) or neovascular age-related macular degeneration (NVAMD) (AMARONE). 2023; https://www.clinicaltrials.gov/study/NCT05919693.
Li P, Wang H, Tian G, Fan Z. Identification of key biomarkers for early warning of diabetic retinopathy using BP neural network algorithm and hierarchical clustering analysis. Sci Rep. 2024;14:15108.
Channa R, Wolf RM, Simo R, Brigell M, Fort P, Curcio C, et al. A new approach to staging diabetic eye disease: staging of diabetic retinal neurodegeneration and diabetic macular edema. Ophthalmol Sci. 2024;4:100420.
Acknowledgements
Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy and intellectual property considerations.
Funding
Medical writing support was provided by Terri Penfold, BSc, and Anna Wydra, MSc, of Callisto, OPEN Health Communications (London, UK), and further medical writing and editorial support by Catherine Wood, PhD, of HCG (London, UK), both funded by Boehringer Ingelheim, in accordance with Good Publication Practice guidelines (www.ismpp.org/gpp-2022).
Author information
Authors and Affiliations
Contributions
All authors were involved in the conception, drafting, reviewing and approval of this review article. The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors and did not receive payment related to the development of this review article.
Corresponding author
Ethics declarations
Competing interests
SS reports receiving financial support from AbbVie, Amgen, Apellis, Bayer, Biogen, Boehringer Ingelheim, EyeBiotech, EyePoint Pharmaceuticals, Janssen Pharmaceuticals, Kriya Therapeutics, Novartis, Novo Nordisk, Ocular Therapeutix, OcuTerra, Optos, Roche, Sanofi and Stealth BioTherapeutics. SS was the Editor-in-Chief of the journal Eye at the time of manuscript submission. CMGC reports serving on the safety monitoring committee for diabetic macular ischaemia clinical trials sponsored by Boehringer Ingelheim and receiving financial support from AbbVie, Bayer, Boehringer Ingelheim, Janssen Pharmaceuticals, Novartis, Roche and Zeiss. CMGC is a member of the Eye editorial board. CCW reports receiving consulting fees/honoraria for ongoing services provided for 4DMT, AbbVie, Adverum, Alcon, Alimera, Alkeus, Allgenesis, AMC Sciences, Annexon, Apellis, Ascidian, Aviceda, Bayer, Biocryst, Bionic Vision, Boehringer Ingelheim, Curacle, Emmecell, EyeBiotech, EyePoint, Genentech, InGel, IVERIC Bio, Janssen, Kiora, Kodiak, Merck, Merit, Nanoscope, Neurotech, NGM, Novartis, Ocular Therapeutix, Ocuphire, OcuTerra, ONL, Opthea, Osanni, Oxular, Perceive Bio, Perfuse, Ray, Regeneron, RegenXBio, Roche, Sandoz, Sanofi, Santen, Stealth, Sylentis, Thea, Therini, Valo, Visgenx and Zeiss; grants for ongoing research support as a Principal Investigator for trials sponsored by 4DMT, Adverum, AffaMed, Alexion, Alimera, Allgenesis, Amgen, Annexin, Annexon, Apellis, Ascidian, Asclepix, Aviceda, Bayer, Boehringer Ingelheim, Chengdu Origen, Clearside, Curacle, EyeBiotech, EyePoint, Genentech, Gyroscope, IONIS, iRENIX, IVERIC bio, Janssen, Kodiak, Kyoto DDD, Kyowa Kirin, Nanoscope, Neurotech, NGM, Novartis, Ocugen, Ocular Therapeutix, OcuTerra, OliX, Opthea, Outlook Therapeutics, Oxular, Oxurion, Perceive Bio, Pykus, Regeneron, RegenXBio, Rezolute, Roche, Shanghai Henlius, Stealth, Skyline and Valo; and stock investments in InGel, ONL, Osanni, Panther, PolyPhotonix, RecensMedical, TissueGen, Visgenx and Vitranu. SI reports receiving financial support from AbbVie, Alcon, Alpha Communications, AMO Japan, Bayer, Bloom Technology, Bonac, Chugai, HOYA, Kowa, Kyowa Kirin, Mylan, Nidec, Nippon Boehringer Ingelheim, Novartis, Otsuka, Santen, Seed, Senju and Wakamoto. QDN reports serving as the Coordinating Investigator for the diabetic macular ischaemia and diabetic retinopathy programmes sponsored by Boehringer Ingelheim. He also serves on scientific advisory boards for Alumis, Boehringer Ingelheim, Genentech, Kriya Therapeutics, Priovant, Regeneron, Rezolute and Tourmaline. MG and NZ are employees of Boehringer Ingelheim.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sivaprasad, S., Cheung, C.M.G., Gliem, M. et al. New targets in diabetic retinopathy: addressing limitations of current treatments through the Sema3A/Nrp1 pathway. Eye (2025). https://doi.org/10.1038/s41433-025-03835-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41433-025-03835-w