Abstract
Background
To evaluate treatment intervals at 12/24 months following initiation of anti-VEGF therapy and to characterise the clinical profile of neovascular AMD (nAMD) patients achieving extended treatment intervals (≥12 and ≥16 weeks).
Methods
National, retrospective, real-world study using data from the validated web-based Fight Retinal Blindness (FRB!) registry. Treatment-naive nAMD eyes managed with approved first-generation intravitreal VEGF inhibitors (ranibizumab, aflibercept 2 mg) and followed for at least 12 months were included. A subanalysis was conducted on eyes receiving a number of injections within range of a treat and extend (TAE) regimen at 12 and 24 months.
Results
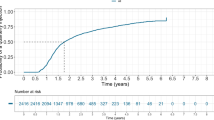
A total number of 1278/557 treatment-naïve nAMD eyes within the TAE range category completed the required follow up at 12/24 months. At 12 months, 39.3% of eyes remained on ≤Q8W, 22.5% >Q8W- < Q12W, 29.1% ≥Q12W- < Q16 and 9.1% ≥Q16W. At 24 months, the distribution was 35.4%, 17.6%, 28.3% and 18.7%, respectively. Mean VA change was not significantly different between groups at both 12 months (≤ Q8W: +4.7, Q8-Q12: +3.5, Q12-Q16: +6.1, ≥Q16W: +4.8 letters) and 24 months (≤Q8W: +5.8, Q8-Q12: +3.7, Q12-Q16: +4.1, ≥Q16W: +3 letters). The percentage of visits with active lesions was similar across groups at both time points, indicating consistent disease control.
Conclusions
Despite receiving a number of injections within a TAE range, a substantial proportion of eyes failed to achieve extended treatment intervals at 12 and 24 months (61.8% and 52.9%, respectively). These results underscore the significant unmet therapeutic need in the management of nAMD with currently approved first-generation anti-VEGF agents.
Similar content being viewed by others
Introduction
Neovascular age-related macular degeneration (nAMD) is the leading cause of legal blindness in older adults in most developed countries [1]. Anti-vascular endothelial growth factor (anti-VEGF) drugs are the gold-standard treatment for nAMD [2] and have significantly contributed to reducing the prevalence of visual impairment at a population level [3, 4]. While randomised clinical trials (RCT) have demonstrated substantial improvements in visual outcomes with anti-VEGF therapies [5], these results have not been consistently replicated in real-world clinical practice [6]. This discrepancy may be attributed to several factors, including the inclusion of broader, unselected patient populations in observational studies, suboptimal treatment adherence due to the high frequency of injections required for disease control, and the limited durability of first-generation anti-VEGF agents [7,8,9]. These challenges contribute to a considerable treatment burden for both patients and healthcare providers, potentially compromising long-term visual outcomes.
The collection of real-world data on AMD management has been facilitated by the widespread adoption of electronic medical records systems. The Fight Retinal Blindness (FRB!) registry [10] is an international consortium health outcomes measurement (ICHOM)-compliant web-based platform that enables the systematic and rapid capture of clinically relevant data fields, thereby supporting the investigation of key clinical questions related to the treatment of nAMD [11,12,13]. Despite these advances, certain aspects remain relatively underexplored, particularly the durability of anti-VEGF therapies and treatment responses under extended dosing intervals in routine clinical settings. This represents a critical gap, as raw observed treatment intervals may reflect undertreatment or poor adherence rather than a favourable therapeutic response [14]. Consequently, additional inclusion criteria are required in the analysis plan to ensure that the eyes under evaluation have received appropriate disease management in routine clinical care. While this approach may reduce the external validity of the findings relative to a broader cohort, it enhances the interpretability and clinical relevance of the results.
Recent advances in anti-VEGF therapy have introduced novel second-generation agents designed to reduce injection frequency and extend treatment intervals by targeting additional molecular pathways involved in vascular stabilisation (i.e. faricimab, blocking VEGF and also angiopoietin-2) [15] or elevating the concentration of pre-existing drugs (i.e. aflibercept 8 mg, compared to the classic 2 mg) [16]. RCTs have demonstrated that both approaches effectively extend treatment intervals while maintaining visual and anatomical outcomes over 24 months [15, 16]. Moreover, dual-pathway inhibition with faricimab has shown potential for enhanced disease control, as evidenced by greater reductions in macular leakage area and retinal hyperreflective foci volume in both RCTs and real-world settings [17, 18] leading to increased durability and improved clinic capacity [19].
To assess the potential clinical benefit of these longer-acting therapies, it is essential to first characterise current treatment patterns with first-generation anti-VEGF agents in routine clinical care. Quantifying the proportion of eyes that fail to achieve extended treatment intervals provides a measure of the unmet therapeutic need and identifies candidates who may benefit from newer agents. This information is critical for informing policy decisions and guiding the integration of second-generation therapies into clinical pathways, which are influenced by local healthcare infrastructure, reimbursement models, and regulatory frameworks. These considerations are particularly relevant in the context of the emerging availability of biosimilar formulations of first-generation anti-VEGF agents.
This study aims to quantify the extent of the unmet need in achieving extended treatment intervals with approved first-generation anti-VEGF agents (ranibizumab, aflibercept 2 mg) in the management of nAMD. Specifically, it evaluates the proportion of eyes that fail to reach 12- or 16-week injection intervals at 12 and 24 months, based on data collected in the national nAMD registry.
Methods
Study design, setting and ethics approval
Retrospective, observational analysis (SL44438) of nAMD-treated eyes included in the FRB! registry nationwide (FRB Spain project) in routine clinical care [10, 13, 20,21,22]. Ethics approval was obtained from the coordinating centre Institutional Review Board (IRB) (Hospital Clinic Barcelona) and all local authorities. The study adhered to the tenets of the Declaration of Helsinki. All patients in ongoing treatment provided their written informed consent to be included in the registry.
Data sources
Data collection was completed using the nAMD module of the FRB! registry, a validated ICHOM-compliant online web-based tool [10, 23]. Data collected included visual acuity (VA), treatment given, and ocular adverse events at baseline and at each subsequent visit. Demographic characteristics (age and sex), prior treatments (including cataract surgery and vitrectomy) or comorbidities were recorded at the baseline visit. Treatment decisions, including the choice of VEGF inhibitor and visit schedule, were driven locally at the physician’s discretion in each centre, thereby reflecting daily clinical practice. Data extraction was performed in May 2023.
Outcomes
The primary objective of this analysis was to describe the treatment and disease burden after anti-VEGF treatment initiation by analysing the treatment intervals and the number of visits/injections at 12 months. Secondary objectives included the 24 months outcomes, the baseline clinical characteristics of treated eyes and the clinical outcomes of eyes achieving and not achieving extended intervals at both timepoints. The visit of the first anti-VEGF injection was considered the baseline visit. Months 12 and 24 visits were the visits that occurred 12 and 24 months (±1.5 months) after the first anti-VEGF injection.
Study cohorts
Two study cohorts were evaluated, the overall cohort and the Treat and extend (TAE) cohort. Inclusion criteria for the overall cohort were nAMD adult (≥18 years) patients treated with an approved anti-VEGF (aflibercept, ranibizumab, brolucizumab) from the start of anti-VEGF treatment (considering the first anti-VEGF injection as the baseline visit, dated before 1st January 2022) and followed-up for at least 12 months (±1.5 months) after the first injection. The TAE cohort was selected as a subpopulation from the overall cohort using additional criteria, such as completion of a correct loading dose (at least 3 injections during the first 16 weeks since baseline); between 6 and 13 injections during the first year (12-months cohort), and between 8 and 24 injections during the first two years (24-months cohort); no treatment intervals greater than 20 weeks (12- and 24-month cohorts); and no gap between visits greater than 365 days during their first 24 months since baseline visit. The analysed eyes were grouped by four-week intervals according to the interval observed between the closest injection at 12 and 24 months and the previous one, in 8 weeks or less (≤ 8 weeks; ≤Q8W), more than 8 weeks but less than 12 weeks (8-12 weeks; >Q8W-<Q12W), 12 weeks or more but less than 16 weeks (≥ 12-16 weeks; ≥Q12W-<Q16W) or 16 weeks or more (≥16 weeks; ≥Q16W). These specific limits could inform suboptimal response estimations to current available drugs and highlight potential unmet needs for novel approved therapies [15, 16].
Statistical analysis
A description of the study variables was provided. Summary statistics for categorical variables included frequency (N) and percentage (%) of each category/modality. Summary statistics for continuous variables included mean, standard deviation (SD), first quartile (Q1), median, and third quartile (Q3). For VA change, 95% confidence intervals (95% CI) are provided.
Results
Baseline characteristics
A total of 1950 eyes from 1629 patients were included in the overall cohort (Supplementary Table 1). From these, the TAE cohort included 1278 eyes which fulfilled the additional selection criteria and had a minimum follow up of 12 months, and 557 eyes were followed up for 24 months. This TAE cohort constituted the core of the analysis. Demographic and baseline characteristics of these patients and eyes are shown in Table 1. Briefly, mean (SD) age was 79.6 (7.6) years and 59% of patients were female. Ranibizumab was the initial anti-VEGF most frequently received (58%). Macular neovascularisation lesion type was classified in 55.8% of the study eyes (n = 714), and the most frequent type was type 1 (44.2%, n = 316) followed by type 2 (27.7%, n = 198), type 3 (21.0%, n = 150) and aneurysmatic type 1 (polypoidal choroidal vasculopathy, 5.4%, n = 39).
Treatment intervals
In the overall cohort, 12 months after anti-VEGF treatment initiation 33.1% of eyes were on a ≤Q8W interval, 17.5% were on a >Q8W-<Q12W interval and 49.4% were on a ≥Q12W interval. At 24 months, 28.1% of eyes were on a ≤Q8W interval, 13.1% were on a >Q8W-<Q12W interval and 58.8% were on a ≥Q12W interval. The main results for this population are shown in Supplementary Table 2.
In the TAE cohort at 12 months of follow-up the percentage (n) of eyes according to their last treatment interval category was: 39.3% (n = 502) on a ≤Q8W interval, 22.5% (n = 288) on a >Q8W-<Q12W interval, 29.1% (n = 372) on a ≥Q12W–Q<16W interval and 9.1% (n = 116) ≥Q16W (≥Q12W = 38.2%) (Fig. 1A, Table 2). At 24 months of follow-up, 35.4% of study eyes (n = 197) were on ≤Q8W, 17.6% (n = 98) were on >Q8W-<Q12W, 28.3% (n = 158) were on ≥Q12W-<Q16W and 18.7% (n = 104) were on ≥Q16W (≥Q12W = 47%) (Fig. 1B, Table 2). The percentage of eyes achieving intervals ≥Q12W was 38.2% and 47% at months 12 and 24. Particularly, 9.1% and 18.7% of patients achieved intervals ≥Q16W at 12 and 24 months, respectively (Fig. 1A, B, Table 2).
The evolution of treatment intervals during the first 12 and 24 months after anti-VEGF treatment initiation in this specific population is graphically presented in Figs. 2 and 3. The most frequent treatment interval was 4 weeks both at month 12 (47%) and at month 24 (27%) followed by 6 weeks at month 12 (26%) and at month 24 (25%). Intervals shorter than Q10W (Q4W, Q6W, Q8W) were more frequent than the longer ones at both follow-up time points (Table 2). The maximum treatment interval (median, Q1-Q3) was 84 days (70–98.8) and 103 days (85–119) at 12 and 24 months, respectively.
Number of injections and visits
In the TAE cohort, at 12 months the median (Q1, Q3) number of injections and visits was 8 (7, 9) and 9 (8, 10), and at 24 months the median number of injections and visits was 13 (12, 15) and 15 (13, 18) (Table 2). The mean (SD) number of injections/visits was 8 (1.3)/9.2 (1.9) at month 12 and 13.8 (2.7)/15.9 (4) at month 24. The number of injections and visits according to their last treatment interval category is shown in Supplementary Tables 3 and 4 (at 12 and 24 months, respectively). In the overall cohort, at 12 months the median (Q1, Q3) number of injections/visits was 7 (6, 8)/8 (7, 10) and at 24 months it was 11 (9, 14)/14 (12, 17) (Supplementary Table 2).
Clinical outcomes according to the last treatment interval category
In the TAE cohort, the mean baseline VA (SD) was similar between groups at month 12: 57 (19), 56.8 (18.7) 56.1 (20.3) and 55.9 letters (21.3) for eyes treated ≤Q8W, >Q8W-<Q12W, ≥Q12W-<Q16W and ≥Q16W, respectively. The mean (95% CI) VA change from baseline to final evaluation was +4.7 (3.1, 6.2) for eyes treated ≤Q8W, +3.5 (1.6, 5.4) for eyes >Q8W-<Q12W, +6.1 (4.3, 7.9) for eyes on ≥Q12W-<Q16W and +4.8 (1.8, 7.8) for eyes on ≥Q16W. Similar results were observed among eyes followed for 24 months: 60.4 (17.1), 59 (17.4) 59.5 (16.4) and 59.2 (18.6) at baseline for eyes treated ≤Q8W, >Q8W-<Q12W, ≥Q12W-<16W and ≥Q16W, respectively. The mean (95% CI) VA change was +5.8 (3.5, 8.1) for eyes treated ≤Q8W, +3.7 (−0.2, 7.6) for eyes >Q8W-<Q12W, +4.1 (1.2, 7) for eyes on ≥Q12W-<Q16W and +3 (−0.3, 6.4) for eyes on ≥Q16W. Additionally, the percentage of eyes with inactive lesion activity at last visit was numerically higher than at all visits for each category at 12 months (all visits vs last visit, inactive: ≤Q8W, 19% vs 30%; >Q8W-<Q12W, 23% vs 34%; ≥Q12W-<16W, 37% vs 54%; ≥Q16W, 28% vs 43%) and at 24 months (all visits vs last visit, inactive: ≤Q8W, 18% vs 25%; >Q8W-<Q12W, 27% vs 39%; ≥Q12W-<Q16W, 35% vs 50%; ≥Q16W, 35% vs 55%) (Supplementary Table 4).
Discussion
This study offers a robust estimation of the unmet therapeutic need associated with classic first-generation anti-VEGF drugs in a national dataset of nAMD treated eyes. By evaluating treatment intervals at 12 and 24 months, the analysis employs these intervals as surrogate indicators of the treatment burden necessary to sustain visual acuity gains under real-world clinical conditions. The findings underscore the potential value of integrating novel pharmacological agents and treatment protocols to alleviate the treatment burden in the management of nAMD at a multicentre, national level.
The results presented in this study characterise national adherence to established treatment guidelines for nAMD, as evidenced by the mean number of injections administered across different treatment interval groups. At 12 months, injections frequencies ranged from 6.8 to 8.9, and at 24 months, from 11.8 to 15.7, aligning with real-world evidence (RWE) from European studies reporting mean injection counts between 4 and 9 range at month 12 [7, 9, 13, 24, 25]. Notably, the 24-month injection rates observed in our cohort slightly exceeded the European range of 6.1 to 11, and were more consistent with those reported in Canada (12.1), Australia (13.0), or the USA (14.3) [13]. These data underscore the persistent treatment burden associated with nAMD management over time, as corroborated by extension studies [25, 26]. Furthermore, the discrepancy between visual outcomes achieved in clinical trials and those observed in routine clinical care -where injection frequency is generally lower- suggests that extended treatment intervals may be artificially prolonged, potentially compromising visual outcomes [7, 24,25,26,27,28,29]. Accordingly, careful evaluation of visual acuity outcomes is essential when interpreting treatment interval efficacy in real world settings. Importantly, our analysis demonstrated comparable VA improvements at both 12 and 24 months across eyes treated at extended intervals (Q12W-<Q16W and ≥Q16W intervals) and those treated at shorter intervals (≤Q8W and >Q8W-<Q12W), indicating that treatment decisions were appropriately guided by lesion activity across all treatment interval groups.
Our analysis revealed that approximately one-third of eyes (35.4%) failed to achieve treatment intervals of ≥8 weeks at 24 months, despite receiving intensive therapy. These findings are consistent with recent evidence reporting that 25% of patients maintained injection intervals of <6 weeks at 24 months, a group categorised as “high treatment burden” eyes [29]. In the same study, an additional 45% of eyes exhibited treatment intervals >6 and <12 weeks at 2 years, resulting in a combined 70% of patients (25 + 45 = 70%), comparable to the 53% observed with <12 week intervals in our cohort. Minor discrepancies between the two studies may be attributed to differences in cohort composition and methodology, including the broader international sample in the referenced study and our inclusion of multiple interval metrics (i.e. most frequent, maximum and final intervals) across a nationally representative dataset.
New generation anti-VEGF therapies are anticipated to prolong injection intervals while maintaining efficacy, thereby reducing treatment burden in nAMD patients. This benefit has already been demonstrated by faricimab [19]. In our series, ranibizumab was the most frequently administered agent, followed by aflibercept 2 mg, both of which have demonstrated favourable visual outcomes at 12 months in previous studies [12, 30,31,32,33]. Randomised clinical trials have shown that newer agents such as brolucizumab [34], faricimab [15], or aflibercept 8 mg [16, 35] achieve non-inferior visual acuity gains compared to aflibercept 2 mg every 8 weeks (Q8W), with extended dosing intervals up to every 16 weeks (Q16W). Furthermore, RWE suggests that intravitreal faricimab may reduce treatment burden in patients requiring frequent injections with conventional anti-VEGF therapies [19, 36]. To date, real-world evidence supports the clinical trial findings for faricimab in treatment-naïve patients with nAMD and DME, demonstrating both anatomical stability and extended durability [35]. Despite these advances, the implementation of newer agents faces several barriers, including the need for updated treatment protocols, clinical guideline revisions, resistance to change in clinical practice, and cost considerations. Therefore, evaluating outcomes with first-generation anti-VEGF therapies remains essential to quantify the current unmet needs and to better define their role within the evolving nAMD treatment landscape.
This study presents some limitations. The external validity of the results reported may be limited as participating centres represent a selection of tertiary referral academic centres and advanced private practices [13], which may not reflect the reality of nAMD management nationwide in Spain or other countries or regions. Differences in treatment practices, healthcare infrastructure, or populations should be considered when interpreting and comparing to other international cohorts. Additionally, other potential confounding variables difficult to address in real-world data may have influenced treatment intervals (i.e. the influence of treatment switch).
In conclusion, this study demonstrates that, despite injection frequency falling within the TAE framework, a substantial proportion of eyes fail to achieve extended treatment intervals at both 12 and 24 months. These findings provide precise estimates of the extent of the unmet therapeutic need in the management of nAMD with currently available first-generation anti-VEGF agents. This evidence is critical for informing clinical decision-making, increasing awareness among healthcare providers, and guiding the development and implementation of more effective strategies aimed at reducing treatment burden and preventing vision loss associated with nAMD.
Summary
What was known before
-
Real-world anti-VEGF therapy outcomes in neovascular AMD are suboptimal compared to randomised clinical trials for a variety of reasons, that include the treatment of unselected study cohorts, the high treatment burden required and the limited duration of first-generation anti-VEGF drugs.
-
The magnitude of the unmet need in terms of percentage of eyes not achieving extended treatment intervals with first generation anti-VEGF drugs is not well established in routine clinical care.
-
It is critical to identify the size of this unmet need, as this information could help clinicians to direct treatment decisions and design management algorithms in the current therapeutic scenario, that encompasses biosimilars and new generation anti-VEGF drugs.
What this study adds
-
This real-world national database study reveals that a high percentage of eyes do not achieve extended treatment intervals (≥12 weeks) at 12 and 24 months, highlighting a significant unmet need in nAMD management with first-generation anti-VEGF drugs.
-
These findings provide a strong rationale for implementing novel longer-lasting anti-VEGF therapies in clinical practice to reduce treatment burden while maintaining disease control in a significant percentage of eyes that fail to achieve extended treatment intervals nationwide.
-
This data can inform discussions with stakeholders about potential benefits of integrating new treatments into clinical pathways, illustrating the limits of biosimilars in terms of treatment intervals and setting the scene to guide future research on strategies directed to improve nAMD management and patient outcomes.
References
Bourne RRA SJ, Saylan M, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–e60.
Finger RP, Daien V, Eldem BM, Talks JS, Korobelnik J-F, Mitchell P, et al. Anti-vascular endothelial growth factor in neovascular age-related macular degeneration – a systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmol. 2020;20:294.
Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153:209–13.
Skaat A, Chetrit A, Belkin M, Kinori M, Kalter-Leibovici O. Time trends in the incidence and causes of blindness in Israel. Am J Ophthalmol. 2012;153:214–21.
Agarwal A, Aggarwal K, Gupta V. Management of neovascular age-related macular degeneration: a review on landmark randomized controlled trials. Middle East Afr J Ophthalmol. 2016;23:27–37.
Rao P, Lum F, Wood K, Salman C, Burugapalli B, Hall R, et al. Real-world vision in age-related macular degeneration patients treated with single anti–VEGF drug type for 1 year in the IRIS Registry. Ophthalmology. 2018;125:522–8.
Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Hykin P, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016;100:1623–8.
Loewenstein A, Berger A, Daly A, Creuzot-Garcher C, Gale R, Ricci F, et al. Save our Sight (SOS): a collective call-to-action for enhanced retinal care across health systems in high income countries. Eye. 2023;37:3351–9.
Pina Marín B, Gajate Paniagua NM, Gómez-Baldó L, Gallego-Pinazo R. Burden of disease assessment in patients with neovascular age-related macular degeneration in Spain: results of the AMD-MANAGE study. Eur J Ophthalmol. 2022;32:385–94.
Gillies MC, Walton R, Liong J, Arnold JJ, McAllister I, Morlet N, et al. Efficient capture of high-quality data on outcomes of treatment for macular diseases: the fight retinal blindness! Project. Retina. 2014;34:188–95.
Figueras-Roca M, Parrado-Carrillo A, Nguyen V, Casaroli-Marano RP, Moll-Udina A, Gillies MC, et al. Treat-and-extend versus fixed bimonthly treatment regimens for treatment-naive neovascular age-related macular degeneration: real world data from the Fight Retinal Blindness registry. Graefes Arch Clin Exp Ophthalmol. 2021;259:1463–70.
Nguyen V, Barthelmes D, Gillies MC. Neovascular age-related macular degeneration: a review of findings from the real-world Fight Retinal Blindness! registry. Clin Exp Ophthalmol. 2021;49:652–63.
Zarranz-Ventura J, Parrado-Carrillo A, Nguyen V, Sararols L, Garay-Aramburu G, Puzo M, et al. Creation of a neovascular age-related macular degeneration national database using a web-based platform: Fight Retinal Blindness Spain. Report 1: Visual outcomes. Clin Exp Ophthalmol. 2022;50:312–24.
Khachigian LM, Liew G, Teo KYC, Wong TY, Mitchell P. Emerging therapeutic strategies for unmet need in neovascular age-related macular degeneration. J Transl Med. 2023;21:133.
Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–40.
Wykoff CC, Brown DM, Reed K, Berliner AJ, Gerstenblith AT, Breazna A, et al. Effect of high-dose intravitreal aflibercept, 8 mg, in patients with neovascular age-related macular degeneration: the phase 2 CANDELA randomized clinical trial. JAMA Ophthalmol. 2023;141:834–42.
Goldberg RA, Kolomeyer A, Nudleman E, Csaky K, Willis J, Gibson K, et al. Faricimab reduces macular leakage vs aflibercept in patients with DME. Invest Ophthalmol Vis Sci. 2023;64:2816.
Maunz A, von, Schulthess E, Patel KM, Chakravarthy U, Bachmeier I, et al. Automated segmentation of hyperreflective foci in diabetic macular edema shows greater volume reduction by faricimab vs aflibercept in phase 3 YOSEMITE and RHINE. Invest Ophthalmol Vis Sci. 2023;64:PB0039–PB.
Wong D, Chhabra R, Ruiz-Medrano J, Hamilton R. Improving clinic capacity with faricimab. EMJ Innov. 2025;9:2–11.
Izquierdo-Serra J, Martin-Pinardel R, Moll-Udina A, Bernal-Morales C, Garay-Aramburu G, Sanchez-Monroy J, et al. Macular neovascularization type influence on anti-VEGF intravitreal therapy outcomes in age-related macular degeneration. Ophthalmol Retin. 2024;8:350–9.
Martin-Pinardel R, Izquierdo-Serra J, De Zanet S, Parrado-Carrillo A, Garay-Aramburu G, Puzo M, et al. Artificial intelligence-based fluid quantification and associated visual outcomes in a real-world, multicentre neovascular age-related macular degeneration national database. Br J Ophthalmol. 2024;108:253.
Puzo M, Calvo-Perez P, Bartol-Puyal F, Sanchez-Monroy J, Martin-Pinardel R, Parrado-Carrillo A, et al. Fight Retinal Blindness SPAIN. Report 3: clinical outcomes of vascular endothelial growth factor inhibitors in low vision eyes with neovascular age-related macular degeneration. A national database study. Eye. 2024;38:3450–8.
Rodrigues IA, Sprinkhuizen SM, Barthelmes D, Blumenkranz M, Cheung G, Haller J, et al. Defining a minimum set of standardized patient-centered outcome measures for macular degeneration. Am J Ophthalmol. 2016;168:1–12.
Monés J, Singh RP, Bandello F, Souied E, Liu X, Gale R. Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world: the need for a change of mindset. Ophthalmologica. 2020;243:1–8.
Holz FG, Tadayoni R, Beatty S, Berger AR, Cereda MG, Hykin P, et al. Determinants of visual acuity outcomes in eyes with neovascular AMD treated with anti-VEGF agents: an instrumental variable analysis of the AURA study. Eye. 2016;30:1063–71.
Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36:1418–31.
Teo KYC, Eldem B, Joussen A, Koh A, Korobelnik JF, Li X, et al. Treatment regimens for optimising outcomes in patients with neovascular age-related macular degeneration. Eye. 2025;39:860–9.
Kiss S, Malangone-Monaco E, Wilson K, Varker H, Stetsovsky D, Smith D, et al. Real-world injection frequency and cost of ranibizumab and aflibercept for the treatment of neovascular age-related macular degeneration and diabetic macular edema. J Manag Care Spec Pharm. 2020;26:253–66.
Boudousq C, Nguyen V, Hunt A, Gillies M, Zarranz-Ventura J, O’Toole L, et al. European unmet needs in the management of neovascular age-related macular degeneration in daily practice: data from the Fight Retinal Blindness! Registry. Ophthalmol Retin. 2024;8:527–36.
Gillies MC, Hunyor AP, Arnold JJ, Guymer RH, Wolf S, Ng P, et al. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2019;137:372–9.
Gillies MC, Nguyen V, Daien V, Arnold JJ, Morlet N, Barthelmes D. Twelve-month outcomes of ranibizumab vs. aflibercept for neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2016;123:2545–53.
Lotery A, Griner R, Ferreira A, Milnes F, Dugel P. Real-world visual acuity outcomes between ranibizumab and aflibercept in treatment of neovascular AMD in a large US data set. Eye. 2017;31:1697–706.
Bhandari S, Nguyen V, Arnold J, Young S, Banerjee G, Gillies M, et al. Treatment outcomes of ranibizumab versus aflibercept for neovascular age-related macular degeneration: data from the Fight Retinal Blindness! Registry. Ophthalmology. 2020;127:369–76.
Dugel PU, Singh RP, Koh A, Ogura Y, Weissgerber G, Gedif K, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128:89–99.
Lanzetta P, Korobelnik JF, Heier JS, Leal S, Holz FG, Clark WL, et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet. 2024;403:1141–52.
Penha FM, Masud M, Khanani ZA, Thomas M, Fong RD, Smith K, et al. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. Int J Retin Vitreous. 2024;10:5.
Acknowledgements
Thanks to Ruben Martin-Pinardel for the statistical support during the preparation of the study protocol and this manuscript. Medical writing assistance was provided by Laura Prieto del Val and Alicia Subtil-Rodriguez from Evidenze Clinical Research during the preparation of the study protocol and this manuscript. The authors would also like to acknowledge Marta Urech and Belén Muñoz for their support during the development of this study. The authors would like to thank all the Fight Retinal Blindness! Spain Investigators that contribute to the registry.
Funding
Funding was provided by Roche Farma S.A.
Author information
Authors and Affiliations
Consortia
Contributions
Conception and design: JZV, PA, PGL; Analysis and interpretation: JZV, GGA, PC, MAZ, CA, PA, PGL, LSR; Data collection: JZV, GGA, PC, MAZ, CA, LSR, FRB SPAIN study group; Obtained funding: JZV; Overall responsibility: JZV, PA, PGL.
Corresponding author
Ethics declarations
Competing interests
All authors have completed and submitted the ICMJE disclosures form. JZV is a grant holder for Novartis, Bayer, Allergan/Abbvie and Roche, and a consultant for Novartis, Bayer, Allergan/Abbvie, Alcon, Alimera Sciences, Bausch and Lomb, Brill Pharma, DORC, Preceyes, Roche, Topcon, and Zeiss; is a member of the Eye editorial board. GG-A receives consulting fees from AJL S.A. and Ulma Medical Tech; payment of honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events of Roche and Ulma Medical Tech; and support for attending meetings and/or travel of AbbVie, Novartis, and J&J. PA and PG-L are employees of Roche Spain. The rest of the authors declare that they have no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was presented in part at the Association for Research in Vision and Ophthalmology (ARVO) annual meeting 2024, May 5-9, Seattle.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zarranz-Ventura, J., Garay-Aramburu, G., Calvo, P. et al. Treatment intervals with first-generation anti-vascular endothelial growth factor drugs: evaluating the unmet need in a real-world neovascular age-related macular degeneration national database. Eye 39, 3306–3313 (2025). https://doi.org/10.1038/s41433-025-03996-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41433-025-03996-8