Abstract
We present a reference genome assembly from an individual male Violet Carpenter Bee (Xylocopa violacea, Linnaeus 1758). The assembly is 1.02 gigabases in span. 48% of the assembly is scaffolded into 17 pseudo-chromosomal units. The mitochondrial genome has also been assembled and is 21.8 kilobases in length. The genome is highly repetitive, likely representing a highly heterochromatic architecture expected of bees from the genus Xylocopa. We also use an evidence-based methodology to annotate 10,152 high confidence coding genes. This genome was sequenced as part of the pilot project of the European Reference Genome Atlas (ERGA) and represents an important addition to the genomic resources available for Hymenoptera.
Similar content being viewed by others
Introduction
We live in a time of unprecedented biodiversity loss (Ceballos and Ehrlich 2023) exemplified by the global decline of insect fauna undeniably associated with anthropogenic stressors (Outhwaite et al. 2022). Insect biodiversity loss puts key ecosystem services, such as pollination (Ollerton 2021) and decomposition (Yang and Gratton 2014), at risk. Although there is strong evidence of insect declines in the recent history (Hallmann et al. 2017; Powney et al. 2019), changes in global climate have also seen patterns of range shift in many taxa (e.g. Kerr et al. 2015; Lehmann et al. 2020; Rollin et al. 2020; Halsch et al. 2021; Skendžić et al. 2021). The European Reference Genome Atlas (ERGA, Mc Cartney et al. 2024 aims to empower research communities to expand the taxonomic coverage of genomic resources, enabling cross taxa analyses to address continent-scale questions, such as those surrounding range shifts, at the genomic level.
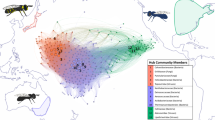
There are currently no annotated, reference quality, genomic resources for the Carpenter bees (Hymenoptera: Apidae). Carpenter bees are classified as a single genus, Xylocopa (Latreille 1802), which contains around 400 species (Gerling et al. 1989; Leys et al. 2000, 2002; Michener 2007), and are considered as essential pollinators (e.g. Vargas et al. 2017; Malabusini et al. 2019). In Europe, the most widespread Xylocopa species is the Violet Carpenter Bee, Xylocopa violacea (Linnaeus 1758) (Vicidomini 1996). This species has a pan-European distribution (Fig. 1, https://www.gbif.org/species/1342108) that also extends to Algeria and Turkey (Gerling et al. 1989; Aouar-Sadli et al. 2008; Tezcan and Skyrpan 2022), Iraq and India (Dar et al. 2016; Bamarni and Elsaiegh 2022).
A Records of X. violacea occurrence in Europe between 1980 and 2023 (GBIF.org, 04 December 2023, https://doi.org/10.15468/dl.3gr8wv). Hexes are coloured by earliest year of occurrence; lighter colours are more recent. Records prior to 1980 not plotted. B A female X. violacea individual (Bautsch, CC0, via Wikimedia Commons.). C The male X. violacea (iyXylViol4) used for DNA sequencing in this study.
In recent years, Xylocopa violacea has exhibited a marked range expansion, with records in Germany (Praz et al. 2022), Czech Republic (Kleprlíková and Vrabec 2020), Poland (Banaszak et al. 2019), and as far north as Sweden (Cederberg and Others 2018) (Fig. 1). The northward expansion of the Violet Carpenter Bee’s range may be attributed to various factors, including climatic changes in Europe (Banaszak et al. 2019). Xylocopa violacea is a solitary bee (Vicidomini 1996), although within the genus there is evidence for several independent transitions to sociality (Gerling et al. 1989; Sless and Rehan 2023). X. violacea also exhibits a lineage specific microbiome (Alberoni et al. 2019; Holley et al. 2022; Handy et al. 2023) and a distinctive venom profile with novel melittin variants that show potential for anticancer applications (von Reumont et al. 2022; Erkoc et al. 2022). There is only a contig-level assembly of the X. violacea genome currently available (Koludarov et al. 2023).
Here, we present a pseudo-chromosomal assembly of the genome of Xylocopa violacea. The genome was sequenced as part of the pilot project of the ERGA (Mc Cartney et al. 2024). The ERGA consortium is pioneering a democratised approach to biodiversity sequencing, and paired a sample ambassador from Malta, where X. violacea is an important and understudied species, with a sequencing centre in the UK order to generate the assembly presented here. The X. violacea genome assembly is characterised by its highly heterochromatic karyotype, a trait also shared by other Xylocopa species (Hoshiba and Imai 1993). This genomic resource fills an important gap in the taxonomy of the Apidae, and also releases the potential to study the expanding population of this important pollinating species at the genomic level (e.g. Formenti et al. 2022; Webster et al. 2022).
Materials and methods
Sample acquisition
A male (iyXylViol4, ERS10526494) and female (iyXylViol2, ERS10526492) Xylocopa violacea individual were collected at Chadwick Lakes, Rabat, Malta (Latitude: 35.894639, Longitude: 14.392165). Samples were chilled to 4 °C, preserved in dry ice, and maintained at −80 °C until shipment to the Earlham Institute, Norwich, UK following Nagoya Protocol, permit ABSCH-IRCC-MT-255778-1. Sample metadata conformed to ERGA sample manifest standards (Böhne et al. 2024) and were submitted to ENA using COPO (Shaw et al. 2020).
DNA library preparation and sequencing
High molecular weight (HMW) DNA was extracted from thorax tissue of an individual male bee (iyXylViol4) using the Qiagen MagAttract HMW DNA Kit, with modifications as described in Mullin et al. (2022). HiFi library preparation and Pacific Biosciences (PacBio) sequencing were carried out following the low-input protocol described in Mullin et al. (2022), (Supplementary Methods) and sequenced on four Sequel II SMRT® Cell 8 M (diffusion loading, 30-h movie, 2-h immobilisation time, 2-h pre-extension time, 60–77 pM on plate loading concentration).
RNA extraction, RNA-seq library preparation and sequencing
RNA extractions were conducted on flash frozen head, thorax, abdomen, and leg tissues from an individual female bee (iyXylViol2) using the Omega EZNA Total RNA Kit I (R6834-01). RNA-seq libraries were then constructed using the NEBNext Ultra II RNA Library prep for Illumina kit (NEB#E7760L) NEBNext Poly(A) mRNA Magnetic Isolation Module (NEB#7490) and NEBNext Multiplex Oligos for Illumina® (96 Unique Dual Index Primer Pairs) (E6440S) at a concentration of 10uM. Libraries were sequenced on an SP flow cell on a NovaSeq 6000 instrument set up to sequence 150 bp paired end reads.
Iso-seq library preparation and sequencing
PacBio Iso-Seq libraries were constructed starting from 234 to 300 ng of total RNA from the 4 tissue specific extractions described above. Reverse transcription cDNA synthesis was performed using NEBNext® Single Cell/Low Input cDNA Synthesis & Amplification Module (NEB, E6421). Samples were barcoded and the library pool was prepared according to the guidelines laid out in the Iso-Seq protocol version 02 (PacBio, 101-763-800), using SMRTbell express template prep kit 2.0 (PacBio, 102-088-900). The Iso-Seq pool was sequenced on the PacBio Sequel II instrument with one Sequel II SMRT® Cell 8 M.
Hi-C library preparation and sequencing
High-throughput/resolution chromosome conformation capture-based (Hi-C) sequencing data was generated from head tissue of male individual iyXylViol4 using the Arima Genome Wide Hi-C kit, the NEBNext Ultra II DNA Library preparation kit, and Kappa HiFi HotStart ReadyMix. The resulting libraries were sequenced on an SP flow cell, on the Novaseq 6000 instrument, sequencing 150 bp paired end reads.
Contig level genome assembly
HiFi reads were extracted from the raw Pacific Biosciences output by the Earlham Institute core bioinformatics group using the Pacific Biosciences SMRTlink pipeline (v10.1.0.119588). Prior to assembly, HiFi reads were trimmed for adapter sequences with Cutadapt (v3.2, Martin 2011). The genome was assembled with hifiasm (v0.18.5, Cheng et al. 2021). Mitochondrial contigs were identified with MitoHifi (v3.0.0, Uliano-Silva et al. 2023), using the Apis mellifera mitochondrial genome (OK075087.1) as a closely related guide. All putative mitochondrial contigs were removed prior to scaffolding, and the MitoHifi best fit mitochondrial sequence was added back into the assembly following scaffolding. Contaminant contigs were identified and removed as the intersect of the outputs of Kraken2 (v2.0.7, Wood et al. 2019), BlobTools (v1.1.1, Laetsch and Blaxter 2017), barnapp (v0.9, Table S1), CAT (v5.2.3,von Meijenfeldt et al. 2019), and FCS-GX (v0.3.0, Astashyn et al. 2024). Assembly completeness was assessed with BUSCO (v5.0.0, Manni et al. 2021) using hymenoptera_odb10. Assembly quality and kmer completeness were assessed with Merqury (v1.3, Rhie et al. 2020). Genome size of the final assembly was estimated using FastK (Table S1) and GeneScopeFK (Table S1).
Hi-C read QC & scaffolding
Raw Hi-C reads were trimmed for adapters using trimmomatic (v0.39, Bolger et al. 2014) with the adapters.fa file from bbmap (v35.85, Bushnell 2014) as input (see Supp. Methods). Hi-C reads were mapped to the draft assembly with Juicer (v1.6, Durand et al. 2016). Following the removal of contigs assigned as contaminant or mitochondrial, Hi-C reads were mapped to the resulting assembly using the Arima Mapping Pipeline (Table S1). The resulting mappings were used to scaffold the decontaminated assembly using YaHS (v1.2a.2, Zhou et al. 2023).
Manual curation of scaffolded assembly
Following scaffolding, trimmed, unfiltered Hi-C reads were mapped to the scaffolded assembly using Juicer (v1.6, Durand et al. 2016). Using these mappings, the scaffolded assembly was manually curated to pseudo-chromosomal level using Pretext-Map (v0.1.9, Table S1) contact maps visualised in PretextView (v0.2.5, Table S1). Inputs for PrextextView (Coverage track, Gap track, Telomere track) were created using the eihic pipeline (Table S1) in curation mode (-c). Following curation, the Rapid Curation Pipeline (Table S1), developed by the GRiT team at the Wellcome Sanger Institute, was used to extract the manually curated assembly in fasta format.
Annotation
Annotation of repetitive DNA content was performed using the EI-Repeat pipeline (v1.3.4, Table S1) which uses third party tools for repeat calling. The repeat content of the iyXylViol4.1 assembly was further classified using srf (Zhang et al. 2023) and TRASH (Wlodzimierz et al. 2023), and visualised using StainedGlass (Vollger et al. 2022). The telomeric repeat landscape was explored using the explore and search functions of tidk (Table S1). Gene models were generated from the iyXylViol4.1 assembly using REAT - Robust and Extendable eukaryotic Annotation Toolkit (Table S1) and Minos (Table S1) which make use of Mikado (Table S1), Portcullis (Table S1) and many third-party tools (listed in the above repositories).
Results & discussion
DNA sequencing
HMW DNA extractions from two 30 mg sections of thorax tissue from a single male Xylocopa violacea individual (iyXylViol4) yielded 829 ng of HMW DNA, with 74–84% of fragments over 40 kb fragment size (Fig. S1). Following library preparation, 2,520,442 PacBio HiFi Reads were obtained (21.8× coverage of the final assembly). The whole head tissue from this individual (98 mg) was used to generate 535,271,589 Illumina paired reads following proximity ligation and Arima High Coverage Hi-C library preparation (see Supp. Results). Following QC, 509,760,108 read pairs remained.
Transcriptome sequencing
Total RNA was extracted from four tissues segments (Head, Thorax, Abdomen, Legs) from a second individual (female, iyXylViol2). These tissues produced 4.3 µg, 3.6 µg, 18.2 µg, 2.6 µg of total RNA respectively. We generated 149,032,417,107,159,638,116,609,061, and 148,189,077 Illumina RNA-seq short reads respectively for the head, thorax, abdomen, and legs. Additional RNA-seq reads, from X. violacea venom gland, were downloaded from SRA (SRR14690757, Koludarov et al. 2023). The same extractions were also used to generate 790,150, 717,956, 977,170, and 999,264 PacBio Iso-Seq long reads for the head, thorax, abdomen, and legs respectively. Cumulatively, this represented an average of 81.76x long-read coverage of the transcriptome.
Genome assembly
The initial contig assembly had 1224 contigs and spanned 1.08 Gb with an N50 of 5.91 Mb (Table 1). Prior to scaffolding, 161 contigs (59.8 Mb) were classified as contaminant content and removed from the assembly. A contig was only classified as contaminant and removed if it was identified in the output of 2 of the following tools: Contigs identified as not within the Insecta by Kraken2 (316), contigs classified as ‘’no-hit’ by blobtools (389), contigs identified as bacterial or archaeal 16 s by barnapp (384), contigs classified as bacterial or viral by CAT (4), or contigs identified as contaminants by FCS-GX (1). For further details see Table S6. 79 mitochondrial candidates (1.7 Mb), identified by MitoHifi, were also removed. With this content removed, the assembly had 984 contigs spanning 1.02 Gb, with an N50 of 5.96 Mb (Table 1).
Scaffolding generated an assembly with 1343 scaffolds spanning 1.02 Gb with an N50 of 6.65 Mb (Table 1). The scaffolded assembly was manually curated to give the final pseudo-chromosomal iyXylViol4.1 assembly (GCA_963969225.2), containing 1300 scaffolds over 1.02 Gb, and an N50 of 11.42 Mb (Fig. 2, Table 1). The consensus mitogenome (21.8 Kb) was added to the assembly following manual curation and annotation. The iyXylViol4.1 assembly contains 17 pseudo-chromosomal units. One of these units has Hi-C telomeric signal at both ends, and the remaining 16 of which have Hi-C telomeric signal at one end. Xylocopa violacea has been suggested to have a karyotype of 16 (Granata 1909), similar to a related species, X. fenestra, (Kumbkarni 1965; Kerr and da Silveira 1972), thus it is possible that two of the remaining super scaffolds in the iyXylViol4.1 assembly correspond to chromosomal arms with insufficient Hi-C signal to be joined. Alternatively, X. appendiculata has a karyotype of 17 chromosomes including a majority of pseudo-acrocentric chromosomal morphologies (Hoshiba and Imai 1993).
A Hi-C contact map (Supp Methods). Scaffolds are ordered by size with the 17 pseudo-chromosomal super scaffolds appearing in the top left half of the map, defined by overlayed lines. Visualisation constructed with multimapping reads (MAPQ = 0). B Merqury kmer spectra, k = 19, single peak representing the haploid male genome of iyXylViol4. C Completeness of the hymenoptera_odb10 BUSCO set (5991 genes).
Following Wallberg et al. (2019), we identified the centromeric signature of low GC% in 6 super scaffolds (Supplementary Methods, Fig. S5). We identified one such region at the centre of the only firmly identified metacentric chromosome (iyXylViol4_SUPER_4). The other 5 candidates all separate putative euchromatic regions bearing many coding annotations, from regions of high repeat content. This pattern of repeat expansion around centromeric sequences has been observed in other bees, such as Austroplebeia australis (Travenzoli et al. 2022), and may help to explain the high levels of interaction between unplaced scaffolds and the pseudo-chromosomal units in the iyXylViol4.1 assembly.
Highly acrocentric karyotypes are well represented within the Xylocopinae, the genus Ceratina exhibits species with karyotypes representing 14–17 chromosomes, with ratios of acrocentric to metacentric chromosomes varying between 16:1, 15:2, and 12:5 (Hoshiba and Imai 1993; Cunha et al. 2021). Such patterns are also common in other, more evolutionarily distant bees: Austroplebeia australis has been shown to have 14 largely heterochromatic chromosome pairs and four that are fully euchromatic (Travenzoli et al. 2022).
Without further investigation, potentially employing ultra-long read technologies, it is not possible to differentiate between N = 16 or N = 17 from the iyXylViol4.1 assembly.
Assembly QC
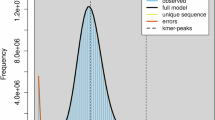
BUSCO analysis of the iyXylViol4.1 assembly showed that it contains 96.5% of the 5991 hymenoptra_odb10 set as complete genes, with only 0.4% complete and duplicated, 0.6% fragmented, and 2.5% missing (Fig. 2, Table S2). The genic content was not impacted by the scaffolding process as the same metrics are recovered in the contig, scaffolded, and manually curated assemblies. The iyXylViol4.1 assembly is QV 63.3 and has a kmer completeness of 98.8% (Table S3).
The iyXylViol4.1 assembly is 1.02 Gb in length. Although this is not outside of the upper limits for known genome sizes from the Apidae e.g. Melipona capixaba 1.38 Gb, (Tavares et al. 2010; Cunha et al. 2021), k-mer based estimation of genome size from iyXylViol4.1 suggests the genome size to be 672 Mb (Table S4, Fig. S4). This estimation is in line with the only prediction from the genus Xylocopa comes from Ardila-Garcia et al. (2010), who report an estimated genome size of 0.69 pg (~675 Mb) for Xylocopa virginica krombein. This species is a member of the North American subgenus Xylocopoides, thought to have diverged from the genus Xylocopa s.l. some 34 mya (Leys et al. 2002), and so using this estimate as a cross validation for the iyXylViol4.1 assembly may not be relevant. The 17 pseudo-chromosomal iyXylViol4.1 super scaffolds (including unloc) are 481.4 Mb in length, representing a large majority of the predicted genome size. As complete reconstruction of the iyXylViol4.1 chromosomes was not feasible in this study, we have included all unplaced scaffolds in the final assembly, as these likely encompass the remaining genomic content.
Repeat content
The majority of the iyXylViol4.1 assembly was masked as repetitive sequence (821.28 Mb, 80.47%) (Table S5). The predominant category was unclassified repeats, with 755.96 Mb (74.08%). This pattern is consistent with pseudo-acrocentric chromosomes with extremely elongated heterochromatic arms which are frequently observed in bees and wasps (Hoshiba and Imai 1993). These have been suggested to be induced by saltatory growth of constitutive heterochromatin after centric fission (Hoshiba and Imai 1993). Bees from the Apinae genus Melipona have recently been shown to exhibit up to 73% heterochromatin content (Pereira et al. 2021). As is seen in iyXylViol4.1, bees from the genus Melipona also have terminal euchromatic regions (Piccoli et al. 2018) which is consistent with the pseudo-acrocentric chromosomal topology derived from X. appendiculata (Hoshiba and Imai 1993), with many chromosomes representing large expansions of heterochromatin repeats around the centromere.
Classification of the repeats within the iyXylViol4.1 assembly showed the ten most abundant satellite repeat units identified by srf (Zhang et al. 2023) to occupy 105.6 Mb of the assembly (Table S6). Further decomposition of the satellite repeats present in the iyXylViol4.1 assembly, using TRASH (Wlodzimierz et al. 2023), revealed the predominant monomeric repeat unit to be a 109mer (Figs. S7, S8, Table S7). This 109mer or a 217mer (approximately double its length) were highly abundant throughout the putative acrocentric chromosomes (Fig. S8) and was repeated with high identity (Fig. S7).
We also observe that the putative centromeric sequences are flanked by a distinct repeat signature. In the metacentric iyXylVio4_SUPER_4, the putative centromere has expansions of a 95mer on either side of it. Regions abundant in this 95mer are also seen in 13 of the 16 putative acrocentric pseudo-chromosomal molecules (Fig. S8), and these often occur in proximity to the location of the regions of low GC% which are putatively centromeric.
Recent studies have shown telomeric repeat motifs in Hymenoptera to be diverse, including complex telomeric layering resulting from numerous site specific retrotransposon insertions (Lukhtanov 2022; Zhou et al. 2022). The iyXylViol4.1 assembly shows that X. violacea has telomeres enriched for the canonical 5 bp ancestral arthropod repeat motif (TTAGG) (Fig. S5). The iyXylViol4.1 assembly also shows that X. violacea has varying sub-telomeric repeat sequences, consistent with ‘Type 2’ telomeres suggested by Lukhtanov and Pazhenkova (2023) (Fig. S6).
Annotation
The iyXylViol4_EIv1.0 annotation of the iyXylViol4.1 assembly contains 10,152 high confidence, protein-coding gene models, coding for 26,577 transcripts (Table S8). This number of annotations is well within the range of those generated for contemporary genome assemblies (Table S9). Using the hymenoptera_odb10 database, this annotation represents 99.75% BUSCO completeness at the protein level, with only 34 BUSCO genes duplicated, 3 fragmented and 12 missing (Table S3). The annotation contains an average of 2.49 transcripts per gene, with a mean transcript cDNA size of 3,238.2 bp (Table S10). The distribution of coding genes is skewed to the distal end of the 16 pseudo-chromosomal super-scaffolds with putative pseudo-acrocentric structure (Fig. S5), supporting the previously suggested topology of highly repetitive pseudo-acrocentric chromosomes expected in Xylocopa species (Hoshiba and Imai 1993; Gokhman 2023).
Conclusion
Here, we present a pseudo-chromosomal genome assembly of the Violet Carpenter bee, Xylocopa violacea. At 1.02 Gb, the assembly is larger than the predicted genome size (672 Mb), but also represents large regions of highly repetitive, putatively heterochromatic, sequence. Such chromosomal architecture is in line with the small amount of karyotypic resources from the genus and is also supported by the iyXylViol4_EIv1 annotation. The repetitive regions we describe are predominantly made up of 109 and 217mers. The annotated assembly we present fills an important taxonomic gap in the genomic resource set representing Hymenoptera and will also provide a genomic basis for future interpretation of the expanding range of this charismatic and economically important species.
Data availability
The data underlying this article are available in the European Nucleotide Archive and can be accessed with the BioProject identifier PRJEB72102. The assembly is available through GenBank under the accession GCA_963969225.2.
References
Alberoni D, Gaggìa F, Baffoni L, Modesto MM, Biavati B, Di Gioia D (2019) Bifidobacterium xylocopae sp. nov. and Bifidobacterium aemilianum sp. nov., from the carpenter bee (Xylocopa violacea) digestive tract. Syst Appl Microbiol 42:205–216
Aouar-Sadli M, Louadi K, Doumandji S-E (2008) Pollination of the broad bean (Vicia faba L. var. major)(Fabaceae) by wild bees and honey bees (Hymenoptera: Apoidea) and its impact on the seed production in the Tizi-Ouzou area (Algeria). Afr J Agric Res 3:266–272
Ardila-Garcia AM, Umphrey GJ, Gregory TR (2010) An expansion of the genome size dataset for the insect order Hymenoptera, with a first test of parasitism and eusociality as possible constraints. Insect Mol Biol 19:337–346
Astashyn A, Tvedte ES, Sweeney D, Sapojnikov V, Bouk N, Joukov V et al. (2024) Rapid and sensitive detection of genome contamination at scale with FCS-GX. Genome Biol 25:60
Bamarni RZ, Elsaiegh MA (2022) A survey and phenotypic study of carpenter bee species recorded in Kzo village and its environs/Dohuk Governorate–Iraq. NTU J. Agric Vet Sci 2:1–4
Banaszak J, Cibicka WB, Twerd L (2019) Possible expansion of the range of Xylocopa violacea L. (Hymenoptera, Apiformes, Apidae) in Europe. Turk Zool Derg. 43:650–656
Böhne A, Fernández R, Leonard JA, McCartney AM, McTaggart S, Melo-Ferreira J et al. (2024) Contextualising samples: Supporting reference genomes of European biodiversity through sample and associated metadata collection. bioRxiv: 2023.06.28.546652. https://doi.org/10.1101/2023.06.28.546652
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Bushnell B (2014) BBMap: A Fast, Accurate, Splice-Aware Aligner. Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States)
Ceballos G, Ehrlich PR (2023) Mutilation of the tree of life via mass extinction of animal genera. Proc Natl Acad Sci USA 120:e2306987120
Cederberg B, Others (2018) The carpenter bees Xylocopa valga and X. violacea-climate refugees or labour migrants in Sweden (Hymenoptera: Apidae). Entomol Tidskr 139:65–72
Cheng H, Concepcion GT, Feng X, Zhang H, Li H (2021) Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods 18:170–175
Cunha MS, Cardoso DC, Cristiano MP, de Oliveira Campos LA, Lopes DM (2021) The Bee Chromosome database (Hymenoptera: Apidae). Apidologie 52:493–502
Dar S, Mir G, Parry M, Sofi MA, Padder SA (2016) Nest distribution and nesting habits of Xylocopa violacea (Donovan), Fabricius (Hymenoptera: Apidae) in Kashmir valley. J Exp Zool India 9:155–162
Durand NC, Shamim MS, Machol I, Rao SSP, Huntley MH, Lander ES et al. (2016) Juicer Provides a One-Click System for Analyzing Loop-Resolution Hi-C Experiments. Cell Syst 3:95–98
Erkoc P, von Reumont BM, Lüddecke T, Henke M, Ulshöfer T, Vilcinskas A et al. (2022) The pharmacological potential of novel melittin variants from the honeybee and solitary bees against inflammation and cancer. Toxins 14
Formenti G, Theissinger K, Fernandes C, Bista I, Bombarely A, Bleidorn C et al. (2022) The era of reference genomes in conservation genomics. Trends Ecol Evol 37:197–202
Gerling D, Velthuis HHW, Hefetz A (1989) Bionomics of the large carpenter bees of the genus xylocopa. Annu Rev Entomol 34:163–190
Gokhman VE (2023) Chromosome study of the hymenoptera: history, current state, perspectives. Biol Bull Rev 13:247–257
Granata L (1909) Le divisioni degli spermatociti di ‘Xylocopa violacea’. L Biologica Torino 2:1–12
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H et al. (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS One 12:e0185809
Halsch CA, Shapiro AM, Fordyce JA, Nice CC, Thorne JH, Waetjen DP et al. (2021) Insects and recent climate change. Proc Natl Acad Sci USA 118:e2002543117
Handy MY, Sbardellati DL, Yu M, Saleh NW, Ostwald MM, Vannette RL (2023) Incipiently social carpenter bees (Xylocopa) host distinctive gut bacterial communities and display geographical structure as revealed by full-length PacBio 16S rRNA sequencing. Mol Ecol 32:1530–1543
Holley J-AC, Jackson MN, Pham AT, Hatcher SC, Moran NA (2022) Carpenter Bees (Xylocopa) Harbor a Distinctive Gut Microbiome Related to That of Honey Bees and Bumble Bees. Appl Environ Microbiol 88:e0020322
Hoshiba H, Imai H (1993) Chromosome evolution of bees and wasps (Hymenoptera, apocrita) on the basis of C-banding pattern analyses. Jpn J Entomol 61:465–492
Jackman SD, Vandervalk BP, Mohamadi H, Chu J, Yeo S, Hammond SA et al. (2017) ABySS 2.0: resource-efficient assembly of large genomes using a Bloom filter. Genome Res 27:768–777
Kerr JT, Pindar A, Galpern P, Packer L, Potts SG, Roberts SM et al. (2015) Climate change impacts on bumblebees converge across continents. Science 349:177–180
Kerr WE, da Silveira ZV (1972) Karyotypic evolution of bees and corresponding taxonomic implications. Evolution 26:197–202
Kleprlíková L, Vrabec V (2020) Bee spread continues-new records of Xylocopinae (Hymenoptera: Apidae) in the Czech Republic. In: Conference paper, 11th Workshop on Biodiversity, Jevany, researchgate.net, p
Koludarov I, Velasque M, Senoner T, Timm T, Greve C, Hamadou AB et al. (2023) Prevalent bee venom genes evolved before the aculeate stinger and eusociality. BMC Biol 21:229
Kumbkarni CG (1965) Cytological Studies in Hymenoptera: Part II: Cytology of parthenogenesis in the carpenter-bee, Xylocopa fenesterata (Fabre). Cytologica 30:222–228
Laetsch DR, Blaxter ML (2017) BlobTools: Interrogation of genome assemblies. F1000Res 6:1287
Latreille PA (1802). Histoire naturelle, générale et particulière des crustacés et des insectes. de l’imprimerie de F. Dufart.
Lehmann P, Ammunét T, Barton M, Battisti A, Eigenbrode SD, Jepsen JU et al. (2020) Complex responses of global insect pests to climate warming. Front Ecol Environ 18:141–150
Leys R, Cooper SJ, Schwarz MP (2000) Molecular phylogeny of the large carpenter bees, genus Xylocopa (Hymenoptera: apidae), based on mitochondrial DNA sequences. Mol Phylogenet Evol 17:407–418
Leys R, Cooper SJB, Schwarz MP (2002) Molecular phylogeny and historical biogeography of the large carpenter bees, genus Xylocopa (Hymenoptera: Apidae). Biol J Linn Soc Lond 77:249–266
Linnaeus C (1758). Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis, 10th edn. Laurentii Salvii: Holmiae.
Lukhtanov VA, Pazhenkova EA (2023) Diversity and evolution of telomeric motifs and telomere DNA organization in insects. Biol J Linn Soc Lond 140:536–555
Lukhtanov VA (2022) Diversity and evolution of telomere and subtelomere DNA sequences in insects. bioRxiv: 2022.04.08.487650. https://doi.org/10.1101/2022.04.08.487650
Malabusini S, Palamara Mesiano M, Zanovello D, Giuliani C, Fico G, Giovanetti M et al. (2019) Flower selection of Xylocopa violacea: aromatic and ornamental plants as resources in a botanic garden. In: Landscape management for functional biodiversity, IOBC-WPRS, pp 41–45
Manni M, Berkeley MR, Seppey M, Simão FA, Zdobnov EM (2021) BUSCO Update: Novel and Streamlined Workflows along with Broader and Deeper Phylogenetic Coverage for Scoring of Eukaryotic, Prokaryotic, and Viral Genomes. Mol Biol Evol 38:4647–4654
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12
Mc Cartney AM, Formenti G, Mouton A, Ciofi C, Waterhouse RM, Mazzoni CJ et al. (2024) The European Reference Genome Atlas: piloting a decentralised approach to equitable biodiversity genomics. bioRxiv: 2023.09.25.559365v4. https://doi.org/10.1101/2023.09.25.559365
von Meijenfeldt FAB, Arkhipova K, Cambuy DD, Coutinho FH, Dutilh BE (2019) Robust taxonomic classification of uncharted microbial sequences and bins with CAT and BAT. Genome Biol 20:217
Michener CD (2007) The Bees of the World. Johns Hopkins University Press
Mullin VE, Stephen W, Arce AN, Nash W, Raine C, Notton DG et al. (2022) First large‐scale quantification study of DNA preservation in insects from natural history collections using genome‐wide sequencing. Methods Ecol Evol 14:360–371
Ollerton J (2021) Pollinators and Pollination: Nature and Society. Pelagic Publishing Ltd
Outhwaite CL, McCann P, Newbold T (2022) Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 605:97–102
Pereira JA, Travenzoli NM, de Oliveira MP, de Azevedo Werneck H, Salomão TMF, Lopes DM (2021) Molecular cytogenetics in the study of repetitive sequences helping to understand the evolution of heterochromatin in Melipona (Hymenoptera, Meliponini). Genetica 149:55–62
Piccoli MCA, Bardella VB, Cabral-de-Mello DC (2018) Repetitive DNAs in Melipona scutellaris (Hymenoptera: Apidae: Meliponidae): chromosomal distribution and test of multiple heterochromatin amplification in the genus. Apidologie 49:497–504
Powney GD, Carvell C, Edwards M, Morris RKA, Roy HE, Woodcock BA et al. (2019) Widespread losses of pollinating insects in Britain. Nat Commun 10:1018
Praz C, Müller A, Hermann M, Neumeyer-Funk R, Bénon D, Amiet F et al. (2022) Swiss National Apoidea Databank. Version 1.5
von Reumont BM, Dutertre S, Koludarov I (2022) Venom profile of the European carpenter bee Xylocopa violacea: Evolutionary and applied considerations on its toxin components. Toxicon X 14:100117
Rhie A, Walenz BP, Koren S, Phillippy AM (2020) Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol 21:245
Rollin O, Vray S, Dendoncker N, Michez D, Dufrêne M, Rasmont P (2020) Drastic shifts in the Belgian bumblebee community over the last century. Biodivers Conserv 29:2553–2573
Shaw F, Etuk A, Minotto A, Gonzalez-Beltran A, Johnson D, Rocca-Serra P et al. (2020) COPO: a metadata platform for brokering FAIR data in the life sciences. F1000Res 9:495
Skendžić S, Zovko M, Živković IP, Lešić V, Lemić D (2021) The impact of climate change on agricultural insect pests. Insects 12:440
Sless T, Rehan S (2023) Phylogeny of the carpenter bees (Apidae: Xylocopinae) highlights repeated evolution of sociality. Biol Lett 19:20230252
Tavares MG, Carvalho CR, Soares FAF (2010) Genome size variation in Melipona species (Hymenoptera: Apidae) and sub-grouping by their DNA content. Apidologie 41:636–642
Tezcan S, Skyrpan I (2022) New Locality Records For Xylocopa (Hymenoptera: Apidae: Xylocopinae) Fauna of Turkey. Біологічні студії /. Stud Biologica 16:3–12
Travenzoli, Cunha NM, Teixeira MS, Brito LV, Oldroyd RM, Campos LAO B et al. (2022) Cytogenetic characterization of Austroplebeia australis: evolutionary hints from a stingless bee outside the Neotropical region. Apidologie (Celle) 53:1–8
Uliano-Silva, Ferreira JGRN M, Krasheninnikova, Darwin Tree of Life Consortium K, Formenti G, Abueg L et al. (2023) MitoHiFi: a python pipeline for mitochondrial genome assembly from PacBio high fidelity reads. BMC Bioinforma 24:288
Vargas P, Liberal I, Ornosa C, Gómez JM (2017) Flower specialisation: the occluded corolla of snapdragons (Antirrhinum) exhibits two pollinator niches of large long-tongued bees. Plant Biol 19:787–797
Vicidomini S (1996) Biology of Xylocopa violacea (Hymenoptera): In‐nest ethology. Ital J Zool 63:237–242
Vollger MR, Kerpedjiev P, Phillippy AM, Eichler EE (2022) StainedGlass: interactive visualization of massive tandem repeat structures with identity heatmaps. Bioinformatics 38:2049–2051
Wallberg A, Bunikis I, Pettersson OV, Mosbech M-B, Childers AK, Evans JD et al. (2019) A hybrid de novo genome assembly of the honeybee, Apis mellifera, with chromosome-length scaffolds. BMC Genomics 20:275
Webster MT, Beaurepaire A, Neumann P, Stolle E (2022) Population Genomics for Insect Conservation. Annu Rev Anim Biosci 6:e13095
Wlodzimierz P, Hong M, Henderson IR (2023) TRASH: Tandem Repeat Annotation and Structural Hierarchy. Bioinformatics 39:btad308
Wood DE, Lu J, Langmead B (2019) Improved metagenomic analysis with Kraken 2. Genome Biol 20:257
Yang LH, Gratton C (2014) Insects as drivers of ecosystem processes. Curr Opin Insect Sci 2:26–32
Zhang Y, Chu J, Cheng H, Li H (2023) De novo reconstruction of satellite repeat units from sequence data. Genome Res 33:1994–2001
Zhou C, McCarthy SA, Durbin R (2023) YaHS: yet another Hi-C scaffolding tool. Bioinformatics 39:btac808
Zhou Y, Wang Y, Xiong X, Appel AG, Zhang C, Wang X (2022) Profiles of telomeric repeats in Insecta reveal diverse forms of telomeric motifs in Hymenopterans. Life Sci Alliance 5:202101163
Acknowledgements
We acknowledge Fiona Fraser (Earlham Institute, Norwich) and Michael Quail (Wellcome Sanger Institute, Hinxton) for valuable conversations and advice in developing Hi-C library preparation. The authors would also like to acknowledge the GRiT (Wellcome Sanger Institute, Hinxton), particularly Jo Wood, Tom Mathers, Dominic Absolon, Camilla Santos, Michael Paulini for invaluable mentorship in Hi-C scaffolding and curation. The authors also acknowledge Kamil Hepak (Norwich Bioscience Institutes, Scientific Computing) for significant HPC support.
Funding
The authors acknowledge support from the Biotechnology and Biological Sciences Research Council (BBSRC), part of UK Research and Innovation, Core Capability Grant BB/CCG2220/1 at the Earlham Institute and its constituent work packages (BBS/E/T/000PR9818 and BBS/E/T/000PR9819), and the Core Capability Grant BB/CCG1720/1 and the National Capability at the Earlham Institute BBS/E/T/000PR9816 (NC1—Supporting EI’s ISPs and the UK Community with Genomics and Single Cell Analysis), BBS/E/T/000PR9811 (NC4—Enabling and Advancing Life Scientists in data-driven research through Advanced Genomics and Computational Training), and BBS/E/T/000PR9814 (NC 3 - Development and deployment of versatile digital platforms for ‘omics-based data sharing and analysis). Authors also acknowledge support from BBSRC Core Capability Grant BB/CCG1720/1 and the work delivered via the Scientific Computing group, as well as support for the physical HPC infrastructure and data centre delivered via the NBI Computing infrastructure for Science (CiS) group. AV and NV acknowledge funding from the BioCon_Innovate Research Excellence Grant (I18LU06-01) from the University of Malta. BMvR acknowledges funding from the DFG (RE3454/6-1).
Author information
Authors and Affiliations
Contributions
Language used to describe roles below uses the CRediT Taxonomy (credit.niso.org). AV acted as ERGA sample ambassador, and with NV and BvRM, initiated the Conceptualisation of this study; WJN, SMcT, KG, and WH designed the sequencing strategy, assembly of the genome, and all analyses. WJN conducted Data Curation throughout the project; GK and DS curated data during genome annotation; DK, AP, and FS curated data for ENA upload through COPO. WJN conducted all Formal Analysis outside of genome annotation, which was conducted by GK and DS. Funding acquisition was conducted by AV, SMcT, and WH. Primary Investigation was conducted by WJN; NI Prepared IsoSeq libraries and Illumina RNA-Seq libraries; TB Sequenced Illumina RNA-seq libraries, PacBio IsoSeq libraries, and PacBio low-input HiFi libraries; AMa prepared Hi-C libraries. AD Developed and improved the Omega EZNA Total RNA extraction protocol Methodology; NI Developed and improved the low-input HiFi library preparation protocol methodology; WJN and AMa developed and tested the Hi-C library preparation methodology. WJN and SMcT conducted overall Project administration; CW and KB Coordinated the project from sample submission to data delivery; AMcC, GF, and AMo Conceptualised and administrated the ERGA Pilot Project. AV and NV delivered Resources by collecting the individuals sequenced. KG led the development of resource data production capability for reference-grade assembly and annotation. WJN wrote code to deploy Software as part of the genome assembly project; DS and GK developed and deployed the software used for genome annotation FS, DK, and AP developed and maintain the COPO data brokering software. WH contributed Supervision to the whole project, KG provided leadership responsibility for nucleic acid extraction, short-read sequencing, and long-read sequencing; CW Provided supervision and oversight of all project management activities; LC Provided supervision and oversight for Illumina RNA-seq library preparation. WJN conducted Validation on all assemblies generated, and generated the Visualisations used in the publication. WJN led the Writing – Original Draft with contributions from AV, NV, SMcT and WH. All authors contributed to Writing – review & editing of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Research Ethics statement
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associate editor: Aurora Ruiz-Herrera.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nash, W.J., Man, A., McTaggart, S. et al. The genome sequence of the Violet Carpenter Bee, Xylocopa violacea (Linnaeus, 1785): a hymenopteran species undergoing range expansion. Heredity 133, 381–387 (2024). https://doi.org/10.1038/s41437-024-00720-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41437-024-00720-2