Abstract
Hypertension is associated with systemic inflammation. The activation of the sympathetic nervous system is critically involved in the pathogenesis of hypertension. Brain perivascular macrophages (PVMs) can be affected by circulating inflammatory cytokines, and the contribution of brain PVMs to sympathoexcitation has been demonstrated in a heart failure model. We thus investigated whether brain PVMs contribute to the development of hypertension through sympathoexcitation. Stroke-prone spontaneously hypertensive rats (SHRSP) developed hypertension over an 8-week period from 4 to 12 weeks of age. The number of brain PVMs and plasma interleukin-1β levels significantly increased at the ages of 8 and 12 weeks in SHRSP compared with normotensive Wistar–Kyoto rats (WKY). To determine the contribution of brain PVMs to blood pressure elevation, we intracerebroventricularly injected liposome-encapsulated clodronate, which eliminates macrophages by inducing apoptosis, into 8-week-old rats; we then assessed its effects in 10-week-old rats. Clodronate treatment attenuated the increase in mean blood pressure in SHRSP but not in WKY. Clodronate treatment reduced the depressor effect of hexamethonium, an index of sympathetic activity; it also reduced neuronal activity in sympathetic regulatory nuclei such as the hypothalamic paraventricular nucleus and rostral ventrolateral medulla and reduced the expression of cyclooxygenase-2 and prostaglandin E2, a downstream pathway in activated macrophages, in SHRSP but not in WKY. Furthermore, clodronate treatment attenuated the increase in blood pressure and renal sympathetic nerve activity in response to an acute intravenous injection of interleukin-1β in WKY. In conclusion, brain PVMs contribute to the development of hypertension via sympathetic activation. PVMs may be activated by increased levels of circulating interleukin-1β.
Similar content being viewed by others
Introduction
Accumulating evidence has indicated that hypertension is associated with systemic inflammation [1,2,3,4]. The levels of blood-borne proinflammatory cytokines are increased in hypertensive patients and animal models compared with those in normotensive controls [5,6,7,8]. The activation of the sympathetic nervous system plays a crucial role in the development of hypertension [3, 4, 9]. As a link between systemic inflammation and the sympathetic nervous system, the systemic administration of proinflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) induce sympathetic activation and blood pressure elevation by acting on the brain [10,11,12]. However, how endogenous circulating proinflammatory cytokines are involved in the development of hypertension through the sympathetic nervous system remains unclear.
Proinflammatory cytokines (molecular weight of approximately 8–52 kDa) are too large to cross the blood-brain barrier (BBB). However, these cytokines can affect the components of the BBB, such as endothelial cells and brain perivascular macrophages (PVMs), which are located in the perivascular space surrounding vessels, as well as macrophages located in the meninges [13]. Here, it should be noted that brain PVMs are resident macrophages and are distinguished from infiltrating nonresident macrophages. Microglia are brain-resident myeloid cells that are located in the brain parenchyma, not in the perivascular space [13]. In response to circulating cytokines, endothelial cells and brain PVMs produce prostaglandin E2 (PGE2) through the upstream biosynthetic enzyme cyclooxygenase-2 (COX-2) [14,15,16]. PGE2 can enter the brain parenchyma and activate the neurons that express PGE2 receptors. The paraventricular nucleus (PVN) in the hypothalamus and the rostral ventrolateral medulla (RVLM) in the brainstem are the major nuclei that regulate sympathetic activity [17,18,19]. PGE2 receptors are expressed in the PVN and RVLM, and brain PGE2 can lead to sympathoexcitation and blood pressure elevation through neuronal activation in these nuclei [20, 21]. Importantly, brain PVMs have been demonstrated to be more sensitive than endothelial cells to circulating proinflammatory cytokines in the production of PGE2 [14, 15]. Therefore, we hypothesized that brain PVMs affected by endogenous circulating proinflammatory cytokines contribute to the development of essential hypertension through sympathetic activation.
To investigate the role of brain PVMs in the regulation of sympathetic activity and blood pressure, we selectively eliminated the brain PVMs via the intracerebroventricular administration of clodronate-containing liposomes in a rat model of essential hypertension. Clodronate liposomes are phagocytosed by brain PVMs, and subsequently, released clodronate inhibits mitochondrial ADP/ATP translocase to induce macrophage apoptosis [22, 23].
Methods
Animals and experimental protocols
All animal experiments were approved by the Committee on the Ethics of Animal Experiments, Kyushu University Graduate School of Medical Sciences and were conducted in accordance with the Guidelines for Animal Experiments of Kyushu University. Male stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar–Kyoto rats (WKY) were purchased from SLC Japan (Hamamatsu, Japan). The animals were housed in a room with controlled temperature (23 ± 1 °C) and a 12-h light/dark cycle and were fed a standard diet with free access to drinking water.
Protocol 1: time-dependent changes in plasma proinflammatory cytokines and brain PVMs in SHRSP and WKY
SHRSP exhibited normal blood pressure at 4 weeks of age [24]. We measured the systolic blood pressure of SHRSP and WKY at the ages of 4, 8, and 12 weeks, which were determined to reflect the prehypertensive phase, the developing phase of hypertension, and the established phase of hypertension, respectively (n = 6 for each group), by the tail-cuff method. The rats were sacrificed after their blood pressure was measured at the ages of 4, 8, and 12 weeks, and their brains and blood samples were collected to immunostain brain PVMs and to measure the plasma levels of proinflammatory cytokines in each phase of hypertension development.
Protocol 2: effects of centrally administered clodronate liposomes in SHRSP and WKY
A radiotelemetry transmitter was implanted in SHRSP and WKY at 7 weeks of age to measure blood pressure and heart rate. A week after telemetry implantation (8 weeks of age), we measured the blood pressure and heart rate for 24 h and then intracerebroventricularly administered vehicle (PBS) or clodronate liposomes, which induces selective apoptosis of brain PVMs. The group assignments were as follows: (1) WKY treated with vehicle (WKY-vehicle, n = 5), (2) WKY treated with clodronate liposomes (WKY-CLOD, n = 5), (3) SHRSP treated with vehicle (SHRSP-vehicle, n = 7), and (4) SHRSP treated with clodronate liposomes (SHRSP-CLOD, n = 7). In a preliminary experiment, we confirmed that the number of brain PVMs in the clodronate-treated SHRSP was significantly lower on days 3–14 but returned to the 8-week level on day 28 (Supplementary Fig. 1), as previously reported [23]. We measured the blood pressure and heart rate of conscious, unrestrained rats for 24 h at 9 and 10 weeks of age. A power spectral analysis of heart rate variability was performed to evaluate autonomic activity. Briefly, the following spectral bands were defined: very low frequency (VLF; 0.05–0.2 Hz), low frequency (LF; 0.2–0.8 Hz), and high frequency (HF; 0.8–3.0 Hz). Sympathetic nerve activity was expressed as LF power in normalized units (LFnu), and parasympathetic nerve activity was expressed as HF power in normalized units (HFnu), and these parameters were calculated by LF/(total power−VLF power) and HF/(total power−VLF power), respectively. The LF/HF ratio reflects the sympathovagal balance. After 24 h of blood pressure and heart rate recording at 10 weeks of age, a ganglionic blocker (hexamethonium, 40 mg/kg) was administered via the tail vein under 2% isoflurane inhalation anesthesia to evaluate the sympathetic activity that contributes to blood pressure maintenance; the maximal decrease in mean blood pressure induced by hexamethonium indicates sympathetic activity [25,26,27]. Then, the rats were sacrificed to collect their brains and blood samples. We assessed the activity of the sympathetic nervous system by measuring renal norepinephrine levels and neuronal activity in the PVN and RVLM, and performed power spectral analysis of heart rate variability and hexamethonium-induced blood pressure decrease. The expression levels of PGE2 and COX-2 as a downstream pathway of the activation of brain PVMs were also evaluated by immunostaining.
Protocol 3: effects of centrally administered clodronate liposomes on responses to the intravenous injection of IL-1β in WKY
Clodronate liposomes or vehicle was intracerebroventricularly injected into WKY at 8 weeks of age (n = 5 for each). A week after the intracerebroventricular injection, at 9 weeks of age, we recorded blood pressure, heart rate, and renal sympathetic nerve activity (RSNA) in rats under urethane anesthesia before and after intravenous injections of IL-1β (500 ng/kg; Pepro Tech, Rocky Hill, NJ, USA). The recording was maintained for 2 h after the IL-1β injection, and the peak responses of mean blood pressure, heart rate, and RSNA to IL-1β injection were compared with the baseline values. The RSNA responses were described as the percent change from the baseline value.
Intracerebroventricular injection of clodronate liposomes
The rats were anesthetized with 2% isoflurane inhalation and were fixed in a stereotaxic frame. A small hole was drilled in the skull (coordinate: 1.5 mm lateral and 1.0 mm posterior to bregma, 3.7 mm deep from the skull surface) [28]. Liposome-encapsulated clodronate [50 μL of 7 mg/mL, Clophosome (neutral); FormuMax Scientific Inc., Palo Alto, CA, USA] or liposome-encapsulated PBS (FormuMax Scientific Inc.) was infused into the right lateral ventricle using a 50-μL Hamilton syringe for 30 min. After injection, the syringe was removed, and the hole was closed with a tissue adhesive (Vetbond; 3 M, St. Paul, MN, USA).
The intracerebroventricular administration of liposomes containing clodronate, a bisphosphonate, has been well established and is widely used to eliminate brain PVMs [16, 29]. Clodronate liposomes administered intracerebroventricularly are phagocytosed by PVMs, not by microglia within the brain parenchyma, and induce apoptosis of brain PVMs [13]. Blood monocytes and splenic macrophages are unaffected by the intracerebroventricular injection of a small amount of clodronate liposomes [23]. To confirm the selectivity of their action on brain PVMs, we differentially counted blood leukocytes in another cohort of SHRSP of the same age that received an intracerebroventricular injection of clodronate liposomes or vehicle at the same dose we used.
Specific methods
The Supplementary information describes detailed methods for blood pressure measurement by tail-cuff plethysmography, plasma cytokine and renal norepinephrine assays, immunohistochemistry, telemetry implantation, spectral analysis of heart rate variability, and preparation for recording blood pressure, heart rate, and RSNA before and after IL-1β injection.
Statistical analysis
All the results are presented as the mean ± standard error of the mean. The data were analyzed using the t-test or ANOVA followed by the Tukey post test. Statistical significance was accepted for p < 0.05. Prism 7 (GraphPad Software Corp, San Diego, CA, USA) was used to perform the data analysis.
Results
Increase in plasma IL-1β levels and the number of brain PVMs during the developing phase of hypertension in SHRSP
In Protocol 1, we evaluated the time course of blood pressure, plasma inflammatory cytokine levels, and the number of brain PVMs in SHRSP and normotensive WKY. Systolic blood pressure, as measured by the tail-cuff method, was within the normal range in both SHRSP and WKY at 4 weeks of age, although it was statistically higher in SHRSP. SHRSP developed hypertension over 8 weeks from 4 to 12 weeks of age (Fig. 1a), as reported previously [24]. Based on our own results and those of others, we considered the period from the age of 4–12 weeks to be the developing phase of hypertension in SHRSP. Plasma IL-1β levels were significantly higher in SHRSP than in WKY at 4, 8, and 12 weeks of age (Fig. 1b). Plasma TNF-α, IL-6, and IL-10 levels were very low or undetectable in both groups at 4, 8, and 12 weeks of age (data not shown). Brain PVMs were mainly found in association with vessels larger than 10 μm (Supplementary Fig. 2), as previously demonstrated [29]. The number of brain PVMs did not differ between the groups at 4 weeks, but it increased significantly in SHRSP compared with WKY at 8 and 12 weeks of age (Fig. 1c–e). These results suggest that the plasma IL-1β levels were increased from the prehypertensive phase and that subsequently, brain PVMs were increased in the developing phase of hypertension in SHRSP.
The time course of blood pressure, plasma IL-1β levels, and brain PVMs in WKY and SHRSP. a, b Systolic blood pressure and plasma IL-1β levels at 4, 8, and 12 weeks of age. c Representative pictures of immunostaining for brain PVMs. Arrowheads indicate PVMs shown in red (CD163). Nuclei are shown in blue (DAPI). Scale bar: 100 μm. d, e The number of brain PVMs juxtaposed to vessel per vessel length (d) and the number of brain PVMs per field area (e) at 4, 8, and 12 weeks of age. n = 6 for each group. *p < 0.05 vs. WKY
Effects of brain PVM elimination on blood pressure
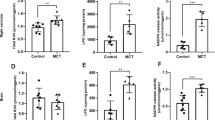
In Protocol 2, we aimed to investigate the role of brain PVMs in the development of hypertension. Therefore, we eliminated brain PVMs via the intracerebroventricular injection of clodronate liposomes, which in turn induced macrophage apoptosis, into SHRSP and WKY at 8 weeks of age. The number of PVMs was significantly increased in the SHRSP-vehicle group compared with the WKY-vehicle group, and clodronate treatment decreased the number of brain PVMs significantly in both WKY and SHRSP 2 weeks later, at 10 weeks of age (Fig. 2a). Plasma IL-1β levels were significantly higher in the SHRSP-vehicle group than in the WKY-vehicle group at 10 weeks of age (Fig. 2b). Clodronate treatment did not affect plasma IL-1β levels in either WKY or SHRSP. Interestingly, clodronate liposomes attenuated the elevation of mean blood pressure, as measured by the telemetry system, in SHRSP but not in WKY (Fig. 2c). The increase in mean blood pressure from 8 to 10 weeks of age was significantly smaller in the SHRSP-CLOD group than in the SHRSP-vehicle group (Fig. 2d). Systolic blood pressure measured by telemetry at 8 weeks of age in Protocol 2 (WKY-vehicle, 122 ± 1.8 mmHg, n = 5; SHRSP-vehicle, 156 ± 2.4 mmHg, n = 7) correlated well with that measured by the tail-cuff method at 8 weeks of age in Protocol 1. These telemetry data supported the tail-cuff measurements in Protocol 1. In another cohort of SHRSP that received an intracerebroventricular injection of clodronate liposomes or vehicle, the number and percentage of blood monocytes 2 weeks after injection in clodronate-treated SHRSP did not differ significantly compared with that in vehicle-treated SHRSP (vehicle, 62.9 ± 14.9 /mL, 2.50 ± 0.34%, n = 5; CLOD, 50.2 ± 6.3 /mL, 2.58 ± 0.27%, n = 6).
The effects of intracerebroventricular injection of clodronate on the brain PVM expression, plasma IL-1β levels, and blood pressure in WKY and SHRSP. a The number of brain PVMs evaluated by immunostaining. Clodronate significantly decreased brain PVMs in both WKY and SHRSP. b Plasma IL-1β levels. c Time-course of mean blood pressure after clodronate or vehicle administration. d Δmean blood pressure from before to 2 weeks after clodronate or vehicle administration. WKY-vehicle, n = 5; WKY-CLOD, n = 5; SHRSP-vehicle, n = 7; SHRSP-CLOD, n = 7. *p < 0.05 as indicated; †p < 0.05, SHRSP-vehicle vs. WKY-vehicle; #p < 0.05, SHRSP-CLOD vs. SHRSP-vehicle. CLOD clodronate
Effects of brain PVM elimination on sympathetic activity
A power spectral analysis of heart rate variability revealed that LFnu and the LF/HF ratio, indexes of sympathetic activity, were increased, whereas HFnu, an index of parasympathetic activity, was decreased in the SHRSP-vehicle group compared with the WKY-vehicle group (Fig. 3a). Clodronate treatment decreased LFnu and the LF/HF ratio and increased HFnu in SHRSP but not in WKY. The hexamethonium-induced decrease in the mean blood pressure, which is an index of the contribution of sympathetic activity to the maintenance of blood pressure, was significantly greater in the SHRSP-vehicle group than in the WKY-vehicle group, and it was attenuated by clodronate treatment in SHRSP but not in WKY (Fig. 3b). Renal norepinephrine levels, another index of sympathetic activity, were significantly higher in the SHRSP-vehicle group than in the WKY-vehicle group (Fig. 3c) and tended to be lower in the SHRSP-CLOD group than in the SHRSP-vehicle group; however, this trend did not reach statistical significance. Based on these data, sympathetic activity was increased in SHRSP compared with WKY, and this increase was ameliorated by brain PVM elimination induced by the intracerebroventricular injection of clodronate liposomes.
The effects of clodronate treatment on the indexes of the sympathetic activity in WKY and SHRSP. a Power spectral analysis of heart rate variability examined in the frequency domain. b Decreases in mean blood pressure by intravenous injection of hexamethonium. c Renal norepinephrine levels. WKY-vehicle, n = 5; WKY-CLOD, n = 5; SHRSP-vehicle, n = 7; SHRSP-CLOD, n = 7. *p < 0.05 as indicated. LFnu low-frequency components normalized units, HFnu high-frequency components normalized units, LF/HF the ratio of LF/HF
Effects of brain PVM elimination on neuronal activity in the nuclei regulating sympathetic activity
The PVN in the hypothalamus and the RVLM in the brainstem are the major nuclei that regulate sympathetic activity, and neural activation in these nuclei can increase sympathetic activity. The expression of c-Fos, a marker of neuronal activity, was significantly increased in the PVN in the SHRSP-vehicle group compared with the WKY-vehicle group. This c-Fos expression was decreased significantly by clodronate treatment in SHRSP, but not in WKY (Fig. 4a, c). Similar to what was observed in the PVN, c-Fos expression in the RVLM was significantly increased in the SHRSP-vehicle group compared with the WKY-vehicle group, and it was decreased by clodronate treatment in SHRSP, but not in WKY (Fig. 4b, d).
The effects of clodronate treatment on neuronal activity in nuclei regulating the sympathetic activity in WKY and SHRSP. a, b Representative pictures of immunostaining for c-Fos, a marker of neuronal activation, labeled in green color in PVN and RVLM. Scale bar: 200 μm. c, d The number of c-Fos positive neurons in PVN and RVLM. WKY-vehicle, n = 5; WKY-CLOD, n = 5; SHRSP-vehicle, n = 7; SHRSP-CLOD, n = 7. *p < 0.05 as indicated. PVN paraventricular nucleus, 3 V third ventricle, RVLM rostral ventrolateral medulla, Amb ambiguus nucleus, IO inferior olive
Effects of brain PVM elimination on PGE2 and COX-2 expression
Brain PVMs produce PGE2, and brain PGE2 can lead to sympathoexcitation and blood pressure elevation. Therefore, we evaluated the expression of PGE2 and its upstream biosynthetic enzyme COX-2 in brain PVMs by immunostaining. Double immunostaining for PVMs (CD163) and PGE2 showed that the number of brain PVMs was increased significantly in the SHRSP-vehicle group compared with the WKY-vehicle group but was decreased in the SHRSP-CLOD group compared with the SHRSP-vehicle group (Fig. 5a, b). In parallel with the changes in the number of PVMs, the colocalization of PGE2 with brain PVM immunostaining was also increased in the SHRSP-vehicle group compared with the WKY-vehicle group, and it was decreased in the SHRSP-CLOD group compared with the SHRSP-vehicle group (Fig. 5c). PGE2 that was not colocalized with PVM immunostaining did not differ among the four groups (Fig. 5d). Consistent with the changes in PGE2 expression, double immunostaining for CD163-positive PVMs and COX-2 showed that the number of CD163-positive COX-2 cells was increased in the SHRSP-vehicle group compared with the WKY-vehicle group, whereas they were decreased after clodronate treatment in the SHRSP group (Fig. 5e–h). These results indicate that the brain PVM-derived expression of PGE2 and its biosynthetic enzyme COX-2 was increased in SHRSP compared with WKY; clodronate treatment reduced these expression levels in SHRSP.
The effects of clodronate treatment on the expression of PVMs, PGE2, and COX-2 in the brain in WKY and SHRSP. a Representative pictures of double immunostaining for PVMs (red, CD163) and PGE2 (green). Scale bar: 20 μm. b–d The number of CD163+ PVMs, CD163+/PGE2+ cells, and CD163-/PGE2+ cells evaluated by immunostaining. e Representative pictures of double immunostaining for PVMs (red, CD163) and COX-2 (green). Scale bar: 20 μm. f–h The number of CD163+ PVMs, CD163+/COX-2+ cells, and CD163-/COX-2+ cells evaluated by immunostaining. Note that the number of CD163+ PVMs in Fig. 5b is the same as Fig. 2a because the same brain sections were used for immunostaining for PGE2 antibody in addition to CD163 as double immunostaining. Arrows indicate double-positive cells (a, e). WKY-vehicle, n = 5; WKY-CLOD, n = 5; SHRSP-vehicle, n = 7; SHRSP-CLOD, n = 7. *p < 0.05 as indicated
Effects of brain PVM elimination on the responses of blood pressure and sympathetic nerve activity to an acute intravenous injection of IL-1β in WKY
Protocol 3 aimed to determine whether the increased circulating levels of IL-1β during the developing phase of hypertension in SHRSP contribute to blood pressure elevation with sympathoexcitation through brain PVMs. Therefore, we evaluated the responses of blood pressure and sympathetic nerve activity to an acute intravenous injection of IL-1β in WKY treated with an intracerebroventricular injection of clodronate liposomes or vehicle. The intravenous injection of IL-1β induced significant and persistent increases in blood pressure, heart rate, and RSNA. These responses began ~10 min after IL-1β administration. The peak responses occurred 20–60 min after IL-1β injection and remained at levels higher than baseline during the observation time, which was 2 h after the injection. These responses to IL-1β administration were significantly decreased in clodronate-treated WKY compared with vehicle-treated WKY (Fig. 6a, b). The number of PVMs was significantly decreased in clodronate-treated WKY compared with vehicle-treated WKY (Fig. 6c).
The effects of clodronate treatment on the responses of blood pressure, heart rate, and sympathetic nerve activity to acute intravenous injection of IL-1β. a Representative recordings of blood pressure, heart rate, and integrated renal sympathetic nerve activity (RSNA) in WKY intracerebroventricularly administered with vehicle (left panel) and clodronate (right panel). b The peak responses of mean blood pressure, heart rate, and RSNA to the IL-1β injection. c The number of brain PVMs evaluated by immunostaining in rats after the experiment with IL-1β injection. WKY-vehicle, n = 5; WKY-CLOD, n = 5. *p < 0.05 vs. WKY-vehicle
Discussion
The major findings of this study are as follows: (1) the number of brain PVMs and the levels of circulating IL-1β, which can activate brain PVMs, were increased in the developing phase of hypertension in SHRSP; (2) the elimination of increased brain PVMs by the intracerebroventricular injection of clodronate liposomes attenuated blood pressure elevation in SHRSP, and clodronate-treated SHRSP had a lower level of sympathetic activity with decreased neuronal activity in the sympathetic regulatory nuclei, namely, the PVN and RVLM, compared with that in vehicle-treated SHRSP; (3) the expression of PGE2, which can induce neuronal activation in the PVN and RVLM, was present in brain PVMs and was decreased by clodronate treatment in SHRSP; and (4) clodronate treatment in WKY attenuated the elevation in blood pressure and sympathetic nerve activity in response to an acute intravenous injection of IL-1β. These findings demonstrate that brain PVMs contribute to the development of hypertension via sympathetic activation. Brain PVMs may be activated, at least in part, by increased circulating IL-1β.
This is the first study to demonstrate that brain PVM expression is increased in associated with blood pressure elevation during the developing phase of hypertension in SHRSP, an animal model of essential hypertension. The increase in the number of brain PVMs in SHRSP at 12 weeks of age, which represents the established phase of hypertension [24], was consistent with a previous study in 15- to 20-week-old SHRSP [30, 31]. In the present study, 4-week-old SHRSP did not exhibit an increase in blood pressure or the number of brain PVMs. However, 8-week-old SHRSP exhibited hypertension with an increased number of PVMs, and 12-week-old SHRSP exhibited further increases in blood pressure and the number of PVMs. Therefore, we eliminated brain PVMs with an intracerebroventricular injection of clodronate liposomes at 8 weeks of age to investigate the role of brain PVMs in blood pressure elevation. Clodronate treatment decreased the number of brain PVMs as previously reported [16, 23] and significantly attenuated sympathoexcitation and blood pressure elevation in SHRSP. This sympathoinhibitory effect of brain PVM elimination in SHRSP was consistent with the results of a previous study that used postmyocardial infarction rats with sympathoexcitation [16]. Although the systemic administration of clodronate liposomes can also affect peripheral macrophages and monocytes [32, 33], in the present study, to eliminate brain PVMs specifically, we intracerebroventricularly injected clodronate liposomes at ~1/8 of the intravenous dose used for the systemic elimination of macrophages according to the data sheet obtained from the supplier. We confirmed that the intracerebroventricular injection of this small amount of clodronate liposomes eliminated only brain PVMs without affecting monocytes in the blood, as previously reported [23]. Therefore, we conclude that the peripheral action of clodronate liposomes can be excluded; thus, brain PVMs contribute to the development of hypertension via sympathetic activation.
The PVN and RVLM contain presympathetic neurons, and the activation of these nuclei causes sympathoexcitation and blood pressure elevation [17,18,19]. In the present study, neuronal activity evaluated by c-Fos expression in the PVN and RVLM was increased in SHRSP compared with WKY and was decreased by the intracerebroventricular injection of clodronate liposomes in SHRSP, which supports the finding that clodronate treatment attenuates the increase in sympathetic activity in SHRSP. We focused on PGE2 and its upstream biosynthetic enzyme COX-2 as potential mechanisms underlying the clodronate liposome-induced decreases in neuronal activity in the PVN and RVLM in SHRSP. In brain PVMs and endothelial cells, COX-2, which can be induced by blood-borne proinflammatory cytokines such as IL-1β, catalyzes the synthesis of PGE2, and PGE2 can enter the brain to activate the neurohumoral system [14,15,16]. There are four subtypes of PGE2 receptors (i.e., EP1, EP2, EP3, and EP4), and both brain PGE2-induced sympathoexcitation and blood pressure elevation are mediated by the brain PGE2 EP3 receptor and not by other subtypes of the PGE2 receptor [20, 34]. In particular, EP3 receptors in the PVN and RVLM have been shown to be critically involved in sympathoexcitation and the pressor response [20, 21].
In the present study, PGE2 and COX-2 were expressed in brain PVMs, and the expression levels of PGE2 and COX-2 in PVMs were increased in SHRSP rats compared with WKY but, in parallel with PVM expression, were decreased by clodronate treatment in SHRSP. Consistent with our results, previous studies have demonstrated that most COX-2-expressing cells are localized to brain PVMs, not to endothelial cells [14, 15]. PGE2 inhibits inhibitory GABAergic neurotransmission in the PVN [35, 36] and increases reactive oxygen species levels in the RVLM and glutamate levels in PC12 neuronal cells [21], which can cause the activation of PVN and RVLM neurons. Taken together, our findings suggest that increased PGE2 derived from COX-2 in brain PVMs activates PVN and RVLM neurons, likely via PGE2 EP3 receptors, which results in sympathoexcitation and blood pressure elevation. Because this COX-2/PGE2 hypothesis of the effect of PVMs was not directly tested in the present study, it would be interesting to investigate whether intracerebroventricular pretreatment with a COX-2 inhibitor or a PGE2 receptor blocker has the same effect as those of clodronate liposomes on sympathetic activity and blood pressure in SHRSP.
Circulating IL-1β levels were increased in SHRSP compared with WKY, whereas the levels of other proinflammatory cytokines, such as TNF-α and IL-6, were extremely low or undetectable, consistent with a previous report [37]. Therefore, we investigated whether increased circulating IL-1β levels might contribute to sympathoexcitation and blood pressure elevation through brain PVMs (Protocol 3). The intravenous injection of IL-1β increased blood pressure and RSNA in WKY, which is consistent with previous studies [10,11,12]. Furthermore, we demonstrated for the first time that the IL-1β-induced sympathoexcitation and pressor response are attenuated significantly by brain PVM elimination. These results indicate that increased circulating IL-1β may be, at least in part, responsible for sympathoexcitation and blood pressure elevation via brain PVMs. This concept is supported by previous findings that IL-1β intravenous administration results in COX-2 induction in brain PVMs and neuronal activation in the PVN and RVLM [11, 14, 38].
The contribution of increased circulating IL-1β and brain PVMs to the increase in sympathetic activity and blood pressure deserves further discussion. In Protocol 1, 4-week-old SHRSP with a similar number of PVMs as WKY exhibited blood pressure in the normal range despite the significant increase in circulating IL-1β levels, whereas the acute intravenous injection of IL-1β increased sympathetic activity and blood pressure through the normal number of PVMs in WKY in Protocol 3. Therefore, the response of PVMs to acute and chronic increases in circulating IL-1β levels may be different. A previous study demonstrated that, after myocardial infarction, rats exhibit sympathetic excitation associated with an increase in circulating proinflammatory cytokines and cerebrospinal fluid PGE2 without a change in the number of brain PVMs, and the increased sympathetic activity and cerebrospinal fluid PGE2 levels are attenuated in myocardial infarction rats treated with an intracerebroventricular injection of clodronate liposomes [16]. Their findings suggest that the exposure of existing PVMs to the higher circulating levels of proinflammatory cytokines following myocardial infarction can easily account for the increased PGE2 production and sympathetic activity. This response might be akin to the acute response to IL-1β in WKY with a normal number of PVMs, which can be explained by an acute increase in circulating proinflammatory cytokines after coronary ligation in myocardial infarction rats [39]. Although our data indicate that an increased number of PVMs contributes to PGE2-mediated sympathetic activation in SHRSP, this sympathoexcitatory pathway through PVMs in other models does not always require an increase in the number of PVMs.
Independent of brain PVMs, there are other central mechanisms by which circulating IL-1β might increase sympathetic nerve activity and blood pressure. Blood-borne IL-1β also acts on the subfornical organ, one of the circumventricular organs that lack BBB and brain PVMs, to increase neuroexcitatory substances such as the renin-angiotensin components angiotensin-converting enzyme and angiotensin II type 1a receptor and the inflammatory element COX-2 in the PVN [11, 12]. These changes in neuroexcitatory substances and the sympathetic response to IL-1β can be significantly reduced by subfornical organ lesions [10, 11]. Furthermore, pericytes and astrocytes in the BBB can also be affected by systemic inflammation, which leads to neuronal activation [40, 41]. These mechanisms may act in concert with the PVM COX-2/PGE2 mechanism to contribute to the sympathetic/hemodynamic response to increased blood-borne IL-1β.
The present study has some limitations. First, we did not address the mechanisms responsible for the increase in the number of brain PVMs and their origin in SHRSP. Early studies have suggested that brain PVMs are derived from the bone marrow and are continuously replaced by blood monocytes [42, 43]. However, controversially, a recent study demonstrated that brain PVMs originate from yolk sac-derived progenitors that are seeded in the brain during early embryogenesis [44]. It has also been demonstrated that negative regulator of reactive oxygen species (NRROS), a transmembrane protein in the endoplasmic reticulum, can control brain-resident macrophage development [45]; NRROS-mutant mice express higher levels of CD45 and CD206, which are characteristically expressed by PVMs, in the brain. As NRROS negatively regulate reactive oxygen species via facilitating NADPH oxidase 2 (NOX2) degradation [46], the dysfunction of NRROS may be responsible for an increase in NOX2 expression. Previous studies have shown that NOX2 expression is increased in several tissues including the PVN, aorta, and renal arteries in adult (≥ 14 weeks old) spontaneously hypertensive rats (SHR) compared with age-matched WKY [47,48,49] and that superoxide levels in the aorta, which are similar in 6-week-old WKY and SHR, increase in an age-dependent manner and are significantly higher in SHR than in age-matched WKY at 9 and 12 weeks [50]. Taken together, we speculate that genetic or epigenetic mutations (or defects) in NRROS may be present in SHRSP, which may cause an increase in the number of brain PVMs in SHRSP. It may be possible that the increase in cerebrovascular pressure during the developing phase of hypertension in SHRSP, which does not occur in postmyocardial infarction rats, induces epigenetic changes in NRROS. Second, there is a possibility that some of the CD163-positive cells might not be brain PVMs. Although CD163 is widely used as a marker for brain PVMs in rats [14,15,16, 51], activated microglia may also express CD163 [52, 53]. In particular, CD163-positive cells that were not juxtaposed to a brain vessel may been be activated microglia. We therefore showed the number of CD163-positive cells juxtaposed to a vessel relative to the vessel length rather than the number of CD163-positive cells relative to the field area to demonstrate the quantitative changes in PVMs. Third, the mechanisms that increase plasma IL-1β levels are undetermined even though the major cellular sources of circulating IL-1β are peripheral monocytes and macrophages [54, 55]. Finally, we did not investigate the effects of brain PVM elimination on blood pressure in the established phase of hypertension in SHRSP. Previous studies have demonstrated that the expression of intercellular adhesion molecule-1 (ICAM-1), an increase in which may be associated with BBB dysfunction in the brain, is similar in SHRSP and WKY at 6 to 16 weeks of age, whereas it is increased in SHRSP compared with WKY at 18 to 23 weeks of age [56, 57]. These previous reports suggest that young SHRSP (8 to 10 weeks old), which we treated with clodronate liposomes in the present study, may have preserved BBB function, whereas adult older SHRSP may have an impaired BBB. Therefore, in older SHRSP with established hypertension, circulating inflammatory cytokines may affect the brain parenchyma due to BBB leakage, and PVM elimination by clodronate treatment may not result in significant decreases in blood pressure. This hypothesis is supported by the finding that increased blood pressure was not affected by the intracerebroventricular injection of clodronate liposomes in other hypertension models with impaired BBB, such as angiotensin II-infused mice and BPH/2 J mice [29, 58]. Future studies are necessary to clarify these issues.
In conclusion, brain PVMs contribute to the development of hypertension via sympathetic activation. Increased circulating IL-1β may cause brain PVM-induced sympathoexcitation and blood pressure elevation.
References
Solak Y, Afser B, Vasiri ND, Aslan G, Yalcin CE, Covic A, et al. Hypertension as an autoimmune and inflammatory disease. Hypertens Res. 2016;39:567–73.
Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med. 2011;17:1402–9.
Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, Demkow U. Brain inflammation and hypertension: the chicken or the egg? J Neuroinflammation. 2015;12:85.
Marvar PJ, Lob H, Vinh A, Zarreen F, Harrison DG. The central nervous system and inflammation in hypertension. Curr Opin Pharm. 2011;11:156–61.
Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin-1β in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. 1997;129:300–8.
Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–54.
Lataro RM, Silva CAA, Tefé-Silva C, Prado CM, Salgado HC. Acetylcholinesterase Inhibition attenuates the development of hypertension and inflammation in spontaneously hypertensive rats. Am J Hypertens. 2015;28:1201–8.
Li HB, Qin DN, Cheng K, Su Q, Miao YW, Guo J, et al. Central blockade of salusin β attenuates hypertension and hypothalamic inflammation in spontaneously hypertensive rats. Sci Rep. 2015;5:11162.
Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens. 2016;10:457–66.
Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62:118–25.
Wei SG, Yu Y, Felder RB. Blood-borne interleukin-1β acts on the subfornical organ to upregulate the sympathoexcitatory milieu of the hypothalamic paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2018;314:R447–R458.
Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65:1126–33.
Faraco G, Park L, Anrather J, Iadecola C. Brain perivascular macrophages: characterization and functional roles in health and disease. J Mol Med (Berl). 2017;95:1143–52.
Serrats J, Schiltz JC, Garcia-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106.
Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22:5606–18.
Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–9.
Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–56.
Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–57.
Hirooka Y, Kishi T, Ito K, Sunagawa K. Potential clinical application of recently discovered brain mechanisms involved in hypertension. Hypertension. 2013;62:995–1002.
Zhang ZH, Yu Y, Wei SG, Nakayama Y, Nakayama K, Felder RB. EP3 receptors mediate PGE2-induced hypothalamic paraventricular nucleus excitation and sympathetic activation. Am J Physiol Heart Circ Physiol. 2011;301:H1559–69.
Rezq S, Abdel-Rahman AA. Rostral ventrolateral medulla EP3 receptor mediates the sympathoexcitatory and pressor effects of prostaglandin E2 in conscious rats. J Pharm Exp Ther. 2016;359:290–9.
Lehenkari PP, Kellinsalmi M, Näpänkangas JP, Ylitalo KV, Mönkkönen J, Rojers MJ, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharm. 2002;61:1255–62.
Polfliet MM, Goede PH, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. A method for the selective depletion of perivascular and meningeal macrophages in the central nervous system. J Neuroimmunol. 2001;116:188–95.
Tanase H, Yamori Y, Hansen CT, Lovenberg W. Heart size in inbred strains of rats. Part 2. Cardiovascular DNA and RNA contents during the development of cardiac enlargement in rats. Hypertension. 1982;4:872–80.
Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension. 2007;50:360–7.
Li P, Gong JX, Sun W, Zhou B, Kong XQ. Hexamethonium attenuates sympathetic activity and blood pressure in spontaneously hypertensive rats. Mol Med Rep. 2015;12:7116–22.
Yamazato M, Ishida A, Yamazato Y, Nakamura T, Ohya Y. Intracerebroventricular administration of bone marrow-derived cells attenuates angiotensin II-initiated neurogenic hypertension in rats. Hypertens Res. 2018;41:828–38.
Nakano M, Hirooka Y, Matsukawa R, Ito K, Sunagawa K. Mineralocorticoid receptors/epithelial Na(+) channels in the choroid plexus are involved in hypertensive mechanisms in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2013;36:277–84.
Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126:4674–89.
Nakamura T, Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, et al. Pioglitazone exerts protective effects against stroke in stroke-prone spontaneously hypertensive rats, independently of blood pressure. Stroke. 2007;38:3016–22.
Liu Y, Jacobwitz DM, Barone F, McCarron R, Spatz M, Feuerstein G, et al. Quantitation of perivascular monocytes and macrophages around cerebral blood vessels of hypertensive and aged rats. J Cereb Blood Flow Metab. 1994;14:348–52.
Thang LV, Deml SL, Crawford R, Kaminski NE, Swain GM, van Rooijen N, et al. Macrophage depletion lowers blood pressure and restores sympathetic nerve α2-adrenergic receptor function in mesenteric arteries of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2015;309:H1186–1197.
Côté CH, Bouchard P, van Rooijen N, Marsolais D, Duchesne E. Monocyte depletion increases local proliferation of macrophage subsets after skeletal muscle injury. BMC Musculoskelet Disord. 2013;14:359.
Ariumi H, Takano Y, Masaumi A, Takahashi S, Hirabara Y, Honda K, et al. Roles of the central prostaglandin EP3 receptors in cardiovascular regulation in rats. Neurosci Lett. 2002;324:61–64.
Ferri CC, Ferguson AV. Prostaglandin E2 mediates cellular effects of interleukin-1β on parvocellular neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2005;17:498–508.
Khazaeipool Z, Wiederman M, Inoue W. Prostaglandin E2 depresses GABA release onto parvocellular neuroendocrine neurones in the paraventricular nucleus of the hypothalamus via presynaptic receptors. J Neuroendocrinol. 2018;30:e12638.
Chiba T, Itoh T, Tabuchi M, Nakazawa T, Satou T. Interleukin-1β accelerates the onset of stroke in stroke-prone spontaneously hypertensive rats. Mediators Inflamm. 2012;2012:1–11.
Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J Neurochem. 1998;70:452–66.
Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol. 2004;286:H2264–71.
Vasilache AM, Qian H, Blomqvist A. Immune challenge by intraperitoneal administration of lipopolysaccharide directs gene expression in distinct blood-brain barrier cells toward enhanced prostaglandin E2 signaling. Brain Behav Immun. 2015;48:31–41.
Sun J, Zheng JH, Zhao M, Lee S, Goldstein H. Increased in vivo activation of microglia and astrocytes in the brains of mice transgenic for an infectious R5 human immunodeficiency virus type 1 provirus and for CD4-specific expression of human cyclin T1 in response to stimulation by lipopolysaccharide. J Virol. 2008;82:5562–72.
Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–2.
Valliéres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23:5197–207.
Goldmann T, Weighofer P, Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17:797–805.
Wong K, Noubade R, Manzanillo P, Ota N, Foreman O, Hackney FriedmanBA, et al. Mice deficient in NRROS show abnormal microglial development and neurological disorders. Nat Immunol. 2017;18:633–41.
Noubade R, Wong K, Ota N, Rutz S, Eidenschenk C, Valdez PA, et al. NRROS negatively regulates reactive oxygen species during host defence and autoimmunity. Nature. 2014;509:235–9.
Song XA, Jia LL, Cui W, Zhang M, Chen W, Yuan ZY, et al. Inhibition of TNF-α in hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by inhibiting neurohormonal excitation in spontaneously hypertensive rats. Toxicol Appl Pharm. 2014;281:101–8.
Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, et al. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–7.
Dong J, Wong SL, Lau CW, Lee HK, Ng CF, Zhang L, et al. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur Heart J. 2012;33:2980–90.
Wu R, Millette E, Wu L, de Champlain J. Enhanced superoxide anion formation in vascular tissues from spontaneously hypertensive and desoxycorticosterone acetate-salt hypertensive rats. J Hypertens. 2001;19:741–8.
Galea I, Felton LM, Waters S, van Rooijen N, Perry VH, Newman TA. Immune-to-brain signalling: the role of cerebral CD163-positive macrophages. Neurosci Lett. 2008;448:41–46.
Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–97.
Borda JT, Alvarez X, Mohan M, Hasegawa A, Jean S, Aya P, et al. CD163, a marker of perivascular macrophages, is up-regulated by microglia in simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am J Pathol. 2008;172:725–37.
Kahlenberg JM, Dubyak GR. Differing caspase-1 activation states in monocyte versus macrophage models of IL-1β processing and release. J Leukoc Biol. 2004;76:676–84.
Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. IL-1β and IL-18: inflammatory markers or mediators of hypertension? Br J Pharm. 2014;171:5589–602.
Ishida H, Takemori K, Dote K, Ito H. Expression of glucose transporter-1 and aquaporin-4 in the cerebral cortex of stroke-prone spontaneously hypertensive rats in relation to the blood-brain barrier function. Am J Hypertens. 2006;19:33–39.
Takemori K, Murakami T, Kometani T, Ito H. Possible involvement of oxidative stress as a causative factor in blood-brain barrier dysfunction in stroke-prone spontaneously hypertensive rats. Microvasc Res. 2013;90:169–72.
Foulquier S, Namsolleck P, van Hagen BT, Milanova I, Post MJ, Blankesteijn WM, et al. Hypertension-induced cognitive impairment: insights from prolonged angiotensin II infusion in mice. Hypertens Res. 2018;41:817–27.
Acknowledgements
This work was supported by JSPS Grants-in-Aid for Scientific Research Grant numbers JP17K16012 and JP17K09508.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HT received honoraria from Daiichi Sankyo, Inc., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Boehringer Ingelheim Japan, Inc., Novartis Pharma K.K., Bayer Yakuhin, Ltd., Bristol-Myers Squibb K.K., and Astellas Pharma Inc. and research funding from Actelion Pharmaceuticals Japan, Daiichi Sankyo, Inc., and Astellas Pharma Inc. All other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Iyonaga, T., Shinohara, K., Mastuura, T. et al. Brain perivascular macrophages contribute to the development of hypertension in stroke-prone spontaneously hypertensive rats via sympathetic activation. Hypertens Res 43, 99–110 (2020). https://doi.org/10.1038/s41440-019-0333-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41440-019-0333-4
Keywords
This article is cited by
-
Establishment of a HFpEF model using female Dahl salt-sensitive rats: a valuable tool for elucidating the pathophysiology of HFpEF in women
Hypertension Research (2025)
-
All Three Supersystems—Nervous, Vascular, and Immune—Contribute to the Cortical Infarcts After Subarachnoid Hemorrhage
Translational Stroke Research (2025)
-
Esaxerenone: blood pressure reduction and cardiorenal protection without reflex sympathetic activation in salt-loaded stroke-prone spontaneously hypertensive rats
Hypertension Research (2024)
-
Neuroimmunology of Cardiovascular Disease
Current Hypertension Reports (2024)
-
Contribution of afferent renal nerve signals to acute and chronic blood pressure regulation in stroke-prone spontaneously hypertensive rats
Hypertension Research (2023)