Abstract
Some studies have shown that a higher ideal cardiovascular health score (CVHS) predicts a lower incidence of arterial stiffness. Few studies have used multiple measurements of CVHS to examine the impact of CVHS on arterial stiffness. The current study aimed to identify the long-term patterns in CVHS trajectory and to explore the association between CVHS trajectory and arterial stiffness. The study cohort consisted of 18,854 participants from the Kailuan Study who were followed up for five physical examinations over 8.10 years. Five discrete CVHS trajectories were identified among the participants: low-stable (8.10%), low-moderate (6.84%), moderate-low (23.46%), moderate-stable (39.83%), and elevated-stable (21.77%). After adjustment for confounding factors, generalized linear model analysis showed that CVHS trajectory group correlated negatively with brachial–ankle pulse wave velocity (baPWV). Compared with the low-stable group, the low-moderate group, moderate-low group, moderate-stable group, and elevated-stable group had B values of −41.81, −24.11, −86.79, and −169.54, respectively. We also used logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for CVHS trajectory groups and arterial stiffness. In fully adjusted models, ORs were 0.76 (95% CI: 0.62–0.94) for the low-moderate group, 0.80 (95% CI: 0.67–0.97) for the moderate-low group, 0.51 (95% CI: 0.42–0.62) for the moderate-stable group, and 0.23 (95% CI: 0.18–0.29) for the elevated-stable group compared with the low-stable group. The results were consistent across a number of sensitivity analyses. In conclusion, the higher long-term attainment and the improvement of CVHS were negatively associated with baPWV and could reduce the risk of arterial stiffness. Our study emphasizes the importance of optimizing CVH throughout life to prevent the incidence of arterial stiffness.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVD) remain a leading cause of death worldwide. A systematic analysis of the Global Burden of Disease Study 2016 showed that the largest number of deaths at level 2 (17.6 million) among all noncommunicable diseases was caused by CVD [1]. Modifiable factors such as smoking, high salt intake, deficient physical activity, abnormal blood lipids, abdominal obesity, and a history of hypertension or diabetes were associated with the incidence of CVD [2, 3]. To target the prevention of CVD, the American Heart Association (AHA) has proposed the ideal cardiovascular health (CVH), which was defined as the simultaneous presence of four ideal health behaviors (nonsmoking, body mass index <25 kg/m2, physical activity at goal levels, and a healthy diet consistent with current guideline recommendations) and three ideal health factors (untreated total cholesterol (TC) <200 mg/dL, untreated blood pressure (BP) <120/80 mmHg, and fasting blood glucose (FBG) <100 mg/dL) [4]. To capture individual-level changes in CVH, Huffman et al. [5] created a composite, individual-level Cardiovascular Health Score (CVHS) based on seven metrics of ideal CVH. Previous studies have revealed that CVHS was inversely associated with the incidence of CVD [6, 7]. Considering the concept of CVH, AHA highlighted the importance of “primordial prevention”, which focused on the prevention of risk factors that may result in the development of subclinical atherosclerosis and other myocardial and vascular changes over the course of years to decades [4]. Therefore, it makes sense to prevent clinical events by avoiding the adverse levels of risk factors.
Arterial stiffness, which directly damaged the cardiovascular system by lowering arterial elasticity, was the cardiovascular organic subclinical damage that was increasingly taken as a surrogate for monitoring CVD [8,9,10]. The pathological progression of arterial stiffness was a slow and dynamic process taking years to decades [9]. Arterial stiffness was related to factors such as aging, hypertension, hyperlipidemia, diabetes mellitus, obesity, smoking, physical activity, and dietary habits [11,12,13,14,15,16].
Studies have reported that ideal CVH could prevent the progression of arterial stiffness [17,18,19]. However, with aging and lifestyle modification, the ability of individuals to attain ideal CVH metrics may change. Few studies have focused on the impact of the deviation from ideal CVH on the development of arterial stiffness, especially in Asian populations. The trajectory model, which groups a given exposure according to homogeneity, was built based on multiple repeated-measurement values [20, 21] and was able to assess long-term CVH changes. Based on the data from the Kailuan Study (registration number: ChiCTR-TNC-11001489), we explored the association between ideal CVHS trajectory and arterial stiffness.
The objectives of the current study were (1) to identify subgroups of participants with similar trajectories in CVHS during the long-term follow-up; (2) to describe and analyze the characteristics of different CVHS trajectory groups; and (3) to investigate the effect of different ideal CVHS trajectories on the presence of arterial stiffness. We hypothesized that distinct ideal CVHS trajectory patterns would exist among the participants and that the higher CVHS trajectories would be associated with a lower probability of arterial stiffness compared with no improvement or decline in CVHS attainment.
Materials and methods
Participants
The Kailuan Study is an ongoing prospective cohort study (trial registration #: ChiCTR-TNC-11001489) [22] that started in 2006. Conducted in the Kailuan community of Tangshan city, a large, littoral, modern city in the southeast of Beijing, China, the Kailuan Study aims to investigate risk factors associated with common chronic diseases, such as myocardial infarction, stroke, and cancers. In brief, between June 2006 and October 2007 (visit 1), a total of 101,510 adult participants (81,110 males and 20,400 females, aged 18–98 years old) were recruited from 11 affiliated hospitals of this company. Participants received an administered questionnaire and underwent physical examinations and laboratory tests upon enrollment. During 2008–2009 (visit 2), 25,337 adult participants who were not part of visit 1 completed the above survey and were recruited into the Kailuan Study. Then 10,519 adult participants who were not part of visit 1 or visit 2 were successfully recruited into the Kailuan Study during 2010–2011 (visit 3). All of the participants were biennially followed with the same strategy to update the state of their health, i.e., in the years 2012 and 2013 (visit 4) and in the years 2014 and 2015 (visit 5).
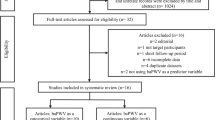
Of the above 137,366 participants, 97,462 participants who had taken part in at least three Kailuan physical examinations were included in our study. Among them, 30,364 participants underwent brachial–ankle pulse wave velocity (baPWV) detection between 2010 and 2018. We excluded 1131 participants with a diagnosis of myocardial infarction or stroke prior to baPWV detection. We also excluded 4,416 participants with any missing data on CVH for three or above time points. We further excluded 5,963 participants with data of third physical examinations at the time point after baPWV detection. The remaining 18,854 participants were included in the final analysis (Fig. 1). The study was performed according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of the Kailuan General Hospital.
Definitions of ideal CVH
Data on smoking status, physical activity and salt intake were collected using a standardized questionnaire. As information on their diets was not available, the amount of salt used during cooking was used as a surrogate marker to replace vegetables [23]. Height was measured to the nearest 0.1 cm using a tape measure, and weight was measured to the nearest 0.1 kg using calibrated platform scales. BMI was calculated as weight (kg) divided by the square of height (m2). Blood samples were collected from the antecubital vein after an overnight fast. FBG and TC were tested using a Hitachi 747 autoanalyzer (Hitachi; Tokyo, Japan) at the central laboratory of the Kailuan General Hospital. BP was measured as detailed elsewhere [24]. CVHS was calculated and classified as “ideal” = 2, “intermediate” = 1 and “poor” = 0 as described by Huffman [5] and detailed in Supplementary Table 1. All missing values were input to be intermediate status, except in the event in which status was known at both the visits immediately before and after the visit in question. If poor status was observed at both neighboring visits, then poor status was input for the missing visit. Similarly, if any ideal status was observed at both neighboring visits, then it was input for the missing visit [19].
Arterial stiffness measurement
BaPWV correlated significantly with carotid-femoral pulse wave velocity (cfPWV) [25], which was the gold standard measurement of arterial stiffness [26]. Because baPWV was simpler and more convenient to perform, it was more often applied in clinical practice than cfPWV in Asian countries [27, 28]. BaPWV and ankle–brachial index (ABI) were measured by a BP-203RPE III networked arteriosclerosis detection device (OMRON healthcare (China) Co., Ltd). The procedure was performed by specially trained nurses between 7 a.m. and 9 a.m. on the examination day, following the manufacturer’s recommendations as detailed elsewhere [29]. The temperature of the examination room was kept between 22 and 25 °C, and the study participants were told to refrain from smoking and drinking caffeinated or alcoholic beverages for at least 3 h and from aerobic exercise for at least 30 min before the baPWV measurement. Larger values of the left-and right-side baPWV were used in further analysis. BaPWV ≥1400 cm/s was considered arterial stiffness [30]. Smaller values of the left- and right-side ABI were used in further analysis.
Covariate assessment
Data on demographic variables such as age, sex, alcohol consumption, education level, the average monthly income of each family member, and medical history (myocardial infarction, stroke, and active treatment such as antihypertensive drugs, hypoglycemic drugs or lipid-lowering drugs) were collected using standardized questionnaires. Heart rate and other biochemical indices were assessed as detailed elsewhere [24].
Statistical analysis
CVHS trajectories were modeled among 18,854 Kailuan Study participants who had CVHS measured at three or more time points. Latent mixture models within the SAS Proc Traj were used to identify subgroups that shared a similar underlying trajectory of CVHS. Model fit was assessed using the Bayesian Information Criterion. Continuous variables were presented as mean ± standard deviation (SD) and were compared by ANOVAs. Categorical variables were described as percentages and were compared using chi-square tests (χ2 tests). Generalized linear model analysis was performed to evaluate the relationship between the trajectory of CVHS and baPWV after adjusting for age, sex, baseline CVHS, education level, the average monthly income of each family member, alcohol consumption, antihypertensive drugs, hypoglycemic drugs, lipid-lowering drugs, and follow-up time. In this model, we designed a dummy variable for the independent variable CVHS trajectory groups and used the low-stable CVHS trajectory group as the reference group. Then, we calculated beta-values for CVHS trajectories and baPWV by the maximum likelihood ratio. Multivariable logistic regression was used to investigate the association of CVHS trajectories with risk of arterial stiffness by calculating the crude odds ratio (OR) and adjusted OR, with 95% their confidence intervals (CIs), after adjusting for the aforementioned covariates.
To test the robustness of our findings, we performed several sensitivity analyses. First, we limited the analysis to participants with complete data on the seven metrics of ideal CVH at five or more physical examinations (n = 2,654). Second, we excluded participants who took antihypertensive drugs, hypoglycemic drugs, or lipid-lowering drugs respectively. Two-sided P-values of <0.05 were considered significant. Statistical analysis was performed using SAS, version 9.3 (SAS Inc., North Carolina).
Results
Baseline characteristics
The baseline characteristics of the participants are shown in Table 1. During a mean (±SD) follow-up of 8.10 (±2.29) years, five discrete trajectories were identified among the participants (Fig. 2): low-stable (8.10%), low-moderate (6.84%), moderate-low (23.46%), moderate-stable (39.83%), and elevated-stable (21.77%). The participants in the low-stable group were the oldest, least educated, and more likely to have other traditional CVD risk factors. It had the highest percentage of men and the lowest average monthly income of each family member. With increasing CVHS, the above factors progressed in the other direction.
Trajectory of ideal cardiovascular health score of the participants. The red line indicates low-stable group; the green line indicates low-moderate group; the blue line indicates moderate-low group; the black line indicates moderate-stable group; the yellow line indicates elevated-stable group. The x-axis refers to the number of physical examinations of the participants. The y-axis refers to the CVHS of the participants
Negative relationship between CVHS trajectory and baPWV
The CVHS trajectory negatively correlated with baPWV after adjustment for the confounding factors (Table 2). Compared with the low-stable group, the low-moderate, moderate-low, moderate-stable, and elevated-stable groups had B values of −41.81, −24.11, −86.79, and −169.54, respectively, adjusting for age, sex, baseline CVHS, education level, average monthly income of each family member, alcohol consumption, antihypertensive drugs, hypoglycemic drugs, lipid-lowering drugs, and follow-up time.
Association of CVHS trajectory with the risk of arterial stiffness
CVHS trajectory was significantly associated with arterial stiffness (Table 3). The prevalence of arterial stiffness decreased as the CVHS trajectory improved, ranging from the low-stable group to the elevated-stable group. After adjusting for potential confounding factors, ORs (95% CIs) were 0.76 (0.62–0.94) for the low-moderate group, 0.80 (0.67–0.97) for the moderate-low group, 0.51 (0.42–0.62) for the moderate-stable group, and 0.23 (0.18–0.29) for the elevated-stable group compared with the low-stable group (P for trend <0.001).
Sensitivity analysis
To eliminate the influence of the missing values processing method on the results of our study, we performed a sensitivity analysis limiting the participants with complete data of the seven metrics in the five visits. The results were consistent with our main findings (Table 4). We also observed that there were no changes in the results of our study after excluding the participants with antihypertensive drugs, hypoglycemic drugs or lipid-lowering drugs (supplementary Fig. 1 and supplementary Table 2).
Discussion
Of the 18,854 Kailuan participants with CVHS evaluated serially in five visits, we found heterogeneous trajectories. Five distinct CVHS trajectories were identified and were significantly associated with the presence of arterial stiffness. CVHS trajectory was negatively associated with baPWV after adjusting for confounding factors. Participants who exhibited elevated-stable CVHS attainment showed the lowest odds of arterial stiffness incidence. Significantly, an increase in CVHS attainment was associated with a lower probability of arterial stiffness compared with no improvement or a decline in CVHS attainment.
Since ideal CVH was defined by the AHA in 2010, a cross-sectional study using a single measurement of CVHS found that CVHS was negatively related to PWV [31]. Although previous studies [17, 19] have examined the negative association between CVHS change and PWV, they only measured CVHS twice at a long time interval, which may be insufficient to assess the effect of CVHS attainment on PWV accurately. In addition, Zheng et al. [29] calculated cumulative CVHS over three visits and identified a negative linear relationship with baPWV. However, none of the above studies took into account the effects of long-term variation tendency in CVHS on PWV. However, we have established five CVHS trajectories that provide a more reliable understanding of long-term development trends in CVHS, which overcame the limitations of previous studies.
We found that CVHS trajectory was negatively associated with baPWV. The BaPWV of the elevated-stable trajectory, in which the CVHS attainment maintained scores of 11.00, was 169.54 cm/s lower than that of the low-stable trajectory. Interestingly, although CVHS attainment of the low-moderate trajectory was lower than that of the moderate-low trajectory initially, baPWV of the low-moderate trajectory increased slower than in the moderate-low trajectory, with the improvement of CVHS attainment in the low-moderate trajectory. Our findings suggest that we should highlight the influence of long-term health lifestyle changes on baPWV, which is an independent predictor of the risk of developing CVD and all-cause mortality [32, 33].
Consistent with previous studies, our study showed that higher CVHS attainment was associated with a lower prevalence of arterial stiffness, a marker of subclinical cardiovascular change. In a cross-sectional study, Na Yan et al. found that the risk of arterial stiffness was reduced by 27% with each 1-point increase in CVHS [34]. The EVIDENT study, which included 1,365 adult participants, also observed that higher CVH metrics were associated with lower arterial stiffness in middle-aged and elderly subjects of both sexes [35]. A study in the Kailuan cohort demonstrated that participants with higher cumulative CVHS had a lower prevalence of arterial stiffness [29]. Our study extends these findings and provides a unique perspective to explore the association between heterogeneous timing changes in CVHS attainment and arterial stiffness. Of the five trajectories, the lowest incidence of arterial stiffness was identified in the elevated-stable trajectory. In addition, after adjusting for confounding factors, the risk of arterial stiffness in the low-moderate CVHS trajectory with improvement in CVHS attainment during follow-up was similar to that in the moderate-low CVHS trajectory, which had a higher CVHS attainment at baseline. The results were also consistent after excluding participants with antihypertensive drugs, hypoglycemic drugs or lipid-lowering drugs. The results of our current study emphasize the importance of optimizing CVH throughout life to prevent the incidence of arterial stiffness, which is increasingly recognized as a surrogate end point for CVD [8,9,10].
Some strengths of this study are worth detailing. The sample of our study was large, with 18,854 participants followed for ~8.10 years. A trajectory model was created to examine long-term patterns of CVHS and compare the heterogeneity among different trajectories. Therefore, we could accurately assess the association between CVH and arterial stiffness. Some limitations also need to be taken into consideration. First, a method of handling missing values was used in the major model in the current study. However, missing values of any of the seven metrics had no effect on the results of this study, as the results of the sensitivity analysis were in line with our overall findings. Second, the physical activity questionnaire was not strictly consistent with the AHA criteria. However, these data accurately recorded the amount of the participants’ daily exercise. Thus, the results were unlikely to be biased. Third, the amount of daily salt intake was used to replace a measure of a healthy diet. A previous study demonstrated that salt intake correlated with healthy dietary habits significantly [23]. The results showed that the healthy diet was the food with the least amount of salt intake. High salt intake was also an important risk factor for CVD and stroke [23]. Fourth, in our current study, baPWV ≥ 1,400 cm/s was considered arterial stiffness. However, there is a lack of Chinese epidemiological data supporting this number. Yamashina et al. found that a cutoff point of 1,400 cm/s of baPWV could serve as an indicator of either atherosclerotic cardiovascular risk or severity of atherosclerotic vascular damage [30]. Finally, it should be noted that the participants in this study were all from the Kailuan community. Therefore, the generalization of the current results to other populations may need to be confirmed.
Conclusions
Our study showed that long-term CVHS attainment was negatively associated with baPWV. Higher long-term CVHS attainment was associated with a lower probability of arterial stiffness. In addition, the improvement of CVHS attainment could reduce the risk of arterial stiffness. The current study warns us that we should focus on the influence of long-term healthy lifestyles and factors of arterial health to achieve the goal of primary prevention of CVD.
References
GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–210.
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. INTERHEART study investigators. effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study):case-control study. Lancet. 2004;364:937–52.
Khanji MY, van Waardhuizen CN, Bicalho VVS, Ferket BS, Hunink MGM, Petersen SE. Lifestyle advice and interventions for cardiovascular risk reduction: a systematic review of guidelines. Int J Cardiol. 2018;263:142–51.
Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L. American Heart Association Strategic Planning Task Force and Statistics Committee et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613.
Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988-2008) and projections to 2020:results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125:2595–602.
Wilsgaard T, Loehr LR, Mathiesen EB, Løchen ML, Bønaa KH, Njølstad l, et al. Cardiovascular health and the modifiable burden of incident myocardial infarction: the Tromsø Study. BMC Public Health. 2015;15:221.
Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–83.
Bonet J, Vicente A. Arterial stiffness, organic subclinical damage and cardiovascular risk factor. Med Clin. 2009;133:137–8.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. European network for non-invasive investigation of large arteries. expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
Veerasamy M, Ford GA, Neely D, Bagnall A, MacGowan G, Das R, et al. Association of aging, arterial stiffness, and cardiovascular disease: a review. Cardiol Rev. 2014;22:223–32.
Rosenberg AJ, Lane-Cordova AD, Wee SO, White DW, Hilgenkamp TIM, Fernhall B, et al. Healthy aging and carotid performance: strain measures and β-stiffness index. Hypertens Res. 2018;41:748–55.
Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension a systematic review. Hypertesion. 2009;54:1328–36.
Aatola H, Hutri-Kähönen N, Juonala M, Viikari JS, Hulkkonen J, Laitinen T, et al. Lifetime risk factors and arterial pulse wave velocity in adulthood: the cardiovascular risk in young Finns Study. Hypertension. 2010;55:806–11.
Im JA, Lee JW, Shim JY, Lee HR, Lee DC. Association between brachial-ankle pulse wave velocity and cardiovascular risk factors in healthy adolescents. J Pediatr. 2007;150:247–51.
Loehr LR, Meyer ML, Poon AK, Selvin E, Palta P, Tanaka H, et al. Prediabetes and diabetes are associated with arterial stiffness in older adults: The ARIC Study. Am J Hypertens. 2016;29:1038–45.
Recio-Rodríguez JI, Rodriguez-Sanchez E, Martin-Cantera C, Martinez-Vizcaino V, Arietaleanizbeaskoa MS, Gonzalez-Viejo N. EVIDENT Investigators group et al. Combined use of a healthy lifestyle smartphone application and usual primary care counseling to improve arterial stiffness, blood pressure and wave reflections: a randomized controlled trial (EVIDENT II Study). Hypertens Res. 2019;42:852–62.
Aatola H, Hutri-Kahonen N, Juonala M, Laitinen TT, Pahkala K, Mikkilä V, et al. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the cardiovascular risk in young finns study. J Am Heart Assoc. 2014;3:e000532.
Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, et al. Healthy lifestyle change and subclinical atherosclerosis in young adults: coronary artery risk development in young adults (cardia) study. Circulation. 2014;130:10–7.
Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, et al. Ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation. 2015;132:1979–89.
Niyonkuru C, Wagner AK, Ozawa H, Amin K, Goyal A, Fabio A. Group-based trajectory analysis applications for prognostic biomarker model development in severe TBI: a practical example. J Neurotramua. 2013;30:938–45.
Nagin DS. Analyzing developmental trajectories: a semiparametric, group based approach. Psychol Methods. 1999;4:139–57.
Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–6.
Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813.
Wu SL, Huang ZR, Yang XC, Zhou Y, Wang AX, Chen L, et al. Prevalence of ideal cardiovascular health and Its relationship with the 4-year cardiovascular events in a northern chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012;5:487–93.
Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hyoertens. 2009;27:2022–7.
Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–46.
Munakata M, Nunokawa T, Tayama J, Yoshinaga K, Toyota T. Brachial-ankle pulse wave velocity as a novel measure of arterial stiffness: present evidences and perspectives. Curr Hypertens Rev. 2005;1:223–34.
Kawai T, Ohishi M, Onishi M, Ito N, Takeya Y, Maekawa Y, et al. Cut-off value of brachial-ankle pulse wave velocity to predict cardiovascular disease in hypertensive patients: a cohort study. J Atheroscler Thromb. 2013;20:391–400.
Zheng X, Zhang R, Liu X, Zhao H, Liu H, Gao J, et al. Association between cumulative exposure to ideal cardiovascular health and arterial stiffness. Atherosclerosis. 2017;260:56–62.
Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hieayama Y, et al. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–22.
Crichton GE, Elias MF, Robbins MA. Cardiovascular health and arterial stiffness: the Maine-Syracuse Longitudinal Study. J Hum Hypertens. 2014;28:444–9.
Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y. Collaborative Group for J-BAVEL(Japan Brachial-Ankle Pulsewave Velocity Individual Participant Data Meta-Analysis of Prospective Studies) et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. 2017;69:1045–52.
Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27.
Yan N, Zhou Y, Wang Y, Wang A, Yang X, Russell A, et al. Association of ideal cardiovascular health and brachial-ankle pulse wave velocity: a cross-sectional study in Northern China. J Stroke Cerebrovasc Dis. 2016;25:41–8.
García-Hermoso A, Martínez-Vizcaíno V, Gomez-Marcos MÁ, Cavero-Redondo I, Recio-Rodriguez JI, García-Ortiz L. Ideal cardiovascular health and arterial stiffness is spanish adults-The EVIDENT Study. J Stroke Cerebrovasc Dis. 2018;27:1386–94.
Acknowledgements
We thank the staff and participants of the Kailuan study for their important contributions.
Funding
The work for this article was supported by the national natural science foundation of China (81873896) to YY.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, R., Xie, J., Yang, R. et al. Association between ideal cardiovascular health score trajectories and arterial stiffness: the Kailuan Study. Hypertens Res 43, 140–147 (2020). https://doi.org/10.1038/s41440-019-0341-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41440-019-0341-4
Keywords
This article is cited by
-
Association between the trajectory of ideal cardiovascular health metrics and incident chronic kidney disease among 27,635 older adults in northern China–a prospective cohort study
BMC Geriatrics (2024)
-
Temporal relationship between arterial stiffness and blood pressure variability and joint effect on cardiovascular disease
Hypertension Research (2024)
-
Exposure to organochlorine pesticides and polychlorinated biphenyls, adherence to an ideal cardiovascular health, and arterial stiffness among Chinese adults
Environmental Geochemistry and Health (2024)
-
Association of cardiovascular health score trajectory and risk of subsequent cardiovascular disease in non-diabetic population: a cohort study
BMC Public Health (2023)
-
Time spent in a better cardiovascular health and risk of cardiovascular diseases and mortality: a prospective cohort study
Journal of Translational Medicine (2023)