Abstract
A significant relationship has been established between central hemodynamics and renal microvascular damage. We hypothesized that the increase in the ankle-brachial index (ABI) with age is due to increased arterial stiffness and wave reflection and is thus associated with the pathogenesis of the renal small artery in patients with chronic kidney disease (CKD). We recruited 122 patients with CKD (stages 1–5) who underwent renal biopsy and ABI measurements between 2010 and 2013. Renal small artery intimal thickening (SA-IT) severity was assessed by semiquantitative grading. The median age was 47 years, with a range of 15–86 years (47% women). The median estimated glomerular filtration rate (eGFR) was 62 mL/min/1.73 m2. Compared with patients with the lowest 1–3 SA-IT index quartile, those with the highest quartile of the SA-IT index were older in age had higher mean arterial pressure, ABI, brachial-ankle pulse wave velocity, and lower eGFR. ABI was positively associated with SA-IT severity and inversely associated with eGFR. Multivariate logistic regression analyses showed that ABI was significantly associated with the highest quartile of the SA-IT index (odds ratio per SD increase in ABI, 1.83; 95% confidence interval, 1.08–3.26) and low eGFR (<60 mL/min/1.73 m2) (odds ratio per SD increase in ABI, 1.74; 95% confidence interval, 1.03–3.03). In conclusion, a high normal ABI was associated with severe renal small artery intimal thickening and low eGFR in patients with CKD.
Similar content being viewed by others
Introduction
The ankle-brachial index (ABI) represents the ratio of systolic blood pressure (SBP) at the ankle to that at the brachial artery. In general practice, ABI is measured exclusively to assess flow-limiting lower extremity peripheral arterial disease (PAD). A low ABI (≤0.90) has been used as a diagnostic test for PAD and as a marker of systemic atherosclerosis. An abnormally high ABI (>1.4) is related to medial calcification and is often associated with lower extremity PAD. A lower ABI (<1.0) and an abnormally high ABI are independent predictors of cardiovascular events and overall and cardiovascular-related mortality [1]. Based on data obtained from older individuals or high-risk patients, normal ABIs are defined as values ranging from 1.00 to 1.39; ABI decreases with age, most likely due to the increased prevalence and progression of lower extremity PAD. However, using a screened Japanese cohort, we reported that ABI was lowest in participants aged < 40 years, increased with age until 60 years, and decreased thereafter [2]. We, along with others, have shown that the ABI and ankle SBP are associated with increased aortic pulse wave velocity (PWV) and brachial-ankle PWV [3, 4]. Another study found that the ABI was positively associated with the augmentation index in patients aged ≤ 45 years without a history of coronary artery disease and stroke [5]. These results suggest that the ABI increases with age due to increased arterial stiffness and/or wave reflection but decreases when flow-limiting atherosclerotic arterial stenosis develops in the arteries of the lower limbs. In line with this hypothesis, a high normal ABI (1.20–1.39) was associated with target organ damage, such as proteinuria and cerebral small vessel disease, in the healthy Japanese population [4, 6].
The pulsatile aspects of central hemodynamics affect the small artery structure and function of vital organs. The kidneys are vulnerable to pulsatile pressure and flow because of a low impedance to maintain an extraordinary volume of blood flow [7]. A close relationship between arterial stiffening and wave reflection and small artery damage in the kidneys as manifested by albuminuria/proteinuria and a loss of kidney function has been reported [8]; however, less is known about the role of central hemodynamics on biopsy-proven renal small artery sclerosis. Herein, we examined the relationship between ABI and renal SA-IT in renal biopsy specimens.
Methods
Study participants
In this cross-sectional and observational study, we consecutively studied 167 patients with CKD who underwent renal biopsy because of renal function impairment or urinalysis abnormalities at University Hospital of the Ryukyus between June 1, 2010, and March 31, 2013. Patients with missing baPWV/ABI data (n = 43) and an ABI ≤ 0.9 (n = 2) were excluded. Finally, 122 patients were enrolled in the study. No patients had an ABI ≥ 1.4. The study protocol was approved by the ethics review board of the University of the Ryukyus. All study patients provided written informed consent.
Histological diagnosis based on renal biopsy specimens
Based on renal biopsy findings, the following diagnoses were made in 122 patients: immunoglobulin A nephropathy (n = 62), benign nephrosclerosis (n = 15), lupus nephritis (n = 8), membranous nephropathy (n = 7), diabetic nephropathy (n = 6), obesity-related nephropathy (n = 3), tubulointerstitial nephritis (n = 3), non-immunoglobulin A mesangial proliferative glomerulonephritis (n = 3), minimal change disease (n = 2), antineutrophil cytoplasmic antibody-related nephritis (n = 2), IgA vasculitis (n = 1), and other miscellaneous diseases (n = 10).
Semiquantitative assessment of renal small arteries
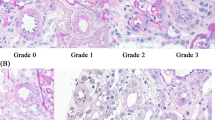
We evaluated the renal small arteries, including the large interlobular artery, with more than three layers of smooth muscle cells and a lumen diameter of 200–300 μm, and the arcuate arteries of the kidney as previously described [9]. The media-to-lumen ratio of the small arteries, which is often used as an index of small vessel disease [10,11,12], is difficult to use in renal biopsy specimens because it is not commonly available in the tangential view. Therefore, we examined the degree of fibrous intimal thickening compared with the thickness of the adjacent medial wall as previously reported [9, 13]. We assessed the degree of intimal thickening compared with the thickness of the adjacent medial wall and semiquantitatively graded intimal thickening in the small arteries of each specimen as follows: grade 0, absence of thickening; grade 1, intimal thickening of less than half the adjusted medial thickness; grade 2, intimal thickening of more than half and/or less than the adjusted medial thickness; and grade 3, intimal thickening of more than the adjusted medial thickness. Based on this grading, we calculated the mean grade of renal SA-IT for each patient according to the following formula: SA-IT index = (n0 × 0 + n1 × 1 + n2 × 2 + n3 × 3)/N (n = total number of small arteries in each specimen). Here, n1, n2, and n3 indicate the number of small arteries showing intimal thickening of grade 1, grade 2, and grade 3, respectively. We defined the highest quartile of the SA-IT index (SA-IT index > 2.0) as the severe SA-IT index. The representative microphotographs of each graded SA-IT are shown in Fig. 1. All histological analyses were performed by an experienced nephrologist (KK) who was blinded to patient information.
Ankle-brachial index and brachial-ankle pulse wave velocity measurement
The ABI and baPWV were measured on the day before renal biopsy. There were measurements using a validated automatic device (BP-203RPE, form PWV/ABI; Omron-Colin, Tokyo, Japan), which simultaneously measured pulse volume in the brachial and ankle arteries using the oscillometric method with bilateral arm and ankle BP. The ABI and baPWV were measured after the patients had rested in the supine position for at least 5 min. The ABI was defined as the ankle SBP/brachial SBP ratio, for which the higher value of the brachial SBP between the right and left arms was used. The ABI of the leg with the highest value was used.
Data collection
Hypertension was defined as an SBP ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg or the use of antihypertensive medication. Pulse pressure (PP) was calculated as SBP−DBP. Body mass index (BMI) was calculated as body weight/height squared (kg/m2). Fasting blood samples were used to measure the serum total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, triglyceride, uric acid, creatinine, hemoglobin A1c (HbA1c), and glucose levels. Urinary protein and creatinine levels were measured using first spot morning urine samples. Proteinuria was assessed by the urinary protein-to-creatinine ratio (with both protein and creatinine levels measured in grams). Diabetes mellitus (DM) was defined as a fasting plasma glucose level ≥ 7.0 mmol/L, HbA1c level ≥ 6.5%, and/or use of any medication for diabetes. Dyslipidemia was defined as a serum LDL-cholesterol level of at least 3.60 mmol/L, triglyceride level of at least 1.69 mmol/L, HDL-cholesterol level < 1.04 mmol/L, and/or the use of medications for dyslipidemia. The estimated glomerular filtration rate (eGFR) was calculated using the Japanese equation [14]. We used the Schwarz formula for eGFR in five patients aged <18 years [15]. A low eGFR was defined as an eGFR < 60 mL/min/1.73 m2. Smoking history was determined by an interview.
Statistical analysis
All values were expressed as medians (interquartile ranges) for continuous variables or percentages for categorical variables. We compared clinical characteristics using the Mann–-Whitney U test for continuous variables and the chi-squared test for categorical variables. Two-sided tests with significance levels of P values < 0.05 were used. To determine the optimal cutoff value of ABI and baPWV for predicting severe SA-IT index and low eGFR, the receiver operating characteristic curve was plotted, and the area under the curve was calculated. Multivariate logistic regression analysis was used to identify the independent factors associated with the severe SA-IT index after adjustment for age, sex, mean arterial pressure (MAP), DM, eGFR, proteinuria, ABI, and baPWV (continuous variables and dichotomized variables by the cutoff value). We also conducted multivariate logistic regression analysis to identify the independent factors associated with low eGFR and proteinuria (≥1.0 g/g Cre) after adjustment for confounding factors. Data were analyzed using JMP 12 software (SAS Institute Inc., Cary, North Carolina, USA).
Results
Clinical characteristics by SA-IT index
The median age of all patients was 47 years (36–61 years), and 47% were male (Table 1). The median eGFR was 62 mL/min/1.73 m2 (45–88 mL/min/1.73 m2), and the median ABI was 1.16 (1.09–1.22). The prevalence was 52% (n = 64) for CKD stages 1 and 2 and 48% (n = 58) for CKD stages 3–5. Of the patients, 16% had nephrotic-range proteinuria (>3.5 g/g Cre). Compared with patients in the lowest 1–3 SA-IT index quartile (mild SA-IT index), those in the highest SA-IT index quartile (severe SA-IT index) were older and had higher SBP, MAP, PP, ABI and baPWV, HbA1c, prevalence of hypertension and DM and lower eGFR.
Clinical characteristics by ABI
The cutoff value that predicted a severe SA-IT index was 1.23 for ABI (area under the curve, 0.65; sensitivity, 39%; and specificity, 86%). Compared with patients with lower ABI (<1.23), those with higher ABI were older and had higher BMI, SBP, MAP, PP, HbA1c, triglyceride, SA-IT index, prevalence of hypertension, and use of antihypertensive agents and renin–angiotensin–aldosterone system inhibitors, more severe SA-IT index and lower eGFR and HDL-cholesterol level (Table 2).
Relationship of ABI and baPWV with SA-IT index, eGFR, and proteinuria
Univariate analyses revealed that the ABI was positively correlated with baPWV (ρ = 0.18, P = 0.043). ABI, baPWV, and PP were positively correlated with the SA-IT index (ABI, ρ = 0.36, P < 0.001; baPWV, ρ = 0.58, P < 0.001; PP, ρ = 0.28, P = 0.002) and inversely correlated with eGFR (ABI, ρ = –0.33, P < 0.001; baPWV, ρ = −0.59, P < 0.001; PP, ρ = –0.21, P = 0.023) (Fig. 2). baPWV (ρ = 0.32, P < 0.001) and PP (ρ = 0.22, P = 0.014), but not ABI (ρ = 0.16, P = 0.086), were positively correlated with proteinuria. Proteinuria was positively correlated with the SA-IT index (ρ = 0.31, P < 0.001) and inversely correlated with eGFR (ρ = −0.25, P = 0.006).
Multivariate logistic regression analysis showed that ABI and baPWV were associated with a severe SA-IT index, even after adjustment for potential confounding factors (Table 3, Model 1). The cutoff value that predicted a severe SA-IT index was 13.19 m/s for baPWV (area under the curve, 0.76; sensitivity, 92%; and specificity, 55%). The results were the same when ABI and baPWV were used as dichotomous variables (Table 3, Model 2). The association between ABI and severe SA-IT index remained significant when PP was used instead of baPWV.
We also evaluated the factors associated with low eGFR (<60 mL/min/1.73 m2) and proteinuria (>1 g/g Cre) using multivariate logistic regression analyses. Cutoff values that predicted a low eGFR were 1.14 and 13.9 m/s for ABI (area under curve, 0.64; sensitivity, 72%; and specificity, 55%) and baPWV (area under curve, 0.84; sensitivity, 79%; and specificity, 78%), respectively. Both ABI and baPWV, but not the presence of a severe SA-IT index, were significantly associated with low eGFR after adjustment for age, sex, MAP, heart rate, BMI, DM, and proteinuria (Table 4). A higher baPWV, but not ABI, was associated with a low eGFR. Any ABI, baPWV and presence of severe SA-IT index were not associated with proteinuria after adjustment for age, sex, MAP, heart rate, BMI and DM and eGFR (Supplementary Table S1).
Discussion
To our knowledge, this observational study is the first to demonstrate that the ABI is positively correlated with the biopsy-proven severity of renal SA-IT in patients with CKD. We found a positive association between ABI and the severity of renal SA-IT even after adjustment for important covariates, including age, sex, MAP, DM, eGFR, proteinuria and baPWV. A high normal ABI was also associated with a low eGFR. These findings suggest that an elevated ABI might be a predictor of renal small artery remodeling and low eGFR in patients with CKD.
Several studies have reported that ankle BP is associated with aortic PWV and wave reflections. In the general population in the Czech post-MONICA study, increased ankle SBP was associated with aortic PWV, independent of brachial BP, and ankle SBP was more closely related to aortic PWV than femoral-ankle PWV [3]. Central PP, but not brachial PP, was positively correlated with ABI and baPWV in patients with type 2 DM [16]. In younger subjects (<45 years) without a history of heart attack or stroke, the ABI was positively correlated with the aortic augmentation index [5]. Therefore, we hypothesized that the ABI is positively associated with aortic stiffness and/or wave reflection and increases with age until flow-limiting arterial stenosis develops in the lower extremities. In this study, univariate analysis revealed a positive relationship between the ABI and baPWV; however, this association was not noted after adjustment for age and sex, BMI, height, MAP, heart rate, DM, baPWV, and current smoking status (Supplementary Table S2). Multivariate regression analysis revealed that age and MAP were positively associated with the ABI after adjustment for confounding factors. Structural alterations in small arteries are major determinants of MAP and contribute to an increase in the amplitude of wave reflection [17]. Early age-related hemodynamic change is dominated by increased MAP and central wave reflection, rather than by aortic stiffening [8]. Before the age of 60 years, the augmentation index increases dramatically, whereas aortic and peripheral PWV change modestly. After the age of 60 years, the augmentation index plateaus despite progressive increases in central PWV. These results indicate that the age-related increase in wave reflection, as a result of small artery remodeling, may predominately affect the increase in ABI.
Because evaluation of the structure of the small arteries in humans requires an invasive procedure, less is known about the role of central hemodynamics on biopsy-proven renal small artery structural alterations. In hypertensive subjects, a media-to-lumen ratio of subcutaneous small resistance arteries was positively correlated with both aortic PWV and aortic augmentation index; this relationship was independent of age and blood pressure [18]. In the relatively younger population (≤65 years) of normotensive and hypertensive subjects, SBP, DBP, and MAP, but not PP, were positively correlated with the media-to-lumen ratio of the small gluteal subcutaneous arteries [19]. These results indicate that an increase in wave reflection, rather than an increase in arterial stiffness, may predominately affect small artery structural changes in the younger population.
baPWV was significantly associated with a severe SA-IT index in univariate analysis, but the relationship disappeared after adjustment for several confounding factors, including age (Table 3, Model 1). When age was removed from the explanatory variables, baPWV and ABI were significantly associated with a severe SA-IT index (OR per SD increase in ABI, 1.92; 95% CI, 1.16–3.36; OR per SD increase in baPWV, 2.48; 95% CI, 1.28–5.12). These results suggest that baPWV was associated with a severe SA-IT index but was confounded by age.
The presence of structural alterations in the small arteries may cause impairment of organ vasodilator reserve [20] and is associated with ischemic organ damage, including renal failure [11]. However, in this study, neither eGFR nor proteinuria was associated with SA-IT index severity in multivariate analyses. Although numerous studies have demonstrated modest associations between arterial stiffness and reduced kidney function [21], the relationship between arterial stiffness and kidney function in the earlier stages of CKD remains controversial [22, 23]. Only a limited number of studies have focused on long-term renal outcomes. Previous studies showed that biopsy-proven arteriosclerosis had minor or no significant impacts on the prediction of CKD progression [24,25,26]. Rule et al. showed that the prevalence of biopsy-proven nephrosclerosis, defined by a histological pattern of sclerosis and atrophy, increased linearly with age even after adjustment for several confounding factors, including albuminuria and GFR, in healthy living kidney donors [24]. After adjustment for age and sex, hypertension and albuminuria, but not GFR, were associated with nephrosclerosis. Age was the strongest predictor of nephrosclerosis, and hypertension and albuminuria, but not GFR, were associated with biopsy-proven nephrosclerosis. GFR is the product of the number of nephrons and the average single-nephron GFR. Numerous conditions, such as obesity, hyperglycemia, extracellular fluid volume conditions, and dietary protein intake, also affect single-nephron GFR [27]. A decline in nephron number caused by nephrosclerosis might be compensated by increased single-nephron GFR, which maintained the total GFR [28]. Scaling of GFR to body surface area may not account for age-related changes in physiological demand for GFR [24]. The serum creatinine-based GFR-estimating equation may not be an appropriate marker for kidney function. Microalbuminuria is a marker of microvascular kidney damage and is associated with aortic stiffness [29]. However, the degree of proteinuria reflects the degree of glomerular injury but not small artery injury in patients with primary glomerular disease because renal small arteries are not the primary sites of injury [29]. This may explain why proteinuria was not associated with the severity of the SA-IT index and ABI in this study. Further large cohort studies with longer observation periods are required to examine the relationship between subclinical age-related organ damage (including biopsy-proven arteriosclerosis and arterial stiffness) and clinical manifestation (GFR and albuminuria/proteinuria).
In the present study, the ABI was positively associated with the biopsy-proven severity SA-IT index. This finding should be interpreted with caution. ABI increases with age until 60 years but decreases thereafter [2]. In older and high-risk patients, the ABI decreases with age because of the increased prevalence and progression of atherosclerosis [30]. We evaluated the ABI and baPWV values in 4 age groups (<35, 35–49, 50–64, and 65≤ years) in this study and found that the ABI was lowest at 35 years and younger and increased with age until 50–64 years but decreased thereafter, whereas baPWV increased linearly with age, as we previously reported [2]. The relationship between age and frequency of borderline low ABI (0.91–0.99) showed a J-curve relationship: 10% in patients <35 years, 0% in those 35–49 years, 3% in those 50–64 years, and 16% in those 65≤ years. We assume that the ABI decreased with age over 65 years due to the presence of flow-limiting arterial stenosis in the lower limbs. In this state, some of the patients may have advanced atherosclerosis and arterial rigidity even in normal to borderline-low ABI. In patients <65 years, the prevalence of the severe SA-IT index was significantly higher in patients with higher ABI (>1.16, median) than in those with lower ABI (38% vs. 13%, respectively; P = 0.003). The prevalence of low eGFR was significantly higher in patients with higher ABI than in those with lower ABI (56% vs. 33%, respectively; P = 0.016). In patients ≥65 years, however, the prevalence of severe SA-IT index was lower (but not significant) in patients with higher ABI than in those with lower ABI (50% vs. 67%, respectively; P = 0.46). The prevalence of low eGFR was not different between patients with higher and lower ABI (70% vs. 67%, respectively; P = 0.88). Thus, our results are only applicable to individuals without lower limb arterial stenosis.
There are several potential limitations of this study. We speculate that the major determinant of the ABI in younger individuals is wave reflection rather than arterial stiffening, but we did not measure the indices of wave reflection, such as the augmentation index. Further studies including pulse wave separation analysis are needed to identify the exact determinants of the ABI in younger populations. This was a cross-sectional observational study; therefore, we were unable to demonstrate a causal relationship underlying the association between the ABI and severity of the SA-IT index. We used the oscillometric method to measure ABI. The oscillometric method tends to overestimate ABI when the Doppler-derived ABI is in the low range. However, the correlation between Doppler-derived and oscillometry-derived ABIs of healthy subjects or subjects with mild PAD has been acceptable in most studies [30]. Because we recruited patients who underwent renal biopsy, the results of this study might not be generalizable. Primary diseases might affect renal small artery structural changes. Further large clinical studies are required to examine primary disease-dependent effects on renal small artery structural changes. We could not exclude the effect of sampling bias on the accuracy of the assessment of structural changes in the renal small arteries due to the small amount of tissue obtained to minimize injury to the kidney. However, the SA-IT index was significantly correlated with arteriosclerotic risk factors, such as BP, DM, eGFR, and proteinuria.
In conclusion, a high normal ABI was associated with severe renal SA-IT and low eGFR in patients with CKD.
References
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45:S5–S67.
Ishida A, Miyagi M, Kinjo K, Ohya Y. Age- and sex-related effects on ankle-brachial index in a screened cohort of Japanese: the Okinawa Peripheral Arterial Disease Study (OPADS). Eur J Prev Cardiol. 2014;21:712–8.
Wohlfahrt P, Krajčoviechová A, Seidlerová J, Galovcová M, Bruthans J, Filipovský J, et al. Lower-extremity arterial stiffness vs. aortic stiffness in the general population. Hypertens Res. 2013;36:718–24.
Ishida A, Nakachi-Miyagi M, Kinjo K, Iseki K, Ohya Y. A high normal ankle-brachial index is associatedwith proteinuria in a screened cohort of Japanese: the okinawa peripheral arterial disease study. J Hypertens. 2014;32:1435–43.
Khaleghi M, Kullo IJ. Aortic augmentation index is associated with the ankle-brachial index: a community-based study. Atherosclerosis. 2007;195:248–53.
Kinjo Y, Ishida A, Kinjo K, Ohya Y. A high normal ankle-brachial index combined with a high pulse wave velocity is associated with cerebral microbleeds. J Hypertens. 2016;34:1586–93.
O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–4.
Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–60.
Miyagi T, Kohagura K, Ishiki T, Kochi M, Kinjyo T, Kinjyo K, et al. Interrelationship between brachial artery function and renal small artery sclerosis in chronic kidney disease. Hypertens Res. 2014;37:863–9.
Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, et al. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation. 2001;103:1238–44.
Rizzoni D, Porteri E, Boari GEM, De Ciuceis C, Sleiman I, Muiesan ML, et al. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–5.
Ooi QL, Tow FKN-FH, Deva R, Alias MA, Kawasaki R, Wong TY, et al. The microvasculature in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1872–8.
Furuichi K, Shimizu M, Yuzawa Y, Hara A, Toyama T, Kitamura H, et al. Nationwide multicenter kidney biopsy study of Japanese patients with hypertensive nephrosclerosis. Clin Exp Nephrol. 2018;22:629–37.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37.
Jung C-H, Jung S-H, Kim K-J, Kim B-Y, Kim C-H, Kang S-K, et al. Differential associations of central and brachial blood pressure with carotid atherosclerosis and microvascular complications in patients with type 2 diabetes. BMC Cardiovasc Disord. 2014;14:23.
Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007–21.
Muiesan ML, Salvetti M, Rizzoni D, Paini A, Agabiti-Rosei C, Aggiusti C, et al. Pulsatile hemodynamics and microcirculation: evidence for a close relationship in hypertensive patients. Hypertension. 2013;61:130–6.
Schiffrin EL, Deng LY. Relationship between small-artery structure and systolic, diastolic and pulse pressure in essential hypertension. J Hypertens. 1999;17:381–7.
Safar ME, Rizzoni D, Blacher J, Muiesan ML, Agabiti-Rosei E. Macro and microvasculature in hypertension: therapeutic aspects. J Hum Hypertens. 2008;22:590–5.
Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for improving and standardizing vascular research on arterial stiffness. Hypertension. 2015;66:698–722.
Hermans MMH, Henry R, Dekker JM, Kooman JP, Kostense PJ, Nijpels G, et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol. 2007;18:1942–52.
Upadhyay A, Hwang S-J, Mitchell GF, Vasan RS, Vita JA, Stantchev PI, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol. 2009;20:2044–53.
Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–7.
Furuichi K, Shimizu M, Yuzawa Y, Hara A, Toyama T, Kitamura H, et al. Clinicopathological analysis of biopsy-proven diabetic nephropathy based on the Japanese classification of diabetic nephropathy. Clin Exp Nephrol. 2018;22:570–82.
Yamanouchi M, Hoshino J, Ubara Y, Takaichi K, Kinowaki K, Fujii T, et al. Clinicopathological predictors for progression of chronic kidney disease in nephrosclerosis: a biopsy-based cohort study. Nephrol Dial Transplant. 2019;34:1182–8.
Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020;16:51–64.
Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017;376:2349–57.
Ito S. Cardiorenal syndrome: an evolutionary point of view. Hypertension. 2012;60:589–95.
Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–909.
Acknowledgements
We are grateful to Morihiro Ota, Masato Nohara, and Sakiko Kina-Hatoma for their skillful technical assistance in histological preparation and staining.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zamami, R., Ishida, A., Miyagi, T. et al. A high normal ankle-brachial index is associated with biopsy-proven severe renal small artery intimal thickening and impaired renal function in chronic kidney disease. Hypertens Res 43, 929–937 (2020). https://doi.org/10.1038/s41440-020-0443-z
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41440-020-0443-z
Keywords
This article is cited by
-
Ankle-brachial index predicts renal outcomes and all-cause mortality in high cardiovascular risk population: a nationwide prospective cohort study in CORE project
International Urology and Nephrology (2022)
-
Combination of low ankle-brachial index and high ankle-brachial index difference for mortality prediction
Hypertension Research (2021)
-
Structural changes in renal arterioles are closely associated with central hemodynamic parameters in patients with renal disease
Hypertension Research (2021)