Abstract
Baroreflex regulates blood pressure and heartbeat through specific mechanosensitive baroreceptors. However, the current knowledge is derived only from animal experiments. No data about human aortic baroreceptors have been reported so far. Therefore, in this study, we performed extended histological, proteomics and transcriptomics analyses of the aortic arch to identify potential human baroreceptors. Three healthy human aortic arches from autopsies, six abdominal aortic aneurysms and four control abdominal aortic tissue samples from our vascular biobank were analysed. For histological analyses, antibodies against various neuronal markers were used. Laser capture microdissection and macrodissection were performed to selectively analyse nerves in the adventitia of the human aorta using proteomics and RNA sequencing. Histological analysis revealed a heterogeneous distribution of nerves in the adventitia along the entire aortic arch, predominantly in the ascending aorta up to the left subclavian artery. Proteome analysis identified three putative human baroreceptors PIEZO1, TRPV2, and TRPM4. Transcriptomics confirmed that these ion channels do not originate from cells within the aortic wall but presumably from the cell body of the vagus nerve. Interestingly, these ion channels were also detected in the healthy abdominal aorta and abdominal aneurysm without any significant differences in their abundance. Our study identified, for the first time, putative baroreceptors in the human aortic arch. Further studies are necessary to validate our current results and elucidate the role of these putative baroreceptors in the human aortic arch.

Similar content being viewed by others
Introduction
Baroreflex describes the principal neuronal regulatory mechanism of negative feedback control of cardiovascular function by the central nervous system [1, 2]. The heartbeat and blood pressure are regulated in response to the activity of specific arterial baroreceptors located in the carotid sinus and aortic arch [2]. The cell bodies of carotid baroreceptors are located in the petrosal ganglia, and the central process terminates in the nucleus of the solitary tract (NTS). In the case of aortic baroreceptors, the cell bodies are located in the nodose ganglia, and the peripheral process forms part of the vagus nerve, innervating the aortic arch and terminating in the NTS [2, 3]. Increased blood pressure leads to increased activity of the neurons containing mechanosensitive ion channels called baroreceptors, which in turn leads to increased parasympathetic activity, slowing down the heartbeat through the pacemaker cells in the sinoatrial node [3]. Concomitantly, sympathetic activity is inhibited, decreasing the vascular resistance. Thus, baroreflex and baroreceptors are necessary to maintain blood pressure within a physiological range [1,2,3].
Arterial baroreceptors are located in the plasma membrane of sensory neurons innervating the carotid sinus and aortic arch [4]. Some authors have stated that aortic baroreceptors might be dominant in regulating the heart rate [4,5,6]. Nothing is known about transmembrane proteins that might serve as putative baroreceptors in the human aortic arch. Thus, it is essential to identify baroreceptors in the human aortic arch to elucidate their contribution to regulating blood pressure in order to endeavour efficient drug therapy. So far, the knowledge of aortic baroreceptors has been derived only from animal models [3, 7, 8]. The animal studies suggest that ion channels such as epithelial sodium (Na+) channels (ENaCs), acid-sensing ion channels (ASICs), transient receptor potential (TRP) ion channels, and piezo ion channels are involved in the mechanotransduction performed by arterial baroreceptors [9,10,11,12,13,14,15,16,17,18,19]. All of these ion channels might contribute to arterial baroreflex. However, no data exists on whether these mechanosensitive proteins are expressed in the human aortic arch.
ENaCs and ASICs are members of the amiloride-sensitive degenerin channel (DEG) family and have mainly been suggested to act as mechanosensors [8, 10, 11, 18,19,20]. Their role and function have been mainly investigated in the carotid sinus [11, 12, 21, 22]. ASIC mRNA and protein have been detected in the aortic baroreceptor terminals in various animal models [8, 11, 13, 16]. TRP ion channels also respond to membrane stretching [8, 13, 15, 16]. TRPV and TRPC have been detected in animal baroreceptor neurons [8, 23, 24]. Recently, novel mechanosensitive ion channels, piezo-type mechanosensitive ion channel component 1 and 2 (PIEZO1 and PIEZO2), have been discovered in mouse neuronal cells, having an important biological function in mechanosensation [9, 18, 19]. The knockout of both PIEZO1 and PIEZO2 in a mouse model was able to completely turn off the baroreceptor reflex [9].
Taken together, numerous ion channels have been assumed to contribute to the arterial baroreflex in animal experiments [8,9,10,11,12,13,14,15,16,17,18,19]. However, no data exist on whether and which ion channels also play a similar role in the human aortic arch. Therefore, in this study, we performed extended histological, proteomics, and transcriptomics analyses of the healthy aortic arch and abdominal aorta, as well as aortic abdominal aneurysm, in order to identify putative baroreceptors in humans.
Methods
Tissue
In this study, various segments from three aortic arches (formalin fixed paraffin embedded (FFPE) tissue: n = 6, fresh frozen tissue n = 16, including upper and lower part) from the autopsy of patients without cardiovascular comorbidities (Department of Pathology and Molecular Pathology, University Hospital Zurich, Switzerland), six abdominal aortic aneurysms from our vascular biobank (Department of Vascular Surgery, University Hospital Zurich, Switzerland) [25], and four control non-aneurysmal abdominal aortic tissues obtained from the Department of Visceral Surgery and Transplantation (University Hospital Zurich, Switzerland) were used. The local ethics committee approved tissue sample collection and analysis (Cantonal Ethics Committee Zurich, Switzerland; BASEC-Nr. 2020-00378 and 2024-02101). All tissue samples were divided into various segments, some were then directly frozen and stored at −80 °C, whereas others were fixed in 4% formaldehyde for 24 h, decalcified, and embedded in paraffin (FFPE). The entire aortic arch was divided into consecutive circular segments (Supplementary Fig. 1). One of these healthy aortic arches was completely processed for FFPE and used for subsequent histology. For the other two healthy aortic arches, every other segment was processed as FFPE and the rest was fresh-frozen in cryopreservative solution and stored at −80 °C until analysis [25].
Histology and immunohistochemistry
For histological analysis, FFPE blocks were cut into 5 µm tissue sections, mounted on standard slides (SuperFrost, Thermo Fisher) and conventional stains were applied using haematoxylin-eosin (HE) and Elastica van Gieson (EvG).
For immunohistochemistry (IHC), the tissue sections were mounted on slides coated with poly-L-lysine (SuperFrost Plus, Thermo Fisher) and stained using rabbit or mouse-specific HRP/DAB (ABC) detection IHC kit (abcam) and antibodies against various nerve markers, such as tyrosine hydroxylase (abcam, ab207673, 1:1000), neurofilament (abcam, ab204893, 1:300), protein gene product 9.5 (abcam, ab15503, 1:200), vesicular glutamate transporter 1 and 2 (abcam, ab305253, 1:1000; ab227805, 1:1000; ab211869, 1:50; ab305253, 1:75), brain-derived neurotrophic factor (abcam, ab108319, 1:500), myelin basic protein (abcam, ab133620, 1:300), and calcitonin-gene related peptide (abcam, ab47027, 1:500).
Tyrosine hydroxylase (TH) is present in catecholaminergic neurons, converting tyrosine into L-3,4-dihydroxyphenylalanine (L-DOPA). Protein gene product 9.5 (PGP9.5), also known as ubiquitin C-terminal hydrolase L1, is a deubiquitinating enzyme confined to neurons. Both TH and PGP9.5 are considered general markers of nerves. Neurofilaments (NF) are intermediate filaments within the cytoplasm of neuronal extensions. Vesicular glutamate transporters 1 and 2 (VGLUT 1 and 2, respectively) are glutamate transporters and detect sensory neurons. Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors found in the peripheral nerves. Myelin basic protein (MBP) shows myelinated nerve fascicles and calcitonin-gene-related peptide (CGRP) is selective for afferent nerves.
Regarding the putative baroreceptors detected by proteomics analysis, the following antibodies were used: anti-piezo-type mechanosensitive ion channel 1 (anti-PIEZO1; Invitrogen, mouse, 1:500), anti-transient receptor potential ion channels V2 and M4 (anti-TRPV2 and anti-TRPM4, Osenses, rabbit, 1:2000 and Origene, mouse, 1:50).
All stained tissue samples were digitalised using ZEISS Axioscan 7 (Zurich, Switzerland). Following digitalisation, the number and area of the nerves within the adventitia were determined using free Software QuPath 0.4.3 (QuPath developers, University of Edinburgh).
Laser capture microdissection
Laser Capture Microdissection (LCM) was performed as described before [26]. Five FFPE aortic arch tissue blocks from our biobank [25] were cut to a thickness of 10 µm, mounted on PEN membrane glass slides (Thermo Fisher LCM0522), and dried at room temperature overnight. For each sample, four to six tissue sections were used. The slides were stained with cresyl violet, and LCM was performed using the Arcturus Cellect LCM System (Thermo Fisher). From each of the five FFPE tissue samples, nerve fascicles within the adventitia and areas without nerves as controls were excised (Supplementary Fig. 2).
Macrodissection of FFPE samples
For macrodissection experiments, FFPE samples of abdominal aortic aneurysm (AAA) (n = 6), healthy aortic arch (n = 6, two segments from each aortic arch), and healthy abdominal aortic tissues (n = 4) were used. For each sample, four sections of 20 µm thickness were cut on a microtome (HM340E, Thermo Fisher) and mounted on standard microscope slides. The tunica adventitia was dissected under a light microscope (AE2000, Motic) with a scalpel and transferred to a 1.5 ml Eppendorf tube.
Macrodissection of fresh samples
Four segments were used, each from two fresh frozen aortic arch samples. In addition, the outer and inner curvatures were treated separately for each segment. The adventitial layer was scraped of the arterial vessel using a scalpel. The proteins from the 16 fresh tissue samples were isolated with RIPA buffer (Thermo Fisher) and total RNA was extracted with TRIZOL reagent (Agilent).
Proteomics
Proteome analysis was conducted by the Functional Genomics Center Zurich, Proteomics Group (Dr Paolo Nanni). For the samples obtained from LCM, areas of interest on the FFPE slides were identified and transferred onto the LCM cap. After excision, the tissue was inserted into a 1.5 ml centrifuge tube and frozen at −20 °C until further processing. The excised tissue was treated with a commercial proteomics kit (Thermo Fisher). The tissue obtained from macrodissection (FFPE samples) was lysed with 4% SDS and heated at 95 °C for 60 min. High-intensity ultrasound was applied for tissue disruption, and the samples were again heated at 95 °C for 30 min. The following steps were the same way for proteins obtained from macrodissection of the fresh-frozen samples using RIPA buffer, as these did not require tissue lysis because the protein was already isolated. This included the addition of Tris(2-carboxyethyl)phosphine and 2-chloroacetamide to reduce and alkylate the proteins. The samples were incubated for 30 min at 30 °C and diluted with pure ethanol to a final concentration of 60%. Carboxylated magnetic beads were added, and after 30 min at room temperature, the beads were washed thrice with 80% ethanol. Trypsin in 50 mM tetra-ethyl-ammonium bromide (TEAB) was then added for enzymatic digestion overnight at 37 °C. The remaining peptides were extracted from the beads with H2O.
For all proteomics experiments (LCM, macrodissection with fresh as well as FFPE samples), the samples were loaded on EvoTip columns (Evosep Biosystem, Denmark), and proteomics was performed on an Evosep One LC coupled to a TIMS TOF Pro mass spectrometer (Functional Genomics Center Zurich (FGCZ), Switzerland). Analysis was performed using DIA-NN software, and only proteins with a number of peptides >1 were included. For the LCM proteomics data, classification and pathway analysis were conducted with the open PANTHER database [27, 28].
RNA extraction and transcriptomics
The RNA was isolated from the adventitia of the same fresh-frozen tissue used for proteomics, applying TRIZOL reagent (Thermo Fisher) according to the manufacturer’s protocol. The RNA quality and degree of degradation were determined on the TapeStation 4150 (Agilent) using the corresponding RNA ScreenTape Assay in accordance with the manufacturer’s protocols. Library preparation and RNA sequencing were conducted by the Functional Genomics Center Zurich, Transcriptomics Group, using paired-end sequencing on NovaSeq X (Illumina). Raw sequencing data were provided by the SUSHI interface [29]. Further processing steps in SUSHI included the use of several packages such as FastQC for quality control of the raw sequencing data, STAR to align the reads to a reference genome, FeatureCounts to estimate read abundance, and CountQC for quality control after counting reads.
Data analysis
For differential expression analysis, the linear model approach from the Bioconductor package DESeq2 and EdgeR2 was used [29, 30]. The Benjamini–Hochberg algorithm was used for multiple testing corrections by calculating the False Discovery Rate (FDR, adjusted P-value) [31]. All other statistical analyses were performed using R software v4.4.1 or IBM’s SPSS software version 29 (SPSS Inc., Chicago, IL, USA). An independent t-test and a Levene test of equality of variances were used. All statistical analyses were two-sided, with P < 0.05 as the significance level.
Results
Histological and immunohistochemical analysis of peripheral nerves
First, several antibodies were applied and optimised in order to detect the different types of peripheral nerves (Fig. 1). Under optimised conditions (antigen retrieval solution and pH, antibody dilution and incubation time), anti-TH and anti-PGP9.5 were able to detect all nerves within the arterial wall. However, TH appeared to have less background than PGP9.5. The antibody against BDNF could not be optimised appropriately, as it has a heavy background independent of the staining conditions. The staining against VGLUT1 also had a strong background, with almost all cells positive. The antibody staining against CGRP was weak and unspecific and could not be clearly assigned to the nerves. The staining of MBP worked well, however, we found only sporadic spots within the aortic wall. Following an extended immunohistochemical optimisation of various peripheral nerves and nerve endings in the human aorta and aortic arch, TH, NF and VGLUT2 were chosen, being able to unambiguously stain nerves in the human aortic arch (Fig. 2). We detected not only large nerve fascicles but also individual nerve fibres. Some of the TH-positive nerve fascicles had various diameters of up to 760 µm. Furthermore, several of these nerve fascicles ran alongside the blood vessel and thus had a round shape. Others, running circumferential, had an elongated or linear form (Fig. 2).
Optimised immunohistochemical staining of nerves in the human aortic arch (n = 3). Different antibodies were used against various types of peripheral nerves. VGLUT1 and 2 vesicular glutamate transporter 1 and 2 (dilution 1:1000 and 1:75), TH tyrosine hydroxylase (dilution 1:1000), NF neurofilament (dilution 1:300), BDNF brain-derived neurotrophic factor (dilution 1:500), MBP myelin basic protein (dilution 1:300), PGP9.5 protein gene product 9.5 (dilution 1:200), CGRP calcitonin gene-related peptide (dilution 1:500)
In order to gain a more detailed insight into the frequency of nerve appearance within the human aortic arch, quantitative analysis was conducted by counting the number of TH-positive nerves (Fig. 3A, B). Furthermore, the TH-stained areas were determined (Fig. 3C). Some segments (5–7, see Fig. 3D and Supplementary Fig. 1) also contained parts of the brachiocephalic, left common carotid and left subclavian arteries. These selective parts were excluded from the analysis, focusing only on the aortic arch (Supplementary Fig. 3). Some segments (particularly segments 8–12) contained very few nerves. However, the detected nerve fascicles were markedly larger than those in the other segments. This was particularly visible when calculating the TH-positive areas (Fig. 3C). Segments 1–6, which are part of the ascending aorta until the left common carotid artery, seemed to have markedly more nerves than the remaining segments 7–12, starting with the left subclavian artery towards the descending aorta. Interestingly, no differences in nerve distribution were observed between the lower and upper aortic arch.
Quantification of nerve distribution along the aortic arch using tyrosine hydroxylase (TH) staining. A Example of the image quantification. The blue line indicates the separation between the outer and inner curvature. The nerves (TH-positive staining) are indicated by red lines. B Quantification plot showing the number of nerves in the individual segments (see Supplementary Fig. 1). C Quantification plot focusing on the TH-stained area. D A schematic image of the aortic arch with the individual segments analysed in the study. The blue dashed line displays the part of the aortic arch with the most nerves and nerve endings
Laser capture microdissection
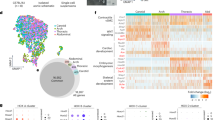
As shown in Supplementary Fig. 2, nerve fascicles and the neighbouring area without nerves were removed using Laser Capture Microdissection and analysed by proteomics. In total, more than 3000 proteins were identified using LCM (Fig. 4A). Interestingly, 334 proteins were found to be significantly more abundant in the nerves than in the control adventitial tissue (FDR < 0.05, log2FC threshold of 0.5, number of peptides >1). In this group, many neuron-specific proteins, such as neurofilament polypeptides, neural cell adhesion molecules, peripherin, and Schwann cell-specific proteins like myelin-related proteins, were identified. These 334 significantly abundant proteins in the nerves were further analysed in the PANTHER database according to their molecular function, biological process, cellular component, protein class and pathway. In the cellular compartment analysis, 9 proteins were found to belong to the axon, 8 to synapses, 9 to neuron projections and 6 to the neuronal cell body (Supplementary Table 1). In addition, in the biological process analysis, processes belonging to the nervous system were present, such as axonogenesis, chemical synaptic transmission, myelination, neuron projection development, synaptic signalling, and synapse organisations (Supplementary Table 2). For the pathway analysis, serotonin receptor-mediated signalling, serotonin degradation, adrenaline and noradrenaline biosynthesis, alpha and beta-adrenergic receptor signalling, axon guidance, dopamine receptor-mediated signalling pathway, GABA receptor signalling, ionotropic and metabotropic glutamate receptors, muscarinic and nicotinic acetylcholine receptor and synaptic vesicle trafficking pathways were implicated (Supplementary Table 3).
Proteomics. A Proteome analysis - Diagram showing the total number of proteins present in the LCM samples and the number of proteins found only in the group with nerves and without nerves. B Number of proteins detected in the various experimental approaches: LCM (laser capture microdissection), FFPE (macrodissection from formalin-fixed in paraffin-embedded samples), and Fresh (fresh frozen tissue samples). C Proteome analysis - raw protein abundance in the adventitia of the FFPE aortic tissue samples. PIEZO1 piezo-type mechanosensitive ion channel 1, TRPV2 and TRPM4 transient receptor potential ion channels V2 and M4, AoA aortic arch, hAo healthy abdominal aorta, AAA abdominal aortic aneurysm. The table shows the mean ± standard deviation of the individual detected putative mechanosensitive ion channels
Surprisingly, using laser capture microdissection, we did not identify any baroreceptor candidates in the nerve areas or surrounding regions.
Proteomics of adventitia from macrodissected FFPE aortic tissue samples
As described above, LCM did not lead to the detection of any potential mechanosensitive receptors in the nerves within adventitia. However, because several publications on animal models have described baroreceptors in the aortic arch, we analysed in the next step the entire adventitia [4, 7, 8, 11, 12, 16].
In order to compare the protein expression also in other sections of human aortas, we performed a macrodissection of FFPE tissue samples from the healthy aortic arch, healthy abdominal aorta and abdominal aortic aneurysm. Using whole adventitia, 5.631 ± 584 proteins from FFPE specimens could be identified (Fig. 4B). Interestingly, among these proteins, three putative baroreceptors with a number of peptides >1 were detected: PIEZO1, TRPM4 and TRPV2 (Fig. 4C). Surprisingly, these ion channels and possible baroreceptor candidates were found not only in the aortic arch but also in the adventitia of the healthy abdominal aorta as well as in the abdominal aortic aneurysm. Considering the protein abundance (Fig. 4C and the corresponding table), PIEZO1 had similar protein abundance in all three study groups (Fig. 4C). In contrast, a significantly increased amount of TRPV2 was observed in AAA compared to the aortic arch or healthy aorta. For TRPM4, the lowest protein abundance was observed in the abdominal aorta in the absence of cardiovascular comorbidities (Fig. 4C).
Proteomics of adventitia from macrodissected fresh aortic arch samples
In order to further improve the sensitivity of our analysis, we focused on the adventitia of the fresh-frozen samples. Using this approach, we divided the aortic arch into upper and lower segments and analysed these segments separately. Using fresh tissue, over 6000 proteins were identified by proteome analysis (Fig. 4B). Considering the number of peptides >1, the same putative baroreceptors, PIEZO1, TRPV2 and TRPM4, were also found in the adventitia of fresh frozen aortic tissue samples (Table 1). Interestingly, no significant differences were observed between the protein abundances of the upper and lower halves of the same segment (FDR < 0.05, log2FC threshold of 0.5).
Transcriptomics of adventitia from the macrodissected fresh aortic arch samples
Finally, transcriptome analysis of the same fresh frozen aortic arch tissue samples used in proteomics was performed. Except for ASIC4 and the alpha-subunit of ENaC, all other expressed mRNAs of the potential mechanosensitive proteins were members of the transient receptor potential ion channel family (Table 2). Particularly high expression was observed for TRPP2. On the contrary, low expression was observed for TRPC3, TRPV5, and the mucolipin TRP channel MCOLN3. Similar to the comparison of the protein abundances, no significant differential expression of the detected ion channel genes between the upper and lower part of the aortic arch (FDR < 0.05, log2FC threshold of 0.5) was observed.
Interestingly, no expression was found for the three putative baroreceptors PIEZO1, TRPV2 and TRPM4, identified in the proteome analysis.
Immunohistochemical analysis of the putative baroreceptors PIEZO1, TRPV2 and TRPM4
In order to localise and ascertain the distribution of the above-identified putative baroreceptors in the human aortic arch, an extensive immunohistochemical analysis of PIEZO1, TRPV2 and TRPM4 was performed. However, even if every staining was positive in the control tissue (mouse brain), we were not able to reliably identify any potential baroreceptors in the aortic arch by immunohistochemistry. The reasons for failing to detect these baroreceptors are discussed in the section Discussion.
Discussion
In the current study, we identified putative baroreceptors that have not yet been described in the human aortic arch. Our results demonstrated a heterogeneous distribution of nerves of different sizes along the aortic arch located in the adventitia, particularly in the ascending aorta and up to the left subclavian artery. There was no accumulation of nerves in the inner curvature of the aortic arch. Using extended proteome analysis, we identified three putative human baroreceptors, PIEZO1, TRPV2 and TRPM4.
Extended IHC analysis of nerves within the human aortic arch wall revealed neuronal fascicles of various sizes exclusively in the adventitia. In particular, an accumulation of nerves was observed in the left subclavian artery, corresponding with the findings in animal models of rabbits and mice [32, 33]. Furthermore, the aortic depressor nerve containing the aortic baroreceptors is located between the left subclavian and the left common carotid artery [8, 18, 33]. Regarding different types of nerves, we were able to detect the sensory nerves using the VGLUT2 antibody. The circumstance that the aortic depressor nerve is appointed between the subclavian and left common carotid arteries also explains the fact that most nerves along the aortic arch were located in the ascending artery up to segment 7 (see also Fig. 3D) [33]. Thus, we assume that the putative human baroreceptors in this area, especidally between left subclavian and left common carotid arteries. However, they are not located exclusively in the inner aortic curvature but alongside the entire circumference of the aortic arch. This assumption is in line with the results of Min et al. [33], who demonstrated the distribution of sensory neurons around the aortic arch by using specific VGLUT2 (marker of sensory neurons) and PIEZO2 transgenic mouse model, showing the branching of the left aortic depressor nerve at the sides of the aortic arch between the left subclavian and common carotid arteries.
Using proteomics of the microdissected nerve fascicles, we did not detect any potential baroreceptors in the aortic arch. We assume that the nerve fascicles, containing a composite of nerve fibres, do not contain these mechanosensitive proteins. They are probably located only at the end of the unmyelinated nerve fibres [3]. In contrast, after analysing the entire adventitia, three putative baroreceptors, PIEZO1, TRPV2, and TRPM4 were detected. These results confirm that the mechanosensitive receptors are not located in the nerve fascicles but in the single nerve fibres, most likely at their terminals. This might be the reason why the immunohistochemical analysis did not identify any of these receptors. Therefore, they are difficult to identify if the staining is not clear and when the antibodies have not yet been established in humans. Only if we were fortunate and cut exactly through these neuronal terminals would we be able to detect these putative baroreceptors also by immunohistochemistry. It is also to mention that the FFPE section are only 5 µm thick, and the nerve terminals have a diameter of about 10 µm. The separate analysis of the inner and outer curvatures of the aortic arch did not demonstrate any significant differences in the abundance of these ion channels. Again, these results confirm the equal distribution of the putative baroreceptors around the aortic arch without any specific hotspots.
We were also interested in the possible presence of these baroreceptors in the abdominal aorta in order to evaluate their specificity for the aortic arch. Therefore, we included healthy abdominal aorta and abdominal aortic aneurysm in the analysis because we have already found an increased number of neuronal fascicles in the diseased aorta [34]. Surprisingly, we detected the putative baroreceptors PIEZO1, TRPV2 and TRPM4 in the abdominal aorta. In addition, no significant differences were observed between healthy and diseased abdominal aorta. Interestingly, only three mechanosensitive receptors have been identified. The consequence of this finding is that PIEZO1, TRPV2 and TRPM4 seem to have a general pattern of appearance along the human aorta. Proteome analysis was performed because nerve endings in the adventitia of the aortic arch do not contain any nuclei as they are located in the brainstem. In order to verify whether these potential baroreceptors are expressed by other cells with their nuclei present in the adventitia of the aorta, we performed an additional transcriptome analysis of the same samples. Interestingly, different baroreceptor candidates, previously described in the animal models, have been identified [9,10,11,12, 14, 16, 18]. In particular, various TRP ion channels were detected. It has already been reported that TRP channels are expressed in SMCs and ECs and participate in regulating vascular tone [35, 36]. Aberrant expression of TRP channels was associated with vascular dysfunction and metabolic syndromes. Regarding TRPV2, the expression of this ion channel has already been found in vascular cells, not yet, however, in human vasculature, only in various animal models [37]. The expression of TRPM4 has been detected in e.g. SMCs, however, again, only in animals [38]. The expression of PIEZO channels has been so far associated with cardiovascular development [39]. In contrast, our results demonstrated no expression of PIEZO1, TRPV2 or TRPM4 at the mRNA level in humans. It is not surprising because the cell nuclei of the sensory neurons containing the putative mechanosensitive receptors of the baroreflex are located in the cell bodies within the brain stem. It has been stated that most of the RNA detected in neuron fibres belongs to ribosomes, mitochondria and cytoskeleton [40]. In general, RNAs can be transported along the nerve fibres by RNA-binding proteins (RBPs) [41]. However, the transport efficiency depends on the mRNA lengths and distance from the cell nuclei. All three putative baroreceptors detected in the study have a large mRNA ranging from 2.4 to 7.6 kbp. Thus, we assume that our baroreceptor candidates are either translated in the cell bodies and transported as proteins along the neuronal fibres [42] or the turnover rate for these receptors is so low that no RNA could be detected, even if those would be transcribed in the nerve endings.
Consequently, these three putative baroreceptors are not expressed in any cells within the adventitia of the aortic arch and are presumably associated with nerves whose cellular bodies are in the brainstem. The appearance of these three baroreceptors in the abdominal aorta may lead to the conclusion that these mechanosensitive ion channels are not specific for a particular function, such as, for instance, baroreflex. They are most likely to be expressed in the human aorta. However, little is known about, where the nerves containing the mechanosensitive ion channel lead. In the aortic arch but not in the abdominal aorta, the ion channels transmit probably the signal to the baroreflex centre in the brainstem. The aortic arch is innervated by the aortic depressor nerve and the abdominal aorta is innervated primarily by the abdominal aortic plexus [1, 2, 4].
Regarding mechanosensitive baroreceptors, more than 30 different ion channels have been described in the aortic arch of various animal models [8,9,10, 13, 14, 17,18,19, 21], such as ENaCs [10, 12, 43], ASIC [8, 11, 13, 16], TRPs [8, 13, 15, 16], and PIEZOs [9, 18, 19]. Interestingly, in the human aortic arch, we detected only the family of TRP ion channels, namely TRPV2, TRPM4, and PIEZO1 of the recently discovered PIEZO mechanosensitive family of ion channels.
TRPs belong to a large family of cellular sensors responding to various stimuli such as e. g. taste, temperature, osmotic stress, membrane stretching, and others [8, 15, 23, 44, 45]. TRPMs and TRPVs have been identified in various animal models as baroreceptors [8, 15, 46]. The mammalian TRPM family consists of 8 genes, TRPM1-8 (melastatin 1–8). Thereby, TRPM4, detected in our study, was shown to be permeable to monovalent cations and activated by intracellular Ca2+ ions, leading to a pronounced voltage modulation [47]. Furthermore, TRPM4 has been described as being able to transduce mechanical stimuli into a change in vascular tone [48]. Other researchers have reported that TRPM4 is activated by membrane stretch [49]. However, the role of TRPM4 in the baroreflex has not yet been described. The TRPV family (vanilloids 1–6) consists of six members. TRPV1-4 are sensitive to various physical stimuli such as stretching, shear stress or osmolarity [45]. They play important role in the regulation of normal and pathological cellular functions in the vasculature. Thereby, TRPV2 has been detected in a wide range of tissues and organs, including neurons, pancreatic β-cells, smooth muscle, and endothelial cells [24]. However, the relevance of TRPV2 and its function in vascular cells, particularly in the context of baroreflex, remain yet to be elucidated.
PIEZO1 was another putative baroreceptor detected in the present study. This ion channel is a promising candidate because PIEZOs have already been described to act as mechanosensitive baroreceptors gated by force, stretch sensation or shear stress [9, 19, 50, 51]. There are two known PIEZO ion channels, PIEZO1 and 2. They have already been described to play various roles in the pathogenesis of hypertension, affecting vascular cells, baroreflex, and the juxtaglomerular apparatus [8, 9, 51]. Furthermore, PIEZO1 has been detected in the cellular membrane, thus being a promising baroreceptor candidate [9]. The knockout of both PIEZO1 and PIEZO2 in a mouse model completely eliminated the baroreceptor reflex. Interestingly, we did not detect PIEZO2 in the human aorta. It seems that in humans, only PIEZO1 is acting as a putative mechanosensitive baroreceptor. In a rat model of hypertension, Cui et al. [52] emphasised the importance of PIEZO1 in decreasing blood pressure by injecting this protein directly into the bloodstream in a rat model of hypertension. Further studies are necessary to elucidate the role of PIEZO1 as a potential baroreceptor in the human aortic arch.
Study Limitations: Obtaining healthy human aortic arch tissue samples without cardiovascular comorbidity is challenging, limiting the sample size in our study to only three individuals, which is relatively small. Therefore, in order to enhance statistical power, we utilised different segments from the available specimens. Consequently, we analysed various segments, including FFPE and fresh-frozen samples (n = 6 and 16). The three putative baroreceptors - PIEZO1, TRPV2, and TRPM4 - were detected in all analysed samples and segments. Nevertheless, further research with more specimens and patients is needed to explore the role of these ion channels in baroreflex function and to fully elucidate their functional significance. In the next study, we intend to analyse the expression of these baroreceptors along the entire arterial tree in order to decipher their distribution in all human arteries.
Conclusions
In this study, we identified for the first time, putative baroreceptors in the human aortic arch. We found an equal distribution of nerves exclusively in the adventitia along the entire aortic arch, predominantly in the ascending aorta up to the left subclavian artery. Through proteome analysis, we identified three potential human baroreceptors PIEZO1, TRPV2, and TRPM4. Using transcriptomics as a process of elimination, no mRNA expression of these three ion channels was detected. Consequently, we hypothesise that these proteins originate from baroreceptor neurons with their cell bodies in the nodose ganglion, which is responsible for the baroreflex. Interestingly, we also found these ion channels in both healthy and diseased abdominal aortas. We assume that PIEZO1, TRPV2, and TRPM4 are generally expressed along the human aorta. Their function, however, is determined by the cell type they are expressed and in the case they are expressed in neurons, their function depends on which path the neuronal fibres take. Further studies are necessary to confirm our current results and determine the extent to which these three ion channels act as baroreceptors in the human aortic arch.
References
Thomas GD. Neural control of the circulation. Adv Physiol Educ. 2011;35:28–32.
Volpe M, Trimarco B, Ricciardelli B, Vigorito C, Rengo F, Veniero AM, et al. [Interrelation between the autonomic nervous system and the baroreceptors in hypertension]. Boll Soc Ital Cardiol. 1981;26:2105–17.
Yang H, Tenorio Lopes L, Barioni NO, Roeske J, Incognito AV, Baker J, et al. The molecular makeup of peripheral and central baroreceptors: stretching a role for Transient Receptor Potential (TRP), Epithelial Sodium Channel (ENaC), Acid Sensing Ion Channel (ASIC), and Piezo channels. Cardiovasc Res. 2022;118:3052–70.
Ferguson DW, Abboud FM, Mark AL. Relative contribution of aortic and carotid baroreflexes to heart rate control in man during steady state and dynamic increases in arterial pressure. J Clin Invest. 1985;76:2265–74.
Mancia G, Ferrari A, Gregorini L, Valentini R, Ludbrook J, Zanchetti A. Circulatory reflexes from carotid and extracarotid baroreceptor areas in man. Circ Res. 1977;41:309–15.
Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Denervation of carotid baro- and chemoreceptors in humans. J Physiol. 2003;553:3–11.
Lu HJ, Nguyen TL, Hong GS, Pak S, Kim H, Kim H, et al. Tentonin 3/TMEM150C senses blood pressure changes in the aortic arch. J Clin Invest. 2020;130:3671–83.
Reutersberg B, Pelisek J, Ouda A, de Rougemont O, Rossler F, Zimmermann A. Baroreceptors in the Aortic Arch and Their Potential Role in Aortic Dissection and Aneurysms. J Clin Med. 2022;11:1161.
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60.
Drummond HA, Welsh MJ, Abboud FM. ENaC subunits are molecular components of the arterial baroreceptor complex. Ann N. Y Acad Sci. 2001;940:42–47.
Fronius M, Clauss WG. Mechano-sensitivity of ENaC: may the (shear) force be with you. Pflug Arch. 2008;455:775–85.
Li YL, Zhang D, Tu H, Muelleman RL. Altered ENaC is Associated With Aortic Baroreceptor Dysfunction in Chronic Heart Failure. Am J Hypertens. 2016;29:582–9.
Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, et al. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–97.
Nilius B, Honore E. Sensing pressure with ion channels. Trends Neurosci. 2012;35:477–86.
Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218.
Sharif-Naeini R, Dedman A, Folgering JH, Duprat F, Patel A, Nilius B, et al. TRP channels and mechanosensory transduction: insights into the arterial myogenic response. Pflug Arch. 2008;456:529–40.
Takahashi K, Naruse K. Stretch-activated BK channel and heart function. Prog Biophys Mol Biol. 2012;110:239–44.
Tu H, Zhang D, Li YL. Cellular and Molecular Mechanisms Underlying Arterial Baroreceptor Remodeling in Cardiovascular Diseases and Diabetes. Neurosci Bull. 2019;35:98–112.
Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, et al. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science. 2018;362:464–7.
Glazebrook PA, Schilling WP, Kunze DL. TRPC channels as signal transducers. Pflug Arch. 2005;451:125–30.
Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron. 1998;21:1435–41.
Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene. 2016;579:95–132.
Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24.
O’Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflug Arch. 2005;451:193–203.
Pelisek J, Yundung Y, Reutersberg B, Meuli L, Rossler F, Rabin L, et al. Swiss Vascular Biobank: Evaluation of Optimal Extraction Method and Admission Solution for Preserving RNA from Human Vascular Tissue. J Clin Med 2023;12:5109.
Amini P, Ettlin J, Opitz L, Clementi E, Malbon A, Markkanen E. An optimised protocol for isolation of RNA from small sections of laser-capture microdissected FFPE tissue amenable for next-generation sequencing. BMC Mol Biol. 2017;18:22.
Mi H, Thomas P. PANTHER pathway: an ontology-based pathway database coupled with data analysis tools. Methods Mol Biol. 2009;563:123–40.
Thomas PD, Ebert D, Muruganujan A, Mushayahama T, Albou LP, Mi H. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 2022;31:8–22.
Hatakeyama M, Opitz L, Russo G, Qi W, Schlapbach R, Rehrauer H. SUSHI: an exquisite recipe for fully documented, reproducible and reusable NGS data analysis. BMC Bioinforma. 2016;17:228.
Qi W, Schlapbach R, Rehrauer H. RNA-Seq Data Analysis: From Raw Data Quality Control to Differential Expression Analysis. Methods Mol Biol. 2017;1669:295–307.
Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84.
Liu J, Song N, Wang Y, Walker J, Yu J. A single baroreceptor unit consists of multiple sensors. Sci Rep. 2021;11:23111.
Min S, Chang RB, Prescott SL, Beeler B, Joshi NR, Strochlic DE, et al. Arterial Baroreceptors Sense Blood Pressure through Decorated Aortic Claws. Cell Rep. 2019;29:2192–201.e2193.
Mohanta SK, Peng L, Li Y, Lu S, Sun T, Carnevale L, et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature. 2022;605:152–9.
Hu XQ, Zhang L. Role of transient receptor potential channels in the regulation of vascular tone. Drug Discov Today. 2024;29:104051.
Solano AS, Lavanderos B, Metwally E, Earley S. Transient Receptor Potential Channels in Vascular Mechanotransduction. Am J Hypertens. 2025;38:151–60.
Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol. 2011;203:99–116.
Earley S. TRPM4 channels in smooth muscle function. Pflug Arch. 2013;465:1223–31.
Shah V, Patel S, Shah J. Emerging role of Piezo ion channels in cardiovascular development. Dev Dyn. 2022;251:276–86.
Garat J, Di Paolo A, Eastman G, Castillo PE, Sotelo-Silveira J. The Trail of Axonal Protein Synthesis: Origins and Current Functional Landscapes. Neuroscience. 2025;567:195–208.
Goering R, Arora A, Pockalny MC, Taliaferro JM. RNA localization mechanisms transcend cell morphology. Elife. 2023; 12:e80040.
Leterrier C, Dargent B. No Pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Semin Cell Dev Biol. 2014;27:44–51.
Ben-Shahar Y. Sensory functions for degenerin/epithelial sodium channels (DEG/ENaC). Adv Genet. 2011;76:1–26.
Desai BN, Clapham DE. TRP channels and mice deficient in TRP channels. Pflug Arch. 2005;451:11–18.
Liedtke W, Kim C. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell Mol Life Sci. 2005;62:2985–3001.
Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417.
Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407.
Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: performing under pressure and going with the flow. Physiology. 2014;29:343–60.
Morita H, Honda A, Inoue R, Ito Y, Abe K, Nelson MT, et al. Membrane stretch-induced activation of a TRPM4-like nonselective cation channel in cerebral artery myocytes. J Pharm Sci. 2007;103:417–26.
Mousavi SAR, Dubin AE, Zeng WZ, Coombs AM, Do K, Ghadiri DA, et al. PIEZO ion channel is required for root mechanotransduction in Arabidopsis thaliana. Proc Natl Acad Sci USA 2021;118:e2102188118.
Nagase T, Nagase M. Piezo ion channels: long-sought-after mechanosensors mediating hypertension and hypertensive nephropathy. Hypertens Res. 2024; https://doi.org/10.1038/s41440-024-01820-6.
Cui CP, Xiong X, Zhao JX, Fu DH, Zhang Y, Ma PB, et al. Piezo1 channel activation facilitates baroreflex afferent neurotransmission with subsequent blood pressure reduction in control and hypertension rats. Acta Pharm Sin. 2024;45:76–86.
Acknowledgements
The authors gratefully acknowledge the Functional Genomics Center Zurich (FGCZ) of the University of Zurich and ETH Zurich, particularly the Proteomics Group (Dr Paolo Nanni), for their support in proteomics analysis.
Funding
This research project was funded by the Swiss National Science Foundation (grant number 310030_213001). The tissue samples used in this study were obtained from the Swiss Vascular Biobank supported by the Department of Vascular Surgery (Director Professor Alexander Zimmermann, University of Zurich, Switzerland). Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yundung, Y., Pelisek, J., Maccio, U. et al. Identification of putative baroreceptors in human aortic arch by histological and omics analyses. Hypertens Res 48, 2083–2094 (2025). https://doi.org/10.1038/s41440-025-02217-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41440-025-02217-9