Abstract
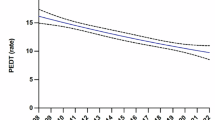
A phase II single-arm trial was conducted from June 2017 to October 2018 to evaluate the efficacy and safety of transcutaneous posterior tibial nerve stimulation (TPTNS) for premature ejaculation (PE) treatment. Twelve men with PE and no prior treatment were enrolled, one was withdrawn and 11 subjects provided data for the main outcome. TPTNS consisted of 30-min sessions of the application of 20 Hz with a pulse amplitude of 200 µsec. The intensity was adjusted based on individual sensibility. The participants received 3 weekly sessions for 12 consecutive weeks. Follow-up continued for 9 months after therapy completion. The main outcome was a threefold increase in the intravaginal ejaculation latency time (IELT) at week 12. Eleven patients completed therapy, and 54.5% (p = 0.037) showed tripled baseline IELT scores at week 12. The IELT increased 4.8-fold, 6.8-fold, and 5.4-fold at weeks 12, 24, and 48, respectively. One episode of constipation was reported, and one patient reported a sensation of heat in the leg during one therapy session. The findings suggest that TPTNS therapy delays ejaculation in patients with lifelong premature ejaculation, with no serious secondary effects. Controlled trials with larger sample sizes are needed to verify these results.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Porst H, Montorsi F, Rosen RC, Gaynor L, Grupe S, Alexander J. The premature ejaculation prevalence and attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. Eur Urol. 2007;51:816–24.
Sridharan K, Sivaramakrishnan G, Sequeira RP, Al-Khaja KA. Pharmacological interventions for premature ejaculation: a mixed-treatment comparison network meta-analysis of randomized clinical trials. Int J Impot Res. 2018;30:215–23.
Jin K, Deng L, Qiu S, Tu X, Li J, Bao Y, et al. Comparative efficacy and safety of phosphodiesterase-5 inhibitors with selective serotonin reuptake inhibitors in men with premature ejaculation: A systematic review and Bayesian network meta-analysis. Medicine. 2018;97:e13342.
Althof SE. Psychosexual therapy for premature ejaculation. Transl Androl Urol. 2016;5:475–81.
Russo A, Capogrosso P, Ventimiglia E, La Croce G, Boeri L, Montorsi F, et al. Efficacy and safety of dapoxetine in treatment of premature ejaculation: an evidence-based review. Int J Clin Pr. 2016;70:723–33.
Castro R, Cruz N, Cabello F, García F, Fernández A, Larrazábal M, et al. Diagnóstico, tratamiento y seguimiento de la eyaculación precoz: recomendaciones de experto. Rev Internacional de Andrología. 2017;15:70–77.
Althof SE, McMahon CG, Waldinger MD, Serefoglu EC, Shindel AW, Adaikan PG, et al. An update of the International Society of Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation (PE). J Sex Med. 2014;11:1392–422.
Giuliano F. Neurophysiology of erection and ejaculation. J Sex Med. 2011;8:310–5.
Alwaal A, Breyer BN, Lue TF. Normal male sexual function: emphasis on orgasm and ejaculation. Fertil Steril. 2015;104:1051–60.
El-Hamd MA, Saleh R, Majzoub A. Premature ejaculation: an update on definition and pathophysiology. Asian J Androl. 2019;21:425–32.
Ferran J, Puigvert A, Castro P. Eyaculación prematura. Rev Int Androl. 2010;8:28–50. 2010;8(1):28–50
Perissinotto MC, DʼAncona CA, Lucio A, Campos RM, Abreu A. Transcutaneous tibial nerve stimulation in the treatment of lower urinary tract symptoms and its impact on health-related quality of life in patients with Parkinson disease: a randomized controlled trial. J Wound Ostomy Cont Nurs. 2015;42(Jan-Feb):94–9.
Sucar-Romero S, Escobar-del Barco L, Rodríguez-Colorado S, Gorbea-Chávez V. Estimulación del nervio tibial posterior como tratamiento de la disfunción del piso pélvico. Revisión de la bibliografía. Ginecol Obstet Mex. 2014;82:535–46.
McMahon CG. The design and methodology of premature ejaculation interventional studies. Transl Androl Urol. 2016;5:508–25. https://doi.org/10.21037/tau.2016.03.28.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). 5th ed. Washington, DC: American Psychiatric Association; 2013.
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26.
Symonds T, Roblin D, Hart K, Althof S. How does premature ejaculation impact a man’s life? J Sex Marital Ther. 2003;29:361–70.
Fleming T. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–51.
Porto R. The impact of premature ejaculation on quality of life of the patient, the partner and the couple. Sexologies. 2013;22:e65–e70.
Zulkifli H, Saharuddin A. Premature ejaculation and quality of life among men attending Klinik Kesihatan Jaya Gading, Kuantan. Int J Public Health Res. 2018;8:878–84.
Tuken M, Culha MG, Serefoglu EC. Efficacy and safety of dapoxetine/sildenafil combination tablets in the treatment of men with premature ejaculation and concomitant erectile dysfunction-DAP-SPEED Study. Int J Impot Res. 2019;31:92–96.
McMahon CG, Jannini E, Waldinger M, Rowland D. Standard operating procedures in the disorders of orgasm and ejaculation. J Sex Med. 2013;10:204–29.
Waldinger MD, Zwinderman AH, Schweitzer DH, Olivier B. Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int J Impot Res. 2004;16:369–81.
Castiglione F, Albersen M, Hedlund P, Gratzke C, Salonia A, Giuliano F. Current pharmacological management of premature ejaculation: a systematic review and meta-analysis. Eur Urol. 2016;69:904–16.
Acknowledgements
The authors would like to thank Jenny Gutiérrez, Andrés Gallego, and Cristina Amaya for their contributions in the execution of this research study.
Funding
This work was funded by the clinical center where this research was developed, and the devices where purchased without any help from the developer industry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors are the employees of the clinical center where this research was developed, and there is no relation to the pharmaceutical or technology industry.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uribe, O.L., Sandoval-Salinas, C., Corredor, H.A. et al. Transcutaneous electric nerve stimulation to treat patients with premature ejaculation: phase II clinical trial. Int J Impot Res 32, 434–439 (2020). https://doi.org/10.1038/s41443-019-0196-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41443-019-0196-x
This article is cited by
-
Pelvic physical therapy for male sexual disorders: a narrative review
International Journal of Impotence Research (2025)
-
What Is the Role of Ejaculation Latency in the Diagnosis of Premature Ejaculation and Does the Ejaculation Latency Threshold Matter?
Current Sexual Health Reports (2025)
-
Effect of cranial electrotherapy stimulation (CES) in treatment of premature ejaculation: a randomized clinical trial
Middle East Current Psychiatry (2024)
-
Low frequency neuromuscular electrical stimulation applied to the bulbospongiosus muscle prolongs the ejaculation latency in a rat model
International Journal of Impotence Research (2024)
-
New technologies developed for treatment of premature ejaculation
International Journal of Impotence Research (2024)