Abstract
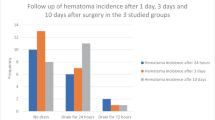
Surgical site infections remain a devastating complication of inflatable penile prosthesis (IPP) implantation. We evaluated a novel surgical protocol combining chlorhexidine-alcohol skin preparation with fibrin sealant-mediated hemostasis and no drain placement, hypothesizing that it would reduce infection and hematoma rates. Between January 2020 and December 2023, 103 men underwent primary IPP placement with a protocol of 2% chlorhexidine gluconate/70% isopropyl alcohol (ChloraPrep®) skin antisepsis and intraoperative application of Evicel® fibrin sealant to corporotomy suture lines, subcutaneous tissues, and pump pocket. This group was compared with 115 historical controls who received povidone iodine skin preparation only and routine surgical drains between January 2015 to December 2019. The primary endpoint was IPP infection at 12 months; secondary endpoints included hematoma rate, operative time, length of stay, and patient satisfaction. No infections occurred in the study cohort (0/103), compared to 3.5% (4/115) of controls (p < 0.001). Hematoma incidence was 1.0% (1/103) versus 5.2% (6/115) in controls (p = 0.03). Mean operative time (52.4 ± 13.8 min vs. 58 ± 18 min, p = 0.12) was similar between groups, while same-day discharge rates were higher in the study cohort (94.7 vs. 88.0%, p = 0.03). Combining chlorhexidine-alcohol skin antisepsis with fibrin sealant hemostasis and eliminating drains is a viable option to reduce infections and hematomas in IPP surgery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Carson CC, Mulcahy JJ, Harsch MR. Long-term infection outcomes after original antibiotic impregnated inflatable penile prosthesis implants: up to 7.7 years of followup. J Urol. 2011;185:614–8.
Eid JF, Wilson SK, Cleves M, Salem EA. Coated implants and “no touch” surgical technique decreases risk of infection in inflatable penile prosthesis implantation to 0.46%. Urology. 2012;79:1310–5.
Licht MR, Montague DK, Angermeier KW, Lakin MM. Cultures from genitourinary prostheses at re-operation: questioning the role of staphylococcus epidermidis in periprosthetic infection. J Urol. 1995;154:387.
Lotan Y, Roehrborn, McConnell JD, Hendin BN. Factors influencing the outcomes of penile prosthesis surgery at teaching institution. Urology. 2003;62:918.
Henry GD, Wilson SK, Delk JR 2nd, Carson CC, Wiygul J, Tornehi C, et al. Revision washout decreases penile prosthesis infection in revision surgery: a multicenter study. J Urol. 2005;173:89–92.
Darouiche RO, Wall MJ Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26.
Swenson BR, Hedrick TL, Metzger R, Bonatti H, Pruett TL, Sawyer RG. Effects of preoperative skin preparation on postoperative wound infection rates: a prospective study of 3 skin preparation protocols. Infect Control Hosp Epidemiol. 2009;30:964–71.
Desouky E, Tsambarlis P, Levine LA. Comparing 0.05% chlorhexidine gluconate monotherapy to conventional antibiotic irrigation in de-novo penile prosthesis implantation: a two-center prospective randomized controlled non-inferiority study (preliminary results). Transl Androl Urology. 2024;13:1905–11.
Garber BB, Bickell M. Delayed postoperative hematoma formation after inflatable penile prosthesis implantation. J Sex Med. 2015;12:265–9.
Braun AE, Swerdloff D, Sudhakar A, Patel RD, Gross MS, Simhan J. Defining the incidence and management of postoperative scrotal hematoma after primary and complex three-piece inflatable penile prosthesis surgery. Int J Impot Res. 2025;37:82–86.
Rezaee ME, Towe M, Osman MM, Huynh LM, El-Khatib FM, Andrianne R, et al. A multicenter investigation examining American urological association recommended antibiotic prophylaxis vs nonstandard prophylaxis in preventing device infections in penile prosthesis surgery in diabetic patients. J Urol. 2020;204:969–75.
Moldovan H, Antoniac I, Gheorghiță D, Safta MS, Preda S, Broasca M, et al. Biomaterials as haemostatic agents in cardiovascular surgery: review of current situation and future trends. Polymers. 2022;14:1189.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453-7.
Althof SE, Corty EW, Levine SB, Levine F, Burnett AL, McVary K, et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology. 1999;53:793–9.
Kapadia BH, Elmallah RK, Mont MA. A randomized, clinical trial of preadmission chlorhexidine skin preparation for lower extremity total joint arthroplasty. J Arthroplasty. 2016;31:2856–61.
Karpman E, Griggs R, Twomey C, Henry GD. Dipping Titan implants in Irrisept® solution (0.05% chlorhexidine gluconate) and exposure to various aerobic, anaerobic, and fungal species. J Sex Med. 2023;20:1025–31.
Vintimilla DR, Chambers L, Mauffrey C, Parry JA. Just add water? Chlorhexidine’s antimicrobial properties are minimally affected by dilution in saline compared to water. Eur J Orthop Surg Traumatol. 2020;30:613–5.
Irrisept Chlorhexidine Gluconate 0.05% Irrigation Solution, 450 mL Bottle [price catalog]. Northfield, IL: Medline Industries, Inc.; 2024. [Accessed May 24, 2024]. Available from: https://www.medline.com.
Sadeghi-Nejad H, Ilbeigi P, Wilson SK, Delk JR, Siegel A, Seftel AD, et al. Multi-institutional outcome study on the efficacy of closed-suction drainage of the scrotum in three-piece inflatable penile prosthesis surgery. Int J Impot Res. 2005;17:535–8.
Spotnitz WD, Burks S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion. 2008;48:1502–16.
Sebbane N, Abramovitz I, Kot-Limon N, Steinberg D. Mechanistic Insight into the anti-bacterial/anti-biofilm effects of low chlorhexidine concentrations on enterococcus faecalis-in vitro study. Microorganisms. 2024;12:2297.
Moukhtar Hammad MA, Barham DW, Stanford DI, Amini E, Jenkins L, Yafi FA. Maximizing outcomes in penile prosthetic surgery: exploring strategies to prevent and manage infectious and non-infectious complications. Int J Impot Res. 2023;35:613–9.
Mandava SH, Serefoglu EC, Freier MT, Wilson SK, Hellstrom WJ. Infection retardant coated inflatable penile prostheses decrease the incidence of infection: a systematic review and meta-analysis. J Urol. 2012;188:1855–60.
Scardino M, Martorelli F, D’Amato T, Fenocchio G, Simili V, Grappiolo G, et al. Use of a fibrin sealant within a blood-saving protocol in patients undergoing revision hip arthroplasty: effects on post-operative blood transfusion and healthcare-related cost analysis. Int Orthop. 2019;43:2707–14.
Mirheydar HS, Palazzi KL, Parsons JK, Chang D, Hsieh TC. Hospital-based trends in penile prosthetic surgery. J Sex Med. 2015;12:1092–8.
Andino JJ, Leelani N, Sato R, Shin Y, Rojanasarot S, Furtado T, et al. Association between surgeon procedure volume and reoperation rates for penile prosthesis implantation. J Sex Med. 2025;22:916–23.
Schulz S, Khan A, Evans J, Charles BR, Parnes N, Scanaliato JP. Estimated cost of fibrin glue. J Orthop Bus. 2024. https://doi.org/10.55576/job.v4i2.56
Schindl M, Függer R, Götzinger P, Langle F, Zitt M, Stattner S, et al. Randomized clinical trial of the effect of a fibrin sealant patch on pancreatic fistula formation after pancreatoduodenectomy. Br J Surg. 2018;105:811–9.
Funding
The authors have no funding to declare.
Author information
Authors and Affiliations
Contributions
Ali Fathollahi Writing- Original draft preparation, Data curation, Formal Analysis, Writing- Review & Editing. Shirin Razdan: Review & Editing, Validation, Methodology. Sanjay Razdan: Providing the data, Writing- Review & Editing. All authors discussed the results and contributed to the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
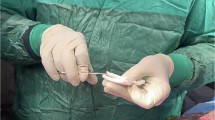
This study was performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. Ethical approval was granted by the Institutional Review Board of HCA Florida Kendall Hospital (IRB-41-2930). Informed consent was obtained from all individual participants included in the study. Written informed consent for publication of the clinical images (Fig. 1) was obtained from the participant.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fathollahi, A., Razdan, S. & Razdan, S. Zero infection protocol in inflatable penile prosthesis surgery: a prospective cohort study using chlorhexidine-alcohol skin preparation and fibrin sealant hemostasis. Int J Impot Res (2025). https://doi.org/10.1038/s41443-025-01174-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41443-025-01174-8
This article is cited by
-
Response to Comment on: Zero infection protocol in inflatable penile prosthesis surgery: a prospective cohort study using chlorhexidine-alcohol skin preparation and fibrin sealant hemostasis
International Journal of Impotence Research (2026)
-
Comment on: Zero infection protocol in inflatable penile prosthesis surgery: a prospective cohort study using chlorhexidine-alcohol skin preparation and fibrin sealant hemostasis
International Journal of Impotence Research (2025)