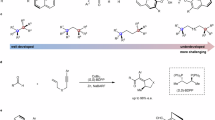
Abstract
Chirality constitutes an inherent attribute of nature. The catalytic asymmetric synthesis of molecules with central, axial, and helical chirality is a topic of intense interest and is becoming a mature field of research. However, due to the difficulty in synthesis and the lack of a prototype, less attention has been given to planar chirality arising from the destruction of symmetry on a single planar ring. Herein, we report the catalytic asymmetric synthesis of planar-chiral dianthranilides, a unique class of tub-shaped eight-membered cyclic dilactams. This protocol is enabled by cinchona alkaloid-catalyzed (dynamic) kinetic resolution. Under mild conditions, various C2- or C1-symmetric planar-chiral dianthranilides have been readily prepared in high yields with excellent enantioselectivity. These dianthranilides can serve as an addition to the family of planar-chiral molecules. Its synthetic value has been demonstrated by kinetic resolution of racemic amines via acyl transfer, enantiodivergent synthesis of the natural product eupolyphagin, and preliminary antitumor activity studies.
Similar content being viewed by others
Introduction
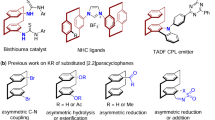
Chirality, an inherent geometric property of any three-dimensional object that cannot coincide with its mirror image, plays a crucial role in a range of disciplines including chemistry, medicine, materials, and life sciences (Fig. 1A)1. Central chirality, which is based on sp3 hybridized stereogenic centers (X = C, Si, P, S, etc.) with four different groups, constitutes the most common one. Conformational chirality, in addition, arises when a particular molecular conformation is sufficiently stable. For instance, the restricted rotation of a σ‐bond leads to axial chirality2,3,4, and steric repulsion between the terminal aromatic rings of helicenoids results in helical chirality5,6. Currently, the catalytic asymmetric synthesis of molecules with central, axial, and helical chirality is a topic of intense interest and is becoming a mature field of research. In sharp contrast, planar chirality arising from the destruction of symmetry on a single planar ring has received less attention7. Metallocenes8,9,10,11,12,13, cyclophanes14,15,16,17,18,19,20,21, and some rigid cycloalkenes22,23,24,25,26,27,28 are typical planar-chiral structures. With recognition of the importance of planar chirality, particularly in chiral ligand/catalyst discovery29,30,31,32,33, several catalytic enantioselective synthesis methods have been developed34,35,36,37,38,39. This is the case for assembling planar-chiral ferrocenes via enantioselective C−H activation by You, Zhou, and others40,41,42,43,44,45. Despite these achievements, difficulties in synthesis and the lack of prototypes have greatly limited the application of planar chirality, and the catalytic asymmetric synthesis of planar-chiral compounds is still in its infancy.
Dianthranilides, dibenzo[b,f][1,5]diazocine-diones, are a unique class of eight-membered cyclic lactams (Fig. 1B)46. The secondary cis-amide groups and the particular tricyclic ring system make dianthranilide an attractive scaffold for use in supermolecule, materials chemistry, and pharmaceuticals47,48,49. For example, the natural alkaloid eupolyphagin exhibited a promising cytotoxic effect on cancer cell lines50. Analogous to rigid V-shaped Tröger′s base in structure and geometry51, dianthranilide adopts a tub-shaped dominant conformation and therefore is a C2-symmetric planar-chiral molecule52,53. However, the unlocked flexible conformation allows fast interconversion of the two enantiomers (S and R forms) at ambient temperature. As a result, dianthranilides are usually racemic, and their catalytic asymmetric synthesis poses a daunting challenge. To our knowledge, in 2017, Tan et al. reported the only example of enantioselective synthesis of a dianthranilide derivative54. Their strategy was to construct the key axially chiral intermediate followed by the formation of an eight-member lactam via axial-to-planar chirality transfer. Given their many intriguing features and easy accessibility, dianthranilides could serve as an additional prototype of planar-chiral molecules. Herein, we set our goal to develop direct catalytic asymmetric methods to access conformationally stable planar-chiral dianthranilides with the ability to achieve chiral induction via asymmetric catalysis. To obtain rigid conformers, inhibiting boat-to-boat ring inversion is key55,56. We assume that N-alkylated dianthranilides 3-5 are more conformationally stable and that their catalytic asymmetric synthesis could be enabled by dynamic kinetic resolution (DKR) or kinetic resolution (KR) (Fig. 1C)57,58,59,60.
Results
Reaction development
To determine its feasibility, dianthranilide 1a was chosen as the model substrate, and Morita–Baylis–Hillman (MBH) adduct 2a was selected as the alkylating agent. To our delight, upon the catalysis of various commercially available cinchona alkaloids, asymmetric dialkylation readily occurred, allowing the formation of conformationally stable 3a via DKR (Table 1). In the presence of cinchonidine (C1), the alkylation-enabled DKR afforded product 3a in 45% yield with 18% ee (entry 1). The solvent effect was then investigated, and acetonitrile was identified as the solvent of choice (entries 2–4). Consequently, catalyst screening was carried out in acetonitrile (entries 5–9). While all catalysts were effective, quinine (C5) and quinidine (C6) were found to be most effective in terms of yield and enantioselectivity (entries 8–9). Notably, quinine and quinidine afforded different enantiomers of 3a.
Substrate scope
With the best conditions, the substrate scope of this DKR reaction was subsequently studied (Fig. 2). First, the generality of the MBH adducts was tested (3b−f). The excellent yields and ee values indicated that various ester groups were well tolerated. Next, we turned our attention to the scope of accessing dianthranilides 1. The reaction was applicable to a range of symmetrically substituted dianthranilides, leading to the formation of dialkylated products 3 g−v in generally excellent yields and enantioselectivities. Neither the position (from the C3- to C6-position) nor the electron nature (electron-donating or electron-withdrawing) of the substituents had an obvious effect on this reaction. In addition, di-substituted and phenyl-fused substrates were employed, which delivered corresponding products 3w−y in excellent yields and ee values. Unsymmetric dianthranilides are challenging substrates for DKR, since intriguing regioisomers may be created in the 1st alkylation, thus affecting the enantioselectivity. Remarkably, when unsymmetric dianthranilides were subjected, the reaction readily occurred, giving the non-C2-symmetric products 3z and 3aa. Considering the consistently excellent enantioselectivity of both the C2- and C1-symmetric products, it can be concluded that this cinchona alkaloid-catalyzed alkylation had good face selectivity and was not affected by steric hindrance. The absolute configurations of these N-alkylated products were assigned on the basis of X-ray crystallographic analysis of 3 m.
To elucidate the reaction mechanism, control and kinetic experiments were performed (Fig. 3). Under racemic conditions, enantioenriched monoalkylation product 1a′ underwent a 2nd N-alkylation reaction with the MBH adduct 2a to afford product 3a with a constant ee (Fig. 3A). In addition, racemization experiments of 1a, 1a′ and 3a were conducted to investigate their configurational stabilities (Fig. 3B). The low rotational barrier (23.3 kcal/mol) indicated a dynamic interconversion in 1a. On the other hand, the monoalkylation product 1a′ was stable enough, with an inversion barrier of 28.2 kcal/mol, while that of dialkylation product 3a was 32.6 kcal/mol61. Therefore, the monoalkylation product 1a′ should be the key intermediate, and the 1st N-alkylation is the DKR and enantio-determining step. In addition, kinetic studies were conducted to explore the reaction pathway. As shown in Fig. 3C, the formation of dialkylation product 3a was fast. While the ee of 3a was maintained at the same high level, substrate 1a was always racemic. This further verified the previous speculation that dialkylation is a DKR process. Furthermore, presynthesized racemic 1a′ was used under standard conditions (Fig. 3D). Enantioenriched 3a was obtained initially, and its ee decreased over time. The ee of substrate 1a increased over time. These results indicated a distinct KR process. To demonstrate this concept, the KR of racemic 1a′ was carried out (Fig. 3E). Under standard conditions, racemic 1a′ reacted smoothly with 0.6 equivalents (eq.) of 2a. The corresponding 3a was obtained in 49% yield with 95% ee, and 1a′ was recovered in 48% yield with 99% ee.
Considering that the excellent s-factor (s = 205) is comparable to that of enzyme catalysis, this KR reaction is synthetically useful. In this context, we prepared several mono-substituted dianthranilides and subjected them to the KR. The results are summarized in Fig. 4. The variation of the MBH adducts was tested. Various ester groups, such as -CO2tBu, -CO2Et, -CO2nBu, -CO2iBu, and -CO2Bn, were well tolerated, affording alkylated products 5a−e and recovered 4a in good yields with excellent ee. Furthermore, dianthranilides bearing substituents on the phenyl ring were also compatible, but the enantioselectivity of products 5 f−j decreased to some extent. The generality of the N-substituents was then investigated. In addition to the substituted benzyl groups (5k−m), allyl (5n), 2-ethoxy-2-oxoethyl (5o), and phenyl (5p) groups were also applied. Notably, dianthranilide substrates featuring acyl groups of N-substitutions had flexible conformations, thus providing DKR results (5q & 5r).
Based on the above experimental results and previous reports on asymmetric amide N-alkylations with MBH adducts62,63,64,65, a plausible reaction mechanism and transition state have been proposed to elucidate the origin of the high stereoselectivity of these reactions (Fig. 5). Taking the synthesis of dialkylation product (R)-3a as an example, the catalyst quinidine (C6) reacts with MBH adduct 2a in an SN2′ fashion to form the chiral adduct C6′, CO2, and tert-butoxide anion. Then, the resulting C6′ distinguishes the two enantiomers of substrate 1a by hydrogen-bonding interactions. The conformationally matched (R)-1a forms a dominant hydrogen bonding network with C6′ via transition state TS-R. However, the same hydrogen bonding network will cause additional steric hindrance for the mismatched (S)-1a (TS-S). As a result, (R)-1a undergoes asymmetric N-alkylation accompanied by the regeneration of catalyst C6, while (S)-1a racemizes due to its low rotational barrier. In the case of excessive alkylation reagents, the monoalkylated product 1a′ generated in situ will rapidly undergo a second alkylation reaction, delivering dialkylation product 3a. The preliminary DFT calculations support our hypothesis, and this transition state model is also applicable to the KR (see Supplementary Figs. 242–244 and Source Data for details).
Finally, to highlight the synthetic utility, scaled-up DKR, and KR experiments were conducted (Fig. 6A). Under standard conditions, the DKR of racemic 1a furnished dialkylated product 3a in 95% yield with 98% ee. Notably, one of these two allyl groups can be selectively removed with NaOH, delivering monoalkylated intermediate 1a′ with a maintained ee. Further, acylation with BzCl gave product 6a, which was proven to be a potential chiral acylation reagent in the subsequent investigations. Moreover, the KR of racemic 4a readily occurred with a perfect s-factor, affording (S)-4a and the (R)-5a with excellent enantioselectivity. More importantly, the dealkylation of (R)-5a can in turn generate (R)-4a. In this way, the direct resolution of (±)-4a can be achieved, allowing access to its two enantiomers with a single chiral catalyst. In addition, these synthesized dianthranilides could serve as potential chiral reagents for acyl transfer (Fig. 6B). Direct acylation of 1a′ and 4a afforded compounds 6 with maintained ee values. Treatment of 6 with racemic 3,3-dimethylbutan-2-amine regenerated 1a′ and 4a with an excellent recycling rate of 95%. At the same time, the KR of (±)-7 was achieved with moderate to good selectivity. This preliminary attempt indicated that conformationally stable planar-chiral dianthranilides could serve as a promising platform for enantioselective synthesis. To further demonstrate the practicality of this protocol, the enantiodivergent synthesis of the natural product eupolyphagin was accomplished (Fig. 6C). Starting from the commercially available 2-iodo-6-methoxyaniline 9, racemic intermediate 10 can be obtained in 5 steps according to Tan’s procedures54. Then, the projected KR served as the key step to afford the highly enantioenriched precursor (S)-10 and alkylation product (R)-11. Dealkylation of (R)-11 led to the (R)-10 precursor. Subsequent deprotection of the methyl group with BBr3 afforded the natural products (+)-eupolyphagin and (-)-eupolyphagin in good yields.
Moreover, we selected a few products (3a, 3 f, 3 g, 3 h, 3j, 3 m, 3p and 3q for DKR; 5a, 5e, 5 f, 5 g, 5 h, 5n and 5o for KR) for anticancer activity evaluation of cell viability via the CTG assay for A2780 (ovarian cancer), HeLa (cervical carcinoma), HT-29 (colon cancer), LoVo (colon cancer), MV-4-11 (acute monocytic leukemia), and U87-MG (astrocytoma) human cancer cell lines (see the Supplementary Table 16 for details). Compounds 3a (A2780 cells, IC50 = 13.89 μM; HeLa cells, IC50 = 37.28 μM; HT-29 cells, IC50 = 28.41 μM; LoVo cells, IC50 = 36.25 μM; MV-4-11 cells, IC50 = 21.00 μM; U87-MG cells, IC50 = 47.68 μM), 3q (A2780 cells, IC50 = 16.24 μM; Hela cells, IC50 = 61.51 μM; HT-29 cells, IC50 = 54.07 μM; LoVo cells, IC50 = 53.60 μM; MV-4-11 cells, IC50 = 38.79 μM; U87-MG cells, IC50 = 109.33 μM), and 5 g (A2780 cells, IC50 = 10.58 μM; HeLa cells, IC50 = 16.80 μM; HT-29 cells, IC50 = 15.06 μM; LoVo cells, IC50 = 14.75 μM; MV-4-11 cells, IC50 = 14.27 μM; U87-MG cells, IC50 = 25.43 μM) exhibited significant and broad anticancer potency (Fig. 6D). Consequently, these planar-chiral dianthranilides have the potential to become lead anticancer compounds. Further structure-activity relationship-studies are ongoing in our laboratory.
In conclusion, we have accomplished the catalytic enantioselective synthesis of planar-chiral dianthranilides. In contrast to the well-studied central, axial, and helical chirality, less attention has been paid to planar chirality due to the difficulty in synthesis and the lack of a prototype. Herein, we report a highly efficient (dynamic) kinetic resolution protocol for the synthesis of tub-shaped dianthranilides, which serve as an addition to the family of planar-chiral molecules. Under the catalysis of cinchona alkaloids, various C2- or C1-symmetric planar-chiral dianthranilides have been readily prepared in high yields and with excellent enantioselectivities. The preliminary attempt at the kinetic resolution of racemic phenylethylamine via acyl transfer demonstrated that conformationally stable planar-chiral dianthranilides can serve as a promising platform for enantioselective synthesis. Using this method, the enantiodivergent synthesis of the natural product eupolyphagin was accomplished. Further applications and other related investigations along this line are ongoing and will be reported in due course.
Methods
General procedure for DKR
Substrate 1 (0.10 mmol) and catalyst C5 (10 mol%) were dissolved in CH3CN, and MBH ester 2 (0.24 mmol) was added. The reaction mixture was stirred for 6 h at room temperature. The solvent was removed in vacuo and the crude product was separated by flash column chromatography on silica gel (petroleum ether/ethyl acetate = 4:1) to afford 3.
General procedure for KR
Substrate 4 (0.10 mmol) and catalyst C6 (10 mol%) were dissolved in CH3CN, and MBH ester 2 (0.06 mmol) was added. The reaction mixture was stirred for 6 h at room temperature. The solvent was removed in vacuo and the crude product was separated by flash column chromatography on silica gel (petroleum ether/ethyl acetate = 3:1 – 1:1) to afford recovered (S)-4 and (R)-5.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the data relating to the characterization of products, experimental protocols, and computational studies are available within the article and its Supplementary Information. The data for the crystal structure 3 m reported in this paper were deposited at the Cambridge Crystallographic Data Center (CCDC) under the deposition number CCDC 2271258. Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. Further data supporting the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Carreira, E. M. & Kvaerno, L. Editors. Classics in Steroeselective Synthesis. (John Wiley & Sons, Inc., 2009).
Cheng, J. K., Xiang, S. H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Mei, G.-J., Koay, W. L., Guan, C.-Y. & Lu, Y. Atropisomers beyond the C–C axial chirality: Advances in catalytic asymmetric synthesis. Chem 8, 1855–1893 (2022).
Carmona, J. A., Rodriguez-Franco, C., Fernandez, R., Hornillos, V. & Lassaletta, J. M. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 50, 2968–2983 (2021).
Wang, Y., Wu, Z. G. & Shi, F. Advances in catalytic enantioselective synthesis of chiral helicenes and helicenoids. Chem Catal. 2, 3077–3111 (2022).
Liu, W., Qin, T., Xie, W. & Yang, X. Catalytic enantioselective synthesis of helicenes. Chem. Eur. J. 28, e202202369 (2022).
Lopez, R. & Palomo, C. Planar chirality: a mine for catalysis and structure discovery. Angew. Chem. Int. Ed. 61, e202113504 (2022).
Wang, Y. et al. Recent advances in transition-metal-catalyzed enantioselective syntheses of planar chiral ferrocenes. Chin. J. Org. Chem. 35, 1399 (2015).
Zhu, D.-Y., Chen, P. & Xia, J.-B. Synthesis of planar chiral ferrocenes by transition-metal-catalyzed enantioselective C−H activation. ChemCatChem 8, 68–73 (2016).
Gao, D. W., Gu, Q., Zheng, C. & You, S. L. Synthesis of planar chiral ferrocenes via transition-metal-catalyzed direct C-H bond functionalization. Acc. Chem. Res. 50, 351–365 (2017).
Huang, J., Gu, Q. & You, S. Synthesis of planar chiral ferrocenes via transition-metal-catalyzed direct C-H bond functionalization. Chin. J. Org. Chem. 38, 51 (2018).
Yoshida, K. & Yasue, R. Planar-chiral ferrocene-based N-heterocyclic carbene ligands. Chem. Eur. J. 24, 18575–18586 (2018).
Liu, C.-X., Gu, Q. & You, S.-L. Asymmetric C–H bond functionalization of ferrocenes: new opportunities and challenges. Trends Chem. 2, 737–749 (2020).
Hassan, Z., Spuling, E., Knoll, D. M., Lahann, J. & Brase, S. Planar chiral [2.2]paracyclophanes: from synthetic curiosity to applications in asymmetric synthesis and materials. Chem. Soc. Rev. 47, 6947–6963 (2018).
Chen, J. F., Ding, J. D. & Wei, T. B. Pillararenes: fascinating planar chiral macrocyclic arenes. Chem. Commun. 57, 9029–9039 (2021).
Felder, S., Wu, S., Brom, J., Micouin, L. & Benedetti, E. Enantiopure planar chiral [2.2]paracyclophanes: synthesis and applications in asymmetric organocatalysis. Chirality 33, 506–527 (2021).
Tanaka, K., Sagae, H., Toyoda, K., Noguchi, K. & Hirano, M. Enantioselective synthesis of planar-chiral metacyclophanes through rhodium-catalyzed alkyne cyclotrimerization. J. Am. Chem. Soc. 129, 1522–1523 (2007).
Araki, T., Noguchi, K. & Tanaka, K. Enantioselective synthesis of planar-chiral carba-paracyclophanes: rhodium-catalyzed [2+2+2] cycloaddition of cyclic diynes with terminal monoynes. Angew. Chem. Int. Ed. 52, 5617–5621 (2013).
Gagnon, C. et al. Biocatalytic synthesis of planar chiral macrocycles. Science 367, 917–921 (2020).
Wang, D., Shao, Y. B., Chen, Y., Xue, X. S. & Yang, X. Enantioselective synthesis of planar-chiral macrocycles through asymmetric electrophilic aromatic amination. Angew. Chem. Int. Ed. 61, e202201064 (2022).
Yu, S., Bao, H., Zhang, D. & Yang, X. Kinetic resolution of substituted amido[2.2]paracyclophanes via asymmetric electrophilic amination. Nat. Commun. 14, 5239 (2023).
Hou, X.-L., Huang, H. & Wong, H. N. C. Planar dehydro[8]annulenes and their ramifications: substituted tribenzo[a,c,e]cyclooctenes and tetrabenzo[a,c,e,g]cyclooc. Synlett 2005, 1073–1089 (2005).
Han, J. W., Li, X. & Wong, H. N. Our expedition in eight-membered ring compounds: from planar dehydrocyclooctenes to tub-shaped chiral tetraphenylenes. Chem. Rec. 15, 107–131 (2015).
Han, J.-W., Peng, X.-S. & Wong, H. N. C. Synthesis of tetraphenylene derivatives and their recent advances. Nat. Sci. Rev. 4, 892–916 (2017).
Tang, M. Y. & Yang, X. Y. Catalytic enantioselective synthesis of inherently chiral molecules: recent advances. Eur. J. Org. Chem. 26, https://doi.org/10.1002/ejoc.202300738 (2023).
Tomooka, K., Komine, N., Fujiki, D., Nakai, T. & Yanagitsuru, S.-I. Planar chiral cyclic ether: asymmetric resolution and chirality transformation. J. Am. Chem. Soc. 127, 12182–12183 (2005).
Tomooka, K., Uehara, K., Nishikawa, R., Suzuki, M. & Igawa, K. Enantioselective synthesis of planar chiral organonitrogen cycles. J. Am. Chem. Soc. 132, 9232–9233 (2010).
Igawa, K. et al. Catalytic enantioselective synthesis of planar-chiral cyclic amides based on a Pd-Catalyzed asymmetric allylic substitution reaction. J. Am. Chem. Soc. 137, 7294–7297 (2015).
Fu, G. C. Applications of planar-chiral heterocycles as ligands in asymmetric catalysis. Acc. Chem. Res. 39, 853–860 (2006).
Zhang, W., Butt, N. & Liu, D. The design and synthesis of planar chiral ligands and their application to asymmetric catalysis. Synlett 25, 615–630 (2014).
Han, J.-W., Chen, J.-X., Li, X., Peng, X.-S. & Wong, H. N. C. Recent developments and applications of chiral tetraphenylenes. Synlett 24, 2188–2198 (2013).
Huang, H., Hau, C. K., Law, C. C. & Wong, H. N. Hydroxytetraphenylenes, a new type of self-assembling building block and chiral catalyst. Org. Biomol. Chem. 7, 1249–1257, (2009).
Xie, E., Huang, S. & Lin, X. Design of planar chiral phosphoric acids with a [2.2]paracyclophanyl backbone as organocatalysts for the highly enantioselective aza-friedel-crafts reaction. Org. Lett. 21, 3682–3686 (2019).
Shibata, T., Chiba, T., Hirashima, H., Ueno, Y. & Endo, K. Catalytic enantioselective synthesis of chiral tetraphenylenes: consecutive inter- and intramolecular cycloadditions of two triynes. Angew. Chem. Int. Ed. 48, 8066–8069 (2009).
An, Y. et al. Enantioselective synthesis of both axially and planar chiral ferrocenes via axial-to-planar diastereoinduction. Org. Lett. 24, 7294–7299 (2022).
Luo, Y. et al. A new saddle-shaped aza analog of tetraphenylene: atroposelective synthesis and application as a chiral acylating reagent. CCS Chem. 4, 2897–2905 (2022).
Luo, Y. et al. Inherently chiral 6,7-diphenyldibenzo[e,g][1,4]diazocine: enantioselective synthesis and application as a ligand platform. CCS Chem. 5, 982–993 (2023).
Lv, X. et al. Access to planar chiral ferrocenes via N-heterocyclic carbene-catalyzed enantioselective desymmetrization reactions. ACS Catal. 12, 2706–2713 (2022).
Wei, S., Chen, L.-Y. & Li, J. Enantioselective synthesis of planar chiral macrocyclic metacyclophanes by Pd-catalyzed C–O cross-coupling. ACS Catal. 13, 7450–7456 (2023).
Liu, L. et al. Synthesis of planar chiral isoquinolinone-fused ferrocenes through palladium-catalyzed C-H functionalization reaction. Chin. Chem. Lett. 32, 239–242 (2021).
Liu, C.-X. et al. Kinetic resolution of planar chiral metallocenes using Rh-catalysed enantioselective C–H arylation. Nat. Synth. 2, 49–57 (2022).
Liu, C. X. et al. Explicit mechanism of Rh(I)-catalyzed asymmetric C-H arylation and facile synthesis of planar chiral ferrocenophanes. J. Am. Chem. Soc. 145, 4765–4773 (2023).
Ye, J. et al. Enantioselective assembly of ferrocenes with axial and planar chiralities via palladium/chiral norbornene cooperative catalysis. JACS Au 3, 384–390 (2023).
Zhou, L. et al. Synthesis of planar chiral ferrocenes via enantioselective remote C-H activation. Nat. Chem. 15, 815–823 (2023).
Zhang, A.-A. et al. Planar chiral 2-(trifluoromethyl)quinoline-fused ferrocenes via palladium(0)-catalyzed C-H functionalization of trifluoroacetimidoyl chlorides. Green Synth. Catal. 2, 311–314 (2021).
Ramle, A. Q. & Tiekink, E. R. T. Synthetic strategies and diversification of dibenzo[1,5]diazocines. Org. Biomol. Chem. 21, 2870–2888 (2023).
Gordon-Wylie, S. W. et al. Exploring hydrogen-bonded structures: synthesis and X-ray crystallographic screening of a cisoid cyclic dipeptide mini-library. Cryst. Growth Des. 4, 789–797 (2004).
Bieszczad, B. et al. Influence of halogen size on the supramolecular and energy landscape of the THF solvates of the halogen derivatives of dianthranilide. CrystEngComm 22, 5389–5399 (2020).
Gillman, P. K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 151, 737–748 (2007).
Jiang, H. L. et al. New isocoumarins and alkaloid from Chinese insect medicine, Eupolyphaga sinensis Walker. Fitoterapia 83, 1275–1280 (2012).
Rúnarsson, Ö. V., Artacho, J. & Wärnmark, K. The 125thanniversary of the tröger’s base molecule: synthesis and applications of tröger’s base analogues. Eur. J. Org. Chem. 2012, 7015–7041 (2012).
Olszewska, T., Gdaniec, M. & Połoński, T. Planar chiral dianthranilide and dithiodianthranilide molecules: optical resolution, chiroptical spectra, and molecular self-assembly. J. Org. Chem. 69, 1248–1255 (2004).
Olszewska, T., Gdaniec, M. & Połoński, T. Enantiomeric resolution of N,N′-dimethyldithiodianthranilide through diastereomeric silver(I) complex. circular dichroism spectra, racemization barrier, and molecular self-assembly. J. Org. Chem. 73, 4859–4864 (2008).
Wang, Y. B., Zheng, S. C., Hu, Y. M. & Tan, B. Bronsted acid-catalysed enantioselective construction of axially chiral arylquinazolinones. Nat. Commun. 8, 15489 (2017).
Li, J. H. et al. Organocatalytic enantioselective synthesis of seven‐membered ring with inherent chirality. Angew. Chem. Int. Ed. 63, e202319289 (2024).
Zhang, D., Zhou, J., Qin, T. & Yang, X. Asymmetric synthesis of saddle-shaped eight-membered azaheterocycles via (dynamic) kinetic resolution. Chem Catalysis 4, 100827 (2024).
Pellissier, H. Organocatalyzed dynamic kinetic resolution. Adv. Synth. Catal. 353, 659–676 (2011).
Ma, G. & Sibi, M. P. Catalytic kinetic resolution of biaryl compounds. Chem. Eur. J. 21, 11644–11657 (2015).
Liu, W. & Yang, X. Recent advances in (Dynamic) kinetic resolution and desymmetrization catalyzed by chiral Phosphoric acids. Asian J. Org. Chem. 10, 692–710 (2021).
Liu, W., Wang, D., Zhang, D. & Yang, X. Catalytic kinetic resolution and desymmetrization of amines. Synlett 33, 1788–1812 (2022).
Oki, M. Recent advances in atropisomerism. Top. Stereochem. 14, 1–81 (1983).
Liu, T.-Y., Xie, M. & Chen, Y.-C. Organocatalytic asymmetric transformations of modified Morita–Baylis–Hillman adducts. Chem. Soc. Rev. 41, 4101 (2012).
Li, S. L. et al. Atroposelective catalytic asymmetric allylic alkylation reaction for axially chiral anilides with achiral morita-baylis-hillman carbonates. J. Am. Chem. Soc. 140, 12836–12843 (2018).
Mei, G.-J. et al. Rational design and atroposelective synthesis of N–N axially chiral compounds. Chem 7, 2743–2757 (2021).
Zhou, Q.-Y. & Li, X. Atroposelective construction of axially chiral enamides via N-allylic alkylation. Chem. Commun. 58, 4727–4730 (2022).
Acknowledgements
Financial support from the National Natural Science Foundation of China (22371265 and 22208302) and the Natural Science Foundation of Henan Province (222300420084) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
C.-Y.G., C.L., Z.-Y. L., and X.X. performed and analyzed the experiments. M.-C.W. participated in the early development of the project. S.Z. and D.W. performed the DFT calculations. M.H. and L.H. performed and analyzed the anticancer tests. G.-J.M. conceived and designed the project. G.-J.M. supervised the project overall. All authors prepared this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guan, CY., Zou, S., Luo, C. et al. Catalytic asymmetric synthesis of planar-chiral dianthranilides via (Dynamic) kinetic resolution. Nat Commun 15, 4580 (2024). https://doi.org/10.1038/s41467-024-48947-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-48947-1
This article is cited by
-
Chiral medium-sized rings beyond central chirality
Nature Reviews Chemistry (2025)
-
Organocatalytic asymmetric synthesis of Tröger’s bases
Nature Communications (2025)