Abstract
Organic electrochemical transistors have emerged as a promising alternative to traditional 2/3 electrode setups for sensing applications, offering in-situ transduction, electrochemical amplification, and noise reduction. Several of these devices are designed to detect potentiometric-derived signals. However, potentiometric sensing should be performed under open circuit potential conditions, allowing the system to reach thermodynamic equilibrium. This criterion is not met by conventional organic electrochemical transistors, where voltages or currents are directly applied to the sensing interface, that is, the gate electrode. In this work, we introduce an organic electrochemical transistor sensing configuration called the potentiometric‑OECT (pOECT), which maintains the sensing electrode under open circuit potential conditions. The pOECT exhibits a higher response than the 2-electrode setup and offers greater accuracy, response, and stability compared to conventional organic electrochemical transistors. Additionally, it allows for the implementation of high-impedance electrodes as gate/sensing surfaces, all without compromising the overall device size.
Similar content being viewed by others
Introduction
Potentiometry is probably the most straightforward electrochemical sensing technique1, measuring the open circuit potential (OCP) shift of a sensing electrode upon its interaction with the molecule of interest. Most potentiometric sensors rely on the classical 2-electrode setup for OCP measurements2,3,4, where the electrochemical potential of the sensing electrode, known as the working electrode (WE), is measured with respect to a reference electrode (RE) in an open circuit configuration. The most common and commercially available potentiometric sensors are the ion-selective electrodes (ISEs)5 that usually employ ion-selective membranes6,7. Potentiometric sensors can also detect the presence of other biologically relevant analytes when they are functionalized with surface-immobilized recognition elements such as enzymes8, aptamers9 or antibodies10,11,12,13. Such potentiometric sensors have been extensively developed for several decades, with efforts focused on improving stability, sensitivity, robustness, miniaturization, and maintenance-free operation while providing a calibration-free system14,15,16. However, several challenges hinder the widespread application of potentiometric sensors. One of them is the need for a stable and well-defined RE, crucial to ensure sensor reproducibility, long-term stability, minimum potential drift, fouling resistance, and biocompatibility (especially in biological applications)17. Another obstacle concerns miniaturization, which remains an important goal in analytical device development due to the potential benefits in terms of reduced sample volume, improved spatial resolution, and portability. However, when the sensing electrode gets smaller, the electrical noise increases due to an increase in impedance. Miniaturizing a RE proves to be even more demanding than any WE due to the requirement of maintaining a protected and stable inner solution for the RE material, enclosed inside a micrometric container, alongside a micrometric ionic bridge. Moreover, materials commonly used for constructing REs, such as Ag- or Hg-based compounds, are not biocompatible, adding an extra layer of complexity, especially in biosensing applications.
The organic electrochemical transistor (OECT) has emerged as an alternative bioelectronic circuit element, enabling the development of RE-free and miniaturized neural interfaces18 and sensors19 for disease biomarkers that provide intrinsic amplification and noise reduction20,21,22. The OECT is a three-terminal device that uses an aqueous or solid electrolyte as the dielectric in contact with the channel. The channel is made of an organic mixed ionic and electronic conductor (OMIEC)23, connecting the Source (S) and Drain (D) electrodes, across which a voltage is applied (VDS). The current measured (IDS) reflects the conductivity of the OMIEC. The doping state of the OMIEC, hence the IDS, can be finely modulated by the voltage (VGS) applied across the electrolyte between a third electrode, the Gate (G), and the S. Therefore, the electrochemical properties of the G directly affect the magnitude of the IDS. The vast knowledge available for the functionalization methods developed for the WE of classical potentiometric sensors can be adapted for the G in an OECT. In such an OECT-based chemical sensor, the recognition event occurs on the G, analogous to the WE in a 2-electrode setup. The electrochemical potential of the G can change upon the binding of species such as ions, nucleic acids, and proteins, altering the charge distribution at the G24,25. These small variations in G electrochemical potential are then translated by the OECT into large changes in the IDS.
There are several reasons why the OECT can be superior and/or a complementary device to the classical 2-electrode setup while also overcoming the abovementioned limitations to develop a RE-free, miniaturized potentiometric sensor with even higher sensitivity. The intrinsic voltage-to-current conversion process allows OECTs to perform potentiometric sensing26 with in-situ amplification, increasing the signal-to-noise ratio27,28,29. This feature has enabled the detection of analytes at attomole-femtomole concentrations using OECTs25,30,31. OECTs can be fabricated in various geometries and on substrates using photolithography processes32 or even simple roll-to-roll33 and inkjet printing34, with good reproducibility35 and long-term stability36,37. Furthermore, the devices show high fouling resistance27, and if necessary, the OMIEC can be kept outside the sensing environment by using a floating gate system38,39. This maintains the OECT channel in its isolated electrolyte, ensuring channel material stability40, while only the G is exposed to the complex sample. In the 2-electrode setup, the RE is usually in direct contact with the sensing environment, exposing it to contaminants. While double junctions can be used, such a design impedes miniaturization and scalability.
While OECTs offer several advantages for potentiometric sensing, they are limited by one important condition, which is also the main requirement of potentiometric sensing. Since the sensing electrode (i.e., WE) is used as the G of the OECT, it is exposed to currents and voltages when modulating the OMIEC doping state. However, in classical 2-electrode potentiometric sensing, the measurement must be conducted in an OCP configuration with negligible current applied to the WE. The current flow/voltage biasing in OECT operation is unsuitable for potentiometric sensing because the G cannot reach thermodynamic equilibrium41,42. In this case, the OECT input signal can become unreliable, making the device output inaccurate. Moreover, especially for biosensing, the voltage range that can be applied to the OECT is limited because the high current passing through the sensing interface (gate current, IGS) can damage the sensing layer43,44 (which usually contains biorecognition molecules), or trigger other parasitic faradaic reactions26. Due to a combination of these factors, the OECT, in its current configuration, is prone to inaccurate or inconsistent sensing readouts and cannot be reliably used for potentiometric sensing.
To overcome these problems and use the OECTs at their maximum performance for biosensing, we introduced an OECT sensing configuration called potentiometric-OECT (pOECT). In this configuration, we maintain the sensing electrode in OCP conditions by minimizing any current flow/voltage bias at this interface, bringing the OECT as close as possible to the ideal conditions of an electrochemical cell. This was achieved by treating the gating system of the OECT as a 3-electrode cell and splitting the G into two different electrodes (Fig. 1). The first electrode is the sensing gate (GS), which has an electrochemical potential sensitive to the target concentrations and functions as a RE for the applied gate voltage. The second electrode is the gating gate (GG), which functions as a CE and is responsible for actively applying the doping voltage. We demonstrate the superiority of the pOECT over the classical 2-electrode system and the OECT operated in the traditional configuration in three distinct sensing scenarios with different device architectures: (1) classical potentiometric sensing, where we sense ions such as Na+, Cl−, and H+ as representative examples; (2) a combined n- and p-type pOECT for the measurement of pH fluctuations; (3) a multichannel pOECT used to monitor barrier tissue integrity. We also discuss how to choose the most appropriate class of OMIEC to maximize the sensor response. Our results show that the pOECT guarantees a higher response than the 2-electrode setup while maintaining the same accuracy despite having no RE. The pOECT exhibits higher response, accuracy, and stability in sensing compared to the conventional OECT and has excellent flexibility in component design and low operational complexity. The setup is compatible with a broad range of sensing interfaces (e.g., high-impedance electrodes) and complex configurations (e.g., parallel channels, n- and p-type channels operating simultaneously) without requiring specific device optimization in terms of geometry or materials.
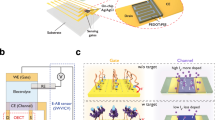
Comparison of the (a) OECT, b gate referenced-OECT, and c pOECT configurations. In the top schematic, the black circle represents the electrolyte, and the dashed line represents the high-impedance connection of the potentiostat. The pink legend represents the cable labeling for the VDS application, and the purple legend represents the cable labeling for the VGS/VSG application. The 3D illustrations of the setups are in the middle panel, with G, GG, and GS placed vertically with respect to the channel. The red spheres represent the ionic flux of the leakage current. Microscopy images of the microfabricated devices at the bottom panel depict the planar G, GG, and GS. The channel size is 100 × 10 µm, and G, GG, and GS size is 500 × 500 µm. G and GS represent any type of sensing interface, while GG represents any conducting material that can act as a counter electrode.
Results and discussion
The pOECT configuration
The top panel in Fig. 1a presents the electrical connections of a conventional OECT, as an electrochemical cell. To apply VDS, the D is connected to the WE1, while the S is connected to a combined RE1/CE1. The electrochemical potential of the D is defined with reference to S (due to the RE1 connection), and the current flowing between WE1 and CE1 is the IDS. Simultaneously, VGS is applied between the G (used as the sensing electrode in OECTs), which is connected to the WE2, and the S, connected to the RE2/CE2. The potential of the G is defined with respect to S due to the RE2 connection, and for this reason, we can consider the gating system as source-referenced. The current between G and S is IGS, also called leakage current.
The roles of S and G for the VGS application can be swapped, resulting in the S being the WE2 and the sensing interface (G) being a combined RE2/CE2, as shown in Fig. 1b. We name this configuration gate referenced-OECT. The gate voltage should now be called VSG, and the gate current is ISG – to maintain an accurate nomenclature. The gate referenced-OECT will work exactly like the regular OECT, with the same IDS and IGS values, but with only the sign of the gating voltage reversed. However, having re-wired the S-G connection, we gain an extra degree of freedom. The G, which is now a combined RE2/CE2, can be decomposed into two independent electrodes, i.e., RE2 and CE2 (Fig. 1c). The sensing electrode remains connected to RE2. A new electrode is introduced, which can be any type of counter electrode, such as a Pt coil (Fig. 1c-middle) or an Au electrode (Fig. 1c-bottom), and connected to CE2. Together, RE2 and CE2 represent the new “gating system”. This configuration is referred to as the “potentiometric-OECT” (pOECT). The pOECT does not require any additional connections. While the conventional OECT operation involves shorting the RE2 and CE2 cables and connecting them to the same electrode (S), in the pOECT, these cables are separated and linked to electrodes with distinct functions.
Both RE2 and CE2 gate the channel but serve different roles. RE2 (sensing electrode) serves as the reference point for the electrochemical potential of the S. CE2, on the other hand, actively provides the voltage and current to bring the S to the required potential difference with respect to RE2, thereby doping/de-doping the channel. Since this electrode provides the necessary current to gate the channel, we eliminate any electrochemical stress from the sensing electrode interface. Considering their distinct functions, we designate RE2 as the sensing gate (GS), and CE2 as the gating gate (GG). In the pOECT configuration, the IDS is modulated solely by electrochemical potential variations of GS. A few other OECTs have used an electrochemical cell-type gating system to control the doping state of the channel45,46,47, yet these setups have entirely differently purposes and working mechanisms compared to the pOECT.
In Fig. 1 (middle and bottom), we present schematics and microscope images of the same channel gated in these three configurations. Like in the OECT, the new gating system (GS and GG) can be either vertical (Fig. 1-middle), or the pOECT can be miniaturized, consolidating all the device components into a single substrate by fabricating planar GG and GS, thereby maintaining the overall device size in the micrometer range (Fig. 1-bottom). The additional electrode introduced has, therefore, no impact on device geometry but offers significant advantages in device performance and sensor accuracy, as described below.
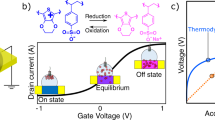
Figure 2a-left shows the transfer curves of an n-type enhancement-mode (see Supplementary Fig. 1 for the polymer chemical structure) device wired in the conventional way (dotted lines, “OECT”), and reconfigured as described in Fig. 1c (solid lines, “pOECT”). First, to characterize the behavior of the pOECT setup as a transistor, we used non-polarizable Ag/AgCl electrodes as G and GS, and a Pt coil as the GG. On the right-hand side of Fig. 2a, we report the evolution of electrochemical potentials of the OECT and pOECT terminals during the acquisition of transfer characteristics (see Supplementary Discussion 1 for a detailed explanation of this experiment). Since the applied voltages are the same for the OECT and the pOECT (except for the sign of the gate voltage; VSG = −VGS), the electrochemical potential profiles overlap when a non-polarizable electrode is used as G/GS. Consequently, the corresponding transfer curves are identical for both configurations, demonstrating no loss or improvement of transistor performance in the pOECT configuration compared to the classical one.
Transfer curves (left) and electrochemical potentials of the OECT/pOECT components during the corresponding voltage scan (right) when (a) the G and GS is a non-polarizable Ag/AgCl electrode or (b) a polarizable Au electrode (Φ = 5 mm). The measurements obtained with the pOECT configuration are reported with solid lines while dotted lines are for the OECT. The devices have an n-type OMIEC in the channel. The applied gate voltage is reported as VSG for the pOECT and –VGS for the OECT. c Transfer curves (left) and electrochemical potentials of the device components during the corresponding voltage scan (right) when the pOECT is operated with either a minimally polarizable GG as a Pt coil (solid lines) or a highly-polarizable GG (up to almost 1 V vs. Ag/AgCl) as Au electrode, Φ = 1.6 mm, (dotted lines).
The advantage of the pOECT becomes evident when using a polarizable electrode as the G of the OECTs. Figure 2b shows the transfer curves of the n-type OECT/pOECT gated with a polarizable vertical Au electrode, with a diameter of 5 mm, used as G or GS, along with the electrochemical potentials of device components. Polarizable electrodes, such as Au, are commonly used as the functionalized biosensing surfaces of OECTs, with the size of the G/Gs chosen to be similar to several biosensing applications reported in the literature25,30. In the case of the OECT (dotted lines), there is a clear voltage drop on the G during the VGS scan, polarizing the G to positive potentials. This, in turn, drags the S and D also toward positive potentials, preventing the OMIEC from reaching a high doping state. Conversely, for the pOECT (solid lines), no voltage drop occurs on the GS across the entire biasing range. This allows the S and D to be linearly brought toward doping potentials without any distortion. As a result, the transfer curves of OECT and pOECT look dramatically different, with the pOECT channel achieving much larger current modulation than that of the OECT. Despite the use of a polarizable electrode as the GS, the pOECT behaves as if the channel is gated with a non-polarizable electrode.
When using a polarizable gate electrode, obtaining the same IDS modulation with the OECT necessitates applying a much higher VGS (×2.5) up to 1.26 V (Supplementary Discussion 2). This marks the first advantage of the pOECT: it enables the safe use of polarizable gate electrodes to attain stable and full modulation of the channel. Secondly, in the pOECT, negligible current (pA or less) flows through the GS due to the high impedance of the RE2 connection (100 TΩ). We thus secure a nondestructive biasing method for the sensing interface, which often contains the biorecognition units that may be damaged due to the leakage current (IGS). Moreover, high voltages applied for better modulation in the OECTs push the sensing electrode to electrochemical potentials, which can be detrimental to the biofunctionalized gate electrode (0.9 V vs. Ag/AgCl, Supplementary Fig. 4). Note that although the GS non-polarizability is not an absolute condition, in most practical sensing applications, this issue does not arise for the pOECT (see Supplementary Discussion 3 for a detailed explanation of the leakage currents in the pOECT and the possible GS polarization in a few specific cases).
In the pOECT, we bypass any possible polarization of the GS by redirecting the polarizing current to the GG. Importantly, the choice of the GG in the pOECT design does not affect device characteristics. In Fig. 2c-right, we show the electrochemical potentials of two pOECTs operated with different GG electrodes. One GG is a large and minimally polarizable electrode, such as a Pt coil (solid lines), and the other is a highly-polarizable electrode, such as a small Au (Φ = 1.6 mm, dotted lines). Both devices effectively prevent polarization on the GS, resulting in identical electrochemical potential profiles of S, D, and GS, as well as transfer curves (Fig. 2c-left). This result underscores the versatility of the pOECT, as any material can be employed as the GG without imposing limitations based on its polarizability/size or specific electrochemical potential. The absence of specific requirements for the newly introduced electrode in our configuration streamlines device fabrication and design. The results depicted in Fig. 2 are derived from devices equipped with vertical gating electrodes. A similar enhancement in device performance can also be achieved with fully microfabricated devices, as demonstrated in Fig. 1-bottom and Supplementary Fig. 8.
The operation of the pOECT as a sensor
Since the pOECT renders any sensing electrode non-polarizable, hence, eliminating interferences arising from its capacitance, it serves as an ideal platform for pure potentiometric sensing. To demonstrate how pOECT operates as a potentiometric sensor and benchmark its capabilities, we focus on ion sensing, that is, the most representative type of potentiometric sensing. We chose ions as the target instead of other biomarkers, such as proteins or nucleic acids, because ion sensing can be easily described by the thermodynamic Nernst equation. This allows us to compare the pOECT and OECT experimental behavior with the theoretically expected one (Nernstian behavior). Secondly, the potentiometric detection mechanism of OECTs for such biomarkers, despite their much lower limit of detection (LoD) compared to that of ion sensors, is still under debate and not uniquely defined by a thermodynamic equation48,49. Therefore, to analyze the performance of this configuration, we opted to work with a well-established sensor surface. Nevertheless, surface potential changes resulting from protein or DNA binding range from 10 to 150 mV for LoD and saturation concentrations, respectively25,50,51,52. These values are in the same range as those reported here for the ISEs. Despite the different origins of the surface potential change (ion capture or DNA/protein binding), the response of the pOECT remains the same since the device detects these changes as a simple equivalent gating voltage variation. Therefore, the conclusions drawn regarding the pOECT’s operation in ion sensing and its advantages extend to the detection of any other target through a potentiometric mechanism.
In Fig. 3a, we schematically represent how the 2-electrode setup and the OECT are merged into a single configuration in the pOECT. Using a Na+ ISE as the GS, we obtain a Na+ Ion-Selective pOECT (Na+-IS-pOECT). To demonstrate the modality of the platform, here we used a p-type OMIEC (see the chemical structure in Supplementary Fig. 1) operating in enhancement mode. Figure 3b shows the transfer curves of the Na+-IS-pOECT when the GS is exposed to different concentrations of Na+. An increase in the Na+ concentration in the sensing chamber increased the IDS. Figure 3c depicts the electrochemical potential changes that the device terminals undergo during Na+ detection. A higher cation concentration shifts the electrochemical potential of the GS to higher positive potentials (ΔOCP), which consequently drags the entire channel (S and D) to a new potential in the same direction and of the same amount as ΔOCP. At this new potential, the p-type OMIEC channel is in a more oxidized/doped state, resulting in the higher IDS observed in Fig. 3b.
a Combination of a 2-electrode potentiometric setup with the OECT to obtain a reference electrode-free IS-pOECT. b Na+ sensing with a Na+-IS-pOECT, where the Na+-ISE is the GS, and a Pt coil is the GG. GS is located in a separate chamber connected to the rest of the device via a microfluidic channel. The pOECT is a p-type enhancement-mode device, and its channel current varies with Na+ concentrations. c The changes in the electrochemical potentials of the Na+-IS-pOECT terminals during device operation when [Na+] = 0 M (left) and [Na+] = 100 mM (right). ΔOCP represents the electrochemical potential shift of the GS or S due to variations in [Na+]. d Na+ sensing with a Na+-ISE in a 2-electrode setup. The plot reports the electrochemical potential of the ISE at different Na+ concentrations. The inset shows the corresponding calibration curve. e A schematic of the sensing-induced changes in the electrochemical potentials of the pOECT terminals (GS, S, and D) at constant VDS and VGS. The target detected by the Gs alters its potential from EGS to EGS*. f Left: Normalized IDS of the Na+-IS-pOECT at various Na+ concentrations when operated in the nA regime. Right: Calibration curves of the conventional 2-electrode potentiometric setup (ISE) and the Na+-IS-pOECT operated in the µA or the nA regime. The channel current or electrode potentials was normalized to the 0 M NaCl readout of the corresponding device.
The origin of the ΔOCP is reported in Fig. 3d, showing the real-time Na+-induced change in the ISE potential monitored with respect to a RE (Ag/AgCl). The electrochemical potential of the ISE (\({{{{\rm{E}}}}}_{{{{\rm{WE}}}}}\)) follows the Nernst equation, Eq. (1), which translates increasing concentrations of Na+ in the electrolyte to a log-linear increase in electrochemical potential.
where \({{{\rm{k}}}}\) is a specific constant of the electrochemical cell that depends on the setup and represents the sum of potential differences at all of the other interfaces other than the ISE, \({{{\rm{R}}}}\) is the gas constant (8.316 J mol−1 K−1), \({{{\rm{T}}}}\) is the temperature in K, \({{{\rm{F}}}}\) is the Faraday constant (96485 C mol−1), \(\pm {{{\rm{n}}}}\) is the charge of the ion detected by the ISE\(,\,{{{{\rm{a}}}}}_{{{{{\rm{M}}}}}^{\pm {{{\rm{n}}}}}}\) and \(\left[{{{{\rm{M}}}}}^{\pm {{{\rm{n}}}}}\right]\) are the activity and the concentration of the target ion in solution, respectively. The sensitivity of the ISE for a monovalent ion is theoretically calculated as 59 mV/dec in standard conditions but can be less depending on the quality, storage conditions, and history of the ISE. When we use the ISE as the GS of the pOECT, the electrochemical potential change reported in Fig. 3d becomes the input signal for the device (\({{{{\rm{E}}}}}_{{{{\rm{WE}}}}}={{{{\rm{E}}}}}_{{{{{\rm{G}}}}}_{{{{\rm{S}}}}}}\)) and is implicitly reflected in an equivalent electrochemical potential shift (ΔOCP) of the whole channel, where it will be amplified as a current output. Figure 3e describes the working mechanism of the pOECT.
In Fig. 3f-left, we report the normalized response (NR) of the Na+-IS-pOECT calculated as the change in IDS at different VSG in the nA current regime. Figure 3f-right reports the corresponding calibration curves of the pOECT working in the nA or µA current regime and the conventional 2-electrode potentiometric setup. NR values of the 2-electrode setup are calculated from the change in the OCP of the GS, and the maximum NR obtained is 1.4 for the highest Na+ concentration. The relatively low NR values can be explained by the Nernst equation (constant sensitivity at 59 mV/dec) and the device operation, which is limited by the voltage regime. On the other hand, the NR of the IS-pOECT is much higher because it is obtained in the current regime, which can span across several orders of magnitude and can also be tuned by selecting the VSG that provides the highest signal shift from the baseline current (0 M), as shown in Fig. 3f-left. The NR of the pOECT working in the µA regime reaches 8.5 for the highest Na+ concentration. When accurate electronics are available to operate the pOECT in the nA regime, where the device shows higher transconductance efficiency, the NR for the highest Na+ concentration can reach up to 35.
The performance comparison of the pOECT versus the conventional OECT
The Nernst equation, Eq. (1), describes the electrochemical potential that the sensing electrode reaches in OCP conditions, i.e., without applied current or voltage. These conditions represent an equilibrium state for the electrode, describing the system’s thermodynamics rather than its kinetics. The monitoring of this thermodynamic state is the analytical signal in a classical OCP measurement using a 2-electrode setup (Fig. 3d) and is also what is aimed to be used as input signal in an OECT and pOECT (Fig. 3e). For this reason, we consider the accuracy of the setups compared in this work (2-electrode, OECT and pOECT) as the agreement between the measured electrochemical potential of the sensing interface and the theoretical expected Nernstian behavior.
To compare the performance of the OECT and pOECT, we created a 5 mm-diameter pH-sensitive electrode, that is, an electrodeposited film of polyaniline (PANI), which will be used as the sensing interface (G and GS, respectively). We chose to work with PANI as the sensing electrode because using the commercially available electrodes (pH-meter or the Na+-ISE) as the G of the OECT deteriorates them, as discussed above. In Fig. 4a, we report the OECT and pOECT transfer curves recorded when the PANI-G and PANI-GS were exposed to buffer solutions with a difference of 1 pH unit (6–7). While the pOECT shows a significant current modulation due to the pH variation, the OECT has no response. The two insets of Fig. 4a report the IDS and IGS of the OECT on a zoomed scale, almost identical in terms of order of magnitude, demonstrating no OMIEC modulation and no signal amplification. The sensing interface of the OECT (i.e., the G) is immediately polarized due to the application of VGS with 63% of the VGS dropping on G (Fig. 4b-left). This drop causes the loss of the initial Nernstian potential, which represented the analytical input signal, making the device output inaccurate. On the other hand, with the pOECT, the GS potential remains completely unperturbed (Fig. 4b-middle). Since the thermodynamics of the electrode used as GS remains unaltered, the behavior of the pOECT is ruled by the Nernst equation. In Fig. 4b-right, we present the electrochemical potential changes of the PANI-GS pH-pOECT at different pH values. The pH-sensitive interface maintains its expected Nernstian response, exhibiting a −59 mV/pH slope, which is then amplified by the pOECT as an output current. These experiments demonstrate that the pOECT preserves the Nernstian behavior of the sensing electrode at the same level as a 2-electrode setup, leading to a transistor that provides the maximum accuracy.
a Transfer curves of an n-type OECT and pOECT operated at two different pH values with a pH-sensitive PANI electrode as the G or GS. The applied gate voltage is reported as VSG for the pOECT and –VGS for the OECT. Inset: IDS and IGS vs. VGS of the OECT (scaled). The sensing electrode (G or GS) is located in a separate chamber connected to the rest of the device via Ag/AgCl floating gates. b Electrochemical potentials of the n-type OECT (left) and pOECT (middle) components monitored during the transfer curve recorded at pH = 6. Right: the electrochemical potential of the GS measured during the acquisition of the transfer curves at different pH. c Relationship between the sensing interface (G and GS) polarization and the electrode diameter/area in a pOECT and OECT bearing the same n-type channel. G and GS are Au electrodes with varying diameters (Φ) and GG is an Au electrode of 1.6 mm-diameter. d 50 consecutive transfer curves with pOECT and OECT, where the G/GS is a 3 mm-diameter and GG is 1.6 mm-diameter Au electrode. e The 50th (final) transfer curve of the first cycling set (black) and the transfer curves recorded during the second set of cycling tests for the OECT. f Normalized response at VSG = −VGS = −0.55 V of the pOECT and OECT during one set of transfer curves and two consecutive sets of transfer curves respectively. g Transfer curves of an n-type pOECT recorded at different pH values (4, 7, and 10). The device has a pH-sensitive glass electrode as the GS and a Pt coil as the GG. GS is located in a separate chamber connected to the rest of the device via Ag/AgCl floating gates. The pOECT is an n-type enhancement-mode device. Inset: corresponding calibration curve.
To construct an OECT with comparable accuracy as the pOECT, the G area could be increased to reduce its polarization. However, the geometry required to attain the same pOECT performance exceeds any reasonable size for a small, portable device. In Fig. 4c, we report how the GS size can be reduced to a diameter as small as 170 µm while keeping the sensing electrode polarization to a very low value (1 mV). With an OECT, it is necessary to use a G with a 4 cm diameter to minimize the electrode polarization to the same level as the pOECT, requiring a sensing electrode area of 55,000 times larger (Supplementary Discussion 4). This would be necessary to claim an accurate OECT potentiometric measurement. The pOECT allows for the use of smaller sensing interfaces, a significant advantage over the OECT in terms of device miniaturization while increasing device performance and ensuring accuracy.
Since the GS is not polarized, the pOECT output response remains constant upon consecutive biasing cycles. In contrast, the OECT shows deviations with a continuous drift of the transfer curve (Fig. 4d). Figure 4e shows the OECT output during a second set of biasing cycles. The second set of transfer curves differs from the first one due to the leakage current (IGS), which permanently alters the electrochemical potential of the gate (+97 mV, Supplementary Fig. 10). In Fig. 4f, we plot the OECT and pOECT IDS at constant operating voltages during the entire duration of these biasing experiments, evidencing the unstable and non-reproducible OECT response compared to the remarkable stability of the pOECT channel current.
In addition to these performance improvements, the pOECT demonstrates exceptional modularity, enabling the integration of virtually any type of potentiometric sensing interface as the GS, including materials with high impedance. This capability surpasses traditional OECTs, which cannot accommodate high-impedance materials as the G. One extreme example of such materials is the commercially available pH-meter glass electrode. The glass membrane of pH-meter glass electrodes, responsible for the sensing mechanism, has a resistance typically between 50 and 500 MΩ53, which would drop the entire VGS on the glass membrane itself if it was used as the G of the OECT. Moreover, since the pH-meter is not designed to work with current or voltage applied, the highly sensitive and delicate glass membrane would be damaged by the flow of IGS, and the thermodynamic equilibrium of the H+ ions bound on the membrane would be disrupted. Figure 4g illustrates the large current change of the pH-pOECT to a pH increase from 4 to 10, with an NR reaching a maximum of 200 (Fig. 4g-inset) along with an output signal spanning three orders of magnitude (nA to µA).
These ion and pH sensing experiments prove that the pOECT combines the accuracy of the 2-electrode setup with the amplification capability of the OECT, all without a conventional RE. Accuracy is the most important trait of all sensors, which is guaranteed for potentiometric-OECT sensors through this configuration. We note that the lowest electrochemical potential change upon ion binding to an ISE is about ±10 mV. This is also the lowest value recorded upon protein or nucleic acid binding to the gate of an OECT25,50,51,52. This value translates into an LoD in the micromolar range for ion sensors, and the physical reason that limits the LoD of ISEs has been discussed by A. J. Bard et al.54, and an LoD in the attomolar range for protein sensors. An effective strategy to further improve the LoD for OECT-based potentiometric sensors is to optimize the chemistry of the sensing interface for amplifying the input signal, i.e., surface potential change25. Moreover, the Nernstian or Super-Nernstian sensitivity (59 mV/dec or >59 mV/dec, respectively, for a monovalent ion) is an intrinsic property of the ion-sensitive material and cannot be improved by either the OECT or the pOECT, as often improperly claimed in studies reporting OECT-based ion sensors (see Supplementary Discussion 5 about OECTs with reported Super-Nernstian response). In fact, as shown for the pOECT, the electrochemical potential modulation of the GS does not exceed the expected Nernstian response (Fig. 4b-right). However, only with the pOECT, it is possible to retain any Nernstian or Super-Nernstian sensitivity of the sensing material, which is converted into an amplified current output signal. Given that in most OECT-based sensing applications, polarizable electrodes are used as the G, we emphasize the inaccuracies in these measurements arising from G polarization. Importantly, without a GG, the changes observed in the transfer characteristics, due to a parasitic polarization, can be falsely attributed to a biorecognition event. In Supplementary Discussion 6, we report other thermodynamically related advantages of the pOECT over the state-of-the-art OECTs used in potentiometric sensing.
Choosing the OMIEC for the pOECT sensor
When developing a pOECT-based sensor, selecting the right OMIEC (n-type or p-type) and device operation (enhancement or depletion mode) is important to achieve the highest signal amplification. The OMIEC type should be selected based on the direction of the electrochemical potential shift of the sensing surface upon its interactions with the target. For example, a positive electrochemical potential shift of the ISE-based GS due to Na+ binding (ΔOCP, Fig. 3c) is translated as a shift of the transfer curve towards the negative VSG direction, without any distortion (Fig. 3b). The magnitude of this transfer curve shift corresponds precisely to the ΔOCP of the GS (ΔOCP = ΔVSG), as shown in Fig. 5a-top. In fact, when we take the transfer curve recorded at 0 M Na+ as a reference point and horizontally shift all the other curves of the sensing experiment by a VSG value corresponding to their own ΔOCP with respect to the 0 M condition (\(\Delta {{{\rm{OC}}}}{{{{\rm{P}}}}}_{{{{\rm{x}}}}}={{{{\rm{OCP}}}}}_{{\left[{{{{\rm{Na}}}}}^{\!+}\!\right]}_{{{{\rm{x}}}}}}-{{{{\rm{OCP}}}}}_{{\left[{{{{\rm{Na}}}}}^{\!+}\!\right]}_{0{{{\rm{M}}}}}}\)), we find that all the curves collapse onto a single plot (Fig. 5a-bottom). If we use the same Na+-selective GS, this time with an n-type channel, the transfer curve still shifts towards negative VSG (Fig. 5b). The electrochemical potential distribution of the pOECT terminals is consistent with those reported in Fig. 3c. An increase in Na+ concentrations leads to increased channel potentials at which the n-type material becomes more de-doped at the given VSG, reducing the IDS. The pure electrochemical potential contribution to the sensing signal is remarked in the inset of Fig. 5b. Figure 5c summarizes and compares the sensing-induced changes in the transfer curves of the two types of devices (n- and p-type) operated with the same Na+- selective GS. Regardless of the OMIEC type employed in the pOECT, the transfer curve shifts towards negative VSG (positive VSG) for positive ΔOCP (negative ΔOCP) of the GS. For a p-type channel, positive ΔOCP causes a current increase, leading to an NR that can easily overcome 1, thus providing high amplification. For an n-type channel, instead, this type of sensing causes a current decrease, leading to low amplification, i.e., the NR values are limited between 0 and 1 (Fig. 5d). For the detection of the same target (Na+), with the same pOECT configuration, and the same pOECT working mode (enhancement), the maximum NR is 35 for the p-type (Fig. 3f), while it cannot exceed 1 for the n-type channel (Fig. 5d).
a Top: Transfer curves of the p-type Na+-IS-pOECT at [Na+] = 0 and 100 mM (as reported in Fig. 3b). The gating voltage is referred to with respect to the electrochemical potential of GS at different [Na+] (vs. GS, xM = 0 M and 100 mM). The double arrow highlights the VSG distance between the two curves, corresponding to the difference in the OCP of the GS in the two conditions (ΔOCP). Bottom: transfer curves at different Na+ concentrations, back shifted by a ΔVSG = ΔOCP where the 0 M condition is the reference point. This back shift makes the gating voltage to be referenced with respect to the GS at 0 M (vs. GS, 0 M). b Transfer curves of the n-type Na+-IS-pOECT at different Na+ concentrations. The inset reports the transfer curves back shifted by a ΔVSG = ΔOCP at 0 M Na+. c Transfer curves of an n-type and p-type Na+-IS-pOECT at [Na+] = 0 and 100 mM. The target interactions with the GS, which causes an increase in the GS electrochemical potential, lead to a negative shift of the transfer curves. d Normalized IDS of the n-type Na+-IS-pOECT recorded at different Na+ concentrations. e Left: Transfer curves of the n-type Cl−-IS-pOECT at different Cl− concentrations Right: Calibration curves of the conventional 2-electrode potentiometric setup (ISE) and the pOECT. f Left: Transfer curves of a PEDOT:PSS based Na+-IS-pOECT at different Na+ concentrations. Right: normalized IDS of the device.
If we use this “low performing” n-type OMIEC to detect anions instead of cations, the device performs as effectively as the p-type Na+-IS-pOECT (Fig. 5e-left). An increase in Cl− concentration turns ON the pOECT, and thus, the NR values are no longer constrained between 0 and 1 and are similar to those achieved with the p-type Na+ sensors for the same range of concentrations and at the same nA working regime (Fig. 5e-right). The similarity in NR values follows the Nernst equation; the slope of the electrochemical potential shift of the GS is the same but opposite in direction, since Na+ and Cl− have the same charge but opposite signs.
This comparison of two devices with similar transconductance efficiencies suggests that detecting increasing concentrations of cations is better accomplished with p-type OMIECs, while detecting increasing concentrations of anions is more effective with n-type OMIECs, since, in both cases, we have a progressive turn-ON behavior of the OMIEC, main requirement for maximizing the NR. This conclusion extends beyond ion detection and can be applied to any biosensing event where a target-recognition unit binding induces a potentiometric change. It is then important to know in advance the polarity of the electrochemical potential shift of the sensing electrode to couple it with an OMIEC that can be doped as a result of the potential shift.
A popular channel material for OECTs is poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS), which is used in p-type depletion-mode devices25,55. The main disadvantage of PEDOT:PSS based OECT sensors is that a depletion-mode device can only be turned OFF, and hence, the maximum NR that can be achieved is 1. Figure 5f shows how this limit can be overcome in the pOECT configuration where the Na+-ISE-based GS increases the electrochemical potential of the channel with an increase in Na+ concentration, leading to a doped film. In this case, the maximum NR for the highest cation concentration is 4, which is not as high as the enhancement-mode p-type pOECT but is still above the limit. The NR is lower because the highest sensitive region of this transistor is close to the OFF state of PEDOT:PSS. The intrinsic doping provided by the PSS- matrix does not allow complete de-doping without reaching an electrochemically unstable region that damages the polymer.
Complementary channel pOECT sensor for exclusive turn-ON operation
When monitoring parameters such as pH or other ions, we usually work in an environment with a constant concentration of the target (set point), equivalent to, for example, the physiological concentration in the healthy state. When using sensors that can perform continuous monitoring, our interest lies in tracking positive or negative fluctuations of the target. Since positive or negative concentration changes can cause an increase or decrease of the electrochemical potential of the Gs, the pOECT channel will either turn ON or OFF, depending on the OMIEC type employed, p or n. As demonstrated above, the pOECT will be more sensitive to fluctuations that turn ON the OMIEC and less sensitive to fluctuations that turn it OFF. To achieve the highest sensitivity for fluctuations in both directions, it is thus necessary to combine an n-type and a p-type channel, both gated by the same GS.
The conventional device type that combines n-type and p-type channels is the complementary amplifier56,57,58. Despite the good sensor performance of OECT-based complementary amplifiers, this configuration requires direct gating using the sensing electrode, which, as discussed earlier, adversely affects the sensor’s thermodynamic behavior. Moreover, given that the two OMIECs are in series and share the same drain voltage (VDD), the relative geometry and thickness of the OECT channels must be optimized so that the current output of both channels is similar in absolute value. This usually means a small channel for the p-type and an interdigitated and thick channel for the n-type device. Another constraint is that since the two materials are gated using the same voltage, their threshold voltage should be matched to avoid overlapping ON states of the OMIECs. Furthermore, the channels should share common gate voltages for the OFF state, conditions that are challenging to achieve using the current library of OMIECs59.
The pOECT configuration can overcome these challenges and monitor all the variations of a target concentration by combining p-type and n-type channels patterned on one substrate operated using the same GS and GG (Fig. 6a). In contrast to a complementary amplifier, in the complementary pOECT, all applied voltages (VDS and VSG for n-type and p-type) are independent. This allows us to obtain similar IDS values in the ON states if needed (in our case, VDS1 = 100 mV and VDS2 = 10 mV) without optimizing the device geometry. In addition, two independent gating voltages can be applied (VSG1 and VSG2), removing any threshold voltage matching requirement, allowing to work efficiently in an OFF state for both channels at a specific electrochemical potential of the GS (set point). In Fig. 6a, we depict the complementary pOECT with a pH-sensitive PANI electrode as the GS. We used Ag/AgCl electrodes as floating gates that establish communication between the sensing chamber and the channels’ chamber. The two Ag/AgCl electrodes play the role of a floating gate and not of conventional reference electrodes. The integration of the floating gates here is equivalent to that of a salt bridge or a microfluidic channel. The same floating gate configuration can also be achieved by using two microfabricated planar Au electrodes instead of Ag/AgCl39. Figure 6b shows that it is possible to keep both channels in the OFF state when the pH is at the set point (7 in this example) by selecting the appropriate VSG1 and VSG2. When the pH increases, the electrochemical potential of the GS decreases (Fig. 6b-inset), and the n-type channel turns ON, while the p-type channel, which was already OFF, remains in an OFF state. When the pH decreases, the positive electrochemical shift of the GS drags the pOECT channels to positive potentials, and only the p-type device turns ON. Working in conditions where both channels are OFF when the system is at the set point allows us to maximize the response to pH shifts in both directions, as shown in Fig. 6c. The complementary pOECT can reach an average NR of 44 for a pH shift of 3 units in both directions, while the classical 2-electrode setup can only reach 1.6. Figure 6b-inset evidences the retention of the Nernstian behavior of the PANI pH-sensitive electrode (GS) in this complementary setup (59 mV/pH). The sensor performance of each pOECT can be further improved by changing the device geometry (see Supplementary Fig. 11).
a Top: a visual representation of the complementary channel pOECT. The GS is kept in a separate electrolyte with a varying pH, while the channel and GG are in a second chamber with an electrolyte of constant composition (1× PBS). Two Ag/AgCl electrodes are used as floating gates to connect the two chambers. Bottom: a schematic of the setup where each channel is operated using the same GS and GG and has its independent drain voltage (VDS1, VDS2) and gate voltage (VSG1, VSG2). The dashed lines represent the high-impedance connections of the potentiostat. The floating gate is not represented. b The transfer curves of the n-type (left) and p-type (right) pOECT when the GS is exposed to electrolytes with different pH values. The inset reports the electrochemical potential of the GS during the measurement, demonstrating the maintained Nernstian response. c Normalized response of the n-type pOECT, the p-type pOECT, and the conventional 2-electrode setup to varying pH values. The error bars correspond to the standard deviation of 3 measurements.
Multichannel pOECT for cell monitoring at constant OMIEC thermodynamic conditions
The devices demonstrated above used the pOECT configuration to amplify the electrochemical potential changes an external electrode (GS) undergoes during sensing. While the pOECT is not designed for faradaic or impedimetric sensing at the gate contact due to the constant OCP condition of this electrode, it is possible to use this configuration to perform such sensing directly at the channel/electrolyte interface. Here, we report a peculiar example of OECT-based impedimetric sensing, where the pOECT provides a fundamental advantage over the traditional OECT. OECTs can measure the transepithelial/transendothelial electrical resistance of barrier-forming cells with high sensitivity, which is a widely accepted electrical parameter to assess tissue health, generating a label-free platform for screening drug toxicity60,61,62,63. In this application, the cells are grown on top of the channel; hence, they partially block the ion flow toward the OMIEC, which is necessary for its doping/de-doping. The permeability of the tissue over time is thus indirectly assessed through IDS, by monitoring how easily the G can inject ions inside the OMIEC, passing across the cell layer. However, for accurate measurements of the tissue permeability, the channel must be biased at the same electrochemical potential, which means that it is at constant thermodynamic conditions and under a constant driving force for ion injection during the entire experiment. This requirement can be achieved only if the electrochemical potential of the G remains constant across all the measurements.
The straightforward solution to bias the OECT channel always at the same and reproducible electrochemical potential is to use a non-polarizable electrode such as the common Ag/AgCl pellet as the G. However, Ag+ released from the pellet into the cell media is toxic for the cells64 (Supplementary Figs. 12–13). A Pt electrode, which has a low polarizability, could be a nontoxic alternative (Supplementary Figs. 12–13). However, since this electrode is directly exposed to the cell media, and cell media composition changes throughout the lifetime of the tissue, it cannot guarantee a constant electrochemical potential (Supplementary Fig. 14). Isolating the G from the cell media and placing it in its own electrolyte with a porous glass frit could be a solution; hence, a regular Ag/AgCl RE or a Pt wire in a separate compartment could be used. However, the glass frit can be easily contaminated with species in the complex cell media65, leading to a variation of the junction potential and a non-constant G biasing. Moreover, the Ag+ ions of the Ag/AgCl RE can still leak in the cell media through the frit - even though at a lower rate – being toxic to the cells14,65,66 (Supplementary Figs. 12–13). A leakless Ag/AgCl RE seems to be the only and best option as the G which features all the necessary requirements (constant electrochemical potential and no toxic compound leakage, Supplementary Figs. 12–13). However, because of the high impedance of the solid electrolyte that prevents leakage or internal contamination, leakless Ag/AgCl RE cannot be used directly as a G in a regular OECT (Supplementary Fig. 15). In Supplementary Discussion 7, we provide experimental evidence for these claims summarized above.
Furthermore, in experiments involving cells, increasing the number of devices is crucial. Probing the behavior of the cells on a single micrometric spot might lead to over- or under-estimation of the system behavior due to the low statistical relevancy of sampling a surface containing a few tens of cells, compared to the several thousand present in the whole cell culture. Instead of handling multiple individual devices, it is more convenient to work with a single chip containing multiple sensors (n) and operate these devices in parallel. Unfortunately, with a conventional OECT, increasing the number of devices progressively increases the IGS, which leads to strong G polarization and low reproducibility.
The pOECT allows for the integration of high-impedance gate electrodes, including the leakless RE (Supplementary Fig. 16) as well as the fabrication of multiple micronscale channels patterned on a small area (6 mm2, Supplementary Fig. 17). Figure 7a shows the device design with multiple channels gated by the same GG (CEα), and their doping potential is defined with respect to a common GS (REα, leakless Ag/AgCl). The number of transistor channels is virtually unlimited, as long as the GG is capacitive enough not to be polarized at too high or low potentials (due to the \({{{{\rm{I}}}}}_{{{{{\rm{G}}}}}_{{{{\rm{S}}}}}}\)). Considering the small size of the pOECT channels, a regular CE, for example, at Pt wire, easily serves the purpose.
a Top: A visual representation of multichannel pOECT where a leakless RE is used as GS and a Pt wire is used as GG. Bottom: A schematic of a multichannel pOECT setup with n channels, each operated at an individual VDS,n but a common VSG. The dashed line represents the high-impedance connection of the potentiostat. b Top: schematic representation of the cell layer grown on top of one pOECT channel before and after trypsin addition. Middle: The applied VSG pulse profile. Bottom: A representative pOECT output in the case of healthy tissue (green) and when the cells are exposed to trypsin (yellow). The measurements were conducted in parallel using multiple pOECT channels. The scale bar in the microscopy images is 100 µm. c Top: The change in the cut-off frequency of four pOECT channels triggered by the trypsin addition (marked with an arrow). Bottom: The average cut-off frequency change (standard deviation from four pOECT channels displayed as shaded area) after compensating for the delay time due to trypsin diffusion.
In Fig. 7b and Supplementary Fig. 17a, we show the healthy layer of epithelial cells covering the pOECT channels (PEDOT:PSS). When a VSG pulse is applied, the equilibrium IDS is reached slowly as the cells present a tight barrier against the injection of ions towards the channel. When trypsin is added to the media, the proteins responsible for forming the barrier tissue and those responsible for the adhesion to the substrate are attacked (Supplementary Fig. 17b). This leads to the progressive detachment of the cells and, hence, easier movement of ions that penetrate the channel in a much shorter time. The response time of the pOECT channels can be modeled with an exponential equation \({{{{\rm{I}}}}}_{{{{\rm{DS}}}}}={{{{\rm{I}}}}}_{{{{\rm{DS}}}} \_ \min }+\Delta {{{{\rm{I}}}}}_{{{{\rm{DS}}}}} \cdot \exp (-\left({{{\rm{t}}}}-{{{{\rm{t}}}}}_{0}\right)/{{{{\rm{\tau }}}}}_{{{{\rm{OFF}}}}})\) from which a time constant (\({{{{\rm{\tau }}}}}_{{{{\rm{OFF}}}}}\)) can be extracted and the cut-off frequency of the device can be calculated (\({{{{\rm{f}}}}}_{{{{\rm{CO}}}}}=1/2{{{\rm{\pi }}}}{{{{\rm{\tau }}}}}_{{{{\rm{OFF}}}}}\)). In Fig. 7c-top, we report the normalized \({{{{\rm{f}}}}}_{{{{\rm{CO}}}}}\) change for four cell-interfacing pOECT channels belonging to the same culture, as trypsin is added to the media. All channels show a sigmoidal frequency change with an initial latency, a fast frequency increase due to progressive cell detachment, and a final saturation when the cells are completely detached. The variability between the speeds of each channel at the beginning of the growth phase is due to the different diffusion rates of trypsin toward the cells, which is related to their differentiation. After compensating for this variable delay by horizontally shifting the curves, according to the method described in Supplementary Fig. 18, we obtain the average \({{{{\rm{f}}}}}_{{{{\rm{CO}}}}}\) change reported in Fig. 7c-bottom.
The multiple pOECT setup coupled with a leakless RE allows to perform a reliable measurement with multiple simultaneous replicates on different locations of the cell culture, providing data of statistical relevance. This sensor design can be used to monitor the behavior of other systems, such as supported lipid bilayers67, and study kinetic interactions between biological interfaces and different drugs for long periods of time in safe conditions. For a more advanced analysis, each pOECT can be independently gated (still using a common GS and GG) to perform different techniques (impedimetric and/or faradaic) and monitor different parameters simultaneously. For example, other than the cut-off frequency, some of the pOECT channels can be functionalized with specific enzymes for the faradaic detection of metabolites68,69,70, whose quantification through OECT/pOECT may require a different bias than the one used for the cut-off frequency.
We have introduced an OECT sensing configuration, called the pOECT, to overcome some possible limitations of the classical OECT when employed for potentiometric sensing while leveraging its high potential as an amplifier and RE-free system. In the pOECT, the gating system is no longer a single electrode that has to both perform the sensing and provide the voltage to modulate the doping state of the semiconductor. These tasks are performed by two independent electrodes, namely, GS and GG, giving a very broad design flexibility for the device component selection. GS works as a RE for applying the gating voltage, while GG works as a CE. If a sensing electrode, traditionally used as WE in a 2-electrode setup or as a G in an OECT, is connected as the GS, the pOECT becomes the most accurate electrolyte-gated transistor for potentiometric sensing with a target response much higher than that of the 2-electrode setup and the standard OECT. This configuration allows to preserve the accuracy of a classical 2-electrode setup (which is the maximum achievable), providing at the same time an intrinsic signal amplification (a typical characteristic of the OECTs), all without requiring a standard RE, advantageous for miniaturization and the biocompatibility of the device.
In contrast to the OECT, the pOECT prevents any polarization of the sensing interface (GS), which is key for ensuring sensor accuracy. By removing any interference from the sensing electrode capacitance, this configuration preserves the thermodynamic behavior of the electrode, rendering the transistor solely responsive to the electrochemical potential of the GS. It eliminates any possible current/voltage-related degradation or parasitic reactions at the sensing electrode, enhancing device stability. The pOECT allows for the use of various potentiometric sensing electrodes, including high-impedance ones, without causing damage, a capability not achievable with conventional OECTs. Moreover, since the operation is not influenced by the size of the Gs, a small sensing electrode can be used when necessary, offering advantages in terms of both cost reduction and miniaturization.
One feature of the pOECT configuration is its modularity. With the pOECT it becomes possible to couple n- and p-type OMIECs to create a complementary device that maximizes sensor output without any restriction on the OMIEC geometry or thermodynamic properties and operational voltages. Besides potentiometric sensing, pOECT can be employed for impedimetric and faradaic sensing, ensuring higher accuracy than the OECT. pOECT allows continuous and real-time sensing on multiple devices in parallel, increasing the statistical relevancy of the experiments. This is a particularly important device design criterion for applications interfacing with living cells. Multi-marker analysis can be done from a single chip, revealing correlations between various analytes that cells consume or produce during their lifetime. The diverse range of experiments conducted in this work underscores the pOECT setup as an accountable and higher-performing edition of the electrolyte-gated transistor operating in potentiometric mode. The pOECT configuration eliminates the errors associated with OECT sensors, propelling the transistor-based sensor technologies toward the translational value they deserve.
Methods
Materials
Sodium chloride (NaCl), ammonium sulfate ((NH4)2SO4), Phosphate Buffered Saline (PBS, pH 7.4), ethylene glycol, sodium dodecylbenzenesulfonate, (3-glycidyloxypropyl) trimethoxysilane, chloroform, hydrochloric acid, aniline, buffer solutions pH = 4, 7 and 10, potassium hexacyanoferrate, (III), potassium hexacyanoferrate (II), phosphoric acid, boric acid, acetic acid, potassium nitrate, and propidium iodide (PI) were purchased from Sigma–Aldrich. Trypsin (TrypLETM), Dulbecco’s Phosphate Buffered Saline containing Ca2+ and Mg2+ (1× DPBS), 1× DPBS (Ca2+ and Mg2+ free), Dulbecco׳s Modified Eagle׳s Media (DMEM), fetal bovine serum (FBS), glutaMax, penicillin–streptomycin (P/S), Bovine Albumin Serum (ABS) were purchased from Thermo Fisher. Calcein AM was purchased from Life Technologies, paraformaldehyde (PFA) from Electron Microscopy Sciences, Triton X-100, phalloidin-rhodamine and gold mounting medium from Invitrogen, Madin-Darby canine kidney cells (MDCKII) from ECACC, CLEVIOS PH1000 (PEDOT:PSS) from Heraeus. All aqueous solutions were prepared with ultrapure water (Millipore Milli-Q). The n-type OMIEC p(C6NDI-T)29 and the p-type OMIEC p(g3C2T2-T)71 were synthesized according to procedures reported previously. The chemical structures of the polymers are shown in Supplementary Fig. S1.
Device fabrication
The single channel OECTs/pOECTs (W · L = 100 · 10 µm) with various OMIEC patterning protocols were fabricated according to a procedure reported previously71. Large channel OECTs/pOECTs capable of reaching mA currents were obtained by using commercially available interdigitated electrodes. The PANI film was electropolymerized on a Au electrode (Φ = 5 mm) using a procedure reported in literature72. Na+ and Cl− ISE were purchased from NT Sensors, Pt coil/wire, Au electrodes and Ag/AgCl glass RE from als-japan, AgCl pellet from Warner Instruments, leakless Ag/AgCl from eDAQ, glass pH-meter from Metrohm, interdigitated electrodes (ED-IDE3-Au) from MicruX Technologies. For interfacing the pOECT channels with epithelial cells, pOECT devices were first sterilized using 70% ethanol for 30 min, rinsed with water, and dried inside biosafety cabinet. To improve the adhesion of cells, pOECT devices were plasma activated using plasma cleaner 20 W for 20 s. MDCKII cells were then seeded on the device and incubated at 37 °C in a humidified atmosphere with 5% CO2 until a barrier tissue was formed, which appeared typically after 2–3 days.
Device operation, characterization, and sensing measurements
All electrochemical measurements were performed with a BioLogic VSP-300 multichannel potentiostat. The transfer curves of the OECT/pOECT were recorded by connecting the devices according to the schematics reported in Supplementary Fig. 2. Specifically, two channels of the multichannel potentiostat were used, the first one for the application of VDS and the second one for the application of VGS or VSG. The instrument can be operated in either floating or grounded mode. The same device operation can also be obtained by combining a source measure unit (SMU) for the application of VDS and a single channel potentiostat for the application of VGS or VSG.
If otherwise stated, the applied VDS for all measurements was +100 mV for the n-type and −100 mV for the p-type devices, while the gate voltage was cyclically scanned multiple times (100 mV/s), according to the ranges reported on each plot until the IDS stabilized. A glass well with a PDMS base was placed on top of the OECT/pOECT channel and filled with the electrolyte (100 mM (NH4)2SO4 or 1× PBS), and G/GG (or the GS when the single chamber configuration was used) was also placed in the same solution.
The OECT and pOECT characterization (no sensing) was done with the OECT-single chamber and pOECT-single chamber setup with 1× PBS electrolyte. For sensing measurements, the sensing electrode (G or GS) was kept in a separate compartment from the OMIEC (and GG when pOECT was used). Since ion concentration/composition variations in the electrolyte can significantly alter the behavior of the OMIEC73, the separation of the channel from the sensing interface enables maintaining a constant electrolyte composition in the OMIEC compartment. This allows us to eliminate any interference from OMIEC and measure solely the effect of the change of electrochemical potential of the GS on output characteristics. The pOECT Na+ and Cl− sensing measurements were performed with the pOECT-microfluidic setup, where the two compartments were connected through a microfluidic tubing to establish the ionic connection. 100 mM (NH4)2SO4 was used as the electrolyte in both chambers. Consecutive aliquots of NaCl from a standard solution were added to the sensing chamber to increase the concentration of the target ion. The 2-electrode OCP measurement for Na+ sensing was performed by measuring the open circuit voltage between the Na+-ISE (WE) and the Ag/AgCl (RE) when the two electrodes were placed in the same electrolyte (100 mM (NH4)2SO4) and consecutive aliquots of NaCl from a standard solution were added to the electrolyte. (NH4)2SO4 was selected as the electrolyte instead of the more common 1× PBS since it contains only minimal traces of Na+ and Cl−, which are otherwise present in PBS.
The measurements related to pH sensing were performed with the OECT-floating gate and pOECT-floating gate setup, where the two compartments were connected through two Ag/AgCl RE to establish the ionic connection. 1× PBS was used as the electrolyte in the OMIEC chamber, and different buffer solutions were added to the sensing chamber. For sensing measurements where the pH variations were of 3 units (4, 7, and 10), commercially available buffer solutions were used. For sensing measurements where the pH variation was of 1 unit (6 and 7), a universal buffer (Britton-Robinson Buffer: 0.01 M H3PO4, 0.01 M H3BO3, 0.01 M CH3COOH in 0.1 M KNO3 adjusted to the desired pH with NaOH(aq)) was used. The floating gate and the microfluidic setup are identical in terms of device performance and reported to show the system’s versatility.
The measurement related to the barrier tissue monitoring was performed with the pOECT-single chamber setup, with a Pt wire as GG and a leakless Ag/AgCl RE as GS. The pOECTs with cells were removed from the incubator and washed 3 times with DPBS after removing the cell media. 100 µL of 1× DPBS (Ca2+ and Mg2+ free) was added as the electrolyte. A constant VDS = −0.1 V was applied while the VSG was continuously pulsed (3 s) between 0 and −0.3 V for the whole experiment, with no interruption. After about 100 s of initial pulsing, to establish a baseline, 100 µL of trypsin solution was added to the initial volume of 100 µL DPBS. The pulse recording was performed for 1 h; however, after 15 min, the cells completely detached.
The electrochemical potential measurements of the OECT/pOECT components were performed by measuring the open circuit voltage of each electrode connected to the WE of the potentiostat with respect to a common Ag/AgCl RE if the components were in the same electrolyte. The measurement of n components of the OECT/pOECT was performed in parallel during the device operation by utilizing n synchronized potentiostat channels.
The cyclic voltammetry (CV) measurements were performed according to the schematics reported in Supplementary Fig. 5d with an Ag/AgCl RE and Pt coil CE, by cyclically applying the voltage range reported in each plot, repeated until IWE stabilization. The scan rate was 100 mV/s. The electrolyte was 1× PBS with 5 mM potassium hexacyanoferrate (III) and 5 mM potassium hexacyanoferrate (II).
The current flowing through the RE connection was measured by using the zero resistance amperometry (ZRA) technique and an ultra-low current module (BioLogic) where one side of the RE cable was connected to the RE/CE, and the other side was connected to the WE of the potentiostat, and a constant 0 V was applied.
To quantify the device response in the ion sensing measurements and minimize the device-to-device variations, we reported the normalized response of the pOECT as NR (IDS). This parameter is also used as a metric to compare the performance of the pOECT in different conditions. It is calculated by normalizing the IDS change after target exposure to the sensing unit with respect to the blank solution:
where \({{{{\rm{I}}}}}_{{{{\rm{DS}}}},{{{{\rm{M}}}}}^{\pm }}\) is the pOECT IDS current after interaction of the GS with the target \({{{{\rm{M}}}}}^{\pm }\) at a specific bulk concentration, and \({{{{\rm{I}}}}}_{{{{\rm{DS}}}},{{{\rm{blank}}}}}\) is the pOECT IDS current before interaction of the GS with the target. The NR values reported in the calibration curves correspond to the voltages where the device showed the highest response (highest NR).
An analogous normalization was performed for the 2-electrode OCP measurement, reported as NR (V):
Since it is not possible to directly compare the signal (IDS vs EWE) or the sensitivity (ΔIDS/ΔC vs Δ EWE/ΔC) of the pOECT and the classical 2-electrode setup, due to different working regimes (current regime vs voltage regime), we compared their performance in terms of NR.
All performance comparisons were conducted in analogous conditions. For the pOECT vs 2-electrode setup with WE size = GS size and for the OECT vs pOECT with G size = GS size and OECT channel size = pOECT channel size.
To quantify the device response in the cell barrier tissue monitoring and minimize the device-to-device variations, the cut-off frequency change was normalized (\({{{\rm{NR}}}}({{{{\rm{f}}}}}_{{{{\rm{CO}}}}})\)) to a range from 0 to 1, where 0 corresponds to the lowest cut-off frequency \(({{{{\rm{f}}}}}_{{{{\rm{CO}}}},\min })\) and 1 to the highest (\({{{{\rm{f}}}}}_{{{{\rm{CO}}}},\max }\)):
Cell culture
Madin-Darby Canine Kidney cells (MDCK II) were routinely maintained in DMEM media at 37 °C in a humidified atmosphere of 5% CO2. The media was supplemented with 5% fetal bovine serum (FBS), 2 mM Glutamax, and 1% PenStrep 100× (10,000 U mL−1 Penicillin, 10,000 µg mL−1 Streptomycin).
Assessment of cytotoxicity in different reference electrodes
For a general evaluation of the cytotoxicity on various reference electrodes (pellet, platinum, leakless, and glass), two characteristics of cells were evaluated after their culture in contact with the electrodes for 72 h: cell viability using a LIVE/DEAD assay and cell morphology with actin staining. The different electrodes were carefully placed in a 48-well plate, avoiding contact with the bottom of the well, where MDCKII cells (6000 cells/cm2, P.34) were seeded.
Cell viability
After 72 h of culture, cells were incubated with 3 μM, Calcein AM, and 3.5 μM PI in PBS containing Ca2+ and Mg2+, for 10 min at 37 °C and fluorescent images were captured using a 10× objective in an inverted Leica DMi8 microscope. Calcein AM is converted to a fluorescent molecule, green, when it interacts with viable cells, while PI reacts with the nuclei content of dead cells, generating a red signal.
Cell morphology
After 72 h of culture, cells were fixed with 4% (w/v) paraformaldehyde for 15 min and stained with rhodamine-phalloidin for 60 min. Phalloidin binds and stabilizes F-actin in the cell cytoskeleton, and rhodamine works as a marker and allows fluorescent visualization of F-actin with a red signal. This method delineates the cells and allows the visualization of differences in morphology between cells in contact with different electrodes. Cells were imaged using a 20× objective in an inverted confocal laser scanning microscope with Airyscan (Zeiss LSM 880).
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format, they are available from the corresponding author upon request.
References
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (Wiley, 2022).
Bandodkar, A. J. et al. Tattoo-based potentiometric ion-selective sensors for epidermal pH monitoring. Analyst 138, 123–128 (2013).
Anastasova, S. et al. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 93, 139–145 (2017).
Novell, M., Parrilla, M., Crespo, G. A., Rius, F. X. & Andrade, F. J. Paper-based ion-selective potentiometric sensors. Anal. Chem. 84, 4695–4702 (2012).
Bobacka, J., Ivaska, A. & Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 108, 329–351 (2008).
Bakker, E., Pretsch, E. & Bühlmann, P. Selectivity of potentiometric ion sensors. Anal. Chem. 72, 1127–1133 (2000).
Bakker, E., Bühlmann, P. & Pretsch, E. Polymer membrane ion-selective electrodes-what are the limits? Electroanalysis 11, 915–933 (1999).
Ding, J. & Qin, W. Recent advances in potentiometric biosensors. TrAC Trends Anal. Chem. 124, 115803 (2020).
Yang, Y., Yang, X., Yang, Y. & Yuan, Q. Aptamer-functionalized carbon nanomaterials electrochemical sensors for detecting cancer relevant biomolecules. Carbon N Y 129, 380–395 (2018).
Hosu, O., Selvolini, G., Cristea, C. & Marrazza, G. Electrochemical immunosensors for disease detection and diagnosis. Curr. Med. Chem. 25, 4119–4137 (2018).
Ozdemir, M. S. et al. A label-free potentiometric sensor principle for the detection of antibody–antigen interactions. Anal. Chem. 85, 4770–4776 (2013).
Sharafeldin, M., James, T. & Davis, J. J. Open circuit potential as a tool for the assessment of binding kinetics and reagentless protein quantitation. Anal. Chem. 93, 14748–14754 (2021).
Figueiredo, A. et al. Electrical detection of dengue biomarker using egg yolk immunoglobulin as the biological recognition element. Sci. Rep. 5, 7865 (2015).
Zdrachek, E. & Bakker, E. Potentiometric sensing. Anal. Chem. 93, 72–102 (2021).
Hu, J., Stein, A. & Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 76, 102–114 (2016).
Rousseau, C. R. & Bühlmann, P. Calibration-free potentiometric sensing with solid-contact ion-selective electrodes. TrAC Trends Anal. Chem. 140, 116277 (2021).
Sophocleous, M. & Atkinson, J. K. A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors. Sens. Actuators A Phys. 267, 106–120 (2017).
Wu, M. et al. Ultrathin, soft, bioresorbable organic electrochemical transistors for transient spatiotemporal mapping of brain activity. Adv. Sci. 10, e2300504 (2023).
Xie, K. et al. Organic electrochemical transistor arrays for real-time mapping of evoked neurotransmitter release in vivo. Elife 9, e50345 (2020).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Strakosas, X., Bongo, M. & Owens, R. M. The organic electrochemical transistor for biological applications. J. Appl. Polym. Sci. 132, 41735 (2015).
Leleux, P. et al. Organic electrochemical transistors for clinical applications. Adv. Health. Mater. 4, 142–147 (2015).
Paulsen, B. D., Tybrandt, K., Stavrinidou, E. & Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 19, 13–26 (2020).
Gualandi, I. et al. Organic electrochemical transistors as versatile analytical potentiometric sensors. Front. Bioeng. Biotechnol. 7, 354 (2019).
Liu, H. et al. Ultrafast, sensitive, and portable detection of COVID-19 IgG using flexible organic electrochemical transistors. Sci. Adv. 7, eabg8387 (2021).
Gualandi, I. et al. Nanoparticle gated semiconducting polymer for a new generation of electrochemical sensors. Sens. Actuators B Chem. 273, 834–841 (2018).
Salvigni, L. et al. Selective detection of liposoluble vitamins using an organic electrochemical transistor. Sens. Actuators B Chem. 393, 134313 (2023).
Arcangeli, D. et al. Smart bandaid integrated with fully textile OECT for uric acid real-time monitoring in wound exudate. ACS Sens. 8, 1593–1608 (2023).
Koklu, A. et al. Microfluidics integrated n-type organic electrochemical transistor for metabolite sensing. Sens. Actuators B Chem. 329, 129251 (2021).
Guo, K. et al. Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat. Biomed. Eng. 5, 666–677 (2021).
Macchia, E. et al. Ultra-sensitive protein detection with organic electrochemical transistors printed on plastic substrates. Flex. Print. Electron. 3, 034002 (2018).
Chen, S. et al. Recent technological advances in fabrication and application of organic electrochemical transistors. Adv. Mater. Technol. 5, 2000523 (2020).
Berggren, M., Nilsson, D. & Robinson, N. D. Organic materials for printed electronics. Nat. Mater. 6, 3–5 (2007).
Demuru, S. et al. All-inkjet-printed graphene-gated organic electrochemical transistors on polymeric foil as highly sensitive enzymatic biosensors. ACS Appl. Nano Mater. 5, 1664–1673 (2022).
Zabihipour, M. et al. High yield manufacturing of fully screen-printed organic electrochemical transistors. npj Flex. Electron. 4, 15 (2020).
Kim, S.-M. et al. Influence of PEDOT:PSS crystallinity and composition on electrochemical transistor performance and long-term stability. Nat. Commun. 9, 3858 (2018).
Bidinger, S. L., Han, S., Malliaras, G. G. & Hasan, T. Highly stable PEDOT:PSS electrochemical transistors. Appl. Phys. Lett. 120, 073302 (2022).
Tan, S. T. M. et al. High‐gain chemically gated organic electrochemical transistor. Adv. Funct. Mater. 31, 2010868 (2021).
White, S. P., Dorfman, K. D. & Frisbie, C. D. Label-free DNA sensing platform with low-voltage electrolyte-gated transistors. Anal. Chem. 87, 1861–1866 (2015).
Zhang, S. et al. Toward stable p‐type thiophene‐based organic electrochemical transistors. Adv. Funct. Mater. 33, 2302249 (2023).
Mariani, F. et al. Design of an electrochemically gated organic semiconductor for pH sensing. Electrochem. Commun. 116, 106763 (2020).
Mariani, F. et al. Advanced wound dressing for real-time pH monitoring. ACS Sens. 6, 2366–2377 (2021).
Di Franco, C. et al. Extended work function shift of large‐area biofunctionalized surfaces triggered by a few single‐molecule affinity binding events. Adv. Mater. Interfaces 10, 2201829 (2023).
Macchia, E. et al. Single-molecule detection with a millimetre-sized transistor. Nat. Commun. 9, 3223 (2018).
Bonafè, F., Decataldo, F., Fraboni, B. & Cramer, T. Charge carrier mobility in organic mixed ionic–electronic conductors by the electrolyte-gated van der Pauw method. Adv. Electron. Mater. 7, 2100086 (2021).
Ji, X., Lin, X. & Rivnay, J. Organic electrochemical transistors as on-site signal amplifiers for electrochemical aptamer-based sensing. Nat. Commun. 14, 1665 (2023).
Memming, R. Semiconductor Electrochemistry (Wiley, 2015).
Park, J. H. et al. Open circuit (mixed) potential changes upon contact between different inert electrodes–size and kinetic effects. Anal. Chem. 85, 964–970 (2013).
Macchia, E. et al. Large-area interfaces for single-molecule label-free bioelectronic detection. Chem. Rev. 122, 4636–4699 (2022).
Liang, Y., Wu, C., Figueroa-Miranda, G., Offenhäusser, A. & Mayer, D. Amplification of aptamer sensor signals by four orders of magnitude via interdigitated organic electrochemical transistors. Biosens. Bioelectron. 144, 111668 (2019).
Lin, P., Luo, X., Hsing, I. & Yan, F. Organic electrochemical transistors integrated in flexible microfluidic systems and used for label‐free DNA sensing. Adv. Mater. 23, 4035–4040 (2011).
Tao, W. et al. A sensitive DNA sensor based on an organic electrochemical transistor using a peptide nucleic acid-modified nanoporous gold gate electrode. RSC Adv. 7, 52118–52124 (2017).
Colburn, A. W., Levey, K. J., O’Hare, D. & Macpherson, J. V. Lifting the lid on the potentiostat: a beginner’s guide to understanding electrochemical circuitry and practical operation. Phys. Chem. Chem. Phys. 23, 8100–8117 (2021).
Percival, S. J. & Bard, A. J. Ultra-sensitive potentiometric measurements of dilute redox molecule solutions and determination of sensitivity factors at platinum ultramicroelectrodes. Anal. Chem. 89, 9843–9849 (2017).
Bernards, D. A. et al. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 18, 116–120 (2008).
Sun, H. et al. Complementary logic circuits based on high‐performance n‐type organic electrochemical transistors. Adv. Mater. 30, 1704916 (2018).
Romele, P. et al. Multiscale real time and high sensitivity ion detection with complementary organic electrochemical transistors amplifier. Nat. Commun. 11, 3743 (2020).
Wang, Y. et al. Acceptor functionalization via green chemistry enables high‐performance n‐type organic electrochemical transistors for biosensing, memory applications. Adv. Funct. Mater. 34, 2304103 (2024).
Druet, V. et al. A single n-type semiconducting polymer-based photo-electrochemical transistor. Nat. Commun. 14, 5481 (2023).
Tria, S. A. et al. Dynamic monitoring of Salmonella typhimurium infection of polarized epithelia using organic transistors. Adv. Health. Mater. 3, 1053–1060 (2014).
Ferro, M. P. et al. Effect of E cigarette emissions on tracheal cells monitored at the air–liquid interface using an organic electrochemical transistor. Adv. Biosyst. 3, 1800249 (2019).
Ramuz, M. et al. Combined optical and electronic sensing of epithelial cells using planar organic transistors. Adv. Mater. 26, 7083–7090 (2014).
Ramuz, M., Hama, A., Rivnay, J., Leleux, P. & Owens, R. M. Monitoring of cell layer coverage and differentiation with the organic electrochemical transistor. J. Mater. Chem. B 3, 5971–5977 (2015).
Ramuz, M. et al. Optimization of a planar all‐polymer transistor for characterization of barrier tissue. ChemPhysChem 16, 1210–1216 (2015).
Anderson, E. L., Lodge, T. P., Gopinath, T., Veglia, G. & Bühlmann, P. More than a liquid junction: effect of stirring, flow rate, and inward and outward electrolyte diffusion on reference electrodes with salt bridges contained in nanoporous glass. Anal. Chem. 91, 7698–7704 (2019).
Anderson, E. L., Troudt, B. K. & Bühlmann, P. Critical comparison of reference electrodes with salt bridges contained in nanoporous glass with 5, 20, 50, and 100 nm diameter pores. Anal. Sci. 36, 187–191 (2020).
Kawan, M. et al. Monitoring supported lipid bilayers with n-type organic electrochemical transistors. Mater. Horiz. 7, 2348–2358 (2020).
Ohayon, D. et al. Biofuel powered glucose detection in bodily fluids with an n-type conjugated polymer. Nat. Mater. 19, 456–463 (2020).
Pappa, A. M. et al. Direct metabolite detection with an n-type accumulation mode organic electrochemical transistor. Sci. Adv. 4, eaat0911 (2018).
Wustoni, S. et al. In situ electrochemical synthesis of a conducting polymer composite for multimetabolite sensing. Adv. Mater. Technol. 5, 1900943 (2020).
Koklu, A. et al. Convection driven ultrarapid protein detection via nanobody‐functionalized organic electrochemical transistors. Adv. Mater. 34, 2202972 (2022).
Lindfors, T. & Ivaska, A. pH sensitivity of polyaniline and its substituted derivatives. J. Electroanal. Chem. 531, 43–52 (2002).
Savva, A. et al. Influence of water on the performance of organic electrochemical transistors. Chem. Mater. 31, 927–937 (2019).
Acknowledgements
This publication is based upon work supported by King Abdullah University of Science and Technology Research Funding under Award No. ORA-2021-CRG10-4650, Smart Health Initiative REI/1/5130-01-01, and Research Translational Grant REI/1/4577-01.
Author information
Authors and Affiliations
Contributions
L.S. conceived the research, designed all the experiments and methodology, performed the electrochemical measurements, analyzed the data, and wrote the manuscript. P.D.N. and A.K. helped with the experimental design and writing of the manuscript. D.A. supervised experiments involving the ion-selective electrodes and generated all illustrations. J.U. performed the cytotoxicity tests, grew cells on pOECTs, and took cell microscopy images. A.H. supervised the pOECT experiments with cells and introduced the floating gate design. T.C.H.C. and R.S. fabricated some of the transistor channels. A.M. and S.G. synthesized the n-type and p-type OMIECs, supervised by I.M. S.I. conceptualized and supervised the work and wrote the manuscript. All authors were involved in the discussion and participated in manuscript input.
Corresponding author
Ethics declarations
Competing interests
L.S. and S.I. made a patent application for the pOECT invention. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Salvigni, L., Nayak, P.D., Koklu, A. et al. Reconfiguration of organic electrochemical transistors for high-accuracy potentiometric sensing. Nat Commun 15, 6499 (2024). https://doi.org/10.1038/s41467-024-50792-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-50792-1
This article is cited by
-
From Electrostatic Field Modulation to Electrochemical Control: The Evolution of Organic Transistors for Next-Generation Electronics
Transactions on Electrical and Electronic Materials (2026)
-
Elemental composition, crystallinity and electrical studies of Ni–Co phosphate-PVC composite membrane through mechanism of monovalent electrolytes
Discover Chemistry (2026)
-
Organic electrochemical transistors for integrated sensing–memory–computing hardware towards intelligent Internet of Bodies
Nature Reviews Electrical Engineering (2025)
-
Algal polysaccharide Sacran-based conductive nanocomposites for ultrathin flexible and biodegradable organic electrochemical transistors
npj Flexible Electronics (2025)
-
Fiber-based organic electrochemical transistors for re-shaping bioelectronics: integration on and in textiles
npj Flexible Electronics (2025)