Abstract
Many disease resistance genes have been introgressed into wheat from its wild relatives. However, reduced recombination within the introgressed segments hinders the cloning of the introgressed genes. Here, we have cloned the powdery mildew resistance gene Pm13, which is introgressed into wheat from Aegilops longissima, using a method that combines physical mapping with radiation-induced chromosomal aberrations and transcriptome sequencing analysis of ethyl methanesulfonate (EMS)-induced loss-of-function mutants. Pm13 encodes a kinase fusion protein, designated MLKL-K, with an N-terminal domain of mixed lineage kinase domain-like protein (MLKL_NTD domain) and a C-terminal serine/threonine kinase domain bridged by a brace. The resistance function of Pm13 is validated through transient and stable transgenic complementation assays. Transient over-expression analyses in Nicotiana benthamiana leaves and wheat protoplasts reveal that the fragment Brace-Kinase122-476 of MLKL-K is capable of inducing cell death, which is dependent on a functional kinase domain and the three α-helices in the brace region close to the N-terminus of the kinase domain.
Similar content being viewed by others
Introduction
Common wheat (Triticum aestivum L., 2n = 6x = 42, AABBDD) is a widely grown cereal crop consumed by ~30% of the global population. Wheat production is constantly challenged by various fungal diseases, including rusts, Fusarium head blight, and powdery mildew1. Wheat powdery mildew, a foliar disease, is caused by the obligate biotrophic pathogen Blumeria graminis f. sp. tritici (Bgt) and is prevalent across most winter and spring wheat growing areas in the world, leading to significant yield losses2,3,4. Bgt is highly dynamic and co-evolves with wheat crops, which results in rapidly shifts in the virulence patterns of pathogen and ineffectiveness of powdery mildew resistance (Pm) genes used in wheat production5,6. Hence, exploiting and utilizing more effective Pm genes from common wheat and its cultivated or wild relatives are important to control powdery mildew disease7.
Aegilops longissima Schweinf. and Muschl. (2n = 2x = 14, SlSl) is a wild species in the Sitopsis section (S genome). It is highly related to the progenitor of the B genome of common wheat8,9. This diploid species is resistant to various diseases, such as rusts10, eyespot11, and powdery mildew12. The transfer of powdery mildew resistance from Ae. longissima into common wheat was initiated in the 1980s, leading to the creation of a series of wheat-Ae. longissima chromosome translocation lines12,13,14,15.
Pm13 was mapped to the distal part of the short arm of chromosome 3S1, which was transferred from Ae. longissima into wheat chromosome 3BS12. This gene has not been widely used in agriculture despite its highly effective resistance to powdery mildew, which is possibly due to the linkage drag of Pm13 introgression. Efforts have been made to reduce the Ae. longissima chromosomal fragments by promoting homoeologous chromosome pairing in the presence of gene ph1b. Cytogenetic and molecular markers were also developed to trace the critical Ae. longissima chromatin where Pm13 resides13,15,16. However, poor chromosomal synapsis between chromosomes 3Sl of Ae. longissima and the homoeologous wheat partners limits the isolation of the target gene via the genetic recombination-based gene cloning strategies.
In this work, we report the isolation of Ae. longissima-originated Pm13 using approaches that do not rely on genetic recombination, supported by transient and stable transgenic complementation assays. Pm13 encodes a kinase fusion protein with an N-terminal domain of mixed lineage kinase domain-like protein (MLKL_NTD domain) and a C-terminal serine/threonine kinase domain bridged by a brace. Transient over-expression analysis in Nicotiana benthamiana leaves and wheat protoplasts reveals that the fragment Brace-Kinase122-476 of MLKL-K is capable of inducing cell death that is dependent on kinase activity and the three α-helices in the brace region close to the N-terminus of the kinase domain.
Results
Pm13 from Ae. longissima confers resistance to 108 Bgt isolates
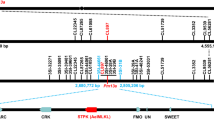
Non-denaturing fluorescence in situ hybridization (ND-FISH) was performed to characterize the chromosomal composition of wheat-Ae. longissima line No.3778, which has a pedigree of R1A/Bainong3217*5 that carries the powdery mildew resistance gene Pm13. Stronger signals of the FAM-labeled probe Oligo-pSc119.2 (green) were observed in the terminal regions of the short arm of chromosome 3B (3BS) compared to Chinese Spring (CS). The Tamra-labeled probe Oligo-pTa535 stained red on the rest part of chromosome 3B, which was similar to CS (Fig. 1a). This indicates that wheat line No. 3778 carries a pair of translocated chromosomes 3SlS-3BS.3BL, where the alien segment 3SlS is introgressed onto a distal location of the wheat chromosome arm 3BS. This result is consistent with the previous cytogenetic and molecular evidence13.
a Non-denaturing fluorescence in situ hybridization (ND-FISH) patterns of Chinese Spring (left) and line No. 3778 (right). Chromosomes of Chinese Spring and No. 3778 were stained with 4’,6-diamidino-2-phenylindole (DAPI) (blue), Oligo-pSc119.2 (green), and Oligo-pTa535 (red). Orange arrows indicate the translocated chromosomes 3SlS-3BS.3BL. The experiment was independently repeated three times with consistent results. Scale bar = 10 μm. b Representative images showing phenotypic effects of Pm13 against 8 out of 108 tested Bgt isolates. Line No. 3778 carrying Pm13, YM18 carrying Pm21, and Chancellor (CC) without any Pm gene were inoculated with the indicated isolates. Leaves are shown sequentially from left to right. c Representative images showing powdery mildew symptoms on susceptible γ-ray irradiation-induced M3 mutants (IT4), the resistant parent line No. 3778 carrying Pm13 (IT0), and the susceptible control XM44 (IT4). Powdery mildew responses of mutants were examined at the M2 and M3 generations. d Physical mapping of Pm13 using chromosomal deletion mutant lines. Dot lines show the positions of markers and corresponding genes in the reference genome of Ae. longissima accession AEG-6782-2. The red marker was developed from the Pm13 candidate transcript NODE_25350. Black and white bars indicate the presence or absence of markers.
Line No. 3778 was challenged with 108 Bgt isolates, including 101 isolates from China and 7 isolates from European countries3,17,18,19. This line was immune (IT 0) to all Bgt isolates tested at the seedling stage (Fig. 1b, Supplementary Data 1). It was also highly resistant to powdery mildew in the field disease test (Supplementary Fig. 1).
Suppressed recombination between Ae. longissima chromosome arm 3SlS and its wheat counterpart 3BS
When inoculated with Bgt isolate BgtYZ01, line No. 3778 showed an immune reaction (IT 0), while wheat cultivar Xumai 44 (XM44) was susceptible (IT 4). The F1 plants from the cross between line No. 3778 and XM44 were resistant (IT 1) (Supplementary Fig. 2). The F2 population, consisting of 238 plants, segregated into 182 resistant plants (IT 0 or 1) and 56 susceptible plants (IT 4), fitting a 3:1 segregation ratio (χ2 = 0.275, P = 0.600). This demonstrates that the seedling resistance of line No. 3778 to isolate BgtYZ01 is controlled by an incompletely dominant gene.
Thirty-seven polymorphic molecular markers were developed based on 57 single-copy or low-copy (E = 10-5) genes from the 0–22 Mb region of chromosome 3Sl of Ae. longissima accession AEG-6782-2 reference genome (Supplementary Data 2). Four co-dominant markers, X1650, X3140, X3990, and X5730, spanning ~16 Mb of 3SlS, were linked to the resistance phenotype (Supplementary Data 3). This suggests that the introgressed chromosomal segment of Ae. longissima 3SlS shows suppressed recombination with its wheat counterpart 3BS, indicating that the No. 3778/XM44 population is not suitable for finely mapping Pm13.
Physical mapping of Pm13 using radiation-induced chromosomal aberrations
A mutant population of line No. 3778 was generated using 60Co-γ-ray irradiation. Ten susceptible mutant lines, designated m1–m10, were identified from 424 M2 families (Supplementary Data 4). The phenotypes of these mutants were confirmed by reassessment of disease resistance with their M3 plants (Fig. 1c). These mutants were genotyped with the 37 molecular markers mentioned above. The presence and absence of these markers defined eight types of structural aberrations representing different sizes of chromosomal deletions (Fig. 1d). These markers failed to amplify any products from the mutant lines m1–m3, suggesting the absence of the Ae. longissima chromosomal segment 3SlS. Mutant lines m4 and m5 harbored chromosomal deletions with different sizes of segments from the proximal side, with breakpoints flanked by markers X2410/X2520 and X2150/X2250, respectively. Mutant lines m7–m10 carried chromosomal segment deletions from the distal side, with breakpoints flanked by markers X4150/X4220, X3940/X3990, X3410/X3520 and X2770/X2820, respectively. Line m6 harbored a chromosomal deletion with breakpoints defined by markers X2020/X2150 and X4150/X4220 (Fig. 1d). Since all mutants were susceptible to isolate BgtYZ01, Pm13 was localized in an interval flanked by markers X2410/X2820.
Markers X2410 and X2820 correspond to Ae. longissima genes AE.LONG.r1.3SG0172410 (Chr3Sl: 3,709,741–3,711,655 bp) and AE.LONG.r1.3SG0172820 (Chr3Sl: 4,446,231–4,448,390 bp), respectively, in the Ae. longissima AEG-6782-2 reference assembly, delimiting Pm13 to a 0.74-Mb interval containing 25 high-confidence genes and 15 low-confidence genes. Four genes were possibly associated with plant disease resistance, including three nucleotide-binding leucine-rich repeat receptor (NLR) genes (AE.LONG.r1.3SG0172500, AE.LONG.r1.3SG0172570 and AE.LONG.r1.3SG0172580), and one receptor-like kinase gene (AE.LONG.r1.3SG0172810).
MLKL_NTD domain-containing kinase gene MLKL-K is the Pm13 candidate
To isolate Pm13, we screened 326 EMS-induced M2 families of line No. 3778 and obtained six independent susceptible mutant lines (m11–m16) (Fig. 2a, Supplementary Data 4). We sequenced the Bgt-infected leaf transcriptomes of the wild-type (WT) line No. 3778 and six susceptible mutants by generating an average of 72.2 million 150-bp paired-end RNA-Seq reads (21.6 Gb) per sample. Sequence reads of the six mutant lines were mapped against the assembled transcriptome of the WT parent to look for transcripts that carry single nucleotide polymorphisms in multiple mutants20.
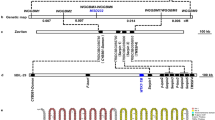
a Schematic representation of transcriptome sequencing analysis of EMS-induced loss-of-function mutants. The de novo assembly of the WT transcriptome was used as a reference. Powdery mildew inoculation of mutants was performed at the M2 and M3 generations (IT4). b The structure of the candidate gene of Pm13. Exons are represented as rectangular bars, and introns are shown as black lines between exons. Predicted protein domains are indicated by different colors: red for N-terminal domain of mixed lineage kinase domain-like protein (MLKL_NTD), blue for brace region, orange for serine/threonine kinase domain, gray for unidentified protein region, and black for 5’ UTR and 3’ UTR. Positions of EMS-type point mutations identified are indicated by short bars. c Schematic diagram of two constructs with the CDS of the Pm13 candidate gene MLKL-K driven by the maize Ubi promoter and its native promoter. These constructs were used to transiently transform epidermal cells of susceptible wheat cv. XM44 and stable transform susceptible wheat cv. Fielder, respectively. d The haustorium index of MLKL-K, Pm21, and the empty vector (EV) in transiently transformed cells challenged by isolate E09. Each red dot represents one bombarded leaf (MLKL-K: n = 17, Pm21: n = 17, and EV: n = 18). Haustorium index values are means ± SD from 17 to 18 biological replicates. Error bars represent standard deviations based on 17 to 18 biological replicates. P values were calculated with a two-tailed Student’s t-test. e Responses of seven independent T0 transgenic lines expressing Pm13 to isolate BgtYZ01 at the adult-plant stage. Line No. 3778 and the recipient Fielder were used as the resistant and susceptible controls. f Responses of the seven corresponding independent T1 transgenic families to Bgt isolate BgtYZ01 at the seedling stage. Symbols ‘+’ and ‘−’ represent siblings from each family with and without transformed MLKL-K, respectively. The experiment was performed with four plants (three transgene-positive and one negative) selected from ten T1 seedlings of each family. Source data are provided as a Source Data file.
This approach revealed a 2006 bp transcript, designated NODE_25350_length_2006_cov_24.294361_g11779_i0 (NODE_25350 for short), which carries the EMS-type point mutations (G to A or C to T) in five out of the six sequenced mutants (Fig. 2a, Table 1). Three out of the four candidate genes within 0.74-Mb interval were retrieved from the assembled transcriptome of the WT parent; however, only the transcript NODE_4312_length_3556_cov_17.726385_g2262_i0 (NODE_4312 for short), which corresponds to the NLR gene AE.LONG.r1.3SG0172580, carries one EMS-type point mutation (G to A) in m13 (Fig. 2a, Table 1). No mutations were found in transcripts that corresponds to the other NLR gene and the receptor-like kinase gene. As EMS mutagenesis usually produces one mutation in 20–40 kb on average in wheat21, the probability of the transcript NODE_25350 (2006 bp) being mutated across five Pm13 mutants by chance is 7.96 × 10−9, while the probability of finding one EMS-type mutation in transcript NODE_4312 (3556 bp) from six Pm13 mutants is 0.12920,22.
We searched for NODE_25350 in the genomes of Ae. longissima accessions AEG-6782-2 and TL05. A putative homologous genomic sequence was found in an unanchored sequence (chrUn: 3,336,888–3,345,975 bp) in accession AEG-6782-2, but no gene was annotated from it. We did not find any homologous sequence or gene that corresponds to NODE_25350 in accession TL05. Orthologous genes corresponding to NODE_25350 were found in other Sitopsis species of Aegilops, including Ae. bicornis.TB01.3S01G0016800, Ae.searsii.TE01.3S01G0016300, Ae.sharonensis.TH02.3S01G0013600, and Ae.speltoides.TS01.3B01G0036300. The collinear positions of these orthologous genes fell into the 0.74-Mb physical interval of Pm13 (Supplementary Fig. 3, Supplementary Data 5). Marker XMLKL-K (Supplementary Data 6) developed from NODE_25350 was present in the WT line No. 3778, but absent in all ten irradiation-induced mutants (Fig. 1d, Supplementary Fig. 4). These results indicate that the corresponding gene of NODE_25350 is located in the Pm13 physical interval.
Primers (Supplementary Data 6) designed on NODE_25350 and conserved sequences of the Sitopsis species of Aegilops were used to isolate the 11,376 bp genomic sequence of MLKL-K from line No. 3778, including a 2637 bp promoter, a 6554 bp encoding region consisting of seven exons and six introns (Fig. 2b) and a 1932 bp terminator. RT-PCR using cDNAs of line No. 3778 and subsequent Sanger sequencing revealed identical transcript sequence to NODE_25350. NODE_25350 contains a 1431 bp open reading frame that encodes a predicted 476 amino acid protein with an N-terminal domain of mixed lineage kinase domain-like protein (MLKL_NTD domain)-containing serine/threonine kinase, designated MLKL-K. Its kinase domain belongs to the LRR_8B subfamily (cysteine-rich receptor-like kinases), which is the most frequent kinase subfamily found in kinase fusion proteins23. The mutations in the five EMS-induced mutants were validated by Sanger sequencing, and they led to five amino acid (AA) substitutions in the kinase domain of MLKL-K (Fig. 2b). Three AA substitutions were aligned to the EMS-induced AA substitutions of kinase-fusion proteins reported before (m13: G231R aligns to G45R in SNN3-D1 and G90E in WTK6-vWA, m16: C334Y aligns to C166Y in Sr43 and C195Y in WTK6-vWA, m15: P461L aligns to P349L in WTK6-vWA), and the other two were adjacent to the reported ones (m12: G308E adjacent to G132D in Pm4 and S170N in WTK6-vWA, m11: G401D adjacent to A234V in Sr43 and T211I in Yr36) (Supplementary Fig. 5)20,24,25,26,27,28,29,30,31,32,33,34,35,36,37. Mutant m14 had no change in the coding sequence (CDS) of MLKL-K, which might have a mutation in the regulatory region or other genes associated with the powdery mildew resistance conferred by Pm13.
We isolated 15 MLKL-K alleles from the cDNAs of 21 Ae. longissima accessions showing immunity to BgtYZ01. These alleles have nucleotide identities of 97.8% to 100% compared to MLKL-K from line No. 3778. These encoded MLKL-Ks exhibited amino acid sequence identities of 96.0% to 100%, compared to MLKL-K from line No. 3778 (Supplementary Data 7).
MLKL-K is sufficient for powdery mildew resistance
Biolistic bombardment-based single-cell transient over-expression of MLKL-K CDS in the epidermal cells of susceptible wheat cv. XM44 significantly reduced the haustorium index (36.8%) of Bgt isolate E09 infection compared to the empty vector as the negative control (67.5%), while over-expressing of the positive control Pm21 resulted in a haustorium index (33.7%) comparable to MLKL-K (Fig. 2d, Supplementary Data 8). Subsequently, the construct containing the MLKL-K CDS under the control of its native promoter (2637 bp) and native terminator (1932 bp) (Fig. 2c) was transformed into the susceptible wheat cv. Fielder via the Agrobacterium-mediated transformation. Seven independent MLKL-K-positive transgenic T0 plants, verified by the diagnostic marker XMLKL-K, showed an immune reaction when inoculated with isolate BgtYZ01, while non-transgenic Fielder plants were susceptible (Fig. 2e, Supplementary Fig. 6). The derived seven T1 families displayed a resistance/susceptibility segregation when challenged by BgtYZ01. Molecular analysis using marker XMLKL-K revealed that the presence of the transgene was associated with the resistant phenotype, and the absence of transgene was associated with the susceptible phenotype (Fig. 2f). Furthermore, all marker-positive T1 plants from T1-line 1 and T1-line 2 were resistant to 20 tested Bgt isolates (Supplementary Fig. 7). The expression of MLKL-K in seven T1 plants was also confirmed by qPCR (Supplementary Fig. 8). Collectively, these results demonstrate that the candidate gene MLKL-K is sufficient for the resistance to wheat powdery mildew and is the causal gene of the Pm13 locus.
Truncated MLKL-K is capable of inducing cell death
T1 transgenic plants expressing MLKL-K were grown until the heading stage in a greenhouse. Comparison of transgene-positive and negative siblings revealed that MLKL-K did not have any apparent deleterious effect on plant development or cause spontaneous cell death in wheat cultivar Fielder (Supplementary Fig. 9a, b). We inoculated MLKL-K donor line No. 3778 and susceptible control XM44 with Bgt isolate E09. At 48 h post-inoculation (hpi), H2O2 accumulation and cell death were observed in the infected epidermal cells of No. 3778 but not in XM44 (Supplementary Fig. 10). This suggests that MLKL-K can prevent the colonization of Bgt by inducing H2O2 accumulation and host cell death.
To study the resistance mechanism of MLKL-K at the biochemical level, we first used AlphaFold v2.0 to predict the three-dimensional structure of MLKL-K. This protein contains an N-terminal MLKL_NTD domain consisting of a four-helix bundle (4HB), a central three-helix-containing brace loop and a C-terminal serine/threonine kinase (STK) domain (Fig. 3a). No signal peptide was predicted for MLKL-K. Localization experiments in wheat protoplasts and epidermis cells of N. benthamiana indicated that MLKL-K might localize in the cytoplasm and nucleus (Supplementary Fig. 11). We then infiltrated Agrobacteria carrying constructs of full-length MLKL-K or different truncated fragments (Fig. 3b) into N. benthamiana leaves independently. Only the fragment Brace-kinase122-476 resulted in strong cell death. The full-length MLKL-K (MLKL-K_FL1-476), single MLKL_NTD domain (MLKL_NTD1-136), brace region (Brace122-217), kinase domain (Kinase218-476) and MLKL_NTD-Brace1-217 did not trigger cell death (Fig. 3c, Supplementary Fig. 12a and 13). The kinase-dead version Brace-Kinase122-476D367A (mutated in the ATP-binding pocket) failed to trigger cell death (Fig. 3d, Supplementary Fig. 14). Similarly, the expression of Brace-Kinase122-476 with point mutations (G231R, G308E, C334Y, G401D and P461L) from five EMS mutants also showed abolished cell death (Supplementary Fig. 15), which correlates with abolished resistance to BgtYZ01 in EMS mutants. These results suggest that Brace-Kinase122-476-induced cell death is dependent on the functional kinase domain and is associated with powdery mildew resistance in wheat.
a, Three-dimensional model of MLKL-K predicted by AlphaFold v2.0. Red: MLKL_NTD domain, blue: brace region, orange: serine/threonine kinase domain, and gray: unidentified protein region. b, Secondary structure of MLKL-K illuminated by cylinders (α-helixes) and arrows (β-sheets). Colors represent protein domains as described above. Black bars represent constructs containing the corresponded MLKL-K expression cassettes. c, d, Cell death in N. benthamiana leaves at 48 h after infiltration with Agrobacterium tumefaciens carrying constructs of 35 S promoter-driven MLKL-K fragments 1 to 10 illuminated above. Quantitative data corresponding to Fig. 3d are presented in Supplementary Fig. 14. EV: empty vector. Both experiments were performed on at least ten N. benthamiana leaves with similar results. e, Relative luciferase activity of wheat protoplasts measured at 16 h after co-transformation with indicated constructs and a construct carrying a luciferase reporter gene. Relative luciferase activity values are means ± SD from three biological replicates. Error bars represent standard deviations of three biological replicates. The relative fluorescence intensity of luciferase was used to assess cell viability. P values were calculated with a two-tailed Student’s t-test. The experiment was independently repeated three times with similar results. Source data are provided as a Source Data file.
We also observed that the intensity of Brace-Kinase122-476-induced cell death appeared to be associated with the number of helices in the brace region. In the transient expression experiments of both N. benthamiana leaves and wheat protoplasts, the kinase domain with three helices (α1-α2-α3-Kinase160-476) induced stronger cell death than the kinase domain with two helices (α2-α3-Kinase174-476), and the kinase domain with only one helix (α3-Kinase194-476) did not trigger any cell death (Fig. 3d, e, Supplementary Fig. 12b and 14). These results suggest that Brace-Kinase122-476 mediated cell death requires a functional kinase domain and the helices in the brace region.
MLKL-K/Pm13 originates from a recent fusion event of the kinase and MLKL_NTD domains
Using the amino acid sequence of the MLKL_NTD domain of MLKL-K/Pm13, we performed a BLAST against the proteomes of the tribe Triticeae and other plant species. The matched proteins were annotated by the NCBI Conserved Domain Search. We found that various types of MLKL_NTD-containing proteins exist in the plant kingdom. MLKL_NTD-PLAC8 (MLKL_NTD fused to PLAC8 domain) is the most common type, widely distributed among plant species across the gymnosperms, eudicots and monocots. Only in Poaceae, we found other types of MLKL_NTD-containing proteins, including proteins containing a single MLKL_NTD domain or two tandem MLKL_NTD domains, MLKL_NTD fused with STK (MLKL-K-like proteins), WD40 domain, BRX domain and NLR proteins (Supplementary Data 9 and 10). This indicates the rapid evolution of MLKL_NTD-containing proteins in Poaceae.
We found that the MLKL-K-like protein family may have expanded and become the major type of MLKL_NTD domain-containing proteins in the tribe Triticeae, which contains 11 to 34 members per species (Supplementary Fig. 16, Supplementary Data 10). Phylogenetic analysis based on the kinase domains showed that MLKL-K-like proteins were clustered into three distinct clades, indicating that the fusion of the kinase and MLKL_NTD domains occurred at least three times during the evolution of the Poaceae plants. MLKL-K/Pm13 and its orthologs were only found in the Sitopsis species of Aegilops, encoded by single-copy genes of the S-genome, while the other two kinase-MLKL_NTD fusion events were observed across different grass species (Supplementary Fig. 16). This suggests that MLKL-K/Pm13 and its orthologs originate from a relatively recent kinase-MLKL_NTD fusion event.
Discussion
The Ae. longissima sourced Pm13 exhibited a wide spectrum of resistance to wheat powdery mildew, as tested by a collection of Bgt isolates from China and the European countries. Suppressed recombination between the alien chromosomal arm 3SlS and the wheat homoeologous chromosomal arm 3BS limits the map-based cloning of Pm13. Irradiation with γ-ray is often used to create small segment translocation lines in wheat38,39. It can also produce different sizes of chromosomal deletions surrounding genes of interest, which we have used to physically map and identify Pm1219. We developed a set of powdery mildew-susceptible chromosomal deletion lines that were used to map Pm13 to a 0.74-Mb interval containing 40 annotated genes in the reference genome of Ae. longissima accession AEG-6782-2. This again proved that γ-ray-mediated chromosomal aberrations could effectively map genes located in suppressed recombination regions.
Several genomics-assisted gene cloning tools have been developed to rapidly isolate disease resistance genes. For example, MutChromSeq, based on chromosome flow sorting, was used to isolate Pm222, Pm434, Lr14a40, and Rph141. Ten NLR genes have been cloned using MutRenSeq, which combines mutagenesis, resistance gene enrichment, and sequencing42,43,44,45,46,47,48. MutChromSeq requests genetic mapping and technically demanding chromosome sorting, while MutRenSeq is suitable only for cloning NLR-type resistance gene. Recently, Lr9/Lr58 and YrNAM were cloned using the transcriptome sequencing-based MutIsoSeq20 and sequencing trait-associated mutations (STAM)49, respectively. Both methods allow direct linkage of the phenotype of disease resistance with its causal transcripts and does not rely on recombination or genetic mapping. They both used full-length transcripts from PacBio Isoseq as references. For the cloning of Pm13, we used Illumina RNA-Seq and de novo transcriptome assembly to generate the reference, which makes the method more cost-effective.
Among the 85 disease resistance genes isolated from wheat and its relatives, 48 genes encode intracellular NLRs, 7 genes encode cytomembrane-localized receptor-like kinases (RLKs), and 16 genes produce different types of kinase fusion proteins (KFPs), including tandem kinases consisting of kinase and pseudokinase, and single or tandem protein kinases fused to other domains, such as integrated NLR, teroidogenic acute regulatory protein-related lipid transfer domain (START), and von Willebrand factor A domain (vWA)20,29,36,50,51,52 These KFPs have emerged as a new type of receptor involved in the innate immunity in the Triticeae species36. MLKL-K/Pm13 encodes a KFP whose putative functional STK domain is fused in the C-terminus of a unique MLKL_NTD domain, which is consistent with the recent report that was published during the consideration of this paper53. Protein kinase proteins are apparent targets of pathogen invasion, and they could duplicate themselves to provide decoys for effectors54. Such decoys work with or integrate with R proteins to convey resistance54,55,56. Phylogenetic analysis shows that the high copy number of MLKL-K-like proteins in the Triticeae plants might result from three domain fusion events and multiple gene duplications. We hypothesize that MLKL-K-like proteins played a role in plant immunity. During the arm race between hosts and pathogens, these MLKL-K-like proteins could be targeted by pathogen virulence effectors. Their MLKL-NTD domains could be the binding sites of effectors, which were fused to a functional kinase domain as an ‘integrated decoy’, resulting in the new resistance gene MLKL-K20,36,56.
Animal MLKLs, Arabidopsis MLKLs (AtMLKLs) and MLKL-Ks share a similar structure of an N-terminal four-helix bundle (4HB) and a C-terminal kinase domain bridged by a brace57. However, both the N-terminal four-helix bundle Animal MLKLs and MLKL-K were annotated as MLKL_NTD (CDD cd21037) using NCBI Conserved Domain Search, while the counterpart of AtMLKLs was annotated as DUF1221 (CDD cl06017). Both animal and Arabidopsis MLKLs have pseudokinase domains that are classified as a dual specificity tyrosine-serine/threonine kinase, while plant MLKL-Ks contain a putatively functional serine/threonine kinase domain. Moreover, MLKL-Ks only occur in Poaceae species. These results indicate that plant MLKL-Ks have evolved independently.
In Arabidopsis, Atmlkl mutants exhibit an increased susceptibility to the obligate biotrophic fungus Golovinomyces orontii, which causes powdery mildew of Arabidopsis. Structural analysis suggests that AtMLKL retains an auto-repressed tetramer state57. During Toll-interleukin-1 receptor domain NLR (TNL)-receptor-triggered immunity, the EDS1-SAG101 complex facilitates AtMLKL clustering at the plasma membrane, leading to 4HB-dependent sustained cytoplasmic Ca2+ influx and subsequent immune responses58. MLKL is the most terminal obligatory component of the necroptosis cell death pathway of human and mouse, which retains a self-inhibition state through intramolecular interaction between the pseudokinase and 4HB/MLKL_NTD domains59. The phosphorylation of the pseudokinase domain of MLKL by an upstream kinase RIPK3 triggers a molecular switch, leading to exposure of the 4HB/MLKL_NTD domain and brace-mediated oligomerization of the 4HB//MLKL_NTD domain. Subsequent plasma membrane translocation and permeabilization trigger cell death59,60,61. The expression of the 4HB plus the brace region of AtMLKLs or the 4HB alone of mouse MLKL was sufficient to induce cell death57,59. We found that the expression of either the 4HB or the 4HB plus the brace region of MLKL-K/Pm13 did not induce cell death, while the expression of Brace-kinase122-476 of MLKL-K/Pm13 induced strong cell death, which relies on a functional kinase domain and the three helices in the brace region. As kinase catalytic activation usually depends on its dimerization-dependent autophosphorylation62,63, the three helices in the brace region of MLKL-K might facilitate dimerization and activation of its kinase domain. Since the full-length MLKL-K did not induce any cell death, it is suggested that the MLKL_NTD domain might prevent dimerization of the kinase domain and maintain an inactively primed state. We hypothesize that MLKL-K/Pm13 might also stay in a self-inhibition state through intramolecular interaction between the 4HB/MLKL_NTD and kinase domains, or an auto-repressed state through intermolecular interactions. Unlike AtMLKLs and animal MLKLs, the putative functional kinase domain of MLKL-K/Pm13, rather than its 4HB/MLKL_NTD domain, is responsible for triggering cell death.
A disease resistance protein is a double-edged sword for host plants. It is able to prevent pathogen invasion but can also cause damage. Therefore, the activity of disease resistance protein needs to be stringently self-regulated. For instance, NLR-mediated resistance, relying on induced cell death upon pathogen infection, maintains a self-inhibition state through intramolecular interactions and can be switched into an activated state upon recognition of pathogen effector64. Yu et al. 36 proposed that KFPs may function through NLR-based and non-NLR-based decoy models. The NLR-based model suggests that KFP may serve as a decoy of an NLR guard. In this case, KFPs are sensors that perceive pathogen effectors and conduct the defense signal to executor NLR, and then trigger cell death. In the non-NLR-based model, KFPs stay in a self-inhibition state until the perception of pathogen effectors36. In this case, KFPs are both sensors that perceive pathogen effectors and executors induce cell death. Our results indicate that MLKL-K might function through the non-NLR-based decoy model, in which MLKL-K stays in a primed self-inhibition state prior to Bgt infection. Its N-terminal MLKL-NTD domain might serve as a decoy, which recognizes the Bgt effector and subsequently activates the kinase domain, initiates defense signal transduction, induces cell death, and ultimately prevents the colonization of the Bgt pathogen.
Methods
Plant materials and growth conditions
Wheat-Ae. longissima chromosome translocation line R1A carries the powdery mildew resistance gene Pm13 on the terminal region of the chromosomal arm 3SlS12,13,14,15. Line No. 3778 was derived from the cross and backcross between R1A and wheat cv. Bainong3217 with a pedigree of R1A/Bainong3217*5. Here, line No. 3778 was crossed with powdery mildew-susceptible cultivar XM44 to generate F1 plants and a segregating F2 population. Yangmai 18 (YM18), which carries Pm21 on the wheat-Dasypyrum villosum translocated chromosome T6AL·6VS, has broad-spectrum resistance to powdery mildew65 and was used as a resistant control. The powdery mildew-susceptible wheat accession Fielder was used as the recipient for genetic transformation. Twenty-one Ae. longissima accessions were ordered from the U.S. National Plant Germplasm System (NPGS) and Genebank Information System of the IPK Gatersleben (GBIS-IPK). Nicotiana benthamiana was used for transient expression assay. Susceptible wheat cultivar Yangmai 23 (YM23) was used for reproducing conidiospores of Bgt. Plants were grown on KLASMAN compost supplied with proper slow-release fertilizer in a greenhouse with ZK-TB18-DH01 LED lighting (~5000 Lux) under long-day conditions (16 h light/8 h dark) at 24 °C.
ND-FISH analysis
Root tips of line No. 3778 and Chinese Spring were treated with nitrous oxide for 2 h and fixed with 90% acetic acid for 10 min66. After washing with double distilled water, root tips were digested with 1% pectolyase and 2% cellulase solution (Yakult Pharmaceutical Industry Co., Ltd, Tokyo, Japan)41. The ND-FISH assay was performed on mitotic metaphase cells using oligo-nucleotide probes, Oligo-pSc119.267, and Oligo-pTa53568, labeled with 6-carboxyflfluorescein (FAM) and 6-carboxytetramethylrhodamine (TAMRA), respectively, followed by staining with 4’, 6-diamidino-2-phenylindole to identify individual chromosomes69. At least three metaphase plates per seedling were analyzed, and FISH images were captured using a Leica DM2500 microscope equipped with a cooled charge-coupled device camera operated with LAS Live software (version 4.6) (Leica, Wetzlar, Germany).
Phenotypic assessments of powdery mildew resistance
Wheat plants, including the parents, F1 (8 plants) and F2 (238 plants), were inoculated with Bgt isolate BgtYZ01 by dusting fungal conidiospores onto leaves at the one-leaf stage. Powdery mildew reactions at the seedling stage were assessed at 7 days post inoculation (dpi). Infection types (ITs) were scored on a 0–4 scale, where, ITs 0, 0; 1 and 2 were regarded as resistant, and ITs 3 and 4 as susceptible70. The resistance spectrum of line No. 3778 carrying Pm13 was examined in seedling tests with three replicates using a set of 108 Bgt isolates held in Institute of Plant Protection and Soil Science, Hubei Academy of Agricultural Sciences, Wuhan, China. Among them, 101 isolates were collected from different regions of China17,18,19 and the other seven (07004, 94202, 96224, 97235, 98013, 98250 and JIW2) were from Europe3. YM18 carrying Pm21 and Chancellor (CC) without any Pm gene were used as the resistant and susceptible controls, respectively. Specifically, leaves of No. 3778, YM18 and CC were cut into segments 3 cm in length and placed in Petri dishes containing 3.0 g/L agar and 50 mg/L 6-benzylaminopurine (6-BA). Line No. 3778 and Fielder were used as the resistant and susceptible controls, respectively. Three biological replicates of each line were adopted. Leaves embed in Petri dishes were inoculated with one Bgt isolate, maintained ex vivo at 18 °C on 16 h light/8 h dark cycle. At 10 dpi, responses to powdery mildew were then assessed using the 0–4 scale as described above. Eight representative isolates were used to reassess the responses of No. 3778, YM18 and CC seedlings (with at least 10 plants for each line) at the one-leaf stage. To detect the adult-plant resistance in the field, flag leaves of No. 3778 were inoculated with Bgt isolate BgtYZ01. YM18 and XM44 were used as the resistant and susceptible controls, respectively.
Molecular marker analysis
Ninety primer pairs were designed according to 57 single copy or low copy genes within the Pm13 translocated chromosomal segment of Ae. longissima accession AEG-6782-2 (physical position: 0–24 Mb)71. Amplification of DNA was performed using the genomic DNA of line No. 3778 and XM44 as templates in a T100 thermal cycler (Bio-Rad, Hercules, CA, USA) with an initial denaturation at 94 °C for 3 min, 35 cycles of 10 s at 94 °C, 30 s at 55 °C, 1 min at 72 °C, and a final extension for 5 min at 72 °C. Reaction mixtures (25 μl) were composed of 1× PCR buffer, 0.2 mM of each dNTP, 2 μM of each primer, 50 ng genomic DNA, and 1 Unit of rTaq DNA polymerase (TaKaRa, Shiga, Japan, Catalog No. R001A). Products amplified were separated in 8% non-denaturing polyacrylamide gels and visualized after silver staining. Thirty-seven polymorphic markers (Supplementary Data 2) were detected with either size differences or present-absent differences. The diagnostic marker XMLKL-K was developed from the identified transcript NODE_25350. Products were amplified with the above PCR conditions and visualized by 1.2% agarose gel electrophoresis.
Genetic analysis and physical mapping
Phenotypes of the F2 population containing 238 plants from the cross between line No. 3778 and XM44 were assessed as described above. Four co-dominant markers, X1650, X3140, X3990, and X5730, spanning ~16 Mb of the chromosomal arm 3SlS, were used to genotype the F2 population. Genotypic and phenotypic results are shown in Supplementary Data 3. The Chi-squared (χ2) test was performed to determine the goodness-of-fit of the observed segregation ratio to the theoretical Mendelian ratio.
About 800 dry seeds of line No. 3778 were irradiated with 60Co-γ-ray with 200 Gy with a dosage rate of 1.0 Gy/min19,39. Irradiated seeds were sown in the field, and 424 M2 families were harvested. About 50 seeds of each M2 families were sown in greenhouse conditions as described above and inoculated with BgtYZ01 at the one-leaf stage. Ten independent susceptible mutants (m1 to m10) were obtained from different M2 families. Then, about 20 M3 one-leaf-stage seedlings from each M2 family were inoculated with BgtYZ01 to confirm their phenotypes. The genomic DNA of each mutant was extracted and used as templates in amplification with the 37 polymorphic markers to physically map Pm13.
Transcriptome sequencing and analysis
About 500 seeds of line No.3778 were soaked in 0.05 M PBS solution for 4 h at 22 °C and then treated with 0.05 M PBS solution containing 0.6% ethyl methanesulfonate (EMS) for 10 h at 22 °C, followed by washing in running water for 4 h. The treated seeds were sown in the field and 326 M2 families were harvested. Approximately 50 seedlings of each M2 family were inoculated with BgtYZ01 as described above. Susceptible mutants were allowed to produce seeds in single pots. About 20 M3 seeds of each independent mutant were re-assessed for their reactions to isolate BgtYZ01. Seedlings of line No. 3778 and six phenotype-confirmed mutants at the four-leaf stage were inoculated with BgtYZ01. As the expression of powdery mildew resistance genes usually peaked at 6 to 24 hpi19,33,65,72,73, we collected the third and fourth leaves at 24 hpi for total RNAs extraction using the RNAiso Plus Kit (TaKaRa, Shiga, Japan, Catalog No. 9109). The quality and quantity of RNA purified were determined using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA, Catalog No. E7530). Qualified RNA samples were used for library construction with the NEBNext Ultra RNA Library Prep Kit (NEB, Ipswich, MA, USA, Catalog No. E7530). RNA-Seq analysis was carried out on an Illumina platform with the PE150 strategy at Novogene Bioinformatics Technology Co., Ltd (Beijing, China).
The RNA-Seq analysis produced an average 72.2 million Illumina reads (2 × 150 bp) for the mutants. Data generated by RNA-Seq of the WT were assembled using SPAdes (v.3.15.3) with default parameters74. The outcome file ‘transcripts.fasta’ containing 201,798 transcripts was used as an input for the remaining steps of the MutIsoSeq pipeline20. Briefly, transposable elements were masked by Repeatmasker (v.4.0.7; http://www.repeatmasker.org) using the TREP database (v.2019)75 as an external library. SeqKit (v2.2.056)76 was used to sort the transcripts from the longest to the shortest. The sorted fasta file was used as a reference to map the Illumina reads from the susceptible mutants using BBMap (v.38.96)77. The resulting sam files were sorted, indexed, and converted to the mpileup format using samtools (v1.6)78. The pileup files were converted to xml files using Pileup2XML.jar and used as inputs to generate the final report using MutChromSeq.jar22.
Gene isolation
The genomic sequence of MLKL-K was amplified from line No. 3778 using the primer pair P1/P2 designed according to transcript NODE_25350 and subsequently sequenced by Sanger sequencing. The upstream and downstream sequences of MLKL-K were isolated through PCR amplification using the primer pairs P3/P4 and P5/P6, respectively, designed based on the conserved sequences of the Sitopsis species of Aegilops. Total RNA was extracted from the third and the fourth leaves of line No. 3778 challenged by Bgt isolate BgtYZ01 (24 hpi) using the RNAiso Plus Kit (TaKaRa, Shiga, Japan, Catalog No. 9109). Total RNA (2 μg) was used for the synthesis of the first-strand cDNA using the PrimeScript™ II 1st Strand cDNA Synthesis Kit (TaKaRa, Shiga, Japan, Catalog No. 6210A). The transcript of MLKL-K was confirmed by RT-PCR and Sanger sequencing. The MLKL-K alleles from different Ae. longissima accessions were amplified from corresponding cDNAs by RT-PCR using the primer pair P7/P8, followed by Sanger sequencing. All amplifications were carried out using the high-fidelity PrimeSTAR GXL DNA polymerase (TaKaRa, Shiga, Japan, Catalog No. R050A).
Sequence and evolutionary analyses
The three-dimensional structure of MLKL-K was predicted by AlphaFold v2.079. The amino acid sequence of the MLKL_NTD domain of MLKL-K was used to perform a BLAST search against the published Triticeae proteomes collected on the WheatOmics website (http://202.194.139.32/)80 and the proteomes of other species in Phytozome website (https://phytozome-next.jgi.doe.gov) (Supplementary Data 9). The domains of the matched proteins were predicted in the conserved domain database of NCBI. In total, 298 putative kinase and pseudokinase domains of plant tandem kinase proteins, Triticeae kinase fusion proteins20,36, Triticeae MLKL-K-like proteins, Sitopsis MLKL-K proteins, Arabidopsis MLKLs57, and human MLKL59 were used for phylogenetic analysis using the neighbor-joining method with a bootstrap 10,000 by MEGA 7 (https://www.megasoftware.net/). Multiple sequence alignment was applied using ClustlW of MEGA 7. The exported Newick tree file was illuminated using iTOL (https://itol.embl.de/).
Single-cell transient assay in wheat leaves
The full-length MLKL-K CDS was amplified from cDNA of line No. 3778 using high-fidelity PrimeSTAR GXL DNA polymerase (TaKaRa, Shiga, Japan, Catalog No. R050A), inserted into the entry vector pDONR207 to produce pDONR207-MLKL-K-CDS, and confirmed by Sanger sequencing with primers provided in Supplementary Data 6. Then, the MLKL-K CDS in pDONR207-MLKL-K-CDS was used to generate the recombinant plasmid pUbi-MLKL-K-CDS using Gateway™ LR Clonase™ II Enzyme mix (Thermo Fisher, Carlsbad, CA, USA, Catalog No. 11791-100). The mixture of the recombinant plasmid pUbi-MLKL-K-CDS and reporter vector pUbi:GUS was co-transferred into leaves of the susceptible wheat cv. XM44 uses a helium-driven particle delivery system (BioRad, Hercules, CA, USA). Briefly, gold particles (BIO-RAD, Hercules, CA, USA, Catalog No. 1652263) coated with the plasmid mixture were introduced into epidermal cells of detached leaves using the particle inflow gun (PIG) system81,82. The constructs carrying Pm21 (positive) and the empty vector (negative) were also co-transferred with pUbi:GUS into XM44 leaves. In each test group, particle bombardment was carried out on 17–18 leaves. Leaves were then inoculated with avirlent Bgt isolate E09 at 4 h after bombardment and stained with the GUS solution at 48 hpi to observe the haustorium structure of Bgt under a microscope. The haustorium index, i.e., the percentage of GUS-staining cells with haustoria among all GUS-stained cells invaded by the Bgt pathogen, was determined to assess whether MLKL-K could provide the powdery mildew resistance transiently. Statistical analysis was done using a two-tailed t-test.
Wheat transformation
The MLKL-K CDS (1431 bp), along with the upstream sequence (2637 bp) and downstream sequence (1932 bp), was synthesized at GENEWIZ (Suzhou, China) and ligated into the binary vector pLGY03. The resultant construct was used for Agrobacterium tumefaciens-mediated transformation of susceptible wheat cv. Fielder83. Seven T0 plants generated were tested for the presence of the transgenic component by PCR using the marker XMLKL-K. Line No. 3778 and non-transgenic Fielder were used as positive and negative controls, respectively. To evaluate the expression level of MLKL-K in transgenic wheat lines, total RNA was extracted from the first leaves of three transgene-positive T1 plants derived from each of the seven T0 plants. The RNA samples were used to synthesize the first strand of cDNA as described above. Quantitative RT-PCR (qPCR) was performed using primer pair QMLKL-K-F/R and above cDNA as a template. Gene actin was used as the endogenous control. Three technical replicates were performed for each cDNA sample. The 2-∆∆Ct method was used to normalize and calibrate transcript values relative to the endogenous actin control84. Line No. 3778 was used as a control to indicate the expression level of native MLKL-K.
Phenotypic assessment of transgenic wheat lines
Firstly, seven T0 transgenic wheat lines at the booting stage were inoculated with isolate BgtYZ01 in a greenhouse, and phenotypes of the flag leaves were observed at 14 dpi. Secondly, ten T1 plants derived from each T0 line were phenotyped by inoculating with BgtYZ01 at the one-leaf stage and genotyped with the diagnostic marker XMLKL-K. Thirdly, T1 lines 1 and 2 containing MLKL-K were obtained after PCR with marker XMLKL-K. Leaf segments of these two lines were used to test reactions to 20 Bgt isolates collected from different regions using the ex vivo method described above. Line No. 3778 and Fielder were used as the resistant and susceptible controls, respectively. Three biological replicates of each wheat line were adopted.
Histochemical detection of H2O2 and cell death in wheat leaves
Line No. 3778 and XM44 were inoculated with avirulent Bgt isolate E09 at the one-leaf stage. At 48 hpi, leaves were treated in 3,3’-diaminobenzidine (DAB) staining solution (1 mg/ml DAB-HCl, 10 mM Na2HPO4, and 0.05% v/v Tween-20) for 8 h and destained in 90% ethanol. At 60 hpi, leaves were collected and immersed in a trypan blue staining solution (2.5 mg/ml trypan blue, 25% lactic acid, and 23% saturated phenol) for 30 min in boiling water. Chloral hydrate solution (2.5 g/ml) was used to destain for 3 d. The above leaf samples were stained with 0.6% Comassie brilliant blue R-250 solution for 10 s to observe Bgt spore and hypha using a Leica DM2500 microscope (Leica, Wetzlar, Germany). In such experiments, the presence of Bgt spores did not consistently associate with H2O2 accumulation and induced cell death in epidermal cells of line No. 3778 and XM44. Only a small proportion of spores triggered such immune responses in the Pm13 donor line No. 3778, while almost no cell death or H2O2 accumulation was observed in the susceptible control XM44. Representative images were taken using a Leica DM2500 microscope (Supplementary Fig. 10).
Transgene-positive and negative siblings of T1-line4 to T1-line7, along with the WT Fielder plants, were grown until the heading stage in a greenhouse with long-day conditions (16 h light/8 h dark, 21 °C). The matured second leaves below flag leaves were used for trypan blue staining following the procedure described above. Stained leaves were scanned on an Epson Perfection V850 Pro scanner. Photos of whole plants were taken using a Canon EOS 6D (W) camera.
Cell death assay on N. benthamiana leaves
The full-length MLKL-K CDS and its partial fragments were amplified from No. 3778 cDNA using high fidelity PrimeSTAR GXL DNA polymerase (TaKaRa, Shiga, Japan, Catalog No. R050A), digested with SmaI and KpnI, and inserted into the vector pS1300-GFP-Nos (C-terminal fused GFP) and pS130085,86 to generate 35 S promoter-driven MLKL-K and truncated fragments with or without C-terminal fused GFP. MLKL-K with N-terminal fused GFP was generated by assembling PCR fragments of GFP (without termination codon) and MLKL-K into pS1300 using the ClonExpress Ultra One Step Cloning Kit (Vazyme, Nanjing, China, Catalog No. C115-01). To create a dead kinase gene Brace-Kinase122-476D367A, the nucleotide A at position 1100 in the MLKL-K CDS was altered to C using overlap extension PCR87, which leads to an amino acid substitution of aspartate (D) with alanine (A) at position 36788,89. Brace-Kinase122-476G401D, Brace-Kinase122-476G308E, Brace-Kinase122-476G231R, Brace-Kinase122-476P461L and Brace-Kinase122-476C334Y were amplified from the cDNA samples of Bgt-susceptible mutants m11, m12, m13, m15 and m16, respectively. The Brace-Kinase122-476 fragments with point mutations were also cloned into pS1300 independently. All constructs were confirmed by Sanger sequencing. Primers used for constructs are listed in Supplementary Data 6. Agrobacterium strain GV3101 carrying the above construct was grown at 28 °C in Luria Bertani (LB) broth, harvested by centrifugation, and suspended in infiltration buffer [2% sucrose, 0.5% Murashige & Skoog basal salts, 10 mM 2-(N-morpholine)-ethanesulfonic acid, pH 5.6, and 200 μM acetosyringone]. The suspension was diluted to OD600 of 0.8 using the above infiltration buffer and incubated for 1–3 h at room temperature. Ten N. benthamiana leaves were infiltrated with each suspension and photographed at 48 h after infiltration. The infiltrated leaves were stained in a trypan blue solution (10 ml lactic acid, 10 ml glycerol, 10 g phenol, 10 mg trypan blue, and 50 ml ethanol, dissolved in 30 ml distilled water) in boiling water for 5 min. The stained leaves were soaked in chloral hydrate solution (2.5 g/ml) until the background disappeared. In the above cell death assay, Agrobacterium strain GV3101 harboring the empty vector pS1300 was used as a negative control.
Western blotting analysis
Using the same method as above, the full-length MLKL-K and its partial fragments were inserted into the vector pS1300-GFP-Nos, which allows for creating fusions with GFP on the C-terminus of target fragments. Agrobacterium strain GV3101 carrying constructs expressing the GFP-fused protein was infiltrated into N. benthamiana leaves. At 20 h post infiltration, total proteins were extracted from 0.1 g of fresh leaves using Plant Protein Extraction Kit (CWBIO, Taizhou, China, Catalog No. CW0885M). The lysates were centrifuged at 12,000 × g for 20 min (4 °C). Supernatants were collected and incubated with 5× SDS loading buffer at 95 °C for 5 min. Heated protein samples were separated by 12% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a PVDF membrane. Immunoblots were detected with an anti-GFP antibody. The primary antibody used for the identification of GFP and GFP fusion proteins was Anti-GFP (Proteintech, Wuhan, China, Catalog No. 66002-1-lg) at a dilution of 1:5000. Following that, the secondary antibody used to detect the primary antibody was HPR-conjugated Affinipure Goat Anti-Mouse IgG (Proteintech, Wuhan, China, Catalog No. SA00001-1) at a dilution of 1:5000. Equal loading was controlled by probing with an anti-Actin antibody.
Quantitative analysis of cell death activity in wheat protoplasts
Protoplasts were isolated from the second leaves of wheat cultivar Fielder. Briefly, the second leaves of 2 weeks Fielder seedlings were harvested, and after removing epidermis, the leaves were placed into protoplast isolation buffer with 15 mg/ml Cellulase R-10 (YAKULT Honsha, Tokyo, Japan, Catalog No. L0012) and 5 mg/ml Macerozyme R-10 (YAKULT Honsha, Tokyo, Japan, Catalog No. L0021), incubated for 3 hours at room temperature in dark with shaking at 60 rpm. Wheat protoplasts were harvested by filtration and centrifugation90. The full-length MLKL-K CDS and its partial fragments were first ligated into entry vector pDONR207 and then recombined into a maize ubiquitin promoter-driven vector, pUbi-GW, using Gateway™ LR Clonase™ II Enzyme mix (Thermo Fisher, Carlsbad, CA, USA, Catalog No. 11791-100). All final constructs were confirmed by Sanger sequencing using primers provided in Supplementary Data 6. The mixture of each construct (10 μg) and the luciferase (LUC) reporter vector (10 μg) were transformed into the protoplasts prepared using the method reported by Saur et al.90. After overnight incubation at 22 °C in darkness, the transformed protoplasts were collected by centrifugation, suspended in 0.2 mL 2× Cell Culture Lysis Buffer (Promega, Madison, WI, USA, Catalog No. E153A), and mixed with an equal volume of Luciferase Assay Substrate (Promega, Madison, WI, USA, Catalog No. E151A). The LUC activity of the samples was immediately detected using GloMax®96 Microplate Luminometer (Promega, Madison, WI, USA). The empty vector pUbi-GW mixed with LUC reporter vector was used as a control. The above experiment was carried out three times. Since only live transformed cells could express luciferase, which emits fluorescence once the substrate is provided, the relative fluorescence intensity of LUC was used to assess cell viability.
Subcellular localization of MLKL-K
Protoplasts were isolated from the second leaves of wheat cultivar Fielder as described above. The Arabidopsis histone gene AtH2B was amplified from Arabidopsis Col-0 cDNA using high fidelity PrimeSTAR GXL DNA polymerase (TaKaRa, Shiga, Japan, Catalog No. R050A), digested with KpnI and BamHI, and inserted into the vector pCAMBIA2300-RFP (C-terminal fused RFP), generating construct pCAMBIA2300-AtH2B-RFP. The constructs pS1300-MLKL-K-GFP-Nos (10 μg) and pS1300-GFP-Nos (10 μg) were separately mixed with 10 μg of pCAMBIA2300-AtH2B-RFP. The mixtures were transformed into the protoplasts prepared using the method reported by Saur et al.90. Fluorescence images were taken at 12 h after transfection using a Nikon confocal microscope (LSM980, Zeiss, city, Germany). GFP fluorescence was excited with a 488 nm laser recorded with 490–552 nm wavelength of light. RFP fluorescence was excited with a 558 nm laser and detected with 560–620 nm wavelength of light. For mCherry, fluorescence was excited with a 587 nm laser and detected with 572–658 nm wavelength of light. For the subcellular localization of MLKL-K in N. benthamiana epidermic cells, Agrobacteria carrying constructs pS1300-MLKL-K-GFP-Nos and pS1300-GFP-Nos were separately co-infiltrated with Agrobacteria carrying construct pS1300-mCherry-Nos into N. benthamiana leaves as described above. At 12 h post infiltration, fluorescence images were acquired following the confocal microscope setting described above.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. Datasets generated and analyzed during the current study are available from the corresponding author upon request. RNA-Seq data from the Bgt-infected leaves of the WT line No. 3778 and its mutants can be found in the European Nucleotide Archive (ENA) under the accession number PRJEB63085. The transcriptome assembly of the WT line No. 3778 was deposited in the ENA under the accession number HCEC01000000. The sequence of Pm13 from wheat line No. 3778 and its alleles from Ae. longissima accessions were deposited in the Genbank of NCBI under the accession numbers OR052510-[OR052527]. The transcriptome assembly of the WT line No. 3778, sequences of the transcript NODE_25350 and the CDS, and the genomic sequence of Pm13 are also available on the DRYAD database [https://doi.org/10.5061/dryad.qjq2bvqmz]. Source data are provided with this paper.
References
Singh, R. P. et al. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 54, 303–322 (2016).
Morgounov, A. et al. Global incidence of wheat rusts and powdery mildew during 1969–2010 and durability of resistance of winter wheat variety Bezostaya 1. Eur. J. Plant Pathol. 132, 323–340 (2012).
Sotiropoulos, A. G. et al. Global genomic analyses of wheat powdery mildew reveal association of pathogen spread with historical human migration and trade. Nat. Commun. 13, 4315 (2022).
Zou, Y. F. et al. Regionalization of wheat powdery mildew oversummering in China based on digital elevation. J. Integr. Agr. 17, 901–910 (2018).
Kunz, L. et al. The broad use of the Pm8 resistance gene in wheat resulted in hypermutation of the AvrPm8 gene in the powdery mildew pathogen. BMC Biol. 21, 29 (2023).
Müller, M. C. et al. A chromosome–scale genome assembly reveals a highly dynamic effector repertoire of wheat powdery mildew. New Phytol. 221, 2176–2189 (2019).
He, H. G. et al. Characterization of a new gene for resistance to wheat powdery mildew on chromosome 1RL of wild rye Secale sylvestre. Theor. Appl. Genet. 134, 887–896 (2021).
Kuluev, A. R., Kuluev, B. R. & Chemeris, A. V. The problem of the origin of subgenomes B, A, and D of bread wheat (Triticum aestivum L.): old facts and new evidences. Biol. Bull. Rev. 13, 148–161 (2023).
Li, L. F. et al. Genome sequences of five Sitopsis species of Aegilops and the origin of polyploid wheat B subgenome. Mol. Plant 15, 488–503 (2022).
Huang, S. Y., Steffenson, B. J., Sela, H. & Stinebaugh, K. Resistance of Aegilops longissima to the rusts of wheat. Plant Dis. 102, 1124–1135 (2018).
Sheng, H. Y. & Murray, T. D. Identifying new sources of resistance to eyespot of wheat in Aegilops longissima. Plant Dis. 97, 346–353 (2013).
Ceoloni, C., Signore, G. D., Ercoli, L. & Donini, P. Locating the alien chromatin segment in common wheat–Aegilops longissima mildew resistant transfers. Hereditas 116, 239–245 (1992).
Cenci, A., D’Ovidio, R., Tanzarella, O. A., Ceoloni, C. & Porceddu, E. Identification of molecular markers linked to Pm13, an Aegilops longissima gene conferring resistance to powdery mildew in wheat. Theor. Appl. Genet. 98, 448–454 (1999).
Cenci, A. et al. Genetic analysis of the Aegilops longissima 3S chromosome carrying the Pm13 resistance gene. Euphytica 130, 177–183 (2003).
Donini, P., Koebner, R. M. & Ceoloni, C. Cytogenetic and molecular mapping of the wheat–Aegilops longissima chromatin breakpoints in powdery mildew-resistant introgression lines. Theor. Appl. Genet. 91, 738–743 (1995).
Li, H. H. et al. Development of novel wheat-Aegilops longissima 3Sl translocations conferring powdery mildew resistance and specific molecular markers for chromosome 3Sl. Plant Dis. 105, 2938–2945 (2021).
Bourras, S. et al. The AvrPm3–Pm3 effector–NLR interactions control both race–specific resistance and host–specificity of cereal mildews on wheat. Nat. Commun. 10, 2292 (2019).
Zeng, F. S. et al. Virulence and diversity of Blumeria graminis f. sp. tritici populations in China. J. Integr. Agr. 13, 2424–2437 (2014).
Zhu, S. Y. et al. Orthologous genes Pm12 and Pm21 from two wild relatives of wheat show evolutionary conservation but divergent powdery mildew resistance. Plant Commun. 4, 100472 (2023).
Wang, Y. J. et al. An unusual tandem kinase fusion protein confers leaf rust resistance in wheat. Nat. Genet. 55, 914–920 (2023).
Slade, A. J., Fuerstenberg, S., Loeffler, D., Steine, M. N. & Facciotii, D. A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat. Biotechnol. 23, 75–81 (2005).
Sánchez-Martín, J. et al. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 17, 221 (2016).
Klymiuk, V., Coaker, G., Fahima, T. & Pozniak, C. Tandem protein kinases emerge as new regulators of plant immunity. Mol. Plant-Microbe Interact. 34, 1094–1102 (2021).
Arora, S. et al. A wheat kinase and immune receptor form host–specificity barriers against the blast fungus. Nat. Plants 9, 385–392 (2022).
Brueggeman, R. et al. The stem rust resistance gene Rpg5 encodes a protein with nucleotide–binding–site, leucine–rich, and protein kinase domains. Proc. Nat. Acad. Sci. USA 105, 14970–14975 (2008).
Brueggeman, R. et al. The barley stem rust-resistance gene Rpg1 is a novel disease-resistance gene with homology to receptor kinases. Proc. Nat. Acad. Sci. USA 99, 9328–9333 (2002).
Chen, S. S. et al. Wheat gene Sr60 encodes a protein with two putative kinase domains that confers resistance to stem rust. New Phytol. 225, 948–959 (2020).
Faris, J. D. et al. A unique wheat disease resistance–like gene governs effector–triggered susceptibility to necrotrophic pathogens. Proc. Nat. Acad. Sci. USA 107, 13544–13549 (2010).
Fu, D. L. et al. A Kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 323, 1357–1360 (2009).
Gaurav, K. et al. Population genomic analysis of Aegilops tauschii identifies targets for bread wheat improvement. Nat. Biotechnol. 40, 422–431 (2021).
Hanks, S. K., Quinn, A. M. & Hunter, T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241, 42–52 (1988).
Klymiuk, V. et al. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat. Commun. 9, 3735 (2018).
Lu, P. et al. A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat. Commun. 11, 680 (2020).
Sánchez-Martín, J. et al. Wheat Pm4 resistance to powdery mildew is controlled by alternative splice variants encoding chimeric proteins. Nat. Plants 7, 327–341 (2021).
Yu, G. T. et al. Aegilops sharonensis genome-assisted identification of stem rust resistance gene Sr62. Nat. Commun. 13, 1607 (2022).
Yu, G. T. et al. The wheat stem rust resistance gene Sr43 encodes an unusual protein kinase. Nat. Genet. 55, 921–926 (2023).
Zhang, Z. C. et al. A protein kinase–major sperm protein gene hijacked by a necrotrophic fungal pathogen triggers disease susceptibility in wheat. Plant J. 106, 720–732 (2021).
Chen, P. D. et al. Radiation-induced translocations with reduced Haynaldia villosa chromatin at the Pm21 locus for powdery mildew resistance in wheat. Mol. Breed. 31, 477–484 (2013).
Zhang, J. et al. Identification of novel chromosomal aberrations Induced by (60)Co–gamma–irradiation in wheat–Dasypyrum villosum lines. Int. J. Mol. Sci. 16, 29787–29796 (2015).
Kolodziej, M. C. et al. A membrane-bound ankyrin repeat protein confers race-specific leaf rust disease resistance in wheat. Nat. Commun. 12, 956 (2021).
Dracatos, P. M. et al. The coiled-coil NLR Rph1, confers leaf rust resistance in barley cultivar Sudan. Plant Physiol. 179, 1362–1372 (2019).
Hewitt, T. et al. A highly differentiated region of wheat chromosome 7AL encodes a Pm1a immune receptor that recognizes its corresponding AvrPm1a effector from Blumeria graminis. New Phytol. 229, 2812–2826 (2021).
Hewitt, T. et al. Wheat leaf rust resistance gene Lr13 is a specific Ne2 allele for hybrid necrosis. Mol. Plant 14, 1025–1028 (2021).
Marchal, C. et al. BED-domain containing immune receptors confer diverse resistance spectra to yellow rust. Nat. Plants 4, 662–668 (2018).
Steuernage, B. et al. Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 34, 652–655 (2016).
Upadhyaya, N. M. et al. Genomics accelerated isolation of a new stem rust avirulence gene–wheat resistance gene pair. Nat. Plants 7, 1220–1228 (2021).
Yan, X. C. et al. High–temperature wheat leaf rust resistance gene Lr13 exhibits pleiotropic effects on hybrid necrosis. Mol. Plant 14, 1029–1032 (2021).
Zhang, J. P. et al. A recombined Sr26 and Sr61 disease resistance gene stack in wheat encodes unrelated NLR genes. Nat. Commun. 12, 3378 (2021).
Ni, F. et al. Sequencing trait-associated mutations to clone wheat rust resistance gene YrNAM. Nat. Commun. 14, 4353 (2023).
Li, H. N. et al. Cloning of the wheat leaf rust resistance gene Lr47 introgressed from Aegilops speltoides. Nat. Commun. 14, 6072 (2023).
Lu, C. T. et al. Wheat Pm55 alleles exhibit distinct interactions with an inhibitor to cause different powdery mildew resistance. Nat. Commun. 15, 503 (2024).
Li, M. M. et al. A membrane associated tandem kinase from wild emmer wheat confers broad-spectrum resistance to powdery mildew. Nat. Commun. 15, 3124 (2024).
Li, H. H. et al. Wheat powdery mildew resistance gene Pm13 encodes a mixed lineage kinase domain-like protein. Nat. Commun. 15, 2449 (2024).
Wang, G. X. et al. The decoy substrate of a pathogen effector and a pseudokinase specify pathogen-induced modified-self recognition and immunity in plants. Cell Host Microbe 18, 285–295 (2015).
Cesari, S., Bernoux, M., Moncuquet, P., Kroj, T. & Dodds, P. N. A novel conserved mechanism for plant NLR protein pairs: the “integrated decoy” hypothesis. Front. Plant Sci. 5, 606 (2014).
van der Hoorn, R. A. & Kamoun, S. From Guard to Decoy: a new model for perception of plant pathogen effectors. Plant Cell 20, 2009–2017 (2008).
Mahdi, L. K. et al. Discovery of a family of mixed lineage kinase domain-like proteins in plants and their role in innate immune signaling. Cell Host Microbe 28, 813–824 (2020).
Shen, Q. C. et al. Cytoplasmic calcium influx mediated by plant MLKLs confers TNL-triggered immunity. Cell Host Microbe 32, 453–465 (2024).
Hildebrand, J. M. et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Nat. Acad. Sci. USA 111, 15072–15077 (2014).
Quarato, G. et al. Sequential engagement of distinct MLKL phosphatidylinositol–binding sites executes necroptosis. Mol. Cell 61, 589–601 (2016).
Tanzer, M. C. et al. Evolutionary divergence of the necroptosis effector MLKL. Cell Death Differ. 23, 1185–1197 (2016).
Dey, M. et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2α substrate recognition. Cell 122, 901–913 (2005).
Rajakulendran, T., Sahmi, M., Lefrancois, M., Sicheri, F. & Therrien, M. A dimerization–dependent mechanism drives RAF catalytic activation. Nature 461, 542–545 (2009).
Wang, J. Z. et al. Ligand–triggered allosteric ADP release primes a plant NLR complex. Science 364, eaav5868 (2019).
He, H. G. et al. Pm21, encoding a typical CC–NBS–LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol. Plant 11, 879–882 (2018).
Zhang, J. et al. Molecular cytogenetic identification of the wheat–Dasypyrum villosum T3DL.3V#3S translocation line with resistance against stripe rust. Plants 11, 1329 (2022).
Cuadrado, A., Vitellozzi, F., Jouve, N. & Ceoloni, C. Fluorescence in situ hybridization with multiple repeated DNA probes applied to the analysis of wheat–rye chromosome pairing. Theor. Appl. Genet. 94, 347–355 (1997).
Komuro, S., Endo, R., Shikata, K. & Kato, A. Genomic and chromosomal distribution patterns of various repeated DNA sequences in wheat revealed by a fluorescence in situ hybridization procedure. Genome 56, 131–137 (2013).
Fu, S. L. et al. Oligonucleotide probes for ND–FISH analysis to identify rye and wheat chromosomes. Sci. Rep. 5, 10552 (2015).
An, D. G. et al. Molecular cytogenetic characterization of a new wheat-rye 4R chromosome translocation line resistant to powdery mildew. Chromosome Res. 21, 419–432 (2013).
Avni, R. et al. Genome sequences of three Aegilops species of the section Sitopsis reveal phylogenetic relationships and provide resources for wheat improvement. Plant J. 110, 179–192 (2022).
Li, M. M. et al. A CNL protein in wild emmer wheat confers powdery mildew resistance. New Phytol. 228, 1027–1037 (2020).
Xie, J. Z. et al. A rare single nucleotide variant in Pm5e confers powdery mildew resistance in common wheat. New Phytol. 228, 1011–1026 (2020).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
Wicker, T., Matthews, D. E. & Keller, B. TREP: a database for Triticeae repetitive elements. Trends Plant Sci. 7, 561–562 (2002).
Shen, W., Le, S., Li, Y. & Hu, F. Q. SeqKit: a cross–platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS One 11, e0163962 (2016).
Bushnell, B. BBMap: a fast, accurate, splice–aware aligner. In Proc. 9th Annual Genomics of Energy & Environment Meeting (LBNL Department of Energy Joint Genome Institute) (2014).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Ma, S. W. et al. WheatOmics: a platform combining multiple omics data to accelerate functional genomics studies in wheat. Mol. Plant 14, 1965–1968 (2021).
Shen, Q. H. et al. Nuclear activity of MLA immune receptors links isolate–specific and basal disease-resistance responses. Science 315, 1098–1103 (2007).
Shirasu, K., Nielsen, K., Piffanelli, P., Oliver, R. & Schulze-Lefert, P. Cell-autonomous complementation of mlo resistance using a biolistic transient expression system. Plant J. 17, 293–299 (1999).
Zhang, S. J. et al. Targeted mutagenesis using the Agrobacterium tumefaciens–mediated CRISPR–Cas9 system in common wheat. BMC Plant Biol. 18, 302 (2018).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆Ct method. Methods 25, 402–408 (2001).
Dong, H. et al. Modulation of guard cell turgor and drought tolerance by a peroxisomal acetate–malate shunt. Mol. Plant 11, 1278–1291 (2018).
Gao, A. L. et al. Pm21 CC domain activity modulated by intramolecular interactions is implicated in cell death and disease resistance. Mol. Plant Pathol. 21, 975–984 (2020).
Russell, H., Barbara, K. & Randall, S. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16, 7351–7367 (1988).
Jiang, Y. H. et al. MAP4K4 associates with BIK1 to regulate plant innate immunity. EMBO Rep. 20, e47965 (2019).
Wang, Y. J. et al. Orthologous receptor kinases quantitatively affect the host status of barley to leaf rust fungi. Nat. Plants 5, 1129–1135 (2019).
Saur, I. M. L., Bauer, S., Lu, X. & Schulze-Lefert, P. A cell death assay in barley and wheat protoplasts for identification and validation of matching pathogen AVR effector and plant NLR immune receptors. Plant Methods 15, 118 (2019).
Acknowledgements
This study was supported by grants from National Key R&D Program of China (2022YFF1001500/2022YFF1001503 to Q.S.), National Natural Science Foundation of China (32171990 to H.H., 32172001 to H.L. and 32372507 to S.G.), Natural Science Foundation of Jiangsu Province (BK20231321 to H.H.) and State Key Laboratory of Plant Cell and Chromosome Engineering (PCCE-KF-2021-05 to H.H. and PCCE-KF-2022-07 to S.Z.), King Abdullah University of Science and Technology (to S.G.K.) and Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences (start-up funding to Y.W.). The authors thank Dr. Genying Li of Crop Research Institution, Shandong Academy of Agricultural Sciences, Jinan, China for providing the binary vector pLGY03.
Author information
Authors and Affiliations
Contributions
H.H., S.G.K., and Y.W. conceived and designed the research. H.H., Z.C., R.F., J.Z., S.Z., J.W., Q.Z., A.G., S.G., L.Z., Y.L. and Y.Z. performed experiments. H.H., Y.W., Z.C. and Q.S analyzed the data. H.H. and Y.W. wrote the manuscript with input from H.L. and S.G.K. H.H. and Z.C. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Matthew Moscou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, H., Chen, Z., Fan, R. et al. A kinase fusion protein from Aegilops longissima confers resistance to wheat powdery mildew. Nat Commun 15, 6512 (2024). https://doi.org/10.1038/s41467-024-50909-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-50909-6
This article is cited by
-
Development and characterization of small translocations between the Pm13 introgression from Aegilops longissima and wheat homoeologous A, B and D chromosomes
BMC Plant Biology (2025)
-
An optimized disease resistance gene cloning workflow for wheat
Nature Communications (2025)
-
An NLR pair in the Pm68 locus confers powdery mildew resistance in durum and common wheat
Nature Communications (2025)
-
Two complementary NLRs from wild emmer wheat confer powdery mildew resistance
Nature Communications (2025)
-
Foliar disease resistance phenomics of fungal pathogens: image-based approaches for mapping quantitative resistance in cereal germplasm
Theoretical and Applied Genetics (2025)