Abstract
DNA-protein crosslinks (DPCs) are toxic lesions that inhibit DNA related processes. Post-translational modifications (PTMs), including SUMOylation and ubiquitylation, play a central role in DPC resolution, but whether other PTMs are also involved remains elusive. Here, we identify a DPC repair pathway orchestrated by poly-ADP-ribosylation (PARylation). Using Xenopus egg extracts, we show that DPCs on single-stranded DNA gaps can be targeted for degradation via a replication-independent mechanism. During this process, DPCs are initially PARylated by PARP1 and subsequently ubiquitylated and degraded by the proteasome. Notably, PARP1-mediated DPC resolution is required for resolving topoisomerase 1-DNA cleavage complexes (TOP1ccs) induced by camptothecin. Using the Flp-nick system, we further reveal that in the absence of PARP1 activity, the TOP1cc-like lesion persists and induces replisome disassembly when encountered by a DNA replication fork. In summary, our work uncovers a PARP1-mediated DPC repair pathway that may underlie the synergistic toxicity between TOP1 poisons and PARP inhibitors.
Similar content being viewed by others
Introduction
DNA is associated with a wide range of proteins that are involved in its organization, regulation, and repair. Dynamic and reversible DNA-protein interactions are critical for maintaining genome integrity; however, DNA-binding proteins can become irreversibly trapped on DNA and form toxic DNA lesions, known as DNA-protein crosslinks (DPCs)1,2,3,4,5. DPCs are induced by various endogenous and exogenous crosslinking agents such as reactive aldehydes, heavy metal ions, ionizing radiation, and UV light, as well as various chemotherapeutics such as topoisomerase poisons3. These bulky lesions stall or inhibit essential processes such as DNA replication and transcription, and thereby represent a major threat to genome integrity. As in theory, any protein in close proximity to DNA can become crosslinked, DPCs constitute an extremely heterogeneous type of lesion. They can differ from one another by the identity of the crosslinked protein, the nature of the covalent bond formed between the DNA and the protein component, and the DNA structure surrounding the protein adduct2,4,6. To counteract the large diversity of DPCs, cells have evolved several specific mechanisms to detect and resolve these lesions during and in the absence of DNA replication4.
Post-translational modifications (PTMs) of DPCs have a crucial role in DPC resolution1. Recent studies using Xenopus egg extracts and human cells have shown that DPCs located on duplex DNA can be repaired both in the presence and the absence of DNA replication. During replication-coupled DPC proteolysis, DPCs are poly-ubiquitylated prior to their degradation7,8,9,10. Upon DPC-replisome collision, the DPC is first ubiquitylated by the E3 ubiquitin ligase TRAIP, which stimulates the bypass of the crosslinked protein by the replicative CMG helicase7,9. Extension of the nascent strand to the DPC activates the DPC protease SPRTN that specifically binds to single-stranded DNA (ssDNA)/double-stranded DNA (dsDNA) junctions9,11. Following CMG bypass, a short stretch of ssDNA is generated downstream of the DPC, which likely targets the E3 ubiquitin ligase RFWD310. DPCs on ssDNA gaps are further ubiquitylated by RFWD3, which promotes DPC degradation by the proteasome9,10. In the absence of DNA replication, DPCs are targeted to proteasomal degradation via the SUMO-RNF4 pathway, during which the DPC is first poly-SUMOylated by the SUMO E3 ligase PIAS4 and subsequently poly-ubiquitylated by the E3 ubiquitin ligase RNF412,13. However, whether other PTMs are involved in DPC sensing and repair and whether/how the structure of the DNA underlying the crosslink (e.g., presence of a DNA break flanking the crosslink) affects the sensing and signaling pathway remains elusive.
The toxicity of DPCs is exploited in cancer therapy as most chemotherapeutic drugs (e.g., nitrogen mustards, platinum compounds, and topoisomerase and DNA methyltransferase poisons) induce DPC formation3. Topoisomerase 1 (TOP1) poisons are commonly used to treat lung, ovarian, and colorectal cancer, as well as leukemia14. These drugs function by trapping TOP1 on DNA, a ubiquitous enzyme that resolves topological stress accumulated during DNA replication and transcription. As part of its catalytic cycle, TOP1 cleaves one DNA strand, resulting in the transient formation of a covalent TOP1-DNA cleavage complex (TOP1cc). TOP1ccs are normally quickly resolved upon religation of the DNA nick. However, TOP1ccs can persist if the DNA is distorted near the cleavage site or when the reaction is exposed to topoisomerase poisons15. Tyrosyl-DNA phosphodiesterase 1 (TDP1) was identified as a key enzyme in TOP1cc repair by directly hydrolyzing the covalent phosphotyrosyl bond that links TOP1 to the 3’ end of an ssDNA break16. Studies have shown that TDP1 is unable to remove full-length TOP1 because the crosslinked protein needs to be processed first to make the covalent bond accessible for TDP1 action17,18,19. The proteasome, yeast Ddi1, and Wss1/SPRTN, have all been implicated in TOP1cc repair by stimulating TOP1 degradation to a short peptide adduct and thus enabling crosslink resolution by TDP120,21,22,23. PTMs such as ubiquitylation play an important role in targeting these proteases to the lesion sites21,22,23,24.
Poly-ADP-ribose polymerase 1 (PARP1) is one of the main sensors of DNA damage25,26. Upon DNA breaks, PARP1 rapidly recognizes and binds to damaged DNA ends, where it attaches poly-ADP-ribose (PAR) chains onto itself (autoPARylation) and other nuclear proteins near the damaged site. While originally thought to occur on acidic residues, recent data showed that upon DNA damage, PARP1-mediated PARylation is also targeted by its co-factor Histone PARylation factor 1 (HPF1) to serine residues using NAD+27,28,29,30,31,32. These PAR chains serve as a recruitment platform for DNA repair factors that contain PAR-binding domains33. The accumulation of negatively charged PAR chains on PARP1 further promotes its release from DNA and thereby allows the recruited repair factors to access and process the lesion. Thus, clinical PARP inhibitors not only inhibit the catalytic activity of PARP1 but also induce PARP1 trapping on DNA, which further blocks the repair of DNA breaks and leads to the formation of cytotoxic DNA double-stranded breaks (DSBs)34,35.
Although PARP1 is primarily activated by DNA breaks, it appears to have a key role in TOP1cc repair by directly binding to TDP1 and mediating its recruitment to damage sites36,37,38,39. Accordingly, cells treated with TOP1 poisons are very sensitive to PARP1 loss and TOP1 poisons combined with PARP inhibitors show synergistic effects both in cells and in patients under clinical trials40,41,42,43,44,45. It was reported that PARP1 PARylates TOP1ccs in vitro, however, the outcome of this PARylation remains unclear46,47,48. Counterintuitively, a recent study showed that extensive TOP1cc PARylation inhibits TOP1cc repair by preventing the premature degradation of TOP1 by the proteasome49. These findings suggest that PARP1 has a multifaceted function in regulating TOP1cc repair. However, whether PARP1 has additional roles in sensing and targeting these lesions for repair remains an open question.
Here, we use Xenopus egg extracts to investigate how PTMs orchestrate the repair of DPCs flanked by DNA gaps and breaks. We show that DPCs on ssDNA gaps can be repaired in the absence of DNA replication in a process that is dependent on the catalytic activity of PARP1. Our data indicate that this novel repair pathway involves direct PARylation of the DPC, which targets it for ubiquitylation and subsequent proteasomal degradation. PARP1-mediated DPC resolution is required to repair TOP1ccs and TOP1cc-like lesions, which are flanked by a DNA break. Consequently, these lesions persist in the absence of PARP1-mediated PAR signaling, leading to the disassembly of the replisome when such lesions are encountered by DNA replication forks. Collectively, our work unravels a novel targeting mechanism of DPCs, which can explain the exquisite synergy between PARP inhibitors and TOP1 poisons.
Results
DPCs on ssDNA gaps are ubiquitylated in a PARP1-dependent manner
To investigate DPC repair mechanisms, we performed DPC pull-down experiments9 (Fig. 1A). To this end, the methyltransferase M.HpaII was crosslinked on different plasmid DNA substrates, which were then incubated in Xenopus egg extracts and recovered via DPC pull-down (Fig. 1A). In contrast to when M.HpaII is crosslinked on dsDNA (pMHdsDNA), M.HpaII crosslinked on ssDNA opposite from a 29 nt gap (pMHssDNA) was rapidly modified and degraded in cytoplasmic Xenopus egg extract (high-speed supernatant, HSS, no DNA replication) (Fig. 1B, compare lanes 2–5 and 6–9, and Supplementary Fig. 1A). Interestingly, these modifications were largely independent of RFWD3 (Fig. 1C, compare lanes 1–4 and 5–8), which otherwise ubiquitylates DPCs on ssDNA in the presence of nucleoplasmic Xenopus egg extract (NPE)10 (Supplementary Fig. 1B). The degradation also occurred in the absence SPRTN (Fig. 1C), which degrades DPCs juxtaposed to ssDNA/dsDNA junctions4,9,11 Thus, to study the pathway acting on pMHssDNA in HSS, we performed our experiments in extracts depleted of RFWD3 and SPRTN, to exclude any residual activities from these enzymes (Supplementary Fig. 1C).
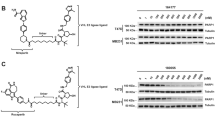
A Schematic of DPC pull-down assay9. At given time points, the DPC plasmid is pulled down under stringent conditions, the DNA is digested by benzonase treatment, and M.HpaII is analyzed via immunoblotting. Note that although the M.HpaII antibody is generated against full-length M.HpaII, it is unlikely to recognize all degradation products. B pMHssDNA or pMHdsDNA were incubated in high-speed supernatant extract (HSS, which is an extract that does not support DNA replication) and recovered by DPC pull-down at the indicated time points and immunoblotted for crosslinked M.HpaII. Molecular weight marker (kDa) is indicated on the left side of the blot and in all subsequent blots presented in this manuscript. C pMHssDNA was incubated in SPRTN- or SPRTN- and RFWD3-depleted HSS and retrieved at the indicated time points by DPC pull-down as in B. D pMHssDNA repair in SPRTN- and RFWD3-depleted HSS. Reactions were supplemented with untagged- or FLAG-tagged recombinant ubiquitin. For each condition, a sample was retrieved at 1 and 10 min, and was first recovered by DPC pull-down (DPC-PD), subsequently by FLAG pull-down (FLAG-PD), and immunoblotted for crosslinked M.HpaII. E pMHssDNA was incubated in SPRTN- and RFWD3-depleted HSS in the presence of the indicated inhibitors. DPCs were recovered via DPC pull-downs at the indicated time points and immunoblotted for crosslinked M.HpaII. Note that the small upshift of M.HpaII signal observed in the presence of Ub.E1i (lanes 5 and 6) is due to M.HpaII PARylation (see Fig. 2D). F pMHssDNA repair in SPRTN- and RFWD3-depleted HSS, which was further mock- or PARP1-depleted in the presence and absence of PARP inhibitor (PARPi). Samples were recovered by DPC pull-down and immunoblotted for crosslinked M.HpaII. The asterisk marks an unspecific band. G Scheme of PARP1. It consists of three main domains: an N-terminal DNA-binding domain (DBD) consisting of zinc-finger (ZF) motifs, a central BRCT domain-containing automodification domain, and a conserved C-terminal catalytic domain (CD). H SPRTN-RFWD3-depleted HSS was further mock- or PARP1-depleted and blotted with PARP1 antibody. PARP1-depleted extracts were supplemented with either buffer (+Buf.), recombinant hPARP1 (+WT), or recombinant catalytically impaired E988K mutant (+E988K). I Add-back rescue experiment using the extracts from H. Samples were recovered by DPC pull-down and immunoblotted against M.HpaII. Source data are provided as a Source Data file.
First, we wanted to address whether the slow-migrating species of M.HpaII are modified with ubiquitin. Hence, we supplemented the reaction with untagged- or FLAG-tagged ubiquitin. The addition of FLAG-ubiquitin shifted the mobility of the modified M.HpaII species (Fig. 1D, compare lanes 1 and 3), which were also specifically precipitated with a FLAG resin (Fig. 1D, lanes 7–8). Moreover, the addition of USP2, which cleaves ubiquitin moieties attached directly to targeted proteins, collapsed the remaining M.HpaII species into the unmodified band (Supplementary Fig. 1D, E). Together, these results show that M.HpaII is ubiquitylated in HSS egg extract independently of RFWD3.
We then tested whether other PTMs — such as SUMOylation or PARylation — could be involved in DPC repair. To this end, we supplemented egg extracts with an E1 ubiquitin-activating enzyme inhibitor (Ub.E1i), SUMO E1 inhibitor (SUMOi), or the PARP1 inhibitor talazoparib (PARPi) (Fig. 1E). As expected, DPC repair was impaired upon blocking de novo ubiquitylation (Fig. 1E, compare lanes 1–4 and 5–8). Strikingly, while SUMOi had no effect on the repair process (Fig. 1E, lanes 9–12), inhibition of PARP1 via PARPi hindered DPC ubiquitylation (Fig. 1E, lanes 13–16), although residual ubiquitylation was still observable at later time points (Fig. 1E, lanes 15–16). A similar effect of PARP inhibition could be observed in undepleted extracts (Supplementary Fig. 1F). This suggested that ADP-ribosylation may orchestrate another important DPC repair route, independent of RFWD3 and SPRTN. To differentiate whether PARP1 trapping at the lesion blocks repair or whether PARP1-mediated PARylation is needed for DPC removal, we depleted PARP1 from egg extracts and compared it to a mock reaction treated with PARPi (Fig. 1F and Supplementary Fig. 1C). Upon PARP1 depletion, we observed a similar effect as with PARPi alone — reduced DPC ubiquitylation and DPC stabilization (Fig. 1F, compare lanes 1–4 to 5–8 and 9–12). These results suggest that the observed repair-blocking effect is due to the loss of PARP1 catalytic activity and not PARP1 trapping.
We next expressed and purified recombinant wild-type (WT) and catalytically impaired E988K mutant PARP1 (Fig. 1G, H) and tested their activity via in vitro autoPARylation assays33,50,51. AutoPARylation of the WT construct was dependent on its co-factor NAD+ and was successfully blocked upon PARPi addition (Supplementary Fig. 1G), confirming that the protein is catalytically active. In contrast, the catalytically impaired mutant did not undergo autoPARylation in the presence of NAD+ (Supplementary Fig. 1H, lanes 3 and 4), although residual ADP-ribosylation could be detected by immunoblotting, which may be consistent with PARP1 E988K’s ability to promote mono- but not poly-ADP-ribosylation (Supplementary Fig. 1I, lane 4 long exposure)51. To validate that DPC repair depends on PARP1 catalytic activity, we performed add-back rescue experiments using the purified PARP1 constructs (Fig. 1H). Importantly, we could partly rescue the effect of PARP1 depletion when egg extracts were supplemented with WT (Fig. 1I, compare lanes 5–8 and 9–12) but not with the catalytically impaired E988K mutant (Fig. 1I, lanes 13–16). Collectively, our results indicate that PARP1-mediated PARylation orchestrates a novel DPC repair pathway via DPC ubiquitylation.
PARP1-dependent DPC PARylation triggers DPC ubiquitylation and resolution
To further study the role of PARP1 in the resolution of pMHssDNA, we generated additional recombinant PARP1 mutants. These included the ∆ZF1-2 DNA-binding mutant (PARP1 lacking the N-terminal DNA-binding domain (AA216-1014)) and a “3SA” mutant (PARP1 S499A/S507A/S519A) in which the three main PARylated serines on PARP1 are mutated to alanine52 (Fig. 2A). HPF1 was shown to be a key regulator of PARP1 activity by switching PARP1 specificity from glutamate/aspartate to serine residues upon DNA damage27,29,52,53. Consistently, the 3SA mutant was efficiently modified in the absence of HPF1 in vitro (Supplementary Fig. 1G, lanes 7 and 8 and 2A, lane 4). However, this was also the case when HPF1 was added to the reaction, suggesting that the PARP1 3SA mutant was still able to undergo serine-driven auto-ADP-ribosylation on different sites (Supplementary Fig. 2A, lane 5), although in cells this mutant exhibits clear auto-ADP-ribosylation inhibition52. To test the effect of these mutants in egg extracts, we immunodepleted PARP1 and performed add-back rescue experiments (Fig. 2B). Similarly to the catalytically impaired mutant, the ∆ZF1-2 mutant could not rescue the effect of PARP1 depletion (Fig. 2C, lanes 9–12 and 17–20), consistent with PARP1 activation relying on its binding to DNA breaks38,54,55. In contrast, we could rescue DPC ubiquitylation and repair with the 3SA mutant (Fig. 2C, lanes 13–16), suggesting that these automodification residues are not required for repairing M.HpaII DPCs on ssDNA gaps.
A Overview table of PARP1 mutants. B SPRTN-RFWD3-depleted HSS was further mock- or PARP1-depleted and immunoblotted with PARP1 antibody. PARP1-depleted extracts were also supplemented with either buffer (+Buf.), DNA-binding deficient mutant (+ΔZF1-2), automodification-deficient mutant (+3SA), or catalytically impaired mutant PARP1 (+E988K). C Add-back rescue experiment using the extracts from B. DPCs were recovered by DPC pull-down and monitored via blotting against M.HpaII. The asterisk denotes an unspecific band. D pMHssDNA was incubated in RFWD3-SPRTN-depleted HSS in the presence of PARPi or E1 ubiquitin-activating enzyme inhibitor (Ub.E1i) alone or in combination. Samples were recovered via DPC pull-down and immunoblotted with either M.HpaII or Poly/Mono-ADP Ribose antibody (α-PAR). E pMHssDNA was incubated in SPRTN-RFWD3-depleted HSS, which was then either mock- or PARP1-depleted in the presence of Ub.E1i where indicated. DPC degradation and PARylation were monitored as in D. F pMHssDNA and pMHdsDNA were incubated in SPRTN-RFWD3-depleted HSS supplemented with PARGi where indicated. DPC degradation and PARylation were monitored as in D. Source data are provided as a Source Data file.
Next, we addressed whether PARP1 directly PARylates the DPC. To this end, we first treated egg extracts with PARPi and/or Ub.E1i. Either inhibitor treatment resulted in M.HpaII stabilization, confirming that both ubiquitylation and PARylation are essential for DPC repair (Fig. 2D, M.HpaII blot). Remarkably, we could detect PARylation signal at the size of M.HpaII in the untreated reaction (Fig. 2D, lane 1), suggesting that the crosslinked protein is directly PARylated. The PAR signal was further stabilized upon Ub.E1i treatment (Fig. 2D, lanes 7–9) and abolished in the presence of PARPi (Fig. 2D, lanes 4–6 and 10–12). Similarly, PARP1 depletion resulted in DPC stabilization and the loss of the PARylation signal (Fig. 2E, lanes 7–9). Upon Ub.E1i treatment in PARP1-depleted extracts, we could not detect any M.HpaII ubiquitylation or PARylation (Fig. 2E, lanes 10–12). Together, these experiments reveal that M.HpaII DPCs on ssDNA gaps are directly PARylated by PARP1, which targets the DPC for downstream ubiquitylation and degradation.
To further support that pMHssDNA can be PARylated, we incubated this substrate with recombinant PARP1 in vitro (Supplementary Fig. 2B). Additionally, we also tested two other DPC substrates — the pMHdsDNA substrate and the pmeMHssDNA substrate, in which the lysines of M.HpaII are chemically methylated (Supplementary Fig. 1A)9. Since ubiquitylation occurs on lysines, this DPC cannot be ubiquitylated but can still be efficiently PARylated. In the presence of NAD+, the two gapped DPCs were extensively modified with PAR chains (Supplementary Fig. 2B, lanes 2 and 6), whereas the DPC on dsDNA was modified to a much lesser extent (Supplementary Fig. 2B, lane 4). This finding is consistent with PARP1 recruitment to broken DNA ends, which likely stimulates PARylation of the DPC.
PAR chains are primarily hydrolyzed by PAR glycohydrolase (PARG), ADP-ribosylhydrolase 3 (ARH3), and TARG1. While PARG can reverse long PAR chains, ARH3 removes the terminal ADP-ribose unit from serine residues in the target protein56,57. In contrast, TARG1 is specialized in removing the terminal ADP-ribose from acidic residues58,59,60. As PARylated proteins are rapidly processed by PARG, we sought to inhibit its activity by adding a specific PARG inhibitor to the reaction. Interestingly, upon PARGi treatment, we observed a slight upward shift in the M.HpaII signal along with an increased PAR signal (Supplementary Fig. 2C, lanes 6–10). The PAR signal was specific for the presence of M.HpaII on the gap substrate (Supplementary Fig. 2D, lanes 13–16). Upon PARPi treatment in combination with PARGi, the DPC was stabilized, and both the PAR and ubiquitin signals disappeared (Supplementary Fig. 2C, lanes 11–15). We next addressed whether pre-PARylation of pMHssDNA could bypass the need for PARP1 in egg extracts (Supplementary Fig. 2E). To this end, in vitro PARylated pMHssDNA (Supplementary Fig. 2E, lane 1) was incubated in PARP1-depleted extracts in the presence or absence of PARGi. As expected, in the absence of PARGi, the DPC was readily de-PARylated and stabilized in PARP1-depleted extracts (Supplementary Fig. 2E, lanes 2–5). However, when we supplemented PARGi to the reaction, the DPC was now resolved in the absence of PARP1 (Supplementary Fig. 2E, lanes 6–9). Together, these findings indicate that PARP1-dependent DPC PARylation is critical and sufficient for triggering DPC resolution. Because the in vitro PARylation was done in the absence of HPF1, it also suggests that PARylation on serine residues is not required to trigger DPC repair. Accordingly, HPF1 depletion in egg extracts did not affect M.HpaII ubiquitylation and degradation (Supplementary Fig. 2F, G).
To confirm that the DNA gap triggers DPC PARylation, we compared the repair of pMHssDNA and pMHdsDNA in egg extracts with and without PARGi (Fig. 2F). While the gapped DPC was rapidly PARylated and repaired, M.HpaII on dsDNA remained stable and did not become PARylated even in the presence of PARGi (Fig. 2F, compare lanes 1–6 and 7–12). Taken together, our results indicate that M.HpaII DPCs on ssDNA gaps but not dsDNA are directly PARylated by PARP1, which targets them for subsequent ubiquitylation and resolution.
Plasmid pull-down mass spectrometry (PP-MS) reveals proteasome and PAR-dependent E3 ubiquitin ligase recruitments
To identify which proteins are involved in PARP1-dependent DPC resolution, we performed plasmid pull-down mass spectrometry (PP-MS)9. To this end, a control plasmid (pCTRL) and pMHssDNA were incubated in HSS in the presence or absence of PARGi and PARPi (Fig. 3A). Plasmids and associated proteins were isolated after 5- and 10-min incubation in egg extracts. The purified proteins were subsequently digested into peptides and analyzed by label-free MS.
A Overview of reaction conditions for PP-MS analysis. B Heatmap showing the mean of the z-scored log2 label-free quantitation (LFQ) intensity (i.e., protein abundance) from four biochemical replicates of pCTRL and pMHssDNA incubated in SPRTN-RFWD3-depleted HSS in the presence of the indicated inhibitors. Dynamic proteins, responsive to PARGi and/or PARPi treatment, were selected. C pMHssDNA was incubated in SPRTN-RFWD3-depleted HSS, which was then additionally mock-depleted, PARP1-depleted, or PARP1-depleted and supplemented with recombinant PARP1 (Input, left WB). Plasmids were recovered via plasmid pull-down and protein recruitment to the plasmid was monitored with the indicated antibodies. D pMHssDNA was incubated in SPRTN-RFWD3-depleted HSS, which was further mock-depleted, PSA1-depleted, DDI2-depleted, or PSA1- and DDI2-depleted. Samples were recovered by DPC pull-down and immunoblotted against M.HpaII. Source data are provided as a Source Data file.
As expected, proteins participating in gap-filling DNA synthesis such as Polδ (Pold1, Pold2, Pold3), FEN1, and DNA ligase 1 were all significantly enriched on pMHssDNA compared to pCTRL (Fig. 3B, Supplementary Fig. 3A, B, and Supplementary Data 1). Given that PARP1 binds to intact DNA through its BRCT domain61, it was enriched on pCTRL as well as on pMHssDNA. Yet, while its presence was constant in pCTRL, it decreased between 5 and 10 min in pMHssDNA, consistent with PARP1 activation and subsequent release from DNA via autoPARylation (Fig. 3B and Supplementary Data 1)54. The presence of PARPi resulted in PARP1 trapping and the specific accumulation of HPF1 (Fig. 3B, Supplementary Fig. 3D, and Supplementary Data 1), while PARGi accumulated PARG on DNA and resulted in de-enrichment of PARP1 (Fig. 3B, Supplementary Fig. 3C, and Supplementary Data 1). This is likely due to extensive PARP1 autoPARylation triggering its release from DNA54.
Several E3 ubiquitin ligases were also found enriched and responded to modulation of PARP1 activity, such as HUWE1, TRIP12, CHFR, PARP9-DTXL3 complex, HLTF, TRAFD1, and RNF41 (Fig. 3B, Supplementary Fig. 3B–D, and Supplementary Data 1). Several of these ubiquitin ligases are known interactors of PARP1 and PAR chains. For example, HUWE1 and TRIP12 both possess PAR-binding WWE domains62, whereas CHFR has been shown to bind to PAR via its specific PAR-binding zinc-finger (PBZ) domain63. Furthermore, studies suggest that in response to DNA damage, PARP1 recruits the PARP9-DTX3L E3 ligase complex via PARP9 binding to PARylated PARP164,65. To identify the specific E3 ligases involved, we immunodepleted HLTF, HUWE1, TRIP12, CHFR, and DTXL3 both individually and in combination. However, these depletions did not affect the ubiquitylation pattern of pMHssDNA (data not shown). This could be explained by redundancy across the investigated ligases and/or inefficient depletion of one or more of the E3 ubiquitin ligases.
The MS analysis also revealed strong recruitment of the proteasome to pMHssDNA compared to pCTRL (Fig. 3B). Consistently with our findings, this recruitment was abolished upon the addition of PARPi to the reaction (Fig. 3B, Supplementary Fig. 3D, and Supplementary Data 1), suggesting that PARP1 activity is critical for removal of the crosslinked protein by the proteasome. Interestingly, our PP-MS data also revealed that proteasome recruitment is specific to the ubiquitylated DPC as proteasome enrichment to the pmeMHssDNA substrate was significantly reduced compared to pMHssDNA and was comparable to pCTRL (Supplementary Fig. 3A and Supplementary Data 1). This is in line with our previous findings, which showed that DPC ubiquitylation targets the proteasome to the lesion9. Overall, our results indicate that DPCs on ssDNA gaps are repaired in a PARP1-dependent manner, which may also involve PAR-dependent E3 ligases and the proteasome.
PARylated DPCs are targeted to degradation by the proteasome and DDI2
To investigate whether proteasome enrichment on pMHssDNA is indeed PARP1-dependent, we performed plasmid pull-down experiments and studied the recruitment of two proteasome subunits to the DPC substrate via immunoblotting. As shown in Fig. 3C, proteasome recruitment (PSA2 and PSA3 subunits) was diminished by PARP1 depletion (compare lanes 3–4 to 5–6). This defect was reversed upon the addition of WT PARP1 (lanes 7–8), indicating that M.HpaII PARylation is required for efficient proteasome recruitment.
Next, we studied the role of the proteasome in the repair process. Proteasome depletion (via its PSA1 subunit) or inhibition (MG262 treatment) resulted in ubiquitin chains that persisted during the reaction (Fig. 3D, compare lanes 1–4 and 5–8, Supplementary Fig. 4A, B). Although the DPC was stabilized, DPC degradation still occurred upon proteasome inactivation, suggesting the existence of an additional DPC protease. Therefore, we immunodepleted the proteasome and DDI2 (Supplementary Fig. 4A), whose yeast homolog Ddi1 was recently shown to also degrade DPCs24. While DDI2 depletion alone did not have any significant effect on DPC degradation (Fig. 3D, compare lanes 1–4 and 9–12), the combined depletion of DDI2 and the proteasome led to the accumulation of DPC species of higher molecular weight throughout the reaction (Fig. 3D, compare lanes 5–8 and 13–16), indicating an additive defect in DPC degradation. Next, we attempted to rescue the effect of DDI2 depletion (Supplementary Fig. 4C). Add-back of recombinant human DDI2 rescued DPC proteolysis (Supplementary Fig. 4D, compare lanes 5–8 to lanes 9–12), whereas the catalytic dead version of DDI266 further stabilized the heavily modified forms of the DPC (Supplementary Fig. 4D, compare lanes 5–8 and 13–16). DDI2 also operated as a backup for proteasomal degradation when DPCs on duplex DNA were targeted via the SUMO-RNF4 pathway (Supplementary Fig. 4E) or during replication-coupled DPC repair, which involves TRAIP and RFWD3 (Supplementary Fig. 4F and 4G)9,12. Thus, we conclude that PARylated M.HpaII DPCs are targeted for degradation by the proteasome. Additionally, DDI2 removes DPCs with long ubiquitin chains that have escaped proteasome processing. Notably, DDI2 acts irrespectively of the mechanism that induces DPC ubiquitylation. This aligns with recent data showing that proteins with long ubiquitin chains are targeted to DDI2 for degradation20,66.
PARP1 stimulates TOP1cc resolution in human cells
PARP1 is activated by DNA damage (e.g., DNA nicks, breaks, or gaps) but not by intact DNA38,54,55,61. Consistently, we show above that a DPC on an ssDNA gap but not on dsDNA can be targeted to degradation via PARP1 (Fig. 2F). However, since DPCs on ssDNA do not form spontaneously, we wondered whether PARP1 has another primary DPC target in cells. PARP1 inhibitors are known to increase the sensitivity of human cells to camptothecin40,41,42,43,44, and importantly PARP1 deletion has a similar impact45. We thus speculated that PARP1 might be an important player in the resolution of TOP1ccs, which are a major source of endogenous DPCs that are flanked by a nick.
We first attempted to study TOP1cc formation and repair generated by TOP1 poisons in Xenopus egg extracts. However, in contrast to Topoisomerase 2 (TOP2) poison or inhibitor, TOP1 poisons had no evident effect on DNA replication, even when used at a very high concentration (Supplementary Fig. 4H and 4I). We put forward a potential structural explanation for the inefficient trapping of Xenopus TOP1 by CPT due to a mutation in Xenopus TOP1 that prevents CPT incorporation into its catalytic pocket (Supplementary Fig. 4J and 4L).
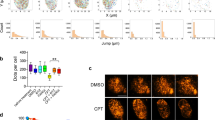
To address whether PARP1 targets TOP1ccs for repair, we measured the accumulation of DNA single-strand breaks (SSBs) in wild-type (WT) and PARP1-/- human RPE-1 cells during incubation with camptothecin, using the alkaline comet assay67 (Fig. 4A–C). Notably, TOP1 degradation via the proteasome is a prerequisite to detect camptothecin-induced SSBs via the alkaline comet assay18. This allowed us to differentiate between intact TOP1ccs (not detected by the alkaline comet assay) and TOP1ccs that have undergone proteolysis (detected via the comet assay) (Fig. 4A). To block the rapid repair of degraded TOP1ccs, we further stabilized the SSBs by removing the SSB repair factor XRCC145.
A Schematic illustrating the current model of TOP1cc repair. B PARP1 deletion suppresses the visibility of camptothecin-induced SSBs. The indicated RPE-1 cells were incubated with 10 µM camptothecin for 1 h and then processed for alkaline comet assays to measure unrepaired SSBs. Data show the median Tail moment of 300 cells combined from three independent experiments (100 cells/experiment). Significant differences were determined by 2-way ANOVA with Sidak’s post hoc multiple comparisons test. CPT denotes camptothecin. **** denotes p-value ≤0.0001. C Proteasome inhibition and/or PARP1 deletion suppresses the visibility of camptothecin-induced SSBs in the alkaline comet assay pre-treated with proteasome inhibitor (50 µM MG132) 2 h prior to and during camptothecin treatment as above. D To generate pFLP, Flp-nick is crosslinked to a plasmid containing the FRT site. Products of Flp-nick incubation with a CTRL (pCTRL) or FRT-site containing plasmid (pFRT) were analyzed on a native agarose gel. E pFLP was incubated in non-replicating egg extracts (1:2 HSS:NPE ratio) in the absence and presence of Ub.E1i. Reaction samples were analyzed by native agarose gel electrophoresis. F Quantification of the experiment shown in E. Error bars represents the SD of the mean. n = 3 independent experiments. Significant differences were determined by a two-tailed unpaired t-test. ** denotes p-value ≤0.01 (p = 0.0061). G Analysis of protein recruitment to pFLP compared to pCTRL via PP-MS. Plasmids were recovered at 10 min after addition in non-replicating egg extracts (1:2 HSS:NPE ratio). The volcano plot shows the difference in the abundance of proteins between the two sample conditions (x-axis), plotted against the p-value resulting from two-tailed Student’s two-sample t-testing (y-axis). Proteins significantly down- or up-regulated (cutoff line represents permutation-based FDR < 5%) are represented in red or blue, respectively. n = 4. H pFLP was incubated in non-replicating egg extracts (1:2 HSS:NPE ratio) in the absence and presence of the indicated inhibitors. Reaction samples were analyzed as in (E). I Quantification of the experiment shown in (H) as in (F). Error bars represent the SD of the mean. n = 3 independent experiments. Significant differences were determined by a two-tailed unpaired t-test. ** denotes p-value ≤0.01 compared to the Mock control (p = 0.0052 for PARPi and p = 0.0061 for Ub.E1i). J Add-back rescue experiment with WT and E988K PARP1 in non-replicating egg extracts (1:2 HSS:NPE ratio). Reaction samples were analyzed as in (E). K Samples from (J) were quantified as in (F). Error bars represent the SD of the mean. n = 3 independent experiments. Significant differences were determined by a two-tailed unpaired t-test. *** and **** denote p-value ≤0.001 and p-value ≤ 0.0001 compared to the Mock control, respectively. ** denotes p-value ≤0.01 compared to the PARP1 depletion control (p = 0.0054). * denotes p-value ≤0.05 compared to Mock control (p = 0.02). L pFLPPK was incubated in non-replicating egg extracts (1:2 HSS:NPE ratio) egg extracts in the absence and presence of Ub.E1i or in PARP1-depleted egg extracts. Reaction samples were as in E. Source data are provided as a Source Data file.
Consistent with XRCC1 promoting the resolution of SSB intermediates, XRCC1-/- RPE-1 cells treated for 1 h with 10 µM camptothecin exhibited a >10-fold increase in SSBs compared to WT cells (Fig. 4B). This was not the case in PARP1-/- RPE-1 cells treated with camptothecin, which exhibited levels of SSBs similar to WT RPE-1 cells (Fig. 4B). Strikingly, the elevated SSBs detected in XRCC1-/- RPE-1 cells were ablated by additional deletion of PARP1 (Fig. 4B). A similar effect was observed if PARP1 was depleted in XRCC1-/- cells via siRNA (Supplementary Fig. 5A, B). These results suggest that PARP1 is required for TOP1cc-detection in alkaline comet assays, and thus functions upstream of TOP1cc degradation during TOP1cc repair. In agreement with this idea, proteasome inhibition similarly prevented the detection of camptothecin-induced SSBs (Fig. 4C). We noticed, however, that proteasome inhibition also reduced the low-level SSBs that accumulated in PARP1-/- cells in the presence of camptothecin, suggesting that a small fraction of TOP1ccs can be processed by the proteasome independently of PARP1 (Fig. 4C). Collectively, these results suggest that PARP1 is required to promote the proteolytic repair of DPCs flanked by a DNA nick, such as TOP1ccs.
Flp-nick DPC as a model substrate for studying TOP1cc repair in Xenopus egg extracts
To mechanistically investigate the role of PARP1 in the resolution of TOP1ccs in Xenopus egg extracts, we generated a TOP1cc-like lesion using the Flp-nick system (FlpH305L). Due to its similarity to TOP1 crosslinking chemistry, the Flp-nick system is used to study TOP1cc-like repair24,68,69,70. One advantage is that the Flp-recombinase enzyme recognizes and crosslinks to Flp recognition target (FRT) sites and can therefore be site-specifically trapped on DNA (Fig. 4D and Supplementary Fig. 5C). Thus, we considered the Flp-nick substrate (pFLP) optimal to understand PARP1’s role in the resolution of TOP1ccs.
Using the same crosslinking chemistry as TOP1, Flp-nick introduced a single nick on plasmid DNA containing the FRT site, resulting in the conversion of closed circular supercoiled (SC) molecules to the open circular (OC) form (Fig. 4D, compare lanes 1–2 to 3–6). The crosslinking efficiency was around 75%, as approximately 25% of the molecules persisted as SC even when we supplemented the reaction with increasing amounts of Flp-nick (Fig. 4D, lanes 3–6). Next, we radiolabeled the FRT-containing plasmid via nick translation prior to Flp-nick crosslinking. The radiolabeled pFLP was then incubated in non-replicating egg extracts in the presence and absence of Ub.E1i (Fig. 4E) to test whether ubiquitylation is important for repair. The conversion of the damaged, OC molecules to SC in the mock reaction indicated that a significant fraction of pFLP underwent full repair (Fig. 4E, lanes 1–6, and 4F). In contrast, the addition of Ub.E1i resulted in repair inhibition, as conversion to SC plateaued at ~25% — corresponding to the uncrosslinked DNA fraction (Fig. 4E, lanes 7–12, and 4F). To test whether ubiquitylation is essential for removing the crosslinked protein, we generated pFLPPK by pretreating the DPC with Proteinase K, which degrades Flp-nick to a short peptide adduct and therefore should bypass the need for protein degradation (Supplementary Fig. 5D). pFLPPK was then incubated in egg extracts where it was readily repaired both in the presence and absence of the inhibitor (Supplementary Fig. 5D), indicating that ubiquitin signaling is dispensable once the protein is degraded. Thus, pFLP is likely targeted to proteolysis by ubiquitylation.
Next, we sought to use an unbiased approach to identify the main factors that are recruited to pFLP and trigger its resolution in egg extracts. To this end, we performed a PP-MS analysis and compared protein recruitment to pFLP and a control plasmid (pCTRL). As shown in Fig. 4G, the TDP1-mediated repair pathway appeared to operate during pFLP repair as TDP1 was found as one of the most significantly enriched proteins along with other factors previously reported to be involved in TOP1cc resolution — including PNKP, XRCC1, and LIG371,72,73, as well as SPRTN and the proteasome2,4,6 (Fig. 4G and Supplementary Data 2). We next asked whether TDP1 is important for pFLP repair by immunodepleting it from egg extracts (Supplementary Fig. 5E). TDP1 depletion resulted in a slight but reproducible delay in pFLP repair, suggesting that TDP1 is involved but not indispensable for repairing the DPC (Supplementary Fig. 5F, G). To further study this, we performed another PP-MS analysis to identify which factors are orchestrating pFLP repair in the absence of TDP1. In agreement with the literature, we noted the strong recruitment of the MUS81 nuclease complex in the absence of TDP1 but were unable to address the functional relevance of this complex on the pFLP substrate74 (Supplementary Fig. 5H and Supplementary Data 3). Overall, our data suggest that TOP1cc-like repair can be recapitulated in Xenopus egg extracts using the Flp-nick recombinase system.
The repair of Flp-nick DPCs is dependent on PARP1
Given our findings on PARP1-mediated pMHssDNA repair and PARP1’s role in TOP1cc resolution in cells, we tested whether alterations of PARP1 activity could also affect the repair process of pFLP. Interestingly, the addition of PARPi to the repair reaction had an inhibitory effect similar to Ub.E1i treatment as the supercoiled fraction persisted at 25% (Fig. 4H, compare lanes 1–6, 7–12, and 13–18, and 4I). In contrast, PARG inhibition did not impact pFLP resolution (Fig. 4H, lanes 19–24, and 4I).
Next, we explored whether the repair process is dependent on PARP1 catalytic activity. To this end, we depleted PARP1 from egg extracts and performed add-back rescue experiments using the purified WT and catalytically impaired E988K PARP1 constructs. Similarly to PARPi treatment, PARP1 depletion resulted in pFLP OC molecule stabilization (Fig. 4J, compare lanes 1–6 and 7–12, and 4K). This effect was significantly rescued when egg extracts were supplemented with WT but not with the catalytically impaired E988K mutant (Fig. 4J, K), indicating that PARP1-mediated PARylation is needed for pFLP repair.
We next addressed whether PARP1 is required for protein removal by monitoring the repair of pFLPPK in PARP1-depleted egg extracts. Similarly to Ub.E1i, PARP1 depletion did not affect the repair of the pre-digested DPC, indicating that PARP1 functions upstream of protein proteolysis during the repair of TOP1cc-like DPCs (Fig. 4L). This is consistent with the repair pathway we identified for the pMHssDNA DPC substrate, during which the protein is removed via PARP1-dependent PARylation and subsequent ubiquitylation. Although we could not raise a good antibody for the Flp recombinase enzyme, we detected direct Flp-nick PARylation via DPC pull-down experiments, which became readily visible in the presence of PARGi (Supplementary Fig. 5I, lanes 13–16). Thus, we conclude that, as seen during pMHssDNA repair, the Flp recombinase substrate appears to first be PARylated and thereby targeted to ubiquitin-mediated degradation and repair. This is also consistent with our findings in cells that suggest that PARP1 operates upstream of TOP1cc degradation. Importantly, neither RFWD3 nor SPRTN depletion impacted pFLP resolution (Supplementary Fig. 5J, K). Thus, PAR-dependent DPC degradation appears to be the primary pathway to target DPCs flanked by a DNA nick.
Inhibition of pFLP repair leads to replisome disassembly during DNA replication
Finally, we wanted to address the impact of impaired pFLP repair caused by PARP1 inhibition during DNA replication. To this end, we replicated pFLP in egg extracts in the presence or absence of PARPi. As seen in Fig. 5A, DNA replication of both pCTRL and pFLP occurred normally in the absence of PARPi, and fully replicated SC molecules rapidly appeared on the gel (lanes 1–5 and 11–15, native gel radiograph). There were no major indications of replication stress nor DNA damage, likely because pFLP was resolved prior to DNA replication during the licensing incubation (Fig. 5A, lanes 11–15, bottom immunoblots). The addition of PARPi did not alter the replication of the control plasmid or show signals of replication stress (Fig. 5A, lanes 6–10). In contrast, when the pFLP substrate was replicated in the presence of PARPi, we observed the appearance of linear species at 15 min (Fig. 5A, lane 16), followed by the generation of well products (WP), which signal increased in intensity over time (Fig. 5A, lanes 16–20, WP and Supplementary Fig. 6A, B). The fraction of fully replicated pFLP plasmids was also reduced compared to the control reaction (Fig. 5A, lanes 16–20, and Supplementary Fig. 6A, B). Importantly, replication of pFLP in the presence of PARPi channeled a strong replication stress response in concurrence with the appearance of the damaged DNA species (Fig. 5A, lanes 16–20, bottom immunoblots). Thus, the inhibition of pFLP resolution via PARPi treatment induced abnormal replication products and replication stress.
A pCTRL and pFLP were replicated in the presence or absence of [α-32P]dATP and PARPi (licensing in one volume of HSS for 60 min followed by the addition of two volumes of NPE). Samples were retrieved at indicated time points following NPE addition and analyzed by native agarose gel electrophoresis (top radiograph) or western blotting (bottom immunoblots). Samples were immunoblotted for p.CHK1 and loading control (ORC2). B Top, leftward, and rightward fork models of replisome disassembly when encountering the Flp-nick crosslink. Bottom, pFLP was replicated in the presence of [α-32P]dATP and with or without PARPi. Samples were retrieved at indicated time points, phenol-chloroform extracted, digested with PstI and SapI, and resolved on a denaturing polyacrylamide gel. C Schematic of pFLP linearization by ScaI and the potential DNA species occurring on a 2D gel upon plasmid replication. D Indicated samples from (B) were linearized by ScaI and run in two dimensions. Source data are provided as a Source Data file.
To understand the impact of inhibiting pFLP repair on replication forks, we analyzed nascent DNA strands using denaturing polyacrylamide gel following SapI and PstI digestion. This approach allowed us to monitor the nascent strands of the leftward fork approaching the DPC at the nucleotide resolution. As seen in Fig. 5B, we observed a ~346 nt DNA fragment during pFLP replication in the presence of PARP inhibitors (PARPi), which was absent in the control condition (compare lanes 1–4 and 8–11). This product corresponds to the nascent leading strand extended up to the nick (leftward fork). It is consistent with CMG “running off” at the end of the break resulting in a nearly blunt DNA end (Fig. 5B and Supplementary Fig. 6C)75. We also detected the formation of fully replicated extended products, which likely originated from the fraction of uncrosslinked plasmids (Fig. 5B, 380 and 387 nt products). Extension products could also be generated if the rightward fork encounters the DPC before the leftward fork (Supplementary Fig. 6C). Considering that the CMG helicase is unloaded at a lagging strand nick75, CMG unloading of the rightward fork could induce subsequent rolling circle DNA synthesis by the leftward fork (Supplementary Fig. 6C). This mechanism would explain the progressive accumulation of extension products between 30 and 180 min (Fig. 5B, lanes 8–14) and the appearance of well products of increasing intensity seen on native agarose gels (Fig. 5A and Supplementary Fig. 6A, B).
To address whether pFLP replication induces rolling circle synthesis, we monitored pFLP replication intermediates via 2-dimensional gel electrophoresis (Fig. 5C). If pFLP replication triggers rolling circle synthesis, the initial double Y arc formed by two DNA replication forks would be converted into a single Y arc — as only one fork would be replicating the plasmid during rolling circle synthesis. As seen in Fig. 5D, the predicted double Y arc visible at 8 min in either condition was rapidly converted in the presence of PARPi into a single Y arc that persisted up to 120 min (red arrows). This observation indicates that pFLP replication in the absence of PARP-mediated repair can indeed trigger rolling circle synthesis (Supplementary Fig. 6C).
We conclude, that in the absence of PARP1-mediated repair, the covalently linked Flp-nick is stabilized and induces replisome disassembly when the lesion is encountered by the replisome on either the leading or lagging strand template (Supplementary Fig. 6C).
Discussion
PTMs are critical for DPC repair1. While both SUMOylation and ubiquitylation are known to target different DPCs to removal/degradation, we present here the existence of another DPC repair pathway orchestrated by PARylation. Our study reveals that M.HpaII DPCs on ssDNA gaps undergo targeted degradation via a replication-independent two-step process. In this process the DPC is first PARylated by PARP1, which subsequently triggers its ubiquitylation and degradation by the proteasome (Fig. 6A). We further show that PAR-mediated DPC resolution is needed for resolving a TOP1cc-like lesion in Xenopus egg extracts and TOP1ccs in cells. Consequently, in the absence of PARP1-mediated repair, TOP1cc-like lesions persist and induce replisome disassembly when a replication fork encounters the lesion (Fig. 6B, C). We discuss the implications of our findings below.
PARP1 as a DPC sensor
Ubiquitylation and SUMOylation play major roles in DPC sensing and repair1. In recent years, PARylation has also been observed on DPCs, however, its regulatory role in DPC repair remained elusive40,49. Since PARP1-mediated PARylation is activated by DNA damage (i.e., presence of ssDNA, nicks, DNA breaks), we rationalized that the PARylation response most probably excludes DPCs on duplex DNA and rather targets DPCs flanked by DNA breaks. Indeed, our data indicate that DPCs located on ssDNA gaps and TOP1cc-like DPCs are directly PARylated by PARP1, while DPCs on intact duplex DNA remain unPARylated (Fig. 2F and Supplementary Fig. 5I). Thus, by sensing the DNA damage juxtaposed to the DPC, PARP1 can modify DPCs via ADP-ribosylation and thereby target them to degradation. Based on our work and the exquisite sensitivity of PARP1 KO cells to TOP1 poisons (Supplementary Fig. 7B)41, we propose that PARP1-mediated DPC targeting is the primary pathway that senses and targets TOP1ccs to repair in a process that can be uncoupled from DNA replication. Consistent with this idea, almost all the SSBs that accumulated in SSB repair-defective XRCC1-/- cells treated with camptothecin required the presence of PARP1 to be visible in alkaline comet assays (Fig. 4A–C), arguing that a large fraction of TOP1-induced SSBs that become abortive require PARP1 for proteolytic processing.
One intriguing question is how PARP1 can detect the nick concealed by TOP1- or the Flp recombinase-DNA interphase76,77. PARP1 is known to scan the genome for DNA damage via the ‘monkey bar’ mechanism61,78. It is possible that, via this process, PARP1 could collide with and partially unfold TOP1 and thereby uncover the DNA nick adjacent to the DPC, triggering PARP1 enzymatic activity to initiate the repair process. Alternatively, an additional enzyme may function upstream of PARP1 to uncover the nick and facilitate PARP1 binding. For instance, the FANCJ helicase has recently been shown to unfold DPCs and thereby promote their proteolysis79. We note that while PARP1 KO cells exhibit severe sensitivity to TOP1 poisons, they remain mostly unaffected by TOP2 poisons41, which induce TOP2 crosslinks flanked by a DSB (Supplementary Fig. 7B). This suggests a specific ability for PARP1 to detect a TOP1cc SSB. Structural studies may eventually unveil the precise mechanism underlying the detection of TOP1ccs and TOP1cc-like lesions by PARP1.
PARP1-mediated DPC PARylation triggers DPC ubiquitylation and proteolysis
While DPC ubiquitylation and degradation can be stimulated via DNA replication or via the SUMO-RNF4 pathway1, our findings reveal the further involvement of PARP1-dependent E3 ubiquitin ligases that specifically respond to DPCs modified by ADP ribosylation (Fig. 3). Notably, the catalytically impaired PARP1 E988K mutant, which is capable of catalyzing mono- but not poly-ADP ribosylation51, failed to rescue DPC resolution (Figs. 1I and 4J). This observation leads us to suspect that the downstream E3 ubiquitin ligase(s) may specifically rely on PAR chains. While we could not identify the E3 ligase(s) that ubiquitylates PARylated M.HpaII DPCs, our PP-MS data show that PAR-dependent E3 ligases, such as TRIP12, HUWE1, and CHFR, are actively recruited to the DPC plasmid and respond to PAR modulation (Fig. 3)62,63. Importantly, genetic data in cells support our findings, as TRIP12 or HUWE1 loss highly sensitizes cells to TOP1 poisons (Supplementary Fig. 7)41. Further investigations are warranted to determine whether TRIP12 and/or HUWE1 play a role in ubiquitylating and degrading TOP1ccs and TOP1cc-like lesions.
We have also identified the 26S proteasome and DDI2 as important factors involved in the degradation of DPCs targeted by PARylation (Fig. 3). Importantly, the recruitment of the proteasome to the DPC substrates was dependent on PARP1-mediated DPC PARylation and subsequent ubiquitylation, as the proteasome was not recruited to a DPC that cannot be ubiquitylated (Fig. 3 and Supplementary Fig. 3). However, in the absence of the proteasome, we noted that DPC degradation still occurred over time suggesting the existence of an additional DPC protease (Fig. 3D). This was also the case during replication-coupled DPC proteolysis or replication-independent DPC repair where, even in the absence of SPRTN and the proteasome, the DPC was still degraded (Supplementary Fig. 4C–E)9,12. We find that the aspartic protease DDI2 provides in part the observed proteolytic activity. Supporting this, the yeast homolog of DDI2 was recently shown to also degrade a variety of DPCs, including Flp-nick DPCs20,24,66. Notably, DDI2 activity in Xenopus egg extracts is only observed on ubiquitylated DPCs when SPRTN and the proteasome are absent, suggesting that DDI2-mediated degradation can operate as a backup mechanism for DPC repair. This agrees with recent findings suggesting that DDI2 acts as a backup to ensure the degradation of proteins that escape proteasome proteolysis66. Thus, DDI2 activity is unlikely to be specific to DPCs but rather needed when the proteasome is unable to degrade its substrate — whether it is a protein or a DPC. Finally, we note that even in the absence of DDI2, SPRTN, and the proteasome, DPC degradation still occurred, which could suggest the possible involvement of another yet-to-be-identified DPC protease.
Multifaced functions of PARP1 in stimulating TOP1cc resolution
It was previously proposed that PARP1 stimulates the recruitment of TDP1 via a direct interaction37. However, TDP1 activity is not limited to TOP1ccs as it has been shown to cleave a broad spectrum of substrates80,81,82. Consistently, our PP-MS analysis revealed that TDP1 is actively recruited to pMHssDNA (Fig. 3B). In this setting, TDP1 recruitment was stimulated in the presence of PARGi, which triggered PARP1 release from the DNA, and ablated in the presence of PARPi, which trapped PARP1 on the plasmid (Fig. 3B). Thus, TDP1 recruitment appears to be stimulated by PARP1-mediated PARylation rather than by the presence of PARP1 itself. Although, no PAR-binding domains are known in TDP1, its recruitment to damaged sites may be dependent on LIG3, with which TDP1 interacts directly18, and which in turn is stably bound to the PAR-binding protein, XRCC183. Thus, in addition to promoting TOP1 proteolysis, PARP1 is likely also stimulating TOP1cc resolution downstream of proteolysis by promoting recruitment of the proteins that mediate SSB repair73 (Fig. 6A).
In a recent study in cells, extensive TOP1cc PARylation was found to recruit the deubiquitylating enzyme USP7, thereby preventing premature ubiquitin-mediated proteasomal degradation49. Although USP7 was found enriched to pMHssDNA, we observed that its presence was not modulated by PAR activity (Supplementary Data 1). Instead, its enrichment pattern across the different conditions correlated with one of its known interactors DNMT184, whose association with DNA depends on the methylation status of the plasmid. Given that PARP1-deficient cells exhibit hypersensitivity to TOP1 poisons, and that TOP1cc repair is suppressed in the presence of PARP inhibitors40,41 (Supplementary Fig. 7A, B), it is more conceivable that PARP1-mediated PARylation promotes rather than inhibits TOP1cc resolution. Supporting this hypothesis, we show that PARGi, which stabilized the PAR chains on the DPC, did not inhibit M.HpaII DPC ubiquitylation and degradation, nor did it affect the resolution of pFLP (Fig. 2F and Supplementary Figs. 2C and 4H). Furthermore, we find that PARP1 is essential in human cells to expose camptothecin-induced SSBs, whose formation is dependent on proteasome activity (Fig. 4A–C). Thus, rather than inhibiting proteolysis, we propose that PARP1, by sensing the DNA nick juxtaposed to TOP1ccs, actively promotes TOP1 ubiquitylation and degradation.
While PARP1 was reported to modify itself and other proteins on several different amino acids, recent studies showed that damage-induced PARylation activity mainly targets serine residues via HPF127,28,29,30,31. Intriguingly, HPF1 depletion from egg extracts did not impact DPC degradation, which suggests that serine-targeted PARylation via HPF1 may be dispensable for DPC repair. However, we note that M.HpaII is an exogenous substrate in the extract, which could explain the lack of functional impact of serine-driven PARylation. Therefore, further investigation is needed to determine whether HPF1 affects PARP1-mediated repair of endogenous DPCs, such as TOP1ccs.
Nucleic acids have also emerged as targets of ADP-ribosylation85,86. In vitro studies showed that DNA breaks can be modified at phosphate groups, while another study reported low levels of adenosine PARylation in human cells87,88,89. However, whether nucleic acid ADP-ribosylation has any biological roles remains unclear. Our results strongly suggest that both M.HpaII and Flp-nick are directly PARylated by PARP1, as the PAR signal on our immunoblots was detected at the size of the crosslinked proteins (Fig. 2 and Supplementary Fig. 5I). We can envision, however, that modification of the DNA component could also contribute to DPC resolution.
Unresolved TOP1cc-like lesions induce replisome disassembly
Numerous clinical trials have investigated the safety and efficacy of combining TOP1 poisons and PARP inhibitors across various cancer types, including ovarian, breast, colorectal, and lung cancers. These results revealed synergistic effects and improved clinical outcomes compared to single-agent therapies42,43,44. The observed synergy between TOP1 poisons and PARPi treatment is also closely associated with a higher frequency of DSBs. These DSBs likely arise due to replication fork collision with unresolved TOP1ccs40,90. Supporting this, our research provides the first direct evidence of replication fork disassembly at a TOP1cc-like lesion during replication in the presence of PARPi (Fig. 5 and Supplementary Fig. 6). Our work shows that when a TOP1cc-like lesion is encountered on the leading strand template, it leads to CMG run-off and single-ended DSB formation, as recently demonstrated on an SSB substrate (Fig. 6B)75. In contrast, when the replisome encounters the DPC on its lagging strand template, it may reach the break without stalling91. At this point, it will be unloaded once the lagging strand is released and no longer occludes the CMG-Cul2LRR1 binding site to the outer face of CMG (Fig. 6C)92,93,94,95. Thus, both scenarios ultimately lead to replisome disassembly at the lesion. Notably, the downstream repair by break-induced replication would differ between leading- and lagging strand template TOP1cc lesions as in one case the 3’ invading DNA end would be obstructed by the DPC (Fig. 6B, C).
In summary, our work provides evidence of replication fork disassembly at a TOP1cc-like lesion and thus sheds light on the possible mechanisms underlying the synergistic toxicity between TOP1 poisons and PARP inhibitors. Further research in this area will contribute to the development of novel targeted therapies to improve cancer treatment.
Methods
Xenopus egg extracts
Egg extracts were prepared using Xenopus laevis (Nasco Cat #LM0053MX, LM00715MX). All experiments involving animals were approved by the Danish Animal Experiments Inspectorate and conform to relevant regulatory standards and European guidelines. The preparation of Xenopus egg extracts was performed as described previously96.
For following pMHssDNA DPC repair in non-replicating extracts, high-speed supernatant (HSS) of egg cytoplasm was added to the DNA substrate (final concentration of 10 ng/µL) and was incubated at room temperature. At the indicated time points, 4.5 µL of reaction was withdrawn and stopped in DPC pull-down buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, mM EDTA pH 8.0, 0.5% IPEGAL-CA630) on ice. After the last time point, the samples were incubated for 45 min rotating at 4 °C and from here on processed as described below for the DPC pull-down procedure.
For pFLP replication reactions, plasmids were first incubated in an HSS (final concentration of 7.5 ng DNA/µL HSS) for 75 min at room temperature to license the DNA, followed by the addition of two volumes of nucleoplasmic egg extract (NPE) to initiate replication. For studying pFLP DPC repair, one volume of HSS and two volumes of NPE were premixed prior to the addition of prelabeled plasmid DNA (final concentration of 7.5 ng/µL). To analyze reaction intermediates, 1 µL of each reaction was added to 6 µL of reaction stop solution (5% SDS, 80 mM Tris-HCl pH 8.0, 0.13% phosphoric acid, 10% Ficoll) supplemented with 1 µL of Proteinase K (20 mg/mL) (Roche). Samples were incubated for 1 h at 37 °C prior to separation by 0.9% native agarose gel electrophoresis and were visualized using a phosphorimager96. Radioactive signal was quantified using ImageJ (NIH, USA).
Where indicated, inhibitors were supplemented to egg extracts 10 min prior to initiating the reaction. Specifically, the Ubiquitin E1 inhibitor (MLN7243; Active Biochem) was added at a final concentration of 200 µM, the PARP inhibitor talazoparib (BMN 673, Selleckchem) was used at a final concentration of 20 µM, while for inhibiting PARGi activity, PDD00017273 (Sigma) was used at a final concentration of 200 µM. Proteasome activity was inhibited via the addition of 200 µM MG262 (Boston Biochem) to extracts (final concentration).
Preparation of DNA constructs
pMHssDNA, pmeMHssDNA, pMHdsDNA, and pMHLeads were previously described in ref. 9 and p4xDPCSUMO was previously described in ref. 12.
To generate pFLP, we first created pFRT by inserting the specific Flp recognition target site sequence into pBS KS(-), by replacing the EcoRI-HindIII fragment with the following sequence: 5’-AAT TCG ATA AGT TCC TAT TCG GAA GTT CCT ATT CTC TAG AAA GTA TAG GAA CTT CAT CA-3’. For the crosslinking reaction, pFRT was mixed with Flp-nick-His6 in reaction buffer (50 mM Tris-HCl pH 7.5, 50 mM NaCl, 20 µg/mL BSA, and 1 mM DTT) and incubated overnight at 30 °C.
For preparing the prelabeled pFLP substrate, we created pBbvCI-FRT by adding an Nt.BbvCI site to the pFRT plasmid. To this end, the following sequence was inserted into pFRT by using the SacI and NotI restriction sites: 5’-CCT CAG CCT CAG GAT GCT GGT ATG TAC ACT GAT GAT AGT GAG TCT TGC ACG C-3’. To generate radiolabeled pFLP, pBbvCI-FRT was first nicked with Nt.BbvCI which cleaves one strand of the plasmid. The DNA was subsequently radiolabeled with [α-32P]dATP via nick translation synthesis by DNA Pol I for 20 min at 16 °C. Radiolabeled pBbvCI-FRT was subsequently crosslinked to Flp-nick-His6 overnight at 30 °C.
To generate pFLPPK, pFLP was treated with Proteinase K (37 °C overnight in the presence of 0.5% SDS) to degrade the crosslinked Flp-nick protein to a short peptide adduct. The plasmid was subsequently recovered by phenol/chloroform extraction and ethanol precipitation.
Antibodies and immunodepletions
Antibodies against PCNA97, PARP198, M.HpaII8, SPRTN9, PSA19, PSA39, RFWD310 ORC299, and TDP1100 were described previously. Rabbit polyclonal antibody against HPF1 was a kind gift of Atsuya Nishiyama101. Monoclonal Poly/Mono-ADP Ribose antibody (Cell Signaling, #83732) and CHK1-pS345 (2341 (133D3) Cell Signaling) are commercially available. PSA2 and DDI2 antibodies were raised by New England Peptide by immunizing rabbits with Ac-CPAEVKDYLAAIA-OH and Ac-CEVLQKSADEADQQKP-OH, respectively.
To immunodeplete RFWD3, SPRTN, PARP1, PSA1, DDI2, HPF1, and TDP1 from Xenopus egg extracts, one volume of Protein A Sepharose Fast Flow (Cytiva) was mixed with either 4 volumes of affinity purified RFWD3 peptide antibody (1 mg/mL), 2 volumes of SPRTN serum antibody, 3 volumes of PARP1 serum antibody, 10 volumes of affinity purified PSA1 peptide antibody (1 mg/mL), 5 volumes of affinity purified DDI2 peptide antibody (1 mg/mL), 3 volumes of HPF1 serum antibody, or 5 volumes of affinity purified TDP1 peptide antibody (1 mg/mL) and incubated overnight at 4 °C. The beads were subsequently washed twice with 500 µL PBS, once with ELB-sucrose (10 mM HEPES pH 7.7, 50 mM KCl, 2.5 mM MgCl2, and 250 mM sucrose), twice with ELB supplemented with 0.5 M NaCl and twice with ELB-sucrose. Five volumes of precleared HSS or HSS-NPE mix were then depleted by mixing with one volume of antibody-bound beads and incubated at room temperature for 15 min. The depletion procedure was repeated three times.
Protein expression and purification
M.HpaII-His6 and LacI-biotin were expressed and purified as previously described8, while lysine-methylated M.HpaII was generated as previously described in ref. 9. Recombinant His-SUMO tagged DDI2 and DDI2D252N proteins were purified as described66. HPF1 was a kind gift of Ivan Ahel and Domagoj Baretic28,32.
General procedure for purification of human PARP1 constructs
Plasmids for expressing human wild-type PARP1 and DNA-binding mutant (∆ZF1-2) (pET-28b-H6-PARP1 and pET-28b-H6-∆ZF-PARP1) were kind gifts from J. M. Pascal. PARP1 E988K, S499A, S507A, and S519A mutations were introduced via Quikchange mutagenesis and confirmed by Sanger sequencing.
To purify wild-type and mutant PARP1, a 3-step purification protocol modified after50 was used. Each PARP1 plasmid was transformed into Rosetta 2 Competent E.coli cells (NEB). Cells were grown at 37 °C to O.D. 0.8-1.0 in LB broth supplemented with 0.1 mM ZnSO4 and moved to 16 °C for overnight induction with 0.2 mM IPTG. Bacteria were harvested by centrifugation and resuspended in 25 mL of lysis buffer (25 mM HEPES pH 8.0, 500 mM NaCl, 0.5 mM TCEP, and 10 mM benzamide). Suspensions were sonicated and cleared by high-speed centrifugation at 40,000 rcf for 1 h at 4 °C. Cleared lysates were loaded on a HisTrap HP column (Cytiva) with binding buffer (25 mM HEPES pH 8.0, 500 mM NaCl and 0.5 mM TCEP) and bound proteins were washed with 50 mL of high-salt buffer (25 mM HEPES pH 8.0, 1 M NaCl, 20 mM imidazole and 0.5 mM TCEP) followed by 50 mL of low salt buffer (25 mM HEPES pH 8.0, 1 M NaCl, 20 mM imidazole and 0.5 mM TCEP). Proteins were eluted with elution buffer (25 mM HEPES pH 8.0, 500 mM NaCl, 400 mM imidazole, and 0.5 mM TCEP). Collected proteins were diluted with no salt buffer (50 mM Tris-HCl pH 7.0, 1 mM EDTA, and 0.1 mM TCEP) to a final NaCl concentration of 375 mM and further purified with a HiTrap Heparin HP column (Cytiva) to remove contaminant DNA from the protein preparations. Proteins were washed with buffer A (50 mM Tris-HCl pH 7.0, 1 mM EDTA, 375 mM NaCl, and 0.1 mM TCEP) and subsequently eluted with an increasing NaCl gradient. Fractions containing the target proteins were collected, concentrated to 3 mL using 30,000 MWCO centrifugal filters (Amicon), and further purified on a HiPrep 16/60 Sephacryl S-200 HR gel filtration column (Cytiva) with 25 mM HEPES pH 8.0, 150 mM NaCl, 1 mM EDTA and 0.1 mM TCEP. The proteins were then flash-frozen in liquid nitrogen and stored at −80 °C.
Purification of Flp H305L
pBAD33-FLP-H305L-H6 for protein expression was a kind gift from Thomas Graham. The plasmid originated from Lotte Bjergbæk laboratory102. FLP-H305L-His6 was purified from T7 Express Competent E.coli cells (NEB). Expression was induced with 0.2% (w/v) arabinose overnight at 18 °C. One liter of culture was harvested and lysed in 25 mL lysis buffer (50 mM Tris-HCl pH 8.0, 300 mM NaCl, 20 mM imidazole, 10% glycerol, 5 mM β-mercaptoethanol and 1 tablet cOmplete Protease Inhibitor Cocktail, EDTA-free (Roche)/50 mL) via sonication. The lysate was centrifuged subsequently at 22,000 rcf for 1 h at 4 °C. The supernatant was bound to 2 mL bead slurry of Ni-NTA resin (Qiagen) for 1 h rotating at 4 °C. The beads were washed with 60 mL wash buffer (50 mM Tris-HCl pH 8.0, 1 M NaCl, 20 mM imidazole, 10% glycerol, and 5 mM β-mercaptoethanol) in total. The protein was eluted from the resin with elution buffer (50 mM Tris-HCl pH 8.0, 300 mM NaCl, 500 mM imidazole, 10% glycerol and 5 mM β-mercaptoethanol) and dialyzed with 20 mM Tris-HCl pH 8.0, 300 mM NaCl, 10% glycerol and 5 mM β-mercaptoethanol overnight and with fresh dialysis buffer for 4 additional hours the following day and stored at −80 °C until use.
Add-back rescue experiments
For PARP1 rescue experiments, depleted egg extracts were supplemented with wild-type or mutant hPARP1 at a final concentration of 15 ng/µL. For rescuing the effect of DDI2 depletion, hDDI2 wild-type or catalytic inactive mutants were added to depleted extracts to a final concentration of 2 ng/µL in the reaction.
In vitro PARP1 autoPARylation and DPC PARylation
To test PARP1 activity, a previously described50 automodification assay was used. Briefly, 1 µM PARP1 and 1 µM duplex DNA (5'-GGG TTG CGG CCG CTT GGG-3') were gently mixed in automodification buffer (20 mM Tris-HCl pH 7.5, 50 mM NaCl, 5 mM MgCl and 0.1 mM TCEP) and incubated at room temperature for 10 min. Where indicated, NAD+ at 5 mM final concentration was added to the reaction mixture and incubated for an additional 30 min. Reactions were stopped in 2x Laemmli sample buffer and resolved by SDS-PAGE.
For PARylation reactions in the presence of HPF1, PARP1 (1 µM final concentration) and HPF1 (10 µM final concentration) were pre-incubated for 10 min prior to the addition of NAD+.
To generate PARylated DPC substrates, 1 µg of human PARP1 was gently mixed with 150 ng of DPC substrate and incubated for 10 min at room temperature in automodification buffer (see above). The reaction was supplemented with NAD+ (5 mM final concentration) and further incubated for 1 h. PARylated DPCs were flash-frozen in liquid nitrogen and stored at −80 °C until use.
DPC pull-downs
DPC pull-downs were performed as previously described9. Briefly, streptavidin-coupled magnetic beads (Dynabeads M-280, Invitrogen; 6 µL per pull-down) were washed twice with 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA pH 8.0, 0.02% Tween-20. Biotinylated LacI was added to the beads (12 pmol per 6 µL of beads) and incubated at room temperature for 40 min. The beads were then washed four times with DPC pull-down buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 2 mM EDTA pH 8.0, 0.5% IGEPAL-CA630) and then stored in the same buffer on ice until needed. At the indicated times, 4.5 µL of reaction was withdrawn and stopped in 300 µL of DPC pull-down buffer on ice. After all time points were taken, 6 µL of LacI-coated streptavidin Dynabeads was added to each sample and incubated for 30–60 min at 4 °C with rotation. The beads were subsequently washed four times with DPC pull-down buffer and then twice with Benzonase buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 2 mM MgCl2, 0.02% Tween-20) before being resuspended in 15 µL Benzonase buffer containing 1 µL Benzonase (Merck Millipore). Samples were incubated for 1 h at 37 °C to allow for DNA digestion and DPC elution, after which the beads were pelleted, and the eluate was mixed with 2x Laemmli sample buffer for subsequent western blotting analysis.
For the FLAG pull-down, half the eluate was retrieved from the DPC-PD and diluted to a volume of 300 µL in Benzonase buffer. Each sample was incubated for 1 h at 4 °C with rotation after the addition of 5 µL of FLAG magnetic beads (Anti-FLAG M2 Magnetic beads, Millipore). The beads were prepared by washing them three times in Benzonase buffer. After incubation, the samples were washed four times with Benzonase buffer before being eluted from the beads by gentle shaking for 10 min at RT in 0.1 M Glycine pH 3.0. Beads were subsequently pelleted, and the supernatant was retrieved and neutralized in 10 mM Tris pH 11.0 before being added to 4x Laemmli buffer. For this experiment, Human Recombinant Ubiquitin or Human Recombinant FLAG-Ubiquitin (R&D Systems), were added to the reaction at a final concentration of 1 µg/mL.
For UbiCREST analysis of isolated DPCs (Supplementary Fig. 1C), pull-down samples were washed with DPC pull-down buffer and then washed and resuspended in 1x DUB reaction buffer and treated with the indicated deubiquitinase(s) (R&D Systems103) at 37 °C for 30 min. The samples were subsequently washed twice with Benzonase buffer and eluted with Benzonase treatment.
Plasmid pull-down
Plasmid pull-downs were performed as described previously104. Briefly, 6 µL streptavidin-coupled magnetic beads (Dynabead M-280, Invitrogen) per pull-down reaction were equilibrated with wash buffer 1 (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA pH 8.0, 0.02% Tween-20) and then incubated with 12 pmol of biotinylated LacI at room temperature for 40 min. The beads were washed four times with pull-down buffer (10 mM HEPES pH 7.7, 50 mM KCl, 2.5 mM MgCl2, 250 mM sucrose, 0.25 mg/mL BSA, 0.02% Tween-20), resuspended in 40 µL of pull-down buffer and stored on ice. At the indicated time points, 8 µL of reaction was gently mixed with the beads. After 30 min incubation at 4 °C, samples were washed twice in wash buffer 2 (10 mM HEPES pH 7.7, 50 mM KCl, 2.5 mM MgCl2, 0.25 mg/mL BSA, 0.03% Tween-20) and resuspended in 2x Laemmli sample buffer. Proteins associated with the chromatin fraction were visualized by Western blotting with the indicated antibodies.
Nascent leading strand analysis
For nascent leading strand analysis, 4 µL of replication reaction was added to 10 volumes of transparent stop buffer (50 mM Tris-HCl, pH 7.5, 0.5% SDS, 25 mM EDTA), and replication intermediates were purified as previously described105,106. DNA was digested with the indicated restriction enzymes and supplemented with 0.5 volumes of denaturing PAGE Gel Loading Buffer II (Life technologies). The digested DNA products were resolved on a 6% polyacrylamide sequencing gel. Samples were visualized using a phosphorimager96.
2D Gel electrophoresis
Purified replication intermediates of pFLP with or without PARPi were digested with ScaI and then subjected to native/native 2D gel electrophoresis. The first dimension involved a 0.4% agarose gel in 1xTBE buffer separated at 0.75 V/cm for 22 h at room temperature. The entire lane was excised and then cast across the top of the second-dimension gel, which consisted of 1% agarose with 0.3 μg/mL ethidium bromide. Electrophoresis of the second dimension was performed in 1xTBE containing 0.3 μg/mL ethidium bromide at 4.5 V/cm for 11 h at 4 °C. The gel was subsequently dried and visualized using a phosphorimager.
Plasmid pull-down mass spectrometry (PP-MS)
pMHssDNA and pmeMHssDNA repair
Plasmid DNA was gently mixed with HSS at 10 ng/µL (final concentration) supplemented with DMSO or the indicated inhibitor. At 5 and 10 min, 8 µL of reaction was withdrawn and plasmids and associated proteins were recovered by plasmid pull-down using LacI-coated magnetic beads as described above. After 30 min incubation at 4 °C, samples were washed twice with wash buffer 1 (10 mM HEPES pH 7.7, 50 mM KCl, 2.5 mM MgCl2, 250 mM sucrose, 0.03% Tween-20), and once with wash buffer 2 (10 mM HEPES pH 7.7, 50 mM KCl, 2.5 mM MgCl2, 250 mM sucrose). Samples were washed one additional time in 50 µL of wash buffer 2 and transferred to a new tube to remove residual detergent. Further processing of the samples for MS analysis was performed as described below.
pFLP repair
Plasmid DNA was bound to LacI-coated magnetic beads for 30 min at 4 °C in pull-down buffer (10 mM HEPES pH 7.7, 50 mM KCl, 2.5 mM MgCl2, 250 mM sucrose, 0.02% Tween-20). After incubation, DPC-coupled beads were washed one time with a pull-down buffer. To start the reaction, beads were dried and gently resuspended in HSS-NPE egg extract mix. At corresponding time points, 8 µL of reaction was withdrawn and mixed with 400 µL of wash buffer 1 (10 mM HEPES pH 7.7, 50 mM KCl, 2.5 mM MgCl2, 250 mM sucrose, 0.03% Tween-20) on ice. Beads were washed one time with ice-cold wash buffer 2 (10 mM HEPES pH 7.7, 50 mM KCl, 2.5 mM MgCl2, 250 mM sucrose). Samples were washed one additional time in 50 µL of wash buffer 2 and transferred to a new tube. Further processing of the samples for MS analysis was performed as described below.
MS sample preparation
Beads were dried and resuspended in 50 µL of ice-cold ABC (50 mM ammonium bicarbonate) supplemented with trypsin (10 ng/µL final concentration). Samples were first incubated on ice for 1 h and then moved to 30 °C overnight to allow protein digestion. Subsequently, TCEP and CAA were added to final concentrations of 5 mM, and digestion was allowed to continue for 1 h. Tryptic peptides were purified on StageTips at high pH107. Quad-layer StageTips were prepared using four punch-outs of C18 material (Sigma-Aldrich, Empore™ SPE Disks, C18, 47 mm). StageTips were equilibrated using 100 μL of methanol, 100 μL of 80% ACN in 200 mM ammonium hydroxide, and two times 75 μL 50 mM ammonium hydroxide solution. Samples were supplemented with 1/5th volume of 200 mM ammonium hydroxide (pH > 10.0), just prior to loading them on StageTip. The StageTips were subsequently washed twice with 150 μL 50 mM ammonium hydroxide and afterward eluted using 80 μl of 25% ACN in 50 mM ammonium hydroxide. All fractions were dried to completion in protein-LoBind tubes (Eppendorf), using a SpeedVac for 2 h at 60 °C, after which the dried peptides were dissolved using 11 μL of 0.1% formic acid, and stored at −20 °C until MS analysis. Samples were prepared in quadruplicates and analyzed by label-free MS.
MS experimental design
Three batches of MS samples were measured. Experiment 1 (“Exp1”); pMHssDNA and pmeMHssDNA repair experiment, corresponding to Fig. 3, Supplementary Fig. 3, and Supplementary Data 1, and RAW file names starting with “20220528”. Experiment 2 (“Exp2”); first pFLP repair experiment, corresponding to Fig. 4 and Supplementary Data 2, and RAW file names starting with “20200819”. Experiment 3 (“Exp3”); second pFLP repair experiment, corresponding to Supplementary Fig. 5H and Supplementary Data 3, and RAW file names starting with “20210103”.
MS data acquisition
All MS samples were analyzed on an EASY-nLC 1200 system (Thermo) coupled to an Orbitrap Exploris™ 480 mass spectrometer (Thermo). Separation of peptides was performed using 20-cm columns (75 μm internal diameter) packed in-house with ReproSil-Pur 120 C18-AQ 1.9 μm beads (Dr. Maisch). Elution of peptides from the column was achieved using a gradient ranging from buffer A (0.1% formic acid) to buffer B (80% acetonitrile in 0.1% formic acid), at a flow of 250 nL/min. The gradient length was 80 min per sample, including ramp-up and wash-out, with an analytical gradient of 60 min ranging from 5% B to 32% B for “Exp1” and “Exp2” or from 5% B to 30% B for “Exp3”. Analytical columns were heated to 40 °C using a column oven, and ionization was achieved using a NanoSpray Flex™ NG ion source. Spray voltage set to 2 kV, ion transfer tube temperature to 275 °C, and RF funnel level to 40%. The full scan range was set to 300–1300 m/z, MS1 resolution to 120,000, MS1 AGC target to “200” (2,000,000 charges), and MS1 maximum injection time to “Auto”. Precursors with charges 2–6 were selected for fragmentation using an isolation width of 1.3 m/z, and fragmented using higher-energy collision disassociation (HCD) with normalized collision energy of 25. Precursors were excluded from re-sequencing by setting a dynamic exclusion of 80 s. MS2 AGC target was set to “200” (200,000 charges), and MS2 maximum injection time to “Auto”. For “Exp1” samples, MS2 resolution was set to 60,000, intensity threshold to 170,000 charges per second, and number of dependent scans (TopN) to 7. For “Exp2” samples, MS2 resolution was set to 30,000, intensity threshold to 360,000 charges per second, and TopN to 12. For “Exp3” samples, MS2 resolution was set to 45,000, intensity threshold to 230,000 charges per second, and TopN to 9.
MS data analysis
All MS RAW data were analyzed using the freely available MaxQuant software108, v. 1.5.3.30. Individual MS experiments were processed in separate computational runs. Default MaxQuant settings were used, with exceptions specified below. For generation of theoretical spectral libraries, the Xenopus laevis FASTA databases were downloaded from UniProt on the 8th of May 2020 (for “Exp2” and “Exp3”) and on the 3rd of September 2021 (for “Exp1” data). In silico digestion of proteins to generate theoretical peptides was performed with trypsin, allowing up to 3 missed cleavages. Allowed variable modifications were oxidation of methionine (default) and protein N-terminal acetylation (default) for all data, and additionally deamidation of asparagine for “Exp2” and “Exp3” data. Maximum variable modifications per peptide were reduced to 3. Label-free quantification (LFQ) using MaxLFQ was enabled109, with “LFQ min. ratio count” increased to 3, and “Fast LFQ” disabled. Stringent MaxQuant 1% FDR control was applied at the PSM- and protein levels (default). Matching between runs was enabled, with an alignment window of 20 min and a match time window of 1 min.
MS data annotation and quantification
The Xenopus laevis FASTA databases downloaded from UniProt lacked comprehensive gene name annotations. Missing or uninformative gene names were, when possible, semi-automatically curated, as described previously10. Quantification of the MaxQuant output files (“proteinGroups.txt”), and all further statistical handling, such as z-scoring for generation of heatmaps, was performed using Perseus software v1.5.5.3110. For quantification purposes, all LFQ-normalized protein intensity values were log2 transformed and filtered for presence in 4 of 4 replicates (n = 4/4) in at least one experimental condition. Missing values were imputed below the global experimental detection limit at a downshift of 1.8 and a randomized width of 0.3 (in log2 space; Perseus default). Statistical significance of differences was in all cases tested using two-tailed Student's two-sample t-testing, with permutation-based FDR control applied to ensure a corrected p-value (i.e., q-value) of <5%, using an s0 factor of 0.5 (for “Exp1” and “Exp3” or 0.1 (for “Exp2”).
Cell culture and alkaline comet assays
The wild-type and mutant RPE-1 cell lines employed here have been described previously111,112. For alkaline comet assays, the indicated cells were incubated for 1 h at 37 °C in the presence of a DMSO vehicle or 10 µM camptothecin, as indicated, and then harvested and processed as described previously18,113. Where indicated, cells were treated with 50 µM MG132 (Sigma) for 2 h prior to, and during, camptothecin treatment as above.
Reproducibility
A minimum of two independent experiments have been conducted for each experimental result shown in this manuscript.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD043107. Source data are provided in this paper.
References
Leng, X. & Duxin, J. P. Targeting DNA-protein crosslinks via post-translational modifications. Front. Mol. Biosci. 9, 944775 (2022).
Kühbacher, U. & Duxin, J. P. How to fix DNA-protein crosslinks. DNA Repair 94, 102924 (2020).
Ide, H., Nakano, T., Salem, A. M. H. & Shoulkamy, M. I. DNA–protein cross-links: formidable challenges to maintaining genome integrity. DNA Repair 71, 190–197 (2018).
Weickert, P. & Stingele, J. DNA–protein crosslinks and their resolution. Annu. Rev. Biochem. 91, 157–181 (2022).
Ruggiano, A. & Ramadan, K. DNA–protein crosslink proteases in genome stability. Commun. Biol. 4, 11 (2021).
Stingele, J., Bellelli, R. & Boulton, S. J. Mechanisms of DNA–protein crosslink repair. Nat. Rev. Mol. Cell Biol. 18, 563–573 (2017).
Sparks, J. L. et al. The CMG helicase bypasses DNA-protein cross-links to facilitate their repair. Cell 176, 167–181.e21 (2019).
Duxin, J. P., Dewar, J. M., Yardimci, H. & Walter, J. C. Repair of a DNA-protein crosslink by replication-coupled proteolysis. Cell 159, 346–357 (2014).
Larsen, N. B. et al. Replication-coupled DNA-protein crosslink repair by SPRTN and the proteasome in Xenopus egg extracts. Mol. Cell 73, 574–588.e7 (2019).
Gallina, I. et al. The ubiquitin ligase RFWD3 is required for translesion DNA synthesis. Mol. Cell 81, 442–458.e9 (2021).
Reinking, H. K. et al. DNA structure-specific cleavage of DNA-protein crosslinks by the SPRTN protease. Mol. Cell 80, 102–113.e6 (2020).
Liu, J. C. Y. et al. Mechanism and function of DNA replication‐independent DNA‐protein crosslink repair via the SUMO‐RNF4 pathway. EMBO J. 40, e107413 (2021).
Sun, Y. et al. A conserved SUMO pathway repairs topoisomerase DNA-protein cross-links by engaging ubiquitin-mediated proteasomal degradation. Sci. Adv. 6, eaba6290 (2020).
Bjornsti, M.-A. & Kaufmann, S. H. Topoisomerases and cancer chemotherapy: recent advances and unanswered questions. F1000Res 8, 1704 (2019).
Pommier, Y., Nussenzweig, A., Takeda, S. & Austin, C. Human topoisomerases and their roles in genome stability and organization. Nat. Rev. Mol. Cell Biol. 23, 407–427 (2022).
Yang, S. W. et al. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc. Natl Acad. Sci. USA 93, 11534–11539 (1996).
Debethune, L. Processing of nucleopeptides mimicking the topoisomerase I-DNA covalent complex by tyrosyl-DNA phosphodiesterase. Nucleic Acids Res. 30, 1198–1204 (2002).
El-Khamisy, S. F., Hartsuiker, E. & Caldecott, K. W. TDP1 facilitates repair of ionizing radiation-induced DNA single-strand breaks. DNA Repair 6, 1485–1495 (2007).
Interthal, H. & Champoux, J. J. Effects of DNA and protein size on substrate cleavage by human tyrosyl-DNA phosphodiesterase 1. Biochem. J. 436, 559–566 (2011).
Yip, M. C. J., Bodnar, N. O. & Rapoport, T. A. Ddi1 is a ubiquitin-dependent protease. Proc. Natl Acad. Sci. USA 117, 7776–7781 (2020).
Fielden, J. et al. TEX264 coordinates p97- and SPRTN-mediated resolution of topoisomerase 1-DNA adducts. Nat. Commun. 11, 1274 (2020).
Lin, C.-P., Ban, Y., Lyu, Y. L., Desai, S. D. & Liu, L. F. A ubiquitin-proteasome pathway for the repair of topoisomerase I-DNA covalent complexes. J. Biol. Chem. 283, 21074–21083 (2008).
Desai, S. D., Liu, L. F., Vazquez-Abad, D. & D’Arpa, P. Ubiquitin-dependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. J. Biol. Chem. 272, 24159–24164 (1997).
Serbyn, N. et al. The aspartic protease Ddi1 contributes to DNA-protein crosslink repair in yeast. Mol. Cell 77, 1066–1079.e9 (2020).
Ray Chaudhuri, A. & Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 18, 610–621 (2017).
Rose, M., Burgess, J. T., O’Byrne, K., Richard, D. J. & Bolderson, E. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front. Cell Dev. Biol. 8, 564601 (2020).
Bonfiglio, J. J. et al. Serine ADP-ribosylation depends on HPF1. Mol. Cell 65, 932–940.e6 (2017).
Suskiewicz, M. J. et al. HPF1 completes the PARP active site for DNA damage-induced ADP-ribosylation. Nature 579, 598–602 (2020).
Langelier, M.-F., Billur, R., Sverzhinsky, A., Black, B. E. & Pascal, J. M. HPF1 dynamically controls the PARP1/2 balance between initiating and elongating ADP-ribose modifications. Nat. Commun. 12, 6675 (2021).
Sun, F.-H. et al. HPF1 remodels the active site of PARP1 to enable the serine ADP-ribosylation of histones. Nat. Commun. 12, 1028 (2021).
Hendriks, I. A., Larsen, S. C. & Nielsen, M. L. An advanced strategy for comprehensive profiling of ADP-ribosylation sites using mass spectrometry-based proteomics*. Mol. Cell. Proteom. 18, 1010–1026 (2019).
Gibbs-Seymour, I., Fontana, P., Rack, J. G. M. & Ahel, I. HPF1/C4orf27 is a PARP-1-interacting protein that regulates PARP-1 ADP-ribosylation activity. Mol. Cell 62, 432–442 (2016).
Kamaletdinova, T., Fanaei-Kahrani, Z. & Wang, Z.-Q. The enigmatic function of PARP1: from PARylation activity to PAR readers. Cells 8, 1625 (2019).
Pommier, Y., O’Connor, M. J. & de Bono, J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 8, 362ps17 (2016).
Murai, J. et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 72, 5588–5599 (2012).
Das, S. K. et al. Poly(ADP-ribose) polymers regulate DNA topoisomerase I (Top1) nuclear dynamics and camptothecin sensitivity in living cells. Nucleic Acids Res. 44, 8363–8375 (2016).
Das, B. B. et al. PARP1–TDP1 coupling for the repair of topoisomerase I–induced DNA damage. Nucleic Acids Res. 42, 4435–4449 (2014).
Langelier, M.-F., Riccio, A. A. & Pascal, J. M. PARP-2 and PARP-3 are selectively activated by 5′ phosphorylated DNA breaks through an allosteric regulatory mechanism shared with PARP-1. Nucleic Acids Res. 42, 7762–7775 (2014).
Zandarashvili, L. et al. Structural basis for allosteric PARP-1 retention on DNA breaks. Science 368, eaax6367 (2020).
Chowdhuri, S. P. & Das, B. B. Top1-PARP1 association and beyond: from DNA topology to break repair. NAR Cancer 3, zcab003 (2021).
Olivieri, M. et al. A genetic map of the response to DNA damage in human. Cells Cell 182, 481–496.e21 (2020).
LoRusso, P. M. et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) in combination with irinotecan in patients with advanced solid tumors. Clin. Cancer Res. 22, 3227–3237 (2016).
Kummar, S. et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 71, 5626–5634 (2011).
Wahner Hendrickson, A. E. et al. A phase I clinical trial of the poly(ADP-ribose) polymerase inhibitor veliparib and weekly topotecan in patients with solid tumors. Clin. Cancer Res. 24, 744–752 (2018).
Demin, A. A. et al. XRCC1 prevents toxic PARP1 trapping during DNA base excision repair. Mol. Cell 81, 3018–3030.e5 (2021).
Yung, T. M. C., Sato, S. & Satoh, M. S. Poly(ADP-ribosyl)ation as a DNA damage-induced post-translational modification regulating Poly(ADP-ribose) polymerase-1-topoisomerase I interaction. J. Biol. Chem. 279, 39686–39696 (2004).
Malanga, M. & Althaus, F. R. Poly(ADP-ribose) reactivates stalled DNA topoisomerase I and induces DNA strand break resealing. J. Biol. Chem. 279, 5244–5248 (2004).
Park, S.-Y. & Cheng, Y.-C. Poly(ADP-ribose) polymerase-1 could facilitate the religation of topoisomerase I-linked DNA inhibited by camptothecin. Cancer Res. 65, 3894–3902 (2005).
Sun, Y. et al. PARylation prevents the proteasomal degradation of topoisomerase I DNA-protein crosslinks and induces their deubiquitylation. Nat. Commun. 12, 5010 (2021).
Langelier, M.-F., Planck, J. L., Servent, K. M. & Pascal, J. M. Purification of human PARP-1 and PARP-1 domains from Escherichia coli for structural and biochemical analysis. 209–226. https://doi.org/10.1007/978-1-61779-270-0_13 (2011).
Rank, L. et al. Analyzing structure–function relationships of artificial and cancer-associated PARP1 variants by reconstituting TALEN-generated HeLa PARP1 knock-out cells. Nucleic Acids Res. gkw859 https://doi.org/10.1093/nar/gkw859 (2016).
Prokhorova, E. et al. Serine-linked PARP1 auto-modification controls PARP inhibitor response. Nat. Commun. 12, 4055 (2021).
Hendriks, I. A. et al. The regulatory landscape of the human HPF1- and ARH3-dependent ADP-ribosylome. Nat. Commun. 12, 5893 (2021).
Zhu, T. et al. Human PARP1 substrates and regulators of its catalytic activity: An updated overview. Front. Pharm. 14, 113715 (2023).
Langelier, M.-F., Planck, J. L., Roy, S. & Pascal, J. M. Structural basis for DNA damage–dependent poly(ADP-ribosyl)ation by human PARP-1. Science 336, 728–732 (2012).
Rack, J. G. M. et al. Mechanistic insights into the three steps of poly(ADP-ribosylation) reversal. Nat. Commun. 12, 4581 (2021).
Schützenhofer, K., Rack, J. G. M. & Ahel, I. The making and breaking of serine-ADP-ribosylation in the DNA damage response. Front. Cell Dev. Biol. 9, 745922 (2021).
Harms, A. & Gerdes, K. Back to the roots: deep view into the evolutionary history of ADP-ribosylation opened by the DNA-targeting toxin-antitoxin module DarTG. Mol. Cell 64, 1020–1021 (2016).
Jankevicius, G. et al. A family of macrodomain proteins reverses cellular mono-ADP-ribosylation. Nat. Struct. Mol. Biol. 20, 508–514 (2013).
Rosenthal, F. et al. Macrodomain-containing proteins are new mono-ADP-ribosylhydrolases. Nat. Struct. Mol. Biol. 20, 502–507 (2013).
Rudolph, J. et al. The BRCT domain of PARP1 binds intact DNA and mediates intrastrand transfer. Mol. Cell 81, 4994–5006.e5 (2021).
Gatti, M., Imhof, R., Huang, Q., Baudis, M. & Altmeyer, M. The ubiquitin ligase TRIP12 limits PARP1 trapping and constrains PARP inhibitor efficiency. Cell Rep. 32, 107985 (2020).
Brodie, S. A. et al. Small molecule inhibition of the CHFR-PARP1 interaction as novel approach to overcome intrinsic taxane resistance in cancer. Oncotarget 6, 30773–30786 (2015).
Yan, Q. et al. BAL1 and its partner E3 ligase, BBAP, link poly(ADP-RIbose) Activation, ubiquitylation, and double-strand DNA repair independent of ATM, MDC1, and RNF8. Mol. Cell Biol. 33, 845–857 (2013).
Richard, I. A., Burgess, J. T., O’Byrne, K. J. & Bolderson, E. Beyond PARP1: the potential of other members of the poly (ADP-ribose) polymerase family in DNA repair and cancer therapeutics. Front. Cell Dev. Biol. 9, 801200 (2022).
Dirac-Svejstrup, A. B. et al. DDI2 is a ubiquitin-directed endoprotease responsible for cleavage of transcription factor NRF1. Mol. Cell 79, 332–341.e7 (2020).
Breslin, C. et al. Measurement of chromosomal DNA single‐strand breaks and replication fork progression rates. 410–425. https://doi.org/10.1016/S0076-6879(05)09024-5. (2006).
Nielsen, I. et al. A Flp-nick system to study repair of a single protein-bound nick in vivo. Nat. Methods 6, 753–757 (2009).
Serbyn, N. et al. SUMO orchestrates multiple alternative DNA-protein crosslink repair pathways. Cell Rep. 37, 110034 (2021).
Jakobsen, K. P. et al. Minimal resection takes place during break-induced replication repair of collapsed replication forks and is controlled by strand invasion. Cell Rep. 26, 836–844.e3 (2019).
Caldecott, K. W. DNA single-strand break repair and spinocerebellar ataxia. Cell 112, 7–10 (2003).
El-Khamisy, S. F. et al. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 434, 108–113 (2005).
Caldecott, K. W. DNA single-strand break repair and human genetic disease. Trends Cell Biol. 32, 733–745 (2022).
Marini, V. et al. MUS81 cleaves TOP1-derived lesions and other DNA–protein cross-links. BMC Biol. 21, 110 (2023).
Vrtis, K. B. et al. Single-strand DNA breaks cause replisome disassembly. Mol. Cell 81, 1309–1318.e6 (2021).
Chen, Y., Narendra, U., Iype, L. E., Cox, M. M. & Rice, P. A. Crystal structure of a Flp recombinase–holliday junction complex. Mol. Cell 6, 885–897 (2000).
Staker, B. L. et al. Structures of three classes of anticancer agents bound to the human topoisomerase I−DNA covalent complex. J. Med Chem. 48, 2336–2345 (2005).
Rudolph, J., Mahadevan, J., Dyer, P. & Luger, K. Poly(ADP-ribose) polymerase 1 searches DNA via a ‘monkey bar’ mechanism. Elife 7, e37818 (2018).
Yaneva, D. et al. The FANCJ helicase unfolds DNA-protein crosslinks to promote their repair. Mol. Cell 83, 43–56.e10 (2023).
Dyrkheeva, N. et al. Human tyrosyl-DNA phosphodiesterase 1 possesses transphosphooligonucleotidation activity with primary alcohols. Front. Cell Dev. Biol. 8, 604732 (2020).
Interthal, H., Chen, H. J. & Champoux, J. J. Human Tdp1 cleaves a broad spectrum of substrates, including phosphoamide linkages. J. Biol. Chem. 280, 36518–36528 (2005).
Pommier, Y. et al. Tyrosyl-DNA-phosphodiesterases (TDP1 and TDP2). DNA Repair 19, 114–129 (2014).
Caldecott, K. W. XRCC1 protein; form and function. DNA Repair 81, 102664 (2019).
Felle, M. et al. The USP7/Dnmt1 complex stimulates the DNA methylation activity of Dnmt1 and regulates the stability of UHRF1. Nucleic Acids Res. 39, 8355–8365 (2011).
Munnur, D. et al. Reversible ADP-ribosylation of RNA. Nucleic Acids Res. 47, 5658–5669 (2019).
Groslambert, J., Prokhorova, E. & Ahel, I. ADP-ribosylation of DNA and RNA. DNA Repair 105, 103144 (2021).
Matta, E., Kiribayeva, A., Khassenov, B., Matkarimov, B. T. & Ishchenko, A. A. Insight into DNA substrate specificity of PARP1-catalysed DNA poly(ADP-ribosyl)ation. Sci. Rep. 10, 3699 (2020).
Munnur, D. & Ahel, I. Reversible mono-ADP-ribosylation of DNA breaks. FEBS J. 284, 4002–4016 (2017).
Talhaoui, I. et al. Poly(ADP-ribose) polymerases covalently modify strand break termini in DNA fragments in vitro. Nucleic Acids Res. gkw675 https://doi.org/10.1093/nar/gkw675 (2016).
Ray Chaudhuri, A. et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat. Struct. Mol. Biol. 19, 417–423 (2012).
Kose, H. B., Larsen, N. B., Duxin, J. P. & Yardimci, H. Dynamics of the eukaryotic replicative helicase at lagging-strand protein barriers support the steric exclusion model. Cell Rep. 26, 2113–2125.e6 (2019).
Villa, F. et al. CUL2 LRR1, TRAIP and p97 control CMG helicase disassembly in the mammalian cell cycle. EMBO Rep. 22, e52164 (2021).
Zhou, H., Zaher, M. S., Walter, J. C. & Brown, A. Structure of CRL2Lrr1, the E3 ubiquitin ligase that promotes DNA replication termination in vertebrates. Nucleic Acids Res. 49, 13194–13206 (2021).
Low, E., Chistol, G., Zaher, M. S., Kochenova, O. V. & Walter, J. C. The DNA replication fork suppresses CMG unloading from chromatin before termination. Genes Dev. 34, 1534–1545 (2020).
Jenkyn-Bedford, M. et al. A conserved mechanism for regulating replisome disassembly in eukaryotes. Nature 600, 743–747 (2021).
Lebofsky, R., Takahashi, T. & Walter, J. C. DNA replication in nucleus-free Xenopus egg extracts. Methods Mol. Biol. 521, 229–252 (2009).
Kochaniak, A. B. et al. Proliferating cell nuclear antigen uses two distinct modes to move along DNA. J. Biol. Chem. 284, 17700–17710 (2009).
Ryu, H. et al. PIASy mediates SUMO-2/3 conjugation of Poly(ADP-ribose) polymerase 1 (PARP1) on mitotic chromosomes. J. Biol. Chem. 285, 14415–14423 (2010).
Fang, F. & Newport, J. W. Distinct roles of cdk2 and cdc2 in RP-A phosphorylation during the cell cycle. J. Cell Sci. 106, 983–994 (1993).
Stinson, B. M., Moreno, A. T., Walter, J. C. & Loparo, J. J. A mechanism to minimize errors during non-homologous end joining. Mol. Cell 77, 1080–1091.e8 (2020).
Kumamoto, S. et al. HPF1-dependent PARP activation promotes LIG3-XRCC1-mediated backup pathway of Okazaki fragment ligation. Nucleic Acids Res. 49, 5003–5016 (2021).
Nielsen, I., Andersen, A. H. & Bjergbæk, L. Studying repair of a single protein-bound nick in vivo using the Flp-nick system. 393–415. https://doi.org/10.1007/978-1-61779-998-3_28 (2012).
Hospenthal, M. K., Mevissen, T. E. T. & Komander, D. Deubiquitinase-based analysis of ubiquitin chain architecture using Ubiquitin Chain Restriction (UbiCRest). Nat. Protoc. 10, 349–361 (2015).
Budzowska, M., Graham, T. G., Sobeck, A., Waga, S. & Walter, J. C. Regulation of the Rev1–pol ζ complex during bypass of a DNA interstrand cross‐link. EMBO J. 34, 1971–1985 (2015).
Räschle, M. et al. Mechanism of replication-coupled DNA interstrand crosslink repair. Cell 134, 969–980 (2008).
Knipscheer, P. et al. The Fanconi anemia pathway promotes replication-dependent dna interstrand cross-link repair. Science 326, 1698–1701 (2009).
Hendriks, I. A. et al. Site-specific characterization of endogenous SUMOylation across species and organs. Nat. Commun. 9, 2456 (2018).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Cox, J. et al. Accurate Proteome-wide Label-free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteom. 13, 2513–2526 (2014).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Hanzlikova, H., Gittens, W., Krejcikova, K., Zeng, Z. & Caldecott, K. W. Overlapping roles for PARP1 and PARP2 in the recruitment of endogenous XRCC1 and PNKP into oxidized chromatin. Nucleic Acids Res. gkw1246. https://doi.org/10.1093/nar/gkw1246 (2016).
Hoch, N. C. et al. XRCC1 mutation is associated with PARP1 hyperactivation and cerebellar ataxia. Nature 541, 87–91 (2017).
Thuresson, A. et al. Novel PNKP mutations associated with reduced DNA single‐strand break repair and severe microcephaly, seizures, and developmental delay. Mol. Genet. Genom. Med. 12, e2295 (2024).
Acknowledgements
We thank members of the Duxin laboratory for their feedback on the manuscript. We also thank Ivan Ahel and Domagoj Baretic for providing HPF1 and the Novo Nordisk Foundation Center for Protein Research protein production facility for purifying LacI‐biotin and PARP1 proteins. We thank Atsuya Nishiyama for providing the HPF1 antibody. The Novo Nordisk Foundation Center for Protein Research is supported financially by the Novo Nordisk Foundation (grant agreement NNF14CC0001). This project has received funding from the Danish Research Independent Fund (DFF; grant agreements # 9060-00030B and 2034-0010B), the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program (grant agreements 715975 and 101126220) and by the Novo Nordisk Foundation (grant no. NNF22OC0074140). Z.F. and A.B.D.S were supported by the Novo Nordisk Foundation (grant no. NNF18CC0033876 and NFF19OC0055875, respectively). Work in the Caldecott lab was supported by an MRC Program grant to KWC (MR/W024128/1).
Author information
Authors and Affiliations
Contributions
Z.F. and J.P.D. conceived the project. Z.F., E.K., U.K., N.B.L., M.O.S., and X.L., performed the experiments in Xenopus under the supervision of J.P.D., I.A.H. analyzed the M.S. data under the supervision of M.L.N., J.W. performed experiments in human cells under the supervision of K.C., and A.B.D.S. and J.Q.S. provided DDI2 proteins. Z.F. and J.P.D. interpreted the results. Z.F. and J.P.D. wrote the manuscript with feedback from E.S.K. and M.O.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Lotte Bjergbaek, Hervé Técher, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fábián, Z., Kakulidis, E.S., Hendriks, I.A. et al. PARP1-dependent DNA-protein crosslink repair. Nat Commun 15, 6641 (2024). https://doi.org/10.1038/s41467-024-50912-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-50912-x
This article is cited by
-
Wss1 and Ddi1 DNA-Protein crosslink repair proteases protect Saccharomyces cerevisiae and Candida albicans against oxidative stress
Scientific Reports (2025)
-
Epigenetic control of topoisomerase 1 activity presents a cancer vulnerability
Nature Communications (2025)