Abstract
There is insufficient data on systemic embolic events (SSEs) in patients with ischemic left ventricular aneurysm (LVA) concerning the impact of anticoagulation therapy. In this retrospective cohort study with 1043 patients with ischemic LVA, SSEs occurred in 7.2% over 2.4 years. After adjusting for relevant factors, the use of anticoagulants was independently associated with a lower incidence of SSE (3.1% vs. 9.0%, P < 0.001; subdistribution hazard ratios (SHR) 0.21, 95% confidence intervals (CI) 0.10-0.44, P < 0.001), with no significant difference in net adverse clinical events (NACEs) (10.6% vs. 13.3%, P = 0.225). Specifically, anticoagulation in patients with apical segment akinesis significantly reduced SSEs (3.9% vs. 13.6%, P = 0.002) and NACE rates (7.8% vs. 19.4%, P = 0.002). Major bleeding rates did not significantly differ between groups (5.6% vs. 3.5%, P = 0.111). These findings highlight the SSE risk in ischemic LVA and suggest potential benefits of anticoagulation, particularly in those with apical segment akinesis. These findings need to be validated in independent datasets.
Similar content being viewed by others
Introduction
Left ventricular aneurysm (LVA) is a severe complication of acute myocardial infarction1,2. Despite the improvement of medical care and the advent of the early reperfusion method, it remains an important entity associated with a risk of ventricular arrhythmias, heart failure, cardiac rupture and left ventricular thrombus (LVT) and subsequent thromboembolism3,4.
The optimal management of patients with post-myocardial LVA formation is a matter of controversy, particularly concerning whether anticoagulation is beneficial5. The 2013 American College of Cardiology Foundation/American Heart Association (ACC/AHA) guideline for the management of ST-elevation myocardial infarction (STEMI) based on Level of Evidence C, gives a class IIb recommendation (“may be considered”) for “anticoagulant therapy in STEMI patients who have left ventricular (LV) anterior apical akinesis or dyskinesis.”6 The weakness of the recommendation and the low level of evidence highlights the scientific uncertainty of the clinical guidance. Indeed, no randomized trials specifically addressing anticoagulation treatment among patients with ischemic LVA have been published. A recent AHA scientific statement also emphasized that routine prophylactic anticoagulation in all patients with myocardial infarction does not appear to be supported by data7. The authors suggested, “any consideration of anticoagulant treatment in patients with acute myocardial infarction with anteroapical akinesis or dyskinesis should weigh and incorporate the perceived risk of thrombus formation and bleeding and involve decision making.”
There have been few non-randomized studies of clinical outcomes, and there is limited evidence on the prognostic significance of anticoagulant use in patients with documented ischemic LVA8. In this work, we have gathered the largest cohort of patients with ischemic LVA to date and analyze the clinical characteristics and prognosis of anticoagulant use among these people.
Results
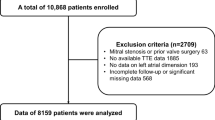
Figure 1 illustrates the flowchart for the patient selection and exclusion process. A total of 1260 patients with a “LVA” on echocardiography reports were selected irrespective of underlying pathology. We excluded 142 patients with non-ischemic LVA, 35 patients with poor original images for diagnosis, and 40 patients without data regarding medications at baseline and lack of follow-up. A total of 1043 patients were finally included in the study analysis.
Baseline characteristics
The mean age at the baseline was 67.1 ± 12.0 years and 727 (69.7%) were men. Prior ischemic stroke/transient ischemic attack (TIA) and prior major bleeding were identified in 170 participants (16.3%) and 23 participants (2.2%), respectively. At a mean follow-up of 2.4 (interquartile range (IQR): 1.0−3.6) years, any systemic embolic events (SSEs) were identified in 75 (7.2%) patients. Table 1 shows the baseline characteristics in the whole population and in patients with or without SSEs.
Overall, the use of anticoagulant agents after initial LVA diagnosis was noted in 30.9% of the patients (Table 1). Patients with or without SSEs did not differ at baseline regarding age (P = 0.570), sex (P = 0.698), prior major bleeding (P = 0.900), estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 (P = 1.000) and other drugs use (all P > 0.05). The group with SSEs had more prior atrial fibrillation (P = 0.051), hypertension (P = 0.001), diabetes mellitus (P = 0.012), prior stroke/TIA (P < 0.001) and less anticoagulant use (P < 0.001) than the group without SSEs.
Echocardiographic evaluation at LVA diagnosis
More than one-half of the population (564/1043, 54.1%) had an LV ejection fraction ≤ 40%. In sixty-seven patients (6.4%) LVT was detected at the time of LVA diagnosis. Table 2 describes the parameters of echocardiographic assessment at the time of LVA diagnosis. The SSEs group had similar LVEF to the non-SSEs group (39.7 ± 9.7% vs. 40.2 ± 9.7% respectively; P = 0.682). Concerning LV wall motion, akinesis in apical segments was detected more frequently in the SSEs group (62.7% vs. 40.3%, P < 0.001). LVT at the time of LVA diagnosis was detected in 14.7% of patients with SSEs and 5.8% in patients without SSEs (P = 0.006).
Factors predictive of systemic embolism events
All the variables related to age, prior atrial fibrillation, hypertension, diabetes mellitus, prior stroke/TIA, anticoagulant use, LVT and akinesis in apical segments had a P value of < 0.05 from the univariable analysis. Multivariable regression analysis identified these parameters as independent predictors of systemic embolic events (Table 3). After multivariable adjustment and considering deaths as a competing risk, the presence of atrial fibrillation (SHR 1.96, 95% CI 1.17−3.26, P = 0.010), history of stroke/transient ischemic attack (SHR 5.76, 95% CI 3.39−9.79, P < 0.001), left ventricular thrombus (SHR 3.82, 95% CI 2.20−6.62, P < 0.001), akinesis in apical segments (SHR 1.83, 95% CI 1.14−2.93, P = 0.011) and anticoagulant use (SHR 0.21, 95% CI 0.10−0.44, P < 0.001) were independently associated with SSEs.
The prognosis of anticoagulant use in patients with LVA
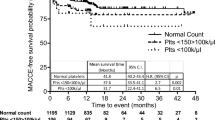
During a follow-up period of 2606.9 patient-year, 130 net adverse clinical events (NACEs) (5.0 per 100 patient-year) were reported among all study populations. Of these, 33 were all-cause deaths, 75 were SSEs, and 43 were major bleeding events. Table 4 shows that patients who had LVA and did not receive anticoagulation demonstrated a higher rate of SSEs than those who received anticoagulation (3.1% vs. 9.0%; P < 0.001). However, the incidence of NACEs did not differ according to anticoagulant use (10.6% in the case with anticoagulant use vs. 13.3% in the case without anticoagulant use; P = 0.225). Similarly, in the Kaplan–Meier survival curve analysis, anticoagulant use was a significant predictor of SSEs (P = 0.001). However, when we analyzed NACEs, all-causes mortality and major bleedings, the event rates were not different according to anticoagulation status (Fig. 2).
Kaplan-Meier event-free survival curve in patients with LVA according to anticoagulation status (without anticoagulant use: n = 721; with anticoagulant use: n = 332): A Net adverse clinical events (B) All-cause mortality (C) Systemic embolism events (D) Major bleeding events. Source data are provided as a Source Data file. Abbreviations: LVA left ventricular aneurysm.
The prognostic significance of anticoagulant use in patients with LVA and apical segment akinesis
The baseline characteristics and echocardiographic evaluation in patients with LVA and apical segment akinesis are shown in Supplementary Table 1. Patients with LVA and apical segment akinesis had a 10.8% rate of systemic embolism events (Table 5). Those with anticoagulant use had significantly lower SSEs than those without (3.9% vs. 13.6%, P = 0.002) and lower NACEs (7.8% vs. 19.4%, P = 0.002). Figure 3 shows the Kaplan–Meier curves according to anticoagulant use in patients with akinesis in apical segments.
Kaplan-Meier event-free survival curve in patients with LVA and apical segment akinesis according to anticoagulation status (without anticoagulant use: n = 309; with anticoagulant use: n = 128): (A) Net adverse clinical events (B) All-cause mortality (C) Systemic embolism events (D) Major bleeding events. Source data are provided as a Source Data file. Abbreviations: LVA left ventricular aneurysm.
Anticoagulant use in patients with LVA and without other clear indications for it
To minimize any potential indication bias, we conducted the same statistical analyses in a subgroup of patients without any indications for anticoagulant use. These indications may include situations such as having atrial fibrillation (N = 173), LVT (N = 67), undergoing treatment or prophylaxis for deep vein thrombosis or pulmonary embolism (N = 97), experiencing recurrent strokes despite antithrombotic therapy (N = 18), prosthetic valves (N = 8), or peripheral embolism without an identified cause (N = 34). More than one indication for anticoagulant use was reported in 78 patients. Supplementary Table 2 shows the clinical outcomes of patients with LVA without other clear indications. Those with anticoagulant use had significantly lower SSEs than those without (0.9% vs. 7.6%, P = 0.008). A numerically lower NACEs were observed in patients treated with anticoagulants (5.4% vs. 11.5%, P = 0.066). Major bleeding events were similar in the 2 groups. Figure 4 shows the Kaplan–Meier curves according to anticoagulant use in patients with akinesis in apical segments.
Kaplan-Meier event-free survival curve in patients with LVA and without a clear indication for anticoagulation according to anticoagulation status (without anticoagulant use: n = 641; with anticoagulant use: n = 112): A Net adverse clinical events (B) All-cause mortality (C) Systemic embolism events (D) Major bleeding events. Source data are provided as a Source Data file. Abbreviations: LVA left ventricular aneurysm.
Multivariable regression analyses were also performed in patients with LVA without clear anticoagulation indications (Table 6). After multivariable adjustment and considering deaths as a competing risk, history of stroke/transient ischemic attack (SHR 9.05, 95% CI 5.09−16.11, P < 0.001), akinesis in apical segments (SHR 1.90, 95% CI 1.07−3.35, P = 0.028) and anticoagulant use (SHR 0.13, 95% CI 0.02−0.97, P = 0.046) were independently associated with SSEs.
Discussion
Investigations that specifically address the clinical outcome and the prognostic significance of anticoagulant use in patients with ischemic LVA are currently absent in the literature. This analysis provided new evidence from a large echocardiographic cohort of patients with ischemic LVA with follow-up data in the modern era. Our key findings are as follows:
-
1.
SSEs rate was achieved in 7.2% of all patients with ischemic LVA over a mean follow-up of 2.4 years.
-
2.
Anticoagulant use, when compared with no anticoagulant use, reduces SSEs, but it does not significantly affect the rate of NACEs.
-
3.
Patients with akinesis in apical segments had a higher risk of systemic embolism, and anticoagulant use improves the clinical outcomes in either SSEs or NACEs.
-
4.
Anticoagulant use in patients without a clear indication of it (such as atrial fibrillation or LVT) resulted in lower SSEs. However, anticoagulation did not achieve a statistically significant benefit on NACEs.
Anticoagulant use in patients with LVA: the issue of embolic complications has yet to be addressed
Persistent myocardial ischemia leads to myocardial tissue injury and triggers an acute inflammatory response and a hypercoagulable state7,9. If infarct expansion leads to LVA, then regional wall motion abnormalities lead to blood stasis, Virchow’s triad is completed, and LVT can occur10,11,12,13. In spite of LVA being considered a risk factor for the development of LVT, prophylactic anticoagulant therapy has not been used in randomized trials in patients with LVA14,15. Hence, anticoagulant use is an unproven strategy to prevent embolic stroke and peripheral embolism in patients with LVA, and Guidelines on both sides of the Atlantic are largely silent on the issue, i.e. the use of anticoagulation in patients with LVA but without other anticoagulant indications6,16.
In our study, 30.9% of patients were on anticoagulation following the LVA diagnosis. SSEs occurred in 7.0% of patients over 2.4 years. More than half the patients had LV ejection fraction ≤ 40%. The SSEs group had a similar LVEF to the non-SSEs group. Both LVT and akinesis in apical segments were detected more frequently in the SSEs group. The presence of atrial fibrillation, history of stroke/transient ischemic attack, LVT, and akinesis in apical segments were independently associated with higher risk, while anticoagulant use was associated with lower risk of embolic complications.
Whether additional antithrombotic regimens should be administered to vulnerable patients after myocardial infarction to prevent LVT occurrence remains a debatable issue. Most available data come from small observational studies and support our findings17,18. Data from patients who underwent LV aneurysmectomy before the area of primary percutaneous coronary intervention in STEMI showed that the incidence of LVT in these patients was very high (48%), and the thrombus was located in antero-apical region in nearly all cases17. Embolism occurred in 5% of the patients with LVA17. Presence of mural thrombus correlated inversely with a duration of anticoagulant therapy17. Moreover, a meta-analysis showed that anticoagulation after acute anterior myocardial infarction resulted in the prevention of thrombus formation (4 studies, 307 patients, odds ratio 0.32, 95% CI, 0.20–0.52) and prevention of systemic embolism in patients with visible thrombus (7 studies, 270 patients, odds ratio 0.14, 95% CI, 0.04–0.52)18. There are poor data regarding embolic complications in LVA patients in the modern era3,8. You et al. reported that LVA is still common (16%) in patients with acute anterior myocardial infarction3. During the 1-year follow-up period, the occurrence of LVT was increased in patients with LVA. Among embolic complications, only ischemic stroke was recorded, while patients with atrial fibrillation were excluded. Patients with LVA had a higher risk of stroke3. Lee et al. reported a large, non-randomized, single-center observational study of 648 patients with ischemic LVA8. Of these patients, 106 patients received anticoagulation (warfarin) and 542 patients did not. Warfarin did not change the patient outcome, either cardiac or cerebrovascular events, including systemic embolism. However, the number of patients who received anticoagulation was relatively small (N = 106), only a minority of LVA patients with identified LVT (43.8%) received anticoagulation, and the authors did not assess the therapeutic range of warfarin (it is possible that a portion of patients did not receive adequate therapy, which may have diminished the benefit of anticoagulation). In our study, a significant proportion of the patients received direct oral anticoagulants (mainly rivaroxaban). No randomized trial has evaluated the efficacy of different anticoagulants in patients with LVA, whereas small randomized trials have shown that rivaroxaban leads to faster thrombus resolution and fewer embolic events in comparison with warfarin in patients with LVT19. The addition of very low-dose rivaroxaban to dual antiplatelet therapy after anterior ST-segment elevation myocardial infarction reduced LVT formation20.
Net clinical benefit of anticoagulants among patients with LVA
Our results demonstrated that the risk of NACEs was not statistically different in patients with or without anticoagulation use (10.6% vs. 13.3%, P = 0.225). The numerically higher rate of major bleeding observed in patients treated by anticoagulation (5.6% vs. 3.5%, P = 0.111) may be a reasonable explanation for it. Since most patients were on antiplatelet treatment and the combination of both antiplatelet and anticoagulant multiplies the risk of bleeding, lower doses of anticoagulants could be another option in these patients. In the aforementioned study by Lee et al. warfarin did not change patient outcomes (a composite of death, nonfatal myocardial infarction, cerebrovascular accident, and systemic embolization was the primary outcome). Unfortunately, detailed bleeds were not investigated in their study. The group of LVA with LVT had a higher incidence of stroke and systemic embolic events during the median follow-up period of just over 3 years. However, fewer than half of the patients in this group received warfarin anticoagulation.
Another important finding of our study was the significant and consistent relationship between apical segment akinesis and the risk of adverse events. Apical segment akinesis is a marker of increased risk of a long-term adverse event, which may persist even after known LVT resolution21,22. That is to say, patients with apical segment akinesis are at a higher risk, which might also influence the clinical outcome of these patients. In our subgroup analysis, the benefits of anticoagulation in reducing embolic events outweighed the effects of other NACEs. Thus, anticoagulant use may be a viable treatment option to be considered20,23. In our previous study involving 7918 patients diagnosed with dilated cardiomyopathy and sinus rhythm, we found that apical LV dysfunction—defined as hypokinesis, akinesis, or dyskinesis present in at least 2 out of 5 apical segments—was associated with an increased risk of incident LVT (HR 2.603, 95% CI: 1.802−3.760, P < 0.001) and embolic events (HR 1.300, 95% CI: 1.004−1.683, P = 0.046)24. Furthermore, among these high-risk patients, the use of anticoagulants was associated with a reduced risk of major adverse cardiovascular events (HR 0.473, 95% CI: 0.269−0.834, P = 0.008), with no observed increase in the risk of major bleeding events (HR 0.679, 95% CI: 0.204−2.262, P = 0.528)24. In another previous study, we enrolled 116 patients with LVT resolution and observed LVT recurrence25. We found that LV aneurysms were an independent predictor of LVT recurrence (HR 2.587, 95% CI 1.199−5.580, P = 0.015), while the use of anticoagulants was associated with a lower LVT recurrence (HR 0.124, 95% CI 0.037−0.413, P = 0.001)25.
Clinical outcomes of patients with LVA and without clear indications for anticoagulation
To minimize indication bias, we did the same statistical analysis in patients without other indications for anticoagulant treatment. Because there is no clear indication for anticoagulation in patients with LVA, clinicians may still decide to administer anticoagulation therapy due to their concern regarding the high risk of embolism in these patients. Therefore, some patients receive anticoagulation therapy under the guidance of physicians. Patients with anticoagulant use had significantly lower SSEs. Again, as in the total population, the incidence of NACEs was numerically lower in those patients, but this finding did not reach statistical significance. Interestingly, in both analyses, the same variables were independently associated with SSEs (history of stroke/transient ischemic attack, akinesis in apical segments, and anticoagulant use). It is also interesting that a history of stroke/transient ischemic attack was the most significant predictor of future SSEs, while the “protection” of anticoagulation was almost exactly the same (as adjusted HRs) in the two cohorts. This observation may not be surprising given the LVA triggers for LVT. So far, few clinical studies have investigated anticoagulation use for patients with LVA and associated SSEs3,8. However, none of these studies examined this association in patients without other indications for anticoagulant treatment. Our analysis showed a persistent association of the same variables including anticoagulation use with SSEs in various groups of patients with LVA, suggesting a true association between anticoagulation use and SSEs in patients with LVA.
Limitations
There are several limitations of our study. First, this study was limited by its retrospective nature, which may give rise to possible undetectable biases. The allocation of the patients to anticoagulation therapy was as per clinicians’ discretionary judgment, and the clinical characteristics of each treatment group were not the same at baseline. It is still possible that unmeasured confounders were not under control. Second, getting information electronic health records to capture events, while not uncommon nowadays, was an imperfect way to adjudicate follow-up events and may have some inherent biases. Using a keyword screen for the specific “left ventricular aneurysm” keyword may miss cases if the coding physician did not key this inaccurately. This may lead to an underestimation of cases. Third, echocardiography is widely used for LVA detection, but cardiac CT, cardiac MRI and echocardiography with ultrasound-enhancing agents may be potentially more sensitive modalities. Unfortunately, these tests are not available to most patients, which can result in LVA being overlooked during diagnosis. Fourth, the percentage of time in the therapeutic range for warfarin could not be determined accurately in this retrospective study. Fifth, the population of patients with LVA was enrolled over a period of more than two decades, during which diagnostic and therapeutic changes may have influenced the prognosis, including systemic embolic events. Sixth, our relatively small sample size could have reduced the statistical power to detect the effects of interest, consequently limiting the generalizability of the conclusions. Lastly, our results suggested anticoagulant use might be beneficial and improve outcomes in patients with LVA and apical segment akinesis. Given the complexity and diversity of anticoagulant strategies, it is difficult to achieve a “one-size-fits-all” anticoagulation approach, which implies variability in outcomes. It has come to our attention that a number of patients were given lower doses of rivaroxaban than the recommended amount. In Asian countries, safety concerns due to the generally low body mass index of the population and differential bleeding tendencies, have resulted in a prevalent preference for lower doses of NOACs. Similar to the J-ROCKET AF dosage, the strategy of using reduced-dose NOACs may provide comparable effects in stroke prevention while also reducing the potential risk of bleeding in the Asian population26. This may help explain the lack of statistical difference in bleeding events. Thus, anticoagulation strategies, including medication objects and dosages, need further study to prevent embolic events and improve clinical outcomes, especially in patients who also received anti-platelet therapy.
In this work, systemic embolic events are still common in patients with LVA. Anticoagulant use can reduce the incidence of systemic embolic events but may not be effective enough to reduce NACEs. Apical segment akinesis is associated with a higher embolic risk and the prognosis of these patients are significantly better during the clinical follow-up if they receive anticoagulant treatment. Strategies of anticoagulant use may be helpful in preventing systemic embolism and improving clinical outcomes in those people. The findings need to be validated in independent datasets.
Methods
Study design
The study complies with the Declaration of Helsinki and the study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University with a waiver of informed consent.
This was a retrospective study from an echocardiography database with 963,201 consecutive transthoracic echocardiograms (TTEs) acquired at the First Affiliated Hospital of Wenzhou Medical University from January 2001 to February 2023. A comprehensive computerized search of all TTE reports in the electronic medical records was conducted using the specific keyword “left ventricular aneurysm”. Initially, all patients with a reported LVA were screened. Patients with incomplete TTEs or poor image, without coronary artery disease, lack of data regarding medications at baseline and lack of data regarding follow-up were excluded from the study. Baseline data such as demographic features, past medical history, medications at discharge, echocardiographic evaluation and follow-up data were obtained from electronic medical records.
Data availability statement
Data supporting the findings of this study are available in the article, its Supplementary information, the source data file and from the corresponding author upon request. Individual participant data are protected by data privacy laws but may be accessed 6 months following publication. To request access, please contact corresponding authors (X-D Zhou, zhouxiaodong@wmu.edu.cn) and all requests will be evaluated within 1 month.
Study protocol
TTE images were analyzed for the presence of LVA. LVA was defined as a thin-walled LV segment that was bulging during both diastole and systole. Ischemic LVA was deemed certain when myocardial infarction had already been diagnosed or at the time of LVA diagnosis. LVT was defined as an echogenic mass adjacent to but distinguishable from the LV endocardium in at least two views. Each echocardiography was conducted and evaluated by two experienced readers with highly specialized expertise, with a minimum of 5 years of experience, and the reports were ultimately determined by consensus. Study coordinators performing the data collection were blinded to the detailed study design. The chief study staff responsible for outcome ascertainment and adjudication based on the clinical medical records and the statistician were masked to detailed study design before the planned analyses.
The patients’ medical records were reviewed retrospectively, including their inpatient and/or outpatient encounters documented in the electronic medical record. We followed the included patients until April 2023, and clinical outcomes at follow-up were then systematically analyzed for the prognosis of anticoagulant use. The follow-up was conducted through regular outpatient visits and phone interviews. Follow-up time was defined as the period from LVA diagnoses to the last clinical follow-up. The primary outcome was a composite of SSEs, including cerebrovascular accident (ischemic stroke or transient ischemic attack), and peripheral artery embolism (acute limb ischemia, renal infarction, splenic infarction, or mesenteric infarction). The secondary outcomes were a composite of NACEs, including all-cause death, SSEs and major bleeding. Major bleeding was defined according to the criteria of ISTH, which was either fatal or accompanied by any of the following criteria: (a) a hemoglobin level drop of 2 g/dL or greater, or documented use of at least 2 units of packed red blood cells, (b) bleeding in a critical anatomical location (intracranial, spinal, ocular, pericardial, articular, intramuscular with compartment syndrome, retroperitoneal)27. Anticoagulant use was based on current guidelines, with shared decision-making between the patient and their physician. Anticoagulants include warfarin and the novel oral anticoagulant (NOAC), such as rivaroxaban and dabigatran, which are available in China.
Statistical analysis
Normally distributed continuous variables were presented as mean ± standard deviation (SD) and non-normally distributed continuous variables were presented as median with IQR. Categorical variables were presented as the number (%) of patients. Comparison between the groups was performed by Student t-test (for normally distributed continuous variables), Mann–Whitney U-test (for non-normally distributed continuous variables) and the Chi-squared test (for categorical variables). Predictors of SSEs or NACEs events were evaluated using univariate and multivariable Cox regression analyses. Variables with a P-value of < 0.1 in the univariate Cox models were entered into the multivariable model. The final multivariable model was created using a forward stepwise method and included only variables significantly associated with SSEs or NACEs. The final model was tested for proportional hazard assumptions using Schoenfeld residuals. We also accounted for the competing risk of all-cause mortality, and competing risks regression was conducted using Fine and Gray’s model to estimate SHR with 95% CI. The cumulative NACEs rate was estimated by the Kaplan–Meier method. A two-tail P < 0.05 was considered statistically significant. All analyses were conducted on SPSS software (SPSS version 23.0 for Windows) and Stata software (Stata version 17.0 for Windows).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Damluji, A. A. et al. Mechanical complications of acute myocardial infarction: a scientific statement from the American Heart Association. Circulation 144, e16–e35 (2021).
Vallabhajosyula, S. et al. Temporal trends and outcomes of left ventricular aneurysm after acute myocardial infarction. Am. J. Cardiol. 133, 32–38 (2020).
You, J. et al. Predictors and long-term prognosis of left ventricular aneurysm in patients with acute anterior myocardial infarction treated with primary percutaneous coronary intervention in the contemporary era. J. Thorac. Dis. 13, 1706–1716 (2021).
Yu, P., Xi, P., Tang, Y., Xu, J. & Liu, Y. Novel analysis of coronary angiography in predicting the formation of ventricular aneurysm in patients with acute myocardial infarction after percutaneous coronary intervention. Front. Cardiovasc. Med. 9, 880289 (2022).
Murphy, J. G., Wright, R. S. & Barsness, G. W. Ischemic left ventricular aneurysm and anticoagulation: is it the clot or the plot that needs thinning? Mayo Clin. Proc. 90, 428–431 (2015).
O’Gara, P. T. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127, 529–555 (2013).
Levine, G. N. et al. Management of patients at risk for and with left ventricular thrombus: a scientific statement from the American Heart Association. Circulation 146, e205–e223 (2022).
Lee, G. et al. Anticoagulation in ischemic left ventricular aneurysm. Mayo Clin. Proc. 90, 441–449 (2015).
Camaj, A. et al. Left ventricular thrombus following acute myocardial infarction: JACC state-of-the-art review. J. Am. Coll. Cardiol. 79, 1010–1022 (2022).
Sumaya, W. & Storey, R. The challenges of antithrombotic therapy in patients with left ventricular thrombosis. Eur. Heart J. 39, 209–211 (2018).
Ebrahimi, M., Fazlinezhad, A., Alvandi-Azari, M. & Abdar Esfahani, M. Long-term clinical outcomes of the left ventricular thrombus in patients with ST elevation anterior myocardial infarction. ARYA Atherosclerosis 11, 1–4 (2015).
Massussi, M., Scotti, A., Lip, G. Y. H. & Proietti, R. Left ventricular thrombosis: new perspectives on an old problem. Eur. Heart J. Cardiovasc. Pharmacother. 7, 158–167 (2021).
Zhou, X. et al. The prevalence, predictors, and outcomes of spontaneous echocardiographic contrast or left ventricular thrombus in patients with HFrEF. ESC Heart Fail 8, 1284–1294 (2021).
Leow, A. S., Sia, C. H., Tan, B. Y., Chan, M. Y. & Loh, J. P. Long-term outcomes and recurrence of left ventricular thrombus after anticoagulation. J. Am. Coll. Cardiol. 76, 484–486 (2020).
Delewi, R., Zijlstra, F. & Piek, J. J. Left ventricular thrombus formation after acute myocardial infarction. Heart 98, 1743–1749 (2012).
Ibanez, B. et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39, 119–177 (2018).
Reeder, G. S. et al. Mural thrombus in left ventricular aneurysm: incidence, role of angiography, and relation between anticoagulation and embolization. Mayo Clin. Proc. 56, 77–81 (1981).
Vaitkus, P. T. & Barnathan, E. S. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J. Am. Coll. Cardiol. 22, 1004–1009 (1993).
Abdelnabi, M. et al. Comparative study of oral anticoagulation in left ventricular thrombi (No-LVT Trial). J. Am. Coll. Cardiol. 77, 1590–1592 (2021).
Zhang, Z. et al. Prophylactic rivaroxaban therapy for left ventricular thrombus after anterior ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 15, 861–872 (2022).
Cavender, M. & Eubanks, G. Preventing left ventricular thrombus formation: another reason to use very low-dose Rivaroxaban?. JACC Cardiovasc. Interv. 15, 873–875 (2022).
Yeung, W. et al. Predicting mortality, thrombus recurrence and persistence in patients with post-acute myocardial infarction left ventricular thrombus. J. Thromb. Thrombolysis 52, 654–661 (2021).
Zhou, X. D., Chen, Q. F. & Shan, P. Reply to the letter regarding the article ‘The prevalence, predictors, and outcomes of left ventricular thrombus in HFrEF’. ESC Heart Fail. https://doi.org/10.1002/ehf2.14941 (2024).
Yao, H., Chen, Q. F., Katsouras, C. S., Lu, Y. & Zhou, X. D. Clinical characteristics of left ventricular thrombus and the use of anticoagulants in patients with dilated cardiomyopathy and sinus rhythm. Eur. J. Intern. Med. 119, 146–148 (2024).
Zhou, X. D. et al. Clinical outcome after left ventricular thrombus resolution: who needs long-term or lifetime use of anticoagulants? J. Am. Heart Assoc. 12, e029070 (2023).
Hori, M. et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—the J-ROCKET AF study. Circ. J.: Off. J. Jpn. Circ. Soc. 76, 2104–2111 (2012).
Schulman, S. & Kearon, C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 3, 692–694 (2005).
Acknowledgements
This study was supported by grants from the Wenzhou Science Technology Bureau Foundation (Y20220081). X.-D. Zhou is supported in part by grants from the Wenzhou Science Technology Bureau Foundation (2022Y0726).
Author information
Authors and Affiliations
Contributions
Q.-F. Chen, C.S. Katsouras and X.-D. Zhou designed the study. M.G. Gong, C.Y. Liu, L.Y. Lian, X.Y. Chen, X.M. Zhu, and C. Chen collected data. Q.-F. Chen and L.G. Wang did the statistical analyses and prepared figures. Q.-F. Chen, C.S. Katsouras, X.F. Feng, W.-H. Lin and X.-D. Zhou reviewed the results, interpreted data, and wrote the manuscript. X.-D. Zhou supervised this study. All authors have made an intellectual contribution to the manuscript and approved the submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks C. H. Sia, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, QF., Wang, L., Katsouras, C.S. et al. Clinical characteristics and prognostic importance of anticoagulant use in ischemic left ventricular aneurysm: a retrospective cohort study. Nat Commun 15, 6883 (2024). https://doi.org/10.1038/s41467-024-51121-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-51121-2