Abstract
Palladium catalyzed tandem reaction represents a one-pot synthetic approach to efficiently synthesize complex functionalized molecules while reducing synthetic steps, aligning with the principles of green chemistry. However, achieving a direct cascade of the aza-Wacker and Povarov reactions in one-pot synthesis presents a challenge due to substrate compatibility issues between the two reactions. In this work, we describe an aza-Wacker/Povarov reaction employing a highly electrophilic palladium catalyst, which effectively converts anilines and 1,6-dienes into hexahydro-cyclopenta[b]quinolines. The optimized conditions yield up to 79%, with a diastereoselectivity > 20:1. Substrate range testing reveals compatibility with various sensitive functional groups, and successful late-stage modifications are performed on several natural products and drug molecules, demonstrating the versatility and practicality of the method. Additionally, a preliminary investigation into the reaction mechanism suggests an aza-Wacker process followed by a Povarov process.
Similar content being viewed by others
Introduction
Transition metal catalysis represents a well-established and continually advancing field of research, providing a robust platform for the incorporation of oxygen- or nitrogen-containing functional groups onto readily available substrates such as alkynes and alkenes1,2,3,4,5. This methodology enables the efficient synthesis of valuable, complex, functionalized molecules in a cost-effective and expeditious manner. There is a challenge to balance the requirement for atom utilization, step economy, the demand for lengthy synthetic routes, reaction selectivity, and yield in the synthesis of complex molecules. Arranging and combining existing elementary ingeniously reactions in one-pot synthesis presents an effective strategy that concurrently addresses the need for rapid synthesis of complex molecular skeletons with high yields by decreasing the number of synthesis steps6,7,8,9,10.
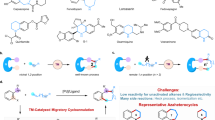
As a classic example of transition metal catalyzed reactions, the aza-Wacker reaction exhibits superior utility and versatility, particularly in the synthesis of natural products and bioactive compounds, with the Heck-type process typically serving as the primary subsequent transformation method (Fig. 1a)11,12,13,14,15,16,17. While the cascade cyclization reaction of dienes via an aminopalladation/Heck process has been reported18,19,20,21,22,23,24,25,26, further exploration is warranted to uncover more diverse subsequent reactions in series with the aza-Wacker process.
To expand upon our previous investigations involving anilines and alkenes27,28,29,30,31, we aimed to diversify the range of reaction modes following the aza-Wacker process in a one-pot synthesis. Povarov reaction, characterized by mild conditions, excellent diastereoselectivity, and robust reaction compatibility, utilizing anilines, aldehydes, and alkenes as starting materials that overlap with the substrates or products of the aza-Wacker reaction, emerged as a promising candidate for the subsequent step following the aza-Wacker process (Fig. 1b)32,33,34,35,36,37,38,39,40,41.
However, to our knowledge, the cascade of the aza-Wacker and Povarov reaction has yet to be demonstrated. Two prominent obstacles contribute to this absence: (I) While there is a degree of substrate overlap between the two reactions, the aniline substrates required for the Povarov reaction are incongruent with the amide (or sulfonamide) substrates typically employed in most aza-Wacker reactions42,43. As an amine source, anilines often prove incompatible with the aza-Wacker reaction, which may stem from factors such as the preferential binding of anilines to palladium over alkenes and the limited reactivity of unactivated terminal alkenes in reaction with anilines. (II) Through Markovnikov aminopalladation/β-H elimination, the aza-Wacker reaction commonly yields ketones, which are inconsistent with the aldehydes essential for the classic Povarov reaction. Examples of Povarov reaction featuring ketones rather than aldehydes as substrates remain rare44, as it typically involves fixed components of anilines, aldehydes, and alkenes45,46,47,48,49,50,51,52,53,54,55,56,57. Drawing upon our group’s extensive research on anilines and alkenes, we possess an inherent advantage in exploring the tandem of the two reactions.
While addressing the aforementioned challenges, it is pivotal for the success of the strategy to identify a suitable catalytic system that amalgamates the two classic reactions into a one-pot synthesis. We propose the utilization of highly electrophilic palladium as a viable solution, which not only facilitates the aza-Wacker process by activating olefins to promote subsequent nucleophilic attack but also facilitates the Povarov cyclization with palladium serves as a Lewis acid. Drawing from recent advances in diene cyclization58,59,60,61,62, we present a palladium-catalyzed reaction of anilines with 1,6-dienes to synthesize hexahydro-cyclopenta[b]quinolines framework. This framework is found in natural products such as isoschizogaline and isoschizogamine63, and the framework has been explored by several research groups44,64,65,66,67,68. This work seamlessly integrates two classic named reactions into one-pot, which is attributed to the utilization of a highly electrophilic palladium catalyst activated by NaBArF4 (Fig. 1c).
Results
Screening of reaction conditions
Initially, we anticipated the necessity of employing a highly electrophilic palladium catalyst for the aza-Wacker reaction involving anilines and terminal olefins69,70,71,72,73. Through screening various additives capable of activating the electrophilic activity of the palladium catalyst, NaBArF4 was identified as an effective activator74,75,76,77,78, leading to the desired reaction and the formation of the target product 3a (see Supplementary Table 1 for more details).
The bidentate nitrogen ligands emerged as the privileged ligands for this reaction. When plain bipyridine L4 and o-phenanthroline L8 were employed, the target product was obtained in 34% and 24% yields, respectively (Fig. 2, Entries 4 and 8). Sterically hindered ligand L9 did not work. To our delight, the use of L5 and L6, featuring electron-withdrawing groups, increased the yields to 54% and 55%, respectively (Fig. 2, Entries 5–6), presumably due to the favorable electronic matching between the electron-deficient bidentate nitrogen ligands and the highly electrophilic palladium salt79. More electron-deficient diazine bidentate nitrogen ligands were synthesized. As anticipated, both the bipyrimidine L7 and bipyrazine L1 successfully promoted the reaction, yielding the target products in 59% and 75% yields, respectively (Fig. 2, Entries 1 and 7). Interestingly, the more electron-deficient L2 or L3 did not work, resulting in trace product formation (Fig. 2, Entries 2–3). This observation suggests a potential failure of these ligands to form compatible complexes with the palladium catalyst, leading to a rapid deactivation of palladium. Further control experiments demonstrate the indispensability of PdCl2 catalyst, L1 ligand, NaBArF4 additive, and 2,5-DTBQ oxidant (Fig. 2, Entries 10–13). Additionally, the absence of Al2O3 was found to decrease the yield (Fig. 2, Entry 14), while the introduction of Lewis acids was not conducive to the reaction (see Supplementary Table 9 for more details). After a comprehensive screening process, the optimized reaction conditions are as follows: PdCl2 (10 mol%), bipyrazine (20 mol%), NaBArF4 (30 mol%), 2,5-di-tert-butyl-1,4-benzoquinone (1.5 equiv.), Al2O3 (300 mg/mmol) in DCM (0.1 M) at 70 °C under N2 for 72 h, affording an isolated yield of 79% with a diastereomeric ratio >20:1 (Fig. 2, Entry 1).
aReaction conditions: 1a (0.2 mmol), 2a (0.2 mmol), PdCl2(10 mol%), L1 (20 mol%), NaBArF4 (30 mol%), 2,5-DTBQ (1.5 equiv.), and Al2O3 (60 mg) in DCM (0.1 M) at 70 oC under N2 atmosphere for 72 h. bYield was determined by 1H NMR of the crude product using CH2Br2 as internal standard. cIsolated yield. ArF = 3,5-(CF3)2C6H3. 2,5-DTBQ = 2,5-di-tert-butylcyclohexa-2,5-diene-1,4-dione.
Substrate scopes
With the optimized reaction conditions established, we investigated the functional group compatibility and substrate scope of the reaction (Fig. 3). A range of para-substituted anilines readily underwent reaction to afford the corresponding products 3a–3h. Substrates, bearing easily removable functional groups, such as iodine atom 1j, proved unsuitable for this transformation. The electron-deficient aniline substrate 1i with trifluoromethyl-substituted exhibited poor reactivity (see Supplementary Table 10 for more incompatible substrates). The reaction exhibited insensitivity to meta-steric hindrance, leading to poor regioselectivity observed for the product of the meta-substituted anilines 3k–3m, 3z–3ag. Anilines bearing ortho-substitution furnished the corresponding target products 3n–3q, 3ah–3ai in moderate yields. Notably, the reaction displayed compatibility with several sensitive functional groups, such as cyclopropyl 3 s, alcohols 3t and 3w, Boc-protected alkylamine 3u, and cyano 3v. Additionally, various aromatic amine substrates participated in the reaction, yielding target products 3aj–3an with moderate to good yields, including 1-naphthylamine, 2-naphthylamine, benzo[b]thiophen-5-amine, dibenzo[b,d]thiophen-3-amine, and benzofuran-5-amine. Intriguingly, these aromatic amine substrates exhibited excellent regioselectivity, affording predominantly single compounds.
aStandard reaction conditions: 1 (0.2 mmol), 2a (0.2 mmol), PdCl2(10 mol%), L1 (20 mol%), NaBArF4 (30 mol%), 2,5-DTBQ (1.5 equiv.), and Al2O3 (60 mg) in DCM (0.1 M) at 70 oC under N2 atmosphere for 72 h. Yields refer to isolated yields of 3. Diastereoisomer rate (d.r.) and regioselectivity rate (r.r.) were determined by 1H NMR. bRegioselective product mixture, overall isolated yield. ArF = 3,5-(CF3)2C6H3.
To further demonstrate the robustness and compatibility of this reaction, late-stage functionalization modifications were conducted on a series of natural products and drug molecules. As expected, anilines derived from the monoterpenoids L-Menthol and L-Borneol, respectively, were efficiently post-modified, yielding the desired products 3ao and 3ap in good yields. Cholesterol is an important molecule in animal cells, and its derived aniline compound can also be successfully modified via this reaction to get 3au. Furthermore, the aniline derivative derived from Vitamin E, known for its susceptibility to oxidation as a plant-derived vitamin, underwent successful modification to yield the target products 3as, under the conditions of the palladium-catalyzed oxidative amination. Both Podophyllotoxin and Galactopyranose-derived anilines, characterized by their oxygen-rich molecular structures, yielded the target products 3aq (24% yield, >20:1 d.r.) and 3ar (29% yield, 6:1 d.r.) with acceptable yields and diastereoselectivity. Tigogenin possesses a spirocyclic structure and is renowned for its cytostatic activity80, whose aniline derivative underwent the reaction smoothly to form product 3at with a yield of 56% and a d.r. value of 11:1.
Substrates on the diene were explored and evaluated in Fig. 4. Compound 2av, featuring a methyl substituent at the ortho-position, and compound 2ay, substituted with a phenyl group at the para-position, underwent the reaction to afford the target molecule 3av and 3ay with good yield and excellent diastereoselectivity. Compound 2ax, featuring a chlorine atom at the ortho-position, underwent the reaction to afford the target molecule 3ax with an acceptable yield and high diastereoselectivity. When the reaction temperature is raised to 80 °C, substrates 3aw, containing an ortho-methoxy group, and 3az, containing a para-trifluoromethyl group, can be separated with moderate yield. It is worth noting that substrates bearing other aromatic substituents are also suitable for this system. For instance, diene substrates substituted with 2-naphthalene or 1-naphthalene yielded the target products 3ba (74% yield, >20:1 d.r.) and 3bb (75% yield, >20:1 d.r.) in good yields and excellent diastereoselectivity respectively. Electron-rich thiophene-substituted dienes can also provide the target product 3bc in acceptable yields. Despite testing diene substrates with different carbon chain lengths, only 1,6-diene and 1,7-diene were suitable for this reaction. The 1,7-diene substrate afforded octahydroacridine 3bd in 30% yield with >20:1 diastereoselectivity.
aStandard reaction conditions: 1b (0.2 mmol), 2 (0.2 mmol), PdCl2(10 mol%), L1 (20 mol%), NaBArF4 (30 mol%), 2,5-DTBQ (1.5 equiv.), and Al2O3 (60 mg) in DCM (0.1 M) at 70 °C under N2 atmosphere for 72 h. Yields refer to isolated yields of 3. Diastereoisomer rate (d.r.) and regioselectivity rate (r.r.) were determined by 1H NMR. bat 80 °C. ArF = 3,5-(CF3)2C6H3.
Synthetic applications
We conducted several synthetic applications using the obtained products (Fig. 5). Initially, the standard reaction was upscaled, yielding 1.10 g of product 3b at a scale of 5 mmol with an isolated yield of 56%. Subsequently, 3b was hydrolyzed with LiOH to afford the corresponding crude diacid product 3be-1, which was further benzyl-modified with benzyl bromide to produce product 3be in a 64% yield over two steps. The trifluoroformylation of 3a yielded the product 3bf, which formed a beautiful monoclinic crystal. The skeleton structure and relative configuration of the three diastereomeric centers were determined via X-ray single-crystal diffraction. Reduction of 3b using lithium aluminum hydride provided the corresponding diol product 3bg in a 79% yield. Subsequent modification of the diol with TBSCl and 2,2-dimethoxypropane afforded products 3bh and 3bi, respectively, in yields exceeding 90%.
Mechanistic investigations
To elucidate the mechanism of the reaction, a series of controlled experiments were designed. In the absence of palladium catalysis, the reaction failed to proceed, with both the aniline and diene almost completely recovered (Fig. 6a). Ketone 4 was introduced into the standard reaction instead of the diene, yielding the corresponding product 3b with a satisfactory yield of 73%. However, the yield drastically decreased in the absence of a palladium catalyst. By comparing Fig. 6a and b, palladium not only catalyzed the aza-Wacker process but also acted as a Lewis acid to promote the cyclization process. The intermediate 4 cannot be obtained without the presence of aniline (Fig. 6c), which excludes the possibility of a Wacker reaction between the small amounts of H2O that may be present in the solvent and the olefin.
a Control experiments with or without palladium catalyst. Standard conditions are the same as those listed in Fig. 2. b Control experiments of intermediate 4 with or without palladium catalyst. c Control experiments with or without 1b. d Control experiments under different equivalent ratios of NaBArF4 and PdCl2. The product yields shown in parentheses were obtained under the following reaction conditions: PdCl2(5 mol%), L1 (20 mol%), NaBArF4 (10 mol%).
Presumably, the nature of this reaction is associated with the activating effect of NaBArF4 on palladium. By fixing the amount of palladium catalyst at 10 mol% and varying the quantity of NaBArF4, it was observed that the reaction failed to proceed in the absence of NaBArF4. Additionally, when the ratio of NaBArF4/PdCl2 equaled 1.0, the reaction yield was low, which resembled that obtained using 5 mol% PdCl2/10 mol% NaBArF4. (Fig. 6d). We hypothesized that under the conditions of 10 mol% PdCl2/10 mol% NaBArF4, only half of the PdCl2 is activated by NaBArF4 and functions as a catalyst. This suggests that two molecules of NaBArF4 react with one molecule of a palladium catalyst, liberating two molecules of NaCl to form LnPd(BArF4)2 active catalytic species.
A series of deuteration experiments are as follows. Surprisingly, when 1b was subjected to standard conditions containing D2O (Fig. 7a), the recovered d-1b were found to be deuterated at the ortho and para positions of the phenyl ring, indicating that anilines are susceptible to Friedel–Crafts attack by certain electrophiles under the reaction conditions. Upon addition of D2O (10 equiv.) to the standard reaction conditions, the resulting product d-3b-1 exhibited deuterium incorporation into four positions a, b, c and d (Fig. 7b). The incorporation of deuterium at a, b positions may originate from the production of d-1b, while the incorporation of c, d positions suggests an interaction between D2O and intermediate 4 (keto-enol tautomerism introduces deuterium atoms).
a Aniline is deuterated under standard conditions. Standard conditions are the same as those listed in Fig. 2. b Control experiments with D2O under standard conditions. c 3b is deuterated under standard conditions. d Intermolecular KIE studies give a KIE value of 1.0.
Further exploration of the deuteration mechanism was conducted by subjecting 3b to standard conditions containing D2O (Fig. 7c). It was found that the recovered d-3b-2 was only deuterated at the a, b positions, while no deuterium atoms were incorporated into the c, d positions. This shows that the Povarov cyclization process is not a reversible process. Otherwise, the intermediate 4 generated by the reversible process will be incorporated with deuterium to obtain d-4’, and then deuterium atoms will be introduced into the c, d positions of the product. Finally, equimolar amounts of aniline and 5d-aniline were subjected to standard conditions to conduct intermolecular KIE experiments, yielding product d-3b-3 (Fig. 7d). The calculated KIE value is 1.0, indicating that the rate-determining step of this reaction does not involve the removal of the ortho-position hydrogen of aniline.
Based on these mechanistic insights, the proposed mechanism unfolds as follows (Fig. 8): initially, the palladium catalyst engages with NaBArF4 and ligand to generate active catalytic species. Following this, species I, formed upon coordination of the palladium species with the diene, undergoes nucleophilic attack by aniline to yield intermediate II. Subsequently, β-H elimination occurs, leading to isomerization and the formation of intermediate IV. The released Pd-H is oxidized to regenerate PdII species. PdII species act as a Lewis acid, facilitating the intramolecular Mannich reaction and subsequent ring closure to furnish intermediate VI. The resulting benzylic carbocation undergoes intramolecular Friedel-Crafts alkylation to afford intermediate VII, which is subsequently deprotonated to yield the target product 3.
Discussion
In conclusion, this study reports an aza-Wacker/Povarov reaction of anilines with 1,6-dienes to obtain the hexahydro-cyclopenta[b]quinolines in moderate to good yields and high diastereoselectivity. Through an investigation into ligand principles and additive screening, we established an efficient reaction system and conducted a preliminary exploration of the reaction mechanism, offering insights into its understanding. The more detailed mechanism awaits further research. Furthermore, we demonstrate the broad applicability of this reaction to diverse substrates, including late-stage functionalization on various natural products and drug molecules. These findings provide robust support for the utilization of this reaction in organic synthesis and offer avenues for further exploration in related fields.
Methods
General procedure for palladium-catalyzed aza-Wacker/Povarov reaction of aryl amines and dienes
To a 25 mL dried Schlenk tube equipped with a magnetic stir bar was added PdCl2 (3.5 mg, 10 mol%), 2,2’-bipyrazine L1 (6.2 mg, 20 mol%), NaBArF4 (53.2 mg, 30 mol%), Al2O3 (60 mg), 2,5-di-tert-butyl-1,4-benzoquinone (66.1 mg, 1.5 equiv.), substituted aniline 1 (0.2 mmol), substituted diene 2 (1.0 equiv.) and DCM (2.0 mL). Under an ice bath, the tube was evacuated and backfilled with N2 three times, after which the tube was sealed and stirred under 70 °C for 72 h. After completion, the mixture was diluted with ethyl acetate and passed through a short pad of Celite. The filtrate was collected. After the removal of the solvent under reduced pressure, the residue was analyzed by 1H NMR to record the dr ratio and rr ratio in CDCl3. The residue was purified by preparative thin-layer chromatography using petroleum ether and ethyl acetate as eluent to provide the analytically pure product. If necessary, about 1 equiv. of TsOH can be added to the crude product before thin layer chromatography purification to combine with incompletely reacted aniline to help purify the target product.
Data availability
Data related to materials and methods, optimization of conditions, experimental procedures, mechanistic experiments, and spectra are provided in the Supplementary Information. All data are available from the corresponding authors upon request. Crystallographic data for the structures of 3bf reported in this paper have been deposited at the Cambridge Crystallographic Data Center under deposition numbers CCDC 2346858 (3bf). Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of the study, including experimental procedures and compound characterization, are available within the article and its Supplementary Information, or from the corresponding author upon request. Coordinates of the optimized structures are provided in the source data file. Source data are provided in this paper.
References
Nájera, C., Beletskaya, I. P. & Yus, M. Metal-catalyzed regiodivergent organic reactions. Chem. Soc. Rev. 48, 4515–4618 (2019).
Neto, J. S. S. & Zeni, G. Transition metal-catalyzed and metal-free cyclization reactions of alkynes with nitrogen-containing substrates: synthesis of pyrrole derivatives. ChemCatChem 12, 3335–3408 (2020).
Hemric, B. N. Beyond osmium: progress in 1,2-amino oxygenation of alkenes, 1,3-dienes, alkynes, and allenes. Org. Biomol. Chem. 19, 46–81 (2021).
Wu, Z., Hu, M., Li, J., Wu, W. & Jiang, H. Recent advances in aminative difunctionalization of alkenes. Org. Biomol. Chem. 19, 3036–3054 (2021).
Zeng, Z., Gao, H., Zhou, Z. & Yi, W. Intermolecular redox-neutral carboamination of C–C multiple bonds initiated by transition-metal-catalyzed C–H activation. ACS Catal. 12, 14754–14772 (2022).
Volla, C. M. R., Atodiresei, I. & Rueping, M. Catalytic C–C bond-forming multi-component cascade or domino reactions: pushing the boundaries of complexity in asymmetric organocatalysis. Chem. Rev. 114, 2390–2431 (2014).
Kashinath, K. & Srinivasa Reddy, D. One-pot quadruple/triple reaction sequence: a useful tool for the synthesis of natural products. Org. Biomol. Chem. 13, 970–973 (2015).
Afewerki, S. & Córdova, A. Combinations of aminocatalysts and metal catalysts: a powerful cooperative approach in selective organic synthesis. Chem. Rev. 116, 13512–13570 (2016).
Heravi, M. M., Zadsirjan, V., Dehghani, M. & Ahmadi, T. Towards click chemistry: multicomponent reactions via combinations of name reactions. Tetrahedron 74, 3391–3457 (2018).
Döndaş, H. A., Retamosa, Md. G. & Sansano, J. M. Recent development in palladium-catalyzed domino reactions: access to materials and biologically important carbo- and heterocycles. Organometallics 38, 1828–1867 (2019).
Kotov, V., Scarborough, C. C. & Stahl, S. S. Palladium-catalyzed aerobic oxidative amination of alkenes: development of intra- and intermolecular aza-Wacker reactions. Inorg. Chem. 46, 1910–1923 (2007).
McDonald, R. I., Liu, G. & Stahl, S. S. Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 111, 2981–3019 (2011).
Thomas, A. A., Nagamalla, S. & Sathyamoorthi, S. Salient features of the aza-Wacker cyclization reaction. Chem. Sci. 11, 8073–8088 (2020).
Shinde, A. H. & Sathyamoorthi, S. Oxidative cyclization of sulfamates onto pendant alkenes. Org. Lett. 22, 896–901 (2020).
Fernandes, R. A. & Gangani, A. J. Palladium-catalyzed oxidant dependent switchable aza-Wacker cyclization and oxidative dimerization of benzimidates. Org. Lett. 24, 7400–7404 (2022).
Paul, D., Mague, J. T. & Sathyamoorthi, S. Sulfamate-tethered aza-Wacker cyclization strategy for the syntheses of 2-amino-2-deoxyhexoses: preparation of orthogonally protected d-galactosamines. J. Org. Chem. 88, 1445–1456 (2023).
Mandal, G. H. & Sathyamoorthi, S. Sulfamate-tethered aza-Wacker strategy for a kasugamine synthon. J. Org. Chem. 89, 793–797 (2024).
Yip, K.-T., Yang, M., Law, K.-L., Zhu, N.-Y. & Yang, D. Pd(II)-catalyzed enantioselective oxidative tandem cyclization reactions. Synthesis of indolines through C−N and C−C bond formation. J. Am. Chem. Soc. 128, 3130–3131 (2006).
He, W., Yip, K.-T., Zhu, N.-Y. & Yang, D. Pd(II)/tBu-quinolineoxazoline: an air-stable and modular chiral catalyst system for enantioselective oxidative cascade cyclization. Org. Lett. 11, 5626–5628 (2009).
Yip, K.-T., Zhu, N.-Y. & Yang, D. Palladium-catalyzed highly diastereoselective oxidative cascade cyclization reactions. Org. Lett. 11, 1911–1914 (2009).
Ramalingan, C., Takenaka, K. & Sasai, H. Pd(II)-SPRIX catalyzed enantioselective construction of pyrrolizines/pyrroloindoles employing molecular oxygen as the sole oxidant. Tetrahedron 67, 2889–2894 (2011).
He, Y.-P., Wu, H., Xu, L., Su, Y.-L. & Gong, L.-Z. Highly enantioselective oxidative tandem cyclization reaction: a chiral ligand and an anion cooperatively control stereoselectivity. Org. Chem. Front. 1, 473–476 (2014).
Du, W., Gu, Q., Li, Y., Lin, Z. & Yang, D. Enantioselective palladium-catalyzed oxidative cascade cyclization of aliphatic alkenyl amides. Org. Lett. 19, 316–319 (2017).
Gu, Q.-S. & Yang, D. Enantioselective synthesis of (+)-mitomycin K by a palladium-catalyzed oxidative tandem cyclization. Angew. Chem. Int. Ed. 56, 5886–5889 (2017).
Ye, C. et al. PdII-catalyzed oxidative tandem aza-Wacker/Heck cyclization for the construction of fused 5,6-bicyclic N,O-heterocycles. Chem.: Asian J. 13, 1897–1901 (2018).
Tian, Q., Liu, Y., Wang, X., Wang, X. & He, W. PdII/novel chiral cinchona alkaloid oxazoline-catalyzed enantioselective oxidative cyclization of aromatic alkenyl amides. Eur. J. Org. Chem. 2019, 3850–3855 (2019).
Ji, X., Huang, H., Wu, W. & Jiang, H. Palladium-catalyzed intermolecular dehydrogenative aminohalogenation of alkenes under molecular oxygen: an approach to brominated enamines. J. Am. Chem. Soc. 135, 5286–5289 (2013).
Ouyang, L. et al. Access to α-amino acid esters through palladium-catalyzed oxidative amination of vinyl ethers with hydrogen peroxide as the oxidant and oxygen source. Angew. Chem. Int. Ed. 56, 15926–15930 (2017).
Liu, C. et al. Palladium-catalyzed cascade cyclization for the synthesis of fused benzo-aza-oxa-[5-6-5] tetracycles. Angew. Chem. Int. Ed. 61, e202215020 (2022).
Liu, C. et al. Palladium-catalyzed 1,1-oxamidation and 1,1-diamination of unactivated alkenyl carbonyl compounds. Org. Lett. 25, 2701–2706 (2023).
Liu, C. et al. Access to amino lactones through palladium-catalyzed oxyamination with aromatic amines as the nitrogen source. ACS Catal. 13, 11339–11344 (2023).
Akiyama, T., Morita, H. & Fuchibe, K. Chiral brønsted acid-catalyzed inverse electron-demand aza Diels−Alder reaction. J. Am. Chem. Soc. 128, 13070–13071 (2006).
Xie, M. et al. Asymmetric three-component inverse electron-demand aza-Diels–Alder reaction: efficient synthesis of ring-fused tetrahydroquinolines. Angew. Chem. Int. Ed. 49, 3799–3802 (2010).
Dagousset, G., Zhu, J. & Masson, G. Chiral phosphoric acid-catalyzed enantioselective three-component povarov reaction using enecarbamates as dienophiles: highly diastereo- and enantioselective synthesis of substituted 4-aminotetrahydroquinolines. J. Am. Chem. Soc. 133, 14804–14813 (2011).
Chen, Z., Wang, B., Wang, Z., Zhu, G. & Sun, J. Complex bioactive alkaloid-type polycycles through efficient catalytic asymmetric multicomponent aza-Diels–Alder reaction of indoles with oxetane as directing group. Angew. Chem. Int. Ed. 52, 2027–2031 (2013).
Yu, J., Jiang, H.-J., Zhou, Y., Luo, S.-W. & Gong, L.-Z. Sodium salts of anionic chiral cobalt(III) complexes as catalysts of the enantioselective Povarov reaction. Angew. Chem. Int. Ed. 54, 11209–11213 (2015).
Leitch, J. A. et al. Photocatalytic reverse polarity Povarov reaction. Chem. Sci. 9, 6653–6658 (2018).
Bisag, G. D. et al. Central-to-axial chirality conversion approach designed on organocatalytic enantioselective povarov cycloadditions: first access to configurationally stable indole–quinoline atropisomers. Chem. Eur. J. 25, 15694–15701 (2019).
Wang, S.-J., Wang, Z., Tang, Y., Chen, J. & Zhou, L. Asymmetric synthesis of quinoline-naphthalene atropisomers by central-to-axial chirality conversion. Org. Lett. 22, 8894–8898 (2020).
Wu, F. et al. Rapid synthesis of luotonin a derivatives via synergistic visible-light photoredox and acid catalysis. J. Org. Chem. 87, 1302–1312 (2022).
Li, C. et al. Enantioselective synthesis of chiral quinohelicenes through sequential organocatalyzed Povarov reaction and oxidative aromatization. Nat. Commun. 14, 3380 (2023).
Ma, S., Fan, H., Day, C. S., Xi, Y. & Hartwig, J. F. Remote hydroamination of disubstituted alkenes by a combination of isomerization and regioselective N–H addition. J. Am. Chem. Soc. 145, 3875–3881 (2023).
Ma, S. & Hartwig, J. F. Progression of hydroamination catalyzed by late transition-metal complexes from activated to unactivated alkenes. Acc. Chem. Res. 56, 1565–1577 (2023).
Chen, S.-Y. et al. Polycyclization enabled by relay catalysis: one-pot manganese-catalyzed C−H allylation and silver-catalyzed Povarov reaction. ChemSusChem 10, 2360–2364 (2017).
Hu, X.-Y., Zhang, J.-C., Wei, W. & Ji, J.-X. Brønsted acid (HNO3)-catalyzed tandem reaction of α-ketoesters and arylamines: efficient synthesis of 1,2-dihydroquinoline derivatives. Tetrahedron Lett. 52, 2903–2905 (2011).
Zhang, J.-C. & Ji, J.-X. Highly efficient synthesis of polysubstituted 1,2-dihydroquinolines via tandem reaction of α-ketoesters and arylamines catalyzed by indium triflate. ACS Catal. 1, 1360–1363 (2011).
Gutiérrez, R. U. et al. Regioselective synthesis of 1,2-dihydroquinolines by a solvent-free MgBr2-catalyzed multicomponent. React. J. Org. Chem. 78, 9614–9626 (2013).
Luo, C. & Huang, Y. A highly diastereo- and enantioselective synthesis of tetrahydroquinolines: quaternary stereogenic center inversion and functionalization. J. Am. Chem. Soc. 135, 8193–8196 (2013).
Gao, Q., Liu, S., Wu, X., Zhang, J. & Wu, A. Coproduct promoted povarov reaction: synthesis of substituted quinolines from methyl ketones, arylamines, and α-ketoesters. J. Org. Chem. 80, 5984–5991 (2015).
Li, G. et al. Enantioselective organocatalytic transfer hydrogenation of 1,2-dihydroquinoline through formation of aza-o-xylylene. Org. Lett. 17, 4125–4127 (2015).
Huang, J., Li, G., Yang, G., Zhao, J. & Tang, Z. Brønsted-acid-catalyzed substrate-controlled and site-selective friedel–crafts alkylation: a new strategy for post-modification of 1,2-dihydroquinolines. Asian J. Org. Chem. 6, 1741–1744 (2017).
El-Harairy, A. et al. A sulfone-containing imidazolium-based brønsted acid ionic liquid catalyst enables replacing dipolar aprotic solvents with butyl acetate. Adv. Synth. Catal. 361, 3342–3350 (2019).
Su, L.-L. et al. Photocatalytic synthesis of quinolines via Povarov reaction under oxidant-free conditions. Org. Lett. 24, 1180–1185 (2022).
Clerigué, J., Ramos, M. T. & Menéndez, J. C. Enantioselective catalytic Povarov reactions. Org. Biomol. Chem. 20, 1550–1581 (2022).
Ren, X.-R. et al. Constructing stable chromenoquinoline-based covalent organic frameworks via intramolecular Povarov reaction. J. Am. Chem. Soc. 144, 2488–2494 (2022).
Masdeu, C., de los Santos, J. M., Palacios, F. & Alonso, C. The intramolecular Povarov tool in the construction of fused nitrogen-containing heterocycles. Top. Curr. Chem. 381, 20 (2023).
Martín-Encinas, E., Lopez-Aguileta, L., Palacios, F. & Alonso, C. Aza-Povarov reaction. A method for the synthesis of fused tetracyclic chromeno[4,3-d]pyrido[1,2-a]pyrimidines. J. Org. Chem. 89, 1099–1107 (2024).
Zhang, D., Liu, J., Córdova, A. & Liao, W.-W. Recent developments in palladium-catalyzed oxidative cascade carbocyclization. ACS Catal. 7, 7051–7063 (2017).
Kanno, S., Kakiuchi, F. & Kochi, T. Palladium-catalyzed remote diborylative cyclization of dienes with diborons via chain walking. J. Am. Chem. Soc. 143, 19275–19281 (2021).
Mondal, S., Ballav, T., Biswas, K., Ghosh, S. & Ganesh, V. Exploiting the versatility of palladium catalysis: a modern toolbox for cascade reactions. Eur. J. Org. Chem. 2021, 4566–4602 (2021).
Tanaka, K., Hattori, H., Yabe, R. & Nishimura, T. Ir-catalyzed cyclization of α,ω-dienes with an N-methyl group via two C–H activation steps. Chem. Commun. 58, 5371–5374 (2022).
Li, F. et al. Photosensitization enables Pauson-Khand–type reactions with nitrenes. Science 383, 498–503 (2024).
Kariba, R. M., Houghton, P. J. & Yenesew, A. Antimicrobial activities of a new schizozygane indoline alkaloid from Schizozygia coffaeoides and the revised structure of isoschizogaline. J. Nat. Products 65, 566–569 (2002).
Jensen, K. L., Dickmeiss, G., Donslund, B. S., Poulsen, P. H. & Jørgensen, K. A. Asymmetric organocatalytic synthesis of complex cyclopenta[b]quinoline derivatives. Org. Lett. 13, 3678–3681 (2011).
Maitland, J. A. P. et al. Switchable, reagent-controlled diastereodivergent photocatalytic carbocyclisation of imine-derived α-amino radicals. Angew. Chem. Int. Ed. 60, 24116–24123 (2021).
Magomedov, N. A. Efficient construction of cyclopenta[b]quinoline core of isoschizozygane alkaloids via intramolecular formal hetero-Diels−Alder reaction. Org. Lett. 5, 2509–2512 (2003).
Huang, H. & Hu, W. An efficient route for the construction of cyclopenta[b]quinoline derivatives via intramolecular cyclopropanation. Tetrahedron 63, 11850–11855 (2007).
Bunce, R. A., Nammalwar, B. & Slaughter, L. M. Divergent reactivity in tandem reduction–Michael ring closures of five- and six-membered cyclic enones. J. Heterocycl. Chem. 46, 854–860 (2009).
Müller, T. E. & Beller, M. Metal-initiated amination of alkenes and alkynes. Chem. Rev. 98, 675–704 (1998).
Hahn, C., Vitagliano, A., Giordano, F. & Taube, R. Coordination of olefins and N-donor ligands at the fragment [2,6-bis((diphenylphosphino)methyl)pyridine]-palladium(II). Synthesis, structure, and amination of the new dicationic complexes [Pd(PNP)(CH2CHR)](BF4)2 (R = H, Ph). Organometallics 17, 2060–2066 (1998).
Hahn, C., Morvillo, P. & Vitagliano, A. Olefins coordinated at a highly electrophilic site − dicationic palladium(II) complexes and their equilibrium reactions with nucleophiles. Eur. J. Inorg. Chem. 2001, 419–429 (2001).
Michael, F. E. & Cochran, B. M. Room temperature palladium-catalyzed intramolecular hydroamination of unactivated alkenes. J. Am. Chem. Soc. 128, 4246–4247 (2006).
Cochran, B. M. & Michael, F. E. Mechanistic studies of a palladium-catalyzed intramolecular hydroamination of unactivated alkenes: protonolysis of a stable palladium alkyl complex is the turnover-limiting step. J. Am. Chem. Soc. 130, 2786–2792 (2008).
Brookhart, M., Grant, B. & Volpe, A. F.Jr. [(3,5-(CF3)2C6H3)4B]-[H(OEt2)2]+: a convenient reagent for generation and stabilization of cationic, highly electrophilic organometallic complexes. Organometallics 11, 3920–3922 (1992).
Krossing, I. & Raabe, I. Noncoordinating anions—fact or fiction? A survey of likely candidates. Angew. Chem. Int. Ed. 43, 2066–2090 (2004).
Yakelis, N. A. & Bergman, R. G. Safe preparation and purification of sodium tetrakis[(3,5-trifluoromethyl)phenyl]borate (NaBArF24): reliable and sensitive analysis of water in solutions of fluorinated tetraarylborates. Organometallics 24, 3579–3581 (2005).
Wozniak, D. I. et al. Comparing interactions of a three-coordinate Pd cation with common weakly coordinating anions. Organometallics 37, 2376–2385 (2018).
Alvarez, S. Coordinating ability of anions, solvents, amino acids, and gases towards alkaline and alkaline-earth elements, transition metals, and lanthanides. Chem. Eur. J. 26, 4350–4377 (2020).
Doll, J. S., Becker, F. J. & Roşca, D.-A. Diazines and triazines as building blocks in ligands for metal-mediated catalytic transformations. ACS Org. Inorg. Au 4, 41–58 (2024).
Zhou, H., Yang, X., Wang, N., Zhang, Y. & Cai, G. Tigogenin inhibits adipocytic differentiation and induces osteoblastic differentiation in mouse bone marrow stromal cells. Mol. Cell. Endocrinol. 270, 17–22 (2007).
Acknowledgements
We would like to thank the National Natural Science Foundation of China (22231002 and 21871095) for the financial support.
Author information
Authors and Affiliations
Contributions
Huanfeng Jiang and Wanqing Wu designed the project and supervised the work. Jiahao Wu designed the project, performed the experiments, analyzed the data, and wrote the paper. Xiangwen Tan supervised the experiments, analyzed the data, and revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, J., Tan, X., Wu, W. et al. Palladium-catalyzed cascade of aza-Wacker and Povarov reactions of aryl amines and 1,6-dienes for hexahydro-cyclopenta[b]quinoline framework. Nat Commun 15, 6776 (2024). https://doi.org/10.1038/s41467-024-51173-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-51173-4