Abstract
Late-stage specific and selective diversifications of peptides and proteins performed at target residues under ambient conditions are recognized to be the most facile route to various and abundant conjugates. Herein, we report an orthogonal modification of cysteine residues using alkyl thianthreium salts, which proceeds with excellent chemoselectivity and compatibility under mild conditions, introducing a diverse array of functional structures. Crucially, multifaceted bioconjugation is achieved through clickable handles to incorporate structurally diverse functional molecules. This “two steps, one pot” bioconjugation method is successfully applied to label bovine serum albumin. Therefore, our technique is a versatile and powerful tool for late-stage orthogonal bioconjugation.
Similar content being viewed by others
Introduction
Bioorthogonal chemistry rendered a general and practical approach to access the selective modification of biological species under mild physiological conditions. Due to its widespread use and impact, bioorthogonal chemistry, together with click chemistry was recognized with the awarding of the 2022 Nobel Prize in chemistry1,2,3,4,5,6,7,8,9. However, clickable handles such as alkynyl, azide, tetrazine, and alkenyl, are barely found in native biomolecules. Introducing these structures is a challenging task due to the vast complexity of biomolecules. Thus, bioconjugations leveraging the in situ orthogonal reactivities of biomolecules that are structurally and functionally diverse and compatible with various functional groups are of great significance and highly desirable.
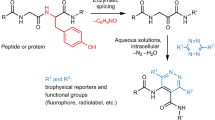
Proteins and peptides are extremely important biological species that participate in vast biological processes and have fascinated the chemical, biological, pharmaceutical, and medicinal communities. Focusing on 20 proteinogenic amino acids, orthogonal chemical modifications of peptides and proteins have recently showcased an enormous potential in drug discovery, proteomic profiling, and clinical diagnostics, and flourished the development of peptide and/or protein engineering10,11,12. Particularly, late-stage modification over de novo synthesis of peptides and/or proteins is wonderfully beneficial for the ideal bioconjugation (Fig. 1A). However, endogenous amino acid residues are mostly nucleophilic residues that pose a formidable task of selectivity when a chemical reaction is introduced to engineer complex peptides and proteins. In this regard, achieving excellent and exquisite selectivity is important for the generation of homogeneous products that neither interact nor interfere with the other residues. Moreover, the inherent sensitive structure of complex biomolecules is extremely susceptible to harsh conditions, such as high temperatures, acidic or basic pH, and metal catalysts. To selectively and compatibly modify peptides and proteins, an orthogonal chemical reaction must be (1) selective toward other potential reactive functional groups, (2) proceed with very fast reaction rates, (3) under physiological pH, and (4) at room temperature or 37 °C. Therefore, with the most ideal chemical bioconjugations, catalysts or additives are not needed, and the reaction components are just mixed into physiological media (Fig. 1B).
Among the canonical amino acids, cysteine (Cys) sharing lower abundance and possessing a nucleophilic thiol group, catches most of the eyes for peptide/protein engineering. Cysteine can undergo alkylation with alkyl halides, alkynylation and alkenylation with ethynyl benziodoxolone, arylation with aryl palladium(II) complexes, or perfluoroaromatic species, Michael addition with α,β-unsaturated carbonyl compounds, and disulfide exchange to form mixed disulfides13,14,15,16,17,18,19,20,21,22. The kaleidoscope of reactivity enabled cysteine as the most common target of interest for diverse applications in protein labeling23, antibody-drug conjugates24,25, peptide-drug conjugates26, and native chemical ligation27,28. Despite significant progress within this field, challenges persist in terms of chemoselectivity, wide scope, fast timescale, and mild reaction conditions, as a result of the inherent structural complexity of peptides and proteins. As part of our ongoing interest in peptide engineering29,30,31,32,33,34,35,36,37, we have been searching for a suitable couple-partner to meet the fast, selective, and compatible bioconjugation requirements. Through massive screening, we found that the synthetically useful thianthrenium salts (TT+) might undergo this selective cross-coupling reaction. Thianthrenium salts have recently attracted considerable attention due to their high reactivity and diversity. Ritter and coworkers reported a highly selective C–H thianthrenation reaction to access aryl and alkenyl thianthrenium salts, which could engage in different transformations and showed large potential in biomolecular modifications38,39,40,41,42,43,44,45,46. Shi and coworkers also developed alkyl thianthrenium salts for diverse cross-coupling reactions47,48. To modify biomolecules, during our submission of this work, Ritter and coworkers described a chemoselective umpolung of thiol to episulfoniums for cysteine bioconjugation through conjugate addition between thiol and vinyl thianthrenium tetrafluoroborate or vinyl tetrafluorothianthrenium tetrafluoroborate followed by a nucleophilic ring opening49.
In this work, we disclose that thianthrenium salts could directly and selectively react with cysteine residues via an SN2 reaction to achieve the orthogonal chemical modification under mild conditions (Fig. 1C). The method proceeds with high chemoselectivity and compatibility, introducing various functional structures, including PEG chains, fluorescent molecules, drugs, carboranes, carbohydrates, and peptides. Additionally, multifaceted bioconjugation is achieved through clickable handles to incorporate structurally diverse functional molecules. Our method is also successfully applied to label bovine serum albumin (BSA).
Results and discussion
To verify our assumption, glutathione and thianthrenium salt 2a were initially chosen as model substrates to evaluate and optimize the reaction conditions (Table 1). The reaction was carried out in a mixed solvent without the need for a catalyst or additive. Organic solvents were used to enhance the solubility of thianthrenium salt. Compared with other solvents, DMSO showed higher conversion and isolated yield (Table 1, entries 1–6), albeit with potential disulfide formation by DMSO oxidation50. Different buffers and diverse pH values were also examined to increase the isolated yield (Table 1, entries 7–19). It was found that Tris-HCl buffer (pH 9.0) generated the desired product 3a in excellent yield and excellent chemoselectivity, even in the presence of free –NH2 and –COOH (Table 1, entry 18). Notably, this method shows great superiority to its previous cysteine alkylation counterparts, such as alkyl halides, by milder reaction conditions, higher yields, and chemoselectivities13,14.
Under the optimized conditions, we explored the methodology to modify the bioactive peptides and further investigated the chemoselectivity when more free active residues were exposed, such as His, Tyr, Trp, Lys, Ser, Arg, Thr, Glu/Asp, and Gln/Asn. Several bioactive peptides were prepared in which cysteine was installed at random positions in the sequences. Linear and cyclic peptides were introduced with different sequence lengths. As shown in Fig. 2, these complex and diverse bioactive peptides suffered from site-selective modifications at cysteine residues in excellent yields (3a–3w). Thianthrenium salts bearing various functional structures, such as alkyne, azide, deuteromethyl, trifluoromethyl, and amino acid, specifically decorated cysteine smoothly even when other polar residues were unmasked under the same spatiotemporal conditions, which showed more versatile modification in comparison with Ritter’s work49. These meaningful results demonstrated possible applications of orthogonal bioconjugation and modification toward cysteine-containing peptides and proteins. Crucially, the mild and highly selective nature of this methodology, as well as its compatibility with biological conditions, rendered it an ideal platform for bioconjugation.
aUnless otherwise stated, the reaction was carried out with peptides 1 (1.0 eq.) and thianthrenium salts 2 (1.0 eq.) in DMSO/Tris-HCl buffer (v/v = 3:1) at room temperature for 1 h. bExtension the reaction to 6 h. Isolated yield after semipreparative HPLC was given. For details, see the Supplementary Information.
Having efficiently and successfully modified bioactive peptides, we further drove the method to construct structurally complex stapled and bridged peptides based on two cysteine residues, considering that cyclization and dimerization peptides exhibited a positive effect on biological properties, including binding affinity and proteolytic stability, as well as pharmacokinetic property (Fig. 3). Four peptide drugs, which were derived from octreotide, argipressin, terlipressin and somatostatin, were synthesized in their reduced forms. Then, the peptides underwent selective cysteine-specific conjugation with a flexible alkyl chain tethered two free thiols together, forging the novel stapled peptides (4a–4e). As expected, bridged peptides were also obtained by coupling two bioactive peptides with one hinge, generating the homologous (4f–4h) and heterologous dimerized hybrid peptides (4i). These stapling and bridging peptides could not be available by dihalogenated alkanes51, thereby rendering this methodology tremendous potential in peptide drug discovery and molecular splicing.
aThe reaction was carried out with peptide 1 (1.0 eq.) and thianthrenium salt 2 (1.0 eq.) in DMSO/Tris-HCl buffer (v/v = 3:1) at room temperature for 1 h. bThe reaction was carried out with peptide 1 (2.0 eq.) and thianthrenium salt 2 (1.0 eq.) in DMSO/Tris-HCl buffer (v/v = 3:1) at room temperature for 1 h. Isolated yield after semipreparative HPLC was given. For details, see the Supplementary Information.
Based on the integration of bioorthogonally useful clickable handles, we harnessed alkynes and azides to achieve multifaceted bioconjugation (Fig. 4). For instance, Cyclo(RGDfC), a sensitive integrin αvβ3 receptor ligand52,53, was selectively installed alkyne and azide at cysteine residues, respectively, generating masked products 3b and 3c. Subsequent classical copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry could enable the diversification of 3b by introducing the PEG chain (7a), fluorescent molecules (7b and 7f), drugs (7d and 7e), and carborane (7c)54,55. Similarly, peptide 3c also proceeded smoothly to access these diverse fluorescence labeling (8b), glycopeptide (8e), peptide-drug, and peptide-peptide conjugations (8a, 8c, 8d, and 8f). This multifaceted bioconjugation is a brilliant method to further expand orthogonal modification.
aUnless otherwise stated, the reaction was carried out with peptide 3b or 3c (1.0 eq.), alkyne 5 or azide 6 (1.0 eq.), THPTA (2.0 eq.) CuSO4•5H2O (1.0 eq.) and sodium ascorbate (2.0 eq.) in DMF/H2O (v/v = 4:1, 1 mL) at room temperature for 1 h. Isolated yield after semipreparative HPLC was given. For details, see the supplementary information.
Encouraged by the superb specificity and selectivity at the peptide level and the power of multifaceted modifications, the strategy was utilized to decorate the more complex proteins. As a proof-of-concept study, we selected BSA, the most abundant carrier protein in blood that contains a single and solvent-exposed free cysteine (Cys-34). BSA was incubated with thianthrenium salt 2c in Tris-HCl/DMSO for 30 min. Then, a second bioconjugation was immediately performed with the crude mixtures through CuAAC click chemistry to introduce fluorescent BODIPY. As shown in Fig. 5, this one-pot multifaceted bioconjugation smoothly furnished fluorescently labeled BSA, which was verified via UPLC-HRMS analysis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Therefore, this orthogonal modification together with click chemistry provides a valuable and versatile platform for selective cysteine bioconjugation under biologically ambient conditions.
In summary, late-stage bioconjugation of peptides and proteins has exposed the need for efficient strategies to enable orthogonal modification and labeling with high selectivity and specificity. We herein presented a chemoselective, fast, mild, and compatible bioconjugation methodology to engineer cysteine residues using synthetically useful thianthrenium salts. The strategy can be used to functionalize bioactive peptides and proteins with various functional groups and further expand to construct structurally complex stapled and bridged peptides. Notably, by introducing clickable handles, the method can achieve multifaceted bioconjugation to invoke PEG chains, fluorescent molecules, drugs, carboranes, carbohydrates, and peptides. In addition, this “two steps, one pot” method was successfully applied to label BSA with BODIPY under mild conditions. We envision that this orthogonal modification will be a valuable tool for chemical biology, material sciences, and drug discovery.
Methods
Synthesis of 3a–3w and 4a–4j
In an oven-dried 10 mL vial with a magnetic stir bar was charged with cysteine-containing peptides (1.0 eq.), alkyl thianthrenium salts (1.0 eq. for 3a–3w and 4a–4d; 0.5 eq. for 4e–4j), DMSO (1.5 mL, 0.1 M) and Tris-HCl buffer (pH 9.0, 0.5 mL). The resulting mixture in the vial was stirred open to air at room temperature for 1 h. Then, the crude reaction mixture was directly purified by Semi preparative HPLC on a Waters 2996 using a Dubhe C18 (10 μm, 20× 250 mm) preparative column, linear gradients using A: MeCN (0.1% CF3COOH) and B: H2O (0.1% CF3COOH).
Synthesis of 7a–7f and 8a–8f
Under argon atmosphere, to a solution of 3b or 3c (0.01 mmol, 1.0 eq.), alkyne derivatives 5 or azide 6 (0.01 mmol, 1.0 eq.) and (THPTA) (0.01 mmol, 1.0 eq.) in DMF (0.8 mL), CuSO4•5H2O in 0.1 mL H2O and sodium ascorbate (0.02 mmol, 2.0 eq.) in 0.1 mL H2O were added successively. The resulting mixture was stirred at room temperature for 3 h. Then, the reaction was identified by LC-MS and the crude reaction mixture was directly purified by Semi preparative HPLC on a Waters 2996 using a Dubhe C18 (10 μm, 20× 250 mm) preparative column, linear gradients using A: MeCN (0.1% CF3COOH) and B: H2O (0.1% CF3COOH).
Labeling of BSA
A solution of alkyl thianthrenium salt 2c (4.2 mg, 10 μmol, 100 eq.) dissolved in DMSO (0.1 mL) was added to a solution of BSA (6.6 mg, 0.1 μmol, 1.0 eq.) in Tris-HCl buffer (pH 9.0, 1.8 mL). The reaction mixtures were stirred for 30 min at room temperature. Then, the mixture of fluorescent BODIPY (4.0 mg, 10 μmol, 100 eq.), THPTA (8.7 mg, 20 μmol, 200 eq.), CuSO4•5H2O (2.5 mg, 10 μmol, 100 eq.), sodium ascorbate (4.0 mg, 20 μmol, 200 eq.) in DMF (0.1 mL) was added to the reaction mixture. After 1 h, an aliquot of each sample (4 µL) was diluted with 5× SDS sample buffer (10 µL) and ddH2O (36 µL). Each sample (10 µL) was loaded onto a 12-well 12% SDS-PAGE gel. The gel was run at room temperature and at 160 V for 70 min. In-gel fluorescence was imaged with a Typhoon FLA 9500 (GE) at 460 nm. Protein modification reaction was monitored and evaluated on a ThermoFisher Orbitrap IQ-X mass spectrometer coupled to an Acquity UPLC Protein BEH C4 column (300 Å, 1.7 um, 2.1 mm × 100 mm). Total mass spectra were reconstructed from the ion series using the intact mass analysis of Thermo BioPharma Finder software according to the manufacturer’s instructions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data relating to the materials and methods, optimization and control studies, experimental procedures, HPLC spectra, NMR spectra, and mass spectrometry are available in the Supplementary Information. Source data are provided in this paper. All data are available from the corresponding author upon request. Source data are provided in this paper.
References
Saxon, E. & Bertozzi, C. R. Cell surface engineering by a modified staudinger reaction. Science 287, 2007–2010 (2000).
Prescher, J., Dube, D. H. & Bertozzi, C. R. Chemical remodelling of cell surfaces in living animals. Nature 430, 873–877 (2004).
Tornøe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A. Stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002).
Agard, N. J., Prescher, J. A. & Bertozzi, C. R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 126, 15046–15047 (2004).
Baskin, J. M. et al. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 104, 16793–16797 (2007).
Laughlin, S. T., Baskin, J. M., Amacher, S. L. & Bertozzi, C. R. In vivo imaging of membrane-associated glycans in developing zebrafish. Science 320, 664–667 (2008).
Blackman, M. L., Royzen, M. & Fox, J. M. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels–Alder reactivity. J. Am. Chem. Soc. 130, 13518–13519 (2008).
Devaraj, N. K., Weissleder, R. & Hilderbrand, S. A. Tetrazine-based cycloadditions: application to pretargeted live cell imaging. Bioconjug. Chem. 19, 2297–2299 (2008).
Boutureira, O. & Bernardes, G. J. L. Advances in chemical protein modification. Chem. Rev. 115, 2174–2195 (2015).
Hoyt, E. A., Cal, P. M. S. D., Oliveira, B. L. & Bernardes, G. J. L. Contemporary approaches to site-selective protein modification. Nat. Rev. Chem. 3, 147–171 (2019).
Tamura, T. & Hamachi, I. Chemistry for covalent modification of endogenous/native proteins: from test tubes to complex biological systems. J. Am. Chem. Soc. 141, 2782–2799 (2019).
Chalker, J. M., Bernardes, G. J. L., Lin, Y. A. & Davis, B. G. Chemical modification of proteins at cysteine: opportunities in chemistry and biology. Chem. Asian J. 4, 630–640 (2009).
Gunnoo, S. B. & Madder, A. Chemical protein modification through cysteine. ChemBioChem 17, 529–553 (2016).
Frei, R. & Waser, J. A highly chemoselective and practical alkynylation of thiols. J. Am. Chem. Soc. 135, 9620–9623 (2013).
Frei, R. et al. Fast and highly chemoselective alkynylation of thiols with hypervalent iodine reagents enabled through a low energy barrier concerted mechanism. J. Am. Chem. Soc. 136, 16563–16573 (2014).
Abegg, D. et al. Proteome-wide profiling of targets of cysteine reactive small molecules by using ethynyl benziodoxolone reagents. Angew. Chem. Int. Ed. 54, 10852–10857 (2015).
Tessier, R. et al. “Doubly orthogonal” labeling of peptides and proteins. Chem 5, 2243–2263 (2019).
Vinogradova, E. V., Zhang, C., Spokoyny, A. M., Pentelute, B. L. & Buchwald, S. L. Organometallic palladium reagents for cysteine bioconjugation. Nature 526, 687–691 (2015).
Rojas, A. J. et al. Divergent unprotected peptide macrocyclization by palladium-mediated cysteine arylation. Chem. Sci. 8, 4257–4263 (2017).
Spokoyny, A. M. et al. A perfluoroaryl-cysteine SNAr chemistry approach to unprotected peptide stapling. J. Am. Chem. Soc. 135, 5946–5949 (2013).
Dai, P. et al. Salt effect accelerates site-selective cysteine bioconjugation. ACS Cent. Sci. 2, 637–646 (2016).
Konievab, O. & Wagner, A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem. Soc. Rev. 44, 5495–5551 (2015).
Cooper, B. M. et al. Peptides as a platform for targeted therapeutics for cancer: peptide–drug conjugates (PDCs). Chem. Soc. Rev. 50, 1480–1494 (2021).
Kang, M. S., Kong, T. W. S., Khoo, J. Y. X. & Loh, T.-P. Recent developments in chemical conjugation strategies targeting native amino acids in proteins and their applications in antibody–drug conjugates. Chem. Sci. 12, 13613–13647 (2021).
Alas, M., Saghaeidehkordi, A. & Kaur, K. Peptide-drug conjugates with different linkers for cancer therapy. J. Med. Chem. 64, 216–232 (2021).
Conibear, A. C., Watson, E. E., Payne, R. J. & Becker, C. F. W. Native chemical ligation in protein synthesis and semi-synthesis. Chem. Soc. Rev. 47, 9046–9068 (2018).
Agouridas, V. et al. Native chemical ligation and extended methods: mechanisms, catalysis, scope, and limitations. Chem. Rev. 119, 7328–7443 (2019).
Bao, G. et al. 1,3-Dipolar cycloaddition between dehydroalanines and C,N-cyclic azomethine imines: application to late-stage peptide modification. Angew. Chem. Int. Ed. 60, 5331–5338 (2021).
Zuo, Q. et al. Cysteine-specific multifaceted bioconjugation of peptides and proteins using 5-substituted 1,2,3-triazines. Adv. Sci. 11, 2308491 (2024).
Bao, G. et al. Dimethyl Sulfoxide/visible-light comediated chemoselective C–S bond formation between tryptophans and thiophenols enables site-selective functionalization of peptides. CCS Chem. 6, 1547–1556 (2024).
Bao, G. et al. Visible-light mediated deoxygenation of carboxylic acid for late-stage peptide modification targeting dehydroalanine. Org. Lett. 25, 8338–8343 (2023).
Yu, C. et al. Michael addition reaction between dehydroalanines and phosphites enabled the introduction of phosphonates into oligopeptides. Org. Lett. 26, 4767–4772 (2024).
Li, Y. et al. NDTP mediated direct rapid amide and peptide synthesis without epimerization. Org. Lett. 24, 1169–1174 (2022).
Liu, Y. et al. Copper(I)-catalyzed late-stage introduction of oxime ethers into peptides at the carboxylic acid site. Org. Lett. 24, 9248–9253 (2022).
Liu, Y. et al. Late-stage peptide modification and macrocyclization enabled by tertiary amine catalyzed tryptophan allylation. Chem. Sci. 15, 11099–11107 (2024).
Guo, X. et al. Novel Feleucin-K3-derived peptides modified with sulfono-γ-AA building blocks targeting pseudomonas aeruginosa and methicillin-resistant staphylococcus aureus infections. J. Med. Chem. 66, 1254–1272 (2023).
Berger, F. et al. Site-selective and versatile aromatic C−H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Chen, J., Li, J., Plutschack, M. B., Berger, F. & Ritter, T. Regio- and stereoselective thianthrenation of olefins to access versatile alkenyl electrophiles. Angew. Chem. Int. Ed. 59, 5616–5620 (2020).
Li, J. et al. Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination. Nat. Chem. 12, 56–62 (2020).
Zhao, D., Petzold, R., Yan, J., Muri, D. & Ritter, T. Tritiation of aryl thianthrenium salts with a molecular palladium catalyst. Nature 600, 444–449 (2021).
Engl, P. S. et al. C−N cross-couplings for site-selective late-stage diversification via aryl sulfonium salts. J. Am. Chem. Soc. 141, 13346–13351 (2019).
Lansbergen, B., Granatino, P., Ritter, T. & Site-selective, C. −H. alkylation of complex arenes by a two-step aryl thianthrenation-reductive alkylation sequence. J. Am. Chem. Soc. 143, 7909–7914 (2021).
Jia, H. et al. Trifluoromethyl thianthrenium triflate: a readily available trifluoromethylating reagent with formal CF3+, CF3•, and CF3− reactivity. J. Am. Chem. Soc. 143, 7623–7628 (2021).
Alvarez, E. M. et al. Late-stage heteroarylation of hetero(aryl)sulfonium salts activated by α-amino alkyl radicals. Angew. Chem. Int. Ed. 60, 13609–13613 (2021).
Ye, F. et al. Aryl sulfonium salts for site-selective late-stage trifluoromethylation. Angew. Chem. Int. Ed. 58, 14615–14619 (2019).
Chen, C., Wang, M., Lu, H., Zhao, B. & Shi, Z. Enabling the use of alkyl thianthrenium salts in cross-coupling reactions by copper catalysis. Angew. Chem. Int. Ed. 60, 21756–21760 (2021).
Chen, C., Wang, Z.-J., Lu, H., Zhao, Y. & Shi, Z. Generation of non-stabilized alkyl radicals from thianthrenium salts for C–B and C–C bond formation. Nat. Commun. 12, 4526 (2021).
Hartmann, P. et al. Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation. Nat. Chem. 16, 380–388 (2024).
Tam, J. P., Wu, C. R., Liu, W. & Zhang, J. W. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J. Am. Chem. Soc. 113, 6657–6662 (1991).
Chalker, J. M. et al. Methods for converting cysteine to dehydroalanine on peptides and proteins. Chem. Sci. 2, 1666–1672 (2011).
Danhier, F., Breton, A. L. & Préat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 9, 2961–2973 (2012).
Nieberler, M. et al. Exploring the role of RGD-recognizing integrins in cancer. Cancers 9, 116 (2017).
Waddington, M. A. et al. An organometallic strategy for cysteine borylation. J. Am. Chem. Soc. 143, 8661–8668 (2021).
Gazvoda, M. et al. Palladium-mediated incorporation of carboranes into small molecules, peptides, and proteins. J. Am. Chem. Soc. 144, 7852–7860 (2022).
Acknowledgements
We are grateful for the financial support from the CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5-074 to R.W., 2021-I2M-1-026 to R.W., 2021-I2M-3-001 to R.W., and 2022-I2M-2-002 to J.X.), the National Natural Science Foundation of China (22307052 to G.B.), the Fundamental Research Funds for the Central Universities (lzujbky-2024-ey10 to W.S.), and the Gansu Science and Technology Program (23JRRA1103 to G.B. and 22JR5RA502 to J.X.).
Author information
Authors and Affiliations
Contributions
W.S. and G.B. conceived the work. G.B. and X.S. performed the most peptide modification experiments. Y.L., Z.H., Q.Z., R.E., T.Y., and K.L. conducted some of the modification experiments and preparation of substrates, and W.S. and R.W. supervised the whole project. G.B. and W.S. wrote the original draft of the manuscript. J.X., W.S., and R.W. reviewed and edited the manuscript. All the authors participated in the data analysis and discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Alexander Spokoyny and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bao, G., Song, X., Li, Y. et al. Orthogonal bioconjugation targeting cysteine-containing peptides and proteins using alkyl thianthrenium salts. Nat Commun 15, 6909 (2024). https://doi.org/10.1038/s41467-024-51217-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-51217-9
This article is cited by
-
Biocompatible ligand balancing in transition metal coordination enables benign in-cell protein arylation
Nature Chemistry (2026)
-
Electro-induced C-H/S-H cross-coupling for the functionalization/macrocyclization of cysteine-containing peptides
Nature Communications (2025)
-
Installation of d3-methyl group to drugs by continuous-flow solid-phase synthesis
Nature Communications (2025)