Abstract
The development of high-performance metal-free organic X-ray scintillators (OXSTs), characterized by a synergistic combination of robust X-ray absorption, efficient exciton utilization, and short luminescence lifetimes, poses a considerable challenge. Here we present an effective strategy for achieving augmented X-ray scintillation through the utilization of halogenated open-shell organic radical scintillators. Our experimental results demonstrate that the synthesized scintillators exhibit strong X-ray absorption derived from halogen atoms, display efficacious X-ray stability, and theoretically achieve 100% exciton utilization efficiency with a short lifetime (∼18 ns) due to spin-allowed doublet transitions. The superior X-ray scintillation performance exhibited by these organic radicals is not only exploitable in X-ray radiography for contrast imaging of various objects but also applicable in a medical high-resolution micro-computer-tomography system for the clear visualization of fibrous veins within a bamboo stick. Our study substantiates the promise of organic radicals as prospective candidates for OXSTs, offering valuable insights and a roadmap for the development of advanced organic radical scintillators geared towards achieving high-quality X-ray radiography.
Similar content being viewed by others
Introduction
X-ray scintillators, capable of converting high-energy X-ray photons into visible light, have witnessed extensive applications in medical imaging, nondestructive inspection, and astronomical exploration1,2,3,4,5. The fundamental requisites for achieving high-efficiency radioluminescence (RL) in X-ray scintillators encompass robust X-ray absorption, high luminescence efficiency, and low self-absorption6,7,8,9. Moreover, a short luminescence lifetime is crucial for numerous applications, including quick-response real-time imaging and micro-computer tomography (micro-CT)10,11,12. Currently, most commercial X-ray scintillators are inorganic compounds like bismuth germanate (BGO) and CsI:Tl, which exhibit outstanding X-ray scintillation properties, but generally suffer from shortcomings such as harsh fabrication conditions, high costs, toxicity, and sensitivity to humidity and oxygen13,14,15,16,17,18,19,20. In contrast, metal-free organic X-ray scintillators (OXSTs) have garnered increasing attention due to their advantages of abundant source materials, ease of processing, low cost, and high mechanical flexibility21,22,23. Despite these inherent advantages, the X-ray scintillation performance of OXSTs currently lags behind their inorganic counterparts and requires further enhancement.
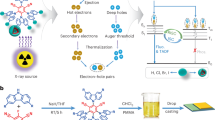
To attain distinguished OXSTs, a variety of materials employing different luminescence mechanisms have been developed, including conventional fluorophores11,24, thermally activated delayed fluorescence (TADF) emitters25,26,27, and room-temperature phosphorescence (RTP) materials21,28,29,30. Upon X-ray excitation, inner-shell electrons of the atoms, typically heavy atoms, are ejected through photoelectric effect and Compton effect (Fig. 1a)29. The ejected high-energy electrons further interact with atoms of the materials, generating numerous secondary electrons and producing electron–hole pairs for RL through radiative decay. According to spin statistics, the recombination of electron–hole pairs generates singlet and triplet excitons at a ratio of 1:331,32. In conventional fluorophores, 75% of triplet excitons are spin-forbidden to produce luminescence (Fig. 1b), resulting in low exciton utilization33. In contrast, TADF– and RTP–based OXSTs can theoretically achieve 100% exciton utilization efficiency34,35,36. TADF materials possess a small singlet–triplet splitting energy (ΔEST), allowing effective utilization of the 75% triplet excitons through an instant reverse intersystem crossing (RISC) process. Organic RTP materials typically incorporate heavy atoms or hetero atoms, which enhance spin-orbital coupling (SOC) for efficient intersystem crossing (ISC) and enable the emission of phosphorescence from originally transition-forbidden triplet excitons. However, the inherent sensitivity to oxygen and the extended microsecond/millisecond scale lifetime of triplet excitons render TADF and RTP materials vulnerable to the external environment, preventing them from practical applications.
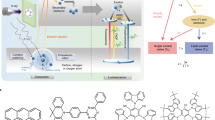
a Schematic representation of the X-ray scintillation process for organic emitters. b, c The RL mechanisms for closed-shell OXSTs through radiative decay from singlet and/or triplet excitons (b) and open-shell OXSTs through radiative decay from doublet excitons (c) after the recombination of holes and electrons. Phos.: phosphorescence. d Chemical structures of TTM-1Cz and TTM-1CzBr.
Luminescent organic radicals have garnered significant attention in recent years due to their promising applications in organic electronics, especially in organic light-emitting diodes (OLEDs)37,38,39,40. Different from closed-shell organic emitters, luminescent organic radicals are a type of open-shell molecules with only one unpair electron in the singly occupied molecular orbital (SOMO). This unique feature enables a doublet spin configuration41,42, allowing for a spin-allowed radiative transition from the first excited doublet state (D1) to ground state (D0), thereby generating doublet emission with 100% exciton utilization efficiency (Fig. 1c). Additionally, doublet excitons are stable under ambient conditions and exhibit short luminescence lifetimes on the nanosecond scale. Considering these characteristics, we speculate that luminescent organic radicals are promising candidates for high-performance OXSTs.
Results
Material synthesis and characterization
To validate this concept, we synthesized a metal-free luminescent organic radical, namely (4-N-carbazolyl-2,6-dichlorophenyl)bis-(2,4,6-trichlorophenyl)methyl radical (TTM-1Cz) (Fig. 1d) using tri(2,4,6-trichlorophenyl)methyl radical (TTM) as electron acceptor and carbazole as electron donor. Since attenuation coefficient of X-ray absorption is proportional to the fourth power of the atomic number21, abundant chloride atoms in TTM are expected to enhance the X-ray absorption ability of TTM-1Cz. To further strengthen X-ray absorption ability, a bromide (Br) atom with a higher atomic number of 35 was incorporated into the TTM-1Cz molecular skeleton to synthesize another luminescent organic radical (TTM-1CzBr). The facile two-step synthetic route furnished the radicals of TTM-1Cz and TTM-1CzBr in gram-scale (Supplementary Fig. 1), demonstrating significant potential for upscaling the synthesis. Their chemical structures were confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), high-performance liquid chromatography (HPLC), and electron spin resonance (ESR) measurements (Supplementary Figs. 2-7).
Basic photophysical properties and theoretical calculations
TTM-1CzBr exhibits a strong absorption band peaking at 372 nm, attributed to a π–π* local transition of TTM unit, accompanied by two relatively weak absorption bands peaking at 542 and 585 nm derived from the intramolecular charge transfer (ICT) from the electron donor 2-bromocarbazole to the electron acceptor TTM (Fig. 2a). The steady-state photoluminescence (SSPL) spectrum of TTM-1CzBr peaks at 612 nm, which would generate bathochromic shift as the solvent polarity increases (Supplementary Fig. 8), indicating its ICT characteristic again. TTM-1Cz displays the similar absorption and SSPL spectra to TTM-1CzBr due to their analogous molecular skeletons, while the spectra of TTM-1CzBr are a little hypsochromically shifted than those of TTM-1Cz, which should be ascribed to the relatively weaker ICT since electronegative Br atom can reduce the electron-donating ability of carbazole43,44. The relatively weak electron-donating ability of 2-bromocarbazole is consistent with cyclic voltammetry (CV) experiments (Supplementary Fig. 9); the oxidation potential of TTM-1CzBr is 0.22 V higher than that of TTM-1Cz. In addition, TTM-1CzBr and TTM-1Cz shows a moderate photoluminescence quantum yield (PLQY) of 40% and 80% in n-hexane, respectively. The two radicals exhibit a fast decay time of ∼40 ns (Fig. 2b), which is several orders of magnitude shorter than those of commercial inorganic X-ray scintillators, TADF- and RTP-based OXSTs.
a, b UV–vis absorption (dashed lines), normalized SSPL spectra (solid lines), PLQYs (inset) (a), and lifetimes (b) in n-hexane at the concentration of 10-5 M under ambient conditions. c, d Calculated energy levels and wavefunctions for the frontier molecular orbitals of TTM-1Cz (c) and TTM-1CzBr (d). e Calculated molecular conformation variation of D0-D1. f Potential energy curves of D0 and D1 for TTM-1CzBr. Abs.: absorption; Fluo.: fluorescence. g Fluorescence stabilities of TTM-1Cz and TTM-1CzBr in PSF (doping concentration: 5 wt%) under the irradiation of a 376-nm pulsed laser at ambient conditions.
To further acquire insight into the structure-property relationship, quantum-chemical calculations of the radicals were performed. As shown in Fig. 2c, d, the highest doubly occupied molecular orbital (HOMO) and lowest singly unoccupied molecular orbital (SUMO) are mainly located on carbazole and TTM groups respectively for TTM-1Cz and TTM-1CzBr, while their SOMO is distributed over the whole molecular skeleton; the spin density calculations indicate the unpaired electrons of TTM-1Cz and TTM-1CzBr are confined to TTM group (Supplementary Fig. 10). Due to the electron-withdrawing effect of Br atom, the calculated HOMO, SOMO and SUMO energy levels of TTM-1CzBr are all lower-lying compared to those of TTM-1Cz. In consistent with experimental absorption results, the calculated D1 states are mainly attributed to a charge transfer transition from HOMO (β) to SUMO, showing weak absorption oscillator strengths of around 0.09. Additionally, TTM-1Cz and TTM-1CzBr display a significant molecular conformation variation between D1 and D0 states with large root of the mean of squared displacement (RMSD) values of 0.82 Å and 0.77 Å (Fig. 2e), respectively. Such large RMSD values would lead to a significant shift of atomic coordinates (ΔQi) between the ground state and excited state, resulting in an obvious Stokes shift of absorption and emission spectra (Fig. 2f). The large Stokes shift and weak absorption oscillator strengths are beneficial for the organic radicals to weaken reabsorption when functioned as OXSTs. Moreover, photostability is another key parameter for luminescent organic radicals. Both of TTM-1Cz and TTM-1CzBr doped in polysulfone (PSF) at a weight doping concentration of 5% (5 wt%) exhibit the excellent photostability with the almost unchanged SSPL intensity for one hour under the persistent irradiation of a 376-nm pulsed laser (Fig. 2g and Supplementary Fig. 11).
X-ray scintillation performance
The extraordinary luminescence characteristics forebode the promising X-ray scintillation performance for the developed radicals, thus their RL properties were systematically characterized. As shown in Fig. 3a, compared to TTM-1Cz (Zmax = 17, Kα = 2.8 keV), TTM-1CzBr (Zmax = 35, Kα = 13.8 keV) exhibits the enhanced X-ray absorption ability owing to the heavy-atom effect. Then the doped films of radical emitters in PSF at different doping concentrations were prepared for X-ray scintillation measurements. PSF was selected as the host matrix because its S = O bonds can form halogen bonds with the radicals, which improves the radicals’ solubility to yield homogeneous films45. The optimized weight ratio for TTM-1Cz/PSF and TTM-1CzBr/PSF films are 5 wt% and 8 wt% respectively (Supplementary Fig. 12). As the thickness of the doped film constantly increases, the RL intensity would increase to the highest value at 0.66 mm and then only show a slow decay at the higher thickness (Supplementary Fig. 13), indicating a weak reabsorption for the organic radicals. The RL spectra of 5 wt% TTM-1Cz/PSF and 8 wt% TTM-1CzBr/PSF films are almost the same with their corresponding SSPL excited at 399 nm (Fig. 3b and Supplementary Fig. 14), which originate from the doublet emission of the radical emitters. The 5 wt% TTM-1Cz/PSF film emits near-infrared RL with a peak wavelength of 725 nm, which is 30 nm red-shifted than that of 8 wt% TTM-1CzBr/PSF film, similar to the case in n-hexane. In addition, the lifetimes of TTM-1Cz and TTM-1CzBr in PSF are 20 and 18 ns respectively, shorter than their lifetimes in n-hexane (Supplementary Fig. 14 and Supplementary Table 1). Although the PLQY of 8 wt% TTM-1CzBr/PSF film is only 10% (Supplementary Table 1), lower than the 20% of 5 wt% TTM-1Cz/PSF film, the stronger X-ray absorption of TTM-1CzBr endows it with more intense RL than that of TTM-1Cz/PSF film (Fig. 3b). Benefiting from high doublet exciton utilization and weak reabsorption of the radical emitters, the X-ray scintillation properties of the two films are comparable to those films of the reported fluorescence46, TADF25,26, and inorganic scintillators46,47,48 (Fig. 3c and Supplementary Fig. 11). Notably, the RL intensity of the radicals is higher than that of the precursors, illustrating the doublet emission of the radicals plays an important role for the high X-ray scintillation performance (Supplementary Fig. 15). In addition, as the dosage of X-rays increases, their RL intensity would linearly enhance (Fig. 3d and Supplementary Fig. 16); the detection limit (DL) of 8 wt% TTM-1CzBr/PSF film is calculatedto be 4.25 μGy s–1, which is lower than the standard dosage for medical X-ray test (5.5 μGy s–1)49. Moreover, both TTM-1Cz/PSF and TTM-1CzBr/PSF films exhibit outstanding X-ray photostability with emission intensity being almost unchanged for 120 on-off excitation circles (Fig. 3e and Supplementary Fig. 17). After X-ray irradiation for 30 min, the 5 wt% TTM-1Cz/PSF experiences ∼10% RL intensity loss, whereas the RL intensity of 8 wt% TTM-1CzBr/PSF film remains the same, illustrating that introducing bromine atoms into radical emitters can strengthen their X-ray photostability. A possible reason might be that the heavy atoms absorb more X-ray to avoid the degradation of the molecular radical center. Therefore, the above-mentioned results forcefully prove that radical emitters are of great potential to serve as high-performance OXSTs, allowing the construction of flexible and large-area scintillation screen.
a Simulated X-ray absorption spectra of TTM-1Cz and TTM-1CzBr. b RL spectra of 5 wt% TTM-1Cz/PSF and 8 wt% TTM-1CzBr/PSF films. c RL intensity comparison of a BGO single crystal film (1 mm thickness), and 2,3,5,6-tetrakis(3,6-di-t-butylcarbazol-9-yl)-1,4-dicyanobenzene (4CzTPN-Bu) (8 wt%), anthracene (8 wt%), TTM-1Cz (5 wt%), TTM-1CzBr (8 wt%) doped PSF films (∼0.1 mm thickness) under the same excitation conditions. d RL spectra of 8 wt% TTM-1CzBr/PSF film under different X-ray dose rates from 0.013 to 278 μGy s–1. Inset: linear behavior of RL intensity for 8 wt% TTM-1CzBr/PSF film as a function of excitation dose rates. e The X-ray photostability for 5 wt% TTM-1Cz/PSF and 8 wt% TTM-1CzBr/PSF films under continuous irradiation (top) and repeated on-off cycles (bottom) at a dose rate of 210 mGy s–1 under ambient conditions.
X-ray imaging applications
By virtue of the excellent X-ray scintillation performance of 8 wt% TTM-1CzBr/PSF film, we adopted it as the scintillation screen for X-ray imaging applications. The prepared doped film is uniform and highly flexible with sufficient light transmission (Fig. 4a, b). For the TTM-1CzBr/PSF scintillation screen, a high resolution of 12.0 lp mm-1 at a modulation transfer function (MTF) of 0.2 is achieved based on MTF calculation using the standard X-ray slanted-edge images (Fig. 4c)50. We then placed various objects between X-ray source and the scintillation screen for X-ray imaging. Under X-ray irradiation, the screw in capsule, the details of fishbone and USAF-1951 test chart can be captured by a commercial digital camera (Fig. 4d). Particularly, the maximum recognizable targets of the X-ray image of USAF-1951 test chart are located at group 4, element 1, which corresponds to a good resolution of 16.0 lp mm−1. Encouraged by the high X-ray imaging quality of the TTM-1CzBr/PSF film, we further applied it in a medical high-resolution micro-CT system equipped with an optical amplifying apparatus (Fig. 4e, f). Delightfully, the fibrous veins of a bamboo stick are visualized under X-ray excitation, illustrating that the doped film is highly homogeneous and the radical emitters have promising applications in commercial X-ray imaging.
a Schematic diagram of the fabrication of thin scintillation screen. b Photographs of the fabricated scintillation screen. c MTF curve of the X-ray image of the screen. d Daylight photographs and X-ray images of a screw in capsule (top), a dried fish (middle) and a USAF-1951 test chart (bottom). e Schematic of a high-resolution micro-CT set-up. f Bright- (left) and dark-field (right) photographs of a bamboo stick. Note: there is a small iron ball on the top of the bamboo stick for positioning during X-ray imaging.
Discussion
In conclusion, we have demonstrated a simple yet highly effective approach to achieving high-performance X-ray scintillation through the use of metal-free organic radicals. Taking advantages of strong X-ray absorption and efficient exciton utilization, the developed organic radicals show comparable RL intensity but significantly shorter luminescence lifetimes compared to commercial inorganic scintillators and benchmark open-shell organic scintillators. The incorporation of heavy atoms into organic radicals not only enhances their X-ray absorption capacity but also improves X-ray photostability. Notably, the brominated organic radical maintains an unaltered scintillation intensity even after 30 min of X-ray irradiation at a high dose rate of 210 mGy s–1. Moreover, the successful application of a flexible and large-area doped film based on the organic radicals in a medical high-resolution micro-CT system demonstrates its capability to visualize the fibrous veins of a bamboo stick. This research provides insights into achieving efficient X-ray luminescence with organic radicals, establishing a foundation for the potential application of OXSTs in commercial radiography.
Methods
Materials and synthesis
All chemicals and solvents, unless otherwise stated, were purchased from commercial suppliers and used as received. Tetrabutylammonium hydroxide (99%), tetrachloro-p-quinone (98%), and 2-bromo-9H-carbazole (98%) were purchased from Energy Chemical. Manipulations involving air-sensitive reagents were performed in an atmosphere of dry argon (Ar). TTM and TTM-1Cz were synthesized according to a literature report40.
1H and 13C-nuclear magnetic resonance (NMR) spectra were recorded on Bruker Ultra Shield Plus 400 MHz instruments with CDCl3 as the solvent and tetramethylsilane (TMS) as the internal standard. The quoted chemical shifts and J values are expressed in ppm and Hz, respectively. The splitting patterns have been designed as follows: s (singlet), d (doublet), dd (doublet of doublets) and m (multiplet). High resolution mass spectrometry (HRMS) was performed on a Bruker autoflex speed MALDI-TOF instrument. HPLC was performed on Agilent 1260 Infinity I HPLC System. ESR was measured using a Bruker A300 instrument.
Synthesis of (2,6-dichloro-4-(2-bromo-N-carbazolyl)phenyl)bis(2,4,6-trichlorophenyl) methyl (HTTM-1CzBr): A mixture of TTM (0.30 g, 0.54 mmol, 1 eq.), 2-bromo-9H-carbazole (0.17 g, 0.70 mmol, 1.30 eq.), anhydrous Cs2CO3 (0.27 g, 0.83 mmol, 1.56 eq.), and DMF (20 mL) was stirred at 160°C for 17 h under Ar atmosphere in the dark. After cooling to room temperature, the resulting mixture was poured into (1 M) hydrochloric acid solution, and the precipitate was filtered. The precipitate was purified by silica gel column chromatography (n-hexane: dichloromethane = 5:1 v/v), obtaining the desired compound HTTM-1CzBr (0.156 g, 37%). 1H NMR (400 MHz, CDCl3, ppm): δ = 8.10-8.08 (d, J = 7.7 Hz, 1H), 7.98-7.96 (d, J = 8.3 Hz, 1H), 7.57-7.56 (d, J = 2.2 Hz, 1H), 7.53-7.52 (d, J = 1.4 Hz, 1H), 7.49-7.41 (m, 5H), 7.39-7.33 (dd, J = 15.5, 8.0 Hz, 2H), 7.32-7.31 (d, J = 2.1 Hz, 1H), 7.29-7.28 (d, J = 2.2 Hz, 1H), 6.86 (s, 1H). 13C NMR (100 MHz, CDCl3, ppm) δ = 140.90, 140.38, 138.70, 138.00, 137.84, 137.24, 134.65, 133.97, 133.91, 130.19, 130.11, 128.57, 128.26, 126.79, 126.65, 124.02, 123.10, 122.71, 121.69, 121.26, 120.50, 119.88, 50.09. HRMS (MALDI-TOF) calculated for C31H14NCl8Br: [M + H]+ m/z: 764.96, found: 764.59.
Synthesis of [2,6-dichloro-4-(2-bromo-N-carbazolyl)phenyl)bis(2,4,6-trichlorophenyl) methyl radical (TTM-1CzBr): The HTTM-1CzBr compound (1.00 g, 1.24 mmol, 1 eq.) was dissolved in 40 mL THF in argon. Then tetrabutylammonium hydroxide in methanol (2.5 mL, 1 M, 2 eq.) was added, which caused an immediate color change from brown to red. The solution was stirred under argon in the dark at room temperature for 2.5 h. Tetrachloro-p-quinone (1.53 g, 6.20 mmol, 5 eq.) was added subsequently and the solution was stirred for another 1 h. After the reaction was finished, the solvent was removed under reduced pressure. The crude product was purified by silica gel column chromatography (petroleum ether: dichloromethane = 10:1 v/v), obtaining the desired radical TTM-1CzBr (1.00 g, 61%). HRMS (MALDI-TOF) calculated for C31H13NCl8Br: [M + H]+ m/z: 763.96, found 763.76.
Photophysical property measurements
Ultraviolet-visible (UV-Vis) and fluorescence spectra were recorded on a Jasco V-750 spectrophotometer and Edinburgh FLS1000, respectively. PLQY was obtained using an Edinburgh FLS980 fluorescence spectrophotometer equipped with an integrating sphere. Fluorescence decay was measured using a picosecond pulse light emitting diode (EPLED-380, wavelength: 376 nm, pulse width: 947.7 ps). X-ray activated RL spectra and X-ray photostability were obtained from an Edinburgh FS1000 and FS5 fluorescence spectrophotometer equipped with a miniature X-ray source (AMPTEK, Inc.). X-ray imaging photographs were acquired with a digital camera (Canon, EOS R5 coupled with EF 100 mm f/2.8 L IS USM) in an all-manual mode.
Theoretical calculations
Density functional theory was used to optimize ground-state structures under UB3LYP/6-31 G(d) level calculated by Gaussian09 D.01 software. Vibrational frequency calculations were performed to verify the minimum nature of the optimized structures. In order to quantitatively measure the geometric changes of the molecule in the ground state, RMSD calculations were performed for the molecules. The spin density of TTM-1Cz and TTM-1CzBr were computed under UB3LYP/6-31 G(d) level.
Data availability
Additional data are available from the corresponding author upon request.
References
Yi, L., Hou, B., Zhao, H. & Liu, X. X-ray-to-visible light-field detection through pixelated colour conversion. Nature 618, 281–286 (2023).
Hong, Z., Chen, Z., Chen, Q. & Yang, H. Advancing X-ray luminescence for imaging, biosensing, and theragnostics. Acc. Chem. Res. 56, 37–51 (2022).
Ou, X. et al. High-resolution X-ray luminescence extension imaging. Nature 590, 410–415 (2021).
Sakhatskyi, K. et al. Stable perovskite single-crystal X-ray imaging detectors with single-photon sensitivity. Nat. Photonics 17, 510–517 (2023).
Hou, B. et al. A swallowable X-ray dosimeter for the real-time monitoring of radiotherapy. Nat. Biomed. Eng. 7, 1242–1251 (2023).
Liang, L. et al. Controlling persistent luminescence in nanocrystalline phosphors. Nat. Mater. 22, 289–304 (2023).
Hajagos, T. J., Liu, C., Cherepy, N. J. & Pei, Q. High-Z sensitized plastic scintillators: a review. Adv. Mater. 30, 1706956 (2018).
Sun, B., Teo, J. Y., Wu, J. & Zhang, Y. Light conversion nanomaterials for wireless phototherapy. Acc. Chem. Res. 56, 1143–1155 (2023).
Chen, Q. et al. All-inorganic perovskite nanocrystal scintillators. Nature 561, 88–93 (2018).
Gandini, M. et al. Efficient, fast and reabsorption-free perovskite nanocrystal-based sensitized plastic scintillators. Nat. Nanotechnol. 15, 462–468 (2020).
Chen, M. et al. Organic semiconductor single crystals for X-ray imaging. Adv. Mater. 33, 2104749 (2021).
Wang, Y. et al. Efficient X-ray luminescence imaging with ultrastable and eco-friendly copper(I)-iodide cluster microcubes. Light Sci. Appl. 12, 155 (2023).
Liang, S. et al. Recent advances in synthesis, properties, and applications of metal halide perovskite nanocrystals/polymer nanocomposites. Adv. Mater. 33, 2005888 (2021).
Yuan, J. W. et al. Highly efficient stable luminescent radical-based X-ray scintillator. J. Am. Chem. Soc. 145, 27095–27102 (2023).
Zhang, Z.-Z. et al. Large-area laminar TEA2MnI4 Single-crystal scintillator for X-ray imaging with impressive high resolution. ACS Appl. Mater. Interfaces 14, 47913–47921 (2022).
Liu, X. et al. Lanthanide(III)-Cu4I4 organic framework scintillators sensitized by cluster-based antenna for high-resolution X-ray imaging. Adv. Mater. 35, e2206741 (2023).
Yao, Q. et al. Achieving a record scintillation performance by micro-doping a heterovalent magnetic ion in Cs3Cu2I5 single-crystal. Adv. Mater. 35, 2304938 (2023).
Xu, L.-J., Lin, X., He, Q., Worku, M. & Ma, B. Highly efficient eco-friendly X-ray scintillators based on an organic manganese halide. Nat. Commun. 11, 4329 (2020).
Jana, A. et al. Perovskite: scintillators, direct detectors, and X-ray imagers. Mater. Today 55, 110–136 (2022).
Zhou, Y., Chen, J. & Bakr, O. M. & Mohammed, O.F. Metal halide perovskites for X-ray imaging scintillators and detectors. ACS Energy Lett. 6, 739–768 (2021).
Wang, X. et al. Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence. Nat. Photonics 15, 187–192 (2021).
Koshimizu, M. Recent progress of organic scintillators. Jpn. J. Appl. Phys. 62, 010503 (2022).
Chen, M., Wang, C. & Hu, W. Organic photoelectric materials for X-ray and gamma ray detection: mechanism, material preparation and application. J. Mater. Chem. C. 9, 4709–4729 (2021).
Chen, H. et al. Highly efficient, low-dose, and ultrafast carbazole X-ray scintillators. Adv. Opt. Mater. 11, 2300365 (2023).
Ma, W. et al. Thermally activated delayed fluorescence (TADF) organic molecules for efficient X-ray scintillation and imaging. Nat. Mater. 21, 210–216 (2022).
Wang, J.-X. et al. Heavy-atom engineering of thermally activated delayed fluorophores for high-performance X-ray imaging scintillators. Nat. Photonics 16, 869–875 (2022).
Wang, X. et al. Halogenated thermally activated delayed fluorescence materials for efficient scintillation. Research 6, 0090 (2023).
Wang, X. et al. Organic phosphorescent nanoscintillator for low-dose X-ray-induced photodynamic therapy. Nat. Commun. 13, 5091 (2022).
Dong, C. et al. Influence of isomerism on radioluminescence of purely organic phosphorescence scintillators. Angew. Chem. Int. Ed. 60, 27195–27200 (2021).
Tang, L. et al. X-ray excited ultralong room-temperature phosphorescence for organic afterglow scintillators. Chem. Commun. 56, 13559–13562 (2020).
Xie, G. et al. Evaporation- and solution-process-feasible highly efficient thianthrene-9,9’,10,10’-tetraoxide-based thermally activated delayed fluorescence emitters with reduced efficiency roll-off. Adv. Mater. 28, 181–187 (2016).
Cai, X. & Su, S. J. Marching toward highly efficient, pure-blue, and stable thermally activated delayed fluorescent organic light-emitting diodes. Adv. Funct. Mater. 28, 1802558 (2018).
Zhang, Q. et al. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 8, 326–332 (2014).
Tao, Y. et al. Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics. Adv. Mater. 26, 7931–7958 (2014).
Xu, S., Chen, R., Zheng, C. & Huang, W. Excited state modulation for organic afterglow: materials and applications. Adv. Mater. 28, 9920–9940 (2016).
Qiu, W. et al. A “flexible” purely organic molecule exhibiting strong spin-orbital coupling: toward nondoped room-temperature phosphorescence OLEDs. J. Phys. Chem. Lett. 13, 4971–4980 (2022).
Ai, X. et al. Efficient radical-based light-emitting diodes with doublet emission. Nature 563, 536–540 (2018).
Guo, H. et al. High stability and luminescence efficiency in donor-acceptor neutral radicals not following the Aufbau principle. Nat. Mater. 18, 977–984 (2019).
Abdurahman, A. et al. Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes. Nat. Mater. 19, 1224–1229 (2020).
Peng, Q., Obolda, A., Zhang, M. & Li, F. Organic light-emitting diodes using a neutral pi radical as emitter: the emission from a doublet. Angew. Chem. Int. Ed. 54, 7091–7095 (2015).
Cui, Z., Abdurahman, A., Ai, X. & Li, F. Stable luminescent radicals and radical-based LEDs with doublet emission. CCS Chem. 2, 1129–1145 (2020).
Liu, C. H., Hamzehpoor, E., Sakai-Otsuka, Y., Jadhav, T. & Perepichka, D. F. A pure-red doublet emission with 90% quantum yield: stable, colorless, iodinated triphenylmethane solid. Angew. Chem. Int. Ed. 59, 23030–23034 (2020).
Reiss, H. et al. Bromination improves the electron mobility of tetraazapentacene. Angew. Chem. Int. Ed. 57, 9543–9547 (2018).
Wang, D., Hu, W., Liu, C., Huang, J. & Zhang, X. Electronic tuning of photoexcited dynamics in heteroleptic Cu(I) complex photosensitizers. J. Phys. Chem. Lett. 14, 10137–10144 (2023).
Wang, J.-X. et al. Triplet-triplet energy-transfer-based transparent X-ray imaging scintillators. Matter 6, 217–225 (2023).
Du, X. et al. Efficient and ultrafast organic scintillators by hot exciton manipulation. Nat. Photonics 18, 162–169 (2024).
Jomkaew, T. et al. Electron and photon responses of CWO scintillation crystal. Radiat. Phys. Chem. 189, 109749 (2021).
Viererbl, L. et al. YAP:Ce scintillator characteristics for neutron detection. IEEE T. Nucl. Sci. 63, 1963–1966 (2016).
Wei, H. et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photonics 10, 333–339 (2016).
Wei, J. et al. Organic room-temperature phosphorescent polymers for efficient X-ray scintillation and imaging. Adv. Photonics 4, 035002 (2022).
Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (22105104 awarded to G.X., 22322106 awarded to Y.T., 22075149 awarded to Y.T., 62075102 awarded to H.L., 22305126 awarded to H.L., and 62288102 awarded to W.H.), the Jiangsu Specially-Appointed Professor Plan, the China Postdoctoral Science Foundation (2023M731774 awarded to G.X.), the National Key Research & Development Program of China (2020YFA0709900 awarded to Q.C.), HuaLi Talents Program of Nanjing University of Posts and Telecommunications (awarded to Y.T.), the Open Research Fund of Songshan Lake Materials Laboratory (2022SLABFN16 awarded to Y.T.), the Project of State Key Laboratory of Organic Electronics and Information Displays, Nanjing University of Posts and Telecommunications (No. GZR2023010029 awarded to G.X.). Some image elements (atomic nucleus in Fig. 1a; hourglass in Fig. 1b; electromagnetic stirrer, dropper, beaker, and culture dish in Fig. 4a; X-ray source and camera in Fig. 4e) created with vectors were designed by Freepik.
Author information
Authors and Affiliations
Contributions
A.L., G.X., Q.C., Y.T. and W.H. conceived the experiments and wrote the paper. A.L., J.Z., D.X., G.X., X.X. and Q.Z. were primarily responsible for the experiments. A.L., Y.L., Z.Z., X.X., Q.P., H.L. and R.C. measured and analyzed the photophysical properties. C.S. and P.L. performed the computational calculations. A.L., X.X., Q.Z. and S.L. fabricated the applications. All authors contributed to data analyses.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, A., Zhang, J., Xiao, D. et al. Efficient metal free organic radical scintillators. Nat Commun 15, 8181 (2024). https://doi.org/10.1038/s41467-024-51482-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-51482-8
This article is cited by
-
Dimensionality-tailored pure organic semiconductor with high hole mobility for low-dose x-ray imaging
Nature Communications (2025)
-
Two-coordinate copper(I) complexes enable efficient X-ray scintillation and imaging
Science China Chemistry (2025)
-
Near-infrared organic scintillators for efficient X-ray imaging via singlet and triplet to doublet energy transfer
Science China Materials (2025)
-
Flash synthesis of high-performance and color-tunable copper(I)-based cluster scintillators for efficient dynamic X-ray imaging
npj Flexible Electronics (2024)