Abstract
The nucleolus, a recognized biomolecular condensate, serves as the hub for ribosome biogenesis within the cell nucleus. Its quantity and morphology are discernible indicators of cellular functional states. However, precise identification and quantification of nucleoli remain challenging without specific labeling, particularly for suspended cells, tissue-level analysis and high-throughput applications. Here we introduce a single-cell laser emitting cytometry (SLEC) for label-free nucleolus differentiation through light-matter interactions within a Fabry–Perot resonator. The separated gain medium enhances the threshold difference by 36-fold between nucleolus and its surroundings, enabling selective laser emissions at nucleolar area while maintaining lower-order mode. The laser emission image provides insights into structural inhomogeneity, temporal fluid-like dynamics, and pathological application. Lasing spectral fingerprint depicts the quantity and size of nucleoli within a single cell, showcasing the label-free flow cytometry for nucleolus. This approach holds promise for nucleolus-guided cell screening and drug evaluation, advancing the study of diseases such as cancer and neurodegenerative disorders.
Similar content being viewed by others
Introduction
The nucleolus is the most paramount structure in the nucleus of eukaryotic cells. It takes the form of a spherical biomolecule condensate encompassing proteins, RNA, and DNA, coordinating ribosome biogenesis and RNA editing1. This membraneless organelle has garnered increased attention with the liquid–liquid phase separation emerging as a new paradigm2,3,4,5. Leveraging its fluid-like dynamics, the nucleolus adeptly adjusts its dimensions, count, and protein composition in response to shifts in cellular homeostasis6,7. Consequently, the nucleolus has evolved into a potent biomarker for disease and a sensitive biosensor for cellular stress8,9,10. For instance, diminutive nucleoli stand as a cellular hallmark of longevity and metabolic health conserved across taxa11,12,13. Conversely, augmented nucleolar size and quantity are recognized as clinically pertinent indicators of adverse prognosis in numerous types of tumors14,15.
Due to the dense condensation of proteins and nuclear acids, nucleolus possesses a relatively higher refractive index (>1.40) in comparison to its surroundings16,17, facilitating its direct visualization through phase contrast microscopy or differential interference microscopy (DIC)18,19,20. Nevertheless, these methods suffer from limitations in specificity and are susceptible to optical phase artifacts (shade-off effect and halo effect)21. These challenges become particularly pronounced when observing suspended or flowing cells, where certain organelles can appear as obscure microspheres, making nucleolus identification difficult without nuclear reference22. Probe-labeled techniques offer a high-level specificity for nucleolus targeting, which requires either particular labeling chemistry (dye, specific antibodies, etc.) or expression of fluorescence-tagged proteins for direct detection23. However, the artificial probes make it challenging to study the native cellular interactions and processes. Furthermore, fluorescence-based techniques often contend with strong background noise.
As an alternative to conventional methodologies, laser emissions have emerged as a promising sensing signal in cellular environments24,25,26,27,28. Such amplified stimulated emissions offer distinctive threshold behavior with high signal-to-noise contrast, unique spectrum, and heightened sensitivity to subtlety29,30,31,32,33,34. However, most laser emissions still fall short in furnishing essential bio-information concerning individual cells and subcellular details. One main reason is the simultaneous excitation of high-order modes along with low-order modes, which usually masks the effective information.
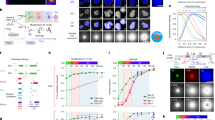
Here we report a single-cell laser emitting cytometry (SLEC) for the label-free characterization of nucleolus. SLEC employs the differentiated refractive index of the nucleolus, enabling lasing within the nucleolar region. It facilitates the assessment of nucleolar inhomogeneity, fluidic-like dynamics, morphology, and quantity through laser emission images and spectrum fingerprints. To establish SLEC, cells were sandwiched between two dielectric mirrors, forming a Fabry–Perot (FP) cavity. A layer of dye-doped polystyrene (PS) was spin-coated on the upper mirror serving as the gain medium (Fig. 1a, see Supplementary Figs. 2, 3). Due to higher refractive index, the nucleolus functions as an optical lens, resulting in a notably reduced lasing threshold compared to its surrounding subcellular components (Fig. 1b). Additionally, the separation of gain medium enhanced the threshold difference by 36-fold relative to the gain medium-labeled cells24, effectively circumventing emissions with high-order modes (Supplementary Note 1). The characteristics of nucleolus can be amplified and converted into lasing signals via the strong laser-matter interactions. Through modulating pump energy, we achieved the mapping of lasing emission from nucleoli, with a threshold of tens of μJ mm−2 and a sub-micrometer spatial resolution (Fig. 1c).
a Experiment setup of the scanning-based SLEC. The cells were sandwiched within a mirror-formed FP cavity. The upper mirror was spin-coated with a dye-doped PS layer, functioning as the gain medium. By adjusting the pump energy, lasing emissions can be achieved only within the nucleolus regions. b Laser threshold and emitting spectrum in regions with and without nucleolus. c Co-localization of the laser emission image with phase contrast image and fluorescence image from nucleolus dye. A549 cells were cultured on the lower mirror as an illustrative example. Scale bar: 5 μm. d Enlarged comparison among phase contrast image, laser emission image and fluorescence image. Scale bar: 5 μm. The lasing emission image indicated distinct subareas attributed to the nucleolus’ inhomogeneity of the refractive index (red arrows indicated). e Configuration of the scanning-based SLEC for tissue sections. f Configuration of the flow-based SLEC for suspended cells. Phase contrast image of a live K562 cell (20X objective), the nucleolus laser emission hyperspectral image and its spectrum. Scale bar: 5 μm. Each nucleolus corresponds to its unique laser peak.
Colocalized with phase-contrast and fluorescent-labeled microscopic images, the laser emission image exhibited excellent alignment with the nucleoli (Fig. 1c, d). The laser emission method offers ultrahigh-contrast imaging and high-spectral resolution. Significantly, the laser emission image contributes additional insights into spatial inhomogeneity and temporal fluid-like dynamics within nucleolus. Importantly, our approach also enabled nucleolus laser emission from tissue sections, facilitating label-free pathological analysis (Fig. 1e). Furthermore, it allowed the laser emission from suspension cells, where identifying nucleoli using phase contrast imaging alone is challenging. Consequently, we developed the label-free laser-emitting flow cytometry (Fig. 1f). The laser spectral fingerprints can be correlated with the number and size of nucleoli within a single cell, owing to the elimination of high-order modes by the gain separation. We envision that this study could provide a multidimensional tool for nucleolus analysis, thereby paving the way for the investigations of nucleolus-relevant fundamental cell biology and disease.
Results
Theoretical analysis and simulation
The nucleoli are considered as biomolecular spherical condensates, including proteins (fibrillarin, etc.), DNA and RNA. With the refractive index of nucleoprotein fibrils ~ 1.54, DNA ~ 1.51–1.55, and RNA ~ 1.50–1.5917,35,36,37, nucleoli have been proven as the main areas with the highest density and refractive index in most mammal cells16,38,39,40. The average number and diameter of nucleoli per nucleus in Hela cells, for instance, are 2–5 and 1–5 μm41. Here, we simplified the model of nucleolus sandwiched in the FP cavity with customized silica microspheres (Fig. 2a). The silica microspheres have diameters of 1, 3, and 5 μm, respectively, with a refractive index of ~1.462.
a Schematic of the silica microspheres between the mirrors. b Electric field within the cavity when there are no microspheres. c Electric fields within the cavity when the microsphere is 1, 3, and 5 μm, respectively. d Theoretical normalized thresholds. e Spectral integrated intensities as a function of pump energy density when the microsphere is 1, 3, and 5 μm, respectively. Insets are the bright-field image of the microspheres and the respective laser emission image. Scale bar: 10 μm. f Threshold characterization (mean ± SD, n = 9, 10, 9). Data are presented as box plots with median, minima, and maxima. g Laser spectra and the laser emission images when one, two, or three microspheres were pumped individually and simultaneously. Scale bar: 10 μm. Source data are provided as a Source Data file.
Here we theoretically analyze the lasing thresholds with and without microspheres in the FP cavity. The threshold of pump energy density is linked to the gain and loss within the cavity and can be expressed as
where L and G denote the loss and gain within the cavity, respectively. Additionally, \({\nu }_{o}\) is the laser frequency, \({T}_{{rt}}\) is the round-trip time that laser travels within the cavity, \({Q}_{o}\) is the Q-factor of the cavity, \(S(r,\, \varphi,\, z)\) and \({\rm H}(r,\, \varphi,\, z)\) are the normalized laser and pump intensity distributions within the PS layer, respectively, described in cylindrical coordinate (r, φ, z). The normalized laser intensity distribution can be obtained through Finite Element Analysis (Fig. 2b, c). The normalized pump distribution can be described by
where \({w}_{p}\) is the spot radius of the pump light, \(\Delta d\) is the thickness of the PS layer. According to Eq. (1), we can theoretically compare the laser thresholds with and without microspheres in the FP cavity (Fig. 2d). It reveals that the inclusion of microspheres can significantly reduce the laser threshold. This reduction in lasing threshold can be attributed to two primary factors. Firstly, microspheres enhance the confinement of lasing fields within the cavity, resulting in lower losses. Secondly, the lens effect of microspheres substantially reduces the laser mode volume within the cavity, which means the laser mode can encompass a larger gain. Consequently, the combined effect of reduced loss and increased gain leads to a lower laser threshold when microspheres are introduced.
We quantified the spectral integrated intensity response in relation to the pump energy, achieving the respective thresholds when encapsulating silica microspheres within the cavity (Fig. 2e). The size of the lasing emission exhibited a direct proportionality to the microsphere diameter (Fig. 2e insets). Specifically, silica microspheres with diameters of 1, 3, and 5 μm exhibited thresholds of ~84.4, 50.2, and 28.5 μJ mm−2, respectively (Fig. 2f). This observation suggests that larger microspheres can exert a more potent lens effect to effectively confine light, consequently yielding significantly lower thresholds. This aligns closely with our theoretical analysis.
An intriguing observation is that the number of laser spectral peaks within a free spectrum range (FSR) corresponds to the number of pumped microspheres. We verified this phenomenon by pumping microspheres with various diameters, individually or simultaneously (Fig. 2g, see Supplementary Figs. 4–6). It is worth noting that the density variations in laser spectral peaks may be attributed to the heterogeneity of the silica microspheres and the uneven distribution of pump energy. Additionally, it is crucial to maintain a moderate pump energy level (e.g., not exceeding 120 μJ mm−2) to avoid high-order lasing modes, which would increase the number of lasing peaks.
Basic characterization of laser emission in spreading cells
Subsequently, we utilized human lung cancer A549 cells to characterize the nucleolus laser emissions. The cells were cultured on the bottom mirror, allowing for cell spreading and subsequent observation through phase contrast imaging. Given that the nucleus is typically enveloped by the endoplasmic reticulum and other organelles, phase contrast imaging facilitated a clear identification of the nucleoli (Fig. 3a). We conducted a comprehensive scan of the entire cell, generating the corresponding laser emission image (Fig. 3b, Supplementary Movie 1). The laser image exhibited a strong alignment with the nucleolus image obtained through phase contrast imaging (Fig. 3c). Moreover, the brilliant laser emission image offered heightened contrast compared to the phase contrast image (Fig. 3d). The spatial resolution of the laser was estimated to be around 1 micrometer, potentially even reaching sub-micrometer levels (see Supplementary Fig. 7).
a Phase contrast image depicting a live A549 cell, with a zoomed-in section highlighting the nucleoli. Scale bar: 10 μm. b Laser emission image within the nucleoli. The separated laser spots were designated as HRIUs within the nucleoli. Scale bar: 5 μm. c Merged image of the phase contrast image and laser emission image. Scale bar: 10 μm. No laser emission was observed when pumping the areas outside of the cell and outside of the nuclei (inset images). d Gray-value profiles along the spatial distance in laser emission image and phase contrast image. e Laser spectrum of the nucleoli within one cell. The nucleoli can be pumped individually or simultaneously to examine their respective spectra. ‘L’, ‘R’ and ‘B’ refer to the top left nucleolus, the top right nucleolus, and the bottom nucleolus, respectively. ‘L + R’ refers to the two nucleoli pumped simultaneously. f, g Far-field laser emission images and hyperspectral images of the lasing modes. The number in the hyperspectral images denotes the Ince-Gaussian mode indices [p,m]. The cells were all pumped under a pump energy of ~ 68.3 μJ mm−2. Scale bar: 5 μm. h Hyperspectral image of nucleoli within cells. Scale bar: 5 μm. i Laser thresholds of areas with various-size nucleoli, cell regions without nucleoli, and regions without cells (n = 45). j Relationship of the laser peak number and nucleolus diameter (n = 94). k Relationship of laser intensity and nucleolus diameter under a fixed pump energy of ~ 68.3 μJ mm−2 (n = 124). Source data are provided as a Source Data file.
Of notable interest were the irregular laser emission patterns observed within larger nucleoli, typically exceeding 3 ~ 4 micrometers (Fig. 3b enlarged). Prior research has highlighted the spatial organization of nucleolar proteins and RNA, resulting in distinct sub-nucleolar domains such as the fibrillar center, the dense fibrillar component, and the granular component. This organization contributes to an uneven distribution of refractive index within the nucleoli42. We hypothesized that these irregular laser emission spots within large nucleoli are mainly attributed to the refractive-index inhomogeneity of nucleoli. As such, we designated each laser spot as a ‘higher refractive index unit (HRIU)’ for lasing emission. Notably, small nucleoli (<3 μm) typically displayed a single HRIU for laser emission, while larger nucleoli exhibited multiple HRIUs. For example, a nucleolus with an irregular shape may be composed of several HRIUs. The laser emission image also showed well-aligned with the HRIUs (see Supplementary Fig. 8). The ability to discern these HRIUs provided insights into finer details of the nucleolus, surpassing what could be achieved through phase contrast imaging alone.
Subsequently, we performed individual and simultaneous pumping of nucleoli, obtaining the respective spectrum of each nucleolus (Fig. 3e). Among the small nucleoli, each nucleolus typically contributes to a single lasing peak (e.g., the red ‘L’ and green ‘R’ peaks in Fig. 3e). Upon simultaneous pumping of small nucleoli, the individual laser spectral peaks collectively appeared (e.g., the ‘L + R’ peak in Fig. 3e). Conversely, larger nucleoli generally generated multiple spectral peaks (e.g., the cyan ‘B’ peak in Fig. 3e). During simultaneous pumping of these nucleoli, these laser spectral peaks overlapped (e.g., the ‘L + R + B’ peak in Fig. 3e). In this context, we posited that these laser spectral peaks may correspond to the HRIUs, implying that the number of laser spectral peaks was directly proportional to the number of HRIUs.
To determine whether the laser emission patterns and spectrum stem from single mode or higher-order modes, we employed a modified spectrometer to image the laser emissions. This setup enabled the laser emission to be spatially imaged according to spectral components through diffraction gratings, resulting in a hyperspectral image of the laser output on the camera24. Figure 3f, g compares the laser emission images to the hyperspectral image. The results indicate that the laser emission image for small nucleoli predominantly emanates from a single transverse laser mode, labeled as the [0,0] mode (see Supplementary Fig. 9). In larger nucleoli, the laser emission image primarily derives from the [0,0] single modes, but higher-order modes, such as [1,1] modes, become more. In theory, the separation of gain medium enabled the primary emissions without high-order modes (see supplementary Note 1). This observation affirms that for small nucleoli, the laser spectral peak number corresponds to the nucleolus number, while in large nucleoli, the laser spectral peak number is not the same but is proportional to the nucleolus count. Thus, in general, the majority of laser spectral peaks within an FSR correspond to the HRIUs. With this rule, we established the hyperspectral image fingerprint of the nucleoli within each cell (Fig. 3h). It’s important to note that during the measurement, the pump energy should not be excessively high (e.g., >120 μJ mm−2) to avoid high-order modes.
We investigated the lasing thresholds within various regions, including the nucleolus regions, cells regions without nucleoli, and regions devoid of cells (Fig. 3i). Upon surpassing the thresholds, the nucleolus regions emitted lasing spectra, characterized by luminous lasing images (see Supplementary Fig. 10). With increasing pump energy, the laser emission is greatly enhanced (Supplementary Movie 2). Notably, the full width at half maximum (FWHM) of the laser spectrum generated around the threshold pump energy was ~0.07 nm. The results revealed a decrease in the threshold with an increase in nucleolus size, ranging from 21–68 μJ mm−2. This value was slightly lower than the threshold obtained from the silica simulation experiment. This difference can be attributed to the additional lens effect generated by the entire cell, aiding in light confinement. This pump energy is comparable to other reported lasers24,26,43,44,45 in biological applications and no cell damage was observed (see Supplementary Fig. 11, Supplementary Table 1). In regions containing cells but no nucleolus, the average threshold was measured at 180 μJ mm−2, while regions without cells exhibited an average threshold of 220 μJ mm−2. The threshold difference between nucleolus and its surroundings can be ~140 μJ mm−2. Subsequently, we conducted a statistical analysis of the laser peak number within nucleoli of varying sizes under a pump energy of 68.3 μJ mm−2 (Fig. 3j). The analysis demonstrated that the laser peak number was directly proportional to the nucleolus diameter. Moreover, the laser peak intensity displayed a proportional relationship with the nucleolus diameter (Fig. 3k). The laser intensity maintained good stability under the pump energy of 68.3 μJ mm−2 within 6 min (18,000 pulses, see Supplementary Fig. 12).
Temporal investigation of the laser emission
Substantial evidence indicates that the nucleolus, as a large-scale biomolecule condensate, is driven by liquid–liquid phase separation. Consistent with the active, multifunctional nature of nucleolus, nucleolus components are liquid-like non-equilibrium, displaying continuous flux within the nucleolus and actively exchanging with the surrounding nucleoplasm2. For instance, the granular components within nucleoli exhibit a viscosity of approximately ~10 Pa·s, akin to the viscosity of honey (2–10 Pa·s)3. Furthermore, emerging evidence suggests that in certain neurodegenerative diseases, the liquid-like properties of the nucleolus tend to transition towards a more solid-like state over time, potentially underpinning pathological mechanisms46.
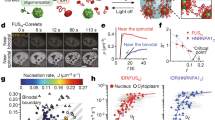
Interestingly, we observed changes in laser emission images within the nucleoli over time (Fig. 4a). We hypothesized that this change is attributed to the flow dynamics within nucleoli, consequently influencing the mobility of HRIUs. Given the laser’s high sensitivity to refractive index changes, the laser image undergoes spatial alterations. The laser spectrum also showed obvious fluctuation (see Supplementary Fig. 13a). To investigate this characteristic, we employed fixed cells as a control group. Conversely, we did not observe distinct changes over time (Fig. 4b). This reinforces the notion that the observed alterations in laser emission images of live cells are linked to dynamic activities within nucleolus. It is paramount to highlight that such changes are imperceptible in phase contrast images. To quantify the laser image alteration, we compared the gray value changes in each pixel within laser images of live cells and fixed cells (see Supplementary Fig. 13b). The gray value fluctuation in live cells was ~ 12.8-fold larger than that in fixed cells (Fig. 4c, see Supplementary Fig. 3c). It’s essential to note that due to the multiple oscillations in the cavity, the laser pattern may not accurately reflect the real spatial changes of the refractive index within nucleoli. Nevertheless, it provides a valuable reference for evaluating the temporal flow-like changes within nucleoli.
a Dynamic changes in laser emission image of a single nucleolus within a live A549 cell over time. Scale bar: 5 μm. b Changes in laser emission image of a nucleolus within a fixed A549 cell. Scale bar: 5 μm. c Quantification of the changes in laser emission images within the live and fixed cells. d Changes in laser emission image of a nucleolus within a ActD-treated cell. Scale bar: 5 μm. e Quantification of the changes in HRIU number and nucleolus size within the ActD-treated cells over time (mean ± SD, n = 5). f Laser emission images during cell mitosis, including prophase, metaphase, anaphase and telophase. Scale bar: 10 μm. g Quantification of the changes in the intensity and peak number of laser spectrum during the mitosis (mean ± SD, n = 9,8,9,7&7). Source data are provided as a Source Data file.
The nucleolus also plays a critical role in sensing various stress, including genotoxic and oxidative stress, heat shock, nutrient deprivation, oncogene activation, and viral infection. To investigate the laser emission under such stress, we treated the cells with Actinomycin D (ActD), known to impede ribosome biogenesis. Initially, multiple lasing spots (HRIUs) were observed emanating from a large nucleolus. Subsequently, a significant reduction in both the number of HRIUs and the size of the nucleolus was noted (Fig. 4d, e). This suggests ActD treatment induces a reduction in the inhomogeneity of refractive index within the nucleolus, indicating a significant change in the fluid properties from complex, non-Newtonian toward simple liquid behavior47. Furthermore, laser emission was characterized when the cells were incubated at 40 °C, indicating its sensitivity to protein translocation from nucleoli to nucleoplasm or cytoplasm (see Supplementary Fig. 14). This underscores the potential of SLEC as a dynamic tool for probing nucleolus responses to various stressors.
Mitosis in higher eukaryotes involves the disassembly and reassembly of the nucleolus, termination of pre-rRNA synthesis, and migration of major nucleolar components48,49. Our investigation delves into the behavior of lasers during mitosis in A549 cells, revealing nuanced changes throughout the stages. Mitosis initiates from prophase, marked by nucleolus disassembly characterized by the loss of RNA polymerase I subunits and dissolution of nucleolar structures (e.g., fibrillar centers). Despite these changes, laser emission remained achievable as chromatin condensed into chromosomes. The laser emission spots became more numerous and intricate compared to the interphase (Fig. 4f-I). The peak number of laser spectrum increased, on the contrary, the average intensity reduced (Fig. 4g). During metaphase, nucleolar proteins concentrated around the chromosomes along the cell’s equator, forming a liquid-like perichromosomal region. At this stage, the equatorial plane of the cell exhibited the highest refractive index. We observed the laser emissions around the equatorial plane (Fig. 4f-II), with a further decrease in average intensity and a slight reduction in the peak number of the laser spectrum. Next, during anaphase, sister chromatids separated and moved towards the centrosome, while nucleolar proteins undergone relocalization within the cytoplasm. Early in anaphase, a reduction in laser emission around the chromosomes is observable (see Supplementary Fig. 15). As a result, achieving laser emission under the same pump energy becomes challenging towards the end of this stage (Fig. 4f-III, Fig. 4g). In telophase, dispersed nucleolar proteins redistributed to the nucleolar organizer regions (NORs), forming prenucleolar bodies. Small laser emission spots emerged within the cells during this phase, accompanied by an increase in intensity and peak number of the laser spectrum (Fig. 4f-IV).
Laser emission from tissues
The nucleolus serves as a pivotal biomarker widely employed in histopathological analyses. we investigated label-free laser emissions at the tissue level. To facilitate comparison, slices of mice colon tumor and healthy tissues (~10 μm thick each) were sandwiched between mirrors. Through adjustment of pump energy, we successfully induced laser emissions from these tissues (Fig. 5a). Contrary to phase contrast imaging, where distinguishing nucleoli can be challenging, laser emissions distinctly highlighted the nucleolar regions. In addition, this method works both fresh tissues and fixed tissues.
Statistical analysis of the laser emissions from mice tumor and healthy tissues demonstrated a significant difference (Fig. 5b). Notably, tumor tissues exhibited a higher proportion of large lasing spots, indicative of enlarged nucleoli. Furthermore, the density of lasing spots was significantly greater in tumor tissues, with nucleoli in these samples showing decreased circularity, indicative of altered nucleolar morphology associated with pathological conditions. Similar results were observed across multiple sample batches (see Supplementary Fig. 16a, b). To validate the results, we compared them with hematoxylin and eosin (HE) stained images (see Supplementary Fig. 16c, d). Our results exhibited similar nucleolar features achieved by HE stains. These underscore the potential of this technique as an effective tool for tissue-level analysis.
Laser emission from suspended cells
The nucleolus is distinctly discernible in phase contrast images when cells are spreading on a substrate. However, this clarity diminishes significantly when cells are in suspension within the medium (see Supplementary Fig. 17). Consequently, without fluorescent labeling, it is difficult to pinpoint the nucleolus within suspended cells. We have demonstrated the robust laser emissions for label-free nucleolus probing even when cells are in suspension (Supplementary Movie 3). To enhance cell localization, we lithographically fabricated a single-cell microwell array on the bottom mirror. This allowed efficient trapping of suspended cells, enabling a co-localized comparison of phase contrast images, fluorescence images, and laser emission images. High-throughput imaging of the nucleolus laser emission was achieved through scanning each cell (Fig. 6a). The results clearly demonstrated a strong correlation between the laser emission image and the fluorescence image, showcasing higher signal contrast and minimal background noise in laser emissions (Fig. 6b). For instance, emissions I, II, and III corresponded to two, three, and four laser spots, respectively, aligning well with the fluorescent spots. Additionally, the respective laser spectrum exhibited laser peaks of two, three, and five (Fig. 6c). It is essential to note that the suspended cells were fixed to meet the staining requirements.
a Single-cell microwell array on the mirror for co-localization analysis and the achieved nucleolus laser emission array. Scale bar: 40 μm. b Phase contrast image, fluorescence image and laser emission image of the fixed suspended A549 cells. Scale bar: 10 μm. c Corresponding spectra obtained from cells I, II, and III. d A live A549 cell in suspension displaying subtly visible nucleoli in the phase contrast image alongside the respective laser emission image. Scale bar: 10 μm. e Laser emission image observed across the entire cell under a pump energy ~ 143.5 μJ mm−2, with the nucleolar area exhibiting the highest laser intensity. Scale bar: 10 μm. f A typical Ince-Gaussian laser mode originating from an entire live suspended A549 cell. Scale bar: 10 μm. g Relationship between the laser spectral peak number and fluorescence-indicated nucleolus number when cells are in suspension (N = 64). h Correlation between lasing threshold and nucleolus diameter observed in both suspended and spreading cell states (mean ± SD, n = 13&13, 7&7, 7&7). i Lasing emission intensity of various-size nucleoli in live and fixed spreading cells (mean ± SD, n = 16&16, 12&12, 11&11). One-way analysis of variance (ANOVA) is used. Data are presented as box plots with median, minima, and maxima. Source data are provided as a Source Data file.
Furthermore, we investigated the laser emissions while the cells remained in a live suspended state. For elucidating depiction, we carefully selected a suspended live A549 cell in which nucleoli were visible under the phase contrast image (Fig. 6d). The laser emission image demonstrated a strong correlation with the nucleolar area, with the spectral peak count aligning with the nucleolus number (see Supplementary Fig. 18a). Notably, the cells were subjected to a pump energy of ~68.3 μJ mm-2. Upon utilizing a higher pump energy (e.g., >121.6 μJ mm−2), the entire cell could be excited, resulting in the generation of Ince-Gaussian lasing modes across the cell due to the lens effect of the cell24. In this case, the nucleolar area exhibited the highest lasing intensity (Fig. 6e, f, see Supplementary Fig. 19), and the spectral peak number increased accordingly (see Supplementary Fig. 18b). This underscores that when cells are in suspension, the nucleolus can be effectively identified through laser emission, even at high pump energies.
We conducted a comparative analysis of the laser spectra with fluorescence images while the cells were in suspension, ultimately establishing a proportional relationship between the peak number of laser spectral and the nucleolus number (Fig. 6g). This correlates with the findings observed when cells were spreading on the substrate. Moreover, our statistics revealed that suspended cells exhibited lower thresholds than their spreading counterparts, likely due to an augmented lens effect in the suspended cell state (Fig. 6h). Notably, both suspended and spreading cells demonstrated reduced thresholds with increasing nucleolus diameter. Furthermore, we observed that live cells generally displayed higher laser emission intensities, both in spreading and suspended states (Fig. 6i). This can be attributed to a refractive index decrease after the chemical fixation of cells, affecting various cellular components including the nucleus, nucleolus, nucleoplasm, and cytoplasm. Notably, the nucleolus experienced the most significant refractive index decrease during the chemical fixation50.
Flow-based SLEC on an integrated chip for nucleolus spectral fingerprinting
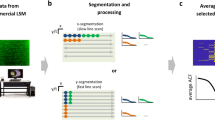
In the aforementioned experiments, we successfully demonstrated that the number of laser spectral peaks and their intensities directly correlated with the count and size of nucleoli. Building on these findings, as proof of concept, we developed the flow-based SLEC as an integrated chip for label-free nucleolus analysis. The SU-8 microfluidic channel was fabricated on the bottom mirror, with corresponding inlet and outlet on the top mirror for fluid flow (Fig. 7a). As cells traverse the pump region, the laser spectra of nucleolus are acquired by the spectrometer. We derived four key parameters for statistical analysis (Fig. 7b): (1) laser spectral peak number, indicative of nucleolus number and size; (2) average intensity of laser peaks, reflecting size and component density; (3) intensity variance of laser peaks, signifying nucleoli variance within each cell; and (4) the low-intensity percentage, identifying small nucleoli. Specifically, we defined nucleoli with lasing intensity less than 3000 as small nucleoli (approximately 1–2 μm in diameter). The pump energy remained consistent at ~ 68.3 μJ mm−2.
a Configuration of the SLEC chip mounted on the microscope system and the schematic of the flow cytometry. Scale bar: 20 μm. b Four main parameters extracted from the nucleolus laser spectrum. c Phase contrast images of the suspension cells K562 and the Caco−2 cells in the suspension state. Scale bar: 20 μm. d Nucleolus laser spectral fingerprints in laser peak number, average laser peak intensity, laser peak intensity variance, and low intensity (<3e3) percentage. (A549: n = 1121, K562: n = 1182, Caco−2: n = 1276, C2C12: n = 1227). e t-SNE visualization of the laser spectral data of nucleolus. Source data are provided as a Source Data file.
We explored four cell types, including A549, C2C12, Caco-2, and the suspension cell K562 (Fig. 7c). Leveraging the flow-based SLEC, we obtained the laser spectral fingerprints of the nucleolus (Fig. 7d). A549 and K562 cells showcased a similar distribution of spectral peak numbers (ranging from 3 to 5), suggesting ~ 3–5 nucleoli within each cell. Additionally, these cells maintained comparable average intensity, indicative of similar nucleolar sizes. However, A549 cells exhibited a larger variance in nucleolus size. Notably, A549 cells with 2-5 spectral peaks demonstrated a 50% prevalence of small nucleoli, while K562 cells showed fewer small nucleoli. K562 cells featured a small subgroup that maintained 6-7 spectral peaks. In contrast, Caco-2 cells predominantly displayed more small nucleoli, with 7-9 spectral peaks and low average intensity. A minor subgroup of Caco-2 cells exhibited fewer but relatively larger nucleoli (~3–5 per cell). Roughly half of C2C12 cells had 2-4 spectral peaks, and the other half had 4-6 spectral peaks. The average size of nucleoli in C2C12 was not substantial. Intriguingly, as the number of nucleoli increased in C2C12, their size and variance gradually decreased. To valid the result, we stained the nucleoli and conducted a statistical characterization based on the fluorescence images (see Supplementary Figs. 20, 21). The results revealed the patterns observed in the lasing fingerprinting align well with those derived from the image analysis.
Given the high-dimensional nature of the raw spectral data (e.g., the dimensions of the spectral peak number can reach up to 11, see Supplementary Fig. 22), we employed the t-SNE (t-distributed stochastic neighbor embedding) method to visualize this data. t-SNE technique maps a set of high-dimensional points to a two-dimensional space, aiming to preserve local relationships such that proximate points remain close while distant ones are appropriately separated51. The details for implementing t-SNE can be found in Methods. We visualized the features of nucleolus laser spectra from the above four cell lines (Fig. 7e). The t-SNE analysis revealed four primary clusters, each corresponding to one of the cell lines. Considering the correlation between laser spectra and nucleolar characteristics within cells, the overlapping regions among these clusters likely indicate similar nucleolar traits. Furthermore, within each cell line (e.g., A549 and K562), we observed subclusters that could potentially represent distinct subgroups within the cells. This observation suggests that the laser spectral fingerprinting possess the granularity to discern subtle variations of nucleolus.
Discussion
The journey of biological cell lasers began more than a decade ago by taking advantage of cavities to amplify cell-light matter interactions. Compared to fluorescence imaging, laser emission offers ultrahigh-contrast imaging with minimal background and unique spectrum information. To obtain excitation and emitting laser signals, transgene-generated green fluorescent protein (GFP) in cells was chosen as the gain medium, showcasing laser emission across entire cells24. In 2017, exogenous fluorescent labeling of nuclear biomarkers paved the way for laser-emission microscopy for cancer analysis, enabling laser emission within nuclear domain33. As an alternative, lasing microspheres and microdisks were embedded into the cells to achieve lasing in cells with desired wavelengths52,53. Instead of labeling cells with fluorescent dyes or micro-nano lasing particles, this study employed subcellular structure with differentiated refractive indices as an indicator to achieve different lasing thresholds within cells. The separation of gain medium with samples further significantly enhanced the threshold difference, leading to label-free selective lasing in nucleolar area. In addition, it enabled the laser emission with primary low-order modes, facilitating the spectrum analysis.
Label-free approach holds paramount importance in the study of subcellular organelles, as it provides a direct means to glean information, particularly concerning the natural kinetics of protein. Nucleolus, as a quintessential model of condensate organelles, exhibits a significant demand for label-free characterization. However, compared to fluorescent labeling, a common challenge is achieving sufficient specificity. Traditionally, morphology and temporal dynamics have been relied upon to aid in the identification of these structures39. More recently, machine learning has been explored to identify endogenous subcellular structures54,55,56,57. The machine learning methods are often more suited to spreading adherent cells. Thomas et al. introduced a label-free imaging flow cytometry approach that employs machine learning for cell classification based on bright-field images of suspended cells58. However, this is limited to cell cycle analysis for DNA content.
Our approach provides specific identification of the nucleolus in both spreading adherent cells and suspended cells, whether they are live or fixed, without extensive big data training. The high sensitivity of the laser emissions can aptly reflect nucleolar inhomogeneity and temporal changes. This enabled us to achieve the laser spectral fingerprints of nucleolus for a diverse range of cells, providing valuable insights into nucleolar characteristics and facilitating broader applications in cellular research. Presently, the SLEC is confined to nucleolus analysis within this experimental setup. We envisage the potential for expanding its capabilities to identify additional cellular structures by precisely adjusting parameters such as pump energy, pump area, and cavity configuration.
Methods
Fabrication of the mirror-based FP Cavity
The FP cavity was formed by two customized dielectric mirrors. The top mirror (Qingdao Novel Beam Technology Co., China) had a high reflectivity in the spectrum range of 490–590 nm and a high transmission at around 480 nm. The bottom mirror (Evaporated Coatings, Inc., USA) had a slightly wider reflection band (485–640 nm). The reflectivities of top and bottom mirrors are 99.8% and 99.9%, respectively, while the transmission of the top mirror at 480 nm (pump wavelength) is ~90.4%. To coat the dye layer on the top mirror, 100 mg PS (Sigma-Aldrich, Singapore) was dissolved in 1.5 mL Toluene, followed by adding 4.5 mg Bodipy-1 (Sigma-Aldrich, Singapore). Then the solution was spin-coated on the top mirror at 2000 rpm for 30 s. The mirror was incubated at 50 °C for 5 min. The thickness of the PS layer was ~1 μm measured by atomic force microscopy. Silicon microbeads with 9.2 μm diameter were used as the spacers to fix the cavity length.
Laser spectroscopic characterization
All the components were integrated into a Nikon Ni-E upright confocal microscopy. A 20X objective was used for the excitation and signal collection. The pulsed laser (EKSPLA NT230, 50 Hz, 5 ns pulse width) with a parameter oscillator (OPO) was used as an optical pump. The excitation was set at 480 nm for Bodipy-1. The laser beam size was around 30 μm. The emission laser from microspheres was split by a beam splitter and sent to a spectrometer (Andor Kymera 328i with 1800 lines per mm grating and Newton 970) and a camera (Photron, FASTCAM Mini UX 50) for spectrum and image capture.
Cell culture
The A549 and C2C12 cells from ATCC were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 100 U mL−1 penicillin/streptomycin. The Caco-2 cells and suspension K562 cells from ATCC were cultured in RPMI 1640 with 10% FBS and 100 U mL−1 penicillin/streptomycin. Cells were incubated in a standard incubator with 5% CO2 at 37 °C. The cells could be used after 3–4 passages.
Cell seeding on the mirror to form the FP cavity
The bottom mirror underwent sterilization involving a wipe-down with 75% ethanol followed by exposure to UV for ~ 1 h. Subsequently, a 2-min plasmonic treatment was applied to render the mirror hydrophilic. The treated mirrors were immersed in a culture medium for 2 h. The cultured cells were treated with Trypsin-EDTA solution and diluted to concentration of 1 × 106–5 × 106 mL−1. Then 100 μL of the cells were dropped onto the mirrors. For cell spreading on the mirror, the mirror with the sedimented cells was incubated for 1–2 days until the cells fully spread. To create the FP cavity, spherical silica spacers were placed on the bottom mirror, and a drop of CO2-independent medium was added. The top mirror was then placed over the bottom mirror to complete the FP cavity. For cells in suspension, without incubation, the sedimented cells were directly sandwiched between the two mirrors. Here 25 μm silica spacers were used to avoid compressing the cells. The entire setup was housed in a compact incubator that could be mounted on the laser setup.
Nucleolus dye staining for co-localization
To facilitate co-localization of cells for comparison between fluorescence images and laser spatial patterns, SU-8 markers with a thickness of 1–2 μm were initially lithographically fabricated on the bottom mirror to serve as physical reference points. Subsequently, cells were cultured on the marked surface. The cells were fixed by cold methanol for 1 min, then treated with Triton X-100 for 20 min. Next, the cells were incubated with the nucleolus staining dye (Nucleolus Bright Red, Dojindo) for 5 min. For the single-cell microwell experiment, cells were first collected, fixed, and stained by repeating the above process. Subsequently, the cells were loaded into the microwells.
Cell scanning
In order to synchronize with the scanning speed, we replaced the pump laser with a high repetition rate pulsed laser (Microlaser SB1-473-3-5, 5 kH). The pump laser was focused to a spot with a diameter of 34 μm. A scanning step of 5 μm was configured using the controller in the Nikon Ni-E upright confocal microscopy. Images were captured using a fast camera (Photron, FASTCAM Mini UX 50) with an exposure time of 2 ms.
Phototoxicity test
The cells were exposed to a pump energy of 68.3 μJ mm−2 for 2 min. Then the top mirror was removed and the cells were further incubated for 2 h. After that, the cells were stained by the Live/Dead Cell Assay (Sigma-Aldrich, Singapore). The SU-8 structures on the mirror were used as references to locate the cells accurately.
Experiment of the cellular stress induction
For drug experiment, the concentration of the ActD (Sigma-Aldrich, Singapore) was ~1 μg mL−1. For heat experiment, the cells sandwiched between the cavity were incubated at 40 °C. To prevent desiccation, the entire microcavity was immersed in the cell culture medium during the experiment.
Tissue experiment
Tumors and healthy tissues for this study were from the p53 + /- mice (female, initially from the Jackson lab). All the mice were bred and maintained under non-specific pathogen-free conditions at the animal research facility of Nanyang Technological University, and were housed in individually ventilated cages under a 12:12 h light-dark cycle. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC), and the tumors are allowed to grow until 3 cm diameter, if the position of the tumor does not cause discomfort or pain to the animal. The tumors that were used in this study were no bigger than 1.5 cm diameter. Tumors and healthy tissues were dissected and then fixed with 4% PFA overnight. They were immersed in 30% sucrose in PBS for 3 days, and then they were freshly frozen by cooling dry ice in methylbutane following by OCT-imbedding in −80 °C. They were cut into 10 µm section. The HE stained tissues were observed subject under a 100X objective by Zeiss microscope.
Fabrication of the single-cell microwell array and microfluidic channels
The fabrication of microstructures followed a conventional lithography process59. Initially, the bottom mirrors were cleaned using isopropyl alcohol and reverse osmosis (RO) water, after which they were subjected to a 2-h heat treatment at 150 °C. Subsequently, a layer of SU-8 (2000.5, KAYAKU) with a thickness of ~ 0.5 μm was spin-coated to enhance adhesion. Then a 35 μm-thick layer of SU-8 was coated for the microwells and flow cytometry channels. The microstructures were then fabricated via an Ultraviolet Maskless Lithography machine (TuoTuo Technology (Suzhou) Co., Ltd.). The microwell array was designed with a diameter of 40 μm. The width of the flow channel at the pump area was designed as 35 μm to allow single-cell passage, while at other locations, the channel width was 200 μm. To prevent cell blockage, pillar filters with a distance of 25 μm were incorporated at the inlet.
Flow cytometry experiment
To establish the inlet and outlet, holes with a diameter of ~ 1 mm were drilled on the top mirror. Following the spin-coating of the dye-doped polystyrene layer onto the top mirror, polyethylene tubes with an outer diameter of ~ 1 mm and an inner diameter of ~ 0.7 mm were inserted into these holes and sealed using epoxy adhesive. Subsequently, the top mirror was placed over the bottom mirror and firmly affixed using epoxy adhesive. To prevent cell blockage within the channel, the cell suspension medium was filtered through a filter with 30 μm micropores. The introduced cells were diluted to a concentration of ~ 1 × 104 mL−1. As proof of concept, considering the repetition rate of the pulsed laser (50 Hz), the flow speed was adjusted to a range of 400–800 μm s−1 by a syringe pump, ensuring that the cells received an adequate number of pump pulses (>2). A 10X objective lens was employed to encompass the entire cell for effective pumping (pump spot diameter: ~72 μm). The camera exposure time was set to ~ 10 ms.
To further increase the throughput, we replaced the pulsed laser with a high repetition rate laser operating at 5 kHz (Microlaser SB1-473-3-5, BrightMicrolaser Ltd., Italy). Additionally, we replaced the spectrometer with a faster one featuring a tunable integration time of 2μs-20s (0.05-500 kHz, AvaSpec-ULS2048XL-EVO, AVANTES Ltd., Netherlands). The flow rate was increased to approximately 60 mm s−1. As a result of these enhancements, the throughput was boosted to 2000 eps.
To mitigate the impact of dye photobleaching, each area for single PS layer was pumped for 5 min. To enhance the lasing duration, multi-PS layers and multiple pumping positions along the channel were investigated. The maximum achievable lasing time can reach 10,350 min (see Supplementary Fig. 23).
t-SNE visualization
We utilized the t-SNE algorithm implemented in Python 3.11 to visualize the raw data acquired from flow cytometry. Key parameters such as peak intensities, peak number, average intensity, and low-intensity percentage were employed to classify and structure the data for the t-SNE visualization.
Statistics and reproducibility
All measurements were taken from distinct samples. The representative results in the figures were from at least 3 independent experiments with similar results. The data were presented by means ± standard deviation (SD). A two-tailed t-test was used to reveal the statistical difference in Supplementary Fig. 14c with Origin 8.0. Pearson’s test was performed to examine the result correlation in Fig. 3i (r = −0.91).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting this study are available within the article and its supplementary files. Any additional requests for information can be directed to the corresponding author and will be fulfilled within 2 weeks. Source data are provided with this paper.
References
Boisvert, F. M., van Koningsbruggen, S., Navascues, J. & Lamond, A. I. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 8, 574–585 (2007).
Lafontaine, D. L. J., Riback, J. A., Bascetin, R. & Brangwynne, C. P. The nucleolus as a multiphase liquid condensate. Nat. Rev. Mol. Cell Biol. 22, 165–182 (2021).
Feric, M. et al. Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697 (2016).
Wei, J. & Yoshimura, S. H. in Phase Separation in Living Cells: Benefits and Risks (ed. R. Kurokawa) 191–206 (Springer Nature Singapore, 2023).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Yoneda, M., Nakagawa, T., Hattori, N. & Ito, T. The nucleolus from a liquid droplet perspective. J. Biochem 170, 153–162 (2021).
Mitrea, D. M. et al. Self-interaction of NPM1 modulates multiple mechanisms of liquid-liquid phase separation. Nat. Commun. 9, 842 (2018).
Corman, A., Sirozh, O., Lafarga, V. & Fernandez-Capetillo, O. Targeting the nucleolus as a therapeutic strategy in human disease. Trends Biochem Sci. 48, 274–287 (2023).
Yang, K., Yang, J. & Yi, J. Nucleolar Stress: hallmarks, sensing mechanism and diseases. Cell Stress 2, 125–140 (2018).
Nicolas, E. et al. Involvement of human ribosomal proteins in nucleolar structure and p53-dependent nucleolar stress. Nat. Commun. 7, 11390 (2016).
Tiku, V. et al. Small nucleoli are a cellular hallmark of longevity. Nat. Commun. 8, 16083 (2017).
Zlotorynski, E. Ageing: live longer with small nucleoli. Nat. Rev. Mol. Cell Biol. 18, 651 (2017).
Tiku, V. & Antebi, A. Nucleolar function in lifespan regulation. Trends Cell Biol. 28, 662–672 (2018).
Zink, D., Fischer, A. H. & Nickerson, J. A. Nuclear structure in cancer cells. Nat. Rev. Cancer 4, 677–687 (2004).
Hein, N., Hannan, K. M., George, A. J., Sanij, E. & Hannan, R. D. The nucleolus: an emerging target for cancer therapy. Trends Mol. Med 19, 643–654 (2013).
Zhang, Q. et al. Quantitative refractive index distribution of single cell by combining phase-shifting interferometry and AFM imaging. Sci. Rep. 7, 2532 (2017).
BARE, R. Refractometry and interferometry of living cells. J. Opt. Soc. Am. 47, 545–556 (1956).
Pederson, T. The nucleolus. Cold Spring Harb Perspect Biol 3, a000638 (2011).
Politz, J. C., Yarovoi, S., Kilroy, S. M., Gowda, K., Zwieb, C. & Pederson, T. Signal recognition particle components in the nucleolus. Proc. Natl. Acad. Sci. USA 97, 55–60 (2000).
Brangwynne, C. P., Mitchison, T. J. & Hyman, A. A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 108, 4334–4339 (2011).
Yin, Z., Kanade, T. & Chen, M. Understanding the phase contrast optics to restore artifact-free microscopy images for segmentation. Med Image Anal. 16, 1047–1062 (2012).
Grah, J. S. et al. Mathematical imaging methods for mitosis analysis in live-cell phase contrast microscopy. Methods 115, 91–99 (2017).
Liu, H. et al. A carbonized fluorescent nucleolus probe discloses RNA reduction in the process of mitophagy. CCS Chem. 4, 2698–2710 (2022).
Gather, M. C. & Yun, S. H. Single-cell biological lasers. Nat. Photonics 5, 406–410 (2011).
Cho, S., Humar, M., Martino, N. & Yun, S. H. Laser particle stimulated emission microscopy. Phys. Rev. Lett. 117, 193902 (2016).
Schubert, M. et al. Monitoring contractility in cardiac tissue with cellular resolution using biointegrated microlasers. Nat. Photonics 14, 452–458 (2020).
Tang, S. J. et al. Laser particles with omnidirectional emission for cell tracking. Light Sci. Appl. 10, 23 (2021).
Qiao, Z. et al. Brain cell laser powered by deep‐learning‐enhanced laser modes. Adv. Opt. Mater. 9, 2101421 (2021).
Wang, Z. et al. Autonomous microlasers for profiling extracellular vesicles from cancer spheroids. Nano Lett 23, 2502–2510 (2023).
Fikouras, A. H. et al. Non-obstructive intracellular nanolasers. Nat. Commun. 9, 4817 (2018).
Chen, Y. C. & Fan, X. Biological lasers for biomedical applications. Adv. Opt. Mater. 7, 1900377 (2019).
Gong, C., Qiao, Z., Zhu, S., Wang, W. & Chen, Y. C. Self-assembled biophotonic lasing network driven by amyloid fibrils in microcavities. ACS Nano 15, 15007–15016 (2021).
Chen, Y. C. et al. Laser-emission imaging of nuclear biomarkers for high-contrast cancer screening and immunodiagnosis. Nat. Biomed. Eng. 1, 724–735 (2017).
Chen, Y. C., Chen, Q., Zhang, T., Wang, W. & Fan, X. Versatile tissue lasers based on high-Q Fabry-Perot microcavities. Lab Chip 17, 538–548 (2017).
Samoc, A., Miniewicz, A., Samoc, M. & Grote, J. G. Refractive‐index anisotropy and optical dispersion in films of deoxyribonucleic acid. J. Appl. Polym. Sci. 105, 236–245 (2007).
Ghasemi, M. et al. Linear and nonlinear optical properties of transfer ribonucleic acid (tRNA) thin solid films. RSC Adv. 12, 8661–8667 (2022).
Wang, L., Yoshida, J., Ogata, N., Sasaki, S. & Kajiyama, T. Self-assembled supramolecular films derived from marine deoxyribonucleic acid (DNA)-cationic surfactant complexes: large-scale preparation and optical and thermal properties. Chem. Mater. 13, 1273–1281 (2001).
Kim, T. K., Lee, B. W., Fujii, F., Kim, J. K. & Pack, C. G. Physicochemical properties of nucleoli in live cells analyzed by label-free optical diffraction tomography. Cells 8, 699 (2019).
Sandoz, P. A., Tremblay, C., van der Goot, F. G. & Frechin, M. Image-based analysis of living mammalian cells using label-free 3D refractive index maps reveals new organelle dynamics and dry mass flux. PLoS Biol. 17, e3000553 (2019).
Liu, X., Oh, S. & Kirschner, M. W. The uniformity and stability of cellular mass density in mammalian cell culture. Front Cell Dev. Biol. 10, 1017499 (2022).
Farley, K. I., Surovtseva, Y., Merkel, J. & Baserga, S. J. Determinants of mammalian nucleolar architecture. Chromosoma 124, 323–331 (2015).
Shan, L. et al. Nucleolar URB1 ensures 3’ ETS rRNA removal to prevent exosome surveillance. Nature 615, 526–534 (2023).
Gong, C. et al. Multifunctional laser imaging of cancer cell secretion with hybrid liquid crystal resonators. Laser & Photon. Rev. 16, 2270036 (2022).
Chen, Y.-C. et al. Chromatin laser imaging reveals abnormal nuclear changes for early cancer detection. Biomed. Opt. Express 10, 838–854 (2019).
Humar, M., Gather, M. C. & Yun, S. H. Cellular dye lasers: lasing thresholds and sensing in a planar resonator. Opt. Express 23, 27865–27879 (2015).
Hetman, M. & Pietrzak, M. Emerging roles of the neuronal nucleolus. Trends Neurosci. 35, 305–314 (2012).
Schmidt, H. B. et al. Oxaliplatin disrupts nucleolar function through biophysical disintegration. Cell Rep. 41, 111629 (2022).
Shishova, K. V., Zharskaya, C. O. & Zatsepina, C. O. The fate of the nucleolus during mitosis: comparative analysis of localization of some forms of pre-rRNA by fluorescent in situ hybridization in NIH/3T3 mouse fibroblasts. Acta Nat. 3, 100–106 (2011).
Leung, A. K. et al. Quantitative kinetic analysis of nucleolar breakdown and reassembly during mitosis in live human cells. J. Cell Biol. 166, 787–800 (2004).
Baczewska, M., Eder, K., Ketelhut, S., Kemper, B. & Kujawinska, M. Refractive index changes of cells and cellular compartments upon paraformaldehyde fixation acquired by tomographic phase microscopy. Cytom. A 99, 388–398 (2021).
Kobak, D. & Berens, P. The art of using t-SNE for single-cell transcriptomics. Nat. Commun. 10, 5416 (2019).
Humar, M. & Yun, S. H. Intracellular microlasers. Nat. Photonics 9, 572–576 (2015).
Martino, N. et al. Wavelength-encoded laser particles for massively multiplexed cell tagging. Nat. Photonics 13, 720–727 (2019).
Jo, Y. et al. Label-free multiplexed microtomography of endogenous subcellular dynamics using generalizable deep learning. Nat. Cell Biol. 23, 1329–1337 (2021).
Ryu, D. et al. Label-free white blood cell classification using refractive index tomography and deep learning. BME Front. 2021, 9893804 (2021).
Cheng, S. et al. Single-cell cytometry via multiplexed fluorescence prediction by label-free reflectance microscopy. Sci. Adv. 7, eabe0431 (2021).
Ounkomol, C., Seshamani, S., Maleckar, M. M., Collman, F. & Johnson, G. R. Label-free prediction of three-dimensional fluorescence images from transmitted-light microscopy. Nat. Methods 15, 917–920 (2018).
Blasi, T. et al. Label-free cell cycle analysis for high-throughput imaging flow cytometry. Nat. Commun. 7, 10256 (2016).
Fang, G. et al. Unidirectional intercellular communication on a microfluidic chip. Biosens Bioelectron. 175, 112833 (2020).
Acknowledgements
The authors thank Wen Sun (Nanyang Technology University, Singapore) and Linwei Shang (Nanjing University of Aeronautics and Astronautics, China) for the discussion of t-SNE. This work was supported by A*STAR MTC IRG-Grant (M21K2c0106-Y.C.) and Nanyang Presidential Postdoctoral Fellowship (G.F.).
Author information
Authors and Affiliations
Contributions
G.F. and Y.C. conceived the project, designed the experiments, and analyzed data. Z.Q. performed the theoretical analysis and simulation. G.F., H.Z., J.X., and Z.X. performed the experiments. T.Z. fabricated the device. L.H. and I.S. offered the tissue section and analyzed data. D.J. designed the experiments and analyzed data. G.F., Z.Q., L.H., and Y.C. wrote the manuscript. Y.C. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yohei Yamamoto, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fang, G., Qiao, Z., Huang, L. et al. Single-cell laser emitting cytometry for label-free nucleolus fingerprinting. Nat Commun 15, 7332 (2024). https://doi.org/10.1038/s41467-024-51574-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-51574-5