Abstract
Histamine is a biogenic amine that is critical in various physiological and pathophysiological processes, including but not limited to allergic reactions, wakefulness, gastric acid secretion and neurotransmission. Here, we determine 9 cryo-electron microscopy (cryo-EM) structures of the 4 histamine receptors in complex with four different G protein subtypes, with endogenous or synthetic agonists bound. Inside the ligand pocket, we identify key motifs for the recognition of histamine, the distinct binding orientations of histamine and three subpockets that facilitate the design of specific ligands. In addition, we also identify key residues responsible for the selectivity of immethridine. Moreover, we reveal distinct structural features as determinants of Gq vs. Gs or Gs vs. Gi coupling differences among the histamine receptors. Our study provides a structural framework for understanding the ligand recognition and G protein coupling of all 4 histamine receptors, which may facilitate the rational design of ligands targeting these receptors.
Similar content being viewed by others
Introduction
Histamine is a widely distributed biogenic amine that is a neurotransmitter in the central nervous system and an important signaling molecule in the immune system, gut, and skin1,2. Histamine is an important metabolite that is formed by L-histidine decarboxylase (HDC), which catalyzes the decarboxylation of L-histidine2,3. Histamine is released most often by mast cells and basophils via degranulation in response to various immune and nonimmune stimuli3,4,5. Moreover, it mediates a wide range of physiological processes, including but not limited to allergic reactions, inflammation, wakefulness, gastric acid secretion, and neurotransmission6,7,8,9. Many histamine functions are mediated by G protein-coupled histamine receptors, which include four members, H1R, H2R, H3R, and H4R7,10,11,12,13. Compared to other G protein-coupled receptors, histamine receptors have low sequence homology, which varies between 16-35% at the protein level14. Drugs targeting H1R, H2R, and H3R have been used to treat allergies, gastroduodenal ulcers, and narcolepsy15,16,17. Indeed, the inflammatory response caused by histamine release has long been thought to be mediated by the histamine H1 receptor and H1 receptor antagonists, often referred to as antihistamines, have been used for years to treat allergies18. Commonly used histamine H1 receptor antagonists, such as diphenhydramine, promethazine, and chlorpheniramine, are the most widely used nonspecific antiallergic drugs19. Moreover, H2 antagonists, such as cimetidine, nizatidine, and ranitidine, are widely used to inhibit the oversecretion of gastric acid for the treatment of peptic acid diseases, including duodenal ulcers, gastric ulcers, gastroesophageal reflux and heartburn20,21. In addition, the clinical use of the H3R antagonist pitrizant for narcolepsy (Waxx®) and obstructive sleep apnea (Ozawade®) has been approved.
Histamine receptors participate in various biological processes by binding to diverse G protein subtypes. For example, H1R is known to activate phospholipase C through the Gq/11 signaling pathway, resulting in increased intracellular DAG, IP3, and Ca2+ concentrations22. Notably, this signaling plays important roles in H1R-mediated atherosclerosis23, capillary permeability24, and airway obstruction25 during asthma. After activation, H2R is known to couple to both the Gs and Gq proteins to stimulate gastric acid secretion, alleviate inflammatory responses26,27,28,29,30, and enhance cardiac output and coronary artery blood flow31. In contrast to H1R and H2R, H3R and H4R primarily couple with Gi/Go proteins. Whereas H3R is known to modulate neurotransmitter release, appetite, and circadian rhythm via Gi/Go coupling30,32,33, H4R is mostly expressed in the immune system and participates in eosinophil chemotaxis and degranulation and leukotriene production in mast cells34,35,36. The structures of inactive H1R, H2R, and H3R, as well as the active state of H1R and H4R, have been recently elucidated37,38,39,40,41. However, there is a lack of structural information regarding the active states of H2R and H3R, as well as agonist recognition, selectivity, and diverse G protein coupling mechanisms.
In this work, we determine nine cryogenic electron microscopy (cryo-EM) structures of the four histamine family receptor subtypes in complex with four different downstream G proteins in the presence of endogenous histamine or other synthetic ligands at resolutions ranging from 2.30 to 3.0 Å. The combination of structural analysis and pharmacological characterization enable us to provide important mechanistic insights into endogenous histamine recognition, synthetic agonist selectivity, receptor activation and diverse G protein coupling profiles within the histamine receptor family.
Results
Structural determination
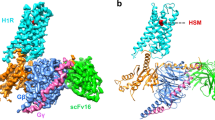
To understand the molecular mechanism of agonist recognition in the histamine receptor (HR) family, we determined nine cryo-EM structures of the four histamine receptor subtypes in complex with four downstream G protein transducers (Gs, Gq, Gi, and Go) in the presence of endogenous ligand histamine or three synthesized agonists, including imetit, immepip, and immethridine, with an overall resolution ranging from 2.30 to 3.0 Å (Fig. 1, Supplementary Figs. 1–4, and Supplementary Tables 1, 2). The EM density maps enabled us to model the ligands, most receptor residues, and heterotrimeric G proteins. The N-terminus, C-terminus, and ICL3 of the four histamine receptors and the α-helix domain of the Gα subunit were not modeled because of poor EM densities (Supplementary Figs. 5, 6).
a–d Cryo-EM density maps (middle) and corresponding models (bottom) of Histamine-bound H1R-Gq (a), Histamine-bound H2R-Gq/Gs (b), Histamine- or Imetit-bound H3R-Gi, and Proxyfan-, or Immethridine-bound H3R-Go (c), Immepip-, or Histamine-bound H4R-Gi (d). Cryo-EM density maps of respective ligands have been shown in gray dotted boxes (top).
A common motif for histamine recognition
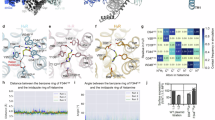
The binding pockets of all four histamine receptors (H1R–H4R) encompassing the putative cryo-EM density of histamine were flanked by TM3 and TM5-TM7 segments. Among the 14 residues constituting the binding pockets of the four histamine receptors responsible for histamine recognition, five residues were structurally equivalent (Fig. 2a). Importantly, in histamine-bound H1R-Gq, H2R-Gs/q, H3R-Gi, and H4R-Gi complexes, an acidic residue D3.32 plays a pivotal role in contacting histamine as described in many GPCRs in recognizing biogenic monoamines. Specifically, in the structures of the histamine-bound H1R-Gq and H2R-Gs/q complexes, the positively charged nitrogen in the ethylamine chain of histamine forms electrostatic interactions with D3.32, while in H3R-Gi and H4R-Gi complexes, D3.32 forms a hydrogen bond with the delta nitrogen in the imidazole ring of histamine, and this interaction is potentially further strengthened by the electrostatic interaction due to the potential protonated status of the delta nitrogen (Fig. 2b). Moreover, two hydrophobic residues, Y/V3.33 and Y6.51 of all HRs, formed interactions with the endogenous agonist histamine (Fig. 2b). These three residues, which are highly conserved in the histamine receptor family, constitute the D3.32-Y/V3.33-Y6.51 motif that is critical for histamine recognition (Fig. 2a and Supplementary Fig. 7a–d). Consistent with these observations, mutations of D3.32A completely impaired the activation of the four histamine receptors in response to stimulation with histamine (Fig. 2c and Supplementary Fig. 7a–d). These three residues, which are highly conserved in the histamine receptor family, constitute the D3.32-Y/V3.33-Y6.51 motif that is critical for histamine recognition. However, it’s worth noting that the functional significance of this motif may vary between subtypes, such as H1R and H2R versus H3R and H4R, due to differences in binding modes. For H1R and H2R, D3.32 and Y6.51 are critical for histamine recognition, as the hydrogen bond contacts. For H3R and H4R, D3.32 is necessary for histamine recognition.
a Barcode representation depicting interaction patterns within the ligand-binding pocket of histamine receptors bound by histamine. Residues of Histamine receptors that interact with histamine are indicated in green circles. The conserved D3.32Y/V3.33 Y6.51 motif is highlighted in orange. b 3D visualization illustrating detailed interactions between histamine and the conserved D3.32Y/V3.33Y6.51 motif in histamine receptors (HRs). Hydrogen bonds are highlighted as red dashed lines. c Heatmap illustrating the effects of mutations in common motif residues within the monoamine recognition pocket of Histamine receptors in response to histamine stimulation. The heatmap displays ΔpEC50 values (ΔpEC50 = pEC50mutant − pEC50wild-type) following histamine stimulation. “ND” denotes no detectable HR activation. The heatmap was generated based on data presented in Supplementary Fig. 7 and Table 7.
Distinct endogenous histamine binding orientation
Interestingly, the orientation of histamine binding to the ligand pockets of H3R and H4R is opposite to that observed with of H1R and H2R, resulting from the rotation of the imidazole ring by ~180° (Fig. 3a). The observed histamine binding modes were further supported by computational molecular dynamics (MD) simulation (Fig. 3b and Supplementary Fig. 8). Notably, two conserved aromatic amino acids in TM7, specifically F7.39 and W7.43, exclusive to H3R and H4R, are replaced by smaller hydrophobic residues I/L7.39 and Y7.43 in H1R and H2R (Fig. 3a, c). F7.39 and W7.43 in H3R and H4R formed strong π-π packings with the imidazole ring and thus could be responsible for the placement of the imidazole ring close to the TM7 of H3R and H4R. Consistent with this hypothesis, mutations such as F7.39A and W7.43A significantly reduced the activation of H3R/H4R in response to histamine stimulation (Fig. 3c, d). In contrast, two phenylalanines, F6.52 and F6.55 in TM6, which were conserved in H1R and H2R but replaced by T/S6.52 and M/T6.55 in H3R and H4R, formed strong π-π interactions with the imidazole ring of histamine in the H1R and H2R structures and could determine the specific orientation of the imidazole ring in the TM5 and TM6 of H1R and H2R (Fig. 3a, c). Additionally, we have generated the I/L7.39A and Y7.43A mutants in H1R (I4547.39A, and Y4587.43A) and H2R (L2747.39A and Y2787.43A), respectively. Together with the F7.39A and W7.43A corresponding position mutants in H3R (F3987.39A and W4027.43A) and H4R (F3447.39A and W3487.43A), we examined the effect of mutations at positions 7.39 and 7.43 on the binding affinities of histamine within the histamine receptor families (H1R–H4R) using radioligand binding assays. Consistent with the hypothesis, the mutations of F3987.39A and W4027.43A in H3R and F3447.39A and W3487.43A in H4R reduced the binding affinities of histamine by more than 30-fold. In contrast, the mutations of I/L4547.39A and Y4587.43A in H1R and L2747.39A and Y2787.43A in H2R had a significantly lesser impact on binding affinities, with reductions around 3-5 times, respectively. This is consistent with our structural observation that the F7.39 and W7.43 in H3R and H4R form pi-pi stacking with the imidazole ring of histamine, whereas in H1R and H2R, the corresponding residues at positions 7.39 and 7.43 do not form pi-pi interactions with the ethylamine chain of histamine. Thus, mutations at these residues have a lesser impact on the binding affinity of histamine to H1R and H2R (Supplementary Fig. 9). Collectively, we speculated that the specific presence of large hydrophobic residue motifs in TM6 and TM7, including the conserved F6.52X2F6.55 in the TM6 of H1R and H2R and F7.39X3W7.43 in the TM7 of H3R and H4R, are determinants of the specific orientation of the histamine imidazole ring.
a Detailed interactions between the imidazole ring and residues F6.52 and F6.55 in H1R and H2R, along with F7.39 and W7.43 in H3R and H4R. b Average root mean square deviation (RMSD) values of histamine in H1R-Gq, H2R-Gs, H3R-Gi, H4R-Gi complexes during triplicate 200 ns molecular dynamics (MD) simulations. The rationality of histamine binding orientations in respective histamine receptor complexes was supported by MD simulation. c Sequence alignments of key residues involved in histamine binding across histamine receptor members. Conserved residues F6.52 and F6.55 in TM6 of H1R and H2R, as well as F7.39 and W7.43 in TM7 of H3R and H4R, are highlighted in red, illustrating their crucial role in determining the specific orientation of histamine’s imidazole ring. Notations for species include h (human), r (rat), and m (mouse). d, e Representative concentration-dependent response curves of H3R (d) and H4R (e), along with their pocket key residue mutants F7.39 and W7.43, in response to histamine by Gαi-Gγ dissociation assay. The summarized pEC50 and Emax values are provided in Supplementary Table 3. Data from three independent experiments are presented as the mean ± SEM (n = 3).
Our findings contrast with those reported in a recently published study, where histamine was observed to bind to H4R in a manner similar to that seen in H1R/H2R42. Importantly, our histamine-bound H4R structures are coupled with heterotrimeric Gi proteins, whereas those resolved by Im et al. involve complexes with Gq. This distinction suggests the possibility that the varying binding modes of histamine to H4R may be influenced by the interaction with different downstream G proteins.
Ligand pocket of H1R and H2R
Structural comparison revealed that the ligand pockets of H1R and H2R are clearly different from those of H3R and H4R. The ligand pockets of H1R and H2R can be divided into two portions: the imidazole group recognition pocket (orthosteric pocket) and the secondary binding pocket (SBP) (Fig. 4a, b and Supplementary Fig. 10a, b). On the other hand, the ligand pockets of H3R and H4R predominantly consist of three portions: the orthosteric pocket, the SBP, and the transmembrane subpocket (TMSP), which is composed of seven residues (Fig. 4a, b).
a 3D visualization showing detailed interactions between Histamine and the pocket residues of histamine receptors (from left to right panel: H1R, H2R, H3R, and H4R). Hydrogen bonds or polar interactions are depicted as red dashed lines, charge-charge interactions are depicted as yellow dashed lines, and red dots shown in the rightmost panel represent water molecules. Note that the primary nitrogen in the ethylamine chain of histamine is protonated and positively charged (marked with +), which forms electrostatic interactions between D3.32 in H1R/H2R (marked with −), and E5.46 in H3R/H4R (marked with −). In addition, the delta nitrogen in the imidazole ring of histamine could undergo protonation (marked with +), potentially enabling an electrostatic interaction with D3.32 in H3R/H4R (marked with −). b Cross-sectional view of the ligand binding pockets in the Histamine-bound H1R-Gq, H2R-Gq, H3R-Gi, and H4R-Gi complexes (from left to right). Residues within the orthosteric pocket (OP), secondary binding pocket (SBP), and transmembrane subpocket (TMSP) are highlighted in brown, dark green, and magenta, respectively. c Barcode representation illustrating residues in the imidazole group recognition pocket (IGRP) of four histamine receptors (HRs). Note that Y6.51 is a common residue among all HRs. H1R and H2R share a similar IGRP, differing only in unique residues: W4.56 and N5.46 in H1R, and D5.42 in H2R. The IGRPs of H3R and H4R are identical.
Approximately 8 residues constitute the orthosteric pocket of H1R and H2R for histamine recognition, of which five residues are nearly identical (Fig. 4b, c). Importantly, the conserved hydrophobic residues in TM6 and TM7, including W6.48, Y6.51, F6.52, and F6.55, formed extensive hydrophobic interactions with the imidazole ring (Fig. 4a). In addition, T3.37 formed a polar interaction with the amine group of the imidazole ring in H1R and H2R. In H1R, W1584.56 and N1985.46 formed hydrophobic and polar interactions with the imidazole ring, respectively (Supplementary Fig. 10c). Conversely, in H2R, D1865.42 formed a charge‒charge interaction with histamine, which was not observed in H1R (Fig. 4a, b and Supplementary Fig. 10d).
Ligand pocket of H3R
In the four H3R complex structures, the ligands histamine, imetit, immethridine, and proxyfan contain imidazole groups with modified tails. In all these structures, the imidazole group is placed in the same position, highlighting the conserved mechanism of imidazole ring recognition in different agonists by H3R (Figs. 4a, 5a). Along with the extended tail length, the ligand pocket gradually moves toward the TMSP (Figs. 4a, b, 5a, b). In total, four hydrophobic residues from TM6 and TM7 and one polar residue from TM3 constituted the orthosteric pocket, in which F3987.39 and W4027.43 form π-π packings with the imidazole group, while Y3746.51 and L4017.42 form hydrophobic interactions with these four ligands (Figs. 4a, b, 5a, b). Specifically, D1143.32 forms both hydrogen bonds and charge‒charge interactions with the nitrogen atom of the imidazole group (Figs. 4a, 5a). Consistent with these observations, the D1143.32A mutation totally abolished the activation of H3R in response to stimulation with these agonists (Fig. 2c).
a 3D visualization showing detailed interactions of Imetit, Immethridine, and Profaxy with the pocket residues of H3R, and Immepip with the pocket residues of H4R. Hydrogen bonds are depicted as red dashed lines. b Cross-sectional view displaying the ligand binding pockets in the Imetit-H3R-Gi, Immethridine-H3R-Go, Profaxy-H3R-Go and Immepip-H4R-Gi complexes (from left to right). Residues in the orthosteric pocket (OP), secondary binding pocket (SBP), and transmembrane subpocket (TMSP) are indicated in brown, dark green, and magenta, respectively.
The presence of additional chemical groups in the agonists imetit, immethridine, and proxyfan revealed that the TMSP is composed of residues from TM3-TM6, which include Y1153.33, C1183.36, Y1674.57, S2035.43, E2065.46, W3716.48 and T3756.52 (Fig. 5a, b). Along with the increase in the ligand tail length, then number of ligand interactions within the pocket also increases. For instance, the tail of histamine only interacts with Y1153.33, C1183.36, and E2065.46, whereas the tail of proxyfan interacts with all seven residues in the TMSP (Fig. 5a, b). Similarly, this TMSP exists in H4R and is composed of eight residues from TM3-TM6 (Fig. 5a, b). The different imidazole ring binding orientations and the presence of the TMSP are unique to H3R and H4R, and these characteristics may assist the design of ligands that preferentially target these two histamine receptors over H1R and H2R.
Mechanism of immethridine selectivity for H3R
In contrast to immepip, which has high affinities for both H3R and H4R, immethridine showed selectivity toward H3R by approximately 300 times that of H4R (Fig. 6a). Compared with that of immepip, the piperidine ring of immethridine was replaced by a pridine ring, which was more rigid (Fig. 6b). Structural comparison of the immethridine-H3R-Go complex and the immepip-H4R-Gi complex structures indicated that the piperidine ring assumed a distorted conformation to accommodate Q3477.42 in the immepip-H4R-Gi complex structure, whereas Q3477.42 is not compatible with the flat conformation of the pridine ring in immethridine (Figs. 5a, 6b, c and Supplementary Fig. 11a, b). Notably, Q3477.42 in H4R was replaced by L4017.42 in H3R (Fig. 6b–d), which formed a suitable hydrophobic interaction with the pyridine ring (Supplementary Fig. 11a, b). The binding poses and interactions of immethridine and immepip within the ligand pockets of H3R and H4R was further confirmed by MD simulation, respectively (Supplementary Fig. 11c, d). Consistent with these observations, the L4017.42 Q mutation of H3R decreased immethridine activity toward H3R by approximately 400-fold, whereas the Q3477.42L mutation significantly increased the immethridine activity toward H4R. In contrast, the L401Q mutation of H3R and the Q347L mutation of H4R had little difference in the effect of immepip (Fig. 6a). Collectively, our structural and pharmacological analysis indicated that the specific interaction between the rigid pyridine ring of immethridine and L4017.42 in H3R is a key determinant of immethridine selectivity for H3R over H4R. The sequence difference between L4017.42 in H3R and Q3477.42 in H4R could be utilized for the design of ligands that specifically target either H3R or H4R.
a Representative concentration-dependent response curves of H3R and its L4017.42Q mutant, and H4R and its Q3477.42L mutant, in response to Immethridine and Immepip using the Gαi-Gγ dissociation assay. The pEC50 and Emax values are provided in Supplementary Table 4. Data from three independent experiments are presented as the mean ± SEM (n = 3). b 2D depiction of contacts between H3R with the ligand Immethridine (left panel) and H4R with the ligand Immepip (right panel). Hydrogen bonds are indicated by red dotted lines. The piperidine ring is highlighted in red shade, while the pyridine ring is highlighted in green shade. c 3D representation showing detailed interaction of immethridine/immepip with the unique L/Q7.42 of H3R (left panel) and H4R (right panel). Hydrogen bonds are depicted as red dashed lines. d Barcode representation illustrating potential interaction patterns in the ligand pockets of H3R (immethridine-bound) and H4R (immepip-bound). Common residues within histamine receptors interacting with ligands are depicted as gray circles, with the unique residue L/Q7.42 highlighted in green for H3R and orange for H4R.
Activation of histamine receptors
Comparing the structure of active H1R with the previously published structures of inactive H1R43, there is a dramatic outward movement of the intracellular part of TM6 (~27.4°) (Fig. 7a). In the extracellular region, there is an inward movement of TM6 (~13.1°) and TM3 (~12.1°) (Fig. 7a). On the cytoplasmic side, H1R contains the conserved D1243.49R1253.50 Y2105.58 (DRY) motif and the N4647.49 P4657.50 XXF/Y4687.53 motif. These motifs are substantially rearranged in active H1R relative to inactive H1R (PDB: 3RZE) (Fig. 7b, c). Moreover, we found that both the F4246.44 and W4286.48 residues in H1R moved downward and that W4286.48 directly contacted histamine (Fig. 7d, e). Structural rearrangements of these two aromatic residues during receptor activation have been reported44. A comparison of the three active state structures of H1R and H2R suggested that the conformations of F6.44 and W6.48 are identical in these two receptors. Similarly, in the active structure of H2R, W6.48 also directly contacted histamine (Fig. 7e). In contrast, the conformations of W6.48 in H3R and H4R are different from those in the other two receptors and do not directly interact with ligands (Fig. 7d, e and Supplementary Fig. 12a).
a Superimposition of active H1R structures (sandy brown) to the inactive H1R structure (dark gray, PDB: 3RZE). Red arrows indicate conformational changes in transmembrane regions (TMs). b, c A superimposition of the DRY (b) and NPxxY (c) motifs between the active H1R structure (sandy brown) and the inactive H1R structure (dark gray, PDB: 3RZE). Conformational changes in the residues are highlighted by red arrows. d Superimpositions of the residues F6.44 and W6.48 are depicted between the active and inactive H1R (left panel), H1R and H2R (middle panel), and H3R and H4R (right panel).The conformational rearrangements of F6.44 and W6.48 in the active H1R and inactive H1R structures are displayed by black arrows. e Cross-sectional view of the ligand-binding pocket in the Histamine-bound H1R-Gq, H2R-Gs, H3R-Gi, and H4R-Gi complexes. Distances between ligands and the traditional toggle switch are indicated by red dashed lines.
ICL3 is a structural determinant for the selective coupling of H2R to Gs and Gq
It has been reported that H2R couples with multiple downstream effectors upon activation, including Gs and Gq. We conducted a detailed exploration of both the Gs and Gq coupling mechanisms of H2R through structural analyses of H2R-Gs and H2R-Gq complexes (Fig. 8a). To assist in determining of H2R-Gq complex, a chimeric Gαsq was employed instead of Gαq (Supplementary Fig. 1d). Among 11 interface contacting residues between Gαsq and H2R, ten residues were identical to those in the Gαq subunit, and one residue differed. Consequently, we replaced these differing residues in Gαsq with the original residues in Gαq and performed MD simulation (Supplementary Fig. 12d, e). The computational simulation suggested that all the aforementioned residues formed interfaces with H2R, with additional contacts formed between Gαq and two residues in H2R (Fig. 8b). All of these interactions were further validated by mutagenesis analysis (Supplementary Fig. 15b). A total of 22 residues spanning TM3, TM5, TM6, Helix 8 (H8), and ICL2 and ICL3 of H2R were observed to form direct interactions with both the Gs and Gq trimers (Fig. 8b). There are more interactions observed in H2R-Gs complex than in the H2R-Gq complex, including additional contacts mediated by ICL2, ICL3, TM5, TM6, and H8 (Fig. 8b). Conversely, in comparison to Gs, Gq exhibited extra contacts with TM6 and a unique contact with TM7 of H2R (Fig. 8b).
a Superposition of H2R-Gs (green-blue) and H2R-Gq (dark gray-wheat) complex based on the transmembrane helices bundle. b Barcode representation illustrates the interaction patterns at the interface between the receptor and their respective G proteins in the cryo-EM structures of H2R-Gs, H2R-Gsq, and computationally simulated H2R-Gq complexes. Common residues are indicated by cyan circles, residues showing unique interaction with Gq are indicated by light green circles, residues showed unique interaction with MD Gq are indicated by purple circles, residues showing unique interaction with Gs are indicated by orange circles, and residues that show no interaction with G proteins are depicted as white circles. c 3D representation illustrating detailed interactions between residues in Gαs (upper panel) and Gαq (lower panel) with the residues of H2R. Hydrogen bonds are shown as red dashed lines. d Average root mean square deviation (RMSD) values of ICL3 in H2R-Gq and H2R-Gs complexes during 200 ns molecular dynamics (MD) simulations. e Detailed interactions between W222ICL3 on ICL3 of H2R and Gs subunit (left panel), and Gq subunit (right panel). Representative conformations of the binding of W222ICL3 in H2R to Gq were obtained from molecular dynamics simulations. Note that D315 and P318 in Gαq are the equivalent residues of R347 and T350 in Gαs. Hydrogen bond is depicted as a red dashed line. f, g Representative concentration-dependent response curves of H2R and its W222ICL3V and W222ICL3A mutants in response to histamine using the Gαs-Gγ dissociation assay (f) and the Gαq-Gγ dissociation assay (g). The pEC50 and Emax values are provided in Supplementary Table 5. Data from three independent experiments are presented as the mean ± SEM (n = 3).
In total, 22 residues that interact with both Gs and Gq were derived from the TM3, ICL2, TM5, ICL3, TM6 and H8 of H2R (Fig. 8b). Three residues from TM3 (R1163.50, A1193.53, and V1203.54), two residues from ICL2 (P123ICL2 and L124ICL2), and five residues from TM5–TM6 (I2055.61, V2085.64, A2095.65, K2316.32, and L2366.37) were common in both H2R-Gs and H2R-Gq complex structures, and one residue from H8 (R2938.48) of H2R form conserved hydrophobic packing with Gq and Gs (Supplementary Fig. 12b, c). In addition, Q2125.68 of H2R formed H-bonds with Q350 of Gαq or R385 of Gαs (Fig. 8C). R2155.71 of H2R formed conserved charge-charge interactions with both Gαq and Gαs (Fig. 8c). We speculated that these common interactions are determinants of H2R coupling to both Gs and Gq subtypes. Notably, N357 of Gαq formed a polar interaction with the backbone carbonyl oxygen of L2917.56 of H2R, which is unique to H2R-Gq but absent in H2R-Gs (Supplementary Fig. 12f). Moreover, K2236.24 and T2266.27 formed additional H-bonds with D315 and the backbone carbonyl oxygen of S320 of Gαq, respectively (Supplementary Fig. 12g). Consistently, Mutations of K2236.24A and T2266.27A had a larger impact on Gq activation than Gs activation in response to histamine stimulation, consistent with these observations (Supplementary Fig. 15b).
Compared with Gαq, Gαs formed more residue contacts with H2R (Fig. 8b). For instance, V128ICL2 of H2R formed extra hydrophobic interaction with A39 of Gαs (Supplementary Fig. 12h). In addition, A2135.69 and K2236.24 in TM5 and TM6 are responsible for more hydrophobic interactions with L394 and T350 of Gαs (Supplementary Fig. 12i). Moreover, R2968.51 of H2R formed additional charge-charge interaction with E392 of Gαs. Interestingly, compared with that in the H2R-Gαq complex structure, the EM density of ICL3 of H2R in the histamine-H2R-Gs structure is more continuous and clear. Therefore, we modeled ICL3 in the histamine-H2R-Gs structure and analyzed its interaction with Gs. W222 in ICL3 of H2R forms a hydrogen bond and a cation-π interaction with T350 and R347 of Gαs, respectively (Fig. 8e). In our computationally simulated H2R-Gq complex, we observed a swinging motion of W222 (Fig. 8d), resulting in the complete abolition of the specific interactions between W222 and D315, as well as P318 in Gαq (D315 and P318 are the equivalent residues of R347 and T350 in Gαs) (Fig. 8e). Consistent with this observation, mutations of W222A and W222V totally abrogated the Gs activity of H2R in response to histamine stimulation, whereas the same mutations showed no significant effects on histamine-induced Gq activity downstream of H2R (Fig. 8f, g). Moreover, we speculated that ICL3 may play an important role in the selectivity of Gs and Gq. The important interaction between these two residues and Gαs is essential to stabilize ICL3 of H2R. Additionally, we compared the outward displacement of TM6 when the H2R receptor couples with Gq or Gs. Consistent with recent reports, we observed that TM6 exhibits a greater outward displacement for receptor coupling to Gs than to Gq45 (Supplementary Fig. 13).
Determinants of Gs and Gi/o coupling differences among different histamine receptors
Whereas histamine receptors H1R and H2R primarily couple to Gs and Gq, H3R and H4R primarily couple with Gi/o. Structural and mutational analysis of H2R-Gs and H2R-Gq revealed that ICL3 is a potential structural determinant for the selective coupling of H2R to Gs and Gq (Fig. 8). An intriguing follow-up question is how different histamine receptor members selectively couple to Gs or Gi/Go proteins. Comparing the structures of H2R-Gs, H3R-Gi/o, and H4R-Gi, we found that six residues of TM3, TM5, and TM6 of H2R, H3R, and H4R were observed to form conserved interactions with both Gs and Gi (Fig. 9a, b). In addition, five residues of ICL2, TM6, and H8 of H2R, H3R, and H4R formed almost identical interactions with both Gs and Gi (Fig. 9b).
a Cartoon and surface representation of the binding area of H2R (left panel), H3R (middle panel), and H4R (right panel) with their respective coupled Gα subunits. Residues within 4.5 Å of H2R or H3R in Gαs or Gαi are highlighted in wheat or salmon, respectively. b Comparison of residues between agonist-bound H2R, H3R, or H4R that contact their corresponding downstream G protein subtypes, Gαs, Gαi, or Gαo, respectively. Residues of HRs that show common interaction with Gs or Gi/o are indicated by cyan circles, residues showing unique interaction with Gs are indicated by orange circles, residues showing unique interaction with Gi/o are indicated by purple circles, and residues that show no interaction with G proteins are depicted as white circles. Residue positions with Ballesteros–Weinstein numbers are shown as superscripts for H2R, H3R, or H4R on the top of the scheme. c Structural comparison of the TM5–TM6 regions of H2R (olive drab), H3R (medium slate blue), and H4R (dark cyan) structures. d Representative concentration-dependent response curves of H3R and H4R, along with their R34.54A, F6.25A and R6.29A mutants, in response to histamine/Proxyfan (H3R) and histamine/Immepip (H4R) using the Gαi-Gγ dissociation assay. The pEC50 and Emax values are provided in Supplementary Table 6. Data from three independent experiments are presented as the mean ± SEM (n = 3).
Interestingly, we found that the extended TM5 in H2R migrates toward TM6 starting from the R2045.60 position by ~15° (Fig. 9c and Supplementary Fig. 14a), allowing substantially more interactions between the TM5 of H2R and Gs than the interactions between the TM5 of H3R or H4R and the Gi/Go proteins (Fig. 9a). Specifically, the H2R-Gs complex exhibited four residues from the TM5 (I2055.61, V2085.64, A2135.69, and I2276.28) of H2R that formed more hydrophobic packings with three residues of the α5 helix (L394, L393, Q384) of Gαs (Supplementary Fig. 14b). Moreover, Q2125.68 and R2155.71 of H2R formed H-bonds or charge‒charge interactions with Q385 and D381 from the α5 helix of Gαs, respectively (Supplementary Fig. 14c). In addition, I2165.72 and W2226.23 of H2R formed hydrophobic packing with Gs (Supplementary Fig. 14d). These interactions were observed only in H2R-Gs complex structures but not in structures of H3R/H4R in complex with Gi/Go proteins. Consistent with these observations, R2155.71A and I2276.28A mutations significantly decreased Gs coupling to H2R (Supplementary Fig. 12c). In contrast, L5.71A and S/R6.28A mutations of structurally equivalent residues in H3R/H4R showed no significant or fewer effects on H3R/H4R coupling (Supplementary Fig. 15c).
Structural comparison of H3R/H4R-Go/Gi structures with the H2R-Gs structure also revealed special structural features of these two receptors responsible for Gi/Go engagements. Unique to H3R, N2.39, Q34.57, and R4.41 formed specific interactions with Gi/Go (Supplementary Fig. 14e). Moreover, common to both H3R and H4R, R34.54, F/V6.25 and R6.29 specifically formed interactions with the α5 helix and α4-β6 turn of Gi/Go (Supplementary Fig. 14f–h). These interactions were not observed in H2R-Gs complex structures. Consistently, mutations of R34.54 F/V6.25 and R6.29 residues significantly decreased histamine and their synthetic agonists induced Gi/Go activity of H3R or H4R (Fig. 9d and Supplementary Fig. 15a). Altogether, our studies suggested that the rotation and more compact packing of the TM5 in H2R with Gs and R34.54-F/V6.25-R6.29 interactions of H3R and H4R with Gi/Go are determinants for the Gs and Gi/o coupling differences among different histamine receptor members.
Discussion
In summary, we have provided a structural landscape of ligand recognition and G protein subtype coupling of histamine receptors by determining 9 structures of the four receptors (H1R–H4R) in complex with four different G protein transducers (Gs, Gq, Gi, and Go) and bound by the endogenous ligand histamine or three other synthesized agonists. Analysis of these structures identified that the presence of the D3.32-Y/V3.33-Y6.51 motif was shared by all histamine receptors, suggesting that this motif plays critical roles in histamine recognition. Interestingly, we observed that the endogenous agonist histamine binds to the ligand pocket of the four histamine receptors in different orientations, which results from the rotation of the imidazole ring by ~180o. Further structural and sequence comparisons and mutational studies enabled us to identify that the conserved F6.52X2F6.55 in the TM6 of H1R and H2R and F7.39X3W7.43 in the TM7 of H3R and H4R are determinants of these distinct orientations. Detailed characterization of histamine binding to H1R and H2R, combined with a structural comparison with the ligand pocket of H3R and H4R to accommodate synthetic agonists, revealed that two specific pockets (orthosteric pocket and SBP) and three specific pockets (orthosteric pocket, SBP and TMSP) were present in H1R and H2R or H3R and H4R, respectively. Residue differences in these pockets may be exploited for the design of specific ligands targeting selected HR members. For example, the substitution of L4017.42 in H3R by Q3477.42 in H4R was the determinant of immethridine selectivity of H3R compared with H4R. Below the ligand pocket, we identified conformational changes in the key structural elements underlying the activation mechanism of H1R. On the cytoplasmic side, we found that ICL3 of H2R is the determinant of Gs/Gq coupling differences. Moreover, more compact packing of TM5 of H2R with Gs and the unique interactions of the R34.54-F/V6.25-R6.29 motif of H3R and H4R with Gi/Go underlies Gs and Gi/o selectivity among the different histamine receptor members. This information may not only further our understanding of the ligand recognition, activation, and G protein coupling mechanisms of the histamine receptor family but may also be helpful for designing selective ligands targeting histamine receptor family members.
Methods
Constructs
DNA sequences encoding human histamine receptors (H1R, H2R, H3R, H4R) were codon-optimized and synthesized for Sf9 insect cell expression and cloned into a pFastBac vector containing an N-terminal bovine prolactin signal peptide followed by a FLAG epitope (DYKDDDD) and an 8×His tag. A fragment of β2AR N-terminal tail (BN)46 was introduced to improve protein expression. One copy of BN for H1R and H3R, and two copies for H2R and H4R were fused at the N-terminus of the receptors with a TEV protease cleavage site. A NanoBiT tethering strategy was used to improve sample quality as described previously47. The LgBiT subunit was fused at the C-terminus of the receptors in different manners (Supplementary Fig. 1a). For H3R, LgBiT was directly fused at the C-terminus. For H1R, LgBiT was fused with a 15-amino acid linker (GSSGGGGSGGGGSSG). For H2R and H4R, residues after A308 and Q378 were truncated respectively and LgBiT was fused with the 15-amino acid linker.
A dominant negative human Gαi1 with four mutations (S47N, G203A, E245A, A326S)48, miniGαo49, miniGαs with two extra mutations (G226A and A366S), and miniGαq50 were cloned into the pFastBac vector. Human Gβ1 with an N-terminal 6×His tag and human Gγ2 were cloned into the pFastBac-Dual vector. The HiBiT subunit was fused to Gβ1 at the C-terminus with a 15-amino acid linker (GSSGGGGSGGGGSSG).
Protein expression and purification
Nb35 and scFv16 were expressed and purified as previously described in refs. 51,52. Briefly, Nb35 was expressed in BL21 (DE3) E.coli cells and purified by Ni affinity chromatography with a C-terminal 6×His tag. scFv16 was expressed in Tni insect cells and purified from the medium by Ni affinity chromatography before the C-terminal 8×His tag was removed by TEV protease (NEB, Cat# P8112S). Nb35 and scFv16 were further purified by size-exclusion chromatography and stored at −80 °C before use.
Histamine receptor-G protein complexes were co-expressed in Sf9 insect cells using the Bac-to-Bac method. Cells were infected with three types of viruses (receptor, Gα and Gβγ) at the ratio of 1:1:1 and cultured for 48 h. Cells were harvested by centrifugation and stored at −80 °C before use.
Purification of the complexes followed a common set of procedures. Frozen cells were thawed in lysis buffer containing 20 mM HEPES pH 7.5, 50 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 25 mU/ml Apyrase (NEB, Cat# M0398S), 1–10 μM agonist, 2.5 μg/ml leupeptin (Sigma-Aldrich, Cat# L2884), 300 μg/ml benzamidine (Sigma-Aldrich, Cat# B6506), 100 μM TCEP (Thermo Fisher, CAS 5961-85-3). The solution was incubated at room temperature for 2 h to facilitate complex formation. After incubation, the sample was centrifuged at 30,700×g for 30 min to collect cell membranes. The membranes were resuspended with a glass dounce homogenizer in solubilization buffer containing 20 mM HEPES pH 7.5, 100 mM NaCl, 0.5% (w/v) LMNG, 0.1% (w/v) CHS, 10% (v/v) glycerol, 10 mM MgCl2, 5 mM CaCl2, 12.5 mU/ml Apyrase, 1–10 μM agonist, 2.5 μg/ml leupeptin, 300 μg/ml benzamidine, 100 μM TCEP and incubated at 4 °C for 2 h. The supernatant was isolated by centrifugation at 38,900×g for 45 min, and then incubated with Ni resin at 4 °C for 2 h. After binding, the resin was washed with 20 column volumes of buffer A consisting of 20 mM HEPES pH 7.5, 100 mM NaCl, 0.05% (w/v) LMNG (Anatrace, Cat# NG310), 0.01% (w/v) CHS (Sigma-Aldrich, Cat# C6512), 1–10 μM agonist, 2.5 μg/ml leupeptin, 300 μg/ml benzamidine, 100 μM TCEP with 20 mM imidazole before bound material was eluted with 5 column volumes of buffer A containing 400 mM imidazole. The eluate was supplemented with 2 mM CaCl2 and incubated with M1 anti-FLAG resin overnight at 4 °C. The resin was washed with 10 column volumes of buffer A supplemented with 2 mM CaCl2, and the protein was eluted with 3.5 column volumes of buffer A containing 5 mM EDTA and 200 μg/ml FLAG peptide. scFv16 was added to the eluate at the molar ratio of 1.3:1 over the receptor-G protein complex. After incubated on ice for 20 min, the sample was concentrated to 0.5 ml with a 100 kD cut-off centrifugal filter and loaded onto Superdex 200 10/300 GL column pre-equilibrated with buffer containing 20 mM HEPES pH 7.5, 100 mM NaCl, 0.00075% (w/v) LMNG, 0.00025% (w/v) GDN, 0.00015% (w/v) CHS, 1–10 μM agonist and 100 μM TCEP. Fractions of the monomeric peak were analyzed by SDS-PAGE and Coomassie staining. Then the desired fractions were pooled and concentrated for cryo-EM studies.
Histamine was used at the final concentration of 10 μM for H1R and H2R in the process of purification, but used at 1 μM for H3R and H4R. Imetit, Immepip, Immethridine, and Proxyfan were used at the final concentration of 1 μM. For the purification of the histamine-H2R-miniGs-Nb35 complex, scFv16 was not added, and Nb35 was added to the lysis buffer at a final concentration of 10 μg/ml. For the purification of the histamine-H2R-miniGq-scFv16 complex, the Superdex 200 column was substituted with a Superose 6 Increase 10/300 GL column to separate the monomeric complex from aggregation.
Cryo-EM sample preparation and data acquisition
For cryo-EM grids preparation of the H1R-Gq, H2R-Gq, H2R-Gs, H3R-Gi, H3R-Go, and H4R-Gi complexes, 3 μl of the purified complexes at ~20 mg/ml were applied onto glow-discharged holey carbon grids (Quantifoil, Au300 R1.2/1.3). Grids were plunge-frozen in liquid ethane using Vitrobot Mark IV (Thermo Fischer Scientific). Cryo-EM imaging was performed on a Titan Krios electron microscope at 300 kV accelerating voltage using a Gatan K3 Summit direct electron detector with an energy filter. Micrographs were collected with a nominal magnification of 81,000× using the EPU software in super-resolution mode at a calibrated pixel size of 1.07 Å and a defocus range of −1.2 to −2.2 μm. Each stack was acquired with an exposure time of 3.5 s and dose-fractionated to 32 frames with a total dose of 55 e-Å−2. A total of 6208 movies, 5078 movies, 5934 movies, 4222 + 2189 movies, 4382 movies, 5122 movies, 4925 + 1303 movies, 5021 movies, and 4464 movies were collected for Histamine-H1R-Gq, Histamine-H2R-Gq, Histamine-H2R-Gs, Histamine-H3R-Gi, Imetit-H3R-Gi, Proxyfan-H3R-Go, Immethridine-H3R-Go, Immepip-H4-Gi complex, and Histamine-H4-Gi complexes, respectively.
Cryo-EM data processing
Image stacks were subjected to beam-induced motion correction using MotionCor253. Contrast transfer function (CTF) parameters were estimated from motion-corrected images using Gctf54.
For the datasets of Histamine-H1R-Gq complex, total of 8,895,197 particles were auto-picked using RELION 3.155 and then subjected to reference-free 2D classification to discard bad particles. After several rounds of 3D classification, two well-defined subsets were selected. Further 3D classification focusing the alignment on the complex, produced one high-quality class accounting for 91,716 particles. These particles were subsequently subjected to 3D refinement, CTF refinement, and Bayesian polishing, which generated a map with an indicated global resolution of 2.66 Å at a Fourier shell correlation (FSC) of 0.143.
For the Histamine-H2R-Gq complex and Histamine-H2R-Gs complex, a total of 6,586,815 and 8,349,077 particles were auto-picked and then subjected to reference-free 2D classification to discard poorly particles, respectively. For the Histamine-H2R-Gq complex, after several rounds of 3D classification, one well-defined subset was selected. Further 3D classification focusing the alignment on the complex, produced one high-quality subset accounting for 118,539 particles. The selected subset was subsequently subjected to 3D refinement, CTF refinement, and Bayesian polishing, which generated a map with an indicated global resolution of 2.97 Å at a Fourier shell correlation (FSC) of 0.143. For the Histamine-H2R-Gs complex, after several rounds of 3D classification, two well-defined subsets were selected. Further 3D classification focusing the alignment on the receptor, produced one high-quality subset accounting for 229,186 particles. These particles were subsequently subjected to 3D refinement, CTF refinement, and Bayesian polishing, which generated a map with an indicated global resolution of 2.56 Å at a Fourier shell correlation (FSC) of 0.143.
For Histamine-H3R-Gi, Imetit-H3R-Gi, Proxyfan-H3R-Go, and Immethridine-H3R-Go complexes, a total of 10,166,561, 6,019,821, 7,515,408, and 7,271,515 particles were auto-picked using RELION 3.1 and then subjected to reference-free 2D classification to discard badly defined particles, respectively. For the Histamine-H3R-Gi complex, after several rounds of 3D classification, four well-defined subsets were selected. Further 3D classification focusing the alignment on the receptor, produced one high-quality class accounting for 319,658 particles. These particles were subsequently subjected to 3D refinement, CTF refinement, and Bayesian polishing, which generated a map with an indicated global resolution of 2.70 Å at a Fourier shell correlation (FSC) of 0.143. For the Imetit-H3R-Gi complex, after several rounds of 3D classification, two well-defined subsets were selected. Further 3D classification focusing the alignment on the complex, produced one high-quality class accounting for 103,738 particles. The selected subset was subsequently subjected to 3D refinement, CTF refinement, and Bayesian polishing, which generated a map with an indicated global resolution of 2.77 Å at a Fourier shell correlation (FSC) of 0.143. For the Proxyfan-H3R-Go complex, after several rounds of 3D classification, one subset was selected. Further 3D classification focusing the alignment on the complex, produced one well-defined class accounting for 85,007 particles. These particles were subsequently subjected to 3D refinement, CTF refinement, and Bayesian polishing, which generated a map with an indicated global resolution of 2.77 Å at a Fourier shell correlation (FSC) of 0.143. For the Immethridine-H3R-Go complex, after several rounds of 3D classification, two subsets were selected. Further 3D classification focusing the alignment on the complex, produced one class accounting for 160,325 particles. The selected subset was subsequently subjected to 3D refinement, CTF refinement, and Bayesian polishing, which generated a map with an indicated global resolution of 2.77 Å at a Fourier shell correlation (FSC) of 0.143.
For the Immepip-H4-Gi complex and the Histamine-H4-Gi complex, a total of 7,742,393 and 6,851,148 particles were auto-picked and then subjected to reference-free 2D classification to discard bad particles, respectively. For the Immepip-H4-Gi complex, after several rounds of 3D classification, one well-defined class was selected. Further 3D classification focusing the alignment on the complex, produced one high-quality subset accounting for 76,732 particles. The selected subset was subjected to 3D refinement, CTF refinement, and Bayesian polishing, which generated a map with an indicated global resolution of 2.63 Å at a Fourier shell correlation (FSC) of 0.143. For the Histamine-H4-Gi complex, after several rounds of 3D classification, one subset was selected. Further 3D classification focusing the alignment on the complex, produced one well-defined subset accounting for 170,852 particles. The selected subset was subjected to 3D refinement, CTF refinement, and Bayesian polishing, which generated a map with an indicated global resolution of 2.30 Å at a Fourier shell correlation (FSC) of 0.143.
Model building and refinement
The models were built based on previously reported structures. Briefly, coordinates of the subunits from reported structures were docked into the density map using UCSF Chimera. Then the model was manually built-in COOT and refined by real-space refinement using PHENIX. First, we built the model of histamine-H4R-Gi-scFv16 based on the M1R crystal structure (PDB: 5CXV) and the Gi-scFv16 crystal structure (PDB: 6CRK). Then, this model was used as a template for the histamine-H3R-Gi-scFv16 complex. For the apo-H3R-miniGo-scFv16 complex, miniGαo derived from the GPR97-miniGo complex (PDB: 7D77) was used to substitute the Gαi subunit before manual adjustment and refinement. These models were docked into the EM maps of other complexes with different ligands and refined with the ligands replaced. For histamine-H1R-miniGq-scFv16 complex, coordinates from antagonist bound H1R crystal structure (PDB: 3RZE), miniGαq from HT2A-miniGq-scFv16 complex (PDB: 6WHA) and Gβγ-scFv16 from histamine-H4R-Gi-scFv16 complex were docked into the EM density to generate an initial model. Coordinates of H1R in the final model was used as a template for H2R in histamine-H2R-miniGs-Nb35 complex, and miniGαs-Nb35 from D1R-miniGs-Nb35 complex (PDB: 7JVQ) and Gβγ from histamine-H4R-Gi-scFv16 complex were combined to complete the initial model. Coordinates of H1R in histamine-H1R-miniGq-scFv16 complex was substituted with refined H2R to generate the initial model for histamine-H2R-miniGq-scFv16. Restraints of all ligands were generated by eLBOW in PHENIX. The model statistics was validated using MolProbity and the details are provided in Supplementary Tables 1, 2. The extent of model overfitting was evaluated by refining the final model against one half-map. FSC curves were calculated between the resulting model and the half map as well as between the resulting model and the other half map for cross-validation.
G-protein dissociation assay
G-protein bioluminescence resonance energy transfer (BRET) probes, including Gα-Rluc (Gαs-Rluc, Gαi-Rluc and Gαq-Rluc, Gβ and Gγ-GFP and the Gα- Gβγ dissociation assay were generated and performed as described. In Gα- Gβγ dissociation assays, Gα-Rluc, Gβ, and Gγ-GFP were used as G-protein BRET probes. HEK293 cells were co-transfected with HR or mutants and the G-protein probes, then plated at 5 × 104 cells per well in 96-well microplates. After a 24-h incubation at 37 °C, cells were washed with Tyrode’s buffer and stimulated with specified ligands at different concentrations. BRET signals were measured using coelenterazine 400a (5 μM) and a Mithras LB940 microplate reader with BRET filter sets. The BRET signal was calculated as the 515 nm/410 nm light emission ratio. HEK293 cells were regularly tested for mycoplasma contamination.
Enzyme-linked immunosorbent assay (ELISA)
The cell surface expression levels of target receptors were assessed by ELISA for G-protein dissociation assay. HEK293 cells, transfected with wild-type Histamine receptor or its mutants, were plated at 1 × 105 cells per well in 24-well plates and incubated for 16 h at 37 °C with 5% CO2. After fixation with 4% paraformaldehyde (w/v) and blocking with 5% BSA (w/v), the cells were incubated with an anti-FLAG primary antibody (1:1000 dilution) at 4 °C for 16 h and then with a secondary goat anti-mouse antibody (1:5000 dilution) for 1 h at room temperature. Following washing, a TMB (3,3’,5,5’-tetramethylbenzidine) solution initiated a color reaction, stopped by 0.25 M HCl. Optical density at 450 nm was measured using a TECAN luminescence counter (Infinite M200 Pro NanoQuant) and correlated with transfection plasmid amounts to determine expression levels. HEK293 cells were regularly tested for mycoplasma contamination.
Molecular dynamics simulation
To evaluate the binding stability of individual ligands within their corresponding Histamine receptor systems, including histamine-H1R, histamine-H2R, histamine-H3R, histamine-H4R, immethridine-H3R, and immepip-H4R, the molecular dynamics (MD) simulations were performed as previously described56. When setting up the MD simulation systems, the protonation states of ionizable residues at pH 7.5 were determined using Gromacs2019.6, which features a built-in protonation prediction analysis function set as a default parameter. All MD input files were generated by the CHARMMGUI website, where the force field parameters of ligands were generated by the CHARMM General Force Field (CGenFF)57. The structures used for MD simulations were embedded into a pre-equilibrated and periodic structure of 1-palmitoyl-2-oleoyl-snglycero-3-phosphatidylcholinemembrane. Subsequently, 0.15 M NaCl was added to balance the charge of the MD structures system using the Monte Carlo Method58. Next, the systems were solvated into periodic hexagonal type TIP3P water box with dimensions of ~80 Å by 80 Å by 80 Å using replacement methods, utilizing the membrane orientation calculated by the Orientations of Proteins in Membranes database. The MD structures systems were first processed for energy minimization for 10,000 steps using the gmx mdrun module in Gromacs2019.659, with the first 5000 steps performed using the steepest descent method and the remaining 5000 steps using the conjugated gradient method. All MD structures systems were heated from 0 to 310 K in the NVT ensemble (number of particles, volume, and temperature are conserved) for more than 1000 ps. Next, production simulations were run at 1 atm in the NPT ensemble (number of particles, pressure, and temperature are conserved) for more than 1000 ps with 10.0 kcal mol−1 Å−2 harmonic restraints. The NVT ensemble, NPT ensemble, and the 200 ns MD production with a time step of 2 fs were performed using Gromacs2019.6. All analyses were completed in Gromacs2019.6 and VMD60.
To examine the effect of the four residues Phe6.52, Phe6.55, Ile7.39, and Tyr7.43 on histamine recognition in H1R, a model with histamine bind to a mutated H1R was generated by replacing residues Phe6.52, Phe6.55, Ile7.39, and Tyr7.43 in histamine-bound H1R with their equivalents in H3R: Thr, Met, Phe, and Trp, respectively, using COOT software. The resultant four-residues base-mutant-H1R (FFIY-mutant-H1R) model was further used for MD stimulation as described above. To further validate the histamine binding orientation in H4R, we conducted MD simulations using a Histamine-H4R model by placing histamine in the opposite orientation within H4R compared to its position in our resolved structure. All analyses were completed in Gromacs2019.6 and VMD60.
To better understand the effect of W222 in ICL2 of H2R on Gs and Gq selectivity in H2R, we performed MD simulation using the histamine-bound H2R-Gs and a Chimera-generated H2R-Gq complex model, respectively. Due to the absence of ICL3 structure in the resolved H2R-Gq complex structure, we first performed homology modeling using SWISS-MODEL. The complete H2R receptor sequence was inputted, and the homology model was generated using the resolved structure of the ICL3-deficient H2R receptor as a template. We extracted the receptor region of the H2R model generated using SWISS-MODEL and the Gq region of the AT1R-Gq complex (PDB: 7F6G)61, and the H2R-Gq complex model for MD simulations was generated by Chimera. All the MD input files were generated, and all these MD simulation systems are executed using the molecular dynamics simulation parameters set at the beginning.
Radioligand binding assay
The human histamine receptors (H1R, H2R, H3R, and H4R) and their mutations were transfected into HEK293 cells for Radioligand binding assay. WT and mutant plasmids were transfected into HEK293 cells using polyethyleneimine (PEI) regent according to the manufacturer’s instructions, and cultured at 37 °C with 5% CO2 for 48 h. The transfected cells were collected using the ice-cold lysis buffer of 50 mM HEPES (pH 7.4) and 2 mM EDTA. The cells were suspended vertically at 4 °C for 20 min and centrifuged at 500 ×g for 10 min. The supernatant was centrifuged at 30,000×g for 30 min at superspeed and ice-cold buffer containing 75 mM Tris (pH 7.4), 1 mM EDTA, and 12.5 mM MgCl2. The total membrane surface protein expression was determined by Bradford protein assay. For the saturation binding assay, the cell membrane (~100 μg) was added with different concentrations of [3H]-Histamine and incubated in a shaking bed at 30°C for 1 h, and the Nonspecific binding curve was determined under the action of 50 μM ligands. The same amount of cell membrane was used for the competitive binding assay. On the basis of the 30 nM isotope ligand, a competitive ligand with gradient concentration was added and incubated in a shaking bed at 30 °C for 1 h. Incubation was in binding buffer [50 mM HEPES (pH 7.4), 10 mM MgCl2, 1 mM EDTA, 0.1% protease-free BSA, and 0.01% ascorbic acid]. The cell membranes were filtered over GF/C glass microfiber filters (Whatman) presoaked in an ice-cold binding buffer of 75 mM tris (pH 7.4), 1 mM EDTA, and 12.5 mM MgCl2 with 0.5% polyethylenimine, which using a 24-well Filter Mate harvester (PerkinElmer), and rinsing three times with 3 mL ice-cold binding buffer to remove free [3H]-Histamine. After drying the filters, 500 μL liquid scintillation was added, and the liquid scintillation count was performed on the PerkinElmer liquid scintillation counter. All data were analyzed by GraphPad Prism 9 (GraphPad Software Inc.). Each measurement was repeated in at least three independent experiments, each in triplicate.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The 3D cryo-EM density maps of Histamine receptors signaling complexes have been deposited in the Electron Microscopy Data Bank under the following accession codes: EMD-39412 (Histamine-H1R-miniGq), EMD-39413 (Histamine-H2R-miniGs), EMD-39414 (Histamine-H2R-miniGq), EMD-39415 (Histamine-H3R-Gi), EMD-39416 (Imetit-H3R-Gi), EMD-39417 (Immethridine-H3R-miniGo), EMD-39418 (Proxyfan-H3R-miniGo), EMD-39419 (Histamine-H4R-Gi), and EMD-39420 (Immepip-H4R-Gi). The atomic models have been deposited in the PDB databases under the following accession codes: 8YN2 (Histamine-H1R-miniGq), 8YN3 (Histamine-H2R-miniGs), 8YN4 (Histamine-H2R-miniGq), 8YN5 (Histamine-H3R-Gi), 8YN6 (Imetit-H3R-Gi), 8YN7 (Immethridine-H3R-miniGo), 8YN8 (Proxyfan-H3R-miniGo), 8YN9 (Histamine-H4R-Gi), 8YNA (Immepip -H4R-Gi). All the raw data from our molecular dynamic simulations have been uploaded to Figshare (https://doi.org/10.6084/m9.figshare.25886296.v1). The relevant raw data from each figure or table in the main manuscript and in the Supplementary Information are provided as a Source Data file. Source data are provided with this paper.
References
Haas, H. L., Sergeeva, O. A. & Selbach, O. Histamine in the nervous system. Physiol. Rev. 88, 1183–1241 (2008).
Garbarg, M., Barbin, G., Feger, J. & Schwartz, J. C. Histaminergic pathway in rat-brain evidenced by lesions of medial forebrain-bundle. Science 186, 833–835 (1974).
Akdis, C. A. & Blaser, K. Histamine in the immune regulation of allergic inflammation. J. Allergy Clin. Immunol. 112, 15–22 (2003).
Metcalfe, D. D., Baram, D. & Mekori, Y. A. Mast cells. Physiol. Rev. 77, 1033–1079 (1997).
Wernersson, S. & Pejler, G. Mast cell secretory granules: armed for battle. Nat. Rev. Immunol. 14, 478–494 (2014).
Fulkerson, P. C. & Rothenberg, M. E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 12, 117–129 (2013).
Gantz, I. et al. Molecular-cloning of a gene encoding the histamine-H2-receptor. Proc. Natl Acad. Sci. USA 88, 429–433 (1991).
Morisset, S. et al. High constitutive activity of native H(3) receptors regulates histamine neurons in brain. Nature 408, 860–864 (2000).
Yokoyama, H. The role of central histaminergic neuron system as an anticonvulsive mechanism in developing brain. Brain Dev. 23, 542–547 (2001).
Parsons, M. E. & Ganellin, C. R. Histamine and its receptors. Br. J. Pharmacol. 147, S127–S135 (2006).
Leconiat, M., Traiffort, E., Ruat, M., Arrang, J. M. & Berger, R. Chromosomal localization of the human histamine H-1-receptor gene. Hum. Genet. 94, 186–188 (1994).
Oda, T., Morikawa, N., Saito, Y., Masuho, Y. & Matsumoto, S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J. Biol. Chem. 275, 36781–36786 (2000).
Lovenberg, T. W. et al. Cloning and functional expression of the human histamine H-3 receptor. Mol. Pharmacol. 55, 1101–1107 (1999).
Thurmond, R. L., Gelfand, E. W. & Dunford, P. J. The role of histamine H-1 and H-4 receptors in allergic inflammation: the search for new antihistamines. Nat. Rev. Drug Discov. 7, 41–53 (2008).
Panula, P. et al. International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol. Rev. 67, 601–655 (2015).
Leurs, R., Smit, M. J. & Timmerman, H. Molecular pharmacological aspects of histamine-receptors. Pharmacol. Therap. 66, 413–463 (1995).
Dauvilliers, Y. et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 12, 1068–1075 (2013).
Simons, F. E. R. Drug therapy - advances in H-1-antihistamines. N. Engl. J. Med. 351, 2203–2217 (2004).
Simons, F. E. R. & Simons, K. J. Histamine and H-1-antihistamines: celebrating a century of progress. J. Allergy Clin. Immunol. 128, 1139 (2011).
Hershcovici, T. & Fass, R. Gastro-oesophageal reflux disease beyond proton pump inhibitor therapy. Drugs 71, 2381–2389 (2011).
Sigterman, K. E., van Pinxteren, B., Bonis, P. A., Lau, J. & Numans, M. E. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst. Rev. 2013, CD002095 (2013).
Nguyen, P. L. & Cho, J. Pathophysiological roles of histamine receptors in cancer progression: implications and perspectives as potential molecular targets. Biomolecules 11, 1232 (2021).
Kimura, S. et al. Relationship between CCL22 expression by vascular smooth muscle cells and macrophage histamine receptors in atherosclerosis. J. Atheroscler. Thromb. 25, 1240–1254 (2018).
Cao, J. et al. H(1)R mediates local anesthetic-induced vascular permeability in angioedema. Toxicol. Appl. Pharm. 392, 114921 (2020).
Kawakami, N., Miyoshi, K., Horio, S. & Fukui, H. Beta(2)-adrenergic receptor-mediated histamine H(1) receptor down-regulation: another possible advantage of beta(2) agonists in asthmatic therapy. J. Pharm. Sci. 94, 449–458 (2004).
Masini, E., Blandina, P., Brunelleschi, S. & Mannaioni, P. F. Evidence for H2-receptor-mediated inhibition of histamine release from isolated rat mast cells. Agents Actions 12, 85–88 (1982).
Clark, R. A., Gallin, J. I. & Kaplan, A. P. The selective eosinophil chemotactic activity of histamine. J. Exp. Med. 142, 1462–1476 (1975).
Frei, R. et al. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J. Allergy Clin. Immunol. 132, 194–204 (2013).
Mazzoni, A. et al. Cutting edge: histamine inhibits IFN-alpha release from plasmacytoid dendritic cells. J. Immunol. 170, 2269–2273 (2003).
Yuan, H. & Silberstein, S. D. Histamine and migraine. Headache 58, 184–193 (2018).
Del Valle, J. & Gantz, I. Novel insights into histamine H2 receptor biology. Am. J. Physiol. 273, G987–G996 (1997).
Yoshimoto, R. et al. Therapeutic potential of histamine H3 receptor agonist for the treatment of obesity and diabetes mellitus. Proc. Natl Acad. Sci. USA 103, 13866–13871 (2006).
Liu, Y. et al. Visualization of the activation of the histamine H3 receptor (H3R) using novel fluorescence resonance energy transfer biosensors and their potential application to the study of H3R pharmacology. FEBS J. 285, 2319–2336 (2018).
O’Reilly, M. et al. Identification of a histamine H4 receptor on human eosinophils-role in eosinophil chemotaxis. J. Recept Signal Transduct. Res. 22, 431–448 (2002).
Zampeli, E. & Tiligada, E. The role of histamine H4 receptor in immune and inflammatory disorders. Br. J. Pharm. 157, 24–33 (2009).
Jemima, E. A., Prema, A. & Thangam, E. B. Functional characterization of histamine H4 receptor on human mast cells. Mol. Immunol. 62, 19–28 (2014).
Shimamura, T. et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 475, 65–70 (2011).
Robertson, M. J. et al. Structure determination of inactive-state GPCRs with a universal nanobody. Nat. Struct. Mol. Biol. 29, 1188–1195 (2022).
Peng, X. et al. Structural basis for recognition of antihistamine drug by human histamine receptor. Nat. Commun. 13, 6105 (2022).
Im, D. et al. Structural insights into the agonists binding and receptor selectivity of human histamine H(4) receptor. Nat. Commun. 14, 6538 (2023).
Wang, C. et al. Measurement and control of quasiparticle dynamics in a superconducting qubit. Nat. Commun. 5, 5836 (2014).
Xia, R. et al. Structural basis of ligand recognition and design of antihistamines targeting histamine H4 receptor. Nat. Commun. 15, 2493 (2024).
Xia, R. X. et al. Cryo-EM structure of the human histamine H-1 receptor/G(q) complex. Nat. Commun. 12, 2086 (2021).
Hua, T. et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 547, 468 (2017).
Carrión-Antolí, A. et al. Antioxidant systems and quality in sweet cherries are improved by preharvest GABA treatments leading to delay postharvest senescence. Int. J. Mol. Sci. 25, 260 (2023).
Zhuang, Y. et al. Structural insights into the human D1 and D2 dopamine receptor signaling complexes. Cell 184, 931–942.e18 (2021).
Duan, J. et al. Cryo-EM structure of an activated VIP1 receptor-G protein complex revealed by a NanoBiT tethering strategy. Nat. Commun. 11, 4121 (2020).
Qi, X. et al. Cryo-EM structure of oxysterol-bound human smoothened coupled to a heterotrimeric Gi. Nature 571, 279–283 (2019).
Garcia-Nafria, J., Nehme, R., Edwards, P. C. & Tate, C. G. Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric Go. Nature 558, 620–623 (2018).
Kim, K. et al. Structure of a hallucinogen-activated Gq-coupled 5-HT2A serotonin receptor. Cell 182, 1574–1588.e19 (2020).
Rasmussen, S. G. et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 (2011).
Koehl, A. et al. Structure of the μ-opioid receptor-Gi protein complex. Nature 558, 547–552 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7, e42166 (2018).
Mao, C. et al. Unsaturated bond recognition leads to biased signal in a fatty acid receptor. Science 380, eadd6220 (2023).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Xiao, P. et al. Tethered peptide activation mechanism of the adhesion GPCRs ADGRG2 and ADGRG4. Nature 604, 771–778 (2022).
Yang, F. et al. Structure, function and pharmacology of human itch receptor complexes. Nature 600, 164–169 (2021).
Acknowledgements
We thank the Cryo-EM Center at the University of Science and Technology of China for the support of cryo-EM data collection. We thank Dr Yongxiang Gao for assistance with cryo-EM data collection. And we thank the Translational Medicine Core Facility of Advanced Medical Research Institute, Shandong University, for technical support. This work was supported by the Ministry of Science and Technology of China grant 2019YFA0904100 and the Natural Science Foundation of China grant T2221005 (both to W.G.), National Science Fund for Excellent Young Scholars (32222038 to P.X.), National Science Fund for Distinguished Young Scholars Grant (82425105 to J.-P.S.), National Key R&D Program of China (2019YFA0904200 to J.-P.S. and P.X.), and Shandong Provincial Natural Science Fund for Excellent Young Scholars (ZR2021YQ18 to P.X.).
Author information
Authors and Affiliations
Contributions
W.G., J.-P.S., and P.X. conceived the project and designed the research with X.Z., G.L., Y.-N.Z., R.Z. and C.-C.Y., X.Z., and G.L. performed protein expression and purification studies, screened cryo-EM grids, collected cryo-EM data, and processed the data under the supervision of W.G. J.-P.S., Y.-N.Z., R.Z., X.L., and C.-C.Y. designed the G protein dissociation assay and analyzed results, X.P., J.S., T.Z., X.S., and L.Y. generated the HRs mutant constructs and performed the cell-based assays. R.Z., C.N., and C.Z. performed MD simulations under the guidance of P.X. and J.-P.S., Y.-N.Z., C.-C.Y., R.Z., and X.Z. finished all the figures under the supervision of P.X., J.-P.S., and W.G. J.-P.S., W.G., and P.X. wrote the manuscript, with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Javier García-Nafría, So Iwata and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Liu, G., Zhong, YN. et al. Structural basis of ligand recognition and activation of the histamine receptor family. Nat Commun 15, 8296 (2024). https://doi.org/10.1038/s41467-024-52585-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-52585-y