Abstract
Acenaphthylene-containing polycyclic aromatic hydrocarbons (AN-PAHs) are noteworthy structural motifs for organic functional materials due to their non-alternant electronic structure, which increases electron affinity. However, the synthesis of AN-PAHs has traditionally required multiple sequential synthetic steps, limiting structural diversity. Herein, we present a tandem C−H penta- and hexaannulation reaction of aryl alkyl ketone with acetylenedicarboxylate. This integrated approach enhances overall efficiency and selectivity, marking a significant advancement in AN-PAH synthesis. Mechanistic studies unveil an orchestrated extension of five- and six-membered rings through C−H activation-annulation and Diels–Alder reaction. Additionally, the tandem annulation reaction can be performed stepwise, further validating the proposed mechanism and increasing the structural diversity of AN-PAHs.
Similar content being viewed by others
Introduction
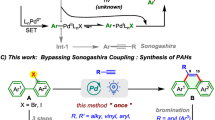
Polycyclic aromatic hydrocarbons (PAHs) featuring non-hexagonal rings have attracted significant interest in the realm of organic optoelectronics1,2,3,4. Among these, acenaphthylene (AN)-containing PAHs stand out as exceptional structural units for organic functional materials, courtesy of their non-alternant electronic structure that enhances electron affinity (Fig. 1a)5,6,7,8,9,10,11,12. Consequently, the efficient synthesis of these compounds has garnered considerable attention. Traditional synthetic routes to AN-PAHs typically begin with the substitution, oxidation or annulation reactions of acenaphthene13,14,15,16. Nevertheless, these methods usually require multistep manipulation, and suffer from limitation of structural diversity. Recently, the advent of transition metal-catalyzed annulations has propelled significant advancements in the synthesis of AN-PAHs through catalytic strategies17,18,19,20. For instance, such PAHs could be obtained through palladium-catalyzed cyclopentaannulation reactions of halogenated naphthalenes with alkynes21,22,23 or 2-halogenated arylboronic acids24,25,26 (Fig. 1b). Despite significant progress, the development of a streamlined approach to access a variety of AN-PAHs, ideally utilizing abundant feedstocks, remains an appealing task in synthetic chemistry.
Aryl ketones are readily accessible chemicals and fundamental building blocks in organic synthesis. In recent years, transition metal-catalyzed ortho-C−H activation and functionalization of aryl ketones have been studied extensively27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44. Among these transformations, the direct C−H annulation reactions of aryl ketones with alkynes have emerged as a versatile strategy for the construction of π-conjugated molecules, as exemplified by the pioneering works of Glorius27, Cheng28, Wang29, Maji45, and our group46,47,48. Despite these advances, most studies have used aryl or alkyl alkynes as substrates for annulation reactions. The aryl or alkyl fragments are often difficult to transform or remove from the formed PAHs, limiting the practical utility of these reactions.
Dimethyl acetylenedicarboxylate (DMAD), an electron-deficient alkyne diester, is widely used in Michael reactions, cyclizations (Diels–Alder and 1,3-dipole) but rarely in transition metal-catalyzed C–H activation-annulations49. Recently, we demonstrated its capability in C–H activation-annulation reaction with naphthalene ketones to generate aromatic polycarboxylic esters, establishing an efficient strategy for the synthesis of graphene-like molecules by utilizing the removability of ester groups as a key point50. Inspired by these studies, we envisaged that its annulation reaction with aryl ketones could rapidly access AN-containing PAHs. In the proposed reaction, a fulvene species, generated from the transition metal-catalyzed C–H activation-annulation of aryl ketone with alkyne, could be a key intermediate. The main challenge lies in effectively coupling the C–H activation-annulation with the Diels–Alder reaction. As proof of concept, we herein disclose a tandem C−H penta- and hexaannulation reaction of aryl alkyl ketones with acetylenedicarboxylates, offering access to a diverse array of AN-PAHs in an atom- and step-economical manner (Fig. 1c). This reaction demonstrates high chemo- and regioselectivity, with the extension of five- and six-membered rings proceeding in an orchestrated pathway.
Results
Optimization of the Reaction Conditions
At the onset of the optimization study, 1-(naphthalen-2-yl)ethan-1-one (1a) was chosen as model substrate to react with DMAD (2a). To our satisfaction, systematic evaluation of reaction parameters led to the highest yield of the desired product 3 at 62% along with the recovery of 1a in 24% yield under an optimized reaction conditions: 1a (0.1 mmol, 1.0 equiv.), 2a (0.4 mmol, 4.0 equiv.), [Cp*RhCl2]2 (5 mol%), AgOTf (20 mol%), CuO (0.3 mmol, 3.0 equiv.), 1-methylcyclohexane-1-carboxylic acid (1-MeCHA) (50 mol%), and DCE (2.0 mL) in a sealed 25 mL Schlenk tube under N2 atmosphere at 150 °C (oil bath) for 16 h (Fig. 2a). The structure of 3 was confirmed by X-ray crystallography (Fig. 2a). Notably, after the reaction, the resulting mixture mainly contained 3 and unconverted 1a. Blank experiments revealed the indispensability of [Cp*RhCl2]2, CuO, and 1-MeCHA for this transformation (Fig. 2b, entries 2–3). [(p-cymene)RuCl2]2 showed negligible catalytic activity (Fig. 2b, entry 4). Replacing AgOTf with AgSbF6 or AgBF4 led to diminished yields of 3, and the reaction ceased completely when AgTFA was used as an additive (Fig. 2b, entry 5). Other copper oxidants such as Cu(OAc)2, Cu2O, and CuBr2 were proven less effective than CuO (Fig. 2b, entry 6). Moreover, this reaction exhibited robustness towards various organic acid additives, as replacing 1-MeCHA with PivOH, MesCO2H or 1-AdCO2H (Fig. 2b, entries 7-8). Furthermore, the choice of solvent significantly influenced the transformation. Replacement of DCE with PhCl or toluene resulted in yields of 3 in 26% or 8% (Fig. 2b, entry 9). The reaction did not occur at all when using THF, DMF or MeCN as solvents (Fig. 2b, entry 10). Additionally, a reaction condition-based sensitivity screening indicated that this process was relatively robust in the face of small changes in concentration and temperature, high oxygen level, moisture, and scale-up (Fig. 2c and Table S1)51.
a Standard reaction conditions: 1a (0.1 mmol), 2a (0.4 mmol), [Cp*RhCl2]2 (5 mol%), AgOTf (20 mol%), CuO (0.3 mmol), 1-MeCHA (50 mol%), and DCE (2.0 mL) in a sealed 25 mL Schlenk tube under N2 atmosphere at 150 °C (oil bath) for 16 h. b Impact of other reaction parameters. Isolated yields are given. c Reaction condition-based sensitivity screening. Me: methyl; Ph: phenyl; Cp*: 1,2,3,4,5-pentamethylcyclopentadiene; OTf: trifluoromethanesulfonate; PivOH: pivalic acid; 1-MeCHA: 1-methylcyclohexane-1-carboxylic acid; DCE: 1,2-dichloroethane.
Substrate Scope
With the optimized reaction conditions in hand, we embarked on a comprehensive exploration of the substrate scope using various aryl ketones 1. As summarized in Fig. 3, 1-(naphthalen-2-yl)ethan-1-ones, equipped with electron donating groups such as methyl, methoxy, phenoxy, benzyloxy, pivaloyloxy, hydroxyl, 4-bromobutoxy, and even methylthio on the naphthyl ring, smoothly underwent this tandem annulation reaction, providing the corresponding AN-PAHs in moderate to good yields (Fig. 3, 3-11). The hydrolysis of compound 3 was achieved by refluxing it in a KOH solution composed of water and THF, resulting in the corresponding tetraacid 3’ in a 96% yield. The versatility of the method was further demonstrated with the successful engagement of substrates featuring halogen substituents (fluoro, chloro, bromo) and electron withdrawing groups (ester and formyl), showing the potential for late-stage modification of the annulation products (Fig. 3, 12–16). The applicability of the reaction extended to naphthalene ketones with aryl or heteroaryl substituents, affording the desired products in moderate yields (Fig. 3, 17–20). Encouragingly, even simple phenyl ketones, such as acetophenone (1s) and 1-(4-methoxyphenyl)ethan-1-one (1t), participated in the reaction to yield products 21 and 22. The structure of 21 was confirmed by X-ray crystallography (Table S7). In addition, the protocol successfully accommodated larger π-conjugated ketones, including 1-(phenanthren-2-yl)ethan-1-one (1u), 1-(pyren-2-yl)ethan-1-one (1v), 1,1’-(pyrene-2,7-diyl)bis(ethan-1-one) (1w), 1-(anthracen-2-yl)ethan-1-one (1x), and 1-(chrysen-2-yl)ethan-1-one (1y), yielding the corresponding AN-PAHs in good yields (Fig. 3, 23-27). Moreover, other electron-deficient alkyne diesters (2b-2c) or diketone (2d) also participated in this reaction, delivering the corresponding products in moderate yields (Fig. 3, 28-30). It is worth to note that the low yields of the above reactions are primarily due to the low conversion of ketone substrates 1. Furthermore, for a comprehensive overview of the substrate scope, details on unsuccessful substrates such as bis(4-(methoxycarbonyl)phenyl)acetylene (2k), methyl 2-butynoate (2n), methyl phenylpropiolate (2o), and 1,2-bis(2-pyridyl)acetylene (2p), are given in Table S2.
Reactions run with 1 (0.1 mmol), 2 (0.4 mmol), [Cp*RhCl2]2 (5 mol%), AgOTf (20 mol%), CuO (0.3 mmol), 1-MeCHA (50 mol%), and DCE (2.0 mL) in a sealed 25 mL Schlenk tube under N2 atmosphere at 150 °C (oil bath) for 16 h. Hydrolysis conditions: 3 (0.1 mmol), KOH (2.0 mmol), and THF/H2O (2.0 mL, 1:1, v/v) in a sealed 25 mL Schlenk tube under N2 atmosphere at 100 °C (oil bath) for 24 h. Me: methyl; Et: ethyl; iBu: iso-butyl; Ph: phenyl; Bn: benzyl; Piv: pivaloyl; Cp*: 1,2,3,4,5-pentamethylcyclopentadiene; 1-MeCHA: 1-methylcyclohexane-1-carboxylic acid; OTf: trifluoromethanesulfonate; DCE: 1,2-dichloroethane.
To further demonstrate the versatility of this protocol, a bidirectional annulation reaction of 1,1’-(naphthalene-2,6-diyl)bis(ethan-1-one) (1z) with 2a was conducted, affording a two acenaphthylene-containing PAH 32 in a synthetically useful yield (Fig. 4a). Subsequently, we conducted density functional theory (DFT) calculations to explore the photophysical and aromatic properties of compound 32. Figure 4c illustrates that the lowest unoccupied molecular orbital (LUMO) of 32 is distributed across the entire molecule skeleton, while the highest occupied molecular orbital (HOMO) predominantly resides along the long axis of 32. The LUMO and HOMO energy levels of 32 are -3.47 and -6.42 eV, respectively, demonstrating promise for semiconductor applications. The UV-vis and fluorescence spectra revealed maximum absorption and emission peaks of 32 at 430 nm and 543 nm (green fluorescence), respectively (Fig. 4d). To further investigate the stereo-electronic structure and aromaticity of 32, we conducted two-dimensional nucleus-independent chemical shift (2D-NICS)52 and anisotropy of the induced current density (ACID)53 analysis (Fig. 4e and f). The results of these calculations indicate that the six-membered rings (rings 1, 2, 3 and 4) exhibit typical aromatic properties, as evidenced by negative 2D-NICS values (Fig. 4b and e) and a clockwise ring current along the perimeter of the chrysene core (Fig. 4f). In contrast, positive 2D-NICS values (Fig. 4e) and an anticlockwise direction of the ring current suggest the anti-aromaticity of the five-membered rings (rings 5 and 6) (Fig. 4b and f). In addition, the UV-vis and fluorescence spectra of other representative products 3, 23, 24, 26, and 27 were measured. As shown in Fig. S9 and Fig. S10, the absorption and emission maxima of these products vary from 369 nm to 499 nm, and 519 nm to 597 nm, respectively, depending on the degree of π-extension and shape of the products. Due to a fusion of a pyrene unit, a remarkable red-shift is observed for both the absorption and the emission maxima of 24 (λabs = 499 nm, λem = 597 nm). To our delight, these compounds exhibit large Stokes shifts, ranging from 70 nm to 150 nm (Table S4). These excellent photophysical properties demonstrate that these compounds may have potential applications in molecular probes.
a Bidirectional annulation reaction of 1z with 2a. Reaction conditions: 1z or 31 (0.1 mmol), 2a (0.4 mmol), [Cp*RhCl2]2 (5 mol%), AgOTf (20 mol%), CuO (0.3 mmol), 1-MeCHA (50 mol%), and DCE (2.0 mL) in a sealed 25 mL Schlenk tube under N2 atmosphere at 150 °C (oil bath) for 16 h. b The structure of 32. c The optimized molecular geometry and molecular orbitals (HOMO and LUMO) of 32, calculated at the B3LYP/6-31 G(d) level of theory. d The absorption and emission spectra of 32 in dichloromethane (1 × 10−5 mol/L). e The two-dimensional nucleus-independent chemical shift (2D-NICS) calculations of 32, calculated at the B3LYP/6-31 G(d) level of theory. f The anisotropy of the induced current density (ACID) plots of 32, calculated at the B3LYP/6-31 G(d) level of theory. The red and blue arrows indicate the clockwise and anticlockwise ring currents, respectively. Me: methyl; HOMO: highest occupied molecular orbital; LUMO: lowest unoccupied molecular orbital; Eg: energy gap.
Mechanism Study
To gain some insight into the reaction mechanism, several control experiments were conducted. Initially, treatment of 1-(1-methylnaphthalen-2-yl)ethan-1-one (1aa) with 2a under the standard reaction conditions afforded product 33 in 7% yield. In contrast, the reaction of 1ab and 2a produced only a byproduct 34 in 38% yield, arising from the trimerization of 2a, with substrates 1ab and 2a being recovered in 92% and 28% yields, respectively (Fig. 5a). These outcomes suggested that the reaction might commence with the C3−H activation and cyclopentaannulation of 1-(naphthalen-2-yl)ethan-1-one to form an intermediate with a five-membered ring. Indeed, a fulvene-containing intermediate 35 was obtained in 44% yield from the reaction between 1-(naphthalen-2-yl)propan-1-one (1ac) and 2a. Simultaneously, the acenaphthylene-containing product 36 was afforded in 17% yield, confirming the above conjecture (Fig. 5b). To clarify the subsequent cyclohexaannulation, compound 35 was subjected to further reaction with 2a in the presence of AgOTf and CuO, excluding [Cp*RhCl2]2, resulting in the desired product 36 in 42% yield (Fig. 5b). The structures of 33 and 36 were confirmed by X-ray crystallography (Table S8 and S9). These findings suggested that the hexaannulation might proceed through a Diels–Alder reaction, and the rhodium catalyst is likely not involved in this step. Subsequently, a series of radical capture experiments were conducted by adding radical scavengers such as 2,2,6,6-tetramethylpiperidine (TEMPO) and 2,6-di-tert-butyl-4-methylphenol (BHT) into the reaction of 1a and 2a. As shown in Table S3, the yield of 3 showed a nonlinear decrease along with the increase of TEMPO addition. However, when BHT was used as a free radical scavenger, the yield of 3 did not decrease significantly, and it could still reach 37% when one equivalent of BHT was added. These experimental results suggest that this reaction may not involve a radical mechanism. Finally, the chemical kinetics of the reaction of 1c and 2a were studied using in situ infrared (IR) measurements (Fig. 5c). Encouragingly, the absorption intensities of 1c, 2a, and 5 could be monitored by in situ IR measurements, tracked by change of the peaks at 1683, 1731, 1540 cm−1, respectively (Fig. 5c, (i)). The kinetic profile showed that this annulation reaction proceeded relatively fast without a discernible induction period, with the majority of 5 formed within 3 h (Fig. 5c, (ii-iii)).
a Reactions of 1aa or 1ab with 2a were performed under standard reaction conditions. b Reaction of 1ac with 2a was performed under standard reaction conditions. Reaction of 35 with 2a was performed without the addition of [Cp*RhCl2]2. c in situ IR experiments of 1c and 2a. Me: methyl; Cp*: 1,2,3,4,5-pentamethylcyclopentadiene; 1-MeCHA: 1-methylcyclohexane-1-carboxylic acid; OTf: trifluoromethanesulfonate; DCE: 1,2-dichloroethane.
Based on the above observations, we propose a mechanism of the reaction between 1a and 2a, involving C–H activation-annulation and Diels–Alder reaction (Fig. 6a). The reaction initiates with a ketone-directed C3–H activation of 1a with Cp*RhX2 (X = OTf or RCO2) to form a five-membered cyclorhodium intermediate I. Subsequently, intermediate I chelates with 2a, leading to the formation of intermediate II. Alkyne insertion and subsequent intramolecular electrophilic attack of the carbonyl unit generate intermediate IV. Transmetalation of intermediate IV with copper carboxylate salt yields intermediate VI and Cp*RhX2 through a transitional intermediate V. beta-Hydrogen elimination of intermediate VI produces intermediate VII. A Diels–Alder reaction of intermediate VII with 2a delivers intermediate VIII, corroborated by a high-resolution mass spectrometry (HRMS) determination (Fig. S7, m/z calcd: 459.1050, found: 459.1046). Finally, intermediate VIII undergoes further oxidative aromatization in the presence of copper or silver salts to produce the desired product 3.
a Proposed mechanism of the tandem annulation reaction. b Stepwise annulation reactions of 1 s with two electronically differentiated alkynes (see part VIII of Supplementary Information for the details). c Derivatization of 44 (see part IX of Supplementary Information for the details). Me: methyl; Et: ethyl; tBu: tert-butyl; Ph: phenyl; Piv: pivaloyl; Ac: acetyl; Cp*: 1,2,3,4,5-pentamethylcyclopentadiene; OTf: trifluoromethanesulfonate; 1-MeCHA: 1-methylcyclohexane-1-carboxylic acid; DPPP: 1,3-bis(diphenylphosphino) propane; DDQ: 2,3-dichloro-5,6-dicyano-1,4-benzoquinone; DCE: 1,2-dichloroethane.
After investigating the reaction mechanism, we speculated that the tandem penta- and hexaannulation reaction could be achieved stepwise by adjusting the reaction conditions. To test this hypothesis, we conducted stepwise annulation reactions of acetophenone (1 s) with two electronically differentiated alkynes (Fig. 6b). Under slightly modified Rh-catalyzed conditions, acetophenone (1 s) reacted with various diphenyl alkynes, affording a series of 1-methylene-2,3-diaryl-1H-indenes (37-43) in good yields. These intermediates then underwent a Diels–Alder reaction with DMAD (2a), producing the desired AN-PAHs (44-50). The structure of 44 was confirmed by X-ray crystallography (Table S10). On the contrary, the Diels–Alder reactions of 37 with thiophene or indole were not achieved likely due to their aromaticity. Additionally, compound 44 was obtained in 60% and 17% yields in the absence of AgOTf or CuO, respectively, and the yield of 44 could still reach 9% in the absence of AgOTf and CuO simultaneously (Fig. S6), which suggests that the hexaannulation does not require an oxidant, but the addition of metal salts, especially copper salts, can significantly promote this process. These results further verified the proposed reaction mechanism and expanded the structural diversity of the AN-PAHs. To demonstrate the practicality of this reaction, several derivatizations of product 44 were conducted (Fig. 6c). Hydrolysis of 44 yielded diacid 51, which further underwent a condensation reaction to produce imide product 52 or a decarboxylation reaction to generate product 53. Finally, treatment of 53 with FeCl3 and DDQ in dichloromethane for 2 h delivered product 54 in a 68% yield. The photophysical properties of 54 were investigated, exhibiting yellow fluorescence and phosphorescence emissions at 544 nm and 524 nm, respectively (Fig. S11). These properties indicate that it may have potential applications in yellow OLED materials.
Discussion
In summary, we have introduced a tandem C–H annulation reaction of aryl alkyl ketones with acetylenedicarboxylates, offering a straightforward and rapid method to access a series of AN-PAHs from abundant feedstocks in a single step. This reaction demonstrates high chemo- and regioselectivity, a broad substrate scope, and excellent functional group tolerance. Mechanism studies has elucidated a comprehensive pathway involving Rh-catalyzed C–H cyclopentaannulation, followed by a Diels–Alder reaction. Additionally, stepwise annulation reactions of acetophenone with two electronically differentiated alkynes verified the proposed mechanism and expanded the structural diversity of AN-PAHs. We anticipate that this work will captivate the synthetic community, offering a valuable toolkit for materials chemists to explore novel acenaphthylene-based organic functional materials.
Methods
General procedure for tandem C−H penta- and hexaannulation reactions
A 25 mL Schlenk tube with a magnetic stir bar was charged with [Cp*RhCl2]2 (3.1 mg, 5 mol%), AgOTf (5.1 mg, 20 mmol%), CuO (24.0 mg, 0.3 mmol, 3.0 equiv.), 1-MeCHA (7.1 mg, 0.05 mmol, 0.5 equiv.), naphthalene ketone 1 (0.1 mmol, 1.0 equiv.) and alkyne 2 (0.4 mmol, 4.0 equiv.) in DCE (2.0 mL) under N2 atmosphere. The vessel was sealed tightly with a screw cap, then stirred at 150 °C in an oil bath for 16 h. The resulting solution was cooled to ambient temperature, diluted with 10 mL of CH2Cl2, filtered through a celite pad, and washed three times with 10 mL of CH2Cl2. The obtained organic extracts were evaporated under reduced pressure and the residue was absorbed into small amounts of silica gel. Purification was performed by column chromatography on silica gel to provide the desired product.
Data availability
The experimental data generated in this study are provided in the Supplementary Information and Source Data file, and also are available from the corresponding author upon request. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2326151 (3), 2326160 (21), 2326156 (33), 2326157 (36), and 2360166 (44). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Rickhaus, M., Mayor, M. & Juríčeka, M. Chirality in curved polyaromatic systems. Chem. Soc. Rev. 46, 1643–1660 (2017).
Pun, S. H. & Miao, Q. Toward negatively curved carbons. Acc. Chem. Res. 51, 1630–1642 (2018).
Stepek, I. A. et al. Construction of heptagon-containing molecular nanocarbons. Angew. Chem. Int. Ed. 60, 23508–23532 (2021).
Fei, Y. & Liu, J. Synthesis of defective nanographenes containing joined pentagons and heptagons. Adv. Sci. 9, 2201000 (2022).
Stuparu, M. C. Corannulene: a curved polyarene building block for the construction of functional materials. Acc. Chem. Res. 54, 2858–2870 (2021).
Liu, Y.-H. & Perepichka, D. F. Acenaphthylene as a building block for π-electron functional materials. J. Mater. Chem. C 9, 12448–12461 (2021).
Ma, L., Han, Y., Shi, Q. & Huang, H. The design, synthesis and application of rubicene based polycyclic aromatic hydrocarbons (PAHs). J. Mater. Chem. C 11, 16429–16438 (2023).
Jellison, J. L., Lee, C.-H., Zhu, X., Wood, J. D. & Plunkett, K. N. Electron acceptors based on an all-carbon donor-acceptor copolymer. Angew. Chem. Int. Ed. 51, 12321–12324 (2012).
Sun, X., Wu, F., Zhong, C., Zhu, L. & Li, Z. A structure-property study of fluoranthene-cored hole-transporting materials enables 19.3% efficiency in dopant-free perovskite solar cells. Chem. Sci. 10, 6899–6907 (2019).
Liu, H., Fu, Y., Tang, B. & Zhao, Z. All-fluorescence white organic light-emitting diodes with record-beating power efficiencies over 130 lmW‒1 and small roll-offs. Nat. Commun. 13, 5154 (2022).
Kumar, R., Chmielewski, P. J., Lis, T., Volkmer, D. & Stępień, M. Tridecacyclene tetraimide: an easily reduced cyclooctatetraene derivative. Angew. Chem. Int. Ed. 61, e202207486 (2022).
Wu, Z. et al. An in-situ cyanidation strategy to access tetracyanodiacenaphthoanthracene diimides with high electron mobilities exceeding 10 cm2V-1s-1. Angew. Chem. Int. Ed. 62, e202307695 (2023).
Amick, A. W. & Scott, L. T. Trisannulated benzene derivatives by acid catalyzed aldol cyclotrimerizations of cyclic ketones. Methodology development and mechanistic insight. J. Org. Chem. 72, 3412–3418 (2007).
Li, H. et al. Tetraazabenzodifluoranthene diimides: building blocks for solution-processable n-type organic semiconductors. Angew. Chem. Int. Ed. 52, 5513–5517 (2013).
Zhylitskaya, H., Cybińska, J., Chmielewski, P., Lis, T. & Stępień, M. Bandgap engineering in π‑extended pyrroles. A modular approach to electron-deficient chromophores with multi-redox activity. J. Am. Chem. Soc. 138, 11390–11398 (2016).
Borstelmann, J., Bergner, J., Rominger, F. & Kivala, M. A negatively curved π-expanded pyracylene comprising a tropylium cation. Angew. Chem. Int. Ed. 62, e202312740 (2023).
Alberico, D., Scott, M. E. & Lautens, M. Aryl−aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 107, 174–238 (2007).
Jin, T., Zhao, J., Asao, N. & Yamamoto, Y. Metal-catalyzed annulation reactions for π-conjugated polycycles. Chem. Eur. J. 20, 3554–3576 (2014).
Hagui, W., Doucet, H. & Soulé, J.-F. Application of palladium-catalyzed C(sp2)−H bond arylation to the synthesis of polycyclic (hetero)aromatics. Chem 5, 2006–2078 (2019).
Jolly, A., Miao, D., Daigle, M. & Morin, J.-F. Emerging bottom-up strategies for the synthesis of graphene nanoribbons and related structures. Angew. Chem. Int. Ed. 59, 4624–4633 (2020).
Bheemireddy, S. R., Hautzinger, M. P., Li, T., Lee, B. & Plunkett, K. N. Conjugated ladder polymers by a cyclopentannulation polymerization. J. Am. Chem. Soc. 139, 5801–5807 (2017).
Qin, L. et al. Diazulenorubicene as a non-benzenoid isomer of peri-tetracene with two sets of 5/7/5 membered rings showing good semiconducting properties. Angew. Chem. Int. Ed. 62, e202304632 (2023).
Liu, B. et al. Bespoke tailoring of graphenoid sheets: a rippled molecular carbon comprising cyclically fused nonbenzenoid rings. J. Am. Chem. Soc. 145, 28137–28145 (2023).
Jackson, E. A., Steinberg, B. D., Bancu, M., Wakamiya, A. & Scott, L. T. Pentaindenocorannulene and tetraindenocorannulene: new aromatic hydrocarbon π systems with curvatures surpassing that of C60. J. Am. Chem. Soc. 129, 484–485 (2007).
Seifert, S., Schmidt, D., Shoyama, K. & Würthner, F. Base-selective five- versus six-membered ring annulation in palladium-catalyzed C−C coupling cascade reactions: new access to electron-poor polycyclic aromatic dicarboximides. Angew. Chem. Int. Ed. 56, 7595–7600 (2017).
Labella, J. et al. Naphthalimide-fused subphthalocyanines: electron-deficient materials prepared by cascade annulation. ACS Mater. Lett. 5, 543–548 (2023).
Patureau, F. W., Besset, T., Kuhl, N. & Glorius, F. Diverse strategies toward indenol and fulvene derivatives: Rh-catalyzed C−H activation of aryl ketones followed by coupling with internal alkynes. J. Am. Chem. Soc. 133, 2154–2156 (2011).
Muralirajan, K., Parthasarathy, K. & Cheng, C.-H. Regioselective synthesis of indenols by rhodium-catalyzed C−H activation and carbocyclization of aryl ketones and alkynes. Angew. Chem. Int. Ed. 50, 4169–4172 (2011).
Tan, X. et al. Rhodium-catalyzed cascade oxidative annulation leading to substituted naphtho[1,8-bc]pyrans by sequential cleavage of C(sp2)−H/C(sp3)−H and C(sp2)−H/O−H bonds. J. Am. Chem. Soc. 134, 16163–16166 (2012).
Zheng, Q.-Z. & Jiao, N. Transition-metal-catalyzed ketone-directed ortho-C−H functionalization reactions. Tetrahedron Lett. 55, 1121–1126 (2014).
Huang, Z., Lim, H. N., Mo, F., Young, M. C. & Dong, G. Transition metal-catalyzed ketone-directed or mediated C−H functionalization. Chem. Soc. Rev. 44, 7764–7786 (2015).
Sambiagio, C. et al. A comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Yang, K., Song, M., Liu, H. & Ge, H. Palladium-catalyzed direct asymmetric C–H bond functionalization enabled by the directing group strategy. Chem. Sci. 11, 12616–12632 (2020).
Mandal, R., Garai, B. & Sundararaju, B. Weak-coordination in C−H bond functionalizations catalyzed by 3d metals. ACS Catal. 12, 3452–3506 (2022).
Kim, J. & Chang, S. Iridium-catalyzed direct C−H amidation with weakly coordinating carbonyl directing groups under mild conditions. Angew. Chem. Int. Ed. 53, 2203–2207 (2014).
Raghuvanshi, K., Zell, D., Rauch, K. & Ackermann, L. Ketone-assisted ruthenium(II)-catalyzed C−H imidation: access to primary aminoketones by weak coordination. ACS Catal. 6, 3172–3175 (2016).
Yang, Y., Gao, P., Zhao, Y. & Shi, Z. Regiocontrolled direct C−H arylation of indoles at the C4 and C5 positions. Angew. Chem. Int. Ed. 56, 3966–3971 (2017).
Kimura, N., Kochi, T. & Kakiuchi, F. Iron-catalyzed regioselective anti-markovnikov addition of C−H bonds in aromatic ketones to alkenes. J. Am. Chem. Soc. 139, 14849–14852 (2017).
Tan, G., You, Q. & You, J. Iridium-catalyzed oxidative heteroarylation of arenes and alkenes: overcoming the restriction to specific substrates. ACS Catal. 8, 8709–8714 (2018).
Hu, Y., Zhou, B., Chen, H. & Wang, C. Manganese-catalyzed redox-neutral C−H olefination of ketones with unactivated alkenes. Angew. Chem. Int. Ed. 57, 12071–12075 (2018).
Chen, S. et al. C2/C4 Regioselective heteroarylation of indoles by tuning C−H metalation modes. ACS Catal. 9, 6372–6379 (2019).
Tan, X. et al. Rhoda-electrocatalyzed bimetallic C−H oxygenation by weak O-coordination. Angew. Chem. Int. Ed. 60, 13264–13270 (2021).
Song, S., Lai, Y., Tuo, Z., Zhong, J. & Zhou, W. Regio- and chemoselective formal (4+1) carbocyclization of chalcones with internal alkynes via rhodium(III) catalysis. Angew. Chem. Int. Ed. 62, e202305983 (2023).
Cao, Y.-X., Wodrich, M. D. & Cramer, N. Nickel-catalyzed direct stereoselective α-allylation of ketones with non-conjugated dienes. Nat. Commun. 14, 7640 (2023).
Sk, M. R., Bhattacharyya, A., Saha, S., Brahma, A. & Maji, M. S. Annulative π-extension by rhodium(III)-catalyzed ketone-directed C−H activation: rapid access to pyrenes and related polycyclic aromatic hydrocarbons (PAHs). Angew. Chem. Int. Ed. 62, e202305258 (2023).
Liu, X., Li, G., Song, F. & You, J. Unexpected regioselective carbon-hydrogen bond activation/cyclization of indolyl aldehydes or ketones with alkynes to benzo-fused oxindoles. Nat. Commun. 5, 5030 (2014).
Yin, J. & You, J. Concise synthesis of polysubstituted carbohelicenes by a C−H activation/radical reaction/C−H activation Sequence. Angew. Chem. Int. Ed. 58, 302–306 (2019).
Yin, J. et al. Acyl radical to rhodacycle addition and cyclization relay to access butterfly flavylium fluorophores. Nat. Commun. 10, 5664 (2019).
Neochoritis, C. G., Zarganes-Tzitzikas, T. & Stephanidou- Stephanatou, J. Dimethy acetylenedicarboxylate: a versatile tool in organic synthesis. Synthesis 46, 0537–0585 (2014).
Yin, J. et al. Programmable zigzag π-extension toward graphene-like molecules by the stacking of naphthalene blocks. Nat. Synth. 2, 838–847 (2023).
Pitzer, L., Sch-fers, F. & Glorius, F. Rapid assessment of the reaction-condition-based sensitivity of chemical transformations. Angew. Chem. Int. Ed. 58, 8572–8576 (2019).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Geuenich, D., Hess, K., Köhler, F. & Herges, R. Anisotropy of the induced current density (ACID), a general method to quantify and visualize electronic delocalization. Chem. Rev. 105, 3758–3772 (2005).
Acknowledgements
This work was supported by National Key R&D Program of China (No. 2021YFA1500100, Y.Y.), National Natural Science Foundation of China (No. 22031007, J.Y., 22171188, Y.Y.), and the Fundamental Research Funds for the Central Universities (YJ202340, G.T., 2023SCUH0080, Y.Y.). We also thank the support from the Analytical and Testing Center of Sichuan University, and Dr. J. Li and Dr. C. Wang from Comprehensive Training Platform Specialized Laboratory, College of Chemistry, Sichuan University.
Author information
Authors and Affiliations
Contributions
J.L. (Li), T.L., Y.Y. and C.Z. performed the experiments and analyzed the data. J.L. (Liu) performed DFT calculations. G.T. and J.Y. designed and directed the project. J.L. (Li), G.T. and J.Y. wrote the manuscript. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Liu, T., Liu, J. et al. Construction of acenaphthylenes via C−H activation-based tandem penta- and hexaannulation reactions. Nat Commun 15, 8319 (2024). https://doi.org/10.1038/s41467-024-52652-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-52652-4