Abstract
Social memory impairment is a key symptom of many brain disorders, but its underlying mechanisms remain unclear. Neuroligins (NLGs) are a family of cell adhesion molecules essential for synapse development and function and their dysfunctions are linked to neurodevelopmental and neuropsychiatric disorders, including autism and schizophrenia. Although NLGs are extensively studied in neurons, their role in glial cells is poorly understood. Here we show that astrocytic deletion of NLG3 in the ventral hippocampus of adult male mice impairs social memory, attenuates astrocytic Ca2+ signals, enhances the expression of EAAT2 and prevents long-term potentiation, and these impairments are rescued by increasing astrocyte activity, reducing EAAT2 function or enhancing adenosine/A2a receptor signaling. This study has revealed an important role of NLG3 in astrocyte function, glutamate homeostasis and social memory and identified the glutamate transporter and adenosine signaling pathway as potential therapeutic strategies to treat brain disorders.
Similar content being viewed by others
Introduction
Social interactions and memory are essential for forming meaningful relationships and for maintaining physical health. Appropriate social behaviors such as cooperation, aggression, avoidance and mating rely on social memory and indeed social memory impairments are a hallmark of many neurodevelopmental and neuropsychiatric disorders, including autism and schizophrenia1,2. Several human and monkey studies have demonstrated the critical role of hippocampus in social memory3,4,5,6. Recent studies in rodents have identified several subregions in the hippocampus, including dorsal CA2, ventral CA1 and dorsal DG, as key loci for social recognition memory storage7,8,9,10,11. However, the role of other cell types in social memory and underlying molecular and synaptic processes within these regions have yet to be determined.
Astrocytes are increasingly recognized as essential and active players in various aspects of brain development and function12,13. Through their complex and intricate processes, astrocytes enwrap and interact with neuronal synapses to regulate several synaptic processes, including synaptogenesis, ion homeostasis, synaptic transmission and plasticity14,15,16,17,18. For example, perturbations of astrocytic function have been shown to affect long-term potentiation (LTP), the most extensively studied form of long-lasting synaptic plasticity widely regarded as the mechanism of learning and memory16,19,20. Consistent with their role in synaptic regulation, astrocytes have been implicated in several forms of memory tasks, including spatial and fear memory16,21,22,23,24,25,26,27. However, the role of astrocytes in social memory and related disorders is largely unknown.
Astrocytes have been shown to exhibit activity-dependent Ca2+ responses27,28,29,30,31 and these Ca2+ transients are thought to induce the release of various gliotransmitters, including glutamate, ATP/adenosine and D-serine32,33,34. These gliotransmitters can activate receptors located on presynaptic terminals and/or postsynaptic membrane to trigger synaptic signaling and this is thought to be the key mechanisms by which synaptic plasticity and memory are modulated16,20. For example, D-serine released from astrocytes can act as a potent ligand at the glycine site of NMDA receptors and facilitate LTP induction35,36,37,38. Other gliotransmitters such as adenosine have also been shown to regulate synaptic plasticity and behavior24,39,40,41,42, but the molecular processes that regulate the release of these gliotransmitters remain unclear.
Neuroligins (NLGs) are a family of cell adhesion proteins known to be involved in several neurodevelopmental and neuropsychiatric disorders, including autism and schizophrenia43,44. The roles of NLGs in neurons have been extensively studied and shown to be critical for synapse maturation and synaptic plasticity through their interactions with presynaptic neurexins45. Recent studies have also shown that NLGs are highly expressed in astroglia and these astrocytic NLGs are required for astrocyte morphogenesis and synapse development18,46. However, whether astrocytic NLGs regulate neurotransmission and plasticity at mature synapses is unknown. In addition, whether the expression of NLGs in astrocytes contributes to learning and memory remains to be determined.
In this work, we specifically manipulate NLG3 in adult ventral hippocampal astrocytes and identify astrocytic NLG3 as a regulator of synaptic plasticity and social memory through modulating astrocyte activity, glutamate homeostasis and adenosine signaling.
Results
Astrocytic deletion of NLG3 impairs social memory
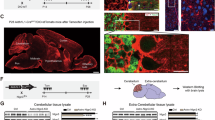
Previous studies have shown that NLG3 is not only expressed in neurons, but also in astrocytes46,47. To confirm this finding, we performed Western blot analysis on the protein lysates prepared from cultured astrocytes. As shown in Supplementary Fig. 1a, NLG3 was expressed in astrocytic cultures from wild type (WT), but not from NLG3 global KO brain. Recent studies have shown that astrocytic NLGs are important for astrocyte morphogenesis and synapse development18,46, but whether they are involved in synaptic plasticity and memory in adults is unknown. To address this question, we genetically ablated astrocytic NLG3 in the adult brain by crossing floxed NLG3 (NLG3 f/f or NLG3 f/Y) mice to a mouse strain expressing CreERT2 under the control of the hGFAP promoter (hGFAP-CreERT2) to restrict the expression of CreERT2 in the astrocytes. The Cre recombinase was inactive until tamoxifen treatment, which was administered after postnatal 2 months to avoid effects on development. First, we confirmed that the floxed NLG3 gene could be efficiently deleted by crossing the NLG3 f/Y mouse with a mouse strain having a ubiquitous expression of Cre (Nestin-Cre, Supplementary Fig. 1b). We also independently verified that the expression of Cre in the hGFAP-CreERT2 mouse line was specific to GFAP positive cells by crossing the hGFAP-CreERT2 mouse with a floxed tdTomato reporter line (Ai9, Supplementary Fig. 1c–j). After knowing that both NLG3 f/Y and hGFAP-CreERT2 mice behaved as intended, we then crossed the two mouse lines to generate NLG3 f/Y; hGFAP-CreERT2 double positive mice (Fig. 1a). Without tamoxifen treatment, the level of NLG3 protein in the total brain lysate showed no differences between NLG3 f/Y and NLG3 f/Y; hGFAP-CreERT2 littermates; however, 7 weeks after the tamoxifen treatment (daily i.p. injection for 7 days), the amount of NLG3 protein was significantly reduced in NLG3 f/Y; hGFAP-CreERT2 mice (referred to as GFAP-NLG3 KO) compared to NLG3 f/Y littermates (referred to as GFAP-NLG3 WT) (Fig. 1b, Supplementary Fig. 1k,l). The levels of other proteins in the hippocampal lysate, including NLG1 and NLG2 were not significantly altered in the GFAP-NLG3 KO mice (Supplementary Fig. 1k,l).
a Strategy for generation of astrocytic NLG3 KO mice. b Significantly reduced NLG3 protein in NLG3 f/Y; hGFAP-CreERT2 mice treated with tamoxifen compared to NLG3 f/Y; hGFAP-CreERT2 mice without tamoxifen or NLG3 f/Y mice with tamoxifen (n = 4 mice for each group, p = 0.000412). c Three-chamber social test consisting of Stage 1, Stage 2 and Stage 3. d Both GFAP-NLG3 WT (n = 10 mice) and GFAP-NLG3 KO (n = 13 mice) mice displayed no preference for either chamber 1 (C1) or chamber 2 (C2) during Stage 1. e Both GFAP-NLG3 WT (n = 10 mice, p < 0.001) and GFAP-NLG3 KO (n = 13 mice, p < 0.001) mice preferred stranger 1 (S1) over empty cage (E) during Stage 2. f GFAP-NLG3 WT (n = 10 mice, p < 0.001), but not GFAP-NLG3 KO mice (n = 13 mice), preferred stranger 2 (S2) over stranger 1 (S1) during Stage 3. g AAV virus constructs and timeline of virus injections and tests. h Significantly reduced NLG3 protein in NLG3 f/Y mice injected with GFAP-EGFP-Cre virus compared to those injected with GFAP-EGFP virus (n = 3 mice, p = 0.00923). i AAV virus injections to both ventral and dorsal hippocampus used for (j, k). j Both GFAP-EGFP (n = 8 mice, p < 0.001) and GFAP-EGFP-Cre (n = 9 mice, p < 0.001) injected NLG3 f/Y mice preferred S1 over E during Stage 2. k GFAP-EGFP (n = 8 mice, p < 0.001), but not GFAP-EGFP-Cre (n = 9 mice) injected NLG3 f/Y mice preferred S2 over S1 (p = 0.00113 for memory index) during Stage 3. Data represent mean ± SEM; two-tailed t test for (b, h), right panel of (f) and (k); two-way ANOVA with Tukey post hoc multiple comparisons for (d, e, j), and left panel of (f, k); **p < 0.01, ***p < 0.001. Source data are provided as a Source Data file.
To determine the functional consequences of astrocytic NLG3 ablation, we performed behavioral tests. In the open field test, the GFAP-NLG3 KO and GFAP-NLG3 WT mice showed no differences in travel distance, movement speed or relative time spent in the center vs periphery area (Supplementary Fig. 2a–c). In the elevated plus maze test, the two genotypes also showed similar travel distance, movement speed, and time and entries spent in the closed vs open arms (Supplementary Fig. 2d–g). These results suggest that the astrocytic ablation of NLG3 does not affect locomotor activity or anxiety-related behavior. In the three-chamber social test (Fig. 1c) that consists of habituation (stage 1), social interaction (stage 2) and social discrimination (stage 3), GFAP-NLG3 KO and GFAP-NLG3 WT mice showed no preference for the left or right empty cage in stage 1 (Fig. 1d), and similar preference for stranger 1 (S1) over the empty cage (E) in stage 2 (Fig. 1e). However, in the social recognition memory test at stage 3, GFAP-NLG3 KO mice showed no significant preference for S1 or S2 (Fig. 1f), suggesting that astrocytic deletion of NLG3 specifically impaired social recognition memory, but not sociability. This memory impairment was not likely due to a deficit in recognizing novelty, because GFAP-NLG3 KO mice showed normal performance in a novel object recognition test (Supplementary Fig. 2h). To examine whether the odor acuity was altered in the GFAP-NLG3 KO mice, we performed the buried food and social odor test, but found no significant differences between the two genotypes (Supplementary Fig. 2i,j), suggesting that the basal olfactory function is not affected by the astrocytic deletion of NLG3. GFAP-NLG3 KO mice were also not altered in short-term contextual fear memory (Supplementary Fig. 2k, l). As a comparison, we also examined the behavior phenotypes of NLG3 global KO mice. Consistent with a previous report48, the global NLG3 KO mice displayed hyperactivity in both open field and elevated plus maze test, normal sociability but impaired social recognition memory, and decreased olfactory performance in the buried food test (Supplementary Fig. 3a–j). These results suggest that in contrast to the global deletion, the astrocytic deletion of NLG3 resulted in selective impairments in social recognition memory without affecting sociability, locomotor activity or social odor acuity.
Astrocytic NLG3 in ventral hippocampus is required for social memory
Previous studies have shown that hippocampal neurons are critically important for social recognition memory7,8,9,49,50,51,52, but whether astrocytes in the hippocampus are also involved is unknown. We therefore deleted astrocytic NLG3 specifically in the hippocampus by local injections of AAV virus containing GFAP-EGFP-T2A-Cre to the hippocampus of adult NLG3 f/Y brains (Fig. 1g). 8 weeks post injections, immunostaining showed that EGFP signals were colocalized with GFAP, but not with NeuN, indicating that Cre was specifically expressed in astrocytes (Supplementary Fig. 4a). Western blot analysis of protein lysates extracted from isolated hippocampi showed that NLG3 protein level was significantly reduced in GFAP-Cre expressing mice compared to EGFP control (Fig. 1h). We first examined the mice in which astrocytic NLG3 was deleted in both dorsal and ventral hippocampus (Fig. 1i; Supplementary Fig. 4b), and found that social memory, but not sociability, was impaired in these mice (Fig. 1j,k). The locomotor activity was not altered in these mice (Supplementary Fig. 4c–e). Therefore, astrocytic deletion of NLG3 in adult hippocampus is sufficient to cause social memory deficits. To determine whether ventral or/and dorsal hippocampus is involved in social memory, we next restricted the GFAP-EGFP-T2A-Cre virus injections to either region of adult NLG3 f/Y brains. The three-chamber test 8 weeks post injections showed that social memory was impaired in astrocytic NLG3 deletion restricted to the ventral (Fig. 2a–c), but not dorsal hippocampus (Fig. 2d–f). Sociability was not affected by either manipulation. For this reason, we focused the rest of the study on ventral hippocampal CA1 area. Previous studies have also identified the ventral CA1 region to contain engram cells for social recognition memory9 and is the major output of dorsal CA2 and DG, both of which are shown to be involved in social memory7,51,52. To confirm that the social memory deficit was due to the lack of NLG3, we re-introduced WT NLG3 cDNA back to the ventral hippocampus by co-injecting AAV virus containing Cre-dependent CMV-DIO-NLG3-HA, together with GFAP-EGFP-T2A-Cre virus in adult GFAP-NLG3 KO brains (Fig. 2g, h). As shown in Fig. 2i–k, re-expression of NLG3 in GFAP-NLG3 KO mice was sufficient to improve social memory in GFAP-NLG3 KO mice without affecting locomotor activity or anxiety-like behavior (Supplementary Fig. 4f–i). To determine whether AAV GFAP-Cre virus may have targeted neurons including adult-born neurons in the hippocampus, we performed immunostaining for doublecortin (DCX) on ventral hippocampus injected with the GfaABC1D-mCherry virus and showed that mCherry signals were colocalized with S100β, but not with DCX in the dentate gyrus (DG), suggesting that GFAP-Cre was not likely to affect adult-born neurons (Supplementary Fig. 5a, b). In addition, we locally injected AAV virus containing CaMKIIα-EGFP-T2A-Cre to the ventral hippocampus (including DG) of adult NLG3 f/Y brains to specifically delete NLG3 in excitatory neurons and showed that the neuronal deletion of NLG3 was not sufficient to impair social recognition memory (Supplementary Fig. 5c–g). These data suggest that ventral, but not dorsal, hippocampal astrocytic NLG3 is indispensable for social memory.
a AAV virus injections to ventral hippocampus. b Both WT (n = 11 mice, p < 0.001) and NLG3 f/Y (n = 8 mice, p < 0.001) mice injected with GFAP-EGFP-Cre virus in ventral hippocampus preferred S1 over E during Stage 2 of three-chamber test. c WT (n = 11 mice, p = 0.004), but not NLG3 f/Y (n = 8 mice) mice injected with GFAP-EGFP-Cre virus in ventral hippocampus preferred S2 over S1 during Stage 3 (memory index p = 0.0151). d AAV virus injections to dorsal hippocampus. e Both WT (n = 10 mice, p < 0.001) and NLG3 f/Y (n = 15 mice, p < 0.001) mice injected with GFAP-EGFP-Cre virus in dorsal hippocampus preferred S1 over E during Stage 2. f WT (n = 10 mice, p = 0.002) and NLG3 f/Y (n = 15 mice, p < 0.001) mice injected with GFAP-EGFP-Cre virus in dorsal hippocampus preferred S2 over S1 during Stage 3. g AAV virus constructs and injection strategy for re-introducing WT NLG3-HA in ventral hippocampal astrocytes of GFAP-NLG3 KO mice. h Increased NLG3 protein in GFAP-NLG3 KO mice expressing NLG3-HA compared to those expressing EGFP (n = 3 mice, p = 0.0382). i AAV virus (same as in g) injections to ventral hippocampus used for (j, k). j Both NLG3-HA (p = 0.001) and EGFP (p < 0.001) expressed GFAP-NLG3 KO mice preferred S1 over E (n = 8 mice for each group) during Stage 2. k NLG3-HA, but not EGFP expressing GFAP-NLG3 KO mice preferred S2 over S1 during Stage 3 (p = 0.002 for sniffing time, p = 0.0106 for memory index, n = 8 mice for each group). Data represent mean ± SEM; two-tailed t test for right panels of (c, f, h, and k); two-way ANOVA with Tukey post hoc multiple comparisons for (b, e, j), and left panels of (c, f, and k); *p < 0.05, **p < 0.01, ***p < 0.001. Source data are provided as a Source Data file.
Astrocytic NLG3 is required for activity-dependent activation of astrocytes
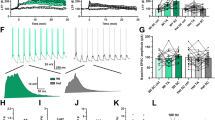
To investigate how astrocytic deletion of NLG3 affects social memory, we examined the structural and functional properties of astrocytes in the hippocampus. Since perturbations of NLGs in the developing brain affects astrocyte morphogenesis46, we first examined the number and morphology of astrocytes but found no differences between GFAP-NLG3 KO and WT mice (Supplementary Fig. 6). We also analyzed the number and ultrastructure of excitatory synapses using electron microscopy and showed no changes in synapse density, postsynaptic length and thickness, synaptic cleft or active zone length in GFAP-NLG3 KO mice (Supplementary Fig. 7a–f). These results suggest that astrocytic NLG3 deletion in adult mice has no significant effects on basal astrocyte or synapse morphology. Electrophysiological recording of CA1 astrocytes also showed no differences in resting membrane potentials (RMP), macroscopic currents in response to voltage steps, input resistance and glia-specific inwardly rectifying K+ channel Kir4.1 between GFAP-NLG3 KO and GFAP-NLG3 WT mice (Supplementary Fig. 7g–i). To directly examine astrocytic activity, we conducted Ca2+ imaging by co-injecting rAAV2/5-GfaABC1D-cyto-GCaMP6f and EF1α-DIO-mCherry viruses to the ventral hippocampus of adult NLG3 f/Y; hGFAP-CreERT2 or hGFAP-CreERT2 control mice. One week after the viral injection, the mice were treated with tamoxifen for one week and then seven weeks later, acute hippocampal slices were prepared and astrocyte (identified as mCherry and GCaMP6f positive) Ca2+ signals were imaged using a confocal microscope (Fig. 3a, b; Supplementary Fig. 8a, b). We first analyzed spontaneous Ca2+ signals (GCaMP6f dF/F0) under basal conditions but found no difference between GFAP-NLG3 KO and hGFAP-CreERT2 control mice (Supplementary Fig. 8c–e). We then examined activity-dependent Ca2+ changes in response to high frequency stimulation (HFS, 100 Hz, 1 s). As shown in Fig. 3c–e, hGFAP-CreERT2 control slices showed a significant increase in Ca2+ signals after HFS, whereas GFAP-NLG3 KO slices showed no significant changes. Consistent with previous studies53,54, the HFS-evoked Ca2+ signals in astrocytes were blocked by the purinergic receptor antagonists (50 μM PPADS, 100 μM suramin) and group I/II metabotropic glutamate receptor antagonist (100 μM MCPG), but not by the NMDA receptor antagonist AP5 (50 μM) (Fig. 3f–h). Post-imaging immunostaining showed that GCaMP6f was colocalized with GFAP (Supplementary Fig. 8a, b). To examine whether neuronal Ca2+ signals were also altered, we performed neuronal Ca2+ imaging as described for astrocytes by co-injecting rAAV2/9-CaMKIIα-cyto-GCaMP6f viruses (to restrict GCaMP6f expression in excitatory neurons) to the ventral hippocampus of adult NLG3 f/Y; hGFAP-CreERT2 or hGFAP-CreERT2 control mice. As shown in Supplementary Fig. 8f–i, WT slices showed a significant increase in Ca2+ signals after HFS, whereas GFAP-NLG3 KO slices showed no significant changes. To determine if astrocyte activation is also impaired in behaving animals, we performed in vivo fiber photometry recording of Ca2+ transients in ventral hippocampal astrocytes in live mice expressing rAAV2/5-GfaABC1D-cyto-GCaMP6f (Fig. 3i, j). As shown in Fig. 3k, l, social interaction (sniffing) with a stranger mouse evoked a significant increase in Ca2+ signals in WT, but not in GFAP-NLG3 KO mice. Therefore, astrocytic deletion of NLG3 impaired HFS- and learning-induced astrocyte activation both in vitro slices and in behaving animals.
a, b AAV virus constructs, injection and astrocyte Ca2+ imaging in hippocampal slices. c, d Sample traces and summary data of increased GCaMP6f signals in hGFAP-CreERT2 (top; n = 20 cells, 4 mice; p = 0.00203), but not in GFAP-NLG3 KO (bottom; n = 20 cells, 4 mice) astrocytes after HFS. e Reduced activated astrocytes in GFAP-NLG3 KO (n = 10 slices, 4 mice) compared to hGFAP-CreERT2 (n = 11 slices, 4 mice) in response to HFS (p = 0.00000208). f, g Representative traces and summary data of increased GCaMP6f signals in hGFAP-CreERT2 hippocampal astrocytes treated with ACSF (n = 15 cells, 4 mice; p = 0.00732) or AP5 (n = 8 cells, 3 mice; p = 0.0240), but not with PPADS + suramin (n = 15 cells, 4 mice) or MCPG (n = 15 cells, 3 mice) after HFS. h Reduced activated astrocytes in hGFAP-CreERT2 slices treated with PPADS + suramin (n = 6 slices, 3 mice; p < 0.001) and MCPG (n = 5 slices, 3 mice; p < 0.001) compared to those treated with ACSF (n = 6 slices, 3 mice) or AP5 (n = 6 slices, 3 mice) after HFS. i, j AAV virus construct and injections for in vivo fiber photometry Ca2+ imaging. k, l Representative traces and summary data of increased astrocytic GCaMP6f signals during social sniffing episodes (pink lines) in GFAP-NLG3 WT (n = 8 recordings, 4 mice; p < 0.001), but not in GFAP-NLG3 KO mice (n = 8 recordings, 4 mice). Data represent mean ± SEM; two-tailed t test for (e); two-tailed paired t test for (d, g); one-way ANOVA with Holm-Sidak test for multiple comparisons for (h), and repeated two-way ANOVA with Holm-Sidak test for multiple comparisons for (l); *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars: 10 μm in left panels of (c); 0.5 dF/F0 /5 s in right panels of (c, f); 0.2 dF/F0/60 s in (k). Source data are provided as a Source Data file.
Enhancing astrocytic activity improves social memory
To test whether the reduced astrocyte activity was a cause for the impaired social memory in astrocytic NLG3 deletion mice, we used the designer receptors exclusively activated by designer drugs (DREADD) system to increase astrocyte activity. We bilaterally injected AAV viruses expressing a floxed engineered M3-muscarinic receptor (EF1α-DIO-hM3D(Gq)-mCherry) or control mCherry (EF1α-DIO-mCherry) into the ventral hippocampus of adult NLG3 f/Y; hGFAP-CreERT2 mice (Fig. 4a, b). One week after the viral injection, the mice were treated with tamoxifen for one week and behavioral tests were performed seven weeks later. We confirmed that the expression of hM3D(Gq)-mCherry was restricted to the hippocampus and colocalized with GFAP (Fig. 4c–f). To activate hM3D(Gq), clozapine-N-oxide (CNO, 0.5 mg/kg, i.p.) was injected 20 min before behavior tests (Fig. 4g). In CNO treated mice, the hM3D(Gq)-mCherry expressing mice showed significantly higher social memory index without affecting sociability compared to mCherry expressing mice (Fig. 4h, i). Ca2+ imaging experiments in acute hippocampal slices of hM3D(Gq)-mCherry expressing mice confirmed that Ca2+ signals were increased by CNO (5 μM) compared to DMSO application (Fig. 4j–m, Supplementary Fig. 9a–c). The hM3D(Gq)-mCherry expressing mice showed no differences in locomotor activity, anxiety, olfaction and novel object recognition (Supplementary Fig. 9d–i). Therefore, chemogenetic activation of astrocytes in ventral hippocampus is sufficient to improve social memory in GFAP-NLG3 KO mice. These results indicate that the impaired astrocyte activity underlies social memory deficits in astrocytic NLG3 KO mice.
a, b AAV hM3D-mCherry virus constructs and injection to ventral hippocampus. c Sample image of viral expression of hM3D-mCherry in ventral hippocampus (repeated 5 times with similar results). Magnified images (d) showing greater >90% hM3D-mCherry expression colocalized with GFAP (e, n = 8 sections, 5 mice) and approximately 50% GFAP+ cells expressing hM3D-mCherry (f, n = 7 sections, 5 mice). g Systemic CNO injection and behavior tests for (h and i). h GFAP-NLG3 KO mice expressing hM3D-mCherry (n = 9 mice, p = 0.004) or mCherry (n = 8 mice, p = 0.001) preferred S1 over E during Stage 2 of three-chamber test. i GFAP-NLG3 KO mice expressing hM3D-mCherry (n = 9 mice, p = 0.010), but not mCherry (n = 8 mice) preferred S2 over S1 during Stage 3 of three-chamber test (p = 0.010 for sniffing time, p = 0.0199 for memory index). j–l Sample images, representative traces and summary data of Ca2+ imaging showing that CNO increased astrocytic GCaMP6f signals in hippocampal slices of hM3D-mCherry virus injected GFAP-NLG3 KO mice (DMSO n = 13 cells, 4 mice; CNO n = 13 cells, 4 mice; p = 0.0113). m Increased activated astrocytes in CNO treated hM3D-mCherry virus injected GFAP-NLG3 KO hippocampal slices compared to DMSO treatment (DMSO n = 10 slices, 4 mice; CNO n = 10 slices, 4 mice; p = 0.0000000384). Data represent means ± SEM; two-tailed t test for (l, m), and right panel of (i); two-way ANOVA with Tukey post hoc multiple comparisons for (h), and left panel of (i); Scale bars: 200 μm in panel (c), 50 μm in panel (d), 10 μm in panel (j). Scale bar: 1 dF/F0/30 s in (k). Source data are provided as a Source Data file.
Astrocytic NLG3 regulates astrocyte activity and social memory through astrocytic EAAT2
To investigate how NLG3 regulates astrocytic activity, we first analyzed receptors and transporters known to be expressed in astrocytes and involved in astrocytic Ca2+ signals55,56. Western blot and immunostaining analysis of ventral hippocampus revealed that both total and surface protein level of EAAT2, a member of glutamate transporters predominantly expressed in astrocytes, was significantly upregulated in GFAP-NLG3 KO mice (Fig. 5a–e). The protein level of EAAT1, which is also expressed in astrocytes57,58, was increased in western blot analysis although it was not altered in immunostaining experiments (Supplementary Fig. 10a–d). The protein level of EAAT3, which is predominantly expressed in neurons, was not altered (Supplementary Fig. 10a–d). The expression of purinergic receptors, P2X7 and P2Y1, and mGluR5, key receptors mediating astrocyte Ca2+ signals in response to intense neuronal activity in the hippocampus55,56, were also not altered (Supplementary Fig. 10a, b). In addition, the astrocyte-specific inwardly rectifying potassium channel Kir4.1, a key player in potassium homeostasis, was unchanged (Supplementary Fig. 10a,b). To confirm that the increased EAAT2 was due to astrocytic NLG3 deletion, we again re-introduced WT NLG3 cDNA back to the ventral hippocampal astrocytes of GFAP-NLG3 KO mice (Fig. 2g–k) and examined the expression of EAAT1/2/3. The re-expression of NLG3 reduced EAAT2 levels (Fig. 5d), but had no effect on EAAT1 and EAAT3 (Supplementary Fig. 10e, f). These experiments suggest that astrocytic deletion of NLG3 has a rather specific effect on EAAT2 protein expression. To determine how EAAT2 protein expression is affected, we analyzed hippocampal mRNA levels of EAAT1/2/3 using real-time quantitative PCR and found that although the mRNA level of EAAT1/3 was not altered, the level of EAAT2 mRNA was surprisingly reduced (Supplementary Fig. 10g). To determine whether translational regulation is altered, we treated acute hippocampal slices with mTOR inhibitor 1 (10 μM) for 3 h and then the protein level of EAAT2 was analyzed. As shown in Supplementary Fig. 11a, in ACSF-treated control slices, EAAT2 protein was increased in GFAP-NLG3 KO compared to WT mice, consistent with hippocampal lysate data (Fig. 5a); however, in mTOR inhibitor 1 treated slices, no differences were found between GFAP-NLG3 KO and WT mice. To examine whether mTOR signaling is altered, we analyzed phosphorylated mTOR (p-mTOR) and showed that the level of p-mTOR was increased in GFAP-NLG3 KO compared to WT mice (Supplementary Fig. 11b), suggesting an elevated mTOR signaling in the KO astrocytes. These data suggest that astrocytic NLG3 regulates EAAT2 protein expression via suppression of a mTOR-dependent translational mechanism.
a Increased EAAT2 protein level in GFAP-NLG3 KO (n = 6 mice) compared to GFAP-NLG3 WT mice (n = 6 mice, p = 0.0494). b, c Increased EAAT2 fluorescence intensity in total hippocampus (top, n = 10 sections, 3 mice, p = 0.00754) and GFAP+ cells (bottom, p = 0.0000453) in GFAP-NLG3 KO (n = 36 cells, 3 mice) compared to GFAP-NLG3 WT (n = 35 cells, 3 mice) mice. d Reduced EAAT2 protein level in GFAP-NLG3 KO mice virally expressing WT NLG3-HA compared to control EGFP (n = 4 mice, p = 0.0265). e, f Increased surface EAAT2 fluorescence intensity in total hippocampus (n = 10 sections, 3 mice, p = 0.0260) and in GFAP+ cells (n = 40 cells from 3 mice, p = 0.00000871) in GFAP-NLG3 KO compared to GFAP-NLG3 WT mice. g, h AAV virus construct, iGluSnFR expression and normalized data (h) showing increased iGluSnFR signals in response to social sniffing episodes in GFAP-NLG3 WT (n = 5 recordings, 5 mice), but not in GFAP-NLG3 KO mice (n = 4 recordings, 4 mice). i Reduced iGluSnFR signals after initial social sniffing in GFAP-NLG3 KO mice compared to GFAP-NLG3 WT (n = 5 mice for each genotype, p = 0.0437). Data represent mean ± SEM; two-tailed t test for (c, d, f and i); *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar: 100 μm in left (b, e), 10 μm in right (b and e); Source data are provided as a Source Data file.
Given the critical role of EAAT2 in the clearance of extracellular glutamate, particularly during intense neuronal activity and synaptic plasticity59,60,61, we reasoned that elevated EAAT2 expression may lead to changes in glutamate homeostasis and subsequently impaired astrocyte activity. To examine this possibility, we examined extracellular glutamate by virally expressing the glutamate sensor, GfaABC1D-iGluSnFR (Fig. 5g), in ventral hippocampal astrocytes, followed by in vivo fiber photometry recording before and after social stimulation. As shown in Fig. 5h, i and Supplementary Fig. 11c–h, social interaction (sniffing) evoked a significant increase in iGluSnFR signals in GFAP-NLG3 WT mice, but not in GFAP-NLG3 KO animals. The frequency, but not amplitude, rise/decay time or AUC, of the individual iGluSnFR response events were decreased in GFAP-NLG3 KO compared to WT mice (Supplementary Fig. 11c–g). This social interaction-induced impairment in glutamate rise is consistent with elevated EAAT2 expression in GFAP-NLG3 KO mice and suggests that the reduced glutamate signal may be responsible for impaired astrocyte activation observed in these mice (Fig. 3). To test this possibility, we conducted calcium imaging experiments similar to those as described in Fig. 3a–h, but instead of using HFS, we perfused 10 μM glutamate to evoke astrocytic Ca2+ response. As shown in Fig. 6a–c, glutamate induced a comparable increase of Ca2+ signals in WT and GFAP-NLG3 KO astrocytes. These results indicate that deficiency in extracellular glutamate is a cause of impaired astrocyte activation. To further support this, we tested the effect of TFB-TBOA (50 nM), an inhibitor of EAAT1/2. As shown in Fig. 6d–f, HFS evoked no Ca2+ signals in GFAP-NLG3 KO slices in control ACSF solution, but with TFB-TBOA perfusion, HFS evoked significant Ca2+ signals, which were comparable to those in WT group. Finally, to determine whether excessive EAAT2 expression is a cause of social memory loss in GFAP-NLG3 KO mice, we bilaterally injected TFB-TBOA (50 nM, 1 μl) into the ventral hippocampus 30 min prior to the social recognition test (Fig. 6g). The results showed that TFB-TBOA-treated GFAP-NLG3 KO mice exhibited normal sociality, spent more time exploring the novel strange mouse (Fig. 6h, i), and therefore improved social memory. The open-field experiments showed that TFB-TBOA treatment had no effect on locomotor activity or anxiety of GFAP-NLG3 KO mice (Supplementary Fig. 12a–c). We also locally injected the EAAT2 specific agonist GT949 to ventral hippocampus and showed that it impaired social memory without affecting sociability in WT mice (Supplementary Fig. 12d, e), suggesting that EAAT2 overactivation is sufficient to cause social memory deficit. Taken together, these results suggest that reduced extracellular glutamate, possibly due to excessive expression of astrocytic EAAT2, is responsible for impaired activity-dependent astrocyte activation and social memory in GFAP-NLG3 KO mice.
a, b Increased GCaMP6f fluorescence in both GFAP-NLG3 WT (n = 26 cells, 3 mice, p = 0.00000169) and KO (n = 26 cells, 3 mice, p = 0.00198) astrocytes after (post) glutamate treatment compared to before (pre). c Activated astrocytes in GFAP-NLG3 WT (n = 6 slices from 3 mice) and KO (n = 6 slices from 3 mice) slices treated with glutamate. d, e Increased GCaMP6f signals in response to HFS in GFAP-NLG3 WT (n = 24 cells, 3 mice, p = 0.00540) and KO (n = 24 cells, 3 mice, p = 0.00000000212) astrocytes treated with TBOA, but not in GFAP-NLG3 KO astrocytes treated with ACSF (n = 28 cells, 4 mice). f Activated astrocytes in TFB-TBOA treated GFAP-NLG3 KO (n = 6 slices, 3 mice, p < 0.001) and WT slices (n = 5 slices, 3 mice), compared to ACSF treated GFAP-NLG3 KO (n = 6 slices, 3 mice), g Local TFB-TBOA injections to ventral hippocampus before behavior tests. h GFAP-NLG3 KO mice treated with saline (n = 8 mice, p < 0.001) or TFB-TBOA (n = 8 mice, p < 0.001) preferred S1 over E during Stage 2 of three-chamber test. i GFAP-NLG3 KO mice treated with TFB-TBOA (n = 8 mice), but not with saline (n = 8 mice), preferred S2 over S1 during Stage 3 of three-chamber test (sniffing time p < 0.001, memory index p < 0.001). Data represent mean ± SEM; two-tailed paired t test for (b and e); two-tailed t test for (c) and right panel of (i); one-way ANOVA test with Holm-Sidak test for (f); two-way ANOVA with Tukey post hoc multiple comparisons for (h) and left panel of (i); **p < 0.01, ***p < 0.001. Scale bar: 0.5 dF/F0 /5 s in (a and d). Source data are provided as a Source Data file.
Astrocytic deletion of NLG3 impairs synaptic plasticity through adenosine signaling
How does impaired astrocyte activity in GFAP-NLG3 KO mice affect social memory? It is widely accepted that astrocytes are an integral component of the synapse and that astrocytes regulate brain function through affecting synaptic transmission and plasticity16,20. Therefore, we carried out electrophysiological recordings in ventral hippocampal slices to examine whether synaptic function is altered by astrocytic deletion of NLG3. Basal synaptic strength as judged by input/output curves of fEPSPs and presynaptic function assessed by paired pulse facilitation (PPF) were not altered in either global or hippocampus-specific GFAP-NLG3 KO (Fig. 7a–d). Whole-cell recordings of CA1 neurons also showed that frequency or amplitude of miniature excitatory synaptic currents (mEPSCs) or EPSC ratio mediated by AMPA versus NMDA receptors were not altered in GFAP-NLG3 KO mice (Fig. 7e, f).
a Input/output curve of fEPSPs in GFAP-NLG3 KO (n = 18 slices, 8 mice) and GFAP-NLG3 WT mice (n = 13 slices, 6 mice). b Input/output curve of fEPSPs in NLG3 f/Y mice injected with GFAP-EGFP-Cre (n = 7 slices, 5 mice) or GFAP-EGFP virus (n = 8 slices, 5 mice). c Paired pulse ratio (PPF) in GFAP-NLG3 KO (n = 10 slices, 6 mice) and GFAP-NLG3 WT mice (n = 11 slices, 6 mice). d PPF in NLG3 f/Y mice injected with GFAP-EGFP-Cre (n = 9 slices, 5 mice) or GFAP-EGFP virus (n = 9 slices, 5 mice). e mEPSCs in GFAP-NLG3 KO (n = 9 cells, 3 mice) and GFAP-NLG3 WT mice (n = 10 cells, 3 mice). f AMPAR/NMDAR EPSC ratio in GFAP-NLG3 KO (n = 8 cells, 3 mice) and GFAP-NLG3 WT mice (n = 8 cells, 3 mice). g Impaired LTP in GFAP-NLG3 KO (n = 5 slices, 3 mice) compared to GFAP-NLG3 WT mice (n = 5 slices, 3 mice, p = 0.00000249). h Impaired LTP in NLG3 f/Y mice injected with GFAP-EGFP-Cre (n = 6 slices, 4 mice; p = 0.0175) compared to those injected with GFAP-EGFP virus. i Impaired dorsal hippocampal LTP in GFAP-NLG3 KO (n = 5 slices, 3 mice) compared to GFAP-NLG3 WT mice (n = 5 slices, 3 mice, p = 0.00505). j 50 nM TFB-TBOA (p = 0.009) restored LTP in GFAP-NLG3 KO mice whereas 150 nM TFB-TBOA did not (n = 5 slices, 3 mice for each group). Data represent mean ± SEM; two-tailed t test for (e, f, g, h, and i); repeated two-way ANOVA test with Holm-Sidak test for multiple comparisons for (c and d); One-way ANOVA for (j); *p < 0.05, *p < 0.01, ***p < 0.001. Scale bars: 0.5 mV/25 ms in (a and b), 30 pA/10 s in (e), 50 pA/50 ms in (f), 0.25 mV/50 ms in (g–j). Source data are provided as a Source Data file.
Hippocampal long-term potentiation (LTP), a widely studied form of long-lasting synaptic plasticity, is a key mechanism for memory. Therefore, we compared LTP of field excitatory postsynaptic potentials (fEPSP) between GFAP-NLG3 KO and WT mice. As shown in Fig. 7g, HFS (four trains of 100 Hz, each lasting 1 s) induced a persistent LTP in WT, but this HFS-LTP was abolished in the GFAP-NLG3 KO mice. LTP was also impaired in hippocampus-specific astrocytic NLG3 KO mice created by local injection of AAV expressing GFAP-EGFP-T2A-Cre (Fig. 7h). HFS-LTP was also impaired in dorsal hippocampal slices in GFAP-NLG3 KO mice (Fig. 7i). These results indicate that astrocytic deletion of NLG3 selectively impairs LTP without affecting basal synaptic function. To examine whether the LTP deficit was caused by the excessive expression of EAAT2, we treated the GFAP-NLG3 KO slices with 50 nM TFB-TBOA and found that LTP was restored in these mice (Fig. 7j). Interestingly, the LTP deficit was not rescued by 150 nM TFB-TBOA (Fig. 7j). These experiments suggest that the reduced glutamate concentration is responsible for the LTP deficit. The impaired glutamate concentration may impair LTP induction by directly limiting depolarization of postsynaptic neurons, and consequently the activation of NMDA receptors in response to HFS. To test this possibility, we analyzed post-tetanic potentiation (PTP) after HFS and accumulative fEPSPs during HFS, but found no differences between GFAP-NLG3 KO and WT mice (Supplementary Fig. 12f–h). Therefore, the LTP deficit is not likely caused by changes in postsynaptic neurons, but rather by impaired astrocyte activity, during HFS.
How does a decrease in Ca2+ signals in astrocytes lead to impaired LTP in GFAP-NLG3 KO mice? Previous studies have shown that astrocytes regulate synaptic plasticity through Ca2+-dependent release of gliotransmitters and modulators such as adenosine and D-serine33,34,35,39,62. To test this possibility, we treated hippocampal slices with either adenosine (400 nM) or D-serine (400 nM), and found that adenosine (Fig. 8a), but not D-serine (Fig. 8b), restored LTP in the GFAP-NLG3 KO slices. To directly determine the involvement of the adenosine signaling pathway, we treated the GFAP-NLG3 KO slices with adenosine receptor agonists and found that the A2a receptor agonist (CGS 21680, 30 nM), but not the A1 receptor agonist (CPA, 500 nM), rescued the LTP deficit in GFAP-NLG3 KO slices (Fig. 8c). In addition, the rescue effect of adenosine was blocked by the A2a receptor antagonist SCH 58261 (100 nM, Fig. 8d). These results suggest that the activation of A2a receptor is necessary and sufficient to rescue LTP impairment in GFAP-NLG3 KO slices. Since adenosine can be either directly released from astrocytes or converted from extracellular ATP63, we also tested the effect of ATP treatment on LTP. As shown in Fig. 8e,f, the LTP deficit in GFAP-NLG3 KO slices was rescued by ATP (400 nM) and this rescue effect was blocked by the A2a receptors antagonist SCH 58261 (100 nM). Interestingly, neither bzATP (400 nM), an ATP analog that preferentially activate purinergic receptors (e.g., P2X7), nor ATPγS (400 nM), a non-hydrolyzable ATP, was able to rescue LTP deficit in GFAP-NLG3 KO slices (Fig. 8g). These results are consistent with the idea that it is adenosine, but not ATP, that directly acts on neurons to regulate LTP. As expected, the A2a receptor antagonist, but not adenosine or the A2a receptor agonist, affected LTP in WT mice (Fig. 8h–j). Consistent with electrophysiological data, Western blot analysis showed that HFS-induced protein kinase A activation, which is the downstream target of the A2a receptor, was impaired in GFAP-NLG3 KO mice (Supplementary Fig. 1m–q). Taken together, these results suggest that astrocytic NLG3 regulates LTP via the release of astrocytic ATP/adenosine and subsequent activation of A2a signaling pathway. However, we cannot rule out the possibility that ATP/adenosine is released from other cells including neurons.
a Adenosine restored LTP (ACSF n = 5 slices, 3 mice; adenosine n = 7 slices, 4 mice; p = 0.00394). b D-serine had no effect on LTP (ACSF n = 5 slices, 3 mice; D-serine n = 6 slices, 3 mice). c A2a agonist CGS 21680, not A1 agonist CPA, restored LTP (ACSF n = 5 slices, 3 mice; CPA n = 5 slices, 3 mice; CGS 21680 n = 5 slices, 3 mice; ACSF vs CGS 21680, p = 0.018; CPA vs CGS 21680, p = 0.014). d LTP rescue by adenosine blocked by A2a antagonist SCH 58261 (ACSF n = 5 slices, 3 mice; adenosine + SCH 58261 n = 5 slices, 3 mice). e ATP restored LTP (ACSF n = 5 slices, 3 mice; ATP n = 5 slices, 3 mice; p = 0.0139). f LTP rescue by ATP blocked by A2a antagonist SCH 58261 (ATP n = 5 slices, 3 mice; ATP + SCH 58261 n = 5 slices, 3 mice; p = 0.0311). g ATPγS or bzATP had no effects on LTP (ACSF n = 7 slices, 3 mice; bzATP n = 6 slice, 3 mice; ATPγS n = 5 slices, 3 mice). h Adenosine had no effect on LTP in WT mice (ACSF n = 5 slices, 3 mice, adenosine n = 5 slices, 3 mice). i A2a antagonist SCH 58261 blocked LTP in WT mice (ACSF n = 5 slices, 3 mice; SCH 58261 n = 5 slices, 3 mice; p = 0.000465). j A2a agonist CGS 21680 had no effect on LTP in WT mice (ACSF n = 5 slices, 3 mice; CGS 21680 n = 5 slices, 3 mice). Data represent mean ± SEM; two-tailed t test for (a, b, d, e, f, h, i, and j); one-way ANOVA with Holm-Sidak test for multiple comparisons for right panels of (c and g); *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars: 0.25 mV/50 ms. Source data are provided as a Source Data file.
Increasing adenosine signaling rescues social memory deficits
To investigate the in vivo effect of adenosine signaling in GFAP-NLG3 KO mice, we first measured the level of extracellular adenosine by cannula implantation in the left side of ventral hippocampus followed by microdialysis in freely moving animals. Social interaction (10 min) increased the level of adenosine in WT, but not in GFAP-NLG3 KO mice (Fig. 9a–c). The basal hippocampal adenosine levels were comparable in GFAP-NLG3 WT and KO mice (Supplementary Fig. 13a). To further confirm these results, we used bilateral injections of AAV virus expressing an adenosine probe, CaMKIIα-GRABAdo64, in the ventral hippocampus, and performed in vivo fiber photometry recording (Fig. 9d). While novel object stimulation did not evoke changes in adenosine signals in either GFAP-NLG3 WT or KO mice (Supplementary Fig. 13b), social stimulation evoked a significant increase in adenosine signals in GFAP-NLG3 WT mice, but not in GFAP-NLG3 KO animals (Fig. 9e, f). The social stimulation induced increase in adenosine signals was blocked by the EAAT2 agonist GT949 (Supplementary Fig. 13c). Interestingly, the analysis of individual GRABAdo responses showed no differences in the number, amplitude, rise/decay time or AUC decreased between GFAP-NLG3 WT and GFAP-NLG3 KO mice (Supplementary Fig. 13d–h). These results indicate that, similar to in vitro slices (Fig. 8), astrocytic deletion of NLG3 also impairs adenosine system in behaving animals, and therefore, the social memory deficit in GFAP-NLG3 KO mice could be due to impaired adenosine signaling. To test this possibility, we intraperitoneally injected the A2a receptor agonist CGS 21680 (0.5 mg/kg) into GFAP-NLG3 KO mice and 20 min later behavior tests were performed (Fig. 9g). As shown in Fig. 9h, i, the CGS 21680 treatment significantly improved social recognition memory, but had no effects on locomotor activity in the open field test, latency to locate food and novel object recognition (Supplementary Fig. 14a–e). Therefore, the activation of A2a receptors is sufficient to rescue the social memory deficit in GFAP-NLG3 KO mice consistent with the idea that the memory deficit is caused by impaired adenosine-A2a signaling pathway. To test the necessity of the A2a receptor, we first locally injected the A2a receptor antagonist SCH 58261 bilaterally in the ventral CA1 area (100 nM, 1 μl) and shown that the local inhibition of the A2a receptor also had no effect on sociability but blocked social recognition memory in WT mice (Supplementary Fig. 14f–h). Local SCH 58261 injections in ventral CA1 area had no effect on locomotor activity or anxiety-like behavior (Supplementary Fig. 14i–k). To directly tested the role of neuronal A2a receptors, we genetically deleted the receptors in excitatory neurons in the ventral hippocampus by local injections of AAV viruses expressing CaMKIIa-EGFP-T2A-Cre in floxed A2a (A2a f/f) mice (Fig. 9j–l, Supplementary Fig. 15a). The deletion of the A2a receptor in neurons was sufficient to impair social memory and LTP without affecting sociability (Fig. 9m–p) or local motor performance (Supplementary Fig. 15b–d). Therefore, the neuronal A2a receptors were required for normal social memory. Taken together, we conclude that the impaired neuronal A2a receptor signaling due to insufficient ATP/adenosine release may underlie social memory deficits GFAP-NLG3 KO mice.
a, b Microdialysis measurement of ventral hippocampal adenosine. c Increased extracellular adenosine in GFAP-NLG3 WT (n = 5 mice, p = 0.0144), not in GFAP-NLG3 KO (n = 5 mice), after social stimulation. RFU: Relative Fluorescence Units. ΔRFU: Post-social RFU minus Pre-social RFU. d AAV virus construct and expression of GRABado. e Increased adenosine signals after sniffings in WT (n = 8 recordings, 4 mice), not in GFAP-NLG3 KO mice (n = 8 recordings, 4 mice). f Reduced GRABado signals after sniffings in GFAP-NLG3 KO mice (n = 8 recordings, 4 mice, p = 0.0325). g Systemic CGS 21680 injections. h GFAP-NLG3 KO mice treated with saline (n = 6 mice, p < 0.001) or CGS 21680 (n = 10 mice, p < 0.001) preferred S1 over E. i GFAP-NLG3 KO mice treated with CGS 21680 (n = 10 mice, p < 0.001), not with saline (n = 6 mice), preferred S2 over S1 (memory index p = 0.0111). j, k AAV virus construct and injection for ventral hippocampal neuronal A2a deletion. l CaMKIIα-EGFP-2A-Cre expression in ventral hippocampus and CA1 neurons (repeated 5 times). m WT and A2a f/f mice injected with CaMKIIα-EGFP-Cre virus preferred S1 over E (n = 7 mice, p < 0.001). n CaMKIIα-EGFP-Cre virus injected WT (p < 0.001), not A2a f/f mice, preferred S2 over S1 (n = 7 mice, memory index p = 0.000161). o Reduced A2a receptor protein in A2a f/f mice injected with CaMKIIα-EGFP-Cre virus (n = 5 mice, p = 0.00174). p Impaired LTP in A2a f/f mice injected with CaMKIIα-EGFP-Cre virus (n = 5 slices, 3 mice, p = 0.00117). Data represent mean ± SEM; two-tailed t test for (c, f, o), and right panels of (i, n, and p); two-way ANOVA with Tukey post hoc multiple comparisons for (h, m), and left panels of (i and n); *p < 0.05, **p < 0.01, ***p < 0.001. Scale bars: 200 μm in left panel of (l), 50 μm in right panel of (l), 0.25 mV/50 ms in (p). Source data are provided as a Source Data file.
Discussion
The present study addresses two unresolved questions regarding NLGs and astrocytes. First, although NLGs in neurons are extensively interrogated in the regulation of synaptic plasticity and memory, the role of NLGs in astrocytes in these processes is surprisingly unknown. Second, although astrocytes are increasingly recognized as an active player at the synapse and contribute to several forms of memory, their involvement in social memory, a common deficit in many brain disorders, remains elusive. Therefore, elucidating the role of NLGs in astrocytes may provide deeper understanding of social memory and potential treatment of social deficits in brain disorders. In this study, we have provided evidence that astrocytic NLG3 in the ventral hippocampus regulates social recognition memory via the inhibition of EAAT2 expression, the release of ATP/adenosine and subsequent effects on LTP (Supplementary Fig. 16), revealing a mechanism by which NLGs affect synaptic function and behavior.
Astrocytic NLG3 regulation of social recognition memory is region-dependent
Recent studies have demonstrated that neurons located in the entorhinal cortex-hippocampal circuits are required for social recognition memory7,8,9,10,11,49,50,51,52. However, whether astrocytes within these circuits are involved is unknown. The results from the present study provide direct evidence to support the essential contribution of astrocytes to social recognition memory. First, both global and hippocampus-specific astrocytic deletion of NLG3 impairs social memory without affecting sociability or other behavior responses, including locomotor activity, anxiety-like behavior, novel object recognition or olfactory acuity. This rather selective effect on social memory is clearly distinct from NLG3 global KO mice, which, in addition to social memory deficits, also display hyperlocomotor activity, anxiety-like behavior and impaired odor acuity48. Second, we show that astrocytic deletion of NLG3 restricted to the ventral, but not dorsal, hippocampus is sufficient to cause social memory deficit. This finding has extended the previous studies showing the involvement of pyramidal neurons in the ventral hippocampus in social memory9,52. Our results also indicate that the participation of astrocytes in the formation of social memory is rather quick following social stimulation (given the short interval between social interaction and memory test), which is in contrast with other forms of memory such as contextual fear and inhibitory avoidance, where astrocytes appear to contribute predominantly to long-term and remote memory16,21,22,23,24,25,26,27.
Astrocytic NLG3 regulates activity-dependent activation of astrocytes
Although astrocytic NLGs are essential for astrocyte morphogenesis and synaptogenesis in developing brain18,46, astrocytic deletion of NLG3 in adult GFAP-NLG3 KO mice has no obvious effect on the number and process complexity of astrocytes. In addition, the deletion of NLG3 in mature astrocytes has no effect on the resting membrane electric properties or basal Ca2+ signals as indicated by whole-cell recordings and GCaMP6f fluorescence intensity, respectively. These results suggest that the expression of astrocytic NLG3 is not required for maintaining astrocyte morphology or cellular function under basal conditions, although we cannot rule out the possibility that other members of the NLG family may play a role in these processes. However, astrocytic deletion of NLG3 dramatically impairs astrocyte’s response to intense synaptic activity or social stimulation. HFS evokes Ca2+ transients in ventral CA1 astrocytes in acute hippocampal slices, and these Ca2+ signals are dramatically diminished by astrocytic deletion of NLG3. In addition, social interaction induces astrocytic Ca2+ rise in ventral hippocampus of behaving animals and these Ca2 signals are also absent in GFAP-NLG3 KO mice. Furthermore, enhancing astrocytic activity in the ventral hippocampus is sufficient to rescue social memory deficit in the GFAP-NLG3 KO mice. These results suggest that astrocytic NLG3 regulates social memory through promoting astrocyte activation in response to social stimulation.
How does astrocytic NLGs regulate activity-dependent astrocyte activation? Previous studies demonstrated that astrocytic Ca2+ increase in the hippocampus, particularly during intense neuronal activity, is mediated by purinergic and metabotropic glutamate receptors32,54,65,66,67, and indeed we also show that HFS-induced astrocytic Ca2+ signals in WT mice are inhibited by blocking these receptors. Therefore, it is possible that the impaired Ca2+ response in GFAP-NLG3 KO mice is caused by changes in these receptors. However, this is not likely because the expression of P2X7, P2Y1 and mGluR5, the key receptors responsible for HFS-induced Ca2+ rise in astrocytes in hippocampal CA1 area53,54, is not altered in GFAP-NLG3 KO mice. In addition, glutamate application elicited similar astrocytic Ca2+ response in both WT and GFAP-NLG3 KO slices suggesting that the function of astrocytic glutamate receptors, including mGluRs, is intact in the KO mice. Instead, our results collectively indicate that reduced extracellular glutamate due to excessive expression of EAAT2 is a cause for impaired astrocyte activity. First, we show that the protein level of EAAT2, which is predominantly expressed in astrocytes68, but not other members of glutamate transporters, is elevated in GFAP-NLG3 KO mice and that re-expression of NLG3 in the KO mice normalizes EAAT2 protein level. The expression of EAAT2 is known to be regulated by transcriptional and translational mechanisms69,70. Indeed our data show that while the mRNA level of EAAT2 is decreased, its protein level is elevated in the KO mice and that the increased EAAT2 protein level is normalized by the mTOR inhibitor suggesting that the expression of EAAT2 is predominantly regulated by NLG3 through translational suppression. Indeed, a previous study showed that NLG3 can regulate the mTOR pathway71. Thus, we have identified NLG3 as a potent regulator of EAAT2 protein synthesis through mTOR-mediated translation. Second, we show that social interaction induces a rise in glutamate concentration in the vicinity of astrocytes and this activity-dependent process is significantly diminished in GFAP-NLG3 KO mice. The impaired glutamate rise in the KO mice is likely related to exaggerated glutamate clearance by astrocytes due to excessive expression of EAAT2. In support of this possibility, addition of exogenous glutamate or EAAT1/2 inhibitor rescues Ca2+ response in GFAP-NLG3 KO mice, which also suggests that the signaling process downstream of glutamate to trigger Ca2+ rise in astrocytes is likely normal in these mice. Finally, we show that inhibiting EAAT1/2 improves LTP and social memory in GFAP-NLG3 KO mice, supporting that reduced extracellular glutamate is responsible for plasticity and memory impairments in these mice. These results are also consistent with previous studies showing that EAAT2 is involved in glutamate clearance particularly during synaptic plasticity and that overexpression of EAAT2 impairs LTP and memory59,61. Thus, we conclude that astrocytic NLG3 regulates astrocyte activity during synaptic plasticity and memory through suppressing protein synthesis of EAAT2. It is important to note that although the protein level of astrocytic EAAT2 was increased and TBOA treatment rescued LTP and memory deficits in GFAP-NLG3 KO mice, changes in glutamate response in the KO mice could also be due to changes in glutamate release or clearance from other cell types including neurons.
Astrocytic NLG3 regulates LTP and memory through the release of ATP/adenosine and subsequent activation of neuronal A2a receptors
Although astrocytes regulate LTP via the release of various gliotransmitters, most previous studies have been focused on D-serine, which binds to NMDA receptors to facilitate LTP induction35,36,37,38. However, a surprising finding of the present study is that the effects of astrocytic NLG3 on LTP and memory are mediated by adenosine signaling pathway, but not by D-serine. This is supported by following data. First, in GFAP-NLG3 KO mice, the impaired LTP is rescued by adenosine, not by D-serine, and this rescue effect is blocked by the A2a receptor antagonist. Second, the A2a, but not by A1 receptor agonist, is sufficient to rescue LTP deficit in GFAP-NLG3 KO mice. Third, ATP, but not non-hydrolyzable ATPγS, is able to restore LTP in GFAP-NLG3 KO mice and this effect is blocked by A2a receptor antagonist. Finally, neuronal deletion of A2a receptor in ventral hippocampus impairs LTP and social recognition memory. These findings are significant not only because they identify neuronal A2a receptors as a target of astrocytic NLG3, but also provide insight on adenosine system in synaptic plasticity and memory. Although several previous studies have investigated the involvement of adenosine and its receptors in hippocampal synaptic plasticity62,72,73 and memory74, their definitive involvement in these processes appears to be complex. For example, although pharmacological studies showed that inhibition and activation of A1 receptors facilitates and impairs LTP and memory, respectively72,75,76, suggesting a suppressive role in LTP and memory, genetic deletion of the A1 receptor gene has no clear effects on LTP or memory77,78. Similarly, although pharmacological perturbations of the A2 receptors impair LTP72,79,80,81,82, suggesting that A2 receptors play a facilitatory role in synaptic plasticity, A2a KO mice show no clear effects on hippocampal LTP83. In addition, A2a KO mice surprisingly display improved spatial memory84 as well as enhanced working memory85,86, suggesting an inhibitory role of A2a receptors in memory formation. This inhibitory effect is also seen in transgenic mice where A2a receptor overexpression impairs both spatial memory87 and working memory88. One possible reason for these apparently inconsistent results is that pharmacological manipulations are non-specific and potentially affect multiple (both A1 and A2) receptors with opposing effects. It is also possible that the roles of these receptors in memory regulation are cell type- and brain region-specifics. Therefore, manipulations of adenosine signaling in specific cell types and brain regions are necessary. In the present study, we specifically deleted neuronal A2a receptors in adult ventral hippocampus, and showed that both LTP and social recognition memory are impaired, underscoring the importance of hippocampal neuronal A2a receptors in these processes. In contrast to neuronal A2a receptors, the deletion of astrocytic A2a has been reported to enhance memory85, consistent with cell type-specific effect of adenosine system.
In summary, the present study provides multiple pieces of evidence supporting that astrocytic NLG3 regulates LTP and social memory through the activation of astrocytes by suppressing the expression of astrocytic EAAT2, the release of ATP/adenosine from the astrocytes and subsequent activation of A2a signaling pathway (Supplementary Fig. 16). These results have revealed a mechanism by which NLGs regulates gene expression, astrocyte function, synaptic plasticity, and memory. However, it is important to note that the present study has limitations. One is that because the GFAP promoter driven Cre is not 100% astrocyte-specific in the hippocampus, the behavioral and synaptic changes observed could be a mixture of effects of NLG3 loss in astrocytes and GFAP-positive newborn neurons or neuroprogenitor cells. In addition, the present study aims to delete NLG3 in the adult brain, and therefore the findings we have obtained cannot be extended to the roles of astrocytic NLG3 in synapse formation and astrocyte morphology. Nevertheless, given the wide involvement of NLGs, particularly NLG3, in neurodevelopmental and neuropsychiatric disorders, our results open the possibility to improve social behavior affected in these disorders by modulating astrocyte activity, glutamate homeostasis and adenosine signaling.
Methods
This research complies with all relevant ethical regulations. The experimental procedures including animal use protocols and biosafety are approved by the Hospital for Sick Children (#64589, Toronto, Canada) and Southeast University (#20211101004, Nanjing, China).
Mice
The NLG3 KO and NLG3 floxed (NLG3 f/f, NLG3 f/Y) mice were obtained from the Jackson Lab89. The hGFAP-CreERT2 mice were purchased from National Resource Center of Model Mice (NRCMM) of Nanjing University90. The Ai991 and Nestin-Cre92 mice were obtained from Zi-Long Qiu (Chinese Academy of Sciences). All the mice were genotyped using standard PCR techniques. To generate the astrocytes specific NLG3 knockout mice, the mice carrying the floxed NLG3 gene (NLG3 f/f or NLG3 f/Y) were crossed with hGFAP-CreERT2 mice to obtain NLG3f/Y; hGFAP-CreERT2 and NLG3 f/Y littermates. Because CreERT2 protein cannot enter nucleus without tamoxifen, deletion of NLG3 in astrocytes was achieved by 7 consecutive daily injections (1 mg, i.p.) of tamoxifen (Sigma, Cat# T5648) dissolved in 90% corn oil and 10% ethanol90. The NLG3 f/Y; hGFAP-CreERT2 and NLG3 f/Y littermates treated with tamoxifen were referred to GFAP-NLG3 KO and GFAP-NLG3 WT, respectively. The absence or reduction of NLG3 protein was verified by western blotting of whole brain or dissected hippocampal protein lysate. Floxed A2a (A2a f/f) mice93 were obtained from Jiang-Fan Chen (Wenzhou Medical University and Boston University). Primers used for genotyping mice were as follows: NLG3 f/Y (JAX: 015835) forward: GGGAGTGACTTGCTAGACAAG, reverse: ATGGGTGAGTTGTCCTTAGGC; hGFAP-CreERT2 forward: CCTGGAAAATGCTTCTGTCCG, reverse: CAGGGTGTTATAAGCAATCCC; Nestin-Cre (JAX: 003771) wild type forward: TTGCTAAAGCGCTACATAGGA, common: GCCTTATTGTGGAAGGACTG, transgene forward: CCTTCCTGAAGCAGTAGAGCA; Ai9 (JAX: 007905) wild type forward: AAGGGAGCTGCAGTGGAGTA, wild type reverse: CCGAAAATCTGTGGGAAGTC, mutant forward: CTGTTCCTGTACGGCATGG, mutant reverse: GGCATTAAAGCAGCGTATCC; A2a f/f forward: GGGCAAGATGGGAGTCATT, reverse: ATTCTGCATCTCCCG AAACC; NLG3 KO (JAX: 008394) wild type forward: GGGAGTGACTTGCTAGACAAG, common: ATGGGTGAGTTGTCCTTAGGC, mutant forward: CCATGTCACTACATGCTCTG. Mice were housed two to five in each cage on a 12 h (7:00 to 19:00) light/dark cycle with food and water ad libitum. All experiments were conducted during the light cycle. The experimenters were blind to the genotype of the mice. The NLG3 global KO and floxed NLG3 mice obtained from the Jackson Lab were backcrossed with C57BL/6 J for at least 5 generations upon arrival to the lab. The hGFAP-CreERT2 mice, Nestin-Cre mice and Ai9 mice were all on C57BL/6 J background. To avoid the effect of sex hormones on social interaction, only male mice were used for the present study. At the completion of all animal experiments, the mice were euthanized by CO2 inhalation.
Behavioral procedures
Mice were transported from the housing room to the behavioral room for at least 1 h before behavioral tests. The age of testing mice was 3–5 months. When multiple behavioral tests were required, the interval between the tests was 1–2 days. All the mice used in behavioral tests were age matched male littermates. Clozapine N-oxide (CNO) (0.5 mg/kg, i.p., Sigma, Cat# C0832) or CGS 21680 HCl (0.5 mg/kg, i.p., Sellect, Cat# S2153) was injected 20 min before the onset of behavioral tests. In intracerebral cannula injection experiments, saline or SCH 58261 (100 nM, 1 μl, Sellect, Cat# S8104) or TFB-TBOA (50 nM, 1 μl, MedChemExpress, Cat# HY-107521) were injected 30 min before tests.
Three-chamber sociability and social novelty test
Sociability and social novelty were tested as previously described8,49,94. The three-chamber apparatus consists of three equal-sized rectangular chambers, with each being (20 cm wide × 40 cm long × 22 cm high) in size. The two adjacent chambers were divided by two plexiglass walls, with rectangular doors (5 cm wide × 8 cm high) to allow access of the subject mice into each chamber. The stranger mice were separated from the subject mice by a wire pencil cup (7.5 cm wide × 7.5 cm long × 10 cm high). A heavy cup was placed on the top of the wire pencil cup to prevent climbing by the subject mice. These wire pencil cups were placed on the two outer sides of a three-chamber apparatus. Before testing, stranger mice were habituated to the wire pencil cup for 10 min. Between testing subject mice, the three-chamber apparatus, wire pencil cups and heavy cups were cleaned by 75% ethanol and dried by paper towel. Each testing session consists of three 10-min stages. In the first stage, the subject mice were allowed to explore the chambers for 10 min. In the second stage, a stranger mouse (stranger 1) was placed into one of the wire pencil cups, and the subject mice were allowed to investigate the three chambers for 10 min. After the sociability test stage, the subjects were separated from stranger 1 for 10 min. In the third stage, a novel stranger mouse (stranger 2) was placed into another wire pencil cups, then the subjects were free to investigate the three-chamber box. The time sniffing with two stranger mice was calculated to measure social memory of subject mice. Social sniffing was defined as the mouse’s nose was oriented towards the pencil box containing the unfamiliar mouse and approached within 2 cm. The sniffing was considered to end when the nose moves away. All the stranger mice were male, age matched and unfamiliar with subject mice on a background of C57BL/6 J. All the tests were videotaped by an overhead camera using an Anymaze (Stoelting Co.) or Ethovision XT software (Noldus). The sniffing time with two stranger mice were also recorded by a trained experimenter using a stopwatch and used to measure sociability and social recognition memory of subject mice. A “social memory index” was used to measure social recognition memory48. \({{\rm{Social}}}\; {{\rm{memory}}}\; {{\rm{index}}}=({{{\rm{Time}}}}_{{{\rm{stranger}}}2})/({{\rm{Time}}}_{{{\rm{stranger}}}1}+{{{\rm{Time}}}}_{{{\rm{stranger}}}2})*100-50\).
The open field test
The apparatus contains a square box made from clear Plexiglass (50 cm wide × 50 cm long × 50 cm high). Mice were placed in the corner and allowed to adapt for 2 min and then explore the open field for 10 min. The apparatus was cleaned with 75% ethanol between different subjects. The movement of the mice was recorded by an overhead camera. The distance, mean speed and time spent in the center (25 cm diameter in the center of the box) and peripheral were calculated by an Anymaze or Ethovision XT software.
Elevated plus maze test
The apparatus contains four elements, two open arms (35 cm long × 5 cm wide) and two closed arms (35 cm long × 5 cm wide × 15 cm high walls). These arms were conjoined at a center, and the maze was elevated 60 cm above the ground. Mice were placed in the central platform, facing an open arm of the plus maze. The test lasted 5 min and was recorded by an overhead camera. The apparatus was cleaned with 75% ethanol between different subjects. The distance, mean speed, time spent in and entries to each arm were calculated by an Anymaze software.
Novel object recognition test
A square box made from clear Plexiglass (50 cm wide × 50 cm long × 50 cm high) was used in this test. The subject mice were placed into the box facing to a corner away to the objects. Then the mice were allowed to freely explore two objects for 5 min. The test consists of two trials with the first trial containing object 1 and 2, and the second trail 1 h later, with the object 2 being replaced with a novel object 3. The objects were placed 8 cm apart to the two diagonal corners of the square box. The apparatus was cleaned with 75% ethanol between different subject mice. All the tests were videotaped by an overhead camera. The interaction time was recorded by an experimenter using a stopwatch.
Buried food test
All the mice used in this test were housed individually. To ensure the food was suitable for the test, 2 days before testing, mice were given 1.5 g food pellets each day and allowed to consume the pellet within 24 h. The mice were then deprived of food for 16 h. Before the test, a piece of food pellet was placed 1 cm under the standard bedding at the end of a clean cage (27 cm long × 16 cm wide × 13 cm high). The subject mice were placed facing the wall away from the pellet. The latency to find the pellet and start to eat were recorded by an experimenter using a stopwatch. To control for the activation of food-finding, the test was repeated as described above with a piece of food pellet being visible on the bedding. A fresh cage and bedding were used for each trial.
Olfaction habituation/dishabituation test
Two days before testing, mice were habituated for cotton swabs each day. On the day of testing, subject mice were housed individually in a clean cage for 40 min. Two social odors were then presented to the mice. Each odor was presented in three consecutive trials for 1 min. The social odor was obtained using cotton swabs rubbed along the bottom of a dirty cage. The two dirty cages were maintained with same numbers of mice with same sex. The time of mice spent sniffing the cotton swab was recorded by an experimenter using a stopwatch. The time spent chewing the cotton swab was not included.
Contextual fear memory test
The conditioning chamber contained the foot-shock box (30 × 26 × 30 cm) with the stainless steel bar floors. The duration of the test session, timing, intensity, and duration of the shock were controlled by Freeze Frame software (USA). The subject mouse was allowed to explore the foot-shock box for 300 s before the onset of electric foot-shock (0.7 mA, 2 s). 40 s later, the mouse was given another foot shock (0.7 mA, 2 s), and 60 s later, the mouse was removed from the chamber and returned to their home cages. Approximately 2 h later, subjects were given a 3 min contextual memory test in the same chamber where they were trained. The chamber was cleaned with 10% alcohol after each subject was trained or tested. The behavior of the mice was recorded and analyzed offline with the automated motion detection function of Freeze Frame software (USA).
Viral injections
The rAAV2/9-Ef1α-DIO-mCherry-WPRE-pA (titer: 5.90 × 1012 VG/mL), rAAV2/9-Ef1α-DIO-hM3D(Gq)-mCherry-WPRE-pA (titer: 6.91 × 1012 VG/mL), rAAV2/5-GfaABC1D-cyto-GCaMP6f-SV40-pA (titer: 2.40 × 1012 VG/mL), AAV2/9-CaMKII-GRABAdo (titer: 5.12 × 1012 VG/mL) and AAV2/5-GfaABC1D-iGluSnFR (A184S) (titer: 5.40 × 1012 VG/mL) viruses were purchased from BrainVTA (Wuhan, China). The rAAV2/8-GFAP-EGFP-2A-Cre (titer: 1.64 × 1013 VG/mL), rAAV2/9-short-GFAP-EGFP (titer: 1.79 × 1013 VG/mL), rAAV2/8-CaMKIIa-GFP-2A-Cre (titer: 2.13 × 1013 VG/mL), AAV2/5-CMV-DIO-EGFP (titer: 2.39 × 1013 VG/mL), pAAV2/5-CMV-DIO-NLG3-HA-WPRE (titer: 3.78 × 1013 VG/mL), rAAV2/5-CMV-DIO-EYFP-WPRE-pA virus (titer:3.35 × 1012 VG/mL), rAAV2/5-GfaABC1D-mCherry-WPRE-SV40-pA (titer: 5.17 × 1013 VG/mL) and rAAV2/9-CaMKII-cyto-GCaMP6f (titer: 2.68 × 1013 VG/mL) were purchased from Obio Technology (Shanghai, China). The pAAV2/5-CMV-DIO-NLG3-HA-WPRE construct was made by inserting HA tag sequence at the site 18 bp before the mouse NLG3 cDNA stop codon. All the viruses were aliquoted and stored at −80 °C before use. The procedure for surgery and virus injection was described previously8,94. Briefly, mice were anaesthetized with 4% isoflurane and maintained with 1% isoflurane (RWD, Shenzhen, Cat# 20072102) through an animal anesthesia system (Friends Honesty Life Science 25 Co, LTD, Beijing, Cat# 700100) and placed onto a stereotaxic frame (RWD, Shenzhen, Cat# D01476-002). A midline scalp incision was made followed by craniotomies using a 0.6 mm drill bit. The rAAV viruses were injected into the dorsal hippocampus (AP: − 1.90 mm, DV: − 1.50 mm, ML: ± 1.60 mm relative to bregma) and/or ventral hippocampus (AP: − 3.16 mm, DV: − 4.00 mm, ML: ± 3.25 mm relative to bregma). The virus was infused with a total volume of 0.5 µl bilaterally at a rate of 0.05 µl/minute via a 10 µl microliter needle syringe and micro-injection pump (KD Scientific, Cat# 78–8130). Following infusion, the internal cannula was left in place for 5 min to allow for diffusion. Mice were placed on a heating pad for a full recovery. For DIO virus injection experiments in hGFAP-CreERT2 mice, which required Cre recombinase to enter nucleus, one week after virus injection, tamoxifen was injected for 7 consecutive days (1 mg, i.p.) as described above. All the behavior tests were done 4 to 8 weeks after virus injections. The expression pattern of viruses was confirmed by immunohistochemical staining and imaging using a confocal microscope (Zeiss, LSM 700) 5× objective (2,048 × 2,048 pixels, NA 0.16) and 20× objective (2,048 × 2,048 pixels, NA 0.8).
Optic fiber implantation
For optic fiber implantation, mice were anaesthetized with 4% isoflurane and maintained with 1% isoflurane and placed onto a stereotaxic frame. 2 screws were placed on the skull away from the implantation site. One week following rAAV2/5-Ef1α-DIO-EYFP-WPRE-pA or rAAV2/5-GfaABC1D-cyto-GCaMP6f-SV40-pA virus injection bilaterally to ventral hippocampus (AP: − 3.16 mm, DV: − 4.00 mm, ML: ± 3.25 mm relative to bregma), optical fibers (200 µm diameter, 0.37 NA, secured to 1.25 mm (for fiber photometry) outer diameter ceramic ferrules, Inper, Hangzhou) were bilaterally implanted to the ventral hippocampus (AP: − 3.16 mm, DV: − 3.95 mm, ML: ± 3.25 mm relative to bregma). A layer of dental cement was applied to the skull, and a cap was used for protecting the fiber. Mice were placed on a heating pad for a full recovery. The mice with surgeries were individually kept and all the behavior tests were done 3 to 4 weeks after tamoxifen injections.
Cannula implantation
For cannula implantation used in microdialysis, 3-month-old GFAP-NLG3 WT and GFAP-NLG3 KO mice were anaesthetized with 4% isoflurane and maintained with 1% isoflurane and placed onto a stereotaxic frame. Two screws were placed on the skull and cannula (4 mm length, Eicom, Japan) was implanted in the right side of ventral hippocampus (AP: − 3.16 mm, DV: − 3.00 mm, ML: + 3.25 mm relative to bregma), and capped with a cannula stopper (diameter 0.5 mm, average length 5 mm, Eicom, Japan). A layer of dental cement was applied to the skull to secure the cannula. Mice were placed on a heating pad for a full recovery. The mice with surgery were individually kept and the microdialysis experiments were done 2 weeks after cannula implantation. For cannula implantation used in local drug injections, WT mice were anaesthetized with 4% isoflurane and maintained with 1% isoflurane and placed onto a stereotaxic frame. Two screws were placed on the skull and cannula (RWD, Shenzhen, Cat# 62004, O. D. 0.41 mm, I. D. 0.25 mm,) was implanted in the ventral hippocampus (AP: − 3.16 mm, DV: − 4.00 mm, ML: ± 3.25 mm relative to bregma), a layer of dental cement was applied to the skull to secure the cannula and a cap (RWD, Shenzhen, Cat# 62104) was used for protecting the cannula. Mice were placed on a heating pad for a full recovery. The mice with surgery were individually kept and the experiments were done 2 weeks after cannula implantation. The positioning of cannula was confirmed by immunohistochemical staining after experiments were completed. Mice with cannula misplaced were excluded from analysis.
Immunohistochemistry
The detailed procedures for immunostaining of fixed brain sections were described previously95. In brief, mice were anaesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and perfused with 25 ml phosphate-buffered saline (PBS, Wisent, Cat# 311-010-CL) followed by 25 ml 4% paraformaldehyde (PFA, Sangon Biotech, Cat# A500684) in PBS. The brain was dissected, further fixed in 4% PFA for 24 h and transferred to 30% sucrose until it sank to the bottom of tube. The brain was embedded in Tissue-Tek® O.C.T. compound (Sakura, Cat# 4583), maintained at 4 °C for 30 min and flash-frozen with liquid nitrogen. The brain was kept at −80 °C before cutting into sections at 35 μm at −20 °C (Leica CM1950). The sections were transferred to a glass slide coated with poly-L-lysine (Sigma, Cat# P8920), washed with PBS for three times and permeabilized by 0.3% Triton X-100 in PBS (PBT) for 1 h. The samples were then blocked by 10% FBS in PBT for 1 h at room temperature and incubated with primary antibodies in PBT overnight at 4 °C. The sections were washed three times with PBS, and incubated with appropriate secondary antibodies in PBS at 37 °C for 2 h and DAPI for 15 min in PBS at room temperature before washing and mounting. For surface EAAT2 staining, brain slices were blocked in 10% FBS and incubated with EAAT2 antibody (1:200; Cat# 20848, CST) overnight at 4 °C followed by secondary antibodies, without a prior PBT treatment. Images were obtained on a Zeiss confocal microscopes (LSM 700). Images were analyzed using the Fiji (National Institutes of Health, NIH) and LSM Image Browser software (Zeiss). Other primary antibodies used for immunohistochemical analyses included anti-GFAP (1:200; Cat# 3670, CST), anti-S100β (1:200; Cat# 15146-1-AP, Proteintech), anti-NeuN (1:200; Cat# 26975-1-AP, Proteintech), anti-EAAT1 (1:200, Cat# AB181036, Abcam), anti-EAAT2 (1:200, Cat# 22515-1-AP, Proteintech), anti-EAAT3 (1:200, Cat# 12686-1-AP, Proteintech), anti-p-mTOR (1:200, Cat# 5536, CST) and anti-DCX (1:200; Cat# 4604, CST). Secondary antibodies used for immunohistochemistry included Alexa Fluor 488 Goat Anti–Mouse IgG (1:200; Cat# A-11029, Invitrogen), Texas Red-Conjugated Goat Anti-Mouse IgG (1:200; Cat# T862, Invitrogen), Cy3-conjugated Goat Anti-Rabbit IgG (1:200; Cat# SA00009-2, Proteintech), Alexa Fluor 633 Goat anti-Rabbit IgG (1:200; Cat# A-21070, Invitrogen), DAPI was from Cayman Chemical (1:1000; Cat# 28718-90-3).
Western blot analysis
The mouse whole brain or hippocampi were quickly dissected and homogenized in ice-cold lysis buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 20 mM NaF, and 1% protease inhibitor cocktail and phosphatase inhibitors (Thermo, Cat# A32961) and lysed for 1 h at 4 °C. Debris was removed by centrifugation at 12000 g at 4 °C for 10 min. Proteins were separated on 10% SDS-PAGE and transferred to a PVDF filter. Filters were then blocked with 5% dry milk TBST (20 mM Tris, 9% NaCl, 1% Tween-20, pH 7.6) and incubated with primary antibodies overnight at 4 °C. After washing and incubation with appropriate secondary antibodies, the filters were washed again and developed using an enhanced chemiluminescence method of detection (Thermo, Cat# 34577) and analyzed using the AlphaEaseFC software (Alpha Innotech). Protein loading was controlled by normalizing each tested protein with α-tubulin or GAPDH on the same blot. Primary antibodies used for Western blot analyses included anti-α-tubulin (1:10000; Cat# T9026, Sigma-Aldrich), anti-NLG1 (1:2000; Cat# 129013, Synaptic System), anti-NLG2 (1:2000; Cat# 129203, Synaptic System), anti-NLG3 (1:2000; Cat# HPA003183, Sigma-Aldrich), anti-GluA1 (1:2000; Cat# MAB2263, Millipore), anti-GluA2 (1:2000; Cat# MAB397, Millipore), anti-NR1 (1:2000; Cat# AB9864, Millipore), anti-PKA (1:2000; Cat# 5842, CST), anti-p-PKA (1:2000; Cat# 5661, CST), anti-ERK (1:5000; Cat# 4695, CST), anti-p-ERK (1:5000; Cat# 4370, CST), Anti-adenosine receptor A2a (1:2000; Cat# 05-717, Millipore), Anti-HA (1:2000; Cat# 51064-2-AP, Proteintech), Anti-Kir4.1 (1:2000, Cat# 12503-1-AP, Proteintech), anti-P2X7 (1:2000, Cat# 28207-1-AP, Proteintech), anti-P2Y1 (1:2000, Proteintech, Cat# 67654-1-AP), anti-mGluR5 (1:2000, Cat# AB76316, Abcam), anti-EAAT1 (1:2000, Cat# AB181036, Abcam), anti-EAAT2 (1:2000, Cat# 22515-1-AP, Proteintech), anti-EAAT3 (1:2000, Cat# 12686-1-AP, Proteintech), anti-GFAP (1:2000; Cat# 3670, CST), anti-NeuN (1:2000; Cat# 26975-1-AP, Proteintech), anti-GAPDH (1:4000; Cat# 60004-1-Ig, Proteintech). Secondary antibodies included: goat anti-rabbit (1:2000; Cat# A00098, Genscript) and goat anti-mouse (1:2000; Cat# A00160, Genscript).
Astrocytes culture
Cultured astrocytes were obtained from postnatal day 2–4 mouse pups. The cortical hemispheres were removed in ice-cold 0.1 M PBS and dissociated by 0.25% trypsinization (Wisent, Cat# 325-043-EL) at 37 °C for 20 min with mechanical trituration every 5 min. Cells were centrifuged at 1500 × g for 3 min, resuspended in DMEM (Wisent, Cat# 319-005-CL) with 10% FBS (Wisent, Cat# 086-150), and plated on 6-well plates. The cells were maintained in DMEM supplemented with 10% FBS, penicillin and streptomycin (Wisent, Cat# 450-201-EL). Culture medium was shaken vigorously and changed every 2-3 days. The cells were cultured for 3 generations before being trypsinized and harvested for biochemical analyses.
Astrocyte morphology analysis
WT and NLG3f/Y mice injected with GFAP-EGFP-T2A-Cre virus were used to analyze the morphology of astrocytes. Eight weeks after virus injection, mice were sacrificed and brain sections prepared as described for immunohistochemical analysis. EGFP fluorescent images were collected using a confocal microscope (Zeiss, LSM 700) 40× objectives (2,048 × 2,048 pixels, NA 0.95). Astrocyte soma areas and processes were initially analyzed on Z-stacks of confocal images using a Fiji software and Sholl analysis plugin in Fiji. The parameters used in Sholl analysis was starting radius 10 µm, radius step size 1 µm. The confocal images were further analyzed using a Imaris software (Oxford, UK) for the 3D reconstruction and measurements of astrocyte process volume/length, territory size and number of branched.
Astrocyte cell count
Mouse brain sections were prepared as described for immunohistochemistry and stained for the astrocyte marker GFAP and S100β. Images were collected using a confocal microscope (Zeiss, LSM 700) 20× objectives (2,048 × 2,048 pixels, NA 0.8). GFAP+ and S100β+cell number of stratum oriens (SO) and stratum radiatum (SR) regions in the CA1 area of the hippocampus were counted by an experimenter blind to the genotypes of the mice using a Fiji software.
Microdialysis and adenosine assay
Eicom microdialysis system was used to collect samples from freely-moving mice as per manufacture instruction. Briefly, a dialysis membrane containing probe (Eicom, Cat# AZ-4-01, 4 mm long metal tube with a 1 mm long 50 kD-below permeable dialysis membrane at the end) was carefully inserted into the cannula, and then mice were placed in a blind cage (Eicom, BC-90, 40 cm wide × 40 cm long × 40 cm high,). After 10 min of exploration, subject mice were given a stranger mouse in a wire pencil cage for 10 min. Microdialysis was performed at 1.0 µl/min with artificial cerebrospinal fluid (ACSF) containing (in mM): 147 NaCl, 2.7 KCl, 1.2 CaCl2 and 0.85 MgCl2 (pH, 7.4). The adenosine deaminase inhibitor EHNA hydrochloride (APExBIO, Cat# B6662) was included in the ACSF. Samples were detected using an Adenosine Assay Kit (BioVision, Cat# K327-100) and measured by BioTek image reader (BioTek, Cytation 5).
Intracerebral cannula drug injections
Two- to three-month-old mice were used for intracerebral cannula injection experiments. Mice were anaesthetized and implanted with two guide cannula (RWD, Shenzhen, Cat# 62004, O. D. 0.41 mm, I. D. 0.25 mm) in ventral CA1 region (AP: − 3.16 mm, DV: − 4.20 mm, ML: ± 3.20 mm relative to bregma). Two weeks later, mice were anaesthetized with 4% isoflurane and maintained with 1% isoflurane, and injected with 1 μl SCH 58261 (100 nM) bilaterally at a rate of 0.1 μl/min through a polyethylene tube (RWD, Shenzhen, Cat# 62320, O. D. 0.85, I. D. 0.42 mm) which connected the cannula and syringe. After injection, mice recovered 30 min before behavior tests. Mice were killed for cannula placement verification after the tests.
Slice electrophysiology
The detailed procedures and analysis for acute hippocampal slices recordings were described previously95. All recordings were conducted at the Schaffer collateral-commissural pathway in the ventral hippocampus of 2–5-month-old mice. In brief, the mouse brains were quickly removed, and sagittal hippocampal slices (360 μm) were prepared (Leica, VT1000S) in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 120 NaCl, 3.0 KCl, 1.2 MgSO4, 1.0 NaH2PO4, 26 NaHCO3, 2.0 CaCl2, 11 D-glucose saturated with 95% O2/5% CO2. The slices were recovered at 28 °C in ACSF saturated with 95% O2/5% CO2 for at least 2 h before a single slice was transferred to a submersion chamber perfusion with 95% O2/5% CO2 saturated ACSF. Hippocampus and neurons were visualized using an infrared differential interference contrast microscope (Olympus X51). Synaptic transmission was evoked by stimulation at 0.067 Hz of Schaffer collaterals and recorded with glass pipettes (3–4 MΩ) filled with ACSF (for field potential recordings) or intracellular solution (for whole-cell recordings). For input-output curves of synaptic transmission, the stimulus intensity was increased gradually from 0.1 mA to 0.2 mA, 0.3 mA, 0.4 mA, 0.5 mA. Paired-pulse facilitations were obtained at inter-pulse intervals of 25 ms, 50 ms, 100 ms, 200 ms, 500 ms, 1000 ms. Field LTP at CA1 synapse was induced by four trains of HFS (100 Hz lasting 1 s each with a 10-s intertrain interval). All the drugs used in LTP experiments were added 30 min before HFS stimulation and maintained throughout the recording. The drugs used for recordings included: TFB-TBOA (50 nM, 150 nM), SCH 58261 (100 nM), CGS 21680 HCl (30 nM), Adenosine (400 nM, Cat# A9251, Sigma), ATP (400 nM, Cat# A9187, Sigma), bzATP (400 nM, Cat# B6396, Sigma), ATPγS (400 nM, Cat# B7582, APExBIO), N6-Cyclopentyladenosine (CPA) (500 nM, Cat# C8031, Sigma), D-serine (400 nM, Cat# S4250, Sigma), CNQX (10 μM, Cat# N183, Sigma), Picrotoxin (PTX) (100 μM, Cat# R284556, Sigma), AP5 (50 μM, Cat# 0106, Tocris) and Tetrodotoxin (TTX) (1 μM, Cat# A3109, Sigma). LTP was calculated and statistically evaluated by comparing the averaged responses 50–60 min after the induction protocol with the averaged responses of the entire baseline. PTP was calculated and statistically evaluated by comparing the averaged responses of the first minute after HFS with the averaged responses of the baseline. For whole cell recordings of hippocampal CA1 astrocytes, astrocytes were initially identified morphologically based on the characteristic size and shape of their soma, which was further confirmed by their electrical responses and GFAP-mCherry/EGFP. Astrocytes were recorded under a current clamp model with glass pipettes (6–8 MΩ) filled with the intracellular solution containing (in mM) 130.0 C6H11KO7, 5.0 NaCl, 1 MgCl2, 0.05 EGTA, 10.0 HEPES, 3.0 Mg-ATP, 0.3 Na3GTP (pH 7.5) (280–300 mOsm). Astrocyte resting membrane potentials (RMP), macroscopic currents in response to voltage steps (from −120 mV to +30 mV, 10 mV step), and input resistance were recorded. Astrocyte cell series resistance was monitored throughout experiments by applying a (10 pA) step and the experiment was excluded from analysis if the resistance changed by more than 20%. For whole cell recordings in CA1 neurons, 100 μM picrotoxin was added in the perfused ACSF, and the intracellular solution containing (in mM) 130.0 CsMeSO4, 5.0 NaCl, 1 MgCl2, 0.05 EGTA, 10.0 HEPES, 3.0 Mg-ATP, 0.3 Na3GTP and 5.0 QX-314 (pH 7.5) (280–300 mOsm). For the recording of EPSCNMDAR/EPSCAMPAR ratio, neurons were clamped at −70 mV, 0 mV and 40 mV for 1 min each. The EPSCAMPAR was qualified by adjusting the peak response at −70 mV and the response at the same time point at 0 mV. Similarly, the EPSCNMDAR was evaluated by the responses at +40 mV and 0 mV 40 ms after the onset of electric stimulation. For the recording of miniature EPSCs (mEPSCs), 100 μM picrotoxin and 1 μM TTX were added in the perfused ACSF, neurons were clamped at –70 mV. During whole-cell recordings, cell series resistance was monitored throughout experiments by applying a (−3 mV) step at the end of each sweep and the experiment was excluded from analysis if the resistance changed by more than 20%. All data acquisition and analysis were done using pCLAMP 10.2 (Molecular Devices) and Mini Analysis software (Synaptosoft). In all electrophysiological experiments, n represents the number of slices and normally one or two slices per animal were used.
RNA isolation and quantitative RT-PCR
Mouse vHPC RNA was extracted with Total RNA Isolation Kit (Vazyme Biotech, Cat# RC112-01). A total of 1 µg RNA for each sample was reverse transcribed into cDNA using the standard cDNA Synthesis Kit (Vazyme Biotech, Cat# R111-01/02). The cDNA was then amplified with the SYBR Green Master Mix Kit (Vazyme Biotech, Cat# Q111-02/03) in the CFX96 real-time PCR system (Bio-Rad). The amplification cycle was: 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s, 59 °C for 1 min. The levels of target RNA were adjusted to that of GAPDH. Each reaction was run in triplicate and was analyzed following the ΔΔCt method. Primer information is as follows: EAAT1, forward: GGAAGAACCCTTTCCGCTTTGC, reverse: CACAGCGGAATGTAACTGGCAG; EAAT2, forward: GCCAACAATATGCCCAAGCAG, reverse: GACACCAAACACAGTCAGTGA; EAAT3, forward: GCGATTGGTCGCGGTGATAATG, reverse: CGACAATGACTGTCACGGTGTAC; NLG3, forward: GTTTGCAGCCGCCTAGG, reverse: GGCCACACTGACTTGAACC; GAPDH, forward: AGGTCGGTGTGAACGGATTTG, reverse: TGTAGACCATGTAGTTGAGGTCA.
Ca2+ imaging in hippocampal slices
Two-month-old hGFAP-CreERT2 and NLG3 f/Y; hGFAP-CreERT2 mice were injected with 0.5 μl 1:1 mixed rAAV2/5-GfaABC1D-cyto-GCaMP6f (for astrocytes) or rAAV2/9-CaMKII-cyto-GCaMP6f (for excitatory neurons) and rAAV2/9-Ef1α-DIO-mCherry viruses bilaterally in ventral hippocampus (AP: − 3.16 mm, DV: − 4.00 mm, ML: ± 3.25 mm relative to bregma). One week after virus injection, tamoxifen was injected for 7 consecutive days (1 mg, i.p.). Seven weeks later, ventral hippocampal slices were prepared as described for electrophysiological recordings. After recovery at 28 °C in ACSF saturated with 95% O2/5% CO2, slices were transferred to a recording chamber perfused with 95% O2/5% CO2 saturated ACSF with picrotoxin (100 μM) and imaged at 30 °C. Alive astrocytes were initially identified by mCherry signal, and further confirmed by Ca2+ fluctuations. Ca2+ signals of astrocytes located in the stratum radiatum (SR) area 20–50 μm from vCA1 pyramidal cell soma layer and 50–100 μm from the stimulating electrode were captured and recorded with a confocal microscopy using 20× water immersion objectives (LSM710, 512 × 512 pixels, NA 1.0, ZEISS). For drug treatment experiments, D-AP5 (50 μM), PPADS (50 μM), suramin (100 μM), MCPG (100 μM, Cat# HY-100406, MCE), TFB-TBOA (50 μM) or glutamate (10 μM, without HFS) were added to the perfusion ACSF 20 min before HFS (100 Hz, 1 s) and kept during the entire experiment. The expression of GCaMP6f was further confirmed by immunostaining with the astrocytic marker GFAP. For neuronal Ca2+ imaging experiments, live neurons were initially identified by weak EGFP signals in vCA1 pyramidal cell soma layer and retroconfirmed by a rise in Ca2+ signals following HFS in WT and 10 μM glutamate treatment in GFAP-NLG3 KO slices.
To measure astrocytic Ca2+ alteration by hM3D activation, 0.5 μl 1:1 mixed rAAV2/9-DIO-hM3D-mCherry and rAAV2/5-GfaABC1D-cyto-GCaMP6f viruses were co-injected into ventral hippocampus (AP: − 3.16 mm, DV: − 4.00 mm, ML: ± 3.25 mm relative to bregma) of NLG3 f/Y; GFAP-CreERT2 mice. One week after virus injection, tamoxifen was injected for 7 consecutive days (1 mg, i.p.) and 6 weeks later, the slices were prepared as described earlier. CNO was added in perfusion ACSF at a final concentration of 5 μM. Ca2+ signals of astrocytes expressing both mCherry and GCaMP6 and located in 20–50 μm from vCA1 soma layer in SR area were captured and recorded, DMSO was added to ACSF as control.
Fiber photometry recording in free-moving mice
4–5-month-old GFAP-NLG3 WT and GFAP-NLG3 KO mice were used for fiber photometry Ca2+ imaging experiments. Mice were bilaterally injected with GfaABC1D-GCaMP6f virus, CaMKII-GRABAdo or GfaABC1D-iGluSnFR (A184S) in ventral hippocampal CA1 region as described earlier and one week later implanted with optic fibers (Inper, Hangzhou, FOC-W-1.25-200-0.37-5.0, diameter of 200 µm, numerical aperture 0.37). The sites of optic fiber implanted were 50 μm upper of the injected sites (AP: − 3.16 mm, DV: − 4.00 mm, ML: ±3.25 mm relative to bregma). The fiber photometry system was purchased from ThinkerTech (ThinkerTech, Nanjing, QAXK-FPS-MC-LED). Two weeks after optic fiber implantation, mice were habituated in a clean cage with fresh bedding for 10 min. After the habituation, a clean inverted wire pencil box was placed in the cage (empty cage), and the subject mice were let to explore freely for 5 min. Then a stranger mouse was put into the pencil box, and the subject mice were allowed to explore for additional 5 min. The signals were collected bilaterally by the implanted fiber and an objective as previously described49. Briefly, a 488-nm laser beam (OBIS 488LS, Coherent) was reflected off a dichroic mirror (MD498, Thorlabs), focused with a 10× objective lens (0.3 NA, Olympus Inc., Japan), and then coupled to an optical commutator (Doris Lenses) to record calcium signals. The commutator and implanted fiber were connected by a 2-m optical fiber (200 mm O.D., 0.37 NA). The laser power at the tip of the optical fiber was adjusted to 0.01 mW to decrease laser bleaching. The fluorescence signals were bandpass filtered (MF525-39, Thorlabs). An amplifier was used to convert the photomultiplier tube current output to a voltage signal, which was further digitized at 100 Hz and recorded by a Power 1401 digitizer and Spike2 software (CED, Cambridge, UK). The heatmap and averaged fluorescence signals traces were plotted using a self-developed MATLAB program. The position of the optical fiber tip sites was histologically examined in each mouse after the behavior tests.
Slices and fiber photometry imaging data analysis
For hippocampal slice Ca2+ imaging data, the analysis of astrocyte and neuronal Ca2+ signals was performed with Fiji software. Polygon selections were manually drawn around the approximate astrocyte soma and processes as the region of interest (ROI). Signal intensity values of ROI were measured through ROI manager and then converted to dF/F0 values with the algorithm: dF/F0 = (F-F0)/F0. For each ROI, F0 was the mode of the entire fluorescence recording. For assessing the effect of HFS stimulation (100 Hz, 1 s) or glutamate treatment, the alteration of Ca2+ signal intensity was calculated and statistically evaluated by comparing the mean dF/F0 values 1 min before and 1 min after the HFS stimulation. For assessing the effect of hM3D activation, the alteration of Ca2+ signal intensity was calculated and statistically evaluated by comparing the ratio of dF/F0 values 2 min before and 2 min or 20 min after DMSO/CNO perfusion. Astrocytes within the ROI with changes of dF/F0 equal or greater than 0.5 were defined as activated cells.
For fiber photometry Ca2+ imaging data analysis, the dF/F0 value was calculated by the same method as above. The peak dF/F0 value within 5 s of both injection sites after the onset of each social sniffing behavior was measured, and the first four peak values of both groups were statistically analyzed. For iGluSnFR and GRABAdo fiber photometry imaging data analysis, the alteration of signal intensity was calculated and statistically evaluated by comparing the mean dF/F0 values 30 s before and 1 min after the onset of the first social sniffing behavior, and the first three peak values of both groups were statistically analyzed. For iGluSnFR and GRABAdo event analysis, a fluctuation with peak intensity equal to or greater than 1% was defined an event. The frequency, amplitude, rise/decay time and area under the curve (AUC) of these events were analyzed by the Mini Analysis software.
Transmission electron microscopy (EM) imaging and analysis
Mice were anaesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and perfused with 25 ml PBS followed by 25 ml 2.5% glutaraldehyde (Sigma, G7651). The brains were cut into 300 μm slices using a vibrating microtome, the CA1 region of the brain was removed, and fixed overnight in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). The procedure for EM imaging and analysis was described previously96. In brief, the samples were rinsed with cacodylate buffer several times at 4 °C, postfixed for 2 h with 1% OsO4 in 0.1 M cacodylate buffer, and rinsed twice with distilled water. The samples were then stained overnight with 2% saturated uranyl acetate and rinsed twice with distilled water. The specimens were dehydrated in an increasing ethanol series (30, 50, 70, 85, 95, 100% twice), passed through propylene oxide twice, and embedded in Epon812 (SPI Science). The embedded block was sectioned into 90 nm thick ultrathin slices using a diamond knife on a Leica UC7 ultrathin microtome, and approximately 20 sections were gathered into a group and attached to a grid. The grids were stained again with 2% saturated uranyl acetate in 50% ethanol and then with 1% lead citrate (pH 12). Finally, the grids were examined and images collected using a transmission electron microscopy (Hitachi H-7650). A total of 26 thin sections (500 or 4 μm2 per section) from 4 animals for each genotype were randomly obtained from the SR region of ventral hippocampus and analyzed. Paired postsynaptic density (PSD) and presynaptic active zone were used to identify excitatory synapses. The synapse number, PSD thickness, PSD length, active zone length and synaptic cleft length were measured with a Fiji software.
Statistical analysis
All the averaged data in the graphs were presented as mean ± s.e.m. and statistically evaluated by two tailed t test, two tailed paired t test, or ANOVA (one-way ANOVA, two-way ANOVA or repeated measures-RM, wherever appropriate) followed by Holm-Sidak’s or Tukey post hoc multiple comparisons. p < 0.05 was considered significant (*p < 0.05, **p < 0.01, ***p < 0.001). The p values, sample sizes and statistical methods were indicated in respective figure legends. For p values smaller than 0.001, they were indicated by p < 0.001. The behavioral, electrophysiological, fiber photometry and astrocytic complexity data were analyzed using SigmaPlot 12.5 (Systat) and Graphpad Prism 7 (GraphPad Software). The Western blot, Ca2+ imaging, microdialysis, astrocyte soma and astrocyte number data were analyzed using Graphpad Prism 7.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the main text and Supplementary Information. Source data are provided with this paper as a Source Data file. All data are available from the corresponding author upon request. Source data are provided with this paper.
References
Barak, B. & Feng, G. Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat. Neurosci. 19, 647–655 (2016).
Porcelli, S. et al. Social brain, social dysfunction and social withdrawal. Neurosci. Biobehav. Rev. 97, 10–33 (2019).
Corkin, S. What’s new with the amnesic patient H.M.? Nat. Rev. Neurosci. 3, 153–160 (2002).
Sliwa, J., Planté, A., Duhamel, J. R. & Wirth, S. Independent Neuronal Representation of Facial and Vocal Identity in the Monkey Hippocampus and Inferotemporal Cortex. Cereb. Cortex 26, 950–966 (2016).
Squire, L. R. & Wixted, J. T. The cognitive neuroscience of human memory since H.M. Annu. Rev. Neurosci. 34, 259–288 (2011).
Steinvorth, S., Levine, B. & Corkin, S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from H.M. and W.R. Neuropsychologia 43, 479–496 (2005).
Hitti, F. L. & Siegelbaum, S. A. The hippocampal CA2 region is essential for social memory. Nature 508, 88–92 (2014).
Leung, C. et al. Activation of entorhinal cortical projections to the dentate gyrus underlies social memory retrieval. Cell Rep. 23, 2379–2391 (2018).
Okuyama, T., Kitamura, T., Roy, D. S., Itohara, S. & Tonegawa, S. Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016).
Smith, A. S., Williams Avram, S. K., Cymerblit-Sabba, A., Song, J. & Young, W. S. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry 21, 1137–1144 (2016).
Stevenson, E. L. & Caldwell, H. K. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur. J. Neurosci. 40, 3294–3301 (2014).
Ben Haim, L. & Rowitch, D. H. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 18, 31–41 (2017).
Khakh, B. S. & Sofroniew, M. V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952 (2015).
Allen, N. J. Astrocyte regulation of synaptic behavior. Annu. Rev. Cell Dev. Biol. 30, 439–463 (2014).
Araque, A. et al. Gliotransmitters travel in time and space. Neuron 81, 728–739 (2014).
Papouin, T., Dunphy, J., Tolman, M., Foley, J. C. & Haydon, P. G. Astrocytic control of synaptic function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160154 (2017).
Perea, G., Navarrete, M. & Araque, A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32, 421–431 (2009).
Sakers, K. & Eroglu, C. Control of neural development and function by glial neuroligins. Curr. Opin. Neurobiol. 57, 163–170 (2019).
Bliss, T. V. & Collingridge, G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Haydon, P. G. & Nedergaard, M. How do astrocytes participate in neural plasticity? Cold Spring Harb. Perspect. Biol. 7, a020438 (2014).
Adamsky, A. et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 174, 59–71.e14 (2018).
Gao, V. et al. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc. Natl. Acad. Sci. USA 113, 8526–8531 (2016).
Kol, A. et al. Astrocytes contribute to remote memory formation by modulating hippocampal-cortical communication during learning. Nat. Neurosci. 23, 1229–1239 (2020).
Martin-Fernandez, M. et al. Synapse-specific astrocyte gating of amygdala-related behavior. Nat. Neurosci. 20, 1540–1548 (2017).
Robin, L. M. et al. Astroglial CB(1) receptors determine synaptic D-serine availability to enable recognition memory. Neuron 98, 935–944.e935 (2018).
Suzuki, A. et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 (2011).
Zhang, K. et al. Fear learning induces α7-nicotinic acetylcholine receptor-mediated astrocytic responsiveness that is required for memory persistence. Nat. Neurosci. 24, 1686–1698 (2021).
Cornell-Bell, A. H., Finkbeiner, S. M., Cooper, M. S. & Smith, S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473 (1990).
Hirase, H., Qian, L., Barthó, P. & Buzsáki, G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol 2, E96 (2004).
Schummers, J., Yu, H. & Sur, M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320, 1638–1643 (2008).
Wang, X. et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 9, 816–823 (2006).
Bazargani, N. & Attwell, D. Astrocyte calcium signaling: the third wave. Nat. Neurosci. 19, 182–189 (2016).
Durkee, C. A. & Araque, A. Diversity and Specificity of Astrocyte-neuron Communication. Neuroscience 396, 73–78 (2019).
Parpura, V. et al. Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747 (1994).
Henneberger, C., Papouin, T., Oliet, S. H. & Rusakov, D. A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236 (2010).
Mothet, J. P. et al. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 97, 4926–4931 (2000).
Panatier, A. et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784 (2006).
Yang, Y. et al. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl. Acad. Sci. USA 100, 15194–15199 (2003).
Pascual, O. et al. Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116 (2005).
Martín, E. D. et al. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia 55, 36–45 (2007).
Halassa, M. M., Fellin, T. & Haydon, P. G. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 57, 343–346 (2009).
Falcón-Moya, R. et al. Astrocyte-mediated switch in spike timing-dependent plasticity during hippocampal development. Nat. Commun. 11, 4388 (2020).
Kenny, E. M. et al. Excess of rare novel loss-of-function variants in synaptic genes in schizophrenia and autism spectrum disorders. Mol. Psychiatry 19, 872–879 (2014).
Südhof, T. C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Südhof, T. C. Towards an understanding of synapse formation. Neuron 100, 276–293 (2018).
Stogsdill, J. A. et al. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551, 192–197 (2017).
Boisvert, M. M., Erikson, G. A., Shokhirev, M. N. & Allen, N. J. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22, 269–285 (2018).
Radyushkin, K. et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav 8, 416–425 (2009).
Dang, R. et al. Regulation of social memory by lateral entorhinal cortical projection to dorsal hippocampal CA2. Neurosci. Bull. 38, 318–322 (2022).
Li, J. et al. Auts2 deletion involves in DG hypoplasia and social recognition deficit: The developmental and neural circuit mechanisms. Sci. Adv. 8, eabk1238 (2022).
Lopez-Rojas, J., de Solis, C. A., Leroy, F., Kandel, E. R. & Siegelbaum, S. A. A direct lateral entorhinal cortex to hippocampal CA2 circuit conveys social information required for social memory. Neuron 110, 1559–1572.e1554 (2022).
Meira, T. et al. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun. 9, 4163 (2018).
Perea, G. & Araque, A. Synaptic information processing by astrocytes. J. Physiol. Paris 99, 92–97 (2006).
Tang, W. et al. Stimulation-evoked Ca2+ signals in astrocytic processes at hippocampal CA3-CA1 synapses of adult mice are modulated by glutamate and ATP. J. Neurosci. 35, 3016–3021 (2015).
Semyanov, A., Henneberger, C. & Agarwal, A. Making sense of astrocytic calcium signals - from acquisition to interpretation. Nat. Rev. Neurosci. 21, 551–564 (2020).
Verkhratsky, A. & Kettenmann, H. Calcium signalling in glial cells. Trends Neurosci 19, 346–352 (1996).
Gegelashvili, G., Danbolt, N. C. & Schousboe, A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J. Neurochem. 69, 2612–2615 (1997).
Karlsson, R. M. et al. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology 34, 1578–1589 (2009).
Postnikova, T. Y. et al. Ceftriaxone treatment weakens long-term synaptic potentiation in the hippocampus of young rats. Int. J. Mol. Sci. 22, 8417 (2021).
Takahashi, K., Foster, J. B. & Lin, C. L. Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol. Life Sci. 72, 3489–3506 (2015).
Valtcheva, S. & Venance, L. Astrocytes gate Hebbian synaptic plasticity in the striatum. Nat. Commun. 7, 13845 (2016).
Dias, R. B., Rombo, D. M., Ribeiro, J. A., Henley, J. M. & Sebastião, A. M. Adenosine: setting the stage for plasticity. Trends Neurosci 36, 248–257 (2013).
Cunha, R. A., Vizi, E. S., Ribeiro, J. A. & Sebastião, A. M. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J. Neurochem. 67, 2180–2187 (1996).
Peng, W. et al. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science 369, eabb0556 (2020).
Ahmadpour, N., Kantroo, M. & Stobart, J. L. Extracellular calcium influx pathways in astrocyte calcium microdomain physiology. Biomolecules 11, 1467 (2021).
Khakh, B. S. & McCarthy, K. D. Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol. 7, a020404 (2015).
Perea, G. & Araque, A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci. 25, 2192–2203 (2005).
Lehre, K. P., Levy, L. M., Ottersen, O. P., Storm-Mathisen, J. & Danbolt, N. C. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J. Neurosci. 15, 1835–1853 (1995).
Fontana, A. C. Current approaches to enhance glutamate transporter function and expression. J. Neurochem. 134, 982–1007 (2015).
Olivares-Bañuelos, T. N., Chí-Castañeda, D. & Ortega, A. Glutamate transporters: Gene expression regulation and signaling properties. Neuropharmacology 161, 107550 (2019).
Xu, J. et al. Neuroligin 3 regulates dendritic outgrowth by modulating Akt/mTOR signaling. Front Cell Neurosci 13, 518 (2019).
de Mendonça, A. & Ribeiro, J. A. Endogenous adenosine modulates long-term potentiation in the hippocampus. Neuroscience 62, 385–390 (1994).
Düster, R., Prickaerts, J. & Blokland, A. Purinergic signaling and hippocampal long-term potentiation. Curr. Neuropharmacol. 12, 37–43 (2014).
Chen, J. F. Adenosine receptor control of cognition in normal and disease. Int. Rev. Neurobiol. 119, 257–307 (2014).
Li, Y. et al. Activation of astrocytes in hippocampus decreases fear memory through adenosine A(1) receptors. Elife 9, e57155 (2020).
Ohno, M. & Watanabe, S. Working memory failure by stimulation of hippocampal adenosine A1 receptors in rats. Neuroreport 7, 3013–3016 (1996).
Giménez-Llort, L. et al. Mice lacking the adenosine A1 receptor have normal spatial learning and plasticity in the CA1 region of the hippocampus, but they habituate more slowly. Synapse 57, 8–16 (2005).
Lang, U. E. et al. Emotional instability but intact spatial cognition in adenosine receptor 1 knock out mice. Behav. Brain Res. 145, 179–188 (2003).
Costenla, A. R. et al. Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur. J. Neurosci. 34, 12–21 (2011).
Forghani, R. & Krnjević, K. Adenosine antagonists have differential effects on induction of long-term potentiation in hippocampal slices. Hippocampus 5, 71–77 (1995).
Pagnussat, N. et al. Adenosine A(2A) receptors are necessary and sufficient to trigger memory impairment in adult mice. Br. J. Pharmacol. 172, 3831–3845 (2015).
Rebola, N., Lujan, R., Cunha, R. A. & Mulle, C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57, 121–134 (2008).
d’Alcantara, P., Ledent, C., Swillens, S. & Schiffmann, S. N. Inactivation of adenosine A2A receptor impairs long term potentiation in the accumbens nucleus without altering basal synaptic transmission. Neuroscience 107, 455–464 (2001).
Wang, J. H., Ma, Y. Y. & van den Buuse, M. Improved spatial recognition memory in mice lacking adenosine A2A receptors. Exp. Neurol. 199, 438–445 (2006).
Orr, A. G. et al. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat. Neurosci. 18, 423–434 (2015).
Zhou, S. J. et al. Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res 1303, 74–83 (2009).
Temido-Ferreira, M. et al. Age-related shift in LTD is dependent on neuronal adenosine A(2A) receptors interplay with mGluR5 and NMDA receptors. Mol. Psychiatry 25, 1876–1900 (2020).
Giménez-Llort, L. et al. Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol. Learn Mem. 87, 42–56 (2007).
Tabuchi, K. et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 318, 71–76 (2007).
Hirrlinger, P. G., Scheller, A., Braun, C., Hirrlinger, J. & Kirchhoff, F. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia 54, 11–20 (2006).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Tronche, F. et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99–103 (1999).
Shen, H. Y. et al. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J. Neurosci. 28, 2970–2975 (2008).
Cao, F. et al. Neuroligin 2 regulates absence seizures and behavioral arrests through GABAergic transmission within the thalamocortical circuitry. Nat. Commun. 11, 3744 (2020).
Zhou, Z. et al. The C-terminal tails of endogenous GluA1 and GluA2 differentially contribute to hippocampal synaptic plasticity and learning. Nat. Neurosci. 21, 50–62 (2018).
Guangming, G. et al. Neurexin and neuroligins jointly regulate synaptic degeneration at the Drosophila neuromuscular junction based on TEM studies. Front Cell Neurosci 17, 1257347 (2023).
Acknowledgements
This work was supported by grants from STI2030-Major Projects (2021ZD0204000, W.X. and A.L.; 2022ZD0205900, A.L.), Natural Science Foundation of China (NSFC 91632201, WX; NSFC 31970958, A.L.), Natural Science Foundation of Jiangsu Province (BK20211561, A.L.), Basic Research Project of Leading Technology of Jiangsu Province (BK20192004, A.L.), Natural Science Foundation of China (NSFC 32070811, GG), the Canadian Institutes of Health Research (CIHR PJT155959, CIHR PJT168922, Z.J.), and Canadian Natural Science and Engineering Research Council (NSERC RGPIN341498, RGPIN06295, Z.J.), Canada Research Chair Program (Z.J.), the Hospital for Sick Children Foundation (Z.J.), Shenzhen Science and Technology Innovation Foundation (2021Szvup028, A.L.), and Guangdong Key Project (2018B030335001, W.X.). We thank Prof. Zilong Qiu for providing the Ai9 and Nestin-Cre mice. We thank Prof. Jiangfan Chen for providing the A2a f/f mice. We are grateful to all members of Jia and Xie lab for their technical assistance and comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: Z.J., A.L., R.D., W.X.; Methodology: Z.J., A.L., R.D., W.X.; Investigation: R.D., A.L., Y.Z., X.L., M.W., K.C., Y.M., H.Z., G.G.; Funding acquisition: Z.J., A.L., R.D., W.X.; Supervision: Z.J., A.L., R.D., W.X.; Writing, Review & Editing: Z.J., A.L., R.D., W.X.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Gertrudis Perea, Sung Joong Lee and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dang, R., Liu, A., Zhou, Y. et al. Astrocytic neuroligin 3 regulates social memory and synaptic plasticity through adenosine signaling in male mice. Nat Commun 15, 8639 (2024). https://doi.org/10.1038/s41467-024-52974-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-52974-3
This article is cited by
-
Neuroligin-3 R451C induces gain-of-function gene expression in astroglia in an astroglia-enriched brain organoid model
Cell Regeneration (2025)
-
Social memory maintenance relies on social interaction-induced proteolytic products of neuroligin 1
Signal Transduction and Targeted Therapy (2025)
-
Estradiol Mediates Astrocyte-Neuron Communication in the Hippocampus
Molecular Neurobiology (2025)
-
The Power of Neuroglia in Driving Brain Function
Neurochemical Research (2025)