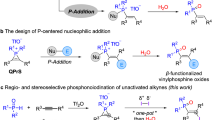
Abstract
Phosphorus chemistry occupies a pivotal position in contemporary organic chemistry but significant synthetic challenges still endure. In this report, a class of electrophilic phosphiranium salts, bearing fluorinated benzyl quaternizing groups, is introduced for the direct synthesis of diversely β-functionalized phosphines. We show that, in comparison with regular quaternary phosphiranium salts, these species display the sought balance of excellent stability and high electrophilic reactivity that allow the unlocking of the C-centered ring-opening reactions with different classes of weak nitrogen-, sulfur- and oxygen protic nucleophiles.
Similar content being viewed by others
Introduction
Developing new synthetic tools is a significant challenge in modern organic chemistry. In the field of phosphine synthesis, halogeno-phosphines have long been considered the most suitable chemical reagents for the electrophilic introduction of a phosphorus atom into organic molecules though C-P bond formation. By contrast, electrophilic P-containing building blocks able at creating C-Nu bonds in reactions with nucleophiles Nu to forge functionalized phosphines have been overlooked for a long time.
Neutral1,2 and cationic3,4,5,6,7,8,9,10,11,12,13 3-membered heterocyclic rings offer an uncommon combination of stability1, synthetic flexibility, regioselective reactivity, atom economy, and very often stereospecificity across their ring-opening transformations. Those heterocycles that are prone to easy ring opening are referred to as spring-loaded rings14, whose reactivity is governed by a synergy of ring-strain15 and C-heteroatom bond polarity to provide the suitable level of electrophilicity16 for easy ring opening into small open-chain heteroelement-containing target molecules or polymers17,18.
In this arena of strained heterocyclic chemistry, phosphacycles as appealing electrophilic P-containing building blocks are under-estimated and this may be considered an anomaly given the long-standing importance of phosphorus in chemical sciences and the related need to propose new approaches and connectivities in the construction of P-containing molecules. This deficiency is primarily due to prohibitive ring-strain energy of only 19.9 kcal.mol−1 far below oxiranes and aziridines (RSE ≈ 27 kcal.mol−1)4,19. Additionally, the phosphorus element has some unfavorable characteristics, such as low electronegativity (EP = 2.19 < EC = 2.55 < EN = 3.04), and susceptibility for hypervalency, alongside beliefs (recently dispelled in phosphiranes)20 about oxidation sensitivity.
Phosphiranes have been comprehensively studied as complexes coordinated with metal carbonyls21,22. Curiously the opening reactions of these yet electrophilically boosted species5,23 by nucleophiles have been largely ignored21,22. In the rarely reported examples, only attacks at phosphorus were described despite the steric bias on the heteroatom brought by metal complexations, leading to exotic P-containing products of little synthetic utility24. Those results therefore highlight the difficulty in controlling a synthetically more useful C-centered ring opening transformation of these electrophilically ambident and inadequately polarized phosphacycles25,26,27.
Quaternization of the pnictogen atom in group 15 three-membered heterocycles is known to increase ring strain23 and electrophilicity16 to a greater extent than the formation of metal complexes. This arguably makes phosphiranium salts the best option for C-selective ring opening in 3-atom phosphacycles chemistry. Curiously the latter species have so far garnered very little curiosity28,29,30 since their discovery about 60 years ago31. Until recently, the only attempts devoted to their opening were made on a P-phenyl, P-methyl triflate salt32. Rapid conversions ensued, thus validating the notion that ring strain drives phosphiraniums to reactivity but again24 nucleophilic attacks did occur on phosphorus32. Increasing the steric bulk of the aryl P-substituent was found instrumental in providing steric protection against nucleophilic attack at phosphorus, as demonstrated by the successful polymerization of a set of tri-t-butylphenylphosphiranes through C-centered ring-opening of transiently formed phosphiraniums33,34. Related to this, it is clear that the two P-substituents in phosphiranium salts provide a steric bias that can control the ring opening selectivity.
By implementing these concepts, previous investigations in our laboratories had established that simple, readily prepared quaternary phosphiranium salts (QPrS)35,36 of type A, derived from the bulky mesityl phosphine enabled facile intermolecular ring-openings with good chemical efficiency and complete carbon selectivity using anilines as nucleophile component, thus establishing a route to functionalized phosphines. In contrast, protic nucleophiles weaker than anilines proved fruitless.
Aware that the nature of the substituents on P also has significant potential for adjusting the electrophilic properties of phosphiraniums, we next sought for alternative EPrS candidates of type B integrating a proper electron-withdrawing group to overcome these limitations while being conducive to a set of original phosphines.
Drawing upon recent research on fluorinated phosphonium salts as highly effective organocatalysts37,38, particularly in the realm of Lewis super acid chemistry39, we surmised that phosphiranium salts resulting from quaternization by polyfluorinated alkylating agents would optimally meet our objectives, and thus enable us to successfully approach ring-opening reactions with weak protic nucleophiles and thus expand opportunities for the synthesis of structurally functionalized phosphines (Fig. 1).
a General statements: 3-membered phosphorus heterocycles ring-opening; b Electrophilic phosphonium cations (EPC) as source of inspiration; c Representative examples of polyluorinated organocatalysts refs. 36,37,38; d This work: Ring-opening of Electrophilic Phosphiranium Cations (EPrS) with weak protic nucleophiles.
Herein, we report the success of this strategy with the preparation of fluorinated phosphiranium substrates and their effective C-centered ring-opening reactions with an extended range of weak nitrogen-, sulfur- and oxygen nucleophiles. The experimental results are convincingly supported by computational data. As a whole, we believe this study definitively grants phosphiranium ions the status of synthetically useful spring-loaded heterocycles.
Results
To support our assumption and ascertain that the introduction of fluorinated benzyl groups would increase electrophilicity, we first compared computationally the reactivity indexes of some representative species. Both the global electrophilicity parameter ω and the energies of the LUMO orbitals for the different optimized model and known35,36 QPrS of type A and targeted EPrS of type B (Fig. 2) were evaluated (for more details, see Supplementary Fig. 4)40. Population analyses were carried out on the optimized structures (optimized in the absence of constraints, at the (PCM:chloroform) ωB97XD/6-31 + G(d,p) level). They were computed at the ωB97XD/6-31 + G(d,p) level. Natural Bond Orbital analysis were computed using NBO keyword (version 3 by default))41.
Starting from the simplest P-Me, Me QPrS A1, the substitution of one methyl group by a benzyl group leads to a marginally more electrophilic QPrS A2. In contrast, its replacement by an aromatic group such as a mesityl in QPrS A3 is more impactful (E LUMO decreased by 0.62 eV, ω increased by 0.23 unit). As anticipated, fluorinated benzyl groups have a huge effect. The P-mesityl, pentafluorinated benzyl substituted EPrS B1 has indeed a low-lying LUMO orbital (−0.58 eV) and higher global electrophilicity (ω = 1.31). Its analog EPrS B2 with one bis-trifluoromethylated benzyl group is the most electrophilic of the series with a low-lying LUMO orbital (at −0.99 eV) and a strong electrophilicity (ω = 1.47).
The A1 EPrS LUMO orbital is centered on the phosphiranium cycle when considering the cation alone, while LUMO + 1 orbital has to be considered in the presence of the triflate counter anion. The empty orbital localized on the phosphiranium carbon atoms also depend on the substitution of the phosphiranium (from LUMO to LUMO + 4). Thus, in addition to the exclusive LUMO orbital, we have also considered an orbital-weighted orbital scheme (ELUMO-mix), mixing the five lowest empty orbitals, with equal contributions, as proposed by Tiznado et al.42. This latter leads to different values with respect to ELUMO, but a similar trend (See Supplementary Fig. 4 for details).
To assess the relative reactivity of QPrS and EPrS on an experimental basis, we then prepared some representative examples of each type. P-methylation (Cond. A) and benzylation (Cond. B) of 1-mesitylphosphirane were easily performed as previously reported and outlined in Fig. 335. After adapting reaction conditions for the triflation step of the fluorinated benzyl alcohols, the corresponding triflates were obtained in excellent yields (95-98%) with small amounts (ca 2–11%) of the unreacted benzyl alcohol and the corresponding dibenzyl ether, resulting from condensation of the parent alcohol on the in situ formed triflate (ratio determined by 1H NMR). Due to their sensitivity, the triflates were directly used in the quaternization step with the parent phosphirane which turned out to be very facile and provided the desired, new EPrS 1c–1f almost quantitatively (95-99%, estimated to be 85-93% pure by 1H NMR, Cond. C).
Similar to QPrS 1a-1b, EPrS 1c–1f appeared to be stable and can be stored neat for months at −20 °C, in the absence of air and water. Likewise, periodic NMR monitoring of CDCl3 solutions of samples of 1c–1f at room temperature showed no signs of decomposition over a week. These are very important observations that demonstrate phosphiranium salts, including the most electrophilic versions to be remarkably stable compounds that should rapidly find wider use in organic synthesis. Crystals suitable for X-ray analysis were obtained for QPrS 1a and EPrS 1f, enabling a comparison of their structural properties. This solid-state analysis revealed two longer C-P bonds of the ring in 1f (1.799(6) Å and 1.784(6) Å) than in 1a (1.779(2) Å and 1.773(3) Å). Compression of the CPC angle (50.5(3)° for 1f vs 51.4(1)° for 1a) was also observed (Fig. 3) suggesting a higher ring strain for the fluorinated salt 1f that is consistent with its stronger electrophilic index (Fig. 2) and greater reactivity (Fig. 4).
a Ring-opening with N-Me aniline 2. b Empirical reactivity scale of QPrS and EPrS 1a–1f. c Ring-opening with hydroxylamines 4a–4e and tosylamide 6. The isolated yields were given.a Product isolated as the corresponding phosphine oxide, after treatment of the crude with H2O2; b nd not detected - although full conversion of the phosphiranium substrate was observed.
The reactivity of QPrS 1a-1b and EPrS 1c–1f was then assessed using N-Me aniline 2 as a benchmark nucleophile (Fig. 4a). In all cases, the reactions were conducted in CDCl3 for monitoring by 1H and 31P NMR and continued until complete conversion. Reactions of phosphiraniums 1a and 1b both proceed at 50 °C, affording the corresponding ring-opened products 3a and 3b in comparable yields of 65% and 64% respectively. Remarkably, in all cases, conversions of the fluorinated phosphiranium substrates 1c–1f were observed at room temperature, validating their high reactivity. This strong reactivity is nevertheless accompanied by a slight drop in yields (40–63%) due to the competitive formation of an opened tertiary phosphine oxide side-product (most likely resulting from reaction with residual traces of water)36. As expected, the best yield (63%), with no visible detection of by-product formation, was obtained with phosphiranium 1f with a 2,6-diCF3-benzyl group, thus confirming the importance of steric protection around phosphorus. It should be mentioned that ring-opening reactions of model substrates 1a and 1e were also tested with water alone. While the reaction was shown to be very slow with 1a at 80 °C, comparatively the reaction of the P-F5-benzyl 1e proceeded faster at rt. In both cases, only the corresponding opened tertiary phosphine oxides were obtained, with no traces of the C-centered ring-opened products with water (i.e. the corresponding β-hydroxyphosphines) being observed.
This set of results maps the reactivity of phosphiraniums 1a–1f with respect to their ring-opening (see Empirical scale established from experimental data that are reaction rates and reaction temperature, Fig. 4b) which is mostly in line with our initial, then computed-supported predictions. Only an inversion of the two most reactive species 1e and 1f is observed, most likely resulting from a combination of steric and electronic factors. Our results clearly show that 1e is the most reactive species of this set of phosphiraniums, while the more congested 1f offers the best compromise between reactivity and selectivity.
After establishing experimental evidence that EPrS are superior electrophilic members, we aimed to expand the range of usable nucleophiles. We first investigated hydroxylamine derivatives as another class of weak aza-nucleophiles (Fig. 4c) based on some similarities with aniline (close nucleophilicity parameter and pKa value). An initial attempt with model P-Me QPrS 1a and O-benzylhydroxylamine 4a validated the potential of this class of nucleophile, with the formation of the expected opened product 5a in 43% yield under relatively harsh reaction conditions (80 °C for 16 h). According to our predictive reactivity scale and in full line with our preliminary results with N-Me aniline, the transformation of F5-benzyl EPrS 1e by 4a appeared much easier, again proceeding at room temperature and giving reaction product 5b in ~50% yield due to the formation of P-oxidized degradation products during the reaction. A very close 42% yield was obtained when performing oxidation of the crude mixture, thus showing that the oxidized side-products were not formed during isolation. Use of the sterically more demanding N-benzyl hydroxylamine 4b logically decreased reactivity, with no conversion of both EPrS 1e and 1f observed at room temperature. However, the desired β-aminophosphines were obtained upon heating at 50 °C for 24 h, in milder conditions and better isolated yields (54% and 49% yields) compared to the analog 5c resulting from reactions with the P-methyl QPrS 1a (38% yield at 80 °C for 140 h). Two additional illustrations of the distinct advantage harnessed by EPrS are the ring openings with hydroxylamine 4c strongly deactivated by a F5-benzyl group and with the O-silylated hydroxylamine 4e, giving the corresponding adducts 5g in 48% yield and 5j in 57% yield after oxidation. In comparison, slightly lower yields of the corresponding phosphines were obtained using the usual work-up. In the latter case, the high reactivity of 1e allowing a room temperature reaction proved decisive for the retention of the sensitive TBDMS group. It is however important to note that the opening products with hydroxylamines proved to be more unstable than in the other series, especially during chromatographic purification. As a result, the open products, whether phosphines or their oxidized form, could not be isolated in completely pure forms.
Remarkably, the ring-opening of 1e, but not of QPrS 1a, readily occurred on reaction with the strongly deactivated tosylamide 6 at 80 °C to afford good yield (79%) of the corresponding adduct 7b, and thus highlights another facet of the synthetic potential of EPrS. Besides the result itself, this experiment is important as additional evidence of the robustness of EPrS, which shows good stability at relatively high temperatures.
Encouraged by this first set of results with nitrogen-based nucleophiles, we were next eager to confront our highly electrophilic EPrS 1d–1f with an extended range of less reactive heteronucleophiles that previously failed to react with the QPrS analogs. As an entry point, we chose thiol derivatives as soft nucleophilic partners (Fig. 5).
As just mentioned above, the first-generation P-Me QPrS 1a proved inert toward thiophenol at 80 °C even after extended reaction times, somehow expectedly, and only slow degradation of substrate 1a was observed. By contrast, engaging F5-benzyl EPrS 1e at gradually increasing temperatures allowed us to establish 80 °C as a threshold temperature for reactivity. To our delight, 31P NMR monitoring revealed complete conversion and appearance of a single diagnostic signal characteristic for a protiophosphonium (i.e. protonated phosphine) adduct after 48 h. An adaptation of the isolation protocol using SiO2 pre-treated with 10%TEA/EtOAc instead af neural alumina (to deprotonate the protiophosphonium intermediate directly resulting from the ring opening into phosphine - see Fig. 7b for the proposed mechanism) resulted in a facile and high-yielding (88%) isolation of thiophosphine 9a.
With an optimal protocol established for this transformation, we next probed the substrate scope. Opening of 3,5-diCF3-, and 2,6-diCF3-EPrS 1d and 1f proceeded equally well but only phosphines 9b could be obtained in 72% yield while subsequent oxidation was found essential to get the bis-2,6-diCF3 product 9c in its P = O form (70%). Gratifyingly, the reaction expanded nicely to naphthyl thiol and diversely substituted thiophenols, except for the dimethylamino thiophenol, which only led to side-products formation. 2-Mercaptobenzothiazole has recently demonstrated high reactivity in various organocatalytic enantioselective opening reactions of strained rings43, and was thus next tested. As we had hoped on these grounds, this nucleophile exhibited a high reactivity substantiated by complete conversion of EPrS 1e within 30 min at room temperature to give the opened phosphine 9i in 71% yield. Aliphatic thiols were found to be more reactive but less selective than their aromatic analogs with the ring-opening reaction of propanethiol occurring at 50 °C and providing thiophosphine 9j in 46% yield. Running the reaction under our standard conditions at 80 °C markedly increased the reaction rate and maintained the reaction profile (40 h at 80 °C vs 88 h at 50 °C, 41% yield). In stark contrast, reaction with benzyl thiol only resulted in decomposition with no trace of adduct 9k being obtained, possibly due to post-reaction cleavage of the sensitive benzylic C-S bond.
To challenge the reactivity of EPrS even more deeply and continue our progressive efforts towards expanding bond creation and molecular diversity through their ring opening, we finally turned our attention to the specific case of oxygenated nucleophiles (Fig. 6). The latter represent the most striking challenge due to both the weak nucleophilicity of alcohols and natural O- to P- affinity.
Preliminary tests with pentafluorobenzyl phosphiranium 1e and p-methoxyphenol rapidly demonstrated the feasibility of the supposedly difficult ring-opening at 80 °C for 72 h. However, the resulting β-alkoxy phosphine turned out to be highly unstable to various isolation protocols and could not be isolated. Oxidation of the reaction crude was then directly performed using standard conditions (H2O2, CH2Cl2, rt), allowing formation and isolation of the stable phosphine oxide 11a in a very satisfactory 66% yield.
The bulkier 2,6-diCF3-EPrS 1f converted at the same rate and afforded the phosphine oxide product 11b in a slightly augmented yield of 70%. The study was then pursued with substrate 1f which satisfyingly led to a similar result with the non-activated phenol, albeit with an expected slower rate (120 h, 67% in 11c). In these three examples, 31P NMR monitoring of the reaction showed that the reaction was very selective as evidenced by the appearance of a very major diagnostic signal. Given the obstacles concerning the use of alcohols as nucleophiles mentioned above, these results arguably are important in phosphiranium chemistry. Logically, we confirmed a complete lack of reactivity with the Me-QPrS substrate 1a.
This optimal protocol was then used to probe the reaction of 1f with aliphatic alcohols. Similarly to thiols, the latter exhibited increased reactivity, with reactions of methanol and cyclohexanol proceeding faster (40 h vs 72 h with phenol derivatives) and providing the corresponding phosphine oxides in high yields (81% and 79% respectively). Allylic alcohol displayed a similar reactivity but only 43% of the corresponding adduct was obtained due to partial decomposition of the adduct.
Last but not least, even p-methoxy benzoic acid which is by far the poorest nucleophile that we have examined, demonstrated a good reactivity profile to deliver after oxidative work-up of the P = O product 11h in 57% yield. This result was obtained after 7 days of reaction at 80 °C, and therefore definitively establishes a robustness of these perflurobenzyl EPrS.
With the scope of the reaction established over three distinct classes of nucleophiles, we then turned our attention to the ring-opening mechanism.
Based on the products obtained and our earlier preliminary mechanistic experiments36, it is reasonable to assume that the reaction proceeds through an SN2-type mechanism with the direct addition of the nucleophile on a ring carbon atom. However, since phosphiranium salts display two electrophilic competing reaction sites and are prone to fragmentations due to their ring strain, other scenarios cannot be precluded44,45. A DFT study was thus performed to get insights into the reaction pathway (Fig. 7). Considering the size of fluorinated QPrS used experimentally, we first confirmed that the computationally less demanding dimethyl substituted A1 constituted a good model for these studies (see Supplementary Table 2 for details).
Four different paths were examined for the interaction between EPrS A1 and aniline chosen as model partners. Our first and preferred approach involves attack of the aniline nucleophile at the CH2 center of the phosphiranium from the back-side of the CH2-P bond in an SN2-like process (Pathway 1, blue). The second one involves the approach of the nucleophile at the cationic P center (Pathway 2, red), when a third path considers the first formation of an aniline-phosphonium complex followed by addition at the CH2 position in an indirect way (Pathway 3, green)44,45. The last approach deals with an attack on the positively charged P center with concomitant ethylene extrusion (Pathway 4, pink).
In agreement with our assumptions, the direct addition at carbon was found to be the most favored of the four considered and is highly exergonic (Fig. 7a, Pathway 1). The calculations also show that pathway 2 lies very high in energy and even if leading to a stable adduct, is not feasible considering the reaction conditions. In contrast, pathway 344,45 turned out to be highly kinetically and thermodynamically disfavored. Last, pathway 4, which involves P-addition with simultaneous extrusion of ethylene32, is more kinetically favorable but thermodynamically disfavored and is therefore not competitive compared to pathway 1. (ΔΔG‡ = 5.8 kcal.mol−1). It is important to note that similar energy profiles apply over the reactions of models QPrS B and EPrS A differently substituted on P with the range of heteronucleophiles used in this study (see Supplementary Table 5 for additional computed values). This supports a uniform reaction mechanism driven by the preferential addition of protic nucleophiles at carbon atom as illustrated in Fig. 7b.
In addition, if the proposed mechanism primarily results in the formation of phosphines of type II, it is important to highlight that monitoring of the QPrS reactions by 31P NMR always shows the formation of protiophosphoniums III resulting from N- to P-proton exchange, even in reactions with the most basic anilines. A basic treatment was thus applied to release the corresponding free phosphines IV. Significant differences in 31P resonances were observed for the protonated-III and free phosphines IV. While in most cases, we assume that the proton is most likely shared between the phosphorus atom and the nucleophile’s heteroatom in III, a clear signal splitting by coupled 31P-1H NMR was detected with the less basic nucleophiles (in particular electron-poor anilines) consistent with protonation at P (with coupling constants 1JP-H measured in the 500–540 Hz range).
By contrast, introduction of fluorinated groups in EPrS decreases the basicity of the corresponding phosphines significantly. Consequently, in reactions involving basic nitrogen nucleophiles and fluorinated EPrS, we only observed formation of type II phosphine intermediates, while species III prevail in the case of F5-aniline, tosylamide, S- and O-nucleophiles. These differing observations are also consistent with the need to adapt the basic treatment depending on the nature and the basicity of the nucleophile used (vide supra).
To complete this study, we elected to take advantage of the high reactivity of 2-mercaptobenzothiazole 8f to perform competition experiments to estimate the relative electrophilicity of phosphiraniums 1f and 1a to gain a quantitative assessment of the observed electrophilicity/reactivity ranking (Fig. 8)46. As outlined in Fig. 8 the experiment was performed with an excess of the two competing electrophiles 1f and 1a and the reference nucleophile 8f. Once the latter was fully consumed, the reaction mixture was analyzed by 1H NMR spectroscopy to determine the relative ratios of the remaining electrophiles 1f and 1a as well as the respective protonated ring-opened products 12a and 12b. A competition constant k of 27 was then determined for 1f (vs. 1a) according to equation 1 (eq. 1). In full correlation with the proposed reactivity scale established from our experimental results (see Fig. 4b), a similar experiment performed to assess the reactivity of EPrS 1e in competition with 1a, revealed a superior competition constant of 44 (eq. 2) that complies with our reactivity scale (Fig. 3).
In summary, we present a class of designer electrophilic phosphiranium salts (EPrS) incorporating fluorinated quaternizing groups. A thorough computational-structural-experimental investigation of their properties has demonstrated their higher electrophilic reactivity over previously described quaternary phosphiranium salts (QPrS). This was substantiated by a much better reactivity in the C-centered ring-opening with nucleophiles, enabling faster processes with previously effective anilines and expansion to a range of heretofore unsuccessful partners, including hydroxylamines, tosylamide, thiols, and alcohols. Besides fundamental aspects, the developed methodology offers an original alternative route to functionalized tertiary phosphines, which have already proved valuable as nonsymmetrical ligands or organocatalysts47,48,49. In comparison to classical approaches which generally exploit either the nucleophilic reactivity of phosphines (mostly through their anions) or the electrophilic reactivity of halogenophosphines, the developed sequence moreover constitutes a convergent synthetic method with possible late-stage diversification (via the quaternization step) and easy deviation from routine diaryl P-substitutions.
Calculations helped for a better understanding of phosphiraniums chemistry and notably demonstrated a uniform C-centered SN2-type mechanism of the ring-opening process. Work is currently underway in our laboratories to further evaluate these fluoro EPrS and to explore the design and reactivity of new highly reactive analogs.
Methods
See Supplementary Information for synthetic details, spectra, computational details and crystallographic data.
General procedure for ring opening reactions
To a solution of phosphiranium salt in CDCl3 (0.075 mmol/mL) was added the nucleophile derivative at room temperature. The mixture was stirred and eventually heated when necessary (see reaction time and temperature specified for each compound). Conversion of the starting material was monitored by 31P NMR. After cooling down to room temperature, the product was isolated using one of the following procedures, depending on the stability of the resulting tertiary phosphine (specified for each compound in the Supplementary Information).
Isolation Procedure A: The crude was filtered through a short pad of basic Al2O3 using EtOAc as eluent, concentrated under reduced pressure and then purified by flash chromatography on silica gel (eluent: Cyclohexane/EtOAc) when necessary.
Isolation Procedure B: The crude was filtered through a short pad of Silica gel (neutralized with 10% Et3N and dried prior to use) using EtOAc or CH2Cl2 as eluent, concentrated under reduced pressure and then purified by flash chromatography on silica gel (eluent: Cyclohexane/EtOAc) when necessary.
Isolation Procedure C: The crude was filtered through a short pad of silica gel (neutralized with 10% Et3N and dried prior to use) using CH2Cl2 as eluent. The filtrate was concentrated to ca 1 mL and then H2O2 (30 % in H2O, 5 equiv) was added. The mixture was stirred for 0.5 h at room temperature and then a saturated solution of NaHSO3 (10 mL/mmol) was added to quench the reaction. After decantation, the aqueous phase was extracted with EtOAc. The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure and then purified by flash chromatography on silica gel (eluent: Cyclohexane/EtOAc) when necessary.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information (experimental procedures, DFT calculations and characterization data). All data are available upon request from the corresponding authors. All the cartesian coordinates of intermediates and transition states are available as a Source Data file. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2336797 (1a) and CCDC 2336798 (1f) Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Yudin, A. K. Aziridines and Epoxides in Organic Synthesis. (Wiley-VCH, Weinheim, 2006).
Stanković, S. et al. Regioselectivity in the ring opening of non-activated aziridines. Chem. Soc. Rev. 41, 643–655 (2012).
Maji, B. Stereoselective Haliranium, Thiiranium and Seleniranium Ion-Triggered Friedel–Crafts-Type Alkylations for Polyene Cyclizations. Adv. Synth. Catal. 361, 3453–3489 (2019).
Ranjith, J. & Ha, H.-J. Synthetic applications of Aziridinium Ions. Molecules 26, 1774–1790 (2021).
Vedejs, E. & Denmark, S. E. Lewis Base Catalysis in Organic Synthesis, 1153–1212. (Wiley-VCH, 2016).
Hartmann, E. & Denmark, S. E. Structural, Mechanistic, Spectroscopic, and Preparative Studies on the Lewis Base Catalyzed, Enantioselective Sulfenofunctionalization of Alkenes. Helv. Chim. Acta 100, e1700158 (2017).
Luo, J., Cao, Q., Cao, X. & Zhao, X. Selenide-catalyzed enantioselective synthesis of trifluoromethyl thiolated tetrahydronaphthalenes by merging desymmetrization and trifluoromethylthiolation. Nat. Commun. 9, 527–535 (2018).
Cai, Y., Liu, X., Zhou, P. & Feng, X. Asymmetric Catalytic Halofunctionalization of α,β-Unsaturated Carbonyl Compounds. J. Org. Chem. 84, 1–13 (2019).
Gieuw, M. H., Ke, Z. & Yeung, Y.-Y. Lewis Base Catalyzed Stereo- and Regioselective Bromocyclization. Chem. Rec. 17, 287–311 (2017).
Cresswell, A. J., Eey, S. T. C. & Denmark, S. E. Catalytic, Stereoselective Dihalogenation of Alkenes: Challenges and Opportunities. Angew. Chem. Int. Ed. 54, 15642–15682 (2015).
Sakakura, A. & Ishihara, K. Stereoselective Electrophilic Cyclization. Chem. Rec. 15, 728–742 (2015).
Landry, M. L. & Burns, N. Z. Catalytic Enantioselective Dihalogenation in Total Synthesis. Acc. Chem. Res. 51, 1260–1271 (2018).
Chung, W.-J. & Vanderwal, C. D. Stereoselective Halogenation in Natural Product Synthesis. Angew. Chem. Int. Ed. 55, 4396–4434 (2016).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Rey Planells, A. & Espinosa Ferao, A. Accurate Ring Strain Energy Calculations on Saturated Three-Membered Heterocycles with One Group 13−16 Element. Inorg. Chem. 59, 11503–11513 (2020).
Baruah, B., Deuri, S. & Phukan, P. Reactivity and regioselectivity in the ring opening of 2-substituted non-activated aziridines: A density functional theory-based analysis. Comp. Theor. Chem. 1027, 197–202 (2014).
Kubisa, P. Hyperbranched polyethers by ring-opening polymerization: Contribution of activated monomer mechanism. J. Polym. Sci. Part A Polym. Chem. 41, 457–468 (2003).
Stewart, I. C., Lee, C. C., Bergman, R. G. & Toste, F. D. Living Ring-Opening Polymerization of N-Sulfonylaziridines: Synthesis of High Molecular Weight Linear Polyamines. J. Am. Chem. Soc. 127, 17616–17617 (2005).
Espinosa, A. & Streubel, R. Computational Studies on Azaphosphiridines, or How to Effect Ring-Opening Processes through Selective Bond Activation. Chem. Eur. J. 17, 3166–3178 (2011).
Gaston, J. J., McCosker, P. M., Yu, H. & Keller, P. A. Predicting phosphirane air stability using density functional theory. J. Phys. Org. Chem. 33, e4110 (2020).
Mathey, F. Chemistry of 3-Membered Carbon-Phosphorus Heterocycles. Chem. Rev. 90, 997–1025 (1990).
Mathey, F. & Regitz, M. Phosphorus-Carbon Heterocyclic Chemistry, The Rise of a New Domain, Chap. 2.1 – Three-membered Rings, 17–55 (Pergamon, 2001).
Rey Planells, A., Espinosa Ferao, A. & Streubel, R. Between Oxirane and Phosphirane: The Spring-loaded Oxaphosphirane Ring. Eur. J. Inorg. Chem. 2021, 348–353 (2021).
Marinetti, A. & Mathey, F. Opening of phosphirane-tungsten complexes by nucleophiles. Tetrahedron 45, 3061–3070 (1989).
Tian, R. et al. The Chemistry of 1-Acylphosphirane Complexes: A Phosphorus Analogue of the Cloke–Wilson Rearrangement. Chem. Eur. J. 23, 13006–13009 (2017).
Xu, Y. et al. An approach to 7-aza-1-phosphanorbornane complexes: strain promoted rearrangement of 1-iminylphosphirane complexes and cycloaddition with olefins. Dalton Trans. 48, 5523–5528 (2019).
Xu, Y. et al. An Approach to Peri-Fused Heterocycles: A Metal-Mediated Cascade Carbonylative Cyclization/Dearomatic Diels−Alder Reaction. Org. Lett. 21, 9512–9515 (2019).
Sølling, T. I., McDonald, M. A., Wild, S. B. & Radom, L. Novel Pi-Ligand Exchange and Insertion Reactions Involving Three-Membered Phosphorus Heterocycles: An ab Initio Investigation. J. Am. Chem. Soc. 120, 7063–7068 (1998).
Tipker, R. M. et al. Configurational Lability at Tetrahedral Phosphorus: syn/anti-Isomerization of a P-Stereogenic Phosphiranium Cation by Intramolecular Epimerization at Phosphorus. Angew. Chem. Int. Ed. 61, e202110753 (2022).
Tipker, R. M. et al. Protonation of P‑Stereogenic Phosphiranes: Phospholane Formation via Ring Opening and C−H Activation. ACS Omega 8, 12565–12572 (2023).
Kortürm, G. & Krieg, P. Spektroskopische Untersuchungen zur Thermo‐, Piezo‐und Photochromie von Bixanthyliden‐(9.9′) und Biflavenyliden‐(4.4′). Chem. Ber. 102, 3033–3045 (1969).
Hockless, D. C. R., McDonald, M. A., Pabel, M. & Wild, S. B. Facile syntheses and interconversions between simple phosphiranium and phosphirenium salts. J. Organomet. Chem. 529, 189–196 (1997).
Kadokawa, J. & Kobayashi, S. New Ring-Opening Polymerization of Phosphorus-Containing Cyclic Monomers. Phosphorus Sulfur Silicon Relat. Elem. 177, 1387–1390 (2002).
For a similar beneficial effect on epi-selenonium reactivity, see, Toshimitsu, A., Nakano, K., Mukai, T. & Tamao, K. Steric Protection of the Selenium Atom of the Episelenonium Ion Intermediate To Prevent both the Racemization of the Chiral Carbon and the Selenophilic Attack of Carbon Nucleophiles. J. Am. Chem. Soc. 118, 2756–2757 (1996).
Gasnot, J. et al. Access to Stable Quaternary Phosphiranium Salts by P-Alkylation and P-Arylation of Phosphiranes. Synlett 31, 883–888 (2020).
Gasnot, J. et al. Taming the Reactivity of Phosphiranium Salts: Site-Selective C-Centered Ring Opening for Direct Synthesis of Phosphinoethylamines. Angew. Chem. Int. Ed. 59, 11769–11773 (2020).
He, R., Wang, X., Hashimoto, T. & Maruoka, K. Binaphthyl-Modified Quaternary Phosphonium Salts as Chiral Phase-Transfer Catalysts: Asymmetric Amination of β-Keto Esters. Angew. Chem. Int. Ed. 47, 9466–9468 (2008).
Zhang, S. et al. Highly Enantioselective Synthesis of Phosphorus-Containing ɛ-Benzosultams by Bifunctional Phosphonium Salt-Promoted Hydrophosphonylation. Chem. Eur. J. 27, 11285–11290 (2021).
Bayne, J. M. & Stephan, D. W. Phosphorus Lewis acids: emerging reactivity and applications in catalysis. Chem. Soc. Rev. 45, 765–774 (2016).
Parr, R. G., Szentpály, L. V. & Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 121, 1922–1924 (1999).
Weinhold F. & Carpenter, J. E. The Structure of Small Molecules and Ions, 227–236 (Plenum, 1988).
Pino‐Rios, R. et al. Proposal of a simple and effective local reactivity descriptor through a topological analysis of an orbital‐weighted Fukui function. J. Comput. Chem. 38, 481–488 (2017).
Wang, Z., Chen, Z. & Sun, J. Catalytic enantioselective intermolecular desymmetrization of 3-substituted oxetanes. Angew. Chem. Int. Ed. 52, 6685–6688 (2013).
Finet, J.-P. Ligand Coupling Reactions with Heteroaromatic Compounds 18, Chap. 4 (Pergamon, 1998).
Oae, S. & Uchida, Y. Ligand-Coupling Reactions of Hypervalent Species. Acc. Chem. Res. 24, 202–208 (1991).
Mayer, R. J. et al. Electrophilic reactivities of cyclic enones and α,β-unsaturated lactones. Chem. Sci. 12, 4850–4865 (2021).
Singewald, E. T. et al. Novel Hemilabile (Phosphinoalkyl)arene Ligands: Mechanistic Investigation of an Unusual Intramolecular, Arene-Arene Exchange Reaction. Organometallics 15, 3062–3069 (1996).
Ulmann, P. A. et al. Reversible Ligand Pairing and Sorting Processes Leading to Heteroligated Palladium(II) Complexes with Hemilabile Ligands. Organometallics 28, 1068–1074 (2009).
Kennedy, R. D. et al. General Strategy for the Synthesis of Rigid Weak-Link Approach Platinum(II) Complexes: Tweezers, Triple-Layer Complexes, and Macrocycles. Inorg. Chem. 52, 5876–5888 (2013).
Acknowledgements
We gratefully acknowledge the French National Research Agency (ANR) for funding (Grant number ANR-19-CE07-0047-01).
Author information
Authors and Affiliations
Contributions
V.D. and C.T. conceived the study and supervised the research. M.A. and M.-J.T. performed the experiments and analyzed the data. N.S.M. performed the crystallographic studies. S.C. and S.L. helped in preparing the manuscript and provided constructive advice. I.C. performed the density functional theory (DFT) calculations. J.P. helped in DFT calculations. V.D. and C.T. wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Arturo Espinosa Ferao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahmad, M., Tranchant, MJ., Comesse, S. et al. Unlocking the C-centered ring-opening of phosphiranium ions for a straightforward entry to functionalized phosphines. Nat Commun 15, 8554 (2024). https://doi.org/10.1038/s41467-024-53003-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-53003-z