Abstract
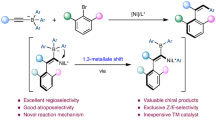
Chiral tertiary alcohols are an important structural motif, however, the general and efficient methodologies for their synthesis are less reported. Herein, we report a Ni(ІІ)-catalyzed asymmetric alkenylation and arylation of aryl ketones with organoborons under air via a 1,5-metalate shift strategy to obtain chiral tertiary allylic alcohols and diaryl alcohols. The reaction demonstrates good functional group tolerance and delivers chiral tertiary alcohols with good to excellent results. Furthermore, this method can be applied to the late-stage modification of drugs and the efficient synthesis of natural products. Notably, the reaction proceeds through an outer-sphere mechanism. The Ni(II) complex functions both as a Lewis acid to activate the ketone and create a chiral environment, and as coordination bridge linking the ketone and the organoboron-derived “ate” complex, facilitating the 1,5-metalate shift without forming a C-Ni bond. This approach contrasts with traditional transition metal-catalyzed nucleophilic addition reactions that involve carbon-metal bond formation.
Similar content being viewed by others
Introduction
Chiral tertiary alcohols, especially allylic alcohols, as an important class of structural motif, are widely present in natural products and drugs and are also important building blocks in organic synthesis and medicinal chemistry (Fig. 1a)1,2,3. The preparation of such compounds has attracted attention for decades4,5,6,7,8,9,10,11,12,13, and the asymmetric nucleophilic addition of organometallic reagents to ketones is considered as one of the most straightforward and efficient routes. Traditional organometallic reagents, such as organozincs4, organomagnesiums5, and organoaluminiums5 are moisture- and air-sensitive, as well as poorly tolerant of functional groups, limiting their applications in the direct transformation of complex molecules. Organoborons are recognized as ideal nucleophiles, due to their ready availability, stability, broad functional group tolerance and applications in a wide range of transformations14,15,16,17,18,19,20,21,22,23,24.
a Typical natural product and drug containing chiral tertiary alcohols. b Transition metal-catalyzed asymmetric alkenylation or arylation of ketones with organoborons (inner-sphere mechanism). c Petasis reaction. d Our design: asymmetric addition to ketones via a 1,5-metalate shift strategy (outer-sphere mechanism).
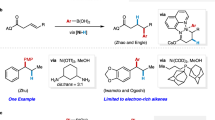
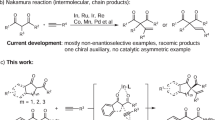
Although ketones are less reactive than aldehydes and the asymmetric alkenylation and arylation of ketones are more challenging, several excellent examples of such transformations with organoborons have been reported. The reaction pathways for those processes can be typically classified into two types: Type I: The carbon–metal species is formed through transmetalation between the transition metal and organoborons. This species then coordinates with the ketone, followed by an addition to the ketone to deliver the chiral tertiary alcohol (Fig. 1b, top). This is a general reaction pathway for the alkenylation and arylation of ketones with organoborons, and several excellent catalytic systems have been developed for this transformation using rare-metal catalysts25,26,27,28,29,30,31,32 or activated ketones as substrates33. However, this transformation has not been applied to the earth-abundant transition metal-catalyzed alkenylation and arylation of unactivated ketones with organoborons, likely due to the low reactivities of unactivated ketones and carbon-metal species derived from earth-abundant transition metal. Type II: The reaction pathway involves oxidative cyclization of Ni(0) with a ketone to afford an oxanickelacycle, followed by transmetalation and reductive elimination to yield the chiral tertiary alcohol (Fig. 1b, bottom). This reaction pathway offers an elegant strategy for the dynamic kinetic alkenylation and arylation of α-racemic ketones in the absence of base. However, this catalytic system requires the use of the air-sensitive and expensive nickel salt Ni(COD)234,35,36,37,38. In view of the importance of chiral tertiary alcohols, it is essential and highly desirable to develop efficient, economical, and simple operation methods for their synthesis.
On the other hand, the Petasis reaction could deliver highly functionalized amines from ketones, amines, and organoborons by transferring a substituent from an organoboron-derived “ate” complex to the in situ formed iminium (Fig. 1c)39. This process is generally referred to as metalate shift40. The Petasis reaction is a powerful transformation due to its broad functional group tolerance, readily available starting materials, and mild, robust reaction conditions. However, reported examples of the Petasis reaction are limited to metalate shifts involving iminium ions, and metalate shifts with ketones have not been reported due to their low reactivity. Inspired by the Petasis reaction and recognizing that a Lewis acid can promote a metalate shift to a C = C bond by coordinating with the alkene40, we aimed to avoid forming low-reactive carbon-metal species derived from the earth-abundant transition metals. Therefore, we designed a chiral Lewis acid-catalyzed asymmetric alkenylation and arylation of ketones with organoborons via a 1,5-metalate shift strategy to synthesize chiral tertiary alcohols (Fig. 1d). We hypothesized that the chiral Lewis acid could not only activate the ketone and provide a chiral environment but also act as a coordination bridge linking the ketone and the oxygen atom of the organoboron-derived “ate” complex. This setup would facilitate the enantioselective attack of the alkenyl or aryl substituent at the boron atom on the ketone, yielding chiral tertiary alcohols via a 1,5-metalate shift without forming a carbon–metal bond. Here, we report a Ni(ІІ)-catalyzed asymmetric alkenylation and arylation of aryl ketones with organoborons via 1,5-metalate shift to access chiral tertiary alcohols.
Results
Reaction optimization
Initially, acetophenone 1a and styrylboronic acid 2a were selected as the model substrates to optimize the reaction conditions in the presence of Ni(OAc)2 (Table 1). After screening various types of ligands L1–L4 (Table 1, entries 1–4), only the Ph-BOX L4 could give the desired product 3a in 24 h (entry 4, 30%, <5% ee). Then, we turned our attention to other BOX ligands, and L5 (R = H) with indenyl groups on oxazoline rings gave the product 3a in 9% yield and with 86% ee (entry 5). Changing the R group at the methylene of the bisoxazoline ligand from H to Ph (L6), 3a was obtained in 55% yield and with 92% ee (entry 6). Subsequently, ligands with R as other aromatic rings were examined (L7–L9, entries 7–9; see Supplementary Table S1 for details), L8 (R = 2-anthryl) gave the best result (entry 8, 66%, 94% ee). Furthermore, other reaction conditions, such as the base, the solvent and the reaction temperature, were also screened with L8; no better results were obtained (see Supplementary Table S2–S4 for details). Then, different nickel salts were investigated with L8, and Ni(ClO4)2‧6H2O gave a similar result to that of Ni(OAc)2 (entry 10 vs. entry 8), but less amount of byproduct was observed in the presence of Ni(ClO4)2‧6H2O (see Supplementary Table S5 for details). Other metal salts, such as FeCl3, CoBr2, Cu(OAc)2, and Zn(OTf)2, were also examined for the reaction, and no desired products were observed (entries 11–14). Then, Ni(ClO4)2‧6H2O was used for the investigation of the concentration of the reaction (entries 15 and 16). The yield of 3a was improved to 95% when the concentration of the substrate in the reaction mixture was increased from 0.05 to 0.2 mol/L (entry 16). To simplify the reaction protocol, complex NiL8(ClO4)2 was preprepared and was subjected to the reaction, almost the same result was obtained (entry 17: 96%, 94% ee vs. entry 16: 95%, 94% ee). To our delight, the reaction proceeded very rapidly and was completed in 1 h, delivering product 3a with 96% yield and with 94% ee (92% isolated yield) (entry 18). In addition, we also tried to reduce the catalyst loading (entries 19 and 20). The desired product 3a was obtained in 83% yield with 5 mol% catalyst (entry 20).
Substrate scope
With the optimized reaction conditions in hand (Table 1, entry 18), the substrate scope was examined (Fig. 2). First, the (hetero)aryl methyl ketones 1 were investigated with respect to styrylboronic acid 2a (Fig. 2, top). When the aryl group in the substrate 1 is a phenyl ring bearing no substituent, an electron-withdrawing or electron-donating group, the reaction delivered the desired alcohols in good results (3a–3o, 71–97%, 83–99% ee). Other aryl methyl ketones, such as naphthyl and piperonyl methyl ketones, gave the corresponding products in good to excellent yields and excellent enantioselectivities (3p–3r, 84–99%, 91–98% ee). Heteroaryl methyl ketones, including challenging pyridinyl and quinolinyl methyl ketones, other phenyl alkyl ketones and cyclic ketones were also tested, and the desired products were obtained in moderate to excellent results (3s–3ae, 53–91%, 76-97% ee).
Subsequently, the scope of alkenylboronic acids 2 with respect to acetophenone 1a was investigated (Fig. 2, middle). For the styrylboronic acids, the substituent on the phenyl ring has little effect on the enantioselectivities of the products (4a–4i, 81-97%, 91-96% ee). 3-Thienyl, 4-phenyl-1,3-butadienyl, 1-pentenyl and 2-cyclohexylethenyl boronic acids provided the corresponding products in excellent results (4j–4m, 93-97%, 91-93% ee). The reaction can also proceed with 1-cyclopentenylboronic acid and (Z)-styrylboronic acid, the corresponding products were also obtained (4n: 64%, 73% ee; 4o: 51%, 89% ee). Furthermore, the substrate scope for the arylation of ketones with arylboronic acids was also investigated (Fig. 2, bottom). The chiral tertiary diaryl alcohols obtained in good results for the phenylation of different aryl methyl ketones (5a–5g, 74–98%, 80–97% ee). The substrate phenyl ethyl ketone was also examined for the reaction, and the corresponding product was obtained in good results (5h, 75%, 85% ee). Next, different arylboronic acids for the arylation of acetophenone 1a were examined, and all delivered the corresponding products in good yields and enantioselectivities (ent-5a and 6a–6e, 69-84%, 85-91% ee). In addition, the alkylation of ketones41 with alkylboronic acids under the standard conditions was examined, and no desired product was observed. The reactions of alkenylation and arylation of the benzaldehyde with organoborons were also conducted under standard conditions, and the corresponding products were obtained in excellent yields and with moderate and poor enantioselectivities (see Supplementary Fig. S4 for details). Finally, the alkenylation of 4-acetylbenzaldehyde, which bears both aldehyde and ketone groups on the aromatic ring, was examined under standard conditions. As a result, the mono-alkenylation product of the aldehyde group was obtained as the major product in 63% yield and with 55% ee, and the double alkenylation product was obtained in 31% yield and with 3.5:1 dr and 98% ee (see Supplementary Fig. S5 for details). The absolute configuration of 3g was determined to be S by X-ray crystallographic analysis, and the other products were assigned accordingly.
The applications
The good functional group tolerance prompted us to apply the methodology to the late-stage modification of several drugs bearing the structural motif of aryl alkyl ketones (Fig. 3a). Drugs, such as Celestolide, Azaperone, Dyclonine hydrochloride, Ebastine, Haloperidol and Acetovanillone derivative, delivered the corresponding products in good yields and enantioselectivities (7a–7f, 70–83%, 89–94% ee). In order to further explore the applications of the methodology, a gram-scale reaction and the synthesis of natural products and other drugs were performed (Fig. 3b, c). The product 3a was obtained in a little higher yield and with no erosion of enantioselectivity when the reaction was performed on a gram-scale (94%, 94% ee). Excitingly, the natural product (S)-1,3,5-Bisabolatrien-7-ol was synthesized from product 8, which was obtained from ketone 1b under the standard conditions, in one step (2 steps in total, 86%, 94% ee), whereas the reported method requires 8 steps from 1b (8 steps, 18%)42. In addition, the natural product (+)-Sydonol was synthesized from ketone 9 with ease via the intermediate 10 (4 steps in total, 53%, 86% ee). The reported synthesis of (+)-Sydonol from Geraniol requires 10 steps, providing the product in 18% yield43. Finally, the drug (R,R)-Clemastine was prepared according to reported method from the product 6b44, which was synthesized from ketone 1e in 80% yield and with 89% ee under the standard conditions.
Mechanistic studies
To gain insight into the reaction pathway, a series of control experiments were designed and carried out (Fig. 4). First, in order to verify if the active intermediate involving the formation of C–Ni bond for the alkenylation of ketones, the following control experiment was carried out (Fig. 4a). It was reported that the [styryl-Ni] species can be generated from styrylbromide and Ni(COD)245. Therefore, styrylbromide 11 was used instead of styrylboronic acid in the presence of 1 equivalent of Ni(COD)2 under a nitrogen atmosphere. As a result, product 3a was not obtained, and only the cross-coupling product 12 between styrylbromide 11 and the solvent TFE was observed. This result indicated that the [styryl-Ni] species were formed46, but the species could not react with the ketone. Furthermore, whether the active intermediate involving the formation of C–Ni bond for the arylation of ketones was also studied (Fig. 4b). [Ar–Ni] complex 14 was preprepared and isolated from 3-bromotoluene 13, Ni(COD)2 and the ligand L847, and was subjected to the reaction in the presence of a halide abstractor (ZnCl2 and AgOTf). As a result, the product 6c was not observed. These results indicated that the products were not generated from the [styryl-Ni] or [Ar–Ni] species, and the generally inner-sphere reaction pathway of Type I for the addition of ketone with organoborons was excluded in this catalytic system (Fig. 1b, top).
a The reaction in the presence of styrylbromide and Ni(0). b The reaction in the presence of [Ar–Ni] species. c The reaction in the presence of Ni(0) in TFE. d The reactions in the presence of Ni(0) in cyclohexane. e The reaction in the presence of Ni(0) or Ni(II) salts. f Radical trapping experiment. g The possible transition state with styrylboronic acid as the example.
Furthermore, to investigate whether the reaction follows the Ni(0)-catalyzed reaction pathway of Type II (Fig. 1b, bottom), the reactions were performed with Ni(COD)2 instead of Ni(II) salt in TFE and cyclohexane, respectively (Fig. 4c and d). As a result, almost identical results were obtained in TFE with those from Ni(II) (86%, 94% ee vs. 92%, 94% ee) (Fig. 4c). However, in cyclohexane, no desired product 3a was observed under either the standard conditions in the presence of Ni(COD)2 and nitrogen or the same conditions as the reported Ni(0)-catalyzed reactions with only different ligand (Fig. 4d). Then, to further verify if the reaction proceeds via the Ni(0)-catalyzed reaction pathway, 4-iodoacetophenone 1g was selected for the reaction with Ni(COD)2 (Fig. 4d). As a result, in addition to the target product 3g, byproducts 3a and 15, which were generated by Ni(0)-catalyzed transformations, were also observed. In comparison, the byproducts 3a and 15 were not observed under the standard conditions. These results suggested that there were no Ni(0) active species in this catalytic system, and the reaction may not undergo a Ni(0)-catalyzed reaction pathway. We speculated that when Ni(COD)2 was used, Ni(II) species were formed via oxidative addition of Ni(COD)2 with TFE, and the reaction proceeded through Ni(II)-catalyzed transformations. Finally, the reaction was performed in the presence of the radical scavenger TEMPO, the product 3a was obtained in 90% yield and 94% ee, indicating that the reaction did not involve a radical intermediate (Fig. 4e)48,49. Therefore, an outer-sphere reaction pathway via a 1,5-metalate shift involving a six-membered ring transition state was proposed (Fig. 4f). For the reaction, the styrylboronic acid-derived “ate” complex was formed from styrylboronic acid with trifluoroethoxyl anion first, which was generated via deprotonation of TFE with base. Next, the Ni(II) complex was used not only as a chiral Lewis acid to activate the ketone, but also as a coordination bridge linking the ketone and the oxygen atom of the styrylboronic acid-derived “ate” complex. This facilitates the attack of the alkenyl group at the boron atom on the ketone. The chiral tertiary alcohol is obtained via a 1,5-metalate shift without the formation of a C–Ni bond.
To further verify the reaction pathway, DFT calculations were carried out. The results of DFT calculations indicated that the proposed outer-sphere pathway via 1,5-metalate shift is reasonable (TS-L8-S, Ea = 20.84 kcal/mol) (Fig. 5a). Then, by comparing BOX ligands L6 and L7 bearing different side arms with L8, it is evident that the activation energy of the reaction barrier decreases with the increase of the length of the side arm (Fig. 5a)50, corresponding to the results of the experiments (Table 1, entries 6–8). In that case, the long side arm, such as 2-anthryl in L8, significantly accelerates the reaction, and the low activation energy further ensures the appropriateness of the outer-sphere reaction pathway. Further IGMH (independent gradient model based on Hirshfeld partition) analysis reveals the interaction between the ligand and substrates (Fig. 5b). Compared with ligand L6 (TS-L6-S), ligand L8 (TS-L8-S) provides more interactions with substrate, for example O–H···π and F···π. Interestingly, to provide these interactions, one of the anthryl groups naturally bends into a cambered surface with 0.044 pm−1 curvature and 22.6 pm radius of curvature (ρ). Finally, a DFT study on the enantioselectivity suggests that the ΔΔG‡ of the reaction is 3.27 kcal/mol, corresponding to 98% ee, and matching the experimental data of 94% ee (Fig. 5c).
To explain why other screened Lewis acids were unable to catalyze the reaction (Table 1, entries 11–14), DFT calculations were conducted with Lewis acid Zn(II) as an example. Different from the Lewis acid nickel, which has a square planar coordination structure, the Lewis acid zinc adopts tetrahedral coordination structures. The steric hindrance between the ligand and the substrates forces the spatial configuration of zinc to distort, as seen in TS-Zn, where the spatial configuration of zinc distorts into a square planar configuration (Fig. 5d). This distortion destabilizes the six-membered ring transition-state, making Lewis acid zinc unsuitable for promoting the reaction.
Discussion
In summary, an efficient Ni(II)-catalyzed asymmetric alkenylation and arylation of ketones with organoborons via a 1,5-metalate shift strategy under air has been developed. The reaction showed good functional group tolerance and delivered the corresponding chiral tertiary allylic alcohols and tertiary diaryl alcohols in high yields and with good to excellent enantioselectivities. The developed methodology can be applied not only to the late-stage modification and synthesis of drugs but also to the efficient synthesis of natural products. The results of control experiments and DFT studies indicated that the reaction proceeds via a Ni(II)-catalyzed outer-sphere process. The Ni(II) complex not only acts as a chiral Lewis acid to activate the ketone and provide a chiral environment but also as bridge coordination to the ketone and the oxygen atom of the styrylboronic acid-derived “ate” complex to facilitate the 1,5-metalate shift. The reaction delivers the chiral tertiary alcohols without the formation of a C–Ni bond, differing from the traditional transition metal-catalyzed nucleophilic addition reactions involving carbon–metal bond formation. This work will inspire researchers to develop novel transformations of organoborons catalyzed by the transition metals.
Methods
General method for the nickel-catalyzed alkenylation and arylation of ketones with organoborons
To a Schlenk tube, the ketone 1 (0.20 mmol, 1.0 equiv), organoboronic acid 2 (0.30 mmol, 1.5 equiv), NiL8(ClO4)2 (19.3 mg, 0.020 mmol, 0.10 equiv), Na3PO4 (65.6 mg, 0.40 mmol, 2.0 equiv) and TFE (1 mL) were added and the reaction mixture was stirred at 70 °C for 1 h. After cooling to room temperature, the solvent was removed under vacuum to give a residue, which was purified by flash column chromatography on silica gel (PE:EA = 6:1) to give the desired product.
Data availability
The data generated in this study are provided in the Supplementary Information file. For the experimental procedures and data of NMR and HPLC analysis, see Supplementary Information. All data are available from the corresponding author upon request. The X-ray crystallographic coordinates for the structure reported in this study has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 2301452 (S-3g). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided in this paper. Source data are provided with this paper.
References
McCluskey, A., Sim, A. T. R. & Sakoff, J. A. Serine-threonine protein phosphatase inhibitors: development of potential therapeutic strategies. J. Med. Chem. 45, 1151–1175 (2002).
Chung, Y.-M. et al. An epigenetic modifier enhances the production of anti-diabetic and anti-inflammatory sesquiterpenoids from Aspergillus sydowii. Biorg. Med. Chem. 21, 3866–3872 (2013).
Lumbroso, A., Cooke, M. L. & Breit, B. Catalytic asymmetric synthesis of allylic alcohols and derivatives and their applications in organic synthesis. Angew. Chem. Int. Ed. 52, 1890–1932 (2013).
Pu, L. & Yu, H.-B. Catalytic asymmetric organozinc additions to carbonyl compounds. Chem. Rev. 101, 757–824 (2001).
Riant, O. & Hannedouche, J. Asymmetric catalysis for the construction of quaternary carbon centres: nucleophilic addition on ketones and ketimines. Org. Biomol. Chem. 5, 873–888 (2007).
Shibasaki, M. & Kanai, M. Asymmetric synthesis of tertiary alcohols and α-tertiary amines via Cu-catalyzed C–C bond formation to ketones and ketimines. Chem. Rev. 108, 2853–2873 (2008).
Liu, Y.-L. & Lin, X.-T. Recent advances in catalytic asymmetric synthesis of tertiary alcohols via nucleophilic addition to ketones. Adv. Synth. Catal. 361, 876–918 (2019).
Ge, L. & Harutyunyan, S. R. Asymmetric nucleophilic addition to ketones and ketimines and conjugate addition reactions. In Catalytic Asymmetric Synthesis (eds Akiyama, T. & Ojima, I.) 4th edn 617–659 (John Wiley & Sons, 2022).
Stymiest, J. L., Bagutski, V., French, R. M. & Aggarwal, V. K. Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature 456, 778–782 (2008).
Shi, S.-L., Xu, L.-W., Oisaki, K., Kanai, M. & Shibasaki, M. Identification of modular chiral bisphosphines effective for Cu(I)-catalyzed asymmetric allylation and propargylation of ketones. J. Am. Chem. Soc. 132, 6638–6639 (2010).
Harper, K. C. & Sigman, M. S. Three-dimensional correlation of steric and electronic free energy relationships guides asymmetric propargylation. Science 333, 1875–1878 (2011).
Yang, Y., Perry, I. B., Lu, G., Liu, P. & Buchwald, S. L. Copper-catalyzed asymmetric addition of olefin-derived nucleophiles to ketones. Science 353, 144–150 (2016).
Huang, S. & Zhou, J. S. Nickel-catalyzed enantioselective reductive arylation of common ketones. J. Am. Chem. Soc. 146, 12895–12900 (2024).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Nambo, M., Noyori, R. & Itami, K. Rh-catalyzed arylation and alkenylation of C60 using organoboron compounds. J. Am. Chem. Soc. 129, 8080–8081 (2007).
Clarke, C., Incerti-Pradillos, C. A. & Lam, H. W. Enantioselective nickel-catalyzed anti-carbometallative cyclizations of alkynyl electrophiles enabled by reversible alkenylnickel E/Z isomerization. J. Am. Chem. Soc. 138, 8068–8071 (2016).
Wu, K. & Doyle, A. G. Parameterization of phosphine ligands demonstrates enhancement of nickel catalysis via remote steric effects. Nat. Chem. 9, 779–784 (2017).
Chen, Y.-G. et al. Nickel-catalyzed enantioselective hydroarylation and hydroalkenylation of styrenes. J. Am. Chem. Soc. 141, 3395–3399 (2019).
Ding, Z., Wang, Y., Liu, W., Chen, Y. & Kong, W. Diastereo- and enantioselective construction of spirocycles by nickel-catalyzed cascade borrowing hydrogen cyclization. J. Am. Chem. Soc. 143, 53–59 (2021).
Li, Y. et al. Modular access to substituted cyclohexanes with kinetic stereocontrol. Science 376, 749–753 (2022).
Liu, Z. et al. A sterically tuned directing auxiliary promotes catalytic 1,2-carbofluorination of alkenyl carbonyl compounds. Angew. Chem. Int. Ed. 62, e202214153 (2023).
Li, L.-J. et al. Recent advances in Mn, Fe, Co, and Ni-catalyzed organic reactions. CCS Chem. 6, 537–584 (2024).
Ma, X. et al. Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift. Nat. Chem. 16, 42–53 (2024).
Sun, B. et al. Dynamic kinetic asymmetric allylation, propargylation and crotylation of ketones using copper catalysis. Nat. Synth. https://doi.org/10.1038/s44160-024-00567-9 (2024).
Shintani, R., Inoue, M. & Hayashi, T. Rhodium-catalyzed asymmetric addition of aryl- and alkenylboronic acids to isatins. Angew. Chem. Int. Ed. 45, 3353–3356 (2006).
Liu, G. & Lu, X. Cationic palladium complex catalyzed highly enantioselective intramolecular addition of arylboronic acids to ketones. A convenient synthesis of optically active cycloalkanols. J. Am. Chem. Soc. 128, 16504–16505 (2006).
Duan, H.-F., Xie, J.-H., Qiao, X.-C., Wang, L.-X. & Zhou, Q.-L. Enantioselective rhodium-catalyzed addition of arylboronic acids to α-ketoesters. Angew. Chem. Int. Ed. 47, 4351–4353 (2008).
Cai, F. et al. Chiral allene-containing phosphines in asymmetric catalysis. J. Am. Chem. Soc. 133, 18066–18069 (2011).
Zhu, T.-S., Jin, S.-S. & Xu, M.-H. Rhodium-catalyzed, highly enantioselective 1,2-addition of aryl boronic acids to α-ketoesters and α-diketones using simple, chiral sulfur-olefin ligands. Angew. Chem. Int. Ed. 51, 780–783 (2012).
Yamamoto, Y., Yohda, M., Shirai, T., Ito, H. & Miyaura, N. Me-BIPAM for the synthesis of optically active 3-aryl-3-hydroxy-2-oxindoles by ruthenium-catalyzed addition of arylboronic acids to isatins. Chem. Asian J. 7, 2446–2449 (2012).
Huang, L. et al. Highly enantioselective rhodium-catalyzed addition of arylboroxines to simple aryl ketones: efficient synthesis of escitalopram. Angew. Chem. Int. Ed. 55, 4527–4531 (2016).
Bartlett, S. L., Keiter, K. M. & Johnson, J. S. Synthesis of complex tertiary glycolates by enantioconvergent arylation of stereochemically labile α-keto esters. J. Am. Chem. Soc. 139, 3911–3916 (2017).
Huang, Y., Huang, R.-Z. & Zhao, Y. Cobalt-catalyzed enantioselective vinylation of activated ketones and imines. J. Am. Chem. Soc. 138, 6571–6576 (2016).
Hirano, K., Yorimitsu, H. & Oshima, K. Nickel-catalyzed alkylation of aldehydes with trialkylboranes. Org. Lett. 7, 4689–4691 (2005).
Cai, Y. & Shi, S.-L. Enantioconvergent arylation of racemic secondary alcohols to chiral tertiary alcohols enabled by nickel/N-heterocyclic carbene catalysis. J. Am. Chem. Soc. 143, 11963–11968 (2021).
Cai, Y., Ruan, L.-X., Rahman, A. & Shi, S.-L. Fast enantio- and chemoselective arylation of ketones with organoboronic esters enabled by nickel/N-heterocyclic carbene catalysis. Angew. Chem. Int. Ed. 60, 5262–5267 (2021).
Wang, Z.-C., Gao, J., Cai, Y., Ye, X. & Shi, S.-L. Chemo- and enantioselective arylation and alkenylation of aldehydes enabled by nickel/N-heterocyclic carbene catalysis. CCS Chem. 4, 1169–1179 (2022).
Ruan, L.-X., Sun, B., Liu, J.-M. & Shi, S.-L. Dynamic kinetic asymmetric arylation and alkenylation of ketones. Science 379, 662–670 (2023).
Candeias, N. R., Montalbano, F., Cal, P. M. S. D. & Gois, P. M. P. Boronic acids and esters in the Petasis–Borono Mannich multicomponent reaction. Chem. Rev. 110, 6169–6193 (2010).
Namirembe, S. & Morken, J. P. Reactions of organoboron compounds enabled by catalyst-promoted metalate shifts. Chem. Soc. Rev. 48, 3464–3474 (2019).
Luo, W., Zhang, L.-M., Zhang, Z.-M. & Zhang, J. Synthesis of W-Phos ligand and its application in the copper-catalyzed enantioselective addition of linear Grignard reagents to ketones. Angew. Chem. Int. Ed. 61, e202204443 (2022).
Yajima, A. et al. Practical synthesis of aromatic bisabolanes: synthesis of 1,3,5-bisabolatrien-7-ol, peniciaculin A and B, and hydroxysydonic acid. Tetrahedron 92, 132253 (2021).
Serra, S. & Cominetti, A. A. A divergent and stereoselective approach to phenolic 1,7-dihydroxy-bisabolane sesquiterpenes: asymmetric total synthesis of (+)-curcutetraol, (+)-sydonol, (+)-sydonic acid, and (+)-7-O-methylsydonic acid. Tetrahedron: Asymmetry 24, 1110–1116 (2013).
Fournier, A. M., Brown, R. A., Farnaby, W., Miyatake-Ondozabal, H. & Clayden, J. Synthesis of (−)-(S,S)-clemastine by invertive N → C aryl migration in a lithiated carbamate. Org. Lett. 12, 2222–2225 (2010).
Hu, X., Cheng-Sánchez, I., Cuesta-Galisteo, S. & Nevado, C. Nickel-catalyzed enantioselective electrochemical reductive cross-coupling of aryl aziridines with alkenyl bromides. J. Am. Chem. Soc. 145, 6270–6279 (2023).
Kundu, D., Maity, P. & Ranu, B. C. Copper-assisted nickel catalyzed ligand-free C(sp2)–O cross-coupling of vinyl halides and phenols. Org. Lett. 16, 1040–1043 (2014).
Ceder, R. M., Granell, J., Muller, G., Font-Bardia, M. & Solans, X. Preparation of five-membered nickelacycles of N-donor ligands by activation of C–X bonds (X = F, Cl, or Br). X-ray crystal structure of [NiBr{2-(CH:NCH2Ph)C6H4}(2,4,6-Me3C5H2N)]. Organometallics 14, 5544–5551 (1995).
Zhang, X., Xie, X. & Liu, Y. Nickel-catalyzed cyclization of alkyne-nitriles with organoboronic acids involving anti-carbometalation of alkynes. Chem. Sci. 7, 5815–5820 (2016).
Wu, D. et al. Alkene 1,1-difunctionalizations via organometallic-radical relay. Nat. Catal. 6, 1030–1041 (2023).
Liao, S., Sun, X.-L. & Tang, Y. Side arm strategy for catalyst design: modifying bisoxazolines for remote control of enantioselection and related. Acc. Chem. Res. 47, 2260–2272 (2014).
Acknowledgements
The National Key R&D Program of China (No. 2023YFA1506700, W.Z.) and the National Natural Science Foundation of China (No. 22001164, Q.Y.; and No. 21991112, W.Z.) supported this work. We thank the Instrumental Analysis Center of Shanghai Jiao Tong University for characterization experiments.
Author information
Authors and Affiliations
Contributions
W.Z. and Q.Y. conceived and designed the experiments. H.W., Q.Y. and J.R. performed the experiments and analyzed the data. Y.L. performed the computational studies. W.Z. and Q.Y. prepared the manuscript with feedback from H.W. Y.L. and J.R. All authors discussed the results and comments on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yuqiang Li who co-reviewed with Dongzhan Zhou; Zhuangzhi Shi, Jianrong Zhou and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wei, H., Luo, Y., Ren, J. et al. Ni(II)-catalyzed asymmetric alkenylation and arylation of aryl ketones with organoborons via 1,5-metalate shift. Nat Commun 15, 8775 (2024). https://doi.org/10.1038/s41467-024-53005-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-53005-x
This article is cited by
-
Lactam enables remote boronate rearrangements to C═N bonds
Communications Chemistry (2026)
-
Recent advances in boron chemistry
Science China Chemistry (2025)