Abstract
Bacterial extracellular vesicles (BEVs) enable communication between bacteria and their natural habitats, including multicellular organisms such as humans. Consequently, the study of BEVs has rapidly gained attention with recent research raising the prospect of developing BEVs as biomarkers and treatments to manage (mal)functioning of natural habitats. Although diverse technologies are available, the composition of their source, their heterogeneity in biophysical and biochemical features, and their multifaceted cargo composition challenges the analysis of BEVs. To map current practices in BEV research, we analyzed 845 publications released in 2015–2021, reporting 3338 BEV-related experiments. The extracted data are accessible via the publicly available EV-TRACK knowledgebase (https://evtrack.org/). We identify the need for transparent reporting, delineate knowledge gaps, outline available best practices and define areas in need of guidance to ensure advances in BEV research and accelerate BEV applications.
Similar content being viewed by others
Introduction
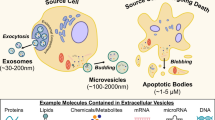
Bacterial extracellular vesicles (BEVs) are nanometer-sized membrane particles transporting a multi-faceted cargo including lipids, nucleic acids, metabolites, proteins, and endotoxins that enable interkingdom communication across species in diverse natural habitats1,2. Different biogenesis routes lead to heterogeneous BEV subsets that vary in membrane and cargo composition. In gram-negative bacteria, BEVs originate either from blebbing of the outer membrane, forming outer membrane vesicles (OMVs) free of cytosolic cargo; or from endolysin-triggered explosive cell lysis, resulting in the release of outer-inner membrane vesicles (OIMVs) and explosive outer-membrane vesicles (EOMVs) encompassing cytosolic cargo3,4. BEVs from gram-negative bacteria mirror the outer membrane and thus present lipopolysaccharide (LPS) on their exterior membrane, enabling host immune cell activation through the Toll-like receptor 4 (TLR4)4,5. Although the biogenesis of BEVs in gram-positive and gram-neutral bacteria is poorly understood, both lysis and blebbing mechanisms exist, forming cytoplasmic membrane vesicles (CMVs) encompassing cytosolic cargo and presenting surface peptidoglycan and lipoteichoic acid (LTA), enabling host immune cell activation through the Toll-like receptor 2 (TLR2)4,5,6.
BEVs orchestrate bacteria–bacteria and bacteria–host interactions to support bacterial survival by (1) the export of bioactive compounds, the neutralization of phages and antibiotics, and bactericidal functions, (2) the delivery of virulence factors and toxins to host cells and competing species, (3) biofilm formation, and (4) inflammatory and immunomodulatory effects in host cells1,3,7,8. Consequently, BEVs are emerging as promising treatment agents and attractive biomarkers with relevance to different domains including medicine, biotechnology, and agriculture. BEVs possess multiple inherent features and can be decorated, which makes them safe and effective acellular candidates for vaccine development, immunotherapy, and drug delivery1,9,10,11,12,13,14. BEVs have been detected in a wide variety of natural habitats, including ocean seawater, plants, and human body fluids unlocking biomarker potential to monitor ecosystem (mal)functioning1,5,6,9,15,16.
However, the complexity and challenges associated with BEV research are considerable. A plethora of methods are available for the preparation and characterization of BEVs2. A diversity of bacterial strains releases heterogeneous subsets of BEVs3,4. The sources from which BEVs are retrieved contain other extracellular particles with comparable biophysical and biochemical features2,6. Lastly, to date no consensus exists on universal BEV markers1,6. Over the past years, the International Society for Extracellular Vesicles (ISEV) developed minimal information guidelines17,18 and endorsed the use of EV-TRACK (https://evtrack.org/), a freely accessible knowledgebase that facilitates transparent reporting of experimental parameters and centralizes knowledge for EV research17,18,19. To enable best practices, it is essential to encourage awareness and to stimulate implementation, but also to ensure the continuous development of tailored guidelines and tools to meet the needs of particular subdomains such as BEV research2,20. Hereto, we record experimental parameters of BEV-related studies published between 2015 and 2021 using the EV-TRACK knowledgebase. We map current practices in BEV research, extract knowledge gaps and propose opportunities for guideline and tool development. Tailoring guidelines and tools to BEV research will ultimately further steer their widespread use and implementation while advancing biological insights and accelerating the translation of BEV-based applications.
Results
Search
We selected all 845 studies reporting BEV preparation from any source between 2015 and 2021 (Supplementary Data 1). 20% of studies report multiple study aims (168/845). The majority of studies evaluate BEV function (65%, 553/845) and biology (15%, 130/845). Other studies analyze BEV cargo composition (24%, 204/845), develop methods (11%, 89/845) or applications (5.9%, 50/845) (Supplementary Fig. 1a). Across these 845 BEV-related studies, heterogeneous nomenclature is applied (Box 1). Outer membrane vesicle (69%, 580/845) is the most commonly used term. The term bacterial extracellular vesicle is reported in 20% of studies (166/845), although its implementation increases over time. Of note, six studies misleadingly implement the term outer membrane vesicle to denote particles released from gram-positive bacteria (Supplementary Fig. 1b, c).
Next, we submitted all studies that were not yet available (98%, 827/845) to the EV-TRACK knowledgebase19. Hereto, studies that reported multiple sources or preparation protocols (i.e., a combination of methods) were divided into multiple entries, resulting in a total of 3338 experiments (with a unique source and/or preparation protocol). For each experiment a checklist of 233 parameters related to source, preparation protocol and characterization method was completed (Supplementary Data 2). Data were curated and integrated into the EV-TRACK knowledgebase. In-depth analysis of this data for all 845 BEV-related studies reveals widespread methodological heterogeneity and variable reporting of experimental parameters (Fig. 1).
Binary heatmap showing the reported experimental parameters (rows of the heatmap, selection of 102 out of 233 parameters for binarity and relevance to experimental results) for each experiment (columns of the heatmap, total n = 3338). The heatmap is divided horizontally into three sections of parameters (preparation, biophysical characterization, and biochemical characterization; indicated in purple, green, and blue and including 57, 32, and 13 parameters, respectively). It is divided vertically in eight blocks according to source (cell culture supernatant (n = 3123, dark green), blood (n = 58, red), feces (n = 53, dark brown), intestinal tissue (n = 19, light brown), milk (n = 3, light blue), saliva (n = 13, dark blue), urine (n = 26, dark yellow) and other (n = 43, dark pink) and in seven blocks according to publication year: 2015 (n = 80, light red), 2016 (n = 73, orange), 2017 (n = 93, light yellow), 2018 (n = 110, light green), 2019 (n = 123, cyan), 2020 (n = 184, purple), and 2021 (n = 182, light pink). For each section, columns (experiments) are sorted according to descending total number of reported experimental parameters. Parameters that were not reported in an experiment appear as a white space in its corresponding column. Ab antibody, BEV bacterial extracellular vesicle, DC density cushion, DG density gradient, DLS dynamic light scattering, DUC differential (ultra-)centrifugation, ELISA enzyme linked immune sorbent assay, FC flow cytometry, NTA nanoparticle tracking analysis, RNA ribonucleic acid, SDS-PAGE sodium dodecyl-sulfate polyacrylamide gel electrophoresis, SEC size exclusion chromatography, TRPS tunable resistive pulse sensing, UF ultrafiltration.
BEV research practices
The vast majority of experiments (94%, 3123/3338) analyze BEVs in media conditioned by bacterial cells under laboratory conditions (Fig. 1), with a preference for bacterial species belonging to the phylum Pseudomonadota. BEVs from gram-negative bacteria are more abundantly analyzed (77%, 2560/3338) compared to BEVs from gram-positive bacteria (16%, 526/3338). Top three studied bacterial species are Escherichia coli (22%, 742/3338), Staphylococcus aureus (7%, 235/3338), and Pseudomonas aeruginosa 6% (201/3338) (Fig. 2a, Supplementary Data 3). BEVs in natural habitats remain minimally explored with 1.7% (58/3338), 0.8% (26/3338), and 1.6% (53/3338) of experiments studying BEVs from blood, urine, and feces, respectively (Figs. 1 and 2a). Of note, BEVs prepared from bacterial species belonging to other environmental sources (marine: 1.7%, 58/3338, plant: 0.3%, 9/3338) are primarily investigated under laboratory conditions.
a Source: BEVs are prepared from bacterial cell culture (93.6%; colors correspond to phyla in cladogram) or other sources (6.4%; gray/white; blood, feces, intestinal tissue, milk, saliva, urine and other) (right). Circular cladogram indicating the taxonomy of studied bacterial species. The outer circle shows studied phyla, with each color representing one phylum. Number of experiments per phylum is represented by the color of the cladogram species level (gray vs. blue for most abundant phylum Pseudomonadota). Number of experiments per species is indicated by the size of the circles on the outer layer of the species level. The three most abundant species are indicated in purple (and with arrow): E. coli, S. aureus, and P. aeruginosa (left). b Preparation: Bar plot indicating number of implemented methods: one (6%), two (48%), or more than two (46%). Chord diagram shows combination of reported preparation methods, with DUC (green) and filtration (blue) as most implemented methods. Other method includes commercial method, tangential flow filtration (TFF), density cushion and precipitation. c Characterization: Bar plot indicating number of implemented methods combined: zero (28.6%), one (38.4%) two (20%), or three or more (13%). Chord diagram shows combination of reported characterization methods, with most studies performing no biochemical (orange) or biophysical characterization (pink). Other method includes PAMP reporter assays, multi-angle light scattering (MALS), confocal microscopy, spectrophotometry. BEV bacterial extracellular vesicle, DLS dynamic light scattering, DUC differential (ultra-)filtration, E. coli Escherichia coli, ELISA enzyme linked immune sorbent assay, NTA nanoparticle tracking analysis, P. aeruginosa Pseudomonas aeruginosa, S. aureus Staphylococcus aureus, SDS-PAGE sodium dodecyl-sulfate polyacrylamide gel electrophoresis, SEC size exclusion chromatography, TRPS tunable resistive pulse sensing.
Among the 845 studies analyzed, 934 unique protocols are reported to prepare BEVs from their source (Fig. 1). Nearly all protocols implement differential (ultra-)centrifugation (DUC) (97%, 3243/3338) with 137 unique combinations of centrifugation steps and final pelleting times recorded. In 88% of experiments performing DUC (2860/3243), the final centrifugation step results in a BEV pellet. Overall, the majority reports centrifugation speed (g-force) (97%, 2782/2860) but only 49% (1403/2860) and 36% (1023/2860) of experiments report centrifugation time and rotor type, respectively (Box 2). Filtration (90%, 3004/3338) and ultrafiltration (28%, 926/3338) are also frequently implemented. Density gradient-based preparation is used in 22% (716/3338) of experiments with adequate reporting of experimental parameters for less than 3% of experiments (2.9%, 21/716) (Fig. 1). The majority (94%, 3133/3338) of experiments sequentially implement two (48%, 1602/3338) or more (46%, 1531/3338) methods. Filtration and DUC are frequently combined (89%, 2965/3338), independent of the source (bacterial cell culture (90%, 2798/3123), blood (66%, 38/58), urine (85%, 22/26), feces (98%, 52/53) and intestinal tissue (100%, 10/10)). DUC is also commonly associated with ultrafiltration (28%, 900/3243) and density gradient centrifugation (22%, 707/3243). Filtration is frequently combined with ultrafiltration (30%, 901/3004) and density gradient centrifugation (22%, 675/3004) (Fig. 2b).
Almost half (46%, 388/845) of studies report storage temperature, with 17% of studies (67/388) storing the BEV preparation at 4 °C, 28% (110/388) at −20 °C and 50% (195/388) at −80 °C. Storage buffer and duration are reported in 60% (509/845) and 5.6% (47/845) of experiments, respectively. Variable storage buffers are reported, predominantly phosphate-buffered saline (PBS) (69%, 349/509) (Supplementary Data 4).
Over one-fourth of experiments (29%, 956/3338) report neither biophysical nor biochemical characterization (Figs. 1 and 2c). 29% (981/3338) and 18% (585/3338) of experiments perform solely biophysical or biochemical characterization, respectively. Only 25% of experiments (816/3338) perform both biophysical and biochemical characterization.
Biophysical characterization is reported in 54% (1797/3338) of experiments, with the majority of experiments reporting microscopy (35%, 1163/3338), followed by nanoparticle tracking analysis (NTA) (21%, 684/3338) and dynamic light scattering (DLS) (12%, 401/3338) (Figs. 1 and 2c). Transmission electron microscopy (TEM) is routinely used (82%, 953/1163), but both widefield and close-up images are included in merely 13% (153/1163) of experiments (Fig. 1, Box 2). 44% (513/1163) of experiments performing qualitative microscopy-based characterization is complemented with quantitative characterization (using NTA, DLS, tunable resistive pulse sensing (TRPS) or flow cytometry (FC)). 15% (513/3338) of experiments thus implement both qualitative and quantitative biophysical characterization (Fig. 2c, Box 2).
For biochemical characterization (performed in 42% of experiments (1401/3338)), western blot is the preferred method (24%, 816/3338), with merely 19% reporting lysate specifics (155/816) (Box 2). This is followed by proteomics (20%, 670/3338) and SDS-PAGE (2.1%, 70/3338) (Figs. 1 and 2c). Lipids or nucleic acids are evaluated in 11% (363/3338) and 3.9% (133/3338) of experiments, respectively. BEV-associated nucleic acids are predominantly analyzed using RT-qPCR assays (34%, 45/133) or RNA sequencing (52%, 69/133), with 30% (40/133) performing 16S rRNA sequencing (Supplementary Data 4). Confirmation of luminal nucleic acid localization is performed in 30% of experiments (i.e., RNAse (22%, 29/133), in combination with proteinase (10%, 13/133) or not). The majority report enzyme (83%, 24/29 and 100%, 13/13) and concentration (69%, 20/29 and 54%, 7/13), but only 24% report treatment time. Data sharing through online databases is rarely reported (proteomics (13%, 89/670) or transcriptomics (7.2%, 5/69)) (Fig. 1).
Quantification of pathogen-associated molecular patterns (PAMPs) is performed using reporter assays for TLR4 (1.9%, 16/845), TLR2 (2.3%,19/845) and NF-kB (1.2%, 10/845). Alternatively, 8.2% of studies (69/845) implement the Limulus amebocyte lysate (LAL) assay to quantify LPS (Supplementary Data 4).
Assess relevance and advance insights by evaluating biophysical and biochemical characteristics
68% of experiments performing biophysical characterization (1216/1797) report particle size (Fig. 1), with the majority implementing NTA (45%, 546/1216). This is followed by EM (36%, 434/1216), and DLS (30% 361/1216) (Figs. 1 and 2c). Average reported particle sizes of BEV preparations for frequently studied phyla Pseudomonadota (63%, 770/1216), Bacillota (14%, 174/1216) and Bacteroidota (3.5%, 43/1216) are, respectively, 118 nm (±78 nm), 158 nm (±277 nm) and 141 nm (±92 nm) (Fig. 3a, Supplementary Fig. 2a). Average reported particle sizes of BEV preparations for commonly analyzed species E. coli (25%, 309/1216), S. aureus (5.4%, 66/1216) and P. aeruginosa (4%, 49/1216) are, respectively, 118 nm (±57 nm), 128 nm (±79 nm), and 147 nm (±75 nm) (Fig. 3a). Overall, BEV preparations derived from gram-negative bacteria (128 nm ± 108 nm) display a smaller average particle size compared to gram-positive BEV preparations (163 nm ± 284 nm (Supplementary Fig. 2a). The average particle size of BEV preparations obtained under laboratory conditions (132 nm ± 144 nm, n = 1300 experiments) is lower compared to BEV preparations from other sources (182 nm ± 127 nm, n = 74 experiments) (Supplementary Fig. 2a). Of note, the reported particle size inherently depends on the settings, the sensitivity and the detection limits of the selected instrument; smaller particle sizes are reported using DLS and EM compared to NTA (Fig. 3a)17,21. Furthermore, methods such as DLS and NTA are not able to distinguish BEVs and other extracellular particles. Overall, results should be interpreted with caution17.
a Size: Average particle size (nm) per experiment for most abundant phylum Pseudomonadota (left) and top three studied species E. coli, S. aureus, and P. aeruginosa (right). Colors indicate method implemented for size measurement. For statistical analysis, a two-sided Mann‐Whitney U test was used. P values smaller than 0.05 were considered statistically significant (**p < 0.01, ****p < 0.0001). Exact p values are provided in the Source data file. b Concentration: Average particle concentration (particles/mL of starting sample) per experiment, plotted on a Log10 scale, for most abundant phyla Pseudomonadota and Bacillota (left) and top two studied species: E. coli and S. aureus (right). Particle concentration is not plotted for Bacteroidota or P. aeruginosa as concentration is only reported for 8 and 3 experiments, respectively. Colors indicate method implemented for concentration measurement. For statistical analysis, a two-sided Mann‐Whitney U test was used. P values smaller than 0.05 were considered statistically significant (**p = 0.0013). c Density: Average particle density (g/mL) per source type: bacterial cell culture (colors indicate the studied phylum; n = 7) or other sources (gray; n = 6). d Molecules: Treemaps indicating the most studied BEV-enriched (left; purple) and BEV-depleted (right; blue) proteins and other molecules for all species studied to recover BEVs from bacterial cells under laboratory conditions, and for the top three studied species: E. coli, S. aureus, and P. aeruginosa. BEV-depleted proteins are not plotted for S. aureus and P. aeruginosa as zero and two proteins are reported, respectively. GroEL (red) and flagellin (orange) are implemented as both BEV-enriched and BEV-depleted. BEV bacterial extracellular vesicle, Crp Cytoplasmic cAMP receptor protein, DLS dynamic light scattering, DnaK chaperone protein, E. coli Escherichia coli, EM electron microscopy, ExoA/U exotoxin A/U, FimA Type-1 fimbrial protein, A, FlaB Flagella filament protein, Gp100 glycoprotein 100, IgIC pathogenicity island protein, Lpp outer membrane prolipoprotein lpp, LUKF-PV F component of Panton-Valentine leucocidin, LPS lipopolysaccharide, LTA lipoteichoic acid, NTA nanoparticle tracking analysis, OmpA/C/F/T outer membrane protein A/C/F/T, P. aeruginosa Pseudomonas aeruginosa, Pba peptidoglycan-binding anchor, Pic protein involved in intestinal colonization, RNAP RNA polymerase, RpoA DNA-directed RNA polymerase subunit alpha, S. aureus Staphylococcus aureus, SepA Shigella extracellular protein A, SigA Shigella IgA protease-like homologue, Stx2a Shiga toxin 2 subunit A, TRP2 tyrosinase-related protein 2, TseF hypothetical protein.
Particle concentration is only reported in 19% of experiments performing biophysical characterization (339/1797), with the vast majority using NTA (90%, 305/339) (Fig. 1). Most experiments quantify particle concentration per mL of starting sample (60%, 203/339). Average reported particle concentrations of BEV preparations for the commonly studied phyla Pseudomonadota (30%, 61/203) and Bacillota (17%, 35/203) are 2.87E12 particles/mL and 9.21E11 particles/mL, respectively (Fig. 3b). For the frequently analyzed bacterial species E. coli (7.1%, 24/339) and S. aureus (6.7%, 23/339), average reported particle concentrations are 2.58E11 particles/mL and 1.25E12 particles/mL, respectively (Fig. 3b). Average particle concentration is higher for gram-negative BEV preparations (3.26E12 particles/mL) compared to gram-positive BEV preparations (4.25E11 particles/mL) (Supplementary Fig. 2b). Similar to particle size, the reported particle concentration inherently depends on the instrument specifics and results should be interpreted with caution17.
Density is reported in less than 2% of experiments performing density gradient centrifugation (1.8%, 13/716) (Fig. 1, Box 2). Densities of BEV preparations obtained under laboratory conditions are reported for E. coli (7.6%, 1/13: 1.16 g/mL), S. aureus (31%, 4/13: 1.096 g/mL (±0.006 g/mL)) and Mycobacterium tuberculosis (15.4%, 2/13: 1.155 g/mL (±0.02 g/mL)). Overall, BEV preparations derived from gram-positive bacteria (1.12 g/mL (±0.032 g/mL)) display lower densities compared to gram-negative bacteria (1.16 g/mL). The average density of BEV preparations obtained under laboratory conditions (54%, 7/13: 1.12 g/mL (±0.034 g/mL)) is lower compared to BEV preparations from other sources (46%, 6/13: 1.14 g/mL (±0.038 g/mL)) (Fig. 3c).
In more than half of the experiments (56%, 780/1401) biochemical characterization is performed to evaluate one or more BEV-enriched proteins, documenting a total of presumably 1242 BEV-enriched proteins. 998 (80%) and 186 (15%) of these are, respectively, used to analyze BEV preparations from gram-negative (77%, 2560/3338) and gram-positive bacteria (16%, 526/3338) (Fig. 1, Supplementary Data 5). Of note, only 5.5% of experiments (77/1401) evaluate three or more BEV-enriched proteins17,18 (Box 2). The vast majority of BEV preparations from bacterial cultures under laboratory conditions is evaluated for outer membrane protein A (OmpA; 16%, 189/1213 proteins) (Fig. 3d). As frequently studied species, E. coli-specific proteins rank in the top ten of BEV-enriched proteins (Shiga toxin 2 subunit A (Stx2a; 5.4%, 65/1213), Type-1 fimbrial protein, A (FimA; 2.6%, 32/1213), antigen-43-alpha (2%, 24/1213), outer membrane prolipoprotein lpp (2%, 24/1213)). For E. coli, S. aureus, and P. aeruginosa, BEV preparations are mainly evaluated for OmpA (28%, 128/462), alpha-toxin (27%, 23/85), and TseF (20%, 6/30), respectively (Fig. 3d). This reflects the most studied proteins for BEV preparations derived from gram-negative (OmpA; 19%, 189/998) and gram-positive bacteria (alpha-toxin; 15%, 28/186) (Supplementary Fig. 2c). For sources other than bacterial cell culture, mainly BEV-enriched molecules LTA (24%, 7/29) and LPS (21%, 6/29) are analyzed (Supplementary Fig. 2c). Only 8.6% of experiments (120/1401) evaluate the presence of proteins associated to other extracellular particles (Box 2), documenting a total of 153 presumably BEV-depleted proteins. 121 of these proteins are reported for BEV preparations from gram-negative but none from gram-positive bacteria (Supplementary Fig. 2c). Of note, while the vast majority of experiments evaluates GroEL and flagellin as BEV-depleted proteins (22% (33/153) and 9.2% (15/153), respectively), some consider them BEV-enriched (1.7% (21/1242) and 3.3% (40/1242), respectively) (Fig. 3d, Supplementary Data 5). For other sources, the principal BEV-depleted proteins are calnexin (38%; 8/21) and apolipoproteins (Apo) A1 (19%, 4/21) and B (19%, 4/21). Well-established markers for eukaryotic EVs (EEVs) are implemented to confirm their absence in BEV preparations: CD9 (4.8%, 1/21), flotillin 1 (FLOT1; 4.8%, 1/21) and Alix (4.8%, 1/21) (Supplementary Fig. 2c). Of note, only one-fourth of experiments using antibodies to evaluate BEV-enriched or BEV-depleted proteins reports antibody specifics (25%, 195/780) (Box 2).
Assess quality by evaluating the EV-METRIC
Each experiment submitted to the EV-TRACK knowledgebase receives an EV-METRIC, which encompasses nine generic components imperative for interpretation and reproduction of extracellular vesicle experiments (Box 2)19. 36% of included experiments (1187/3338) fail to adhere to a single EV-METRIC component (i.e., EV-METRIC of 0%). The average EV-METRIC across all BEV studies is 14.5%, with the highest achieved 87% (Fig. 4a). For experiments studying BEV preparations from bacterial cultures under laboratory conditions (n = 3123) and other sources (n = 215), the average EV-METRIC is, respectively, 14.7% and 12.2% (Fig. 4b). BEV experiments for gram-negative (n = 2560) and gram-positive bacterial species (n = 526) obtain average EV-METRICs of 14% and 18%, respectively (Fig. 4c). Average EV-METRICs for the top three bacterial species used for BEV analysis (E. coli, S. aureus, and P. aeruginosa) are 13.6%, 14.5% and 14.5%, respectively (Fig. 4d).
a Evolution of average EV-METRIC (%) for EEV (cyan) and BEV (purple) studies from 2015 to 2021. Violin and dot plot show EV-METRIC for each included BEV study; number of studies per year are indicated on the x-axis. The highest EV-METRIC is 87%; 36% achieve an EV-METRIC of 0%. b–e Radar charts representing the percentage of experiments that adhere to each of the respective EV-METRIC components according to b source type, c gram staining of studied bacteria, d species type (top three studied species E. coli S. aureus, and P. aeruginosa), and e domain (included experiments for BEVs compared to EEVs). BEV bacterial extracellular vesicle, EEV eukaryotic extracellular vesicle, E. coli Escherichia coli, EM electron microscopy, Pseudomonas aeruginosa, S. aureus Staphylococcus aureus.
Experiments reporting on EEVs, submitted to EV-TRACK between 2015 and 2021 (n = 3499 experiments), receive a higher EV-METRIC, with a mean of 28.5% (Fig. 4a). The highest EV-METRIC for EEV experiments is 100% and less than one-third of EEV experiments (26%, 900/3499) obtains an EV-METRIC of 0%. Overall, each EV-METRIC component is better reported for EEV-related studies, except the implementation of a density gradient: 21% (698/3338) and 14% (486/3499) for BEV and EEV experiments, respectively (Fig. 4e). Upon release of the MISEV2018 guidelines the average EV-METRIC for EEV studies distinctly increases18; the average EV-METRIC of BEV studies plateaus in 2018 (Fig. 4a).
Extracting knowledge gaps and defining tailored recommendations to accelerate BEV research
We identify knowledge gaps to propose a set of tailored recommendations and thereby align available guidelines and tools with the current needs, ensuring high-quality data generation and stimulating transparent reporting and reproducibility in BEV research (summarized in Tables 1 and 2).
The term outer membrane vesicle (OMV) is most commonly reported (Supplementary Fig. 1b). However, this term entails the presence of outer membrane components and should only be utilized to denote particles that bleb from the outer membrane. BEVs can contain components of both outer and cytoplasmic membrane (outer-inner membrane vesicles, OIMV) or only the cytoplasmic membrane (gram-positive bacteria: cytoplasmic membrane vesicles or CMV) and can be induced by cell lysis (explosive OMV or EOMV)3,4. Applying certain nomenclature hence reflects the underlying biogenesis pathways. As much remains to be explored in terms of BEV biogenesis across bacterial species, the term BEV is recommended when multiple subsets are studied, or if biogenesis remains unclear or not specified, especially when exploring natural habitats composed of diverse bacterial species. This term can be contemplated with operational terms (for example, engineered BEVs for BEVs that are modified as treatment vehicle) (Box 1, Table 1).
More than 90% of experiments study BEVs prepared from media conditioned by bacterial cells. 22% of experiments analyze BEV preparations from E. coli, a bacterium that is easily cultivated and commercially available22. Natural habitats of BEVs remain largely unexplored (Figs. 1 and 2a). Molecular techniques however reveal an important discrepancy between the immense bacterial diversity in natural habitats such as multicellular organisms, ocean seawater and soil, and the small fraction that exists in bacterial culture, referred to as the great plate count anomaly23,24,25,26. Further exploration of BEVs in natural habitats will be indispensable to generate insights in BEV biology and function and accelerate the development of BEV-based applications (Table 1).
934 unique protocols are reported to prepare BEVs from their source, with 94% of experiments implementing two or more consecutive steps exploiting biophysical and/or biochemical characteristics (Figs. 1 and 2b). The selection of protocol steps depends on the volume and complexity of the source, other extracellular particles residing in the source, and BEV subsets of interest2. Considering that each protocol prepares BEVs with differential specificity (also referred to as purity) and efficiency (also referred to as recovery)17,18, transparent reporting of experimental parameters (Table 2) is fundamental and thus recommended to ensure reproducible BEV research2,19. While research to define the most optimal storage conditions for BEV preparations is still awaiting, transparent reporting is encouraged since storage further impacts efficiency and specificity27 (Tables 1 and 2). Characterization of BEV preparations may be confounded by bacterial or workflow-induced endotoxin contamination, therefore confirmation of bacterial depletion (e.g., bacterial culture) and implementation of procedural controls (e.g., buffer only) is critical, but to date only reported in one-fifth of analyzed studies (20%, 169/845) (Tables 1 and 2, Supplementary Data 4).
Systematically cataloguing biochemical and biophysical characteristics, supports data validation and contributes insights into BEVs residing in diverse natural habitats. Furthermore, characterization of BEV preparations, using adequate assay controls, allows to identify what is present (presumably BEVs) and what is not (presumably other extracellular particles), in turn supporting data interpretation and conclusion-making2. To comply with MISEV guidelines, complementary biophysical and biochemical characterization should be performed17,18, but more than one-fourth of BEV experiments fail to report biophysical and biochemical characterization. Consequently, biophysical (such as size, density, and particle concentrations) and biochemical (such as BEV-enriched and BEV-depleted proteins) characteristics of BEV preparations remain largely unknown. As an initiating endeavor, we catalogue commonly explored BEV-enriched and BEV-depleted proteins, but dedicated research and research tool (such as antibody) development is required to extend and confirm this catalogue across diverse bacterial species and natural habitats (Table 1). The analysis of other pathogen-associated molecular patterns (PAMPs) is of particular interest and should be considered for future guideline and tool development (Tables 1 and 2).
Since the vast majority of experimental parameters remain underreported, BEV experiments generally receive lower EV-METRICs. With the aim to stimulate an informed dialogue and align guidelines and tools with BEV research we included a set of BEV-dedicated experimental parameters to the EV-TRACK knowledgebase, which will ultimately advance awareness of critical experimental parameters, increase EV-METRICs for BEV experiments and drive transparent reporting and reproducibility (Table 2).
Discussion
Supporting guidelines and tools have been developed for multiple key steps in extracellular vesicle research. These include minimal information guidelines17,18 proposed by the International Society for Extracellular Vesicles (ISEV), the EV-TRACK knowledgebase19 and repositories for sharing data (Vesiclepedia, EVpedia), which have the concerted aim of increasing transparency and reproducibility to advance biological insights and steer applications across multiple research domains. With more than 800 studies analyzed, we reveal that this potential is not fully realized yet, as best practices remain underutilized (as reflected in the low average EV-METRIC of 14.5%) in the fledgling but rapidly developing field of BEVs. Furthermore, whereas the average EV-METRIC of EEV studies distinctly increases since the release of MISEV2018 guidelines, the average EV-METRIC of BEV studies plateaus in 2018 (Fig. 4a). Thus, while general principles of the existing guidelines and tools are applicable, research on BEVs encounters specific challenges and requires tailored recommendations to further mature. Based on the analysis of 3338 experiments studying BEVs, we propose recommendations and identify a set of experimental parameters, to delineate the available guidelines and tools with particular needs for BEV research (Table 2).
Heterogeneous nomenclature is inconsistently implemented, often reflecting a certain underlying biogenesis pathway. As much remains to be explored in terms of BEV biogenesis across bacterial species, a more general term, BEV, is recommended. This is especially the case when multiple subsets are studied, or if biogenesis remains unclear or not specified or natural habitats are explored (Box 1). With more than 90% of included experiments being performed on BEVs prepared from bacterial cell cultures, unfulfilled potential remains in mapping the biophysical and biochemical landscape of BEVs in natural habitats harboring bacterial species that cannot be cultured or that metabolically differ under laboratory conditions. Typically, the exploration of BEVs in these presumably more complex natural habitats compared to bacterial cell cultures, will demand multi-step protocols further increasing the number of experimental parameters2. Transparent reporting will be key to ensure reproducibility and further advance BEV research, and to achieve this, tailored guidelines and tools are fundamental. Hereto, we integrated a set of BEV-specific experimental parameters into the EV-TRACK knowledgebase (Table 2).
Besides experimental parameters, biophysical and biochemical characteristics of BEVs remain largely underreported which hampers the understanding of BEV heterogeneity across different sources. To advance the reporting of biochemical characteristics, we integrated BEV-specific research tools (e.g., PAMP reporter assays) into the EV-TRACK knowledgebase and catalogued commonly explored BEV-enriched and BEV-depleted proteins across abundantly studied phyla and species (Table 2, Supplementary Data 5). Most studied proteins are however often specific to one bacterial species and cannot be implemented as a universal marker for BEVs (Fig. 3d, Supplementary Fig. 2c). In addition, the observation that some proteins are reported as both BEV-depleted and BEV-enriched underpins how little we know. It is pertinent that further research is required to expand and fine-tune this catalogue. We furthermore catalogued particle size and particle concentration of BEV preparations, revealing source-dependent biophysical characteristics, while the density of BEV preparations remains largely unexplored. Cataloguing biophysical and biochemical characteristics of BEVs will be essential to further advance our insights in the presence, function and application potential of BEV subsets across natural habitats. Inherent characteristics of BEVs and their parental bacterium significantly influences their impact on the surrounding natural habitat. BEVs from various origin can either induce immunosuppression or stimulate immune activation1,8,28,29,30. BEV biogenesis is further affected by several aspects, including medium composition, antibiotics and bacterial growth phase, which leads to variations in their molecular cargo3,29,31. For instance, the levels of virulence factors within BEVs can vary depending on the bacterial growth phase, eliciting either a robust pro-inflammatory response or prolonged low-level immune activation3,8,31,32,33.
In conclusion, through mapping the current practices in BEV research, highlighting knowledge gaps and providing recommendations we facilitate the fine-tuning of available guidelines and tools. We enable researchers to easily query all BEV-related publications released between 2015 and 2021 in the EV-TRACK knowledgebase for source, preparation protocols and BEV characteristics (size, density, concentration, BEV-enriched and BEV-depleted molecules) while familiarizing them with experimental guidelines. This effort overall aims to raise awareness on the available guidelines and tools and steer an informed dialogue with BEV researchers to ensure their implementation, since transparency and reproducibility will be indispensable for the development and translation of BEV-based applications.
Methods
We conducted a systematic review of BEV literature using the EV-TRACK knowledgebase (https://www.evtrack.org/)19.
Data sources and search strategy
We searched the academic databases PubMed and EMBASE (see search strategies in Supplementary Methods). Publications were limited to those published between 2015 and 2021 and written in English. For PubMed, searches including both medical subject headings (MeSH) and ‘text words’ were used; the ‘English’ filter was not used a priori. For EMBASE, ‘English’ and ‘Article’ filters were used.
Eligibility criteria
Time period
English-written studies published from 1 January 2015 to 31 December 2021 were included for current analysis.
Study types
Studies reporting on BEVs after preparation from various sources were included. There were no exclusion criteria regarding the extent of the preparation protocol to minimize bias. Studies describing non-bacterial origin EVs and BEVs without prior preparation were excluded. Reviews, comments, editorials, conference abstracts and book chapters were excluded.
Screening, data extraction, and quality assessment
Study screening and selection
The selection process is illustrated in Supplementary Fig. 3. All publications captured by the search were collected in Rayyan. After deduplication, 2919 records were identified and screened by title and abstract by two reviewers (N.D.L. and S.V.D.) before full-text screening. In case of doubt, studies were included for full-text screening. Full-text screening was conducted independently by the individual submitting to EV-TRACK (N.D.L., S.V.D., N.G., A.V.C., M.B., S.C., J.M., P.V.V., M.V.H., and R.V.) and EV-TRACK administrator S. Deville to identify studies fulfilling the inclusion criteria. Full-text screening of studies already submitted to EV-TRACK by independent researchers (n = 18) was performed by N.D.L. When full-text articles could not be retrieved, authors were contacted to provide this. Failure to establish contact or to receive full-text in a timely manner resulted in exclusion. In total 845 publications were included.
Submission to EV-TRACK, data extraction, and quality assessment
All 845 studies were submitted to the EV-TRACK knowledgebase (https://www.evtrack.org/). Studies that included multiple sample types or preparation methods were divided into multiple entries, resulting in 3338 experiments. For each experiment a checklist of 233 parameters related to source, preparation protocol and characterization method was completed (Supplementary Data 2). For studies implementing and referring to the preparation protocol of other studies, this information was included in the checklist, if correctly cited. EV-TRACK submission was revised by N.D.L. or S.V.D. and by EV-TRACK administrator S. Deville. For studies that were already submitted to EV-TRACK by independent researchers (n = 18), data was revised by EV-TRACK administrator S. Deville. Data was extracted from the EV-TRACK knowledgebase EV-TRACK administrator S. Dedeyne. Additional data regarding BEV preparation or characterization not included in the EV-TRACK knowledgebase, was collected in a standardized manner in a spreadsheet matrix (Supplementary Data 4). For all bacterial species identified in the EV-TRACK knowledgebase, bacterial taxonomy was complemented (through taxonomy ID) according to the NCBI taxonomy database (Supplementary Data 3)34. Quality regarding EV preparation and characterization was assessed by the EV-METRIC score of the experiments within the individual studies19.
Data synthesis and analysis approach
Data were synthesized and analyzed by N.D.L. and S. Dedeyne using Microsoft Excel (version 16.88), Graphpad Prism (version 8.4.0), R (version 4.2.2), and R studio (version 2022.07.2) using following R packages: readxl (version 1.4.3), circlize (version 0.4.16), glue (version 1.7.0), stringr (version 1.5.1), fmsb (version 0.7.6), ggtree (version 3.3.3), pheatmap (version 1.0.12), taxonomizr (0.10.6), ggplot2 (version 3.5.1), ape (version 5.8), phytools (version 2.0-3), ggnewscale (version 0.4.10), ggtreeExtra (version 1.5.4), RColorBrewer (version 1.1-3), dplyr (version 1.1.4), ggpubr (version 0.6.0), gridExtra (2.3), grid (version 4.2.2), readr (version 2.1.5) and treemap (version 2.4-4). Statistical calculations were performed using Graphpad Prism (version 8.4.0). Two-sided Mann–Whitney U tests were performed to compare non‐normally distributed continuous variables. P values smaller than 0.05 were considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Data in text are represented by mean value (± standard error). Illustrations were made with Adobe Illustrator (version 28.5).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the article and its supplementary files and are freely available for download on https://www.evtrack.org/. Data can be searched per study (https://evtrack.org/search.php) or the complete dataset for all included studies can be requested from the EV-TRACK administration (evtracksubmissions@gmail.com). Any additional requests for information can be directed to, and will be fulfilled by, the corresponding author. Source data are provided with this paper.
References
Hendrix, A. & De Wever, O. Systemically circulating bacterial extracellular vesicles: origin, fate, and function. Trends Microbiol. 30, 213–216 (2022).
Hendrix, A. et al. Extracellular vesicle analysis. Nat. Rev. Methods Primers 3, 56 (2023).
Toyofuku, M., Nomura, N. & Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 17, 13–24 (2019).
Toyofuku, M., Schild, S., Kaparakis-Liaskos, M. & Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 21, 415–430 (2023).
Tulkens, J. et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 69, 191–193 (2020).
Tulkens, J., De Wever, O. & Hendrix, A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat. Protoc. 15, 40–67 (2020).
Deo, P. et al. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat. Microbiol. 5, 1418–1427 (2020).
Kaparakis-Liaskos, M. & Ferrero, R. L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 15, 375–387 (2015).
Kim, O. Y. et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat. Commun. 8, 626 (2017).
Bai, X., Findlow, J. & Borrow, R. Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert Opin. Biol. Ther. 11, 969–985 (2011).
Micoli, F. & MacLennan, C. A. Outer membrane vesicle vaccines. Semin Immunol. 50, 101433 (2020).
Wang, X., Thompson, C. D., Weidenmaier, C. & Lee, J. C. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 9, 1379 (2018).
Liu, H. et al. Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy. Theranostics 12, 6576–6594 (2022).
Liu, H. et al. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioactive Mater. 14, 169–181 (2022).
Biller, S. J. et al. Bacterial vesicles in marine ecosystems. Science 343, 183–186 (2014).
Ñahui Palomino, R. A. et al. Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV-1 infection of human tissues. Nat. Commun. 10, 5656 (2019).
Welsh, J. A. et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J. Extracell. Vesicles 13, e12404 (2024).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018). J. Extracell. Vesicles 7, 1535750 (2018).
Van Deun, J. et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 14, 228–232 (2017).
De Wever, O. & Hendrix, A. A supporting ecosystem to mature extracellular vesicles into clinical application. EMBO J. 38, e101412 (2019).
Arab, T. et al. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J. Extracell. Vesicles 10, e12079 (2021).
Ruiz, N. & Silhavy, T. J. How Escherichia coli became the flagship bacterium of molecular biology. J. Bacteriol. 204, e00230-22 (2022).
Connon, S. A. & Giovannoni, S. J. High-throughput methods for culturing microorganisms in very-low-nutrient media yield diverse new marine isolates. Appl. Environ. Microbiol. 68, 3878–3885 (2002).
Staley, J. T. & Konopka, A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu. Rev. Microbiol. 39, 321–346 (1985).
Jean-Christophe, L. et al. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin. Microbiol. Rev. 28, 237–264 (2015).
Epstein, S. S. The phenomenon of microbial uncultivability. Curr. Opin. Microbiol. 16, 636–642 (2013).
Gelibter, S. et al. The impact of storage on extracellular vesicles: a systematic study. J. Extracell. Vesicles 11, e12162 (2022).
Pathirana, R. D. & Kaparakis-Liaskos, M. Bacterial membrane vesicles: Biogenesis, immune regulation and pathogenesis. Cell. Microbiol. 18, 1518–1524 (2016).
Chronopoulos, A. & Kalluri, R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene 39, 6951–6960 (2020).
Shen, Y. et al. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12, 509–520 (2012).
Brown, L., Wolf, J. M., Prados-Rosales, R. & Casadevall, A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–630 (2015).
Ellis, T. N. & Kuehn, M. J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74, 81–94 (2010).
Schwechheimer, C. & Kuehn, M. J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619 (2015).
Schoch, C. L. et al. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database 2020, baaa062 (2020).
Acknowledgements
This work was supported by the European Research Council (ERC) for funding received under the European Union’s Horizon 2020 research and innovation program (Grant agreement No. 101045156) (A.H.), European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie (Grant agreement No. 722148) (Q.R.), Ghent University (A.H. and S. Deville) and Research Foundation Flanders (FWO, PhD fellowship) (S.V.D., N.G., and A.V.C.) and (FWO, SBO (grant agreement No. S000319N)) (N.D.L., A.H., and L.V.). We thank all members of the Laboratory of Experimental Cancer Research for discussion, and master students for assisting with EV-TRACK submissions: Margo Bell, Sam Calis, Jodi Moons, Phaedra Van de Vyver, Michiel Van Heusden, and Ruth Vanluchene.
Author information
Authors and Affiliations
Contributions
A.H. and S.V.D. designed the study. S.V.D. performed the database search. Titles and abstracts were screened by N.D.L. and S.V.D. Full-text screening, submission to the EV-TRACK knowledgebase (if the publication was not yet submitted by independent researchers), and collection of additional data not included in the EV-TRACK knowledgebase was performed by N.D.L., S.V.D., N.G., and A.V.C., revised by N.D.L. or S.V.D. and by EV-TRACK administrator S. Deville. Data was extracted from the EV-TRACK knowledgebase by EV-TRACK administrator S. Dedeyne. Data were synthesized and analyzed by N.D.L. and S. Dedeyne. The manuscript was written by N.D.L. and A.H. All authors (N.D.L., S.V.D., N.G., A.V.C., S. Dedeyne, S. Deville, Q.R., O.D.W., L.V., H.D., P.T., and A.H.) reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: N.D.L., S.V.D., N.G., A.V.C., Q.R., S. Dedeyne, S. Deville, O.D.W., and A.H. are affiliated to the Laboratory of Experimental Cancer Research, which coordinated the development of the EV-TRACK knowledgebase19. L.V., H.D., and P.T. declare no competing interests. All authors declare no other competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41467_2024_53279_MOESM7_ESM.xlsx
Supplementary Data 4: Additional experimental parameters: storage, PAMP characterization, 16S rRNA sequencing, bacterial contamination and study aim
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
De Langhe, N., Van Dorpe, S., Guilbert, N. et al. Mapping bacterial extracellular vesicle research: insights, best practices and knowledge gaps. Nat Commun 15, 9410 (2024). https://doi.org/10.1038/s41467-024-53279-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-53279-1
This article is cited by
-
Specific labeling of outer membrane vesicles with antibiotic-conjugated probe reveals early bacterial infections in blood
Nature Communications (2025)
-
Fostering the implementation of liquid biopsy in clinical practice: meeting report 2024 of the European Liquid Biopsy Society (ELBS)
Journal of Experimental & Clinical Cancer Research (2025)