Abstract
Cyclopropanes are not only privileged motifs in many natural products, agrochemicals, and pharmaceuticals, but also highly versatile intermediates in synthetic chemistry. As such, great effort has been devoted to the cyclopropane construction. However, novel catalytic methods for cyclopropanation with two abundant substrates, mild conditions, high functional group tolerance, and broad scope are still highly desirable. Herein, we report an intermolecular electrocatalytic cyclopropanation of alkenyl trifluoroborates with methylene compounds. The reaction uses simple diphenyl sulfide as the electrocatalyst under base-free conditions. And thus, a broad scope of various methylene compounds as well as vinyltrifluoroborates is demonstrated, including styrenyl, 1,3-dienyl, fluorosulfonyl, and base-sensitive substrates. Preliminary mechanistic studies are presented, revealing the critical role of the boryl substituent to facilitate the desired pathway and the role of water as the hydrogen atom source.
Similar content being viewed by others
Introduction
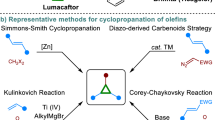
Cyclopropanes have received extensive attention interest as a result of their high ring strain and unique bonding pattern1,2. These strained cyclopropyl moieties constitute not only privileged motifs in natural products, agrochemicals, and pharmaceuticals (Fig. 1A)3,4,5,6,7,8, but also highly versatile intermediates in synthetic chemistry9,10,11,12,13,14. Owing to these pivotal roles, great effort has been devoted to the cyclopropane construction15,16,17,18,19. Conventionally, cyclopropanes are prepared via transition metal-catalyzed carbene insertion into alkenes20,21. This strategy typically relies on metal carbenoids derived from hazardous diazo compounds. As such, alternative carbene precursors have been developed, such as ylides, hydrazones, 1,2-diketones, gem-dihaloalkanes, and others22,23,24,25,26,27,28,29,30,31,32,33,34.
The non-carbenoid approach, such as Kulinkovich reaction35, Corey–Chaykovsky cyclopropanation36, represents an attractive alternative for the synthesis of cyclopropanes. In this context, only a limited number of novel C1 reagents have emerged to undergo [2 + 1] cyclopropanation of alkenes37,38,39,40,41,42,43,44. Among them, methylene compounds are the ideal C1 components for cyclopropyl ring construction, which are safe, abundant, and bench-stable. However, this ideal approach is still challenging. In 2022, Xu discovered an intramolecular electrocatalytic cyclopropanation of alkenes with active methylene compounds using a phenothiazine catalyst (Fig. 1B)45. However, the intermolecular cyclopropanation cannot achieve using this electrocatalytic system. Subsequently, an intermolecular cyclopropanation of alkenes with alkenyl thianthrenium salts in the presence of base was reported by Shu46 and Wickens47, independently (Fig. 1C). These thianthrenium salts have to be pre-made by thianthrenation of olefins using Ritter’s conditions48,49 or electrolysis50 in a divided cell. In 2023, Carreira and co-workers used α-bromo-β-ketoesters and α-bromomalonates as C1 reagents for the intermolecular photocatalytic cyclopropanation of alkenes with α-bromo-β-ketoesters51. Recently, Giri and co-workers disclosed an elegant intermolecular cyclopropanation of olefins with active methylene compounds bearing two electron-withdrawing groups enabled by photosensitized O2 (Fig. 1D)52. Despite these advances, novel catalytic methods for cyclopropanation with two abundant substrates, mild conditions, high functional group tolerance, and broad scope are still highly desirable.
Herein, we detail the discovery on the use of organic electrochemistry53,54,55,56,57,58 as a sustainable synthetic tool for the intermolecular cyclopropanation of alkenyl trifluoroborates with methylene compounds (Fig. 1D). Notably, this approach uses cheap diphenyl sulfide as the electrocatalyst under base-free conditions. In contrast to previous reports, this method allows for cyclopropanation of challenging substrates, such as styrenes, 1,3-dienes, base-sensitive substrates, and methylene compounds bearing a single electron-withdrawing group.
Results
Optimization of the reaction conditions
We chose two commercially available reagents, benzoylacetonitrile 1 and vinyltrifluoroborate 2, as the model substrates to investigate the electrochemical conditions. As illustrated in Table 1, we determined that 1 reacted efficiently with 2 when performed in an undivided cell in CH3CN/H2O (50/1) at room temperature using PhSPh as the catalyst, nBu4NPF6 (1.0 equiv) as the electrolyte, and C(+)/Pt(-) as the electrodes (entry 1). Other electrode materials also worked for this transformation giving cyclopropane 3 in diminished yields (entries 2–4). Notably, switching from Pt to Ni cathodes allowed for a 90% yield of product 3 to be obtained (entry 5). Other solvents such as CH3OH, DMF, DMSO, or CH2Cl2 failed to provide any desired product 3 (entry 6). Using nBu4NBF4 as the electrolyte instead resulted in a reduced yield (entry 7). The desired product 3 could be isolated in 76% yield in the absence of nBu4NPF6 (entry 8), indicating that the vinyltrifluoroborate salt 2 can act as the supporting electrolyte as well. In addition, the optimal loading of vinyltrifluoroborate 2 was determined to be 2.0 equiv (entries 9&10 vs entry 1), since the amounts of trifluoroborate salt could affect the pH of the reaction medium59 and thus further influence the reaction performance. While shorting the reaction time led to incomplete conversion (entry 11), extending the reaction time resulted in a reduced yield probably due to product decomposition (entry 12). Further optimizing the applied current proved that lower yields were observed with a reduced or increased current (entries 13&14). Finally, several control experiments revealed that water, PhSPh, and electricity were all crucial for the success of this cyclopropanation (entries 15–17).
Substrate scope
With the optimized conditions in hand, the scope of this electrochemical cyclopropanation was explored, as outline in Fig. 2. A variety of β‑ketonitriles readily underwent the desired cyclopropanation with vinyltrifluoroborate 2 (Fig. 2A). Pleasingly, aryl halides including para-fluorophenyl, para-chlorophenyl, ortho-bromophenyl were inert under these electrochemical oxidative conditions, providing the desired cyclopropanes 4–6 in good to high yields. β‑Ketonitriles bearing electron-deficient and electron-rich arenes showed similar reactivity, providing the desired products 7–12 in good to excellent yields. Sterically hindered ortho-substituted aryl β‑ketonitriles were less reactive, but 6 and 12 could still be obtained in 68% and 64% yield, respectively. Alkyne and alkene functional groups remained intact under the standard conditions to afford cyclopropanes 13–16 in reasonable yields. Notably, no intramolecular cyclopropanation was observed (15), indicating that the reaction may proceed in a different pathway from Xu’s previous report45. Aryl β‑ketonitriles bearing naphthyl ring, quinoline, pyridine, indole, thiophene, and furan were amenable, leading to the desired products 17–23. In addition, alkyl-substituted β‑ketonitriles also worked smoothly, affording cyclopropanes 24 and 25 in moderate yields.
A Scope of β‑ketonitriles. B Scope of methylene compounds with two EWGs. C Scope of methylene compounds with one EWG. D Scope of cyclic methylene compounds. E Scope of vinyl trifluoroborates. aPhSPh (0.2 mmol 1.0 equiv), 2.5 h, air. bPhSPh (0.2 mmol 1.0 equiv), 4.0 h, under N2. cPhSPh (0.3 mmol 1.5 equiv), 3.0 h, air.
Besides these β‑ketonitriles, the cyclopropanation reaction could be also extended to other active methylene compounds with two electron-withdrawing groups (Fig. 2B). To our delight, a variety of methylene compounds including cyanoacetates (26–28), cyanoacetamides (29 and 30), 2-sulfonyl acetonitriles (31), 2-tosyl acetates (32), bis(sulfonyl)methanes (33), β-ketosulfones (34), 1,3-diketones (35 and 36), β-ketoesters (37), malonates (38), β-ketoamides (39), α-nitroketones (40), and β-keto sulfonyl fluorides (41) reacted smoothly with vinyltrifluoroborate 2 to generate the expected cyclopropanes 26–41. Of note, highly base-sensitive benzyl chlorides (27) were well-tolerated. Moreover, olefins (28, 32), esters (37, 38), ketones (35, 36), amides (29, 30), nitro groups (40), and sulfonyl fluorides (41) survived under our conditions, allowing further diversification of these cyclopropane products via known protocols, such as sulfur(VI)−fluoride exchange (SuFEx) click chemistry60,61,62,63,64,65.
Subsequently, we evaluated the scope with respect to methylene compounds with only one electron-withdrawing group (Fig. 2C). Arylacetonitriles (42), arylacetones (43), and α‑aryl esters (44 and 45) could react successfully with 2. A pyridinyl-substituted acetonitrile was also successfully converted to the desired product 46 in 57% yield. Interestingly, phenylmethanesulfonyl fluoride (PMSF), a well-known serine protease inhibitor, was well-accommodated under the standard conditions to give cyclopropyl sulfonyl fluoride 47. The α‑aminoacetonitriles afforded the 3-membered adduct 48 in 59% yield. Notably, an amino acid Schiff base, which was shown to be unsuitable for previous method47, was also compatible under our electrochemical conditions, leading to cyclopropyl-substituted α‑amino acid derivative 49. Moreover, α-(N-pyrrolyl)ketones were suitable substrates, as illustrated by the isolation of cyclopropane product 50 in 67% yield.
Cyclic methylene compounds would be interesting substrates for our electrocatalytic cyclopropanation, as the resulting spirocyclic molecules may find applications in medicinal chemistry and agrochemical discovery. As shown in Fig. 2D, a variety of cycylic methylenes including 5,5-dimethylcyclohexane-1,3-dione, 1,3-indandione, 4-hydroxycoumarin, benzo[b]thiophen-3(2H)-one 1,1-dioxide, edaravone, 2,4-dihydro-3H-pyrazol-3-ones, 3-phenylisoxazol-5(4H)-one, 2-phenyloxazol-5(4H)-one, 2-coumaranone, 3-coumaranone, 1-indanone, and 2-oxindole were subjected to the electrochemical conditions and delivered the desired spirocyclopropanes 51–62 in moderate to good yields.
We then explored the scope of this electrocatalytic cyclopropanation with respect to alkenyltrifluoroborates (Fig. 2E). Various styrenyl trifluoroborates bearing either electron-donating (-Me) or electron-withdrawing (-Cl, -F, -CF3, -CO2Me) groups furnished the desired cyclopropanes 63–69 in reasonable yields. In addition, thiophene and pyridine motifs were also tolerated to afford the corresponding cycylopropanes 70 and 71. Notably, 1,3-dienyl trifluoroborates were also appropriate coupling partners to give 72 and 73. Alkyl-substituted vinyltrifluoroborates underwent the desired process to provide 74–78. It is worth highlighting that sterically challenging trisubstituted olefins were also applicable to this electrochemical cyclopropanation affording tetrasubstituted cyclopropane 76 and spirocyclopropane 77. Overall, synthetically useful yields could be obtained for most challenging substrates with a catalytic amount of PhSPh, the use of stoichiometric mediator is typically required to achieve better reaction performance.
Mechanistic studies
We envisioned two reaction pathways for the possible mechanism of this electrocatalytic cyclopropanation (Fig. 3A). The first commences by formation of the radical cation from the anodic oxidation of PhSPh (Fig. 3A, pathway A). This sulfide radical cation undergoes a single electron transfer with benzoylacetonitrile 1 to generate electrophilic C-radical I. Radical ipso-addition to vinyltrifluoroborate 2 forms radical intermediate II, which is known under photoredox conditions66. Under the electrochemical conditions, the intermediate II is then trapped by sulfide radical cation via a radical-radical coupling process. The resultant sulfonium intermediate III sets the stage for a subsequent nucleophilic substitution reaction. The intramolecular substitution delivers cyclopropyl trifluoroborate IV, which can be facilely converted to radical V through a one-electron oxidation process. After the C–B bond cleavage, a hydrogen atom transfer (HAT) from water to the alkyl radical V eventually furnishes the final product 3. An alternative reaction pathway involving the formation of a vinyl sulfonium salt intermediate must be considered (Fig. 3A, pathway B). Reaction of sulfide radical cation with vinyltrifluoroborate 2 generates a β-boron radical i, which undergoes a single electron oxidation and C–B bond cleavage to form vinyl sulfonium salt ii and BF366,67, The intermediate ii, a known Michael acceptor, reacts with nucleophile 1 to give sulfur ylide iii. Subsequently, a proton transfer from water produces sulfonium iv. Cyclization of intermediate iv eventually delivers the desired cyclopropane 3.
In this regard, a mixture of alkene-tethered substrate 79 and trifluoroborate 2 was subjected to the electrolysis conditions to trap a putative intermediate 80 on the pathway A (Fig. 3B). No intramolecular cyclopropanation was observed and 15 was obtained instead, indicating the carbon radical intermediate 80 was not formed. A radical clock reaction using cyclopropylethenyl trifluoroborate 81 as radical acceptor further confirmed α-carbon radical I could not be generated from benzoylacetonitrile 1, as no ring-opened product was detected (Fig. 3C). Cyclic voltammetry (CV) experiments displayed no oxidation peak of benzoylacetonitrile 1 at 0–3.0 V (Fig. 3D), ensuring that benzoylacetonitrile 1 could not be oxidized under the standard conditions. Taken together, the pathway A, proceeding via oxidation of benzoylacetonitrile 1 and subsequent radical addition with α-carbon radical I, is unlikely.
On the other hand, pathway B accounts for these experimental studies. CV studies revealed the addition of 2 to the PhSPh solution resulted in the disappearance of the oxidation peak of PhSPh (Fig. 3D), suggesting that the electrochemical generated [PhSPh]•+ was trapped by vinyltrifluoroborate 2. Furthermore, the key intermediate, diphenyl vinyl sulfonium salt 82, was successfully isolated in 45% yield, when the counter anion was switched from PF6– to TfO– (Fig. 3E, eq 1). When this intermediate 82 was subjected to the electrolysis conditions or stoichiometric Cs2CO3, the desired product 3 was isolated in 27% or 90% yield, respectively (Fig. 3E, eq 2). When D2O was used in place of H2O, the deuterated product 52-D was isolated in 57% yield with 96% deuterium incorporation from the cyclopropanation of 1,3-indandione 83 (Fig. 3F, eq 1). In a parallel experiment using CD3CN instead of CH3CN, product 52 was obtained in 60% yield without deuterium incorporation (Fig. 3F, eq 1). When we subjected deuterated 1,3-indandione 83-D to the cyclopropanation conditions, no deuterium was detected in the product 52 (Fig. 3F, eq 2). These outcomes support that water is involved in the reaction process as the terminal hydrogen atom source. Whereas HAT from water is challenging due to the high bond dissociation energy68, deprotonation of water can be feasible with the intermediate iii. These mechanistic studies strongly supported that the electrochemical cyclopropanation followed reaction pathway B, as illustrated in Fig. 3A.
In conclusion, we have developed an electrochemical cyclopropanation of alkenyl trifluoroborates with methylene compounds. This transformation utilizes simple diphenyl sulfide as the electrocatalyst under base-free conditions, and thus shows broad scope with various methylene compounds as well as substituted vinyltrifluoroborates. Notably, challenging substrates, such as styrenyl, 1,3-dienyl, fluorosulfonyl, and base-sensitive substrates, have been successfully employed in this approach.
Methods
General procedure for the cyclopropanation
General procedure A
A 10 mL Schlenk tube equipped with a magnetic stir bar was charged with the methylene substrate (0.2 mmol, 1.0 equiv.), potassium alkenyl trifluoroborate (0.4 mmol, 2 equiv), PhSPh (0.04 mmol, 20% mmol) and nBu4NPF6 (0.2 mmol, 1 equiv.). The Schlenk tube was equipped with graphite plate (10 mm × 10 mm × 2 mm) as the anode and Pt plate (10 mm × 10 mm × 0.1 mm) as the cathode. CH3CN (5.0 mL) and H2O (0.1 mL) were added. The mixture was electrolyzed at a constant current of 8 mA at room temperature for 2.5 h. The solvent was then removed under vacuum to give a residue, which was purified by flash column chromatography on silica gel (eluent, petroleum ether/ethyl acetate = 50:1 − 4:1 V/V) to afford desired products 3–12, 17–18, 21–27, 30–31, 33–35, 40, 43, 51–52, 54, 74.
General Procedure B
A 10 mL Schlenk tube equipped with a magnetic stir bar was charged with the methylene substrate (0.2 mmol, 1.0 equiv.), potassium alkenyl trifluoroborate (0.4 mmol, 2 equiv), PhSPh (0.2 mmol, 100% mmol) and nBu4NPF6 (0.2 mmol, 1 equiv.). The Schlenk tube was equipped with graphite plate (10 mm × 10 mm × 2 mm) as the anode and Pt plate (10 mm × 10 mm × 0.1 mm) as the cathode. CH3CN (5.0 mL) and H2O (0.1 mL) were added. The mixture was electrolyzed at a constant current of 8 mA at room temperature for 2.5 h. The solvent was then removed under vacuum to give a residue, which was purified by flash column chromatography on silica gel (eluent, petroleum ether/ethyl acetate = 50:1 − 4:1 V/V) to afford desired products 13–16, 19–20, 29, 32, 37–39, 41, 46, 50, 53, 57–58, 60, 64–69, 71, 73–75, 77–78.
General procedure C
A 10 mL Schlenk tube equipped with a magnetic stir bar was charged with the methylene substrate (0.2 mmol, 1.0 equiv.), potassium alkenyl trifluoroborate (0.4 mmol, 2 equiv), PhSPh (0.3 mmol, 150% mmol) and nBu4NPF6 (0.2 mmol, 1 equiv.). The Schlenk tube was equipped with graphite plate (10 mm × 10 mm × 2 mm) as the anode and Pt plate (10 mm × 10 mm × 0.1 mm) as the cathode. CH3CN (5.0 mL) and H2O (0.1 mL) were added. The mixture was electrolyzed at a constant current of 8 mA at room temperature for 3 h. The solvent was then removed under vacuum to give a residue, which was purified by flash column chromatography on silica gel (eluent, petroleum ether/ethyl acetate = 50:1 − 4:1 V/V) to afford desired products 28, 32, 36, 41–42, 44–45, 47–49, 53, 56, 59, 61–63, 70, 72, 76.
Data availability
The data supporting the findings of this study, including materials and methods, optimization studies, experimental procedures, mechanistic studies, compound characterization, and NMR, are available within the article and its Supplementary Information files. All data are available from the corresponding author upon request
References
de Meijere, A. Bonding properties of cyclopropane and their chemical consequences. Angew. Chem. Int. Ed. 18, 809–826 (2003).
Talele, T. T. The “Cyclopropyl fragment” is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem. 59, 8712–8756 (2016).
Pietruszka, J. Synthesis and properties of oligocyclopropyl-containing natural products and model compounds. Chem. Rev. 103, 1051–1070 (2003).
Wessjohann, L. A., Brandt, W. & Thiemann, T. Biosynthesis and metabolism of cyclopropane rings in natural compounds. Chem. Rev. 103, 1625–1648 (2003).
Brackmann, F. & de Meijere, A. Natural occurrence, syntheses, and applications of cyclopropyl-group-containing alpha-amino acids. 1. 1-aminocyclopropanecarboxylic acid and other 2,3-methanoamino acids. Chem. Rev. 107, 4493–4537 (2007).
Fan, Y.-Y., Gao, X.-H. & Yue, J.-M. Attractive natural products with strained cyclopropane and/or cyclobutane ring systems. Sci. China Chem. 59, 1126–1141 (2016).
Mizuno, A., Matsui, K. & Shuto, S. From peptides to peptidomimetics: a strategy based on the structural features of cyclopropane. Chem. Eur. J. 23, 14394–14409 (2017).
Ma, S., Mandalapu, D., Wang, S. & Zhang, Q. Biosynthesis of cyclopropane in natural products. Nat. Prod. Rep. 39, 926–945 (2022).
Schneider, T. F., Kaschel, J. & Werz, D. B. A new golden age for donor-acceptor cyclopropanes. Angew. Chem. Int. Ed. 53, 5504–5523 (2014).
Ivanova, O. A. & Trushkov, I. V. Donor-acceptor cyclopropanes in the synthesis of carbocycles. Chem. Rec. 19, 2189–2208 (2019).
Singh, P., Varshnaya, R. K., Dey, R. & Banerjee, P. Donor–acceptor cyclopropanes as an expedient building block towards the construction of nitrogen‐containing molecules: an update. Adv. Synth. Catal. 362, 1447–1484 (2020).
Pirenne, V., Muriel, B. & Waser, J. Catalytic enantioselective ring-opening reactions of cyclopropanes. Chem. Rev. 121, 227–263 (2021).
Xia, Y., Liu, X. & Feng, X. Asymmetric catalytic reactions of donor-acceptor cyclopropanes. Angew. Chem. Int. Ed. 60, 9192–9204 (2021).
Gabbey, A. L., Scotchburn, K. & Rousseaux, S. A. L. Metal-catalysed C-C bond formation at cyclopropanes. Nat. Rev. Chem. 7, 548–560 (2023).
Chen, D. Y., Pouwer, R. H. & Richard, J. A. Recent advances in the total synthesis of cyclopropane-containing natural products. Chem. Soc. Rev. 41, 4631–4642 (2012).
Ebner, C. & Carreira, E. M. Cyclopropanation strategies in recent total syntheses. Chem. Rev. 117, 11651–11679 (2017).
Dian, L. & Marek, I. Asymmetric preparation of polysubstituted cyclopropanes based on direct functionalization of achiral three-membered carbocycles. Chem. Rev. 118, 8415–8434 (2018).
Jin, W. B., Yuan, H. & Tang, G. L. Strategies for construction of cyclopropanes in natural products. Chin. J. Org. Chem. 38, 2324–2334 (2018).
Časar, Z. Synthetic approaches to contemporary drugs that contain the cyclopropyl moiety. Synthesis 52, 1315–1345 (2020).
Maas, G. Ruthenium-catalysed carbenoid cyclopropanation reactions with diazo compounds. Chem. Soc. Rev. 33, 183–190 (2004).
Ford, A. et al. Modern organic synthesis with alpha-diazocarbonyl compounds. Chem. Rev. 115, 9981–10080 (2015).
Zhang, L. A non-diazo approach to alpha-oxo gold carbenes via gold-catalyzed alkyne oxidation. Acc. Chem. Res. 47, 877–888 (2014).
Jia, M. & Ma, S. New approaches to the synthesis of metal carbenes. Angew. Chem. Int. Ed. 55, 9134–9166 (2016).
Ye, L. W. et al. Nitrene transfer and carbene transfer in gold catalysis. Chem. Rev. 121, 9039–9112 (2021).
Zhu, D., Chen, L., Fan, H., Yao, Q. & Zhu, S. Recent progress on donor and donor-donor carbenes. Chem. Soc. Rev. 49, 908–950 (2020).
Cao, L. Y. et al. Molybdenum-catalyzed deoxygenative cyclopropanation of 1,2-dicarbonyl or monocarbonyl compounds. Angew. Chem. Int. Ed. 60, 15254–15259 (2021).
Ni, J., Xia, X., Zheng, W. F. & Wang, Z. Ti-Catalyzed diastereoselective cyclopropanation of carboxylic derivatives with terminal olefins. J. Am. Chem. Soc. 144, 7889–7900 (2022).
Berger, K. E., Martinez, R. J., Zhou, J. & Uyeda, C. Catalytic asymmetric cyclopropanations with nonstabilized carbenes. J. Am. Chem. Soc. 145, 9441–9447 (2023).
Cai, B. G., Empel, C., Jana, S., Xuan, J. & Koenigs, R. M. Catalytic olefin cyclopropanation with In situ-generated dialkyl diazomethanes via Co(II)-based netalloradical catalysis. Acs. Catal. 13, 11851–11856 (2023).
Liu, H. L., Wang, X., Gao, K. & Wang, Z. Catalytic diastereo- and enantioselective cyclopropanation of gem-dihaloalkanes and terminal olefins. Angew. Chem. Int. Ed. 62, e202305987 (2023).
Liu, M., Le, N. & Uyeda, C. Nucleophilic carbenes derived from dichloromethane. Angew. Chem. Int. Ed. 62, e202308913 (2023).
Wang, C., Wu, R., Chen, K. & Zhu, S. Enantioselective synthesis of biscyclopropanes using alkynes as dicarbene equivalents. Angew. Chem. Int. Ed. 62, e202305864 (2023).
Chen, Z. L., Xie, Y. & Xuan, J. Visible Light‐Mediated Cyclopropanation: Recent Progress. Eur. J. Org. Chem. 2022, e202201066 (2022).
Kelly, C. B. et al. Modern Cyclopropanation via Non‐Traditional Building Blocks. ChemCatChem 16, e202400110 (2024).
Kulinkovich, O. G. S. S. V., Vasilevskii, D. A. & Pritytskaya, T. S. Reaction of ethylmagnesium bromide with esters of carboxylic acids in the presence of tetraisopropoxytitanium. Zh. Org. Khim. 25, 2244–2245 (1989).
Corey, E. J. & Chaykovsky, M. Dimethylsulfoxonium methylide. J. Am. Chem. Soc. 84, 867–868 (1962).
Phelan, J. P. et al. Redox-neutral photocatalytic cyclopropanation via radical/polar crossover. J. Am. Chem. Soc. 140, 8037–8047 (2018).
Sayes, M., Benoit, G. & Charette, A. B. Borocyclopropanation of styrenes mediated by UV-light under continuous flow conditions. Angew. Chem. Int. Ed. 57, 13514–13518 (2018).
Shu, C., Mega, R. S., Andreassen, B. J., Noble, A. & Aggarwal, V. K. Synthesis of functionalized cyclopropanes from carboxylic acids by a radical addition-polar cyclization cascade. Angew. Chem. Int. Ed. 57, 15430–15434 (2018).
Bunyamin, A., Hua, C., Polyzos, A. & Priebbenow, D. L. Intramolecular photochemical [2 + 1]-cycloadditions of nucleophilic siloxy carbenes. Chem. Sci. 13, 3273–3280 (2022).
Zhang, X. & Cheng, X. Electrochemical reductive functionalization of alkenes with deuterochloroform as a one-carbon deuteration block. Org. Lett. 24, 8645–8650 (2022).
Hu, J. et al. Photocatalyzed borylcyclopropanation of alkenes with a (diborylmethyl)iodide reagent. Angew. Chem. Int. Ed. 62, e202305175 (2023).
Huai, L., Zhang, L., Wang, Z., Wu, H. & Fang, Y. Cyclopropanation of N-vinylimidesviaa redox-neutral photocatalysed radical addition/anionic cyclisation process. Org. Chem. Front. 10, 1245–1251 (2023).
Xia, D. D. et al. Visible-light-mediated energy transfer enables cyclopropanes bearing contiguous all-carbon quaternary centers. Acs. Catal. 13, 9806–9816 (2023).
Jie, L. H., Guo, B., Song, J. & Xu, H. C. Organoelectrocatalysis enables direct cyclopropanation of methylene compounds. J. Am. Chem. Soc. 144, 2343–2350 (2022).
Liu, M. S., Du, H. W., Cui, J. F. & Shu, W. Intermolecular metal-free cyclopropanation and aziridination of alkenes with XH2 (X=N, C) by thianthrenation. Angew. Chem. Int. Ed. 61, e202209929 (2022).
Kim, M. J. et al. Diastereoselective synthesis of cyclopropanes from carbon pronucleophiles and alkenes. Angew. Chem. Int. Ed. 62, e202303032 (2023).
Berger, F. et al. Site-selective and versatile aromatic C-H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Chen, J., Li, J., Plutschack, M. B., Berger, F. & Ritter, T. Regio, Stereoselective thianthrenation of olefins to access versatile alkenyl electrophiles. Angew. Chem. Int. Ed. 59, 5616–5620 (2020).
Holst, D. E., Wang, D. J., Kim, M. J., Guzei, I. A. & Wickens, Z. K. Aziridine synthesis by coupling amines and alkenes via an electrogenerated dication. Nature 596, 74–79 (2021).
Fischer, D. M., Lindner, H., Amberg, W. M. & Carreira, E. M. Intermolecular organophotocatalytic cyclopropanation of unactivated olefins. J. Am. Chem. Soc. 145, 774–780 (2023).
Poudel, D. P., Pokhrel, A., Tak, R. K., Shankar, M. & Giri, R. Photosensitized O2 enables intermolecular alkene cyclopropanation by active methylene compounds. Science 381, 545–553 (2023).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 57, 5594–5619 (2018).
Little, R. D. A perspective on organic electrochemistry. J. Org. Chem. 85, 13375–13390 (2020).
Liu, J., Lu, L., Wood, D. & Lin, S. New redox strategies in organic synthesis by means of electrochemistry and photochemistry. Acs. Cent. Sci. 6, 1317–1340 (2020).
Zhu, C., Ang, N. W. J., Meyer, T. H., Qiu, Y. & Ackermann, L. Organic electrochemistry: molecular syntheses with potential. Acs. Cent. Sci. 7, 415–431 (2021).
Cheng, X. et al. Recent applications of homogeneous catalysis in electrochemical organic synthesis. Ccs. Chem. 4, 1120–1152 (2022).
Christian, A. & Wamser Equilibria in the System Boron Trifluoride—Water at 25°. J. Am. Chem. Soc. 73, 409–416 (1951).
Dong, J., Krasnova, L., Finn, M. G. & Sharpless, K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 53, 9430–9448 (2014).
Xu, L. & Dong, J. Click chemistry: evolving on the fringe. Chin. J. Chem. 38, 414–419 (2020).
Lou, T. S.-B. & Willis, M. C. Sulfonyl fluorides as targets and substrates in the development of new synthetic methods. Nat. Rev. Chem. 6, 146–162 (2022).
Zeng, D., Deng, W.-P. & Jiang, X. Advances in the construction of diverse SuFEx linkers. Natl Sci. Rev. 10, nwad123 (2023).
Homer, J. A. et al. Sulfur fluoride exchange. Nat. Rev. Method. Prim. 3, 58 (2023).
Zheng, Y., Lu, W., Ma, T. & Huang, S. Recent advances in photochemical and electrochemical strategies for the synthesis of sulfonyl fluorides. Org. Chem. Front. 11, 217–235 (2024).
Fernandez Reina, D. et al. Visible-light-mediated reactions of electrophilic radicals with vinyl and allyl trifluoroborates. Acs. Catal. 7, 4126–4130 (2017).
Kim, H. & MacMillan, D. W. C. Enantioselective organo-SOMO catalysis: the alpha-vinylation of aldehydes. J. Am. Chem. Soc. 130, 398–399 (2008).
Warren, J. J., Tronic, T. A. & Mayer, J. M. Thermochemistry of Proton-Coupled Electron Transfer Reagents and its Implications. Chem. Rev. 110, 6961–7001 (2010).
Acknowledgements
Financial support from the National Natural Science Foundation of China (32171724, S.H.) is gratefully acknowledged. The authors thank the Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Hunan Normal University).
Author information
Authors and Affiliations
Contributions
S.H. designed and directed the project. W.Y., P.-C.X., T.H., and S.S. performed the experiments and analyzed the data. S.H. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xu Cheng, Linbao Zhang and Yunfei Zhang for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yi, W., Xu, PC., He, T. et al. Organoelectrocatalytic cyclopropanation of alkenyl trifluoroborates with methylene compounds. Nat Commun 15, 9645 (2024). https://doi.org/10.1038/s41467-024-54082-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-54082-8