Abstract
Gasdermin E (GSDME) is a pyroptotic cell death effector and a promising target for pyroptotic tissue injury. Here we perform high-throughput screening and demonstrate that methylcobalamin (MeCbl), an endogenous coenzyme form of vitamin B12, is a specific GSDME inhibitor and highly effective against cholestatic liver failure. MeCbl specifically blocks GSDME cleavage by directly binding with GSDME. In cholestasis-, cisplatin- or concanavalin A (Con A)-induced male mouse models, MeCbl significantly suppresses liver transaminase activities and inflammation, alleviates hepatocyte death, and reduces mortality of mice by blocking GSDME cleavage. The conserved Cys180 residue in GSDME is essential for caspase-3/GzmB recognition. MeCbl in base-off conformation coordinates to Cys180 to prevent caspase-3/GzmB-GSDME interactions and thereby GSDME-mediated pyroptosis. In summary, our study discovers MeCbl as a specific GSDME inhibitor that is promisingly to be developed as an effective drug against cholestatic liver failure, and other GSDME triggered sterile inflammation and/or organ failure.
Similar content being viewed by others
Introduction
Programmed cell death mediated by dedicated molecular pathways is essential for organismal host defense and diseases1,2. Accumulating evidence support that pyroptosis is a vital pathological process in both infectious and sterile diseases3. Pyroptotic dying cells appear to flatten and form large ballooning bubbles, leading to the release of inflammatory cytokines and cellular alarmins4. Mechanistically, the inflammatory human caspase-1/4/5 and mouse caspase-1/11 as well as caspase-3, serine protease, mediate proteolytic cleavage of corresponding gasdermins (GSDMs) and liberate N-terminal (NT) fragments binding to phospholipids in inner plasma membrane to form pores, enabling the release of pro-inflammatory cytokines and plasma membrane rupture5,6,7,8. Pyroptosis mediated by gasdermin D (GSDMD) occurs predominantly in monocytes, macrophages, and dendritic cells stimulated by various pathogen-associated molecular patterns (PAMPs). Another pivotal pyroptotic executor GSDME is universally expressed in diverse tissues/organs, and plays crucial roles in both infectious and sterile diseases. Chemotherapy drugs or enterovirus infection can activate caspase-3-GSDME pathway, thereby initiating intestinal or other organ injuries9,10. In addition, granzyme B (GzmB), released from infiltrated cytotoxic lymphocytes, can also cleave GSDME directly, or activate caspase-3-dependent GSDME cleavage to induce autoimmune diseases11, which are also characterized by irreversible tissue damages. All of these emerging evidences indicate that GSDME may represent a key executor in mediating pyroptotic organ damage.
Liver failure, induced by various pathological factors, is observed in up to 20% of patients in intensive care unit (ICU)12. Since this disease lacks efficient therapeutic option except for liver transplantation, patients with liver failure are characterized by poor prognosis and high mortality. Viral infection, cholestatic dysfunction, and drug-induced liver injury (DILI) represent by far the most frequent forms of hepatic injury in secondary or acquired liver failure, which are strong predictors of an unfavorable outcome and prolonged hospitalization13. Apoptotic, necrotic, necroptotic, and ferroptotic cell death have been proposed to underscore various kinds of pathological factors-initiated liver damage14,15. However, therapeutic approaches to exploiting these mechanisms are far from success to protect against liver failure. Our previous findings revealed that under cholesteric conditions, the accumulated high levels of bile acids can promote the assembly of Apaf-1/caspase-4 pyroptosome, activating caspase-3-GSDME-executed pyroptosis and thereby cholestatic liver failure16. Markedly, cholestasis-induced mortality was completely rescued in Gsdme–/– mice, supporting that GSDME-mediated pyroptosis is likely a dominant type of cell death causing liver failure. It is thus reasonable to propose GSDME can be a crucial target against liver damage.
Previous studies have shown that dimethyl fumarate (DMF) can block GSDME-mediated pyroptosis17. However, DMF is not a specific GSDME inhibitor since it can also inhibit GSDMD and cysteine-dependent proteases. There is no evidence that DMF can be effective against liver failure, and in particular, it has a narrow therapeutic window and thus might not expected to be used in conditions of liver failure. Unlike GSDMD, the crystal structure of GSDME has still not been verified and thus it is hard to perform a structure-guided drug design for GSDME. Since the host-derived damage-associated molecular patterns (DAMPs), like bile acids, can directly engage GSDME to trigger pyroptosis, we reasoned that there might have certain endogenous metabolites capable of inhibiting GSDME activation. To this end, we intended to perform a phenotypic screen-based discovery of GSDME inhibitors from an in-house developed bioactive metabolites library containing 829 metabolites with relatively high abundance in human beings.
In this work, we identified methylcobalamin (MeCbl), known as a coenzyme form of vitamin B12, as a specific and effective inhibitor of GSDME cleavage. Mechanistically, MeCbl in base-off conformation coordinates to Cys180 site in GSDME and thereby prevents its recognition and cleavage by caspase-3 or GzmB. More importantly, MeCbl shows powerful effects in combating cholestatic liver failure dependent of GSDME. Considering that MeCbl has been widely used for decades in the clinic to treat hyperhomocysteinaemia and peripheral neuropathy18, our results support the application of MeCbl for the therapy of cholestatic liver failure, characterized by high mortality without efficient therapeutics.
Results
Methylcobalamin is a specific and direct inhibitor of GSDME
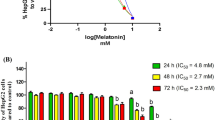
Our previous research found that bile acids as DAMPs can directly engage to trigger pyroptotic cell death of hepatocytes16,19. To identify possible endogenously derived GSDME inhibitors, we devised a liposome leakage assay, which detects the leakage of terbium (Tb3+) from liposomes incubated with recombinant full-length GSDME and active caspase-3, to detect the pore-formation activity of GSDME N-terminal domain (GSDME-NT) (Fig. 1a and Supplementary Table. 1). Dimethyl fumarate (DMF) was used as a positive control since it had been previously validated as a dual inhibitor of GSDMD and GSDME17. We screened 829 compounds from an in-house developed Human Metabolites Library to identify inhibitors that can inhibit liposome leakage by at least 70%. After excluding nonspecific and cytotoxic compounds, 9 hits were initially identified (Supplementary Table. 2) and then subjected to cytotoxicity assay via detecting lactic dehydrogenase (LDH) release in cell supernatant (Fig. 1b, blue dots). All nine compounds barely induced LDH release, indicating these hits had negligible cytotoxicity (Supplementary Fig. 1a). To exclude non-specific hits, we further validated these hits in TNFα/cycloheximide (CHX)-induced HepG2 liver cell pyroptosis model, which was mediated by caspase-3-GSDME pathway20. The strongest inhibitor is code.19A11, which is known as methylcobalamin (MeCbl), a coenzyme form of vitamin B12 widely used to treat hyperhomocysteinaemia and peripheral neuropathy (Fig. 1c, d). Code.18C6 (Melanin) and 20F3 (Myricetin) also inhibited GSDME mediated pyroptosis; however, previous studies showed that both melanin and myricetin directly inhibit caspase-3 activity and thus unlikely representing specific GSDME inhibitors21,22. We thus excluded these two non-specific hits from further research. Of interest, it has been recently found a ferroptosis suppressor protein 1 (FSP1)-dependent non-canonical vitamin K cycle that could potently suppress ferroptosis23, highlighting the potential roles of various vitamins in regulating cell death. MeCbl at even high concentrations up to 100 μM had no inhibitory effect against recombinant caspase-3 activity (Fig. 1e). MeCbl specifically bound to GSDME but not to caspase-3 or GSDMD, which was assessed by microscale thermophoresis (MST) (Fig. 1f). These results support that MeCbl specifically targets GSDME but not upstream caspase or other GSDMs.
a Procedure of terbium (Tb3+)/dipicolinic acid (DPA) fluorescence liposome leakage assay. b Percentage of inhibition on 829 compounds, assayed at 100 μM. Cutoff was 70% inhibition. Dimethyl fumarate (DMF) was used as a positive control. Each point is an individual inhibitor (n = 1). c LDH release assay for cytotoxicity of HepG2 cells pretreated with 9 screening hits at 100 μM before challenged with TNFα/cycloheximide (CHX) for 16 h to induce GSDME-mediated pyroptosis. d Chemical structure of compound 19A11 (methylcobalamin, MeCbl). e 25 ~ 100 μM MeCbl or DMF were treated with 0.2 μM recombinant active-caspase-3 for activity assay. Z-DEVD-FMK was used as positive control. f MST measurements of the binding of 0.2 μM recombinant caspase-3, GSDME, GSDMD with MeCbl or positive control. Values of Kd are listed in the graphs. Data are mean ± SEM (n = 3) of at least two independent experiments for bar charts. Analysis was done using one-way ANOVA (c, e). p < 0.05 is considered significant and p values are provided in graphs, n.s = nonsignificant. All groups were compared to indicated vehicle. Source data are provided as Source Data file.
GSDME has been reported to be cleaved at site 267DMPD270 in humans and 267DMLD270 in mouse by caspase-39. After cleavage, GSDME-NT binds to plasma membrane phospholipids and oligomerizes to form membrane pores that induce pyroptosis. Therefore, inhibition of GSDME cleavage or/and its pore formation activity can potently inhibit liposome leakage and pyroptosis. To uncover the exact mechanism of MeCbl, we first examined liposome leakage by pretreating the full-length GSDME with indicated concentrations of each compound before adding active-caspase-3 and liposome. MeCbl inhibited liposome leakage with an IC50 of 0.47 μM that was lower than the positive control DMF with an IC50 of 0.826 μM (Fig. 2a). DMF but not MeCbl inhibited liposome leakage induced by cleaved GSDMD (Fig. 2b), which was in line with the results from the binding assay (Fig. 1f). Next, we performed a time course assay of liposome leakage and further confirmed that MeCbl (1 μM) pretreatment sustainably inhibited liposome leakage (Fig. 2c), as well as maintained the membrane integrity and diameter length of liposome evidenced from negative staining electron microscopy (Fig. 2d, e). Immunoblotting analysis showed GSDME cleavage by caspase-3 was potently disrupted by MeCbl (Fig. 2f). We further asked whether MeCbl can also impair pore formation activity of GSDME-NT and tested the direct liposome leakage by precleaved GSDME. It was found that MeCbl could not inhibit the pore formation induced directly by precleaved GSDME, suggesting inability of MeCbl to inhibit GSDME-NT pore-formation activity (Fig. 2g). Granzyme B (GzmB) also induces caspase-3-independent pyroptosis by directly cleaving GSDME at the same sites as caspase-36. We found that MeCbl was able to inhibit liposome leakage by blocking GzmB-cleaved GSDME, and MeCbl had no direct effect on GzmB activity (Fig. 2h–j), further supporting that MeCbl directly targets GSDME but not upstream events.
a, b Dose response of MeCbl or DMF on liposome leakage induced by cleaved human GSDME (a) or human GSDMD (b). c, Time course of liposome leakage in the presence of 1 μM MeCbl or DMF. d, e Electron microscopy images of nanodiscs that were pretreated with inhibitors as indicated, recombinant active caspase-3 and GSDME were then added for pore formation (d). Scale bar= 200 nm. Quantitative data of the long diameter of 10 liposomes of each group (e), each point is an individual liposome. f Representative immunoblot of GSDME pretreated with inhibitors as indicated, and then incubated with recombinant active caspase-3 for GSDME activation analysis. g Dose response of MeCbl on liposome leakage induced by precleaved human GSDME. h–j Dose response of MeCbl on liposome leakage induced by human GSDME-NT cleaved by granzyme B (GzmB) (h), representative immunoblot of recombinant GSDME pretreated with inhibitors and then incubated with recombinant active GzmB (i), 25 ~ 100 μM MeCbl was pretreated with 0.4 μM recombinant active GzmB for activity assay. 3,4-dichloroisocoumarin (DCI) was used as positive control (j). Data are mean ± SEM of at least two independent experiments for bar and line charts, n = 3 other than quantitative data of the long diameter of liposomes (e, n = 10). Blots and micrographs are representative of three independent experiments. Analysis was done using one-way ANOVA (e, j) or two-way ANOVA (a–c). p < 0.05 is considered significant to indicated group and p values are provided in graphs, n.s = nonsignificant compared to vehicle. Source data are provided as Source Data file.
Methylcobalamin inhibits GSDME-mediated pyroptosis
Our results indicate that MeCbl is a direct GSDME inhibitor. We thus examined whether and how it prevents GSDME-mediated pyroptosis. To this end, we pretreated human liver cell line HepG2 with indicated compounds before activating caspase-3-GSDME pathway with 200 μM deoxycholic acid (DCA), according to our previous findings of that DCA can initiate caspase-3-GSDME-executed pyroptosis via promoting the assembly of Apaf-1/caspase-4 pyroptosome16. MeCbl drastically attenuated DCA-induced cytotoxicity (LDH release assay) in a dose-dependent manner with an IC50 of 2.25 μM, significantly lower than that of DMF (14.88 μM) (Fig. 3a). Unlike DMF, GSDMD-dependent cytotoxicity was not apparently influenced by MeCbl (Fig. 3b), suggesting MeCbl specifically targets GSDME but not GSDMD. To determine the exact mechanism by which MeCbl inhibits GSDME-mediated pyroptosis, we employed several methodologies. Pyroptotic membrane permeabilization and exposed cell nucleus were assessed by the uptake of membrane-impermeable dye SYTOX green; GSDME-NT-mediated pore formation was tracked by immunofluorescence microscopy using a monoclonal antibody that recognizes both full-length and GSDME-NT; Characteristic pyroptotic bubbles were captured by microscope in DIC mode. MeCbl sustainably maintained HepG2 cell membrane integrity by directly inhibiting GSDME cleavage (Fig. 3c, d), without affecting caspase-3 activity (Fig. 3e). Both MeCbl and DMF completely blocked the appearance of pyroptotic bubbles (red arrow) (Fig. 3f) and GSDME-NT membrane staining (white arrow) (Fig. 3g). MeCbl was also capable of inhibiting perforin (PFR) plus GzmB-induced mouse hepatocyte pyroptosis (Fig. 3h–j), as well as blocking HepG2 pyroptosis induced by chemotherapy drug TNFα/CHX and cisplatin (DDP) by blocking GSDME cleavage (Supplementary Fig. 1b–k), further supporting that the direct interaction of MeCbl with GSDME prevents its cleavage of the latter and represents the primary mechanism of MeCbl in inhibiting pyroptosis. Since caspase-3 activation may also induce apoptotic cell death, we sought to determine whether MeCbl inhibits apoptosis. To this end, human embryonic kidney 293 T (HEK293T) without inherent GSDME expression transfected with GSDME or an empty vector were used. Flow cytometric analysis revealed that both MeCbl and DMF significantly decreased the percentage of 7-AAD positive cells stimulated with DDP, without affecting those of Annexin-V-positive apoptotic cells (Supplementary Fig. 1l–n). In addition, MeCbl and DMF reversed pyroptotic morphology of GSDME-expressing HEK293T cells. In contrast, MeCbl had no effect on typical apoptosis induced in empty vector-expressing HEK293T cells (Supplementary Fig. 1o), supporting MeCbl specifically inhibits GSDME-mediated pyroptosis but not apoptosis.
a, c, d, e HepG2 were pretreated with indicated concentrations of each compound for 2 h before adding 200 μM deoxycholic acid (DCA) for 4 h, the cytotoxicity of HepG2 cells was determined by LDH release assay (a). Pyroptosis was measured by SYTOX green uptake in the presence of 20 μM MeCbl or DMF (c). Representative immunoblotting analysis of caspase-3 and GSDME in HepG2 (d), and caspase-3 activity was assessed with substrate Ac-DEVD-pNA (e). b Bone marrow derived macrophages (BMDMs) were pretreated with indicated concentrations of MeCbl for 2 h before challenged with LPS transfection by Fugene HD for GSDMD-mediated pyroptosis. Cytotoxicity was determined by LDH release assay. f Changes in HepG2 cell morphology were observed with a microscope (scale bar= 10 μm). g Representative images of GSDME localization in HepG2 cells treated as indicated by confocal microscopy (scale bar= 5 μm). h, i Mice primary hepatocytes were pretreated with indicated concentrations of each compound for 2 h before challenged with 40 nM perforin (PFR) and 0.5 μM GzmB simultaneously for 24 h to activate GSDME-mediated pyroptosis, the cytotoxicity of hepatocytes was determined by LDH release assay (h). Pyroptosis was measured by SYTOX green uptake in the presence of 20 μM MeCbl or DMF (i). j Effect of MeCbl on PFR+GzmB-induced LDH release in hepatocytes pretreated with Z-DEVD-FMK (20 μM). k, m WT, Gsdmd-/-, Gsdme-/- hepatocytes were pretreated with indicated inhibitors for 2 h before challenged with Fugene HD/LPS for 16 h (k) or 200 μM DCA for 4 h (m), cytotoxicity was determined by LDH release assay. l, n Representative immunoblotting analysis of GSDMD and GSDME in hepatocytes. Data are mean ± SEM (n = 3) of at least two independent experiments for bar and line charts. Blots and micrographs are representative of three independent experiments. Analysis was done using one-way ANOVA (e) or two-way ANOVA (a–c, h–k, m). p < 0.05 is considered significant to the indicated group and p values are provided in graphs, n.s = nonsignificant compared to vehicle (e) or indicated group (k, m). Source data are provided as Source Data file.
Next, we asked whether all variants of vitamin B12 could inhibit GSDME. To this end, we pretreated recombinant GSDME protein or DCA-, DDP-challenged mouse primary hepatocytes with vehicle, MeCbl, or other three typical forms of vitamin B12, namely hydroxocobalamin (HOCbl), adenosylcobalamin (AdoCbl), or cyanocobalamin (CNCbl). It was observed that MeCbl, but not other forms of vitamin B12, significantly prevented GSDME-mediated liposome leakage and pyroptosis (Supplementary Fig. 1p, q). We speculated that the specific activity of MeCbl may be associated with its strong binding to GSDME, as the MST assay indicated that other three forms of vitamin B12 had very weak binding affinity with GSDME (Supplementary Fig. 1r). As an important co-factor of folate cycle and methionine cycle, MeCbl helps to convert homocysteine into methionine. To exclude the effects of these metabolic intermediates on pyroptosis, we then pretreated hepatocytes with L-methionine (L-Met) or tetrahydrofolic acid (THF) and found neither can block GSDME-mediated pyroptosis (Supplementary Fig. 1s).
Next, to corroborate target specificity, we tested the effects of MeCbl in primary murine bone marrow-derived macrophages (BMDMs) and hepatocytes. The dual gasdermin inhibitor DMF, but not MeCbl, was found effective against cytoplasmic LPS-induced and GSDMD dependent pyroptosis in both wild-type (WT) and Gsdme-/- cells (Fig. 3k, l and Supplementary Fig. 2a, b). In contrast, MeCbl was effectively against LDH release and GSDME activation in WT and Gsdmd-/- cells but not in Gsdme-/- cells (Fig. 3m, n and Supplementary Fig. 2c, d). Collectively, these data support that MeCbl specifically inhibits GSDME dependent pyroptosis in both hepatocytes and macrophages.
Methylcobalamin takes effect via coordinating to Cys180 in GSDME
Our results support that MeCbl suppresses pyroptosis via engaging GSDME. We thus supposed MeCbl may directly bind to specific residues in GSDME. Indeed, coordination compounds containing core metallic elements generally attach to cysteine (Cys) thiol, such as Cys-Fe ligation between HEME and Cytochrome P450 enzymes24. It is noteworthy that MeCbl is a Cobalt-containing porphyrin, and its core structure is extremely similar to HEME. In addition, previous studies have reported the coordination of Cobalt to Cys, in which Cys residue acted as axial ligand of corrin Cobalt of vitamin B1225,26. It has been shown N-acetylcysteine (NAC), which contains a reactive Cys, can inactivate Cys-binding drugs. We thus first preincubated MeCbl or DMF with NAC and found the ability of MeCbl or DMF to inhibit hepatocyte pyroptosis was largely weakened (Supplementary Fig. 3a). Next, we attempted to determine the activity and binding mode of corrin Cobalt of MeCbl in forming potential ligation with Cys. For this purpose, the absorbance spectrum of MeCbl-bound GSDME was recorded, because any change of electronic densities around corrin Cobalt will be reflected in the absorbance spectrum of cobalamin. The spectral record of MeCbl/GSDME showed pronounced differences compared to that of free MeCbl. The characteristic absorption peak at 525 nm was absent and shifted to 460 nm (Supplementary Fig. 3b). This large blue shift from 525 to 460 nm in MeCbl/GSDME complex points to a base-off conformation of MeCbl27,28, in which the nucleotide base of cobalamin dissociates from the Cobalt ion. Afterward, the Cys residues of GSDME may bind to the “lower” surface of corrin ring (so called α-face). In contrast, the absorbance spectrum of HOCbl/GSMDE was found identical to that of free HOCbl (Supplementary Fig. 3c), indicating that HOCbl cannot interact with GSDME. These results are consistent with the established chemistry of cobalamin that the dissociation of DMB-base from MeCbl is sufficiently feasible for coordination of Cys thiol but is extremely difficult to attain for HOCbl or CNCbl. In view that photoreaction would convert MeCbl to HOCbl29, we reasoned that whether such a conversion might disrupt MeCbl/GSDME complex. Indeed, we observed that the inhibition of GSDME cleavage by MeCbl was canceled after photoreaction in converting MeCbl to HOCbl, the latter being unable to inhibit GSDME cleavage (Supplementary Fig. 3d). Our results indicate that MeCbl may bind to GSDME and thereby inhibit its activity via adopting base-off conformation in coordination to the Cys residue of GSDME.
To investigate the potential ligating Cys in GSDME, AlphaFold structure of GSDME (AF-O60443-F1-model_v2) was used as the initial structure in view that the experimental GSDME structure remains still unresolved. After equilibrated with molecular dynamics (MD) simulation, the final structure of GSDME was employed for molecular docking, where the docking center was set to each predictive Cys site (a total of 13 Cys sites) of the protein. Three docking simulations correspond to three potential binding sites, Cys45, Cys180, and Cys371 (Supplementary Fig. 3e–g). Coincidentally, DMF succinates GSDME at these three and additional Cys residues to inhibit GSDME-dependent pyroptosis17. Furthermore, we applied a method coupling LC-MS/MS with fast photochemical oxidation of proteins (FPOP) to analyze MeCbl binding induced conformational changes of GSDME to explore the exact binding site. Peptide oxidation was internally normalized to the total mean oxidation across all replicates detected for all peptides. The FPOP-LC-MS/MS footprint detected the significant difference in oxidation of peptide 318-329 between Control/his-sumo-GSDME and MeCbl/his-sumo-GSDME group, in which we found a significantly reduced oxidation of M324 upon MeCbl binding. By deducting the amino acid sequences of N-terminal tag his-sumo, it indicated that the region near peptide 168-179 is occupied by MeCbl (Supplementary Fig. 3h, i). Furthermore, UHPLC-timsTOF HT mass analysis revealed the incubation with MeCbl but not HOCbl could induce an increase of m/z 944.33 Da (peptide KCGGIV) or 1093.41 Da (peptide KCGGIVGIQ) at Cys180 of GSDME, indicating that MeCbl coordinated to Cys180 in base-off conformation (Fig. 4a, b and Supplementary Fig. 3j, k).
a, b Representative mass spectrometry spectra of MeCbl coordinated to Cys180 in human GSDME peptide KCGGIVGIQTK (b), GSDME incubated with ddH2O was used as negative control (a). The binding mode of MeCbl (base-off conformation) to GSDME was shown in graph b. c LDH release assay of Gsdme-/- hepatocytes overexpressed with indicated GSDME plasmids. Cells were pretreated with 20 μM MeCbl, then challenged with 200 μM DCA for 4 h. d Representative immunoblotting analysis of GSDME cleavage in Gsdme-/- hepatocytes overexpressed with indicated GSDME plasmids and challenged by DCA. e Co-immunoprecipitation (Co-IP) analysis of overexpressed GSDME with cleaved-caspase-3 in Gsdme-/- hepatocytes treated with 200 μM DCA for 1 h. f MST measurements of the binding of 0.2 μM recombinant WT and cysteine mutant GSDME with recombinant human caspase-3. Values of Kd are listed in the graphs. g LDH release assay of Gsdme-/- hepatocytes overexpressed with indicated GSDME-NT plasmids. h Co-IP analysis of endogenous GSDME with cleaved-caspase-3 in WT hepatocytes pretreated with 20 μM MeCbl or DMF, then cells were challenged with 200 μM DCA for 1 h. i MST measurements of the binding of 0.2 μM recombinant WT and cysteine mutant GSDME with MeCbl. Values of Kd are listed in the graphs. j Dose response of MeCbl on liposome leakage induced by WT and cysteine mutant GSDME. Data are mean ± SEM (n = 3) of at least two independent experiments for bar and line charts. Blots are representative of three independent experiments. Analysis was done using two-way ANOVA. p < 0.05 is considered significant to the indicated group and p values are provided in graphs, n.s = nonsignificant compared to vehicle (g) or indicated group (c). Source data are provided as Source Data file.
Next, we sought to investigate the roles of these Cys residues in GSDME-mediated hepatocyte pyroptosis. We transfected WT, C45A, K179A, C180A, C371A, or D270A GSDME plasmids into Gsdme-/- hepatocytes. Cells expression of both GSDME D270A and C180A resisted the cleavage by caspase-3 in DCA stimulated hepatocytes and showed no obvious LDH release (Fig. 4c, d). D270 had been previously demonstrated as the caspase-3-cleavable site; our results suggest that Cys180 might be also essential for caspase-3-cleaved GSDME. To further corroborate this result, we performed a co-immunoprecipitation (Co-IP) assay using a GSDME antibody to determine whether Cys180 is essential for caspase-3 recognition. The cleaved caspase-3 was co-precipitated with GSDME WT, C45A, and C371A, but not GSDME C180A (Fig. 4e). MST assay further confirmed that GSDME C180A disrupted the interaction with recombinant caspase-3 (Fig. 4f). We next asked whether Cys180 is necessary for GSDME-NT activity. In line with previous reports, we confirmed that overexpressed GSDME-NT I217N blocked LDH release in Gsdme-/- hepatocytes30; however, GSDME-NT WT and C180A did not (Fig. 4g). MeCbl was unable to inhibit the GSDME-NT-induced LDH release, further supporting that MeCbl engages GSDME but not its activated form GSDME-NT. In PFR+GzmB-induced pyroptosis, Cys180 is also essential for GzmB recognition and the resultant pyroptosis (Supplementary Fig. 4a–c). Collectively, these results suggest Cys180 in GSDME is essential for caspase-3/GzmB recognition and the subsequent cleavage.
We conducted further assays to verify that Cys180 is essential for MeCbl engaging in prevention of GSDME cleavage. Co-IP assay in WT hepatocytes indicated that MeCbl disrupted caspase-3/GzmB-GSDME interactions (Fig. 4h and Supplementary Fig. 4d). MeCbl was unable to further decrease LDH release induced by DCA or PFR+GzmB in GSDME C180A-expressed hepatocytes (Fig. 4c and Supplementary Fig. 4e). Kd values for cobalamin upon interaction of MeCbl with WT and Cys mutant GSDME were further analyzed by MST. MeCbl had significantly weaker binding affinity for GSDME C180A compared with that of GSDME WT (Fig. 4i). In addition, MeCbl was unable to substantially affect GSDME C180A-induced faint liposome leakage in a dose-dependent manner (Fig. 4j). Together, these results support that Cys180 is the critical residue that MeCbl directly coordinates to GSDME, thereby inhibiting GSDME-mediated pyroptosis.
Methylcobalamin protects against GSDME-initiated acute liver failure
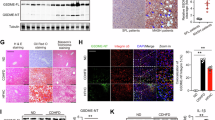
Our recent findings indicate GSDME-mediated pyroptosis plays essential roles in bile duct ligations (BDL) induced acute liver failure. We thus supposed that MeCbl, as a specific and powerful GSDME inhibitor, would be expected to be effective against BDL induced liver failure. To this end, we first examined DCA-induced liver injury in zebrafish. Five days postfertilization (dpf) zebrafish larvaes (n = 6) were treated with vehicle or 20 μM MeCbl before immersed in 400 μM DCA for 48 h, and SYTOX Green nucleic acid stain was used to assess the tissue damage in zebrafish31. MeCbl strongly reduced systemic SYTOX green fluorescence intensity (Fig. 5a), and it is noteworthy that fluorescence in zebrafish liver (red vs yellow circle) was not observed in the presence of MeCbl (Fig. 5b), supporting that MeCbl is effective against DCA-induced systemic and liver damage of zebrafish. Next, we examined whether GSDME-mediated pyroptosis was involved in DCA-induced zebrafish larvae damage. Previous studies demonstrated zebrafish has two GSDME, GSDMEa and GSDMEb, sharing about 50% sequence similarity to human GSDME, but only GSDMEa could trigger TNFα-induced pyroptosis32. As expected, we observed an obvious cleavage of caspase-3 and GSDMEa after DCA immersion (Fig. 5c), supporting GSDMEa might act as an executor inducing systemic and liver damage in zebrafish. MeCbl had strong effect on inhibiting GSDMEa proteolytic processing, and substantially improved survival of zebrafish (n = 60) (Fig. 5c, d). Sequence alignment shows that Cys180 of GSDME is conserved in human, mouse, and zebrafish (highlighted in red) (Fig. 5e), indicating that MeCbl may target Cys180 in GSDMEa to block pyroptosis in zebrafish.
5 dpf zebrafish larvaes were pretreated with 20 μM MeCbl before immersed in 400 μM DCA for 48 h. a–c SYTOX fluorescence probe and immunoblotting analysis were used to investigate the protective effects of MeCbl on DCA-induced systemic and liver injury (liver region was indicated with red or yellow circle, scale bar=250 μm) in zebrafish, n = 6. d Survival analysis of zebrafish after DCA immersion, n = 60. e Sequence alignment of GSDME from human, mouse and zebrafish. In addition, mice were performed with bile duct ligation (BDL) surgery to induce GSDME-mediated acute liver failure. MeCbl (10 mg/kg), DMF (50 mg/kg) were given intragastrically to mice daily before euthanized. f–j Serum ALT, AST, IL-1α, IL-1β, HMGB1 levels in WT and Gsdme-/- mice, n = 6. k Representative immunoblots of caspase-3 and GSDME in the livers of the mice. l Survival analysis of WT and Gsdme-/- mice after BDL and treated as indicated, n = 10. m, n Representative H&E sections of the liver (m) (scale bar = 50 μm). Damaged area was traced with black dash line and analyzed using Image J software (n, n = 6 representative section of six independent mice samples). Data are mean ± SEM of at least two independent experiments. Each point is an individual zebrafish larvae or mouse, number is indicated, n = 6-60 per group. Blots are representative data of six independent samples (c, k). Analysis was done using two-tailed unpaired Student’s t test (a) or two-way ANOVA (f–j, n). Log-rank (Mantel-Cox) test was used for survival analysis (d, l). p < 0.05 is considered significant to indicated group and p values are provided in graphs, n.s=nonsignificant. Source data are provided as Source Data file.
To further verify the protective effects of MeCbl against pyroptotic liver failure, we developed different models of liver failure to mimic various pathological causes inducing liver failure in the clinic. Our previous findings support that cholestatic liver failure was mainly dependent on GSDME-mediated pyroptosis. Moreover, it had been previously verified that DDP induced pyroptosis rather than apoptosis in normal tissues with high expression of GSDME, such as small intestine and lung tissues, leading to chemotherapy-mediated organ injury9. Likewise, concanavalin A (Con A) induced liver injury may be also mediated by GSDME upon GzmB cleavage33. We thus employed these three models to determine the potential protective effects of MeCbl against GSDME-mediated liver failure. Mice were intragastrically treated with vehicle, MeCbl, or DMF daily after models were developed. Serum transaminase ALT/AST activities, IL-1α, IL-1β, and HMGB1 concentrations were drastically reduced by MeCbl treatment (Fig. 5f–j). MeCbl at 10 mg/kg showed much more powerful protective effects against liver failure than that by DMF at 50 mg/kg. As expected, MeCbl blocked GSDME cleavage in mice liver (Fig. 5k), and more importantly, rescued BDL mice from death, with stronger effects in comparison with that caused by DMF (Fig. 5l). Severe cholestasis caused massive hepatocyte death with pronounced damaged area and expansion of the portal tracts, which were substantially attenuated by MeCbl (Fig. 5m, n). To check whether the attenuation of hepatotoxicity was dependent on GSDME inhibition, we compared transaminase activities and inflammatory cytokines levels in WT and Gsdme−/− mice in the presence or absence of MeCbl. Gsdme−/−, as predicted, produced lower ALT/AST, interleukin, and HMGB1 levels than in WT mice, and these indicators were not further reduced by MeCbl (Fig. 5f–j, l). The involvement of GSDME in mediating the beneficial effects of MeCbl against liver injury was further corroborated in the survival assay (Fig. 5l). These results were consistent with those from in vitro experiments, all of which support that MeCbl specifically targets GSDME but not other cell death pathways. Similar results were reproduced in DDP- or Con A-induced liver failure mice; Gsdme−/− mice were resistant to these factors induced-liver injury and mortality; MeCbl potently reduced serum ALT/AST activities, inflammatory cytokines, total bile acids, AKP, indirect bilirubin levels, and the mortality rate of mice, in a GSDME dependent manner (Supplementary Fig. 5). As predicted, MeCbl showed better performance than DMF and particularly in Con A-induced liver failure model.
To verify whether MeCbl directly engages GSDME at Cys180 in vivo, WT (GSDMEWT), C180A (GSDMEC180A) and D270A (GSDMED270A) GSDME plasmids constructed in adenovirus vector were injected into Gsdme-/- mice via tail vein (Fig. 6a). Mice were orally treated with vehicle or MeCbl after BDL surgery. As expected, serum ALT, AST, IL-1α and IL-1β concentrations were strongly reduced in GSDMEWT mice by MeCbl, while GSDMEC180A or GSDMED270A mice were resistant to liver injury and inflammatory response from BDL model (Fig. 6b–e). GSDMEWT, but not GSDMEC180A or GSDMED270A, was recognized and cleaved by caspase-3 which was substantially prevented by MeCbl (Fig. 6f), aligned with their differential effects in hepatic injury (Fig. 6g, h). These results further confirm that Cys180 is the critical residue for both caspase-3 recognition and MeCbl binding and thereby inhibition of GSDME cleavage and activation.
GSDME Cys mutant plasmids constructed in adenovirus vector were injected into Gsdme-/- mice via tail vein, a GSDME expression in mice liver was detected with immunoblotting analysis. Mice were orally given 10 mg/kg MeCbl daily after BDL surgery. b–e Serum ALT, AST, IL-1α and IL-1β levels in serum 5 days after BDL/sham, n = 6. f Co-IP analysis of overexpressed GSDME with cleaved-caspase-3 in Gsdme-/- liver. g, h Representative H&E sections of the liver (g) (scale bar = 50 μm). Damaged area was traced with black dash line and analyzed using Image J software (h, n = 6 representative section of six independent mice samples). Data are mean ± SEM of at least two independent experiments. Each point is an individual mouse, number is indicated, n = 6–10 mice/group. Blots are representative data of six independent samples (a, f). Analysis was done using two-way ANOVA (b–e, h). p < 0.05 is considered significant to indicated group and p values are provided in graphs, n.s=nonsignificant. Source data are provided as Source Data file.
To determine whether MeCbl blocks infection-induced acute liver failure or sepsis, each mouse was injected intraperitoneally with 108 CFU S. typhimurium for 6 h and immediately given intragastrically with MeCbl or DMF. Results revealed that DMF, but not MeCbl, reduced serum ALT/AST activities, and IL-1α, IL-1β, HMGB1 levels, as well as the mice mortality. Gsdme−/− mice were not resistant to liver injury, inflammatory response or mice mortality from S. typhimurium (Supplementary Fig. 6). Acetaminophen (APAP) is another common agent inducing liver failure, likely attributed to hepatocyte necroptosis via RIPK1/RIPK3-MLKL pathway34. APAP dosage dramatically induced liver injury with increased ALT, AST, malondialdehyde (MDA) activities, and reduced GSH levels (Supplementary Fig. 7a–d). GSDME-NT was not observed in APAP challenged mice, and Gsdme−/− or GSDME inhibitor MeCbl could not reverse APAP induced liver injury and the resultant mice mortality (Supplementary Fig. 7e–h). Collectively, all of our results demonstrate MeCbl is a specific inhibitor of GSDME, but not GSDMD, caspases, or necroptotic cell death signals; MeCbl is promisingly to be repurposed as an effective therapeutic drug against liver failure executed by GSDME dependent pyroptosis, which may be dominant in cholestatic, chemotherapeutics, and autoimmune hepatitis triggered liver failure.
Discussion
Pyroptosis has been verified to play important roles in diverse human inflammatory and sterile diseases, such as sepsis, ulcerative colitis, and cancer6,35,36. Since the pioneering identification of GSDMD/E and other gasdermins in executing pyroptosis, increasing evidence indicates that gasdermins, particularly GSDMD/E, can be expected to be promising targets for innovative drug discovery. In particular, GSDME-mediated pyroptosis has been observed in the context of tissue damages in multiple organs9,10. Our previous findings together with panels of other evidence support that GSDME might be a promising target against liver failure16,37,38, which can be seen in many clinical settings and is characterized by high mortality without efficient therapeutic approaches. Here in this study, we contribute to identify MeCbl, an endogenous active form of vitamin B12 and a drug widely used in the clinic for hyperhomocysteinaemia and peripheral neuropathy, as a potent and specific inhibitor of GSDME. More importantly, we provide sufficient evidence supporting that MeCbl, via engaging GSDME, is powerfully effective against liver failure induced by various pathological factors.
Increasing efforts have been endeavored to identify potential inhibitors against gasdermins, which are key executors in pyroptosis and are expected to be promising therapeutic targets for diverse diseases including but not limited to septic shock, cancer, and tissue damage. Necrosulfonamide (NSA), a MLKL inhibitor that blocks necroptosis by covalently binding to unique human Cys86 residue, was found to disrupt the functions of GSDMD-NT to oligomerize and form membrane pores, likely through alkylating Cys191/Cys19239. Similarly, FDA-approved drug disulfiram (DSF) inhibits pore formation of GSDMD-NT by covalent addition of dithiodiethylcarbamoyl (DTC) at Cys191/19240. DMF, a derivative of the Krebs cycle intermediate fumarate, reacts with GSDMD at same cysteine residues to form S(2-succinyl)-cysteine, but this GSDMD succination can prevent both GSDMD cleavage and pore formation17. Pharmacologic inhibitions of pyroptotic cell death by these inhibitors are efficacious in sepsis models or autoimmune diseases. Notably, DMF can also directly bind and block GSDME cleavage. In spite of these advancements in discovery of gasdermin inhibitors, there is no specific GSDME inhibitor up to now. Moreover, DMF at relatively high dose is toxic, possibly due to its non-specific covalent binding to Cys residues of diverse proteins, and thus cannot be used under conditions of organ failure. In this study, we screened from an in-house developed metabolites library and identified MeCbl, an endogenous coenzyme form of vitamin B12, as a potent and specific inhibitor of GSDME without any effects on GSDMD. MeCbl is capable of blocking recombinant active caspase-3/GzmB-GSDME-induced liposome leakage, but has no apparent effect on precleaved GSDME-induced liposome leakage. Further, it inhibited GSDME-mediated pyroptosis of hepatocytes and macrophages by both caspase-3 and GzmB pathways. Our results support the view that MeCbl specifically and directly engages and inhibits GSDME rather than upstream events. Currently, MeCbl is a common drug that has been widely used for decades to treat hyperhomocysteinaemia, pernicious anemia and peripheral neuropathy. As an important coenzyme in vitamin B12-dependent methyltransferases, MeCbl supplementation accelerates homocysteine consumption by enhancing methionine synthesis, decreasing the risks of cardiovascular diseases. It is also indicated that MeCbl can quickly enter nerve cells and promote the regeneration of myelin sheath, accelerating the repair of damaged nerve tissue18. Moreover, MeCbl has no known toxicity even applied at high dosage in the clinic (0.5–6 mg/day), and it appears to be well tolerated, with a safety and tolerability profile similar to that of the placebo41. Moreover, various previous researches support that MeCbl can be used in an off-label manner in addition to its well-established applications in hyperhomocysteinaemia, pernicious anemia, and peripheral neuropathy, with ultra-high dose MeCbl up to 500 mg/day in patients with chronic renal failure and chronic axonal degeneration42,43. All of these results indicate that the effective dosage of MeCbl (10 mg/kg, around 60 mg/day for human beings) applied in this study, despite much higher than its commonly prescribed dosage (1.5 mg/day), is safe and unlikely to cause toxicity. Altogether, our findings support that MeCbl can be recommended for the therapy of GSDME-involved liver injury induced by diverse factors.
In this context, although DMF can also prevent GSDME cleavage in mice, MeCbl has a number of advantages over DMF. The elevation of serum transaminase and total bilirubin have been observed in clinical trials with DMF, with some cases even required hospitalization44, indicating that DMF is unlikely to be prescribed in conditions of liver failure. In contrast, the safety profile of MeCbl in clinical use has been well proven, and therefore MeCbl can be expected to be repurposed as a therapeutic drug against GSDME-mediated liver failure, and possibly other organ damages.
We further unveiled that Cys180 in GSDME was essential for caspase-3/GzmB-GSDME interaction and following GSDME processing (Supplementary Fig. 8). As a highly Cys-reactive drug, DMF succinates GSDME at Cys45, Cys180 and additional sites, and thereby attenuates GSDME-dependent cell death and the generation of GSDME-NT. However, DMF modifies multiple cysteine residues on cysteine-dependent proteases, which is likely to affect diverse proteins, including caspases. This might explain why DMF interfere with many other signal pathways45,46. In contrast, we provide panels of evidence supporting that MeCbl is a specific GSDME inhibitor. MeCbl coordinates to the Cys180 in GSDME, approaching to the protein via α-face of corrin ring and thereby adopting the base-off conformation. Interestingly, MeCbl blocks GSDME-mediated pyroptosis, but barely has any effect on apoptosis when Gsdme genetic deficiency cells were challenged with apoptotic stimulation. In addition, MeCbl specifically interacts with GSDME but not with caspase-3, GzmB or GSDMD. Finally, MeCbl is not able to protect against S. typhimurium- or acetaminophen-induced liver failure with equal dose, both of which induce liver failure independent of GSDME. S. typhimurium activates caspase-1/4/11-GSDMD inflammasome pathway, while acetaminophen toxicity begins with its metabolic conversion to reactive chemical species, N-acetyl-p-benzoquinoneimine (NAPQI), which causes hepatocyte necrosis47. Collectively, it seems that MeCbl is different from other previously identified Cys-reactive compounds, particularly the nonspecific GSDME inhibitor DMF, most of which lack Cys residue specificity. The specificity of MeCbl to interact with Cys180 of GSDME, which is evolutionally conserved in zebrafish, mice, and humans, together with its capability in inhibiting GSDME-mediated pyroptotic cell death across these species, strongly support that MeCbl can either be directly repurposed as a drug, or serve as a leading compound to ignite future development of specific GSDME inhibitory drugs, to prevent GSDME-mediated pyroptosis. Moreover, although we cannot rule out the possibility of other mechanisms contributing to the observed hepatoprotective effects of MeCbl, panels of evidence collected from our study support that GSDME inhibitory effect might represent a dominant mechanism.
In summary, our study supports that MeCbl, a widely prescribed drug, is promisingly repurposed to be an effective therapeutic option to GSDME dependent liver failure, which is characterized with high morbidity and high mortality without efficient clinical therapies. Moreover, the present findings of MeCbl, an endogenous coenzyme form of vitamin 12, engaging with GSDME-mediated pyroptosis, together with other exciting findings about the signaling metabolites involved in diverse cell death signals, may pave a way in innovating metabolic bionics guided drug discovery against cell death signals, for which the endogenous signaling metabolites can serve as leading compounds for drug design48. Although our study mainly focused on the protective role of MeCbl in inhibiting GSDME-mediated liver failure, it is important to note that GSDME is universally expressed in diverse other organs and may represent a key executor in mediating multiple pyroptotic organ damage. For instance, chemotherapy drugs or enterovirus infection induce GSDME-mediated pyroptosis, thus leading to intestinal, lung or other organ injuries10. Likewise, GSDME is found to be cleaved by GzmB upon delivery by cytotoxic lymphocytes, and such cytotoxic lymphocytes participate in various tissue damage in autoimmune diseases11. Therefore, it is reasonable to predict that MeCbl may exert similar protective effects against GSDME-mediated injury of other organs. Therefore, our findings may also ignite future researches in verifying the potential protective benefits of MeCbl against GSDME mediated pyroptotic injury of other organs.
Methods
Plasmids, adenovirus, antibodies and regents
cDNAs for full-length or 1-270aa (NT domain) WT, C45A, K179A, C180A, C371A, D270A, I217N GSDME were synthesized and cloned into pCDNA3.1 vector, and adenovirus-vector-based WT and Cys residues mutant plasmids were all from Sangon Biotech (Shanghai, China). Rabbit polyclonal anti-caspase-3 (WB, 9662) used for human, mouse and zebrafish samples, and rabbit monoclonal anti-GSDMD (WB, 97558) antibodies were from Cell Signaling Technology (Danvers, USA), rabbit monoclonal anti-GSDME (WB/IP, ab215191) used for human, mouse and zebrafish samples, and rabbit monoclonal anti-granzyme B (WB, ab255598) antibodies were from Abcam (Cambridge, USA), rabbit polyclonal anti-GSDME (IF, A7432) was from ABclonal (Wuhan, China), mouse monoclonal anti-caspase-3 (WB/IP, sc-271759) and mouse monoclonal anti-granzyme B (WB/IP, sc-8022) were from Santa Cruz Biotechnology (Dallas, USA), rabbit monoclonal anti-GAPDH (AB0037) was from Abways Technology (Shanghai, China). In-house Human Endogenous Metabolite Compound Library was synthesized by MCE (HY-L030, New Jersey, USA). Recombinant human active caspase-3, human WT, C45A, C180A, C371A His-sumo-GSDME-eGFP were from BioGot (Nanjing, China), recombinant human His-GSDME (CSB-EP006766HU) was from CUSABIO (Wuhan, China), recombinant human active caspase-4 was purchased from Biovision (1084-100, San Francisco, USA), recombinant human His-sumo-GSDMD-eGFP, His-sumo-GSDME were from Detai Bio (Nanjing, China), recombinant human TNFα (300-01 A) was purchased from Peprotech (Cranbury, USA), recombinant perforin/PFR (APB317Hu02) was purchased from Cloud-Clone (Wuhan, China), recombinant granzyme B/GZMB (HY-P75157) was purchased from MCE (NJ, USA). Terbium (Tb3+)-loaded liposomes were purchased from DDSome Lab (Shanghai, China). Methylcobalamin (HY-B0586), hydroxocobalamin (HY-B2209A), adenosylcobalamin (HY-112790), cyanocobalamin (HY-B0315), L-methionine (HY-N0326), tetrahydrofolic acid (HY-14520), dimethyl fumarate (HY-17363), Z-DEVD-FMK (HY-12466), cisplatin (HY-17394), acetaminophen (HY-66005), N-acetylcysteine (HY-B0215) were purchased from MCE (NJ, USA). Deoxycholic acid (D2510), cycloheximide (C7698), dipicolinic acid (P63808), Lipopolysaccharides from Escherichia coli O111: B4 (L2630), concanavalin A (C2010) were purchased from Sigma-Aldrich (St. Louis, USA). 3,4-dichloroisocoumarin (D909910) was from Macklin (Shanghai, China), SYTOX green nucleic acid stain (S7020), lipofectamine 3000 transfection reagent (L3000001) were purchased from Invitrogen (Carlsbad, USA). Fugene HD (E231A) was purchased from Promega (Madison, USA).
Mice models and treatments
Wild-type (WT) six-week-old C57BL/6 J male mice were purchased from Beijing Vital River Laboratory Animal Technology Co., LTD (Beijing, China). Gsdmd-/-, Gsdme-/- male and female mice on C57BL/6 J genetic background were grifts from Prof. Feng Shao at National Institute of Biological Sciences (Beijing, China). Mice were maintained under controlled conditions of humidity (50 ± 10%), light (12/12 h light/dark cycle), temperature (23 ± 2 °C) and given free access to a standard NIH-31 chow-diet (1019018, Xietong Bio, Nanjing, China) and water ad libitum. All mouse experiments were conducted using protocols approved by China Pharmaceutical University Animal Care and Use Committee. Euthanasia of mice were performed by exposure to carbon dioxide, and visually confirming cessation of breath.
For GSDME overexpression in mice liver, 1 × 109 PFU of adenovirus was injected into Gsdme-/- mice via tail vein and mice were used for experiments after 5 days of maintaining under controlled conditions.
Bile duct ligation (BDL) was operated as previously described16. After anesthetized with chloral hydrate solution (0.3 g/kg), a thoracotomy was made and the common bile duct was found and carefully ligated with 7–0 prolene (Ethicon, Somerville, NJ). Mice in sham operation were dissected without ligation, then wiped the incision with alcohol swab and injected with 0.5 mL of 0.9% saline on incision to improve recovery and survival after operation. Kept the mice warm at 37 °C until recovery. After recovery, mice were administered intragastrically daily with MeCbl (10 mg/kg) or DMF (50 mg/kg) formulated in 50% PEG300 and 50% saline, samples were collected for experiments 5 days after surgery.
For chemotherapy-induced liver injury, mice were intraperitoneally injected with 10 mg/kg DDP at day 1 and day 5, after injection at day1, mice were administered intragastrically with MeCbl (10 mg/kg) or DMF (50 mg/kg) daily before euthanized at day 6 for following experiments. In addition, mice were intraperitoneally injected with 20 mg/kg DDP once at day 1 for survival analysis.
For concanavalin A (Con A)-induced autoimmune hepatitis, mice were injected with 20 mg/kg Con A via tail vein, then administered intragastrically with MeCbl (10 mg/kg) or DMF (50 mg/kg) immediately after Con A injection, mice were euthanized after 6 h for sample collection.
For S. typhimurium-induced liver injury, mice were injected intraperitoneally with 108 CFU S. typhimurium per mouse and immediately administered intragastrically with MeCbl (10 mg/kg) or DMF (50 mg/kg) after S. typhimurium injection, mice were euthanized after 6 h for sample collection. Before S. typhimurium injection, S. typhimurium ATCC14028 (22956, CICC) was cultured in sterilized LB liquid medium (bacterial suspension: medium = 3%-5%, v/v) at 50 g and 37 °C for 24 h. Then bacterial suspension was centrifuged at 1300 g for 20 min to collect S. typhimurium pellets, resuspended with saline. 100 μL bacterial suspension was added to 96-well plate and OD600 was read by microplate reader, pure saline was used as control. 1OD600 = 8 × 108 CFU/mL, the bacterial suspension was diluted to injection concentration with saline and stored at 4 °C.
For acetaminophen (APAP)-induced liver injury, mice were injected intraperitoneally with 250 mg/kg APAP for 6 h and immediately administered intragastrically with MeCbl (10 mg/kg) or N-Acety-L-Cysteine (NAC, 300 mg/kg) after APAP injection.
To determine liver failure and inflammation of mice, blood samples were collected and allowed to clot at room temperature. Serum obtained after centrifugation were analyzed with Alanine aminotransferase Assay Kit (C009-2-1), Aspartate aminotransferase Assay Kit (C010-2-1), Direct bilirubin (D-BIL) kit (C019-2-1), Total bilirubin (T-BIL) Kit (C019-1-1), Alkaline phosphatase Assay kit (A059-2-2), Total bile acid Assay kit (E003-2-1), Glutathione Assay kit (A005-1-2), Malondialdehyde Assay kit (A003-1-2) from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Mouse IL-1α (EM011-96), IL-1β (EM001-96) ELISA Kits were from ExCell Bio (Shanghai, China). Mouse HMGB1 (CSB-E08225m) ELISA kit was from CUSABIO (Wuhan, China). To assay Con A-induced autoimmune hepatitis, mouse IFN-γ (EM007-96), IL-6 (EM004-96), MCP-1 (EM018-96), IL-10 (EM005-96) ELISA Kits were from ExCell Bio (Shanghai, China). Mouse perforin (CSB-E13429m), granzyme B (CSB-E08720m) ELISA kits were from CUSABIO (Wuhan, China).
Zebrafish model and treatment
Zebrafish larvaes were from Nanjing Hunter Biotechnology Co., Ltd (Nanjing, China) and all experiments were conformed to the care and use of laboratory animals and ethically approved by China Pharmaceutical University Animal Care and Use Committee. The larvaes were maintained in E3 medium according to standard protocol49. To assess the treatment of MeCbl on systemic and liver damage in zebrafish, 5 dpf zebrafish larvaes were randomly picked and pretreated with 20 μM MeCbl before immersed in 400 μM DCA for 48 h for SYTOX fluorescence detection (n = 6), or immersed in 400 μM DCA for 108 h for survival analysis (n = 60). Zebrafish larvaes were then anesthetized with 200 ng/mL tricaine (L4391, Sigma-Aldrich, St. Louis, USA) and the following experiments were conducted. Euthanasia was performed using a lethal dose of anesthetic (300 mg/L).
High-throughput screen
Fluorescence signal derived from Tb3+ bounding to dipicolinic acid (DPA) in reaction buffer (20 mM HEPES, 150 mM NaCl and 50 μM DPA) was used to detect liposome leakage. 0.4 μM recombinant human His-sumo-GSDME was preincubated with 100 μM 829 compounds from In-house Human Endogenous Metabolite Compound Library dispensed into 96-well plates for 0.5 h before addition of 0.2 μM recombinant active-caspase-3 for another 2 h at 37 °C. 200 μM liposome was then added into the caspase-3-GSDME reaction well for 30 min. The fluorescence intensity of each well was measured at Ex276 nm/Em545 nm using a Synergy H1 Microplate Reader (Bio Tek, Winooski, USA). The inhibition (%) was calculated as 100- ((fluorescencecompound –fluorescencenegative control)/(fluorescencepositive control −fluorescencenegative control)) × 100, where wells with GSDME activation without compounds were used as positive control and wells with liposome alone as negative control, inhibition of 70% was chosen as a threshold.
Cell culture and isolation
Liver hepatocellular cell line HepG2 (ATCC, HB-8065, male) and human embryonic kidney cell line HEK293T (ATCC, CRL-3216, female) were purchased from ATCC (Virginia, USA). Cells were maintained in 5% CO2 at 37 °C and grown in DMEM medium containing 10% Fetal Bovine Serum (FBS), 2 mM L-glutamine, penicillin (50 U/mL) and streptomycin (100 mg/mL) (All medium and supplements were obtained from GIBCO).
Murine bone marrow-derived macrophages (BMDMs) were harvested as previously described16. In brief, mice were euthanized and sterilized with 75% alcohol for 5 min. Peeled the skin of each hind leg, discarded the feet and cut off the hind legs at the hip joint, sterilized with 75% alcohol for 2 min, then washed with sterile PBS. Got the muscles off the legs with forceps and scissors, dredged the legs from both ends. Inserted into bone marrow cavity of femur with a 20 mL syringe, flushed bone cavity with sterile PBS until the bone marrow was rinsed thoroughly. Cells were centrifuged at 500 g for 10 min and cell pellets were resuspended with red blood cell lysis buffer for another 5 min, centrifuged at 500 g for 5 min. The isolated BMDMs were finally resuspended and cultured in 5% CO2 at 37 °C with DMEM/F-12 complete medium containing M-CSF, 10% FBS, penicillin (50 U/mL) and streptomycin (100 mg/mL). The medium was renewed every 3 days and nonadherent cells were eliminated, the cells were ready for assays on 5th day.
For primary mouse hepatocytes isolation, male mice were anticoagulated with heparin sodium and anesthetized with chloral hydrate by intraperitoneal injection. Under anesthesia, opened the abdominal cavity and exposed liver, inserted a catheter into the superior vena cava and perfused liver with buffer I (D’Hanks containing 180 mg/L EGTA and 80 mg/L NaOH) at a flow rate of 4.0 mL/min, followed by buffer II (D’Hanks containing 1 g/L collagenase IV and 444 mg/L CaCl2) at a flow rate of 2.0 mL/min. The perfusion was ended when liver became soft and grainy. Put the liver into the prechilled medium (DMDM medium containing 10% FBS, 10 μM dexamethasone and 100 U/L insulin) quickly for mechanical separation and dissociated the liver cells. Filtered through a 100 μm nylon mesh and centrifuged at 50 g for 5 min, resuspended cells with 5 mL medium and then added 5 mL gradient solution (10×PBS: Percoll=1:9, v/v), gently pipetted to mix, centrifuged at 120 g for 5 min, removed the floating particles and supernatant. Washed and resuspended cells twice with 2 mL medium before seeding into plate. Cells were maintained in 5% CO2 at 37 °C. Refreshed medium after 5 h and cells were ready for experiments after 24 h of seeding.
Cell treatment
Unless otherwise indicated in figure legends, cells were treated with 200 μM DCA for 4 h, 20 μg/mL cisplatin for 16 h, or pretreated with 10 μg/mL cycloheximide (CHX) for 30 min and then synergistically challenged with 20 ng/mL TNFα for 16 h to induce GSDME-mediated pyroptosis. For PFR/GzmB-induced pyroptosis, cells were treated with 40 nM PFR and 0.5 μM GzmB simultaneously in HBSS buffer (10 mM HEPES pH7.5, 4 mM CaCl2 and 0.4% BSA) for 24 h to activate GSDME-mediated pyroptosis. For GSDMD-mediated pyroptosis, cells were primed with 1 μg/mL LPS for 6 h, then 5 μg/mL LPS was transfected into cytoplasm by 0.3% (v/v) Fugene HD with Opti-MEM in 1640 medium for 16 h.
Cell transfection for GSDME overexpression
Primary mouse hepatocytes isolated from Gsdme-/- mice were seeded in a 6-well plate at a density of 8 × 105/well. After Ad-GSDME were completely melted on ice, discarded medium in plate and added 1 mL fresh medium containing 1 × 107 MOI adenovirus/well. Supplemented another 1 mL fresh medium into well after 4 h of infection. After 6 ~ 8 h of infection, replaced with fresh medium, and continued to maintained in 5% CO2 at 37 °C for 48 h.
HEK293T or hepatocytes isolated from Gsdme-/- mice were seeded in a 6-well plate, GSDME or vector plasmids were transfected into cells with Lipofectamine 3000/P3000 (L3000001, Invitrogen, Carlsbad, USA) according to instructions. Briefly, 2 μg plasmid, 4 μL P3000 and 4 μL Lipofectamine 3000 were mixed into 500 μL Opti-MEM medium in order for 30 min, then added up to 3 mL with DMEM medium before transferring to 6-well plate. After 6 h of transfection, replaced the cell culture with fresh DMEM containing 10% FBS, and cells were ready for assay after 48 h of transfection.
Cell cytotoxicity assays
A total of 1 × 105 cells/well in a 96-well plate were treated as indicated in figure legends or overexpressed with GSDME-NT. Pyroptosis was determined by SYTOX Green uptake assay via using SYTOX™ Green nucleic acid stain (S7020, Invitrogen, Carlsbad, USA) according to instructions. Supernatant was collected for cytotoxicity analysis by using Lactic Dehydrogenase Release Assay Kit (C0016, Beyotime Biotechnology, Shanghai, China) according to instructions.
In vivo SYTOX green staining
After zebrafish were immersed by 400 μM DCA (n = 6, 1 larvae/well), 5 μM SYTOX Green nucleic acid stain was directly added into 96-well plate for 30 min in a dark place. After SYTOX staining, the larvae were directly anesthetized with 200 ng/mL tricaine and fluorescence intensity of stationary zebrafish in 96-well plate was detected at Ex488/Em525 nm using a Synergy H1 Microplate Reader (Bio Tek, Winooski, USA). Larvae was fixed in Low Melting Agarose to observe systemic and liver SYTOX green fluorescence. Representative images were captured on Olympus CKX53 inverted fluorescence microscope (Tokyo, Japan).
Recombinant protein incubation assays
The hit MeCbl was evaluated in concentration-response experiments in a dose range of 0.156-10 μM to determine IC50 for inhibition on liposome leakage. The inhibitory activity of four kinds of vitamin B12 was all evaluated at 1 μM. Procedure of liposome leakage assay using recombinant human His-sumo-GSDME was referred to section of high-throughput screen. For GzmB-GSDME-induced liposome leakage, 0.4 μM recombinant human His-sumo-GSDME was preincubated with 0.156–10 μM MeCbl for 0.5 h before addition of 0.4 μM recombinant GzmB for another 2 h at 37 °C. 200 μM liposome was then added for 30 min. For caspase-4-GSDMD-induced liposome leakage, 0.2 μM recombinant human His-sumo-GSDMD was pretreated with indicated concentrations of MeCbl for 0.5 h, then incubated with 0.2 μM active-caspase-4 for another 2 h, 200 μM liposome was then added into the caspase-4-GSDMD reaction system and incubated at 37 °C overnight for final fluorescence measurements. Tb3+/DPA fluorescence (RFU)= ((fluorescenceinhibitor –fluorescencenegative control)/(fluorescencepositive control −fluorescencenegative control)) × 100, where wells with gasdermin activation without compounds were used as positive control and wells with liposome alone as negative control.
For time course assay of liposome leakage, 1 μM MeCbl or DMF was pretreated with 0.4 μM His-sumo-GSDME, then incubated with 0.2 μM active-caspase-3 for another 2 h. Reaction well was finally incubated with 200 μM liposome and fluorescence intensity was detected every 10 min from 0 to 60 min.
Enzyme activity assay
Recombinant caspase-3 activity was detected with a fluorogenic substrate DEVD-AFC (1007, Biovision, San Francisco, USA). 0.2 μM recombinant active-caspase-3 was pretreated with compounds as indicated in reaction buffer (20 mM HEPES, 150 mM NaCl and 50 μM DPA), DEVD-AFC was then added at a final concentration of 200 μM. The reaction mixture was transferred to a 96-well plate and incubated at 37 °C for 30 min. Substrate cleavage was monitored by measuring the fluorescence at Ex400/Em505 nm on a Synergy H1 Microplate Reader (Bio Tek, Winooski, USA). Caspase-3 activity was indicated as relative fold change to control.
For cell lysates sample detection, caspase-3 activity in DCA treated cells were determined following the instructions of Caspase-3 Colorimetric Assay Kit (K106, Biovision, San Francisco, USA) with substrate Ac-DEVD-pNA and detected by a Synergy H1 Microplate Reader (Bio Tek, Winooski, USA). Caspase-3 activity was indicated as relative fold change to control.
Recombinant GzmB activity was detected with substrate Ac-IEPD-AFC (HY-P1092, New Jersey, USA). Briefly, 0.4 μM recombinant GzmB was pretreated with indicated concentrations of MeCbl in assay buffer (50 mM Tris, pH 7.4, 100 mM NaCl, 0.01% Tween 20) for 0.5 h before adding at a final concentration of 200 μM Ac-IEPD-AFC for another 0.5 h. Substrate cleavage was monitored as DEVD-AFC. GzmB activity was indicated as relative fold change to control.
Microscale thermophoresis (MST) assay
Direct binding between molecules were performed by MonolithTM NT.115 instrument (Nano Temper Technology, unich, Germany). Recombinant GSDME WT or Cys mutant proteins, caspase-3 and GSDMD were desalted and fluorescently labeled in advance by using a MonolithTM NT Protein Labeling Kit RED-MALEIMIDE (MO-L004, Nano Temper Technology, Munich, Germany). Gradient concentrations of molecules (0.001, 0.01, 0.1, 1, 10, 100 and 1000 μM) as indicated were incubated with 0.2 μM prelabelled recombinant proteins at a volume ratio of 1:1 for 30 min at room temperature. For measurements of the binding between active-caspase-3/GzmB and GSDME WT/Cys mutant: gradient concentrations of recombinant human active-caspase-3 or granzyme B (0.078, 0.156, 0.3125, 0.625, 1.25, 2.5, 5 μM) were incubated with 0.2 μM prelabelled recombinant GSDME proteins at a volume ratio of 1:1 for 10 min at room temperature. 10 μL molecule/protein-protein mixture was inhaled into capillary and loaded onto MonolithTM NT.115 instrument, then run the MST experiment using 40% LED-power and 80% MST-power. Data were analyzed using the NT Analyses 1.5.41 software. DMSO or ddH2O was used parallelly as Vehicle.
Flow cytometry analysis
For flow cytometry analysis, vector- or GSDME-expressing HEK293T were treated as indicated. In brief, approximately 1 × 106 cells per group were collected with 0.25% typsin without EDTA and centrifuged at 500 g for 5 min. Each group was resuspended by 200 μL 1×binding buffer containing 2.5 μL Annexin V-FITC and 2.5 μL 7-AAD (556547, BD Pharmingen, San Diego, USA) for 20 min at room temperature. 1 × 104 cells were collected and analyzed by a BD FACS Celesta flow cytometer (BD Biosciences, San Jose, USA). The Annexin V-FITC positive and 7-AAD negative cells were indicated as apoptosis, on the contrary, as pyroptosis.
Microscopy imaging of cell death and Immunofluorescence
To observe the morphological changes of apoptotic or pyroptotic cells, cells were seeded in cover-glass bottom dishes and treated as indicated in figure legends. Then, cell images were captured by using an LSM700 confocal microscope in DIC mode (Zeiss, Oberkochen, Germany).
To observe the distribution of GSDME in cytoplasm, cells were treated as indicated and washed with PBS/T (PBS containing Tween-20, 0.05% v/v), then fixed with 4% paraformaldehyde for 20 min before permeabilized with 0.2% Triton X-100 in 3% BSA for 15 min. Blocked with 3% BSA for 1 h at room temperature, then incubated with rabbit polyclonal anti-GSDME (1:100, A7432, ABclonal, Wuhan, China) in 3% BSA overnight at 4 °C. Washed dishes six times with PBS/T, incubated with Donkey Anti-Rabbit IgG(H + L) Antibody, Alexa Fluor 488 (1:1000, A-21206, Invitrogen) for 1 h at 37 °C in 3% BSA, then washed five times with PBS/T and incubated with DAPI Staining Solution (C1005, Beyotime Biotechnology, Shanghai, China) for another 15 min. Washed dishes five times, cell images were acquired on LSM700 confocal microscope (Zeiss, Oberkochen, Germany).
Negative staining electron microscopy
To observe the effect of MeCbl on liposome membrane rupture, 0.4 μM recombinant human His-sumo-GSDME was incubated with 1 μM MeCbl or DMF for 30 min before adding 0.2 μM recombinant active-caspase-3 for another 2 h at 37 °C. 200 μM liposome was then added into the reaction system for 30 min. For sample preparation of negative staining electron microscopy, 5 μL cleaved GSDME-liposome reaction sample was placed onto a glow-discharged carbon-coated copper grid and stained with 1% uranyl formate for 1 min, air dried. The grid was imaged on the JEOL JEM-1400 Flash electron microscope (Tokyo, Japan). At least 10 liposomes were analyzed per group for the quantitative data of the long diameter of liposomes.
Molecular modeling
The AlphaFold structure of GSDME (AF-O60443-F1-model_v2) was used to predict the binding site of MeCbl. In the structure preparation, we eliminated the loop region of 247-280 since this long loop shows very low quality with the pLDDT score <50 that may disrupt the identification of the binding site of MeCbl to GSDME. MD simulation was then conducted to equilibrate the protein structure using our previous protocol50. Next, we identified the binding site of MeCbl to GSDME by using molecular docking (Autodock Vina/1.1.251), where the docking center was set to each Cys site (a total of 13 Cys sites) in the protein with the docking space of 30 Å×30 Å×30 Å. Since the large structure of MeCbl that hinders the molecule bind to the deep pockets of the protein, only three docking simulations succeed in this process that correspond to three potential binding sites distributing on the protein surface (Cys45, Cys180, and Cys371 with the best docking score of -10.2, -6.0 and -5.5 kcal/mol, respectively). Therefore, these three sites were selected for further experimental validation.
Peptide mapping by mass spectrometry
To probe the binding sites of MeCbl on GSDME, 0.4 μM recombinant human GSDME (CSB-EP006766HU) purchased from CUSABIO (Wuhan, China) was incubated with 10 μM MeCbl in 100 μL buffer (20 mM HEPES, 150 mM NaCl and 50 μM DPA) at room temperature for 2 h and protected from light. 0.4 μM recombinant human GSDME was incubated with ddH2O as negative control, or with HOCbl for binding sites analysis between GSDME and cobalamin. All groups were equally prepared with ten incubation samples and mixed for further analysis. Trypsin and Glu C were then added to the mixture overnight, and formic acid was added to adjust the pH down to three to terminate the enzyme digestion. Activated the C18 desalting column with 100 μL 100% acetonitrile, then centrifuged at 400 g for 3 min. Added 100 μL of 0.1% formic acid and centrifuged at 400 g for 3 min for column equilibration. Replaced with new EP tube, added samples to the column, and centrifuged at 400 g for 3 min. Washed the column twice with 100 μL of 0.1% formic acid and centrifuged at 400 g for 3 min, washed once again with 100 μL water at pH10. The eluents were collected with 70% acetonitrile, each sample was lyophilized and stored at −80 °C until further mass spectrometry analysis.
Prepared the mobile phase solution, A (0.1% (v/v) formic acid in ddH2O), B (0.1% (v/v) formic acid and 80% acetonitrile in ddH2O), the lyophilized samples were dissolved with 10 µL solution A, then centrifuged at 14000 g for 20 min at 4 °C, 400 ng supernatant of the samples were injected onto a Thermo Scientific™ UltiMate™ 3000 UHPLC coupled to a timsTOF HT mass spectrometer (BRUKER). Peptides were then separated over 100 μm × 15 cm ReproSil-Pur C18-AQ 1.5-µm silica beads (Beijing Qinglian Biotech Co., Ltd, Beijing, China), at a flow rate of 300 nL/min using a gradient: 0–4 min (5–10% B), 4–46 min (10–24% B), 46–53 min (36% B), 53–54 min (95% B), 54–60 (95% B). The timsTOF HT mass spectrometer was used for liquid quality detection with a Captive Spray source, and the mass spectrum was collected in DDA mode. The scanning range of the mass spectrum was m/z 100-1700, the primary mass spectrum resolution was set to 60,000 (1222 m/z), and the cumulative time was set to 100 ms in the TIMS tunnel. The capillary voltage is set at 1.6 kV and the mobility is 0.6–1.6 cm2/(V). The total cycle time was 1.1 s with 10 PASEF cycles. The mass spectrometry raw data was processed by FragPipe to search against the target protein database. The precursor ion mass tolerance was ±15 ppm; the fragment mass tolerance was ±0.02 Da. The static modification was carbamidomethylation of cysteine; the dynamic modifications were oxidation of methionine, acetylation of N-termini of peptides, and C63H91CoN13O14P or C62H88CoN13O14P of cysteine; a maximum of two missed cleavages were allowed.
FPOP-LC-MS/MS analysis
Sample preparation and LC-MS/MS analysis
A total of 5 μM recombinant His-sumo-GSDME was incubated with 250 μM MeCbl for 1 h, then added 1 mM H2O2 and 200 mM methionine, irradiated with 355 nm ultraviolet pulsed laser (LWUVL355-120uJ, Laserwave, Beijing, China) for 1 min, vortexed and irradiated again for 1 min. Added 20 μL methionine after irradiation to end the reaction. Discarded H2O2 and methionine, added 25 mM NH4HCO3, transferred sample to ultrafiltration centrifuge tube (3 kDa, UFC500396, Millipore, Boston, USA), and centrifuged at 12,000 g for 20 min. Added 200 μL of 25 mM NH4HCO3 and centrifuged again. Added 25 mM NH4HCO3 to ultrafiltration membrane, pipetted up and down. Inserted the tube into a new tube upside down and centrifuged at 2500 g for 5 min. Added quadruple volume of 10 M urea into tube and denatured at 25 °C for 1 h on thermostatic mixer. After denaturation, added 500 μM DTT and reacted at 56 °C for 30 min, then added 550 μM iodoacetamide, protected from light and reacted at room temperature for 30 min, finally added 16 μL DTT and mixed. Transferred the mixture to an ultrafiltration centrifuge tube, centrifuged at 12,000 g for 20 min, discarded solution, added 50 mM NH4HCO3, centrifuged and discarded liquid, then added 50 mM NH4HCO3, centrifuged and discarded solution. 50 mM NH4HCO3 was added to centrifuge tube and inserted upside down into a new tube, centrifuged at 2500 g for 5 min, transferred protein solution to chromatographic sample bottles and added 2 mg/mL Trypsin into sample bottles, reacted at 37 °C for 20 h. Added 10% formic acid to stop enzymatic hydrolysis, then evaporated solvent. Re-dissolved the sample with 100 μL 0.1% formic acid and then desalted with ZipTip (ZTC18S096, Millipore, Boston, USA). After desalting, evaporated sample solvent, added 25 μL 0.1% formic acid to re-dissolve the sample, centrifuged at 12000 g for 10 min, pipetted 22 μL supernatant into a new tube, centrifuged at 12,000 g for 10 min, and pipetted 20 μL supernatant to sample bottles for FPOP-LC-MS/MS analysis. Protein samples were analyzed using Orbitrap Eclipse high-resolution mass spectrometer (FSN40304, ThermoFisher Scientific, Waltham, USA). Mobile phase consisted an aqueous solution containing 0.1% formic acid and aqueous solution containing 0.1% formic acid, 80% acetonitrile, flow rate was 300 nL/min.
FPOP data analysis
Data acquired by LC-MS/MS of oxidized and unoxidized peptides were identified by PEAKS Studio 11 (Bioinformatics Solutions Inc) as previously described. Unoxidized and oxidized peptide peaks were quantified by integration of the selected ion chromatogram peaks of unoxidized and oxidized peptides, respectively, plus 1/2 oxygen atoms. Normalized peptide oxidized (%)= ∑oxidizedpeaks/unoxidized + ∑oxidizedallpeaks.
Cobalamin-GSDME binding mode assay
UV-visible spectroscopy
All measurements were carried out in a UV-2600i spectrophotometers (SHIMADZU, Kyoto, Japan) at room temperature and baseline corrected for reaction buffer (20 mM HEPES, 150 mM NaCl and 50 μM DPA) in a quartz cuvette. To monitor the binding of cobalamin and GSDME, 10 μM MeCbl or HOCbl was preincubated with 10 μM recombinant GSDME protein for 30 min in reaction buffer at room temperature, then transferred to quartz cuvettes to record absorbance spectrum in the range of 320–600 nm, free GSDME was set as negative control, and free MeCbl or HOCbl was used for monitoring the absorbance changes compared to cobalamin-GSDME mixture. Data were exported and the absorbance curves were analyzed using GraphPad Prism 8.2.1 software. Experiments were performed in triplicate.
Cobalamin conversion
10 μM MeCbl was preincubated with 0.4 μM recombinant human His-sumo-GSDME for 30 min, the complex was then illuminated with 60 W bulb with 15–20 cm distance overnight to convert MeCbl to HOCbl. After conversion, 0.2 μM recombinant active-caspase-3 was added for another 2 h at 37 °C. MeCbl-GSDME mixture without such conversion and HOCbl-GSDME incubation mixture were used as comparison groups. All mixtures were subjected to SDS-PAGE electrophoresis for GSDME activation assay.
Co-immunoprecipitation
A total of 5 × 106 overexpressed or WT hepatocytes, or 10 mg liver tissue homogenate were harvested and lysed in 300 μL NP-40 lysis buffer (P0013F, Beyotime Biotechnology, Shanghai, China) containing protease inhibitor cocktail (P8340, Sigma-Aldrich). Lysates with 500 μg protein were diluted into 500 μL NP-40 lysis buffer and used for co-immunoprecipitation assay according to instructions. Briefly, lysates were preincubated with rabbit monoclonal anti-GSDME antibody (ab215191, 1: 100) overnight at 4 °C. 50 μL of Pierce™ protein A/G beads (88802, ThermoFisher Scientific, Waltham, USA) were prewashed three times with PBS/T (0.1% Tween 20), then incubated with lysates-antibody mixture for 6 h at 4 °C. Wash with PBS/T for three times and NP-40 lysis buffer once, the immunoprecipitates were boiled up for 15 min in 100 μL of 2× sample buffer before western blot analysis. Cell or tissue lysates containing 60 μg protein were used to western blot analysis as input.
Western blotting
Cells or homogenized tissue were lysed in RIPA solution containing PIC (P8340, Sigma-Aldrich). Protein contents were quantitated by the BCA assay kit (Beyotime Biotechnology, Shanghai, China) before the addition of XT Sample Buffer (1610791, Bio-Rad) and boiled. A total of 60 μg of protein was subjected to electrophoresis (Bio-Rad), transferred to 2.2 μm polyvinylidene fluoride membranes (1620177, Bio-Rad) and probed with primary antibodies followed by HRP-conjugated Anti-Rabbit IgG (H + L) antibody (ab6721, Abcam) or HRP-Conjugated Anti-Mouse IgG (H + L) antibody (ab6789, Abcam). The immunoreactive bands were captured with HRP substrate (170-5061, Bio-Rad) and detected with iBright CL1000 System (Invitrogen, Carlsbad, USA). Blots were analyzed by iBright Analysis Software Version 3.0.1 (Invitrogen, Carlsbad, USA).
For the detection of GSDME in recombinant protein incubation system, 50 μL incubation mixture was mixed with XT Sample Buffer (1610791, Bio-Rad) and boiled. 15 μL boiled sample was subjected to SDS-PAGE electrophoresis. Immunoblots were probed with indicated antibodies and visualized using HRP-conjugated secondary antibodies with HRP substrate.
Statistical analysis
GraphPad Prism 8.2.1 software was used to perform statistical analysis. All results are based on biological replicates and presented as means ± SEM. Statistical significance of differences between the two groups was analyzed by two-tailed unpaired Student’s t test, between multiple groups by one-way or two-way analysis of variance (ANOVA). Formula of log(inhibitor) vs. normalized response-Variable slope in Dose-response-Inhibition procedure of GraphPad Prism 8.2.1 software was used to obtain the IC50 value and nonlinear curve fit. Log-rank (Mantel-Cox) test was used for survival analysis (n = 60 in zebrafish experiment, n = 10 in mice experiment). p values of <0.05 were considered significant, otherwise indicated as nonsignificant (n.s). One representative image of at least two independent experiments/samples is shown. Flow cytometry analysis were carried out using BD FACS Celesta flow cytometer. Calculation of dissociation constant Kd was carried out using NT Analyses 1.5.41 software; nonlinear curve-fitting of the binding was performed and Kd was calculated automatically. Quantitative data of the long diameter of liposomes (n = 10) and damaged area (%) of H&E histological staining (n = 6) were recorded using Image J software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data associated with this study are present in the paper or the Supplementary Information. Source data are provided with this paper. Source data of each figure, including uncropped blots, are provided in a single Excel document labeled ‘Source Data’. Images, flow cytometry, sequencing data of plasmids and validation reports of recombinant proteins have been deposited to Figshare (https://doi.org/10.6084/m9.figshare.27014824). The raw data of mass spectrometry analysis generated in this study have been deposited in the ProteomeXchange Consortium (iProX partner repository) with the dataset identifier PXD057768. All data are available from the corresponding authors upon request. Source data are provided with this paper.
References
Newton, K., Strasser, A., Kayagaki, N. & Dixit, V. M. Cell death. Cell 187, 235–256 (2024).
Yuan, J. Y. & Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Bio 25, 379–395 (2024).
Liu, X., Xia, S. Y., Zhang, Z. B., Wu, H. & Lieberman, J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov. 20, 384–405 (2021).
Ding, J. et al. Erratum: Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 540, 150 (2016).
Zhou, Z. W. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, 965–974 (2020).
Zhang, Z. B. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020).
Shi, J. J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Hou, J. W. et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 22, 1396–1396 (2020).
Wang, Y. P. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Dong, S. W. et al. Gasdermin E is required for induction of pyroptosis and severe disease during enterovirus 71 infection. J. Biol. Chem. 298, 101850 (2022).
Zhang, Y., Cai, X. B., Wang, B., Zhang, B. H. & Xu, Y. Q. Exploring the molecular mechanisms of the involvement of GZMB-Caspase-3-GSDME pathway in the progression of rheumatoid arthritis. Mol. Immunol. 161, 82–90 (2023).
Perricone, G. et al. Intensive care management of acute-on-chronic liver failure. Intens. Care Med 49, 903–921 (2023).
Lemmer, P., Pospiech, J. C. & Canbay, A. Liver failure-future challenges and remaining questions. Ann. Transl. Med. 9, 734 (2021).
Chen, J. Y., Li, X. P., Ge, C. D., Min, J. X. & Wang, F. D. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 29, 467–480 (2022).
Schwabe, R. F. & Luedde, T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat. Rev. Gastro Hepat. 15, 738–752 (2018).
Xu, W. F. et al. Apaf-1 pyroptosome senses mitochondrial permeability transition. Cell Metab. 33, 424–436 (2021).
Humphries, F. et al. Succination inactivates gasdermin D and blocks pyroptosis. Science 369, 1633–1637 (2020).
Zhang, Y. F. & Ning, G. Mecobalamin. Expert Opin. Inv Drug 17, 953–964 (2008).
Hao, H. P. et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 25, 856–867 (2017).
Rogers, C. et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 8, 14128 (2017).
Amin, S., Thywissen, A., Heinekamp, T., Saluz, H. P. & Brakhage, A. A. Melanin dependent survival of conidia in lung epithelial cells. Int J. Med Microbiol 304, 626–636 (2014).
Shimmyo, Y., Kihara, T., Akaike, A., Niidome, T. & Sugimoto, H. Three distinct neuroprotective functions of myricetin against glutamate-induced neuronal cell death: Involvement of direct inhibition of caspase-3. J. Neurosci. Res 86, 1836–1845 (2008).
Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778–783 (2022).
Dubey, K. D. & Shaik, S. Cytochrome P450-The Wonderful Nanomachine Revealed through Dynamic Simulations of the Catalytic Cycle. Acc. Chem. Res 52, 389–399 (2019).
Uhlich, N. A. et al. Structure and binding of peptide-dendrimer ligands to vitamin B. Chembiochem 11, 358–365 (2010).
Rempel, S., Colucci, E., de Gier, J. W., Guskov, A. & Slotboom, D. J. Cysteine-mediated decyanation of vitamin B12 by the predicted membrane transporter BtuM. Nat. Commun. 9, 3038 (2018).
Kim, J., Gherasim, C. & Banerjee, R. Decyanation of vitamin B by a trafficking chaperone. Proc. Natl Acad. Sci. USA 105, 14551–14554 (2008).
Fleischhacker, A. S. & Matthews, R. G. Ligand trans influence governs conformation in cobalamin-dependent methionine synthase. Biochem.-Us 46, 12382–12392 (2007).
Yamada, K., Gravel, R. A., Toraya, T. & Matthews, R. G. Human methionine synthase reductase is a molecular chaperone for human methionine synthase. Proc. Natl Acad. Sci. USA 103, 9476–9481 (2006).
Wang, X. et al. Characterization of GSDME in amphioxus provides insights into the functional evolution of GSDM-mediated pyroptosis. PLoS Biol. 21, e3002062 (2023).
Wang, Z. et al. Zebrafish GSDMEb cleavage-gated pyroptosis drives septic acute kidney injury in vivo. J. Immunol. 204, 1929–1942 (2020).
Forn-Cuní, G., Meijer, A. H. & Varela, M. Zebrafish in inflammasome research. Cells-Basel 8, 901 (2019).
Zhang, Q. H., Wang, J. J., Huang, F., Yao, Y. L. & Xu, L. Leptin induces NAFLD progression through infiltrated CD8+T lymphocytes mediating pyroptotic-like cell death of hepatocytes and macrophages. Dig. Liver Dis. 53, 598–605 (2021).
Han, Y. et al. Membrane-delimited signaling and cytosolic action of MG53 preserve hepatocyte integrity during drug-induced liver injury. J. Hepatol. 76, 558–567 (2022).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Rana, N. et al. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis (vol 185, pg 283, 2022). Cell 187, 1011–1015 (2024).
Xu, W. F. et al. Gasdermin E-derived caspase-3 inhibitors effectively protect mice from acute hepatic failure. Acta Pharm. Sin. 42, 68–76 (2021).
Lu, N. et al. Inhibiting apoptosis and GSDME-mediated pyroptosis attenuates hepatic injury in septic mice. Arch. Biochem Biophys. 754, 109923 (2024).
Rathkey, J. K. et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 3, eaat2738 (2018).
Hu, J. J. et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020).
Ide, H. et al. Clinical usefulness of intrathecal injection of methylcobalamin in patients with diabetic neuropathy. Clin. Ther. 9, 183–192 (1987).
Koyama, K., Usami, T., Takeuchi, O., Morozumi, K. & Kimura, G. Efficacy of methylcobalamin on lowering total homocysteine plasma concentrations in haemodialysis patients receiving high-dose folic acid supplementation. Nephrol. Dial. Transpl. 17, 916–922 (2002).
Shibuya, K. et al. Safety and efficacy of intravenous ultra-high dose methylcobalamin treatment for peripheral neuropathy: a phase i/ii open label clinical trial. Intern. Med 53, 1927–1931 (2014).
Paolicelli, D., Manni, A., Iaffaldano, A. & Trojano, M. Efficacy and safety of oral therapies for relapsing-remitting multiple sclerosis. Cns Drugs 34, 65–92 (2020).
Schmitt, A. et al. BRD4 inhibition sensitizes diffuse large B-cell lymphoma cells to ferroptosis. Blood 142, 1143–1155 (2023).
Liebmann, M. et al. Dimethyl fumarate treatment restrains the antioxidative capacity of T cells to control autoimmunity. Brain 144, 3126–3141 (2021).
McMillin, M. & Frampton, G. Thrombospondin-1 inhibition reduces nrf2 activity and increases liver injury during acetaminophen-induced hepatotoxicity. Faseb J. 35, S1.02127 (2021).
Zheng, X., Cai, X. Y. & Hao, H. P. Emerging targetome and signalome landscape of gut microbial metabolites. Cell Metab. 34, 35–58 (2022).
Yang, D. et al. Sensing of cytosolic LPS through caspy2 pyrin domain mediates noncanonical inflammasome activation in zebrafish. Nat. Commun. 9, 3052 (2018).
Tang, R. et al. Characterizing the stabilization effects of stabilizers in protein–protein systems with end-point binding free energy calculations. Brief. Bioinform. 23, bbac127 (2022).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Acknowledgements
This work was supported by National Natural Science Foundation of China (grants to 81930109, 82321005 to H.P.H; grants 82173886, 82373886 to L.J.C; grant 82304590 to W.F.X; grant 82204512 to S.C); Jiangsu Funding Program for Excellent Postdoctoral Talent (grant 2022ZB312 to S.C); Shenzhen Basic Research Program (grant JCYJ20220530151415034 to W.F.X); Medical Science and Technology Research Foundation of Guangdong (grant A2023228 to W.F.X). We greatly appreciate Dr. Feng Shao at the National Institute of Biological Sciences (Beijing, China) for kindly providing Gsdmd-/-, and Gsdme-/- mice. We thank Beijing Qinglian Biotech Co.,Ltd for mass spectrometry spectra analysis support, and thank Nanjing Hunter Biotechnology Co., Ltd for zebrafish model of MeCbl treatment, survival curve and phenotype analysis.
Author information
Authors and Affiliations
Contributions
H.P.H., L.J.C., and W.F.X. formulated the original idea and conceived the study. H.P.H., L.J.C., H.Y.S., and W.F.X. designed the experiments, carried out the analysis and interpretation, and wrote and revised the manuscript. H.P.H., L.J.C. supervised the work. L.J.C., H.Y.S., Q.L.Z., W.F.X., Y.W., S.C., Y.X.X., Y.M.Z., Y.H.L., Q.Q.C., C.Z., Y.J.L., X.J., and M.N.Q. performed the experimental studies. All authors contributed to the manuscript revision and review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sergey Fedosov, Yoshihiro Shidoji and Yue Wu for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, W., Wang, Y., Cui, S. et al. Methylcobalamin protects against liver failure via engaging gasdermin E. Nat Commun 16, 1233 (2025). https://doi.org/10.1038/s41467-024-54826-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-54826-6
This article is cited by
-
Multifunctional nanoagent for enhanced cancer radioimmunotherapy via pyroptosis and cGAS-STING activation
Journal of Nanobiotechnology (2025)