Abstract
Wide-bandgap perovskite solar cells (WBG-PSCs) are critical for developing perovskite/silicon tandem solar cells. The defect-rich surface of WBG-PSCs will lead to severe interfacial carrier loss and phase segregation, deteriorating the device’s performance. Herein, we develop a surface reconstruction method by removing the defect-rich crystal surface by nano-polishing and then passivating the newly exposed high-crystallinity surface. This method can refresh the perovskite/electron-transporter interface and release the residual lattice strain, improving the charge collection and inhibiting the ion migration of WBG perovskites. As a result, we can achieve certified efficiencies of 23.67% and 21.70% for opaque and semi-transparent PSCs via a 1.67-eV perovskite absorber. Moreover, we achieve four-terminal perovskite/silicon tandem solar cells with a certified efficiency of 33.10% on an aperture area of one square centimeter.
Similar content being viewed by others
Introduction
Single-junction perovskite solar cells (PSCs) have emerged as one of the most promising candidates for future photovoltaic (PV) technology owing to their remarkable power conversion efficiency (PCE) growth from 3.8% to 26.7% in only one decade1,2,3,4,5,6,7. Due to the advantage of the tunable bandgap of perovskite, researchers have developed perovskite-based tandem solar cells to break the single-junction Shockley-Queisser limit by stacking the wide-bandgap (WBG) perovskite top cell with another narrow-bandgap (NBG) bottom solar cell, including NBG-PSC, silicon solar cell, organic solar cell, etc.8,9,10,11. Among them, perovskite/silicon tandem solar cells are attracting intense research interest because silicon-based solar cells are dominating the photovoltaic industry and seeking efficiency upgrading12,13,14.
Developing efficient and stable WBG-PSCs is critical in determining the performance and stability of perovskite/silicon tandem solar cells10,12,14,15,16,17. The WBG perovskite is typically prepared with mixed Br-I halide components, and the corresponding crystalline film surface suffers issues like disordered crystal packing, component vacancies, and randomly distributed halide components, resulting in a defect-rich crystal surface18,19. The defect-rich crystal surface will cause severe carrier recombination, open-circuit voltage (VOC) loss, and accelerate photoinduced phase separation15,20,21,22,23,24. Due to these undesirable factors, the highest certified efficiency of WBG-PSCs is currently limited to 22.95%25.
In recent years, researchers have spared efforts to remove or passivate the aforementioned crystal surface defects2,18,26,27,28,29,30. Most previous efforts are additive manufacturing methods, i.e., depositing another layer on top of the perovskite active layer. For example, WBG materials29,31, two-dimensional (2D) perovskites32,33,34,35, ultrathin insulated materials36,37, and Lewis acid/base38,39 have been utilized as effective passivation layers. However, these passivation chemicals seem unique for specific perovskite compositions and not universal. Moreover, although the defective outmost surface layer can be passivated by additional chemicals, the residual surface layer in the thickness of tens of nanometers remains defective. These regions with poor crystalline quality can facilitate ion migration, they are prone to causing ion migration and photoinduced phase separation in WBG-PSCs, thereby compromising their stability40,41.
To eliminate the defect-rich layer on the perovskite film’s surface, Lin et al. employed a dry mechanical polishing method to remove the amorphous surface layer, thereby suppressing ion migration and enhancing the stability of perovskite films42. Chen et al. employed a facile approach via tape stripping to mechanically peel off the soft defect-rich region on the perovskite film’s surface43. This represents a universally effective method for enhancing the stability of various perovskite compositions’ stability and PSCs’ stability with different device areas43. Although removing the perovskite surface layer via dry mechanical methods is facile processing, it is suspected to be more likely to cause surface scratches or uneven surface morphology, leading to limited controllability and repeatability in fabricating devices. We propose that wet polishing strategies, which utilize polishing nanoparticles in a liquid medium, can reduce the friction between perovskite and the polishing medium as well as help wash away the removed perovskite particles during the polishing process, thus avoiding scratches and improving polishing reproducibility.
In this work, we developed a surface reconstruction method to remove the defect-rich surface layer of the 1.67-eV WBG perovskite by wet nano-polishing and then passivate the newly exposed surface, which achieved record-breaking single-junction and four-terminal (4T) perovskite/silicon tandem solar cells. We found that the surface reconstruction strategy can improve the contact of the perovskite/C60 interface, release the residual lattice strain of the bulk perovskite film, and form a Br-rich perovskite surface layer, which effectively suppresses the interface carrier loss and the ion migration. Therefore, the VOC and fill factor (FF) have been significantly improved, producing a record efficiency of 24.48% (certified 23.67%) for the opaque PSCs and a record efficiency of 21.95% (certified 21.70%) for the ST-PCSs. The corresponding devices retained 80% of the initial efficiency after operation at the maximum power point under 1-sun illumination for 1505 h, showing enhanced operational stability. Moreover, by physically stacking the as-fabricated ST PCSs with a hybrid-back-contact (hybrid-BC) silicon bottom solar cell, we achieved 4T perovskite/silicon tandem solar cells with a record certified efficiency of 33.10% (1 cm2). This work reveals that the surface passivation of perovskite films with a high density of defects like WBG perovskite films requires a pre-step to reduce the defect density in the initial films, indicating directions for designing surface engineering strategies for further boosting the performance of WBG-PSCs.
Results
Morphology and crystallinity change of perovskite films after removing the defect-rich layer by nano-polishing
The deposition process of the 1.67-eV WBG perovskite films can be found in the experimental section. We found that solution-processed WBG perovskite films usually have relatively rough surfaces that contain many defect-rich regions (Fig. 1a). To remove the defect-rich surface layer, nano-polishing was applied to treat the glass/ITO/NiOx/Me-4PACz/perovskite sample (Supplementary Fig. 1a), where ITO is indium tin oxide and Me-4PACz is [4-(3,6-dimethyl-9H-carbazol-9-yl)butyl]phosphonic acid. The detailed process of the nano-polishing method (Supplementary Fig. 1b) can be found in the experimental section. During the optimization of nano-polishing, we found that dry polishing strategies led to surface abrasions on the perovskite film and severe hysteresis effect for the PSCs. Therefore, we developed a wet polishing protocol utilizing anisole and nanometric abrasive particles (Supplementary Figs. 2–4 and Supplementary Table 1)42. The effect of anisole was providing a liquid medium for polishing and simultaneously helping clear the polished perovskite residues, avoiding scratches on the perovskite surface. Incorporating Al2O3 nanoparticles facilitates a ball-bearing effect between the perovskite film and the polishing substrate, thereby mitigating friction during polishing.
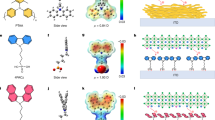
a, b Cross-sectional SEM images of perovskite films before and after nano-polishing treatment. Cross-sectional TEM images of the full solar cell devices based on c control perovskite film and d target perovskite film. High-resolution TEM images of e region I and f region II. g Schematic diagram of the changes in perovskite film before and after nano-polishing treatment. Nano-polishing can remove surface defects (I), restrain phase separation (II), release the residual strain (III), and create the Br-rich interface (IV).
After nano-polishing, a thin layer with a thickness of several tens of nanometers was eliminated from the annealed perovskite film, producing an ultra-smooth perovskite surface (Fig. 1b). Scanning electron microscope (SEM) images in Supplementary Fig. 5 reveal that the perovskite surface turn from rough into ultra-smooth as the treatment time increases. The removal thickness increased from approximately 20 nm to 50 nm and 80 nm for the treatment time of 15 s, 30 s, and 45 s, respectively. In our method, the removal thickness of the surface layer was proportional to the polishing time. Preliminary solar cell performance screening experiments showed that the optimized treatment time of about 30 s with a removal thickness of 50 nm delivered the highest device efficiency. Further extending the nano-polishing time led to a significant drop in the short-circuit current density due to insufficient light absorption of the perovskite active layer. Unless otherwise stated, the treatment time of 30 s was used hereafter to prepare the sample for further characterization. As shown in Supplementary Fig. 6a, b, it reduced the surface roughness from 12.5 nm of the control to 3.3 nm after nano-polishing treatment. The cross-sectional transmission electron microscopy (TEM) images of the as-fabricated PSCs before and after nano-polishing treatment are depicted in Fig. 1c, d and Supplementary Fig. 6c, d. The ultra-smooth surface of nano-polishing treated perovskite film enabled more uniform deposition for the following functional layers, such as C60, bathocuproine (BCP), and Ag, bringing in improved contact between the perovskite film and the C60 layer. High-resolution TEM images (Fig. 1c, e) of the control perovskite film showed that the surface region within the thickness of 40 nm consisted of amorphous substances, which was possibly due to inadequate crystallization at the film surface. After the nano-polishing treatment, the amorphous layer was eliminated, and the surface layer showed high crystallinity (Fig. 1d, f)42,43,44. Region II of the treated device (Fig. 1f) displays the neat lattice fringes with a distance of 6.6 Å that could be assigned to the (001) crystal plane of α-FAPbI345.
The schematic diagram in Fig. 1g summarizes the potential changes in perovskite film after nano-polishing. Previous reports show that inadequate crystallization would produce lattice defects and serious charge loss18. The SEM and high-resolution TEM results proved that nano-polishing treatment can precisely remove the defect-rich region and expose the highly crystalline surface (Effect I). In addition, the defect-accelerated ion migration of perovskite can be suppressed due to the elimination of the defect-rich surface, so nano-polishing also retrains the phase separation (Effect II). Nano-polishing also suppresses the crystallinity inhomogeneity of the film, which can reduce the lattice mismatch between the surface and the bulk, releasing the residual lattice strain (Effect III). Finally, nano-polishing can create a Br-rich surface due to the different hardness of Br-related and I-related perovskites (Effect IV). The experimental evidence that supports these claims will be provided and discussed in the following parts.
Suppression of surface defects and ion migration after nano-polishing treatment
We used X-ray photoelectron spectroscopy (XPS) to check the chemical composition of the perovskite film surface. Compared to the control film, the XPS intensity of Pb0 defects in the perovskite film treated by nano-polishing remarkably decreased (Fig. 2a). X-ray diffraction (XRD) and grazing-incidence wide-angle X-ray scattering (GIWAXS) were employed to evaluate the crystallinity of the control and nano-polishing treated films (Supplementary Fig. 7). The results demonstrated that the XRD peak intensity of the (001) crystal plane of perovskite was considerably enhanced after nano-polishing, which can be attributed to the effective removal of insufficient crystallization regions, leading to improved crystallinity at the film surface and therefore enhanced overall crystallinity of the whole film. GIWAXS results also showed enhanced surface crystallinity.
a The XPS spectra of Pb 4f, b, c PL mapping of the control and nano-polishing treated perovskite films. d Normalized TRPL curves of the control and nano-polishing treated perovskite films. e, f The evolution of PL spectra as a function of illumination time of the control and nano-polishing treated perovskite films. g Schematic diagram of the ion migration measurements for perovskite films. h, i PL intensity of the control and nano-polishing treated perovskite films after aging under the electric field stress. The excitation source for the PL mapping test was a 488 nm laser.
Next, we investigated the effect of nano-polishing on the carrier dynamics and ion migration of perovskite films under the stress of different physical fields. The perovskite film after nano-polishing revealed a noteworthy enhanced photoluminescence (PL) intensity and a minor blue shift of the PL peak (Supplementary Fig. 8a). PL mapping further affirmed the strengthened PL intensity and more uniform PL distribution (Fig. 2b, c). Time-resolved photoluminescence (TRPL) showed that the charge carrier lifetime of nano-polishing treated perovskite film underwent a significant enhancement, with the average lifetime increased from 413 ns of the control to 869 ns of nano-polishing treated sample (Fig. 2d and Supplementary Table 2). These measurements indicated a notable reduction in the non-radiative carrier recombination in the perovskite films.
The evolution of the PL peak of perovskite films as a function of time under simulated sunlight (AM1.5G, 1 sun) irradiation was employed to estimate the light-induced halide components segregation. When the experiment was done in the nitrogen environment, the control film showed a significant redshift of the PL peak from the initial 742 nm to 748 nm after 60 min of illumination. In contrast, the PL peaks of the nano-polishing treated film did not shift (Fig. 2e, f). When exposing the samples to the air under the stress of light and moisture, both the control film and the nano-polishing treated film exhibited a redshift. However, the control film displayed a significant peak splitting, while the nano-polishing treated film maintained a single peak (Supplementary Fig. 8b, c). These results indicate that nano-polishing can suppress the phase segregation of the WBG perovskite film.
The ion migration behavior was also studied by PL mapping after applying a strong electric field to the perovskite films. The device’s current as a function of time after applying a voltage of 30 V was monitored (Fig. 2g and Supplementary Fig. 9). The control device exhibited an abrupt and substantial current increase after 26 min, attributed to ion-migration induced current. In contrast, the device subjected to nano-polishing did not demonstrate this behavior. After subjecting both films to a 30 V bias for 30 min, the PL quenching of the control perovskite film was observed, while the nano-polishing treated perovskite film still demonstrated strong and uniform fluorescence (Supplementary Fig. 10 and Fig. 2h, i). These results demonstrate that nano-polishing can effectively inhibit the ion migration and phase separation of WBG perovskite films, which can be attributed to the elimination of the inadequate crystallization regions on the film surface.
Properties of the nano-polishing treated perovskite film and the perovskite/C60 interface
The manufacture of metal workpieces typically involves a polishing procedure to reduce the residual stress, ultimately enhancing their mechanical strength and fatigue life. Lattice strain strongly modulates the carrier dynamics and the degradation of perovskite thin films. Grazing incident X-ray diffraction (GIXRD) was employed to assess whether nano-polishing can facilitate the release of in-plane residual stress in perovskite films. We measured the GIXRD patterns with a penetration depth of 200 nm and analyzed the lattice strain using the classic sin2φ method, where φ is the angle of the diffraction vector with respect to the sample’s normal direction. We fixed the 2θ and varied the instrument tilt angle ψ to obtain corresponding diffraction patterns46. As ψ varied between 10° and 50°, we observed a leftward shift in the scattering peak for the control film (Fig. 3a). In contrast, the GIXRD peak of the nano-polishing treated film showed almost no shift (Fig. 3b). Generally, sin2φ and 2θ follow a linear relationship, and the slope of the fitting line stands for the magnitude of the residual strain. As shown in Fig. 3c, the fitting line of the control sample exhibited a negative value in the slope, which means the corresponding (012) crystal planes exhibit an enlarged distance. It thus indicates the entire sample is subjected to tensile strain47. In contrast, the nano-polishing treated sample exhibited a significantly reduced negative slope, indicating largely suppressed lattice strain. Using the method described in Supplementary Note 1, it was found that the residual stress of the film before and after nano-polishing treatment is 20.90 MPa and 10.47 MPa, respectively (Fig. 3c and Supplementary Table 3).
GIXRD of the a control and b nano-polishing treated perovskite films. c Linear regression analysis of the GIXRD data. d XPS relative element contents of Br and I of the control and nano-polishing treated perovskite film. e ToF-SIMS results of the full solar cell devices based on the control and nano-polishing treated perovskite films. The element ratio curves of Br and the intensity of I ion. The device structure is glass/ITO/NiOx/Me-4PACz/perovskite/C60/BCP/Ag. f The nanoindentation depth of the control and nano-polishing treated perovskite films. Contact potential difference of the g control and h nano-polishing treated perovskite films. i Energy level alignment of the control and nano-polishing treated perovskite films with C60.
XPS elemental analysis showed that the (Br+I)/Pb ratio of the control and nano-polishing treated samples was 2.76 and 2.96, respectively, indicating that the perovskite film obtained by nano-polishing treatment was closer to the stoichiometric ratio of 3:1. Additionally, the Br/(Br+I) ratio of the control and nano-polishing treated samples was 16.9% and 19.5%, respectively, indicating that the Br content on the perovskite film surface was increased after nano-polishing treatment (Fig. 3d and Supplementary Table 4). Energy dispersive spectroscopy (EDS) also supported the increased Br content at the perovskite surface after nano-polishing (Supplementary Fig. 11 and Supplementary Table 5). Time of flight secondary ion mass spectrometry (ToF-SIMS) was tested to detect the longitudinal element distribution of the control and nano-polishing treated devices. The results showed that the Br content at the perovskite/C60 interface in the target device was higher than that of the control (Fig. 3e and Supplementary Fig. 12). This provides further evidence that the target device formed a Br-rich interface.
To elucidate why nano-polishing produces a Br-rich perovskite surface, we studied the mechanical hardness of the perovskite films. We employed the nanoindentation technique to determine the hardness of perovskite films with different Br contents while keeping the contents of cations (Cs, FA, and MA) unchanged. The results showed that the hardness of the perovskite film with higher Br content was larger than those with higher I content (Supplementary Fig. 13). Therefore, the I-rich species can be preferentially removed during the nano-polishing process due to its lower hardness, resulting in Br-rich perovskite surface (Effect IV in Fig. 1g). The perovskite film’s hardness also increased from 0.82 GPa to 1.03 GPa after nano-polishing treatment (Fig. 3f), further supporting the preferential removal of the soft species by nano-polishing treatment. Additionally, due to the formation of the Br-rich phase, the XPS peaks of both Br 3d and I 3d moved towards lower binding energies (Supplementary Fig. 14).
We further investigated the effect of the Br-rich perovskite surface on the energetics of the perovskite/C60 interface. Kelvin probe force microscopy (KPFM) was employed to measure the distribution of contact potential difference (CPD) between the AFM tip and the perovskite sample. The CPD distribution of the control was uneven, with an average value of −403 mV (Fig. 3g). In contrast, the nano-polishing treated sample showed very uniform CPD with an average value of −561 mV (Fig. 3h). The increased CPD indicated a reduced work function of the perovskite after nano-polishing treatment. We used ultraviolet photoelectron spectroscopy (UPS) to characterize the energy-level alignment at the perovskite/C60 interface. It was found that nano-polishing treatment caused the Fermi level to shift from −4.81 eV to −4.68 eV. The higher Fermi level of the perovskite surface at the n-side of the device would result in a higher built-in voltage (Vbi)29. The conduction band minimum (CBM) of the nano-polishing perovskite film changed from −4.21 eV to −4.31 eV, making better energy-level alignment with the lowest unoccupied molecular orbital (LUMO) of C60 (−4.35 eV)48, which may facilitate electron transfer and improves the FF of the device (Fig. 3i and Supplementary Fig. 15).
Photovoltaic performance of the devices based on surface reconstruction treated perovskite films
We fabricated PSCs using nano-polishing treated WBG perovskite (1.67-eV) and the structural diagram of the device is shown in Supplementary Fig. 16a. The performance of the nano-polishing treated device indicated a significantly increased VOC and FF. This increase should be attributed to the removal of the inadequate crystallization region, ultra-smooth surface morphology, and improved energy level matching with C60. Nano-polishing treatment with a removal thickness of 50 nm yielded the optimal performance of the device (Fig. 4a, b and Supplementary Fig. 17a, b). The champion PCE of the control device was 20.72%, with a JSC of 22.52 mA/cm2, a VOC of 1.140 V, and an FF of 80.70%. After nano-polishing treatment, the champion device exhibited a remarkable increase in both VOC to 1.221 V and FF to 83.65%, leading to a champion PCE of 22.81% with a 2.09% absolute PCE improvement (Fig. 4c). Meanwhile, the device’s hysteresis was decreased (Supplementary Fig. 17c and Supplementary Table 6). The external quantum efficiency (EQE) matched well with the integrated current density determined from the J-V curves. The light absorption loss due to the slightly reduced perovskite thickness can be partially compensated by improved charge transport. Consequently, the integrated current density after nano-polishing exhibited a slight reduction from 21.73 mA/cm² for the control sample to 21.64 mA/cm² after polishing (Supplementary Fig. 18a). From the differentiation of the EQE, the curve’s inflection point was observed at 743 nm, indicating a bandgap of 1.67 eV in the WBG-PSCs (Supplementary Fig. 18b). The prolonged lifetime of transient photovoltage (TPV) suggested reduced non-radiative recombination between the perovskite layer and the electron transport layer, leading to an enhancement in the VOC of the target device. Transient photocurrent (TPC) analyses corroborated an improvement in charge transfer efficiency at the interface of the perovskite and electron transport layer, consequently improving the FF of the device (Supplementary Fig. 19). The carrier dynamics characterization results of the device showed that the non-radiative recombination loss of nano-polishing treated devices was significantly reduced due to the reduced defect density within the perovskite films (Supplementary Fig. 20). The trap density of states (tDOS) (Supplementary Fig. 21) revealed that the deep-level defects of the nano-polished device at 0.4–0.53 eV were significantly lower than those of the control device. As the deep-level defects are mainly related to the surface defects of the device, this result indicates that the nano-polishing greatly reduces the density of defect states on the surface of the perovskite film18,49,50. Additionally, since the control and the nano-polished target samples are only different on the surface, the difference in the tDOS data should be ascribed to the various surface defects.
Statistical box charts for the photovoltaic parameters of a VOC and b PCE of the control and target opaque WBG-PSCs. The box plot denotes the median (center line), 75th (top edge of the box), and 25th (bottom edge of the box) percentiles. c J-V curves of the champion control and target opaque WBG-PSCs. d The J-V curve and SPO of the champion device of opaque WBG-PSCs after surface reconstruction treatment. e Operational stability of the non-passivated WBG-PSCs under MPPT in N2, AM 1.5G, 100 mW cm−2 continuous illumination. f Structure diagram of the 4T perovskite/silicon tandem solar cells. J–V (g) and h EQE of the ST-PSCs, the hybrid-BC silicon solar cells, and the silicon bottom cell working under the perovskite top cell in the 4T configuration.
To prove the universality of the nano-polishing strategy on different perovskite components, we also prepared PSCs with 1.53-eV perovskite. We also found that nano-polishing treated devices obtain an improved PCE with the increase in VOC and FF, resulting in a high efficiency of 25.44% (Supplementary Fig. 22 and Supplementary Table 7).
We also applied the nano-polishing strategy to a large-area (1 cm2) device. The efficiency loss after active area enlargement was reduced from 1.12% for the control device to 0.39% for the nano-polishing treated device (Supplementary Fig. 23 and Supplementary Table 8). The different performance evolution behavior when scaling up the device area can be ascribed to the nano-polishing method enhancing the uniformity of perovskite film5.
The above characterizations indicated that nano-polishing successfully reconstructed the surface of WBG perovskite, exposing the inner high-quality crystal bulk as the new surface. We tried to further passivate the newly exposed surface with piperazinium iodide, achieving the so-called surface reconstruction of WBG perovskites in this work. It was found that the target device after surface reconstruction treatment had a higher VOC and FF than the control device (Supplementary Fig. 24). Finally, a champion PCE of 24.48% was achieved for 1.67 eV PSCs, and the stabilized power output (SPO) was 24.08% (Fig. 4d). It is worth mentioning that the maximum VOC of the surface reconstruction treated device has been improved to 1.29 V, achieving a low VOC loss of 0.38 V, which is comparable with the traditional iodide-dominated perovskite system (Supplementary Fig. 25 and Supplementary Table 9). The best-performing device was sent for third-party certification, and the certified PCE was 23.67% (Supplementary Fig. 26), which sets the current world record for WBG-PSCs (Supplementary Fig. 27)25.
The device stability was significantly improved after surface reconstruction treatment. The device’s long-term stability was assessed under 1 sun of white LED irradiation in nitrogen conditions. After 1505 h, the surface reconstruction treated device maintained 80% of its original PCE, much longer than the control device (746 h) (Fig. 4e).
We combined the WBG-PSCs prepared by surface reconstruction with a hybrid back contact (hybrid-BC) silicon solar cell to construct 4T perovskite/silicon tandem solar cells. The WBG perovskite top cell was changed from opaque to semi-transparent by substituting BCP and Ag with SnO2 and indium zinc oxide (IZO) transparent window layers at the top side of the device (Supplementary Fig. 16b). To determine the performance of the ST-PSCs, we subjected the device to third-party certification testing and achieved a certified efficiency of 21.70% (0.119 cm2) for the ST-PCSs with a JSC of 20.36 mA, a VOC of 1.263 V, and an FF of 84.38% (Supplementary Fig. 28). Concurrently, we have fabricated ST-PSCs with a large area of 1 cm2 (Supplementary Fig. 29 and Supplementary Table 10), and achieved a certified efficiency of 20.39% (Supplementary Fig. 30 and Supplementary Table 11). To construct a 4T perovskite/silicon tandem solar cell, ST-PSC was stacked on top of a hybrid-BC silicon solar cell (Fig. 4f and Supplementary Fig. 31). The sunlight with a shorter wavelength is absorbed by the top cell, and the long-wavelength light reaches the silicon bottom cell. The hybrid-BC silicon solar cell working under the transmitted light was tested and reached a third-party certified PCE of 12.71% (Supplementary Figs. 32 and 33). Therefore, we achieved four-terminal perovskite/silicon tandem solar cells with a record-certified efficiency of 33.10% (Fig. 4g and Table 1). A matched JSC was observed in the external quantum efficiency (EQE) spectra of the perovskite top cell and the silicon bottom solar cell (Fig. 4g).
Discussion
We reconstructed the surface structure of perovskite films using the surface reconstruction method and obtained ultra-smooth surfaces. The surface reconstruction method can effectively remove the defect-rich regions caused by inadequate crystallization on the perovskite film surface, restraining ion migration and releasing the residual stress in perovskite films. Moreover, the surface reconstruction also preferentially removes the soft I-rich halide components, producing a Br-rich perovskite surface, which can adjust the energy level matching. For the 1.67-eV opaque WBG-PSCs, the VOC and FF have significantly increased and achieved a certified efficiency of 23.67%. The corresponding ST-PSCs also produced certified efficiencies of 21.7% and 20.39% for aperture areas of 0.12 and 1.0 cm2, respectively. Moreover, the surface reconstruction method enhances the uniformity of perovskite film, effectively addressing the efficiency loss caused by the enlargement of the device area. The treated device retained 80% of the initial efficiency after operation at the maximum power point under 1-sun illumination for 1505 h, showing enhanced operational stability. Furthermore, we achieved 4T perovskite/silicon tandem solar cells with a certified efficiency of 33.10% (1 cm2), representing a significant advancement of 4T perovskite/silicon tandem solar cells. This work reveals that the surface passivation of perovskite films with a high density of defects like WBG perovskite films requires a pre-step to reduce the defect density in the initial films, indicating directions for designing surface engineering strategies for further boosting the performance of WBG-PSCs.
Methods
Materials
Indium tin oxide coated (ITO) glasses (15 Ω/sq) were purchased from Suzhou ShangYang Solar Technology Co., Ltd. Methylammonium iodide (MAI, 98.0%) and formamidinium iodide (FAI, 99.99%) were purchased from Greatcell Solar. [4-(3,6-dimethyl-9H-carbazol-9-yl)butyl]phosphonic acid (Me-4PACz, >99.0%), lead iodide (PbI2, 99.99%) and piperazinium iodide (PI) were purchased from TCI Chemicals. Nickel oxide nanoparticles (NiOx) were purchased from Advanced Election Technology Co., Ltd (China), and the average nanoparticle size is 7 nm. C60, bathocuproine (BCP), and PbBr2 (≥98.0%) were purchased from Xi’an Yuri Solar Co., Ltd. Cesium iodide (CsI, 99.999%), ethanol (ETH, ≥99.5%), dimethylformamide (DMF, ≥99.5%), dimethylsulfoxide (DMSO, ≥99.9%), anisole (≥99.5%), and isopropanol (IPA, ≥99.5%) were purchased from Sigma-Aldrich.
Perovskite precursor solution
1.67-eV perovskite
The 1.5 M perovskite precursor solution was prepared by dissolving 19.5 mg of CsI, 25.2 mg of MABr, 206.4 mg of FAI, 165.2 mg of PbBr2 and 487.1 mg of PbI2 in 1 mL DMF:DMSO (v:v = 4:1) mixed solvent with a chemical formula of Cs0.05FA0.80MA0.15Pb(I0.75Br0.25)3 to form a 1.67-eV perovskite, where FA was formamidinium and MA was methylammonium. All steps were conducted in the N2-filled glovebox, and the perovskite precursor solution was stirred overnight.
1.53-eV perovskite
The 1.6 M perovskite precursor solution was prepared by dissolving 20.8 mg of CsI, 247.6 mg of FAI, 12.7 mg of MAI, 5.4 mg of MACl, 22.2 mg of PbCl2 and 737.6 mg of PbI2 in 1 mL DMF:DMSO (4:1 in volume) mixed solvent with a chemical formula of Cs0.05FA0.9MA0.05PbI3 + 5% MAPbCl3 to form a 1.53-eV perovskite, and the perovskite precursor solution was stirred overnight.
Device fabrication
1.67-eV PSCs
The glass/ITO substrates were cleaned with a detergent solution and then sequentially ultrasonicated in deionized water, acetone, isopropanol, and ethanol for 20 min, respectively. Then, the glass/ITO substrates were dried with high-pressure nitrogen (N2) and then treated with oxygen plasma for 10 min before use. In this work, NiOx/ Me-4PACz were applied as composite hole transport layers (HTLs). NiOx nanoparticles were dispersed in deionized water at a concentration of 10 mg mL−1 to form NiOx ink. The as-prepared NiOx ink was spin-coated onto the ITO substrate at 4000 r.p.m. for 20 s, then annealed at 150 °C for 10 min in the ambient air. Me-4PACz was dissolved in ethanol with an optimized concentration of 0.33 mg mL−1. The as-prepared SAM solution was spin-coated on the NiOx substrate at 5000 r.p.m. for 30 s, then annealed at 100 °C for 10 min in an N2-filled glove box. The perovskite precursor was spin-coated on the as-prepared substrates with HTLs at 1000 r.p.m. for 8 s, subsequently at 5000 r.p.m. for 30 s, during which 180 μL of anisole was dripped onto the center of film at 6 s before the end of spin-coating. The substrates were immediately transferred to the hotplate and annealed at 100 °C for 15 min. The spin-coating processes were conducted in the N2-filled glovebox with a controlled temperature of 20–23 °C, and the water and oxygen levels should be both less than 1 ppm.
To prepare perovskite film by nano-polishing, the glass side of the sample was first cohered onto the fixed station by paraffin wax with heating at 85 °C. A damping polishing cloth was employed for polishing, and Al2O3 nanoparticles (20–80 nm particle size) were utilized as the polishing solution (1–2 wt.% concentration in anisole). The polishing disc spined at a fixed rate of 5000 r.p.m. for 15–45 s in the nano-polishing process to remove the rough perovskite film surface. At the same time, the sample was controlled to undergo reciprocating motion during the polishing process. The pressure exerted between the perovskite and the polishing cloth was 0.5 N cm−2, and the polishing liquid was dispensed at a rate of 8 mL min−1 during the process. After completing the nano-polishing process, heating was applied again for peptizing at 85 °C. Subsequently, 200 μL mixed solution of IPA and methanol (v:v = =4:1) was used for washing at 4000 r.p.m. for a duration of 30 s.
A solution of 0.4 mg mL−1 PI dissolved in IPA was prepared and heated at 60 °C. The passivation solution of PI was spin-coated at 5000 rpm for 30 s and annealed at 100 °C for 10 min. A washing step by spin-coating IPA at 5000 rpm for 30 was applied to wash off the excess PI on the perovskite surface.
Finally, 25 nm of C60 was thermally evaporated at a rate of 0.2 Å s−1, 7 nm of BCP was thermally evaporated at a rate of 0.15 Å s−1, and 70 nm of silver electrode was thermally evaporated at a rate of 1.0 Å s−1.
ST-PSCs
The fabrication of ST-PSCs was similar to that described for 1.67-eV WBG-PSCs but with a few modifications. To minimize the parasitic absorption of the electron transport layer, the C60 thickness was decreased to 10 nm. To form the transparent front electrode, a buffer layer of ~15 nm of SnO2 was deposited on C60 by atomic layer deposition using a Yunmao mini-150 ALD system at 100 °C using 100 cycles of tetrakis (dimethylamino) tin (400 sccm, 0.3 s pulse, 5.0 s purge) and H2O (250 sccm, 0.1 s pulse, 5.0 s purge). After depositing the SnO2 layer, IZO was deposited as transparent conductive front contact. RF sputtering was performed in Ar (34 ppm) using a power of 25 W. The sheet resistance of the IZO transparent electrode was 110–120 Ω/sq and the thickness was 40 nm. Then, a 70 nm-thick Ag grid was thermally evaporated at a rate of 1 Å/s through a shadow mask. Finally, an anti-reflective coating of MgF2 (2 Å/s, 100 nm, Umicore) was thermally evaporated over the active area.
1.53-eV PSCs
The fabrication of 1.53-eV PSCs was similar to that described for 1.67-eV WBG-PSCs but with a few modifications. For perovskite film fabrication, the perovskite precursor was spin-coated on the as-prepared substrates with HTLs at 1000 r.p.m. for 5 s, subsequently at 5000 r.p.m. for 30 s, during which 200 μL of CB was dripped onto the center of film at 15 s before the end of spin-coating. The substrates were immediately transferred to the hotplate and annealed at 100 °C for 30 min.
Hybrid BC silicon solar cell
The hybrid BC silicon solar cell was obtained from Gold Stone (Fujian) Energy Company Limited and fabricated through the following process. First, the N-type monocrystalline silicon wafer (a) was double-sided polished in 20% KOH solution to remove the damage layer, and then cleaned using the RCA standard process. Subsequently, a low-pressure chemical vapor deposition (LPCVD) coating was applied on the back of the silicon wafer to form a tunneling silicon oxide layer (b1) and a polycrystalline silicon layer (c), followed by high-temperature phosphorus diffusion of the polycrystalline silicon layer to form an N-type semiconductor, thereby forming the first passivation layer (b1) and the first semiconductor (c). Subsequently, SiNx was deposited by plasma-enhanced chemical vapor deposition (PECVD) to cover and protect the first semiconductor and the first passivation layer. The first opening area was generated by the laser. Then the front side of the silicon wafer and the first opening area were textured with an alkaline texturing solution to form a pyramid texture. Intrinsic amorphous silicon (b2) and P-type doped microcrystalline layer (d) were formed by PECVD in the first opening area on the back of the silicon wafer, thus forming the second passivation layer (b2) and the second semiconductor (d). Intrinsic amorphous silicon layer (e) and MgFx anti-reflection layer (f) were deposited on the front side of the silicon wafer. Laser was used to form a second open area arranged at intervals with the first open area above the first semiconductor and then a conductive ITO layer (g) was formed on the back side of the silicon wafer by magnetron sputtering. Laser streaking formed an insulation groove between the first opening area and the second opening area, and the conductive film layer in the insulation groove was etched off so that the first semiconductor and the second semiconductor were insulated. Finally, the Ag electrode (h) was formed by screen printing.
Characterization of perovskite films
Time-of-flight secondary ion mass spectrometry (ToF-SIMS)
ToF-SIMS measurements for the complete device were performed on an ION-TOF GmbH instrument (ToF-SIMS 5). A pulsed 30 keV Bi1+ primary ion beam was utilized to generate the secondary ions. The beam current of the Bi1+ primary ion beam was 1 pA. All the ToF-SIMS results were obtained from a 100 μm × 100 μm area on the sample surface. During the sputtering, a 1 keV oxygen sputter beam was applied to remove the surface material. The oxygen sputter beam was applied on a large 300 μm × 300 μm area with a beam current of 100 nA. The ejected secondary ions were accelerated by a sample bias of 3 kV.
X-ray diffraction (XRD) and Grazing-incidence wide-angle X-ray scattering (GIWAXS)
XRD was performed on a SmartLab X-ray diffractometer (Rigaku Corporation) using a Cu Kα radiation source operating at a voltage of 40 kV and a current of 30 mA. GIWAXS patterns were acquired at XEUSS WAXS/SAXS system, Xenocs, France. A Pilatus 3R 300 K image plate detector was used to capture the scattering patterns. The sample-to-detector distance was 130 mm, the wavelength of the incident X-rays was 1.5418 Å, and the incident angle was 0.5°.
Grazing incident X-ray diffraction (GIXRD)
GIXRD was characterized using a Rigaku SmartLab five-axis X-ray diffractometer at 45 kV and 200 mA, equipped with Cu Kα radiation (λ = 1.54050 Å), parallel beam optics and a secondary graphite monochromator. Before the test, the X-ray diffraction on well-recrystallized LaB6 powders was used for subtle alignment of an instrument, the acceptable LaB6 peak shift was less than 0.01° in 2θ compared to its JCPDF file. For the residual stress tests, a slow scan rate of 0.12° min−1 was carried out to ensure fine structural information.
X‑ray photoelectron spectra (XPS)
XPS was tested by Thermo Fisher Scientific K-alpha+ using an Al Kα source. The X-ray photoelectron spectrometer was calibrated according to the C 1s (284.8 eV). The formula and integration method for calculating elemental ratios by XPS is provided in Supplementary Note 2.
Other film characterizations
The PL spectra were recorded with an FLS1000 spectrometer (Edinburgh Instruments Ltd). Time-resolved photoluminescence (TRPL) was measured using time-correlated single photon counting (TCSPC) equipment (FLS920, Edinburgh Instruments Ltd) with a 375 nm pulsed laser (EPL-375). The excitation laser fluence was fixed at 29.4 nJ/cm−2 with a repetition rate of 0.5 MHz. A laser scanning confocal microscope (Leica TCS SP8) with a pulsed excitation at 488 nm was used to obtain the PL mapping images. Ultraviolet photoelectron spectroscopy (UPS) spectra were collected using a Thermo Fisher Scientific ESCALAB 250XI at a bias of −5 V using a He-Iα (21.22 eV) UV light source. The Kelvin probe force microscopy (KPFM) measurements were carried out in air conditions (Bruker Multimode 8) using frequency-modulation KPFM (FM-KPFM) to probe the contact potential difference (CPD) between the perovskite surface and the tip.
Characterization of devices
Current density-voltage characterizations were measured using a digital source meter (Keithley 2400) under AM 1.5G conditions (EnliTech, AAA solar simulator). The scan rate was 250 mV s−1 (voltage step of 10 mV and delay time of 40 ms). The light intensity was calibrated using an NREL-calibrated Si solar cell, which was equipped with an infrared cut-off filter (KG-5 for ST-PSCs and quartz for silicon solar cells). All J-V measurements were performed via an aperture area of 0.119 cm2 for opaque devices and ST-PSCs with small active area, and 1.013 cm2 for opaque devices with centimeter-scale active area. External quantum efficiencies were carried out on an EQE system (EnliTech, QER666) without any bias light. The operational stability of the PSCs was monitored by a solar cell stability test system, which consisted of a white LED lamp with a power density of 100 mW cm−2 as the light source (Suzhou Derui Keyi Instrument Equipment Co., Ltd). Device performance certification was conducted by the Shanghai Institute of Microsystems and Information Technology of the Chinese Academy of Sciences. The mott-Schottky analysis, trap density of states (tDOS) and transient photovoltage were carried out on a Zahner electrochemical workstation equipped with a transient electrochemical measurement unit (Fast CIMPS). Electrochemical impedance spectroscopy (EIS) was measured on a CHI 660E potentiostat system. Dark J–V curves and space-charge-limited-current (SCLC) data were collected by the Keithley 2400 in the dark.
The test method of 4T tandem solar cell
The 1 cm2 ST-PSCs were tested to obtain the efficiency of the top cell. To test the efficiency of the 158.75 × 158.75 mm2 silicon bottom cell, we fabricated a 300 × 300 mm2 ST-PSC as a light filter. The composition of the perovskite active layer and the thickness of functional layers were the same as that used in 1 cm2 ST-PSCs. The large-area ST-PSC was placed on the top of the hybrid BC silicon solar cell as a filter, and the remaining light traveled through the ST-PSCs was absorbed by the silicon solar cell. The PCE of the hybrid BC silicon solar cell with an area of 158.75 × 158.75 mm2 was tested with a mask aperture area of 209.8 cm2. Finally, the PCE of the 1 cm2 ST-PSCs was added to the PCE of the hybrid BC silicon solar cell under light filtering to obtain the PCE of the 4T tandem solar cell.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available in the following repository: https://doi.org/10.6084/m9.figshare.26940811. The source data is provided with this work.
References
Kojima, A. et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Liu, C. et al. Bimolecularly passivated interface enables efficient and stable inverted perovskite solar cells. Science 382, 810–815 (2023).
Jiang, Q. et al. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 13, 460–466 (2019).
Peng, J. et al. Nanoscale localized contacts for high fill factors in polymer-passivated perovskite solar cells. Science 371, 390–395 (2021).
Li, N. et al. Liquid medium annealing for fabricating durable perovskite solar cells with improved reproducibility. Science 373, 561–567 (2021).
Chen, H. et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 384, 189–193 (2024).
National Renewable Energy Laboratory (NREL). Photovoltaic Research, Best Research-Cell Efficiency Chart. www.nrel.gov/pv/cell-efficiency.html (2024).
He, R. et al. Improving interface quality for 1 cm2 all-perovskite tandem solar cells. Nature 618, 80–86 (2023).
Chen, W. et al. Monolithic perovskite/organic tandem solar cells with 23.6% efficiency enabled by reduced voltage losses and optimized interconnecting layer. Nat. Energy 7, 229–237 (2022).
Al-Ashouri, A. et al. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 370, 1300–1309 (2020).
Liang, H. et al. 29.9%-efficient, commercially viable perovskite/CuInSe2 thin-film tandem solar cells. Joule 7, 2859–2872 (2023).
Mariotti, S. et al. Interface engineering for high-performance, triple-halide perovskite–silicon tandem solar cells. Science 381, 63–69 (2023).
Aydin, E. et al. Enhanced optoelectronic coupling for perovskite/silicon tandem solar cells. Nature 623, 732–738 (2023).
Chin, X. Y. et al. Interface passivation for 31.25%-efficient perovskite/silicon tandem solar cells. Science 381, 59–63 (2023).
Liu, Z. et al. Grain regrowth and bifacial passivation for high-efficiency wide-bandgap perovskite solar cells. Adv. Energy Mater. 13, 2203230 (2023).
Lamanna, E. et al. Mechanically stacked, two-terminal graphene-based perovskite/silicon tandem solar cell with efficiency over 26%. Joule 4, 865–881 (2020).
Chai, W. et al. Graded heterojunction improves wide-bandgap perovskite for highly efficient 4-terminal perovskite/silicon tandem solar cells. Research 6, 0196 (2023).
Ni, Z. et al. Resolving spatial and energetic distributions of trap states in metal halide perovskite solar cells. Science 367, 1352–1358 (2020).
Chen, B. et al. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 48, 3842–3867 (2019).
Wu, H. et al. Pivotal routes for maximizing semitransparent perovskite solar cell performance: photon propagation management and carrier kinetics regulation. Adv. Mater. 35, 2206574 (2023).
Tong, J. et al. Wide-bandgap metal halide perovskites for tandem solar cells. ACS Energy Lett. 6, 232–248 (2020).
Ritzer, D. B. et al. Translucent perovskite photovoltaics for building integration. Energy Environ. Sci. 16, 2212–2225 (2023).
Tong, Y. et al. Wide-bandgap organic–inorganic lead halide perovskite solar cells. Adv. Sci. 9, 2105085 (2022).
Yang, D. et al. 28.3%-efficiency perovskite/silicon tandem solar cell by optimal transparent electrode for high efficient semitransparent top cell. Nano Energy 84, 105934 (2021).
Li, S. et al. A generic strategy to stabilize wide bandgap perovskites for efficient tandem solar cells. Adv. Mater. 36, 2307701 (2024).
Wang, T. et al. Phenethylammonium functionalization enhances near-surface carrier diffusion in hybrid perovskites. J. Am. Chem. Soc. 142, 16254–16264 (2020).
Park, S. M. et al. Engineering ligand reactivity enables high-temperature operation of stable perovskite solar cells. Science 381, 209–215 (2023).
Pan, Q. et al. Atomic faulting induced exceptional cryogenic strain hardening in gradient cell–structured alloy. Science 382, 185–190 (2023).
Li, X. et al. Constructing heterojunctions by surface sulfidation for efficient inverted perovskite solar cells. Science 375, 434–437 (2022).
Wang, C. et al. Solvent-assisted surface modification using metallocene-based molecules for high-efficiency perovskite/silicon tandem solar cells. Adv. Energy Mater. 14, 2401039 (2024).
Yang, Y. et al. Inverted perovskite solar cells with over 2,000 h operational stability at 85 °C using fixed charge passivation. Nat. Energy 9, 1–10 (2023).
Luo, Y. et al. Dissolved-Cl2 triggered redox reaction enables high-performance perovskite solar cells. Nat. Commun. 14, 3738 (2023).
Lee, D. S. et al. Passivation of grain boundaries by phenethylammonium in formamidinium-methylammonium lead halide perovskite solar cells. ACS Energy Lett. 3, 647–654 (2018).
Wang, Z. et al. Efficient ambient-air-stable solar cells with 2D–3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites. Nat. Energy 2, 17135 (2017).
Azmi, R. et al. Damp heat–stable perovskite solar cells with tailored-dimensionality 2D/3D heterojunctions. Science 376, 73–77 (2022).
Zhang, B. et al. A multifunctional polymer as an interfacial layer for efficient and stable perovskite solar cells. Angew 62, 202213478 (2023).
Zhu, Z. et al. Correlating the perovskite/polymer multi-mode reactions with deep-level traps in perovskite solar cells. Joule 6, 2849–2868 (2022).
Xu, J. et al. Anion optimization for bifunctional surface passivation in perovskite solar cells. Nat. Mater. 22, 1507–1514 (2023).
Shen, L. et al. Ion-diffusion management enables all-interface defect passivation of perovskite solar cells. Adv. Mater. 35, 2301624 (2023).
Chen, Y. et al. Nuclei engineering for even halide distribution in stable perovskite/silicon tandem solar cells. Science 385, 554–560 (2024).
Shao, Y. et al. Grain boundary dominated ion migration in polycrystalline organic–inorganic halide perovskite films. Energy Environ. Sci. 9, 1752–1759 (2016).
Lin, Y. et al. Revealing defective nanostructured surfaces and their impact on the intrinsic stability of hybrid perovskites. Energy Environ. Sci. 14, 1563–1572 (2021).
Chen, S. et al. Identifying the soft nature of defective perovskite surface layer and its removal using a facile mechanical approach. Joule 4, 2661–2674 (2020).
Xiao, X. et al. Argon plasma treatment to tune perovskite surface composition for high efficiency solar cells and fast photodetectors. Adv. Mater. 30, 1705176 (2018).
Park, B. W. et al. Stabilization of formamidinium lead triiodide α-phase with isopropylammonium chloride for perovskite solar cells. Nat. Energy 6, 419–428 (2021).
Zhu, C. et al. Strain engineering in perovskite solar cells and its impacts on carrier dynamics. Nat. Commun. 10, 815 (2019).
Wang, H. et al. Interfacial residual stress relaxation in perovskite solar cells with improved stability. Adv. Mater. 31, 1904408 (2019).
Li, T. et al. Inorganic wide-bandgap perovskite subcells with dipole bridge for all-perovskite tandems. Nat. Energy 8, 610–620 (2023).
Jiao, B. et al. Realizing stable perovskite solar cells with efficiency exceeding 25.6% through crystallization kinetics and spatial orientation regulation. Adv. Mater. 36, 2313673 (2024).
Liang, J. et al. Origins and influences of metallic lead in perovskite solar cells. Joule 6, 816–833 (2022).
Acknowledgements
This work was financially supported by the FuXiaQuan Self-created Zone Collaborative Project (3502ZCQXT2023006, Z.W.), the National Natural Science Foundation of China (22179042, L.X.; and U21A2078, Z.W.), and the Natural Science Foundation of Fujian Province (2023J06034, L.X.). We thank Prof. Jianhang Qiu (Institute of Metal Research, Chinese Academy of Science) for his assistance in the ToF-SIMS measurements. We would like to thank the Instrumental Analysis Center of Huaqiao University for providing the various tests.
Author information
Authors and Affiliations
Contributions
Z.W. and X.X. conceived the idea. L.X. and Z.W. supervised the project and process. Z.F. prepared perovskite films and fabricated devices and conducted most of the characterizations. B.D. and Y.J. fabricated devices and carried out the XPS, SCLC, EIS, UPS, KPFM, XRD, and PL mapping. L.Y., H.F., Y.Z., and Y.L. assisted in the preparation of ST-PSCs. L.C., Y.Y., J.Y., and C.T. contributed to improving the overall device efficiency. H.W. performed the measurement and analysis of residual strain. K.L. and X.Y. prepared the large-area perovskite filter sample. J.Z., J.H., and Q.Z. completed the preparation and certification of hybrid-BC silicon solar cells. All authors discussed the results and contributed to the revisions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shengchun Qu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fang, Z., Deng, B., Jin, Y. et al. Surface reconstruction of wide-bandgap perovskites enables efficient perovskite/silicon tandem solar cells. Nat Commun 15, 10554 (2024). https://doi.org/10.1038/s41467-024-54925-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-54925-4
This article is cited by
-
Reproducible Fabrication of Perovskite Photovoltaics via Supramolecule Confinement Growth
Nano-Micro Letters (2026)
-
High-Efficiency Perovskite/Silicon Tandem Solar Cells Based on Wide-Bandgap Perovskite Solar Cells with Unprecedented Fill Factor
Nano-Micro Letters (2026)
-
Towards efficient, scalable and stable perovskite/silicon tandem solar cells
Nature Photonics (2025)
-
Efficiency improvement in silicon and perovskite solar cells through nanofluid cooling using citrate and PVP stabilized silver nanoparticles
Scientific Reports (2025)