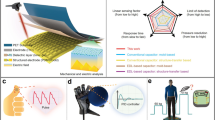
Abstract
The incompatibility of the high sensitivity and wide linear range still restricts the further development of active sensors. Here we report a triboelectric pressure sensor based on water-containing triboelectric elastomer with gradient-based microchannels. Tiny amount of liquid is injected into the triboelectric elastomer and the pressure-induced water bridges can modulate the built-in electric field of the sensor, which enhance the signal linearity near the compression limit. Moreover, it has been found that liquid-solid contact electrification can be enhanced by triggering selective ionic transfer, while the prepared ion-rich interface in the microchannels boosts the sensitivity of the sensor. Hence, an ultra-wide linear range (5 kPa–1240 kPa) with a sensitivity of 0.023 V kPa−1 can be achieved, which is so far the widest linear range of active sensors to our knowledge. Our work can promote the practical application of triboelectric sensors and provide new insights for other sensory devices.
Similar content being viewed by others
Introduction
Pressure and tactile sensors can convert stress signals into quantifiable and observable electrical outputs through a variety of mechanoelectrical conversion mechanisms, while these sensory techniques can be important supports for intelligent robots1,2,3, intelligent medical treatment4,5,6 and other fields. In recent years, a series of breakthroughs have been made for increasing the sensitivity of pressure sensor, including introducing microstructures to the interlayer7,8,9,10, mechanoelectrical conversion mechanism11,12, etc. However, the high sensitivity of the pressure sensor usually exists in a very narrow linear range1,13,14,15,16. The linearity of the pressure sensor and the width of the linear range are related to the compressibility of the active material in the sensor. When the active material reaches the strain limit under applied pressure, the conversion ratio between applied pressure and generated electrical signal is reduced, resulting in the attenuation of the linearity and the discontinuity of the linear range. In order to improve the linearity of sensor, a variety of mixed surface micromorphology such as pyramid type17,18,19, dome type20 and protrusion type8 are adopted, while the multi-layer structure design can also allow the electrical signal to be generated step by step for realizing a high linearity in different pressure ranges21. Therefore, the compatibility between high sensitivity and wide linear range of pressure sensor is a direction worthy of further exploration, while the cooperation of material innovation and structural design is the prerequisite.
On the other hand, the active sensor based on triboelectric effect can achieve high sensitivity and high linearity, as well as self-powered capability under different applied pressures. The triboelectric pressure sensor mainly relies on elastic triboelectric materials, such as fibers22,23, hydrogel24,25 and dielectric elastomer26,27. When the elastic triboelectric materials are compressed to a certain extent, the increase of elastic modulus results in attenuation of linearity and discontinuity in the linear range. Meanwhile, surface charge density is also the key point to the sensitivity of triboelectric sensors. The charge density of elastic and compressible triboelectric materials is usually weaker than that of non-stretchable plastic film materials, such as fluorinated ethylene propylene (FEP) and polytetrafluoroethylene (PTFE)28,29,30,31,32. The use of softer materials or structural improvements can partially overcome the compression limitation of the triboelectric sensors and expand the linear range to a certain extent, while this kind of optimization method may further reduce the triboelectric charge density of the material, leading to the suppression of sensory sensitivity. Therefore, in order to improve the performance of the triboelectric pressure sensor and enhance its linearity, both the mechanical properties of the material and the tribo-electrification capability are needed to take into account.
In this paper, a water-containing triboelectric elastomer is fabricated by applying gradient-based microchannels and ion-rich interface for polydimethylsiloxane (GBM-IR PDMS) film. For the sensor construction, the silver nanowires (AgNWs) electrode is applied on the top of GBM-IR PDMS film with the dual functions of anti-electromagnetic interference shielding layer and grounded electrode, while the FEP film with the output electrode is placed at the bottom of GBM-IR PDMS film to generate sensory signal. Tiny amounts of conductive liquid are injected into the GBM-IR PDMS film, which can gradually form water bridges between grounded electrode and FEP film with the GBM-IR PDMS film approaching its compression limit. These established water bridges can modulate the built-in field generated by electrostatic charges, which ensures that the variation amplitude of voltage output remains stable even after the elastic sensor approaching the compression limit. In addition, for the first time, we demonstrate that selective ion transfer on solid-liquid interface can increase the charge density of solid-liquid electrification, which greatly enhances the bulk charge density inside GBM-IR PDMS film and accordingly improves the sensitivity of sensor. Finally, the prepared triboelectric pressure sensor realizes the co-compatibility of high sensitivity and ultra-wide linear range. This study presents a different approach to achieve synchronous improvement of sensitivity and linearity, promoting the practical process of high-performance triboelectric pressure sensors. This design concept of deformable water bridge can also inspire many design strategies for other sensory devices.
Results
Concept of water-containing GBM-IR PDMS film design and the sensing mechanism
Pressure sensors are typically based on the detection of force-induced changes in resistivity, capacitance, piezoelectricity and triboelectricity, while the first two are passive sensors (Fig. 1a) and the last two are active sensors (Fig. 1b). Passive sensors achieve sensing by detecting the signal gain under the drive of external source and it cannot achieve spontaneous signal generation, resulting in high standby power consumption33,34,35. On the other hand, active sensors have a simpler structure and can generate electrical signals spontaneously based on specific conversion mechanisms (piezoelectric or triboelectric) between force and electricity. For example, triboelectric pressure sensors have the advantages of high linearity and high sensitivity, while they also have narrow linear range and are susceptible to environmental electromagnetic interference. Triboelectric pressure sensor without shielding layer exhibits higher sensitivity, while the lack of anti-interference capability seriously jeopardizes the repeatability of the experiment. The grounded shielding layer is the simplest and effective way to mitigate the electrostatic interference from surrounding environment. In this case, AgNWs are employed as the shielding layer and the grounded electrode for signal output, which is attached on top of the elastic triboelectric material (with the resistance of ~200 Ω sq−1), as shown in Fig. S1. Figures S2 and 1c explain the essential causes of discontinuities in the linear range of traditional triboelectric pressure sensor. For the triboelectric nanogenerator (TENG) output without the elastic internal layer (see Fig. S2), the established potential drop between top grounded electrode and bottom electrode with pre-charged triboelectric material (FEP film) decreases during the approaching motion, resulting in a voltage output with high linearity. After corona polarization, the FEP film shows high surface charge density and good charge retention ability. Surface charged density of the charged FEP films by a contact-separate TENG with the FEP film and Kapton film as triboelectric layers is measured, and the loss of surface charge density within 7 days was only 0.48% (the average of three samples, see Fig. S3). The detailed operation principle of this kind of contact-separation TENG can be seen in previous ref. 36. However, this working mode does not build the relationship between applied pressure and output voltage, since motion of the bottom electrode encounters no resistance without the elastic internal layer. As shown in Fig. 1c, triboelectric elastomer as active layer is utilized to link the applied pressure and the approaching distance of the bottom electrode, which is the typical working principle of traditional triboelectric pressure sensor37,38. In the initial state of compression, the compression deformation of the elastomer reacts to the increase of the pressure with high linearity. However, when the compression approaches its elastic limitation, the Young’s modulus of elastomer significantly increases, resulting in the nonlinear increase of the output voltage with the increase of pressure. As can be seen in Fig. 1d, the width of the linear region is determined by the compression limit of the elastomer.
a Converse mechanism, advantages and limitation of passive sensors. b Converse mechanism, advantages and limitation of active sensors. c Illustrations of traditional triboelectric pressure sensor responding applied pressure and generating voltage output. The rate of change in voltage is limited by the deformation limit. d Illustrations of GBM-IR PDMS-based triboelectric sensor responding applied pressure and generating voltage output. The forming of water bridges overcomes the deformation limit. e, f The relationship of voltage-deformation-pressure. For GBM-IR PDMS-based triboelectric sensor (red line), the linear range is broadened as well as the output voltage is increased.
In our work, it has been found that the problem of compression limit as well as the insufficient linearity can be overcome by injecting tiny amount of liquid into triboelectric elastomer with microchannels (Fig. 1e). The designed elastic triboelectric material is sandwich structure with gradient microchannels, where a trapping layer (water containing) is sandwich in the middle of two buffer layers (water blocking). The structure of interlinking microchannels provides high compressibility, as well as the diffusion path of the conductive liquid. In such a structure, when the triboelectric elastomer is compressed to its deformation limit, the liquid is squeezed and gradually forms water bridges connecting the grounded electrode and the pre-charged FEP film. Due to the conductivity of water bridges, redistribution of the potentials happens between the FEP film and the grounded electrode, which further decreases the established potential drop between grounded electrode and FEP film and generates more voltage signal to compensate the linearity of the sensory signal. Therefore, in this state, the variation amplitude of output voltage is also decided by the formation of water bridges, which can be modulated by the amount of liquid. By injecting the appropriate amount of liquid, the variation amplitude of output voltage can be optimized, achieving the continuity and broadening of the linear range (Fig. 1f).
Fabrication of the GBM-IR PDMS-based triboelectric sensor and liquid behavior
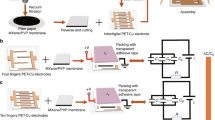
On the basis of the above scheme, the GBM-IR PDMS film is fabricated by using different grain sizes of sugar as sacrificial templates and by applying the surface modification process of ionic infiltration, as shown in Fig. S4 and Method section for details. The dimensions ratio of microchannels in the GBM-IR PDMS film with sandwich structure is 1:6 and the thickness ratio of that is 2:3, for the upper and lower parts with small pore size are buffer layers and the middle part with large pore size is trapping layer. For the pressure sensor utilizing the GBM-IR PDMS film as active layer, the stretchable AgNWs grounded electrode lies on the top and FEP film with copper (Cu) output electrode lies at the bottom of the active layer, which is shown in Fig. 2a for details. The whole structure is finally sealed with silicone (Ecoflex 00-30) to achieve liquid locking and good compressibility, the microstructure and photos of which are shown in Fig. 2b, c, respectively. It is obvious to see the diameters of fine and coarse microchannels and the connectivity between them. Figure 2b(i) is shot from the trapping layer and the diameter of the channel in it is matched to the size of coarse sugar we used, which is about 900 μm, while which of the channel from buffer layer is about 500 μm (Fig. 2b (ii)). Figure 2b(iii) shows the channels on the edge of trapping and buffer layers, which are well-interconnect with each other, providing clear path for water. By using the self-made fixture to keep the device under different strain states, the change rate of the microchannel can reflect the strain, and when the strain reaches 70% (Fig. 2(v) and (iv)), the microchannel is still connected, and there is enough space for tiny water, which is well-support to our theory. The FEP film is pre-charged by corona method for establishing initial potential drop with grounded AgNWs electrode. Figure 2c, d illustrates the anti-interfering property of the AgNWs electrode, while the peak value of the clutter signal with grounded AgNWs electrode is reduced by 92.59%. The sensor can be pressed without damage and perfectly recover to its original shape as shown in Fig. S5.
a Schematic of the composition of a GBM-IR PDMS-based triboelectric sensor. b Scanning electron microscopy (SEM) image of the GBM-IR PDMS film. Scale bar, 500 μm. c Detailed photos of the GBM-IR PDMS-based triboelectric sensor. d, e Illustrations of the shielding property of AgNWs top electrode. The inset is the test method. f Illustrations of the liquid behavior in the GBM-IR PDMS film. Blue ink for easy viewing and marking of liquid diffusion. Source data are provided as a Source Data file.
The diffusion path of liquid inside GBM-IR PDMS film under pressure is displayed in Fig. 2f and Movie S1, while blue ink is used to show the location and diffusion of the liquid. In the initial state, the liquid gathers in the center of the trapping layer with larger microchannels because of the capillary effect (i)39. For fluid transportation in a microchannel, the critical pressure required for fluid transportation is determined by the Laplace pressure (PL) of fluid, which depends on the interface tension of the fluid, advancing contact angles (θ) of fluid on the surrounding surfaces, and dimensions of the microchannel40. In GBM-IR PDMS film, greater pressure is needed for liquid entering the buffer layer on top and bottom with small dimension of microchannel. Therefore, at the beginning of compression, the liquid only spread in the liquid trapping layer (ii). Then, with the decrease of the thickness of the triboelectric elastomer under high pressure, the liquid enters the liquid buffer layers and gradually forms water bridges connecting the upper and lower triboelectric layers (iii). After releasing, the liquid is absorbed back into trapping layer and water bridges are disconnected. The thermal imagines of the sensor with water were photographed, which can distinguish the water and elastic materials due to their different thermal conductivities. The water was injected into the trapping layer, and then heated by microwave for 10 s. Due to the different capacity of water and PDMS, the temperature of the PDMS is higher than the water, so the location of water can be identified, as shown in Fig. S6a, b is the up view and side view of the sensor that was just injected with the liquid, and it can be seen that the water is aggregated in the middle except for part of the water remaining during injection. Figure S6c, d was photographed after ten compression cycles (pressure of 1 MPa), where the water is still trapped at its original position with just a slight expansion. The blue ink was also injected into two elastomer samples respectively. The first one is kept still and the second one is compressed for ten times with a pressure of 1 MPa. Then, both samples are frozen by liquid nitrogen and cut from the middle, as shown in Fig. S6e, f. It can be seen that the water still returns to the trapping layer after several times of compression. The further explanation of the mechanism is mentioned in Fig. S7 and Note S1 in Supplementary Information.
Fabrication of ion-rich microchannel interface by ionic infiltration
More importantly, the electrification of the liquid and inner wall of the microchannels during the extrusion and release of the sensor naturally constitutes a built-in electric field as well as the bulk charge density. The existence of bulk charge density generated by solid-liquid electrification in microchannel can further increase the sensory performance and the ionic infiltration method has been applied to enhance this bulk charge density. In our previous works, it has been found that selective ion migration can compensate the escape of polarized charge during solid-solid contact electrification (CE)41,42. Hence, in this work, ion-rich interfaces are fabricated to study whether the similar ion transfer effect can enhance the electrification ability on solid-liquid interfaces. Ionic infiltration with different ions is performed on the PDMS film samples and the electrification performance of them is characterized, respectively. The cured PDMS samples are immersed in the NaF, NaCl, FeCl3 saturated salt solution for 3 h and then drying in the oven for 8 h, detailed photos of samples (respectively recorded as PDMS, PDMS-NaF, PDMS-NaCl, PDMS-FeCl3) are shown in Fig. S8. The surface potentials on the PDMS samples before and after treatment are measured as shown in Fig. S9, where the highest potential difference appears on the PDMS-FeCl3. The charges for the CE from the interaction between a sequential of deionized water (DI) droplets and PDMS samples are measured in the single-electrode mode with Cu electrode, as shown in Fig. S10. The detailed working principle of this kind of droplet single-electrode TENG can be seen in previous work43. As the droplet number increased, the accumulated charges on the film surface alternatively and asymmetrically decrease (at contacting) and increase (at separating) until reaching a saturation. The highest amount of accumulated transferred charge and the highest amount of transferred charge in single contact at saturation both appear on the PDMS-FeCl3 (Figs. S11 and 3a), which is about 115.14% and 78.01% improvement compared to untreated PDMS, respectively. Then, the PDMS samples contact with FEP film to test their solid-solid electrification performance, as shown in Fig. S12, where the signals of the treated PDMS are all higher than that of untreated PDMS. The contact angle does not change significantly after this kind of treatment, which can maintain the smooth flow of liquid in the microchannel (Fig. S13).
a Single transferred charges of treated and untreated PMDS films after liquid-solid CE saturation. b, c XPS results of PDMS-FeCl3 and untreated PDMS. d Ion distributions of Fe3+ (blue) and Cl- (green) on the PDMS-FeCl3 film before and after liquid-solid CE saturation. e Principle of ion-rich interface enhancing the output voltage of the GBM-IR PDMS-based triboelectric sensor. f Comparison in current of the GBM-IR PDMS-based triboelectric sensor and GBM PDMS-based triboelectric sensor. g Comparison in charge density of the GBM-IR PDMS-based triboelectric sensor and GBM PDMS-based triboelectric sensor. h The output voltage and power density of the GBM-IR PDMS-based triboelectric sensor with various loads. Source data are provided as a Source Data file.
A series of experiments have been done to explore the detailed contribution of iron-rich interface to the liquid-solid electrification. Figure S14 demonstrates the overall attenuated total reflection fourier transform infrared sprctroscopy (ATR-FTIR) of the untreated PDMS and PDMS treated with different saturated salt solutions. Among the wavenumber of 400–1600 cm−1, there are three parts of major characteristic peaks. As it shows, 700–800 cm−1 represents for the Si–C vibrating peaks (−CH3 rocking and Si–C stretching in Si–CH3), 900–1100 cm−1 stands for the Si–O–Si vibrating peaks, and 1200–1300 cm−1 denotes the Si–C vibrating peaks (CH3 deformation in Si–CH3). Obviously, no new peaks emerge, as well as no band shifts to higher or lower wavenumbers. This reveals that the chemical reaction does not occur during the sault solution immersing process, and no new substantial generated. Then, XPS result is performed on FeCl3-treated PDMS, which detects the information of the two elements of Fe and Cl (Fig. 3b, c), but did not produce new bonds. In addition, time-of-flight secondary ion mass spectrometry (TOF-SIMS) in both positive and negative ion modes is used to reveal the complex molecular-ionic chimerism system in the PDMS-FeCl3 film before and after contact with water droplets. As shown in Fig. 3d, the presence of Fe3+ and Cl- suggests adsorption between FeCl3 and the PDMS surface, and the ion distribution indicates that adsorption mainly occurs on the surface. After contact with DI droplets for a while, signal intensity of these two ions both decreases, while the loss of Fe3+ is much more than Cl–. Compared with Fe3+, Cl– can enter deeper layers and have stronger retention. The reason for this phenomenon may be related to the fact that the Si–O–Si have different strengths of coordination to Lewis acid (LA) and Lewis base (LB) ions. Three types of complexes may be formed by Si–O–Si bonding with LA and LB (Fig. S15), and the strength of the coordination bond is related to the size of the bond angle44. The bond angle of Si–O–Si in PDMS is close to 165°, at which the potential of the binding site for LA in configuration A is smaller than that of the binding site for LB in configuration B and C, representing a smaller coordination bond strength with LA ions. Therefore, Cl– as LB ion is more likely to stay on the surface of PDMS film and form a negative ion-rich surface. The characterization of the results shows that the new charges introduced by the selective adsorbed ions can cause the increase of the transferred charge, which makes up for the deficiency of the intrinsic charge in PDMS. The ion migration in materials can be detected by thermal stimulation depolarization currents (TSDC) to better, and the result is revealed as Fig. S16. The activation energy \({E}_{a}\) (calculated by Eq. 2 in Method) of charges excited in FeCl3-treated PDMS film (2.89 eV) is much larger than that of the pure PDMS (0.0019 eV). Activation energy \({E}_{a}\) over 1.5 eV is thought to be as deep traps level of charge, indicating that the ion adsorption and selective ion transfer on the microchannel surface may contribute to the enhancement of the total charge density of device. Moreover, phenomenon that selective ionic transfer and related ion compensation effect can significantly enhance the electrification have been observed on solid-liquid interfaces for the first time, which indicates an effective method for enhancement of the solid-liquid electrification.
Enhancement mechanism of sensor output voltage due to ion-rich interface is explained by Fig. 3e(i–iv). Because of the CE process between the liquid and the GBM-IR PDMS film, the negative ion fragments are distributed on the inner wall of microchannel inside GBM-IR PDMS film, while the positive ions are concentrated in the central liquid. Dispersed distribution of negative charges inside GBM-IR PDMS film leads to the increase of the established potential between grounded top electrode and the bottom output electrode. With pressure applied on top of the sensor, the GBM-IR PDMS film undergoes elastic deformation, resulting in diffusion of the liquid and the neutralized between positive and negative charges in the bulk. Due to the weakening of the built-in electric field strength, the electrical potential on the Cu electrode changes, driving electrons flow to the electrode through an external circuit. Besides, the positive charges in water droplet are discharged as the water contact to the grounded electrode, which allow the unbalanced residual negative charges to dominate the charge density in the bulk region. After pressure unloading, the GBM-IR PDMS film returns to the relaxed state with the separation of positive and negative charges, and the number of negative charges is much more than positive ones. Therefore, enhancement of the initial electric field strength is the source of the boost in the sensor output signal. Additional experiment is designed to confirm the charge leakage from the water, as shown in Fig. S17a. The sensor device is compressed to 1 MPa and released, while the top electrode is either connected to the ground or being suspended. Then, a nickel-coated iron probe with the diameter of 350 μm was stabbed into the center of the elastomer to detect the charges on the water. Eight sensor samples were measured and the results of probed charges can be seen as Fig. S17b and S17c. Hence, it is confirmed that the grounded top electrode can help to remove the charges in the liquid. It is worth to note that the charges we tested in Fig S17b and Fig. S17c is not the absolute value of the charges in the water droplet and can only be used as a reference since the influence of environmental electrostatic signals on the probe is difficult to eliminate and the discharge signal detected by the probe will always have some fundamental waves. The detailed mechanisms of the unbalanced residual negative charges and the clear explanation of the contribution of water and iron-rich interface to the sensor device can be seen in Fig. S18 and Note S2.
As can be seen in Fig. 3f, the comparison in current between sensors utilized GBM PDMS film and GBM-IR PDMS film presents that 123.31% increasing of current occurs after ion-rich treatment. Similarly, 141.41% increasing of transferred charges also happens on sensor utilized GBM-IR PDMS film, as shown in Fig. 3g. The further enhancement occurs inside the microchannels of GBM-IR PDMS film, which is primarily attributed to the selective ion transfer process proceeding to completion by repeated squeezing cycles during sensor operation, leading to a higher accumulated transferred charge at the microchannel interface compared to that of the droplet-TENG. The results illustrate the enhancement brought by the ion-rich interface, contributing to high sensing performance of the sensor. The output voltage with various load resistances (from 1 MΩ to 100 GΩ) are measured and calculated, as illustrated in Fig. 3h. As the load resistance increases, the voltage is augmented., while the power density first increases and reaches the maximum of 1.36 μW m−2 at the load resistance of 100 MΩ, then decreases sharply. These high impedance helps to keep the output signal of sensor stable during operation and avoid distortion of the signal source, while respond time will be extended.
Sensing properties of the water-containing GBM-IR PDMS-based triboelectric sensor
Based on this GBM-IR PDMS film as active layer, a triboelectric pressure sensor is constructed for studying the improvement of detection linearity near the deformation limit. Here, the changing rate of the voltage output (∆V/P) is determined by the formation process of water bridge under compression. Therefore, the amount of injected liquid needs to be optimized for extending the linear range of pressure detection. The output voltage of GBM-IR PDMS-based triboelectric sensor under the applied pressure of 300 kPa with different DI content (0 vol%, 1 vol%, 1.5 vol%, 2 vol%, 2.5 vol%) are measured as shown in Fig. 4a, where the sensor provides the highest output and the maximum detection range with the DI content of 1.5 vol%. The detailed explanation of the principle of water content selection are demonstrate in Figs. S19, S20 and S21 and Note S3. The optimal GBM-IR PDMS-based triboelectric sensor has an ultra-wide linear range from 5 kPa to 1124 kPa (Fig. 4b), which is two orders of magnitude improvement compared with that of the GBM PDMS-based sensor (Fig. 4c and Movie S2). The sensitivity of GBM-IR PDMS-based triboelectric sensor is 0.023 V kPa−1 in wide linear range, which is 228.57% higher than that of the sensor without ion-rich interface. Under the perfectly coordination of the optimal water content, GBM-IR, the sensor achieves the dual function of broadening linear range and sensitivity improvement. Besides, for the reason that linear ranges have different starting points is that before the starting point of the linear range, the signal has a sudden increase process, which is because the elastic modulus of the sensor structure in the initial state is very small, and the sensor does not have the ability of linear change under pressure change. Due to the introduction of water, selective ion transfer can be generated between the ion-rich microchannels and water. Although there is an initial small elastic modulus stage exists, the negative charge center is concentrated on the side near the output electrode and will move to the output electrode after being squeezed, which makes up for the voltage drop caused by the small elastic modulus. The sensitivity of the sensor under different compression rate are revealed in Fig. S22. Within the range of the measurement, the sensitivity of the sensor shows slight fluctuations and the fluctuation amplitude does not exceed 5%. The fluctuation is caused by variation of surface charge density of the device, while the sensitivity is still at a relatively stable value. The motion of motor also introduces destabilizing volatility, such as the instability during acceleration and deceleration process. It is worth noting that even though the sensitivity is generally independent from the compression rate, the excessively high compression rate (over 6 mm/s) is likely to cause the damage to the sensor. In addition, the sensitivity and the width of linear range of previously reported triboelectric sensors with the anti-electromagnetic interference capability are investigated and summarized, as displayed in Fig. 4d45,46,47,48,49,50,51,52,53. Obviously, this GBM-IR PDMS-based triboelectric sensor manifests the widest linear range and its sensitivity is also at the advanced level of the field. Moreover, among all the active pressure sensors (piezoelectric and triboelectric sensors) with and without the consideration of anti-electromagnetic interference, our sensor still exhibits the widest linear range with the relatively high-level sensitivity (Fig. 4e45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68). It is important to note that the triboelectric sensor without anti-interference protection usually can maintain a much higher sensitivity, as can be seen in Fig. 4e, while this is at the expense of environmental adaptability and experimental repeatability. The high sensitivity of these triboelectric senor without anti-interference protection further exacerbates the decay process of linearity near their compression limit and thus, the GBM-IR PDMS-based triboelectric sensor with balanced high sensitivity and wide linear range has better applicability in real applications. Moreover, stress-strain curve is also measured as shown in Fig. 4f, with the linearity occurring only in a small range, further illustrating the importance of the fluid to sense the pressure linearly in the nonlinear compression phase. After over 1000 working cycles under the applied pressure of 1.2 MPa, the output voltage of the sensor remains stable (Fig. 4g), and there is no significant change in the compressibility of the GBM-IR PDMS film (Fig. 4h), which indicates that the prepared sensor has excellent fatigue resistance. Meanwhile, the sensor exhibits a response time of ~33 ms and a recovery time of ~102 ms (see Fig. S23). Furthermore, the static and dynamic force detection capabilities of the GBM-IR PDMS-based sensor are exhibited in Movie S3 and Figs. S24 and S25. The sensor shows high linearity in both detection mode, and as can be seen in the last seconds of the Movie S3 step1, even though the displacement is small, the change of the applied pressure is dramatic and significant. The voltage of our sensor can follow the increase of the applied pressure with a good match, where the water bridge plays the key role.
a The voltage outputs of GBM-IR PDMS-based triboelectric sensor with different content of DI under the pressure of 300 kPa. b Voltage varies with pressure of the optimized GBM-IR PDMS-based triboelectric sensor. The inset is the datapoints at the range of 0–10 kPa. The error bar is obtained by repeating the test for 5 times. c Voltage varies with pressure of the GBM PDMS-based sensor. The inset is the datapoints at the range of 0–2100 kPa. The error bar is obtained by repeating the test for 5 times. d Comparison in the sensory sensitivity and linear range of triboelectric sensor with anti-electromagnetic interference ability. e Comparison in the sensory sensitivity and linear range of active sensor (triboelectric and piezoelectric sensors). The inset is zoom-in y-axis at the range of 0.015–0.025 V kPa−1. f Force varies under the displacement (compression) of GBM-IR PDMS-based triboelectric sensor. The insets are photos of sensor in light compression and heavy compression. g Cyclic compression of GBM-IR PDMS-based triboelectric sensor under square waves, each endures less for 800 ms, over 1000 cycles at 1.4 MPa. The inset is the photos of sensor on the signals at the initial and final cycles. h Enlarged view of the single response signal in the cyclic test. Source data are provided as a Source Data file.
Application in sensor array and bending angle measurement
The applicability of the GBM-IR PDMS-based triboelectric sensor is further demonstrated by integrating it into a 3 × 3 sensor array for spatial mapping of pressures in both tapping and sliding motion. Both the fabrications of GBM-IR PDMS film and AgNWs electrode are scalable, enabling facile large-scale integration. A GBM-IR PDMS film of 5 × 5 cm2 is fabricated and cut into 9 pieces (1.5 × 1.5 mm2 each), while they are finally integrated into a 3 × 3 pixel arrays of sensing matrix with the assistance of acrylic and Cu electrodes (Fig. 5a), for each position corresponding to one output channel. The thickness of liquid trapping layer and liquid buffer layer reduce to ≈0 and 0.5 mm, respectively. The liquid is injected into the GBM-IR PDMS film by fumigation to make sure only tiny amount of liquid exists in the film without contacting with the electrode or FEP film. As can be seen in Fig. 5b, the output performance of thin GBM-IR PDMS-based triboelectric sensor with tiny liquid is still much better than that without liquid. By pressing the sensor in position 2 and measuring the output signals of sensor in channel 2 and 5, significantly differentiated signal strength demonstrates that the cross-talk between the sensing elements is the effectively suppressed by the AgNWs electrode (Fig. 5c). Then the array is tested in response to two kinds of moving objects of taping and sliding using three-axis motors according to the trajectory in Fig. 5d, and the results are shown in Fig. 5e, f, respectively. It can be seen that the sensor array exhibits a recognition function on the pressure loading site, and the signal curves of the sensor array have different shapes for different pressure moving modes, for square waves generated by taping and spike waves generated by sliding. Furthermore, bending experiment is performed with the similar sensor device (size 3 × 5 cm), where the sensor device is placed at the elbow joint and the sensor signals has a distinguishable response between bending angles from 30° to 150° (Fig. 5g). The triboelectric sensor with similar working principle but different operating modes and diversified thickness can be further modified based on its flexible preparation process, which allow this GBM-IR PDMS-based triboelectric sensor to be adapted to various applications, meeting the requirements for wide detection ranges of e-skins, intelligent robots, etc.
a Schematic of the 3 × 3 array sensor. b Comparison in the output voltage of the single pixel sensor with and without DI. c Output signals from channels 2 and 5 when pressing position 2, showing no crosstalk between channels. d Moving trajectory of tapping and sliding mode. e Output voltages of pixels in the moving trajectory of tapping mode. f Output voltages of pixels in the moving trajectory of sliding mode. g Output voltages of bending test. The insets are bending angles when the sensor is placed at the elbow joint and the photo of sensor for bending test. Source data are provided as a Source Data file.
Discussion
In summary, the triboelectric pressure sensor based on GBM-IR PDMS film as the core component is proposed, which demonstrates the compatibility with high sensitivity and ultra-wide linear range. The stretchable AgNWs layer is deposited on the top of the sensor as the grounded electrode, which can form a shielding layer to reduce the clutter amplitude by over 92.59%. The pre-charged FEP film is attached on the surface of output electrode with the triboelectric active layer of GBM-IR PDMS sandwiched between grounded electrode and pre-charged FEM film. The established potential drop between pre-charged FEP and grounded AgNWs electrode is the origin of the output signal for the sensor, while the elastic deformation of the GBM-IR PDMS under different applied pressure can modulate the relationship of the sensory signal (the negative voltage) and the detected pressure. In order to break through the restriction of material compression limit on sensing linearity, tiny amount of liquid is injected into the middle of GBM-IR PDMS film and the conductivity of this liquid can help to modulate the built-in electric field. When the GBM-IR PDMS is compressed near its limitation, the injected liquid gradually forms water bridges to link the AgNWs electrode and FEP film, which ensures the voltage output still change linearly with the increase of applied pressure. As a result, the width of linear range reaches 5–1240 kPa, which is the broadest range achieved by active (triboelectric or piezoelectric) pressure sensor.
Meanwhile, the compression process of tiny liquid inside the microchannels also leads to the contact electrification. This solid-liquid electrification increases the bulk charge density of GBM-IR PDMS film and improves the sensitivity of the sensor. More interestingly, it has been found for the first time that the selective ion transfer process on solid-liquid interface can facilitate the contact electrification process. The total amount of accumulated transferred charges and the transferred charges in single contact at saturation both are increased by 115.14% and 78.01% after an ion infiltration treatment. Accordingly, large number of ions is enriched at the interface of the GBM-IR PDMS through the ionic infiltration of FeCl3 solution, resulting in the high bulk charge density of the GBM-IR PDMS film. In this case, the sensitivity of the prepared sensor reaches 0.023 V kPa−1, which is 228.57% higher than that of the sensor without ion-rich interface. To better demonstrate applicability, a 3 × 3 sensor array for spatial mapping of pressures in both tapping and sliding motion is exhibited, while a bending sensor based on the same the working principle is also designed for detecting the bending angle of joint part. Our work proposes a different strategy to solve the compatibility problem of high sensitivity and wide linear range, which will help to promote the practical application of both triboelectric sensors and other elastic electronics devices. The unbalanced ion-transfer phenomenon observed during solid-liquid contact electrification may also provide new insights in electrochemistry, surface catalysis, and other areas.
Methods
Material
For templets, the fine sugar with the average grain size of 150 μm and the coarse sugar with the average grain size of 900 μm are used. The silicone (Ecoflex 00-30) was produced by Smooth-On. The PDMS was made by Dow Corning. The thickness of the FEP film is 30 μm. The FeCl3 powder (98%, Aladdin), KCl (99%, Alfa Aesar), NaF (AR, Xilong scientific Co., Ltd) were used to configure the saturated solution. The ethanol solution of AgNWs (diameter: 30 nm, length: 20–30 µm, concentration: 10 mg ml−1) was produced by Xianfeng Nano Co., Ltd.
Preparation of the GBM-IR PDMS film
First, small amount of water was added to the sugar and stirred to make the sugar particles sticky with each other, then the fine and coarse sugar were poured into cut acrylic mold with different thickness and cold pressed into shape. After drying in the oven at 60 °C for 5 h, the sugar templets with the thickness of 2 mm (fine sugar) and 3 mm (coarse sugar) were molded. Then the sugar templates were placed layer by layer as sandwich structure and compacted into a whole, ensuring the sugar particles are tightly connected. Next, the whole template was put into the PDMS, which was mixed with the crosslinker at a weight ratio of 10:1 and degassed in a vacuum chamber for 5 min. The PDMS was suck to full fill the space in the templet in a vacuum for 10 min, and then cured at 80 °C for 2 h. After curing, the PDMS with templet was put into the water at 80 °C with agitation for 30 min, ensuring complete dissolution of the template. Obtained PDMS with gradient-based design of branched microchannel was then put into the FeCl3 saturated solution (prepared by dissolving excess FeCl3 powder in water) and totally immersed for 3 h. After drying in oven at 60 °C for 5 h, the GBM-IR PDMS film was fabricated. The porosity was calculated as listed in the Table S1 by the equation \(P=(1-\frac{{\rho }_{0}}{\rho })\times 100\%=(1-\frac{{m}_{D}}{{m}_{W}})\times 100\%\), where P is the porosity, mD is the dry weight and mW is the wet weight.
Preparation of the GBM-IR PDMS-based triboelectric sensor
Part A and Part B of Ecoflex 00–30 were evenly mixed at a weight ratio of 1:1 and poured onto a glass sheet, cured at 60 °C for 30 min. Then, a strip of Cu foil was placed on the surface and the cured Ecoflex was put on a heating plate at 65 °C. At the same time, spraying the AgNWs solution (3.3 mg ml−1) on it. After curing and drying for 30 min, peeled it off and the AgNWs electrode was obtained. Next, the AgNWs electrode was placed on the top of the GBM-IR PDMS film, and the FEP film pre-charge by corona polarization with Cu electrode was placed at the bottom. Each side of the whole structure was dipping in uncured Ecoflex, and then completely curing at 60 °C for 2 h. Finally, tiny amount of DI was injected into middle of the structure by syringes, and the GBM-IR PDMS-based triboelectric sensor was fabricated.
The GBM-IR PDMS film of array sensor was prepared by the same fabrication, while the sugar templet with the thickness of 1 mm was only utilized fine sugar. The FEP films and Cu electrodes were attached to sensing matrix of acrylic, and AgNWs electrode was big enough to wrap the whole sensing matrix.
Characterizations of PDMS samples
The droplet-TENG, which consisted of three layers: PDMS film for contact electrification, a Cu film for electrostatic induction, and an acrylic plate for support, was used to characterized the contact electrification ability of the treated and untreated PDMS samples. Liquid droplets (about 25 μL per drop, with a dropping frequency of about 40 drops per minute) were released from a grounded stainless-steel needle (2 mm diameter) at a fixed height above the polymer surface with a tilted angle of 30°. The droplet height was set as 0.6 cm, which was slightly longer than the long axis length of the droplet hanging at the needle. The entire experiment was conducted in a homemade Faraday cage to isolate electrostatic interference. The contact angles were tested by contact angle measuring machine (CA100C).
TSDC experiment
TSDC were measured using a dielectric impedance spectrometer (Nanocontral Concept 90). Pure PDMS and FeCl3-treated PDMS films are polarized in the external electric field of 2 kV/cm for 5 min at the temperature of 293 K, and then rapidly cooled to a low temperature of 123 K. After freezing the polarization, the depolarization currents of the samples (gold plated on both sides) are measured during heating at a constant rate of 5 K/min. Based on the Arrhenius relation, the current I is given as
where β is the heating rate, SA is the electrode area, \({\tau }_{0}\) is the pre-exponential factor, \({E}_{a}\) is the activation energy, kB is the Boltzmann constant, and P0 is the initial dipolar polarization. Therefore, the activation energy Ea can be estimated from the slope of the ln I−1/T curve
Mechanical characterizations
The loading and unloading of pressure were controlled by a linear motor, movement patterns of pressure was controlled by a three-axis motor. The force is tested by a force gauge (Handpi/HP-10). The static and dynamic force detections were performed by an Electrodynamle test instrument (Instron, E3000).
Characterization and electrical measurements
The SEM photos of GBM-IR PDMS film were shot by scanning electron microscope (Hitachi SU8020). The infrared absorption spectra of the samples were measured by a Fourier transform infrared spectrometer (VERTEX80v, Bruker). The X-ray photoelectron spectrometer (Escalab 250Xi, Thermo Fisher) was used to analyze the surface element composition and molecular structure of the samples. The surface element composition and molecular structure information of the sample was characterized by a time-of-flight secondary ion mass spectrometer (PHI nano TOF II, ULVAC). Voltage, current and charge were measured by a programmable electrometer (Model 6514, Keithley). The surface potentials were measured by an electrostatic voltmeter (Model 341B, Advanced Energy Industries).
Data availability
The data supporting the findings of this study are reported in the main text or the Supplementary Information. Raw data are provided as a Source Data file. Source data are provided with this paper.
References
Pyo, S., Lee, J., Bae, K., Sim, S. & Kim, J. Recent progress in flexible tactile sensors for human‐interactive systems: from sensors to advanced applications. Adv. Mater. 33, 2005902 (2021).
Liu, F. et al. Neuro-inspired electronic skin for robots. Sci. Robot. 7, eabl7344 (2022).
Guo, H. et al. A highly sensitive, self-powered triboelectric auditory sensor for social robotics and hearing aids. Sci. Robot. 3, eaat2516 (2018).
Kang, S.-K. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016).
Su, Q. et al. A stretchable and strain-unperturbed pressure sensor for motion interference–free tactile monitoring on skins. Sci. Adv. 7, eabi4563 (2021).
Boutry, C. M. et al. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 1, 314–321 (2018).
Mannsfeld, S. C. B. et al. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 9, 859–864 (2010).
Bai, N. et al. Graded intrafillable architecture-based iontronic pressure sensor with ultra-broad-range high sensitivity. Nat. Commun. 11, 209 (2020).
Zhang, Y. et al. Localizing strain via micro-cage structure for stretchable pressure sensor arrays with ultralow spatial crosstalk. Nat. Commun. 14, 1252 (2023).
Chen, C. et al. Micro triboelectric ultrasonic device for acoustic energy transfer and signal communication. Nat. Commun. 11, 4143 (2020).
Ji, B. et al. Bio‐inspired hybrid dielectric for capacitive and triboelectric tactile sensors with high sensitivity and ultrawide linearity range. Adv. Mater. 33, 2100859 (2021).
Chen, R. et al. Nonlinearity synergy: an elegant strategy for realizing high-sensitivity and wide-linear-range pressure sensing. Nat. Commun. 14, 6641 (2023).
Liu, W. et al. Piezoresistive pressure sensor based on synergistical innerconnect polyvinyl alcohol nanowires/wrinkled graphene film. Small 14, 1704149 (2018).
Qiu, Z. et al. Ionic skin with biomimetic dielectric layer templated from Calathea Zebrine leaf. Adv. Funct. Mater. 28, 1802343 (2018).
Shi, L. et al. Quantum effect-based flexible and transparent pressure sensors with ultrahigh sensitivity and sensing density. Nat. Commun. 11, 3529 (2020).
Ren, H. et al. Transfer-medium-free nanofiber-reinforced graphene film and applications in wearable transparent pressure sensors. ACS Nano 13, 5541–5548 (2019).
Boutry, C. M. et al. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater. 27, 6954–6961 (2015).
Schwartz, G. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 4, 1859 (2013).
Ruth, S. R. A., Feig, V. R. & Tran, H. Microengineering pressure sensor active layers for improved performance. Adv. Funct. Mater. 30, https://doi.org/10.1002/adfm.202003491 (2020).
Boutry, C. M. et al. A hierarchically patterned, bioinspired e-skin able to detect the direction of applied pressure for robotics. Sci. Robot. 3, eaau6914 (2018).
Yang, J. C. et al. Microstructured porous pyramid-based ultrahigh sensitive pressure sensor insensitive to strain and temperature. ACS Appl. Mater. Interfaces 11, 19472–19480 (2019).
Li, Z. et al. All‐fiber structured electronic skin with high elasticity and breathability. Adv. Funct. Mater. 30, 1908411 (2020).
Xu, H. et al. Fully fibrous large‐area tailorable triboelectric nanogenerator based on solution blow spinning technology for energy harvesting and self‐powered sensing. Small 18, 2202477 (2022).
Pu, X. et al. Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing. Sci. Adv. 3, e1700015 (2017).
Leng, Z. et al. Sebum‐membrane‐inspired protein‐based bioprotonic hydrogel for artificial skin and human‐machine merging interface. Adv. Funct. Mater. 33, 2211056 (2023).
Xiong, J., Thangavel, G., Wang, J., Zhou, X. & Lee, P. S. Self-healable sticky porous elastomer for gas-solid interacted power generation. Sci. Adv. 6, eabb4246 (2020).
Tao, X., Chen, X. & Wang, Z. L. Design and synthesis of triboelectric polymers for high performance triboelectric nanogenerators. Energy Environ. Sci. 10.1039.D3EE01325A https://doi.org/10.1039/D3EE01325A (2023).
Li, S. et al. Contributions of different functional groups to contact electrification of polymers. Adv. Mater. 32, 2001307 (2020).
Tao, X. et al. Large and tunable ranking shift in triboelectric series of polymers by introducing phthalazinone moieties. Small Methods 7, 2201593 (2023).
Li, S. et al. Manipulating the triboelectric surface charge density of polymers by low-energy helium ion irradiation/implantation. Energy Environ. Sci. 13, 896–907 (2020).
Liu, Z. et al. Creating ultrahigh and long‐persistent triboelectric charge density on weak polar polymer via quenching polarization. Adv. Funct. Mater. 33, 2302164 (2023).
Liu, Z. et al. Fabrication of triboelectric polymer films via repeated rheological forging for ultrahigh surface charge density. Nat. Commun. 13, 4083 (2022).
Li, R. et al. Supercapacitive iontronic nanofabric sensing. Adv. Mater. 29, 1700253 (2017).
Hammock, M. L., Chortos, A., Tee, B. C. ‐K., Tok, J. B. ‐H. & Bao, Z. 25th Anniversary article: the evolution of electronic skin (E‐Skin): a brief history, design considerations, and recent progress. Adv. Mater. 25, 5997–6038 (2013).
Liu, R., Wang, Z. L., Fukuda, K. & Someya, T. Flexible self-charging power sources. Nat. Rev. Mater. 7, 870–886 (2022).
Niu, S. et al. Theoretical study of contact-mode triboelectric nanogenerators as an effective power source. Energy Environ. Sci. 6, 3576 (2013).
Wang, S., Lin, L. & Wang, Z. L. Triboelectric nanogenerators as self-powered active sensors. Nano Energy 11, 436–462 (2015).
Park, S. et al. Stretchable energy‐harvesting tactile electronic skin capable of differentiating multiple mechanical stimuli modes. Adv. Mater. 26, 7324–7332 (2014).
De Gennes, P.-G., Brochard-Wyart, F. & Quéré, D. Capillarity and Wetting Phenomena (Springer New York, 2004). https://doi.org/10.1007/978-0-387-21656-0.
Wang, S. et al. Inner surface design of functional microchannels for microscale flow control. Small 16, 1905318 (2020).
Yang, P. et al. Radical anion transfer during contact electrification and its compensation for charge loss in triboelectric nanogenerator. Matter 6, 1295–1311 (2023).
Tao, X. et al. Acid-doped pyridine-based polybenzimidazole as a positive triboelectric material with superior charge retention capability. ACS Nano 18, 4467–4477 (2024).
Zhan, F. et al. Electron transfer as a liquid droplet contacting a polymer surface. ACS Nano 14, 17565–17573 (2020).
Martín‐Fernández, C., Alkorta, I., Montero‐Campillo, M. M. & Elguero, J. Stand up for electrostatics: the disiloxane case. ChemPhysChem 23, e202200088 (2022).
Jiang, X.-Z., Sun, Y.-J., Fan, Z. & Zhang, T.-Y. Integrated flexible, waterproof, transparent, and self-powered tactile sensing panel. ACS Nano 10, 7696–7704 (2016).
Ren, Z. et al. Fully elastic and metal‐free tactile sensors for detecting both normal and tangential forces based on triboelectric nanogenerators. Adv. Funct. Mater. 28, 1802989 (2018).
Wang, L. et al. A metal-electrode-free, fully integrated, soft triboelectric sensor array for self-powered tactile sensing. Microsyst. Nanoeng. 6, 59 (2020).
Zhao, Z. et al. A broad range triboelectric stiffness sensor for variable inclusions recognition. Nano-Micro Lett. 15, 233 (2023).
Zhu, Z. et al. Human nervous system inspired modified graphene nanoplatelets/cellulose nanofibers‐based wearable sensors with superior thermal management and electromagnetic interference shielding. Adv. Funct. Mater. 2315851 https://doi.org/10.1002/adfm.202315851 (2024).
Wang, S. et al. Flexible triboelectric sensor array based on 3D printed bead-on-string sacrificial layer for human-machine interactions. Nano Energy 122, 109318 (2024).
Chen, H. et al. Drug-screening triboelectric nanogenerator-based strain sensor for cardiomyocyte contractility. Nano Energy 109, 108251 (2023).
Xu, P., Liu, J., Liu, X. et al. A bio-inspired and self-powered triboelectric tactile sensor for underwater vehicle perception. npj Flex Electron 6, 25 (2022).
Zou, J. et al. Coupled supercapacitor and triboelectric nanogenerator boost biomimetic pressure sensor. Adv. Energy Mater. 8, 1702671 (2018).
Song, K., Zhao, R., Wang, Z. L. & Yang, Y. Conjuncted pyro‐piezoelectric effect for self‐powered simultaneous temperature and pressure sensing. Adv. Mater. 31, 1902831 (2019).
Zhou, Z., You, C., Li, Z., Xia, W. & Tian, N. Enhancing the piezoelectric sensing of CFO@PDA/P(VDF-TrFE) composite films through magnetic field orientation. ACS Appl. Mater. Interfaces 14, 45679–45687 (2022).
Zhu, M. et al. Highly shape adaptive fiber based electronic skin for sensitive joint motion monitoring and tactile sensing. Nano Energy 69, 104429 (2020).
Huang, X. et al. Piezoelectric nanogenerator for highly sensitive and synchronous multi-stimuli sensing. ACS Nano 15, 19783–19792 (2021).
Tian, G. et al. Rich lamellar crystal baklava-structured PZT/PVDF piezoelectric sensor toward individual table tennis training. Nano Energy 59, 574–581 (2019).
Zhong, J. et al. Smart face mask based on an ultrathin pressure sensor for wireless monitoring of breath conditions. Adv. Mater. 34, 2107758 (2022).
Park, D. Y. et al. Self‐Powered Real‐Time Arterial Pulse Monitoring Using Ultrathin Epidermal Piezoelectric Sensors. Adv. Mater. 29, 1702308 (2017).
Wang, J.et al. A stretchable self-powered triboelectric tactile sensor with EGaIn alloy electrode for ultra-low-pressure detection. Nano Energy 89, 106320 (2021).
Tian, X. et al. Continuous Fabrication of a Highly Integrated, User-Friendly, and Low-Cost Triboelectric Yarn/Fabric for Diverse Sensing Applications. ACS Sustain. Chem. Eng. 11, 16087–16097 (2023).
Zhong, Y. et al. Dome-Conformal Electrode Strategy for Enhancing the Sensitivity of BaTiO 3 -Doped Flexible Self-powered Triboelectric Pressure Sensor. ACS Appl. Mater. Interfaces 16, 1727–1736 (2024).
Xu, R. et al. Laminated triboelectric nanogenerator for enhanced self-powered pressure-sensing performance by charge regulation. ACS Appl. Mater. Interfaces 14, 40014–40020 (2022).
Park, J., Kim, I., Yun, J. & Kim, D. Liquid-metal embedded sponge-typed triboelectric nanogenerator for omnidirectionally detectable self-powered motion sensor. Nano Energy 89, 106442 (2021).
Zhao, Z. et al. Machine-washable and breathable pressure sensors based on triboelectric nanogenerators enabled by textile technologies. Nano Energy 70, 104528 (2020).
Yang, P. et al. Monitoring the Degree of Comfort of Shoes In-Motion Using Triboelectric Pressure Sensors with an Ultrawide Detection Range. ACS Nano 16, 4654–4665 (2022).
Zu, L. et al. Multiangle, self-powered sensor array for monitoring head impacts. Sci. Adv. 9, eadg5152 (2023).
Acknowledgements
This work is supported by the National Natural Science Foundation of China for Distinguished Young Scholar (No. 52322313), National Key R&D Project from Minister of Science and Technology (2021YFA1201601), National Natural Science Foundation of China (No. 62174014), Beijing Nova program (No. 20230484399), Youth Innovation Promotion Association CAS (2021165), Innovation Project of Ocean Science and Technology (22-3-3-hygg-18-hy), State Key Laboratory of New Ceramic and Fine Processing Tsinghua University (KFZD202202), Fundamental Research Funds for the Central Universities (292022000337).
Author information
Authors and Affiliations
Contributions
S.Q. and X.C. designed the GBM-IR PDMS structure. S.Q. and X.C. wrote the paper. Z.L. and J.H. helped prepared the electrodes. P.Y. provided the FEP films. S.Q. conducted the majority of the experiments. N.L. participated in the discussion of PDMS treatment methods. L.D. and X.C. revised the manuscript. All authors reviewed and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Qingshen Jing, Ying-Chih Lai, Jiayi Yang and Junwen Zhong for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qin, S., Yang, P., Liu, Z. et al. Triboelectric sensor with ultra-wide linear range based on water-containing elastomer and ion-rich interface. Nat Commun 15, 10640 (2024). https://doi.org/10.1038/s41467-024-54980-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-54980-x
This article is cited by
-
Phase-shift engineered triboelectric nanogenerators for constant-voltage output and efficient energy harvesting
Communications Materials (2026)
-
Entropy-Driven Cellulosic Elastomer Self-Assembly for Mechanical Energy Harvesting and Self-Powered Sensing
Nano-Micro Letters (2026)
-
Tunable Phase-Engineered Polyhydroxybutyrate Fibrous Mat: An Energy Autonomous, Temperature-Responsive Platform for Wearable Application
Advanced Fiber Materials (2025)