Abstract
The design of functional artificial cells involves compartmentalizing biochemical processes to mimic cellular organization. To emulate the complex chemical systems in biological cells, it is necessary to incorporate an increasing number of cellular functions into single compartments. Artificial organelles that spatially segregate reactions inside artificial cells will be beneficial in this context by rectifying biochemical pathways. Here, we develop artificial cells with all-aqueous droplet-in-droplet structures that separate transcription and translation processes like the nucleus and cytosol in eukaryotic cells. This architecture uses protein-based inner droplets and aqueous two-phase outer compartments, stabilized by colloidal emulsifiers. The inner droplet is designed to enrich DNA and RNA polymerase for transcription, coupled to translation at the outer droplet via mRNA-mediated cascade reactions. We show that these processes proceed independently within each compartment, maintaining genotype-phenotype correspondence. This approach provides a practical tool for exploring complex systems of artificial organelles within large ensembles of artificial cells.
Similar content being viewed by others
Introduction
Bottom-up artificial cells have been studied in the field of synthetic biology, where cellular functions are reconstituted in cell-sized compartments. This research aims to identify the minimal composition of living systems and chemically distinguish them from non-living ones. It also offers an industrial platform for producing beneficial biomolecules while avoiding cellular toxicity and incorporating non-canonical elements.
Molecules for representative cellular functions, such as transcription-translation1, DNA replication2,3,4,5, and cellular division6, have typically been introduced into a wide range of compartments. Ideal compartments should be biocompatible to facilitate biological reactions internally and have the capability of self-organization for growth and division7. One prime example is a liposome, where the lipid bilayer surrounds the aqueous droplet akin to modern cells. Liposomes have been extensively studied as an artificial cell compartment together with other membranous compartments.
On the other hand, membrane-less compartments like coacervates have been proposed to be a possible candidate for protocells before the advent of lipids8,9. These compartments are increasingly receiving attention as artificial cell reactors because of their unique features of molecular permeability, selective molecular enrichment, and crowded interiors10.
These membrane-less compartments are formed through liquid-liquid phase separation (LLPS), which can be classified into two modes: segregative LLPS and associative LLPS. Among the segregative LLPS systems, one of the most well-studied involves a pair of neutral polymers, i.e., Dextran and PEG (polyethylene glycol), which form an ATPS (aqueous two-phase system)11,12. Both Dextran-in-PEG and PEG-in-Dextran droplets can be formed depending on the concentration of both polymers, and it has been shown that DNA, RNA, and most proteinous components for cell-free protein expression are enriched in Dextran-in-PEG droplets13,14,15,16, enabling protein expression inside. Meanwhile, associative LLPS gives coacervates formed through multivalent intermolecular interaction. It has been actively reported recently that low-complexity protein regions form these condensates inside cells and act as membrane-less organelles to control biochemical pathways spatiotemporally in a stimuli-responsive manner17.
The concept of organelles is also starting to be applied to artificial cells18,19,20. These artificial organelles in the artificial cell will offer chemically isolated space for specific reactions, thereby enabling spatial arrangement of modular functions and higher-order organization of complex chemical systems closer to the framework of living organisms. Enzymatic cascade reactions have been demonstrated with compartment-in-compartment systems21. For example, hydrogels with DNA being chemically immobilized are encapsulated in compartments to allow transcription, followed by translation to express protein22,23. Alternatively, self-organizing membranous droplets also serve as the inner compartment, where this inner membrane can be engineered to permit the transfer of small molecules. These molecules can then be used as substrates for the enzymatic reactions in the outer compartments21,24.
Here, we show that the droplet-in-droplet structure can be formed in an all-in-water mixture by the combination of coacervate and ATPS, and transcription and translation can be respectively conducted in the inner droplet and the outer droplet. The droplet-in-droplet structure can be spontaneously formed by mixing three polymers: IDP (intrinsically disordered protein) forming coacervate as the inner droplet and Dextran and PEG forming ATPS as a Dextran-in-PEG outer droplet. The interface between Dextran and PEG is stabilized by proteinous colloids to suppress coalescence, thus maintaining the individuality of the artificial cell compartments. The inner IDP droplet acts as a field for transcription by selective recruitment of template DNA and RNA polymerase. The absence of a physical membrane in the inner droplet enables transcribed mRNA to migrate freely into the surrounding Dex droplet, where it undergoes translation to produce proteins, thereby completing the sequential reaction cascade. This system recreates the spatial hierarchical structure found in eukaryotic cells, where the nucleus and cytosol are distinct compartments. Furthermore, it successfully mimics the roles of these compartments by enabling localized transcription and translation.
Results
Formation and stabilization of droplet-in-droplet structure by LLPS
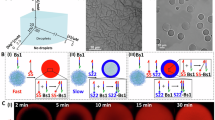
First, we formed a droplet-in-droplet structure by mixing three polymers: IDP (intrinsically disordered protein), Dex (Dextran), and PEG (polyethylene glycol). The IDP construct consists of RGG-BFP-RGG. The RGG domain, derived from LAF-1 in Caenorhabditis elegans, forms membrane-less droplets through coacervation25,26. The BFP component (mTagBFP2, blue fluorescent protein) allows us to visualize these droplets using fluorescence microscopy. While Dex and PEG are known to form segregative LLPS droplets as ATPS (aqueous two-phase system), we found that the addition of IDP to this system resulted in droplet-in-droplet structures. As shown in Fig. 1a, b, the IDP droplet nested inside the Dex droplet, surrounded by a continuous PEG phase.
a Schematic of the preparation process of the surface-stabilized droplet-in-droplet structure. Dextran (Dex) and PEG (polyethylene glycol) undergo segregative LLPS (liquid-liquid phase separation) to form Dex and PEG phases, whose interface is stabilized by PEGylated proteinous colloids as emulsifiers. Inside the droplet of the Dex phase, intrinsically disordered protein (IDP) forms a coacervate droplet. b Confocal microscopy images of each phase and emulsifiers. IDP is genetically fused with BFP for fluorescence observation. The Dex phase and the PEG phase are fluorescently labeled by TRITC-Dex and Atto647N-PEG, respectively. Proteinous colloidal emulsifiers are labeled by SYPRO orange. The scale bar represents 10 µm. The experiment was repeated three times with similar results. c, d Dex droplets’ size tunability by altering the concentration of PEG. Scale bars indicate 20 µm. Significance was assessed using a two-tailed Welch’s t-test: NS (p > 0.05), * (p < 0.05), ** (p < 0.01), *** (p < 0.001), **** (p < 0.0001). Exact p-values are 2.6e-247 (PEG 6.3 wt% vs PEG 7.2 wt%), 9.6e-61 (PEG 5.4 wt% vs PEG 6.3 wt%), and 2.6e-108 (PEG 5.4 wt% vs PEG 7.2 wt%).
However, Dex droplets tend to coalesce, which is problematic in artificial cell compartments; it is crucial to maintain a correlation between each genotype and phenotype. While the addition of amphiphilic molecules can simply stabilize the interface between oil, this is not the case for the interface of Dex and PEG phases because both are aqueous phases27. To address this challenge, the Dex-PEG interface can be stabilized using Pickering emulsions28,29. In this approach, microparticles surround the dispersed phase and prevent coalescence. Following this strategy, we developed protein-based colloidal microgels as emulsifiers by heat-denaturing BLG (β-lactoglobulin). While unmodified BLG colloids can stabilize PEG-in-Dex droplets at neutral pH, they fail to stabilize Dex-in-PEG droplets30. Based on the principle that effective emulsifiers should have a higher affinity for the continuous phase31, we modified the BLG colloids through PEGylation. This modification successfully stabilized the Dex-in-PEG droplets, maintaining their integrity for over 4 h (Supplementary Fig. 1).
While the total volume fractions of the Dex and PEG phases in an ATPS can be controlled by adjusting their concentrations, the incorporation of BLG colloids, which help prevent droplet fusion, allowed us to control the size of each dispersed Dex droplet. By setting a PEG concentration of 6.3 wt% in the presence of BLG colloids, we achieved Dex droplets ranging from 5 to 20 µm in diameter (Fig. 1c). This size range corresponds to that of natural cells, making our system suitable for artificial cell applications.
Localization of transcription in IDP droplet
Next, we aimed to conduct transcription at the inner IDP droplets (Fig. 2a). To achieve this, we localized essential transcription components—RNA polymerase (RNAP) and template DNA—within the IDP droplet. To recruit proteins, including enzymes, into the IDP droplets, Schuster et al. utilized the interaction between peptide tags SYNZIP1 (SZ1) and SYNZIP2 (SZ2), which were fused to IDP and the proteins of interest (POI), respectively25,32. We implemented this approach by attaching the SZ2 tag to T7 RNA polymerase (T7RNAP) and the SZ1 tag to IDP. Enrichment of T7RNAP in the IDP droplet was assessed by labeling either SZ2-tagged T7RNAP or non-tagged T7RNAP with fluorescein (FL), followed by measuring the fluorescence intensity in both the IDP and Dex phases. The environment-dependent effect on FL intensity was assessed to be negligible by testing fluorescence in each polymer-rich solution (Supplementary Discussion and Supplementary Fig. 2). While T7RNAP without the SZ2 tag already demonstrated a 14-fold higher fluorescence intensity in the IDP droplet compared to the Dex droplet, the SZ2-tagged T7RNAP exhibited a 190-fold increase, as illustrated in Fig. 2b, c, and Supplementary Fig. 3. Similarly, we attached an SZ2 tag to the lac repressor (LacR), enabling the DNA coding the lac operator (lacO) sequence to interact with this modified SZ2-LacR. This modification, combined with the SZ1-SZ2 and LacR-lacO interactions, facilitated the recruitment of the lacO DNA to the IDP droplet. This was verified by labeling lacO DNA with FL and analyzing its distribution in the droplet-in-droplet structure both with and without SZ2-LacR (Fig. 2d, e). Consequently, the ratio of fluorescence intensity from the IDP phase to the Dex phase of lacO DNA increased from 7.9 to 45 with the addition of SZ2-LacR. Since this study aims to perform translation in addition to transcription, all these observations were conducted in the presence of cell-free translation components (i.e., PURE frex 2.0 ΔT7RNAP, see “Methods” for details).
a Schematic of transcription in the inner IDP droplet. Enzymes (RNAP, RNA polymerase) and repressors (LacR) are both designed to bind with IDP through SZ1-SZ2 peptide interactions. LacR binds to the lacO sequence placed outside the region to be transcribed, thus recruiting template DNA to the IDP droplet. Transcribed RNA contains a binding site for a molecular beacon (MB) for fluorescence observation. b, c Enrichment of SZ2-tagged RNAP confirmed by fluorescent labeling by fluorescein (FL). Line profiles were extracted from white dashed lines. White bold scale bars at lower right represent 5 µm (b). Exact p-values are 2.6e-14 (SZ2-T7RNAP-FL) and 3.0e-9 (T7RNAP-FL) (c). The experiment was repeated at least three times with similar results. d, e Enrichment of fluorescence-labeled lacO-containing DNA in the presence or absence of SZ2-LacR. Line profiles were extracted from white dashed lines. White bold scale bars at lower right represent 5 µm (d). Exact p-values are 1.8e-13 (FL-lacO DNA + SZ2-LacR) and 6.6e-52 (FL-lacO DNA) (e). The experiment was repeated over three times with similar results. f Time-lapse images of transcription monitored by MB. The scale bar represents 10 µm. See also Supplementary Fig. 7a for line profiles. g Fluorescence intensity of MB was measured in the outer Dex region of droplet-in-droplet structures to quantify transcription activity. Data are presented as mean values ± 2SE. Under each experimental condition, over 250 droplet-in-droplet structures were imaged across four distinct microscopic fields per frame. Significance was assessed using a two-tailed Welch’s t-test: NS (p > 0.05), * (p < 0.05), ** (p < 0.01), *** (p < 0.001), **** (p < 0.0001).
To visualize RNA synthesis in real time, we designed a molecular beacon (MB) system that fluoresces upon RNA binding. The template DNA contained a GFP gene and MB binding site under T7 promoter control, with two upstream lacO sequences. This design enabled us to track transcription activity by monitoring MB fluorescence intensity (Fig. 2f). In the droplet-in-droplet structures, the MB signal in the outer Dex droplet increased steadily until reaching a plateau at ~500 min (Fig. 2g). At this time point, significant fluorescence was detected in both the inner IDP and outer Dex droplets, while controls lacking template DNA showed no fluorescence increase (Supplementary Fig. 4).
To verify that transcription occurs in the IDP phase rather than the Dex phase, we performed bench-top experiments using conventional test tubes alongside microscopic observations. For this verification, we omitted PEG and colloidal emulsifiers to achieve distinct sampling of the Dex phase. The IDP-Dex phase system containing PURE frex 2.0 ΔT7RNAP, SZ2-T7RNAP, SZ2-LacR, and MB was centrifuged and macroscopically separated into a supernatant Dex phase and a sedimented IDP phase (Supplementary Fig. 5a). Since the volume of the IDP phase was too small (about 1/103 of the total volume) to extract directly, we sampled the supernatant Dex phase and compared it to the whole mixture sampled before centrifugation (IDP + Dex phases). By monitoring transcription activities with MB fluorescence, we observed that RNA production over 600 min was twice as high in the combined IDP + Dex sample compared to the Dex-only sample (blue lines, Supplementary Fig. 5c). RT-qPCR analysis of samples collected at different time points aligned with these findings (blue lines, Supplementary Fig. 5e). Given that the IDP phase constituted only 1/103 of the total volume, these results suggest that transcription occurred predominantly in the IDP phase.
Localized translation in outer Dex droplet
We conducted translation in the outer Dex droplet in our droplet-in-droplet system coupled with the transcription in the inner IDP droplet (Fig. 3a). Before transcription-translation experiments, to confirm the partitioning of ribosomes, we labeled them using FL-NHS dye targeting primary amines in ribosomal proteins and observed their locations. Figure 3b, c illustrate that ribosomes are preferentially localized in the Dex phase. The fluorescence intensity was 2.9-fold higher from the outer Dex phase than from the inner IDP phase. The exclusion of ribosomes from the IDP droplet could be attributed to their size (~20 nm), which surpasses the previously reported coacervate’s mesh size from the same IDP (RGG-rich domain from LAF-1) of 3–8 nm33.
a Schematic. Translation at the outer Dex droplet from mRNA synthesized in the inner droplet. By expressing GFP tagged with SNAP-tag in the presence of benzyl guanine-Dextran (BG-Dex), GFP can be fixed to the Dex phase. b, c The location of ribosomes confirmed by fluorescein (FL)-labeled ribosomes (0.1 µM) with confocal microscopy. Line profiles were extracted from white dashed lines. White bold scale bars at lower right represent 5 µm (b). Significance was assessed using a two-tailed Welch’s t-test: NS (p > 0.05), * (p < 0.05), ** (p < 0.01), *** (p < 0.001), **** (p < 0.0001). The exact p-value is 2.4e-52 (FL-ribosome) (c). The experiment was repeated more than three times with similar results. d Time-lapse images of translation. The scale bar represents 10 µm. e GFP expression from Dex droplets with or without inner IDP droplets were compared. Data are presented as mean values ± 2SE. Under each experimental condition, over 3000 Dex droplets were imaged across three distinct microscopic fields per frame.
Before microscopic observations of transcription and translation, the IDP-Dex two-phase system in conventional test tubes was macroscopically separated to examine the localization of translation activity, similar to the method used for identifying transcription sites (Supplementary Fig. 5a). The addition of mRNA to express GFP (orange lines, Supplementary Fig. 5d) resulted in comparable signal increases in both the whole mixture (IDP and Dex phases) and the supernatant Dex phase. This result suggests that the yield of expressed GFP is predominantly from the Dex phase rather than the IDP phase (Supplementary Fig. 5d). The mRNA levels monitored by MB were also comparable in both IDP + Dex sampling and Dex only sampling conditions (orange lines, Supplementary Fig. 5c, e). These observations indicate that, unlike DNA which is designed to be enriched in the IDP phase (leading to enhanced mRNA levels in IDP + Dex phases compared to the Dex-only phase), mRNA is naturally distributed throughout both IDP and Dex phases. This distribution allows translation reactions to occur in the Dex phase.
Based on these results, we aimed to couple transcription and translation in the droplet-in-droplet structure and conducted time-lapse observation under the fluorescence microscope. The protein expression was monitored by GFP fused with an N-terminal SNAP-tag designed to covalently bind with benzyl-guanine-modified Dextran (BG-Dex) so that GFP was partitioned to the Dex phase (Fig. 3d). The effect of this interaction between SNAP-GFP and BG-Dex was assessed by comparing conditions with or without BG-Dex. Although the expression level of SNAP-GFP was independent of the presence of BG-Dex, as detected by measurements with a plate reader, microscopic observations revealed that SNAP-GFP was not partitioned to the Dex phase when BG-Dex was not added (Supplementary Fig. 6).
Figure 3e shows that the fluorescence signal from SNAP-GFP continuously increased at the Dex phase until reaching the plateau at around 400 min, while no signal increase was confirmed if the template DNA was not added. Additionally, Dex droplets were distinguished by the presence or absence of inner IDP droplets. Droplets containing IDP droplets (Fig. 3e, blue line) exhibited ~1.2- to 1.5-fold GFP signal compared to those without (orange line). The enhanced GFP production suggests that the IDP phase accumulates DNA molecules, enhancing the efficiency of transcription and translation. The GFP signal from Dex droplets without the inner IDP droplets originated from gene expression from DNA in the Dex phase.
Assessment of cross-talk between droplets
In artificial cells, it is crucial to ensure that genotype corresponds to phenotype and that phenotype-representing molecules remain confined within each compartment. As the droplets used in this study are membrane-less and molecularly permeable, we investigated how the coupling of genotype (i.e., DNA) with phenotype molecules (i.e., proteins to be expressed) is maintained among droplets.
We prepared two sets of droplets; the first containing DNA encoding for GFP (mStayGold QC2-6 FIQ) fused with a SNAP-tag, and the second containing DNA for RFP (mScarlet-I34) fused with a SNAP-tag. After gently mixing the two droplet populations and transferring them to an observation well, we investigated the exclusive expression of GFP or RFP within each droplet (Fig. 4a).
a Schematic showing how to prepare droplets containing homogeneous genotypes and inquire whether homogeneous phenotypes are expressed in each droplet. b Expression of GFP and RFP at 200 min from the initial image acquisition. The first column at the left end shows the sample prepared in the manner depicted in (a). The second column shows the condition where premixed gfp DNA solution and rfp DNA solution were added. The third and fourth columns show conditions where gfp DNA and rfp DNA are solely added, respectively. The bottom row shows marginal plots, where scatter plots showing GFP and RFP fluorescence from each droplet are combined with histograms for each axis. n values indicate the number of droplets plotted for each condition. The experiment was repeated more than five times with similar results. The scale bar represents 10 µm. See also Supplementary Fig. 7b for line profiles.
As a reference for complete fusion conditions, we analyzed droplets containing a premixed solution of gfp and rfp DNA, which was added before droplet formation. For comparison, we included control droplets containing either gfp DNA or rfp DNA alone. The total DNA concentration was maintained at 0.1 nM across all conditions.
Figure 4b shows that droplets containing either gfp or rfp DNA exhibited distinct protein expression patterns at 200 min, as demonstrated by the orthogonal distribution in the scatter plot. In contrast, droplets formed from premixed gfp and rfp DNA showed a clear correlation between GFP and RFP fluorescence intensities. These findings, along with the control conditions using exclusively gfp or rfp DNA, indicate that individual droplets maintain their genotype-phenotype correspondence throughout the protein expression period, despite their physical proximity.
We further analyzed this orthogonality by transforming the scatter plot data into polar coordinates following subtraction and normalization, with particular emphasis on θ values across conditions (Supplementary Fig. 8a). This analysis was performed on all 86 frames captured at 10-min intervals. We then calculated a symmetry index defined as |θ – 45°| and plotted it against time for each condition (Supplementary Fig. 8b). All conditions exhibited variable symmetry indices during the first 50 min due to noise from low expression levels (represented by r in polar coordinates). After 50 min, these values stabilized, even in the (+) gfp DNA & (+) rfp DNA condition (Supplementary Fig. 8c). This stability persisted throughout the observation period, which extended well beyond the 400-min threshold required for protein expression to reach its plateau (Fig. 3e). These results suggest that the co-expression of GFP and RFP in a small subset of droplets is attributed to time-independent factors, such as initial DNA distribution during droplet formation, rather than time-dependent processes like transcription, translation, or product diffusion.
Discussion
Our study demonstrated that (1) droplet-in-droplet structures can be engineered in an all-aqueous system by combining a coacervate of intrinsically disordered proteins (IDPs) with interface-stabilized Dex-PEG aqueous two-phase systems (ATPS); (2) transcription can be actively carried out within the inner IDP droplet, which acts as a “nucleus”; and (3) translation in the outer Dex droplet can be coupled to transcription, mimicking the “cytosol.” Crucially, Dex droplets with inner IDP droplets showed enhanced protein expression compared to those without. The specific protein phenotypes produced by DNA encapsulated in the inner IDP droplets (Fig. 4) demonstrated that both transcription and translation processes were carried out independently in each droplet. This observation confirms that our membrane-free artificial cell system effectively maintains a correspondence between genotype and phenotype, preserving the individuality of each functional compartment.
The membrane-free design of our system offers important advantages for molecular permeability. Specifically, the membrane-less interface between IDP and Dex phases facilitates the seamless transfer of transcribed mRNA to the outer droplet, triggering translation. This eliminates the need for complex molecular systems such as pores or transporters often required by enclosed compartments like liposomes or polymersomes. The inner IDP droplet was designed to enrich enzymes (RNA polymerase) and template DNAs through the genetic integration of peptide interaction modules. Given the established utility of other orthogonal interacting peptide pairs32 and recent advancements in the mechanism of the coexistence of distinct membrane-less organelles35,36, the introduction of additional modular artificial organelles appears feasible.
Despite the successful compartmentalization of cellular processes, our current system faces certain limitations that require further optimization. Specifically, protein yields in our droplet-in-droplet structure remained at sub-µM levels (Supplementary Fig. 5d), lower than the µM-level yields achieved in standard cell-free expression systems37. This reduction may be attributed to the presence of polymers known to decrease translation efficiency38. To address this limitation, further optimization of the system components would be necessary. For instance, the design of colloidal emulsifiers (size, surface chemistry, and anisotropy) may be improved to stabilize the Dex-PEG phase separation at lower polymer concentrations, potentially mitigating the impact on translation activity. Such adjustments could enhance protein yield and improve the system’s efficiency for future applications.
As a prospect, our membrane-free system offers several possibilities for both practical applications and fundamental research. The membrane-free architecture could enable sustained protein production by facilitating molecular supply. Our observation of SNAP-GFP (50 kDa) diffusion out of the Dex-phase (Supplementary Fig. 6) suggests that smaller molecules (<1 kDa)—including energy sources and building blocks essential for sustainable cell-free protein expression such as NTPs, creatine phosphates, and amino acids—would traverse the Dex-PEG interface more readily. This enhanced permeability could extend the system’s lifespan by facilitating essential component replenishment. Furthermore, the spatial segregation of transcription and translation within our system would allow us to study and modify the relationship between these processes. Although transcription and translation interfere with each other in vitro, natural cells overcome this interference through mechanisms that remain poorly understood39. This system could enable independent adjustment of transcription and translation parameters, providing insights into their interaction and optimization.
Methods
Plasmid DNA and linear template DNAs
Sequence information about plasmids, linear template DNAs, and primers used in this study are listed in Supplementary Data 1. Their sequences were all verified by Sanger sequencing. Plasmid DNA was purified by FastGene PlasmidMini (NIPPON Genetics, FG-90402). Linear DNA was amplified by PCR (KOD One PCR Master Mix, TOYOBO, KMM-101) and purified (FastGene Gel / PCR Extraction Kit, NIPPON Genetics, FG-91202). Primers were purchased from FASMAC.
in vitro transcription
RNA was synthesized by in vitro Transcription T7 Kit (Takara, 6140) and purified by NucleoSpin RNA Clean-up XS (MACHEREY-NAGEL, 740903.50) according to manufacturers’ instructions.
Expression and purification of recombinant proteins
IDP (SZ1-RGG-BFP-RGG) was cloned in a pRSET-B vector with an N-terminal His-rich tag, expressed in T7 Express lysY/Iq Competent E. coli (NEB, C3013I) and purified as previously reported with some modifications25. Fresh colonies on LB agar plate with Carbenicillin (100 µg/ml) were directly inoculated to 1 L of LB liquid media containing the same concentration of Carbenicillin. The culture was incubated at 37 °C with shaking at 200 rpm until the OD600 reached 0.8–1.0 and induced by 1 mM of isopropyl β-D-1-thiogalactopyranoside (IPTG) overnight by shaking at 200 rpm at 18 °C. Cells were then harvested by centrifugation at 7000 × g for 15 min at 4 °C and resuspended by 50 ml of lysis buffer (20 mM Tris-HCl pH = 7.5, 1 M NaCl, 20 mM Imidazole). Cells were disrupted by sonication and incubated in a 60 °C water bath for 1–2 h so that contaminating proteins denature and aggregate. This solution was centrifuged at 15,000 × g for 1 min, and the supernatant was centrifuged at the same condition again. The second supernatant was loaded onto Ni Sepharose 6 Fast Flow (Cytiva) equilibrated with 2CV of lysis buffer at room temperature. The column was washed with over 2CV of wash buffer (20 mM Tris-HCl pH = 7.5, 0.5 M NaCl, 20 mM Imidazole), and the IDP was eluted with elution buffer (20 mM Tris-HCl pH = 7.5, 0.5 M NaCl, 500 mM Imidazole). Fractions of the eluted solution were immediately moved to the 60 °C water bath so as not for IDP to phase separate at room temperature. 3 mL of the purified IDP at 60 °C was loaded onto Econo-Pac 10DG Desalting Prepacked Gravity Flow Column (BIO-RAD) equilibrated with 20 mL of stock buffer (50 mM HEPES-KOH pH = 7.5, 0.5 M NaCl) in an incubator set to 60 °C. The desalted IDP solution was eluted by 4 mL of the stock buffer and moved back to the 60 °C water bath. This stock solution was aliquoted to PCR tubes on a heat block set to 60 °C, flash-frozen with liquid nitrogen, and stored at −80 °C.
SZ2-T7RNAP was cloned in the pBAD vector with an N-terminal His-rich tag and expressed from One Shot TOP10 Chemically Competent (Invitrogen C404003) and purified. A single fresh colony on an LB agar plate with Carbenicillin (100 µg/ml) was inoculated to 5 mL of LB liquid media containing Carbenicillin (100 µg/ml) for pre-culture. This pre-culture was transferred to 1 L of LB liquid media containing Carbenicillin (100 µg/ml). The culture was shaken at 200 rpm at 37 °C until OD600 reached 0.6 and induced by 0.02% L-arabinose (stock solution: 20%) overnight by shaken at 200 rpm at 18 °C. The cells were harvested by centrifugation at 7000 × g for 15 min at 4 °C and resuspended by 50 ml of wash buffer (20 mM Tris-HCl pH = 7.5, 0.5 M NaCl, 20 mM Imidazole, 5 mM 2-mercaptoethanol). Cells were disrupted by sonication and ultracentrifuged (81,000 × g, 4 °C, 20 min). The supernatant was loaded onto Ni Sepharose 6 Fast Flow (Cytiva) equilibrated with 2CV of wash buffer. The following operations were conducted at 4 °C unless otherwise specified. The column was washed with over 2CV of wash buffer, and the protein was eluted with elution buffer (20 mM Tris-HCl pH = 7.5, 0.5 M NaCl, 250 mM Imidazole, 5 mM 2-mercaptoethanol). Fractions of the eluted solution were concentrated by Amicon Ultra Centrifugal Filter, 50 kDa MWCO. This solution was buffer exchanged by Econo-Pac 10DG Desalting Prepacked Gravity Flow Column (BIO-RAD) equilibrated with 20 mL of 2× stock buffer (100 mM HEPES-KOH pH = 7.5, 0.2 M NaCl, 40 mM 2-mercaptoethanol, 2 mM EDTA-NaOH pH = 8.0, 2 mM DTT) at 4 °C. Glycerol was added to the eluted solution as stored at −20 °C. T7RNAP without an SZ2 tag was prepared in almost the same manner, with the exception that this protein was cloned in the pQE30 vector and induced by 1 mM of isopropyl β-D-1-thiogalactopyranoside (IPTG) at OD600 = 0.8–1.0.
SZ2-LacR was cloned in the pBAD vector with a C-terminal 6 × His tag. By replacing the C-terminal region of the original LacR with His tag, this construct is not supposed to form a tetramer (dimerized dimers) but to form a dimer, which is reported to be sufficient for binding lacO DNA. This construct was expressed from One Shot TOP10 Chemically Competent (Invitrogen C404003) and purified. A single fresh colony on an LB agar plate with Carbenicillin (100 µg/ml) was inoculated to 5 mL of LB liquid media containing Carbenicillin (100 µg/ml) for pre-culture. This pre-culture was transferred to 1 L of LB liquid media containing Carbenicillin (100 µg/ml). The culture was shaken at 200 rpm at 37 °C until OD600 reached 0.5 and induced by 0.02% L-arabinose (stock solution: 20%) for 4 h by shaken at 200 rpm at 30 °C. The cells were harvested by centrifugation at 7000 × g for 15 min at 4 °C and resuspended by 50 ml of lysis buffer (20 mM Tris-HCl pH = 7.5, 1 M NaCl, 20 mM Imidazole). Cells were disrupted by sonication and ultracentrifuged (81,000 × g, 4 °C, 20 min). The supernatant was loaded onto Ni Sepharose 6 Fast Flow (Cytiva) equilibrated with 2CV of wash buffer (20 mM Tris-HCl pH = 7.5, 0.5 M NaCl, 20 mM Imidazole). The following operations were conducted at 4 °C unless otherwise specified. The column was washed with over 2CV of wash buffer, and the protein was eluted with elution buffer (20 mM Tris-HCl pH = 7.5, 0.5 M NaCl, 500 mM Imidazole). Fractions of the eluted solution were concentrated by Amicon Ultra Centrifugal Filter, 10 kDa MWCO, and applied to a size exclusion column, Superdex 200, 10/200GL (Cytiva, 17-5175-01) equilibrated with stock buffer (200 mM Tris-HCl pH = 7.5, 200 mM KCl, 1 mM EDTA, 1 mM DTT). Fractions within the single peak were collected and concentrated again by Amicon Ultra Centrifugal Filter, 10 kDa MWCO. This stock solution was aliquoted to PCR tubes on ice, flash-frozen with liquid nitrogen, and stored at −80 °C.
Preparation of BLG colloids and their PEGylation
Colloidal microgels of BLG (beta-lactoglobulin) were prepared according to Gonzalez-Jordan et al. 30. The solution of 4.4 mM CaCl2 and 40 mg/ml BLG (Sigma-Aldrich, L3908) dissolved in Milli-Q water was incubated at 80 °C for 15 h, during which the solution turned to white turbid to form BLG colloids. For PEGylation, this BLG colloid solution (10 mg/ml) was mixed with 16.3 mg/ml mPEG2k-NHS (NANOCS, PG1-SSA-2k) freshly dissolved in DMSO (initial concentration: 81.5 mg/ml) and 50 mM HEPES-KOH pH = 8.3 and incubated for 1.5 h. After that, the solution was washed with 40 mM HEPES-KOH pH = 7.6 by Amicon Ultra Centrifugal Filter, 100 kDa MWCO repeatedly for four times. Finally, the same buffer was added until the volume reached a quarter of the initial volume to make BLG concentration back to 40 mg/ml and stored at 4 °C. The turbidity was measured at OD700 by NanoDrop (Thermo Scientific) to be 0.2 at 1 mm of optical path length.
Preparation of Atto647N-PEG
Atto647N dye-labeled PEG was prepared from PEG20k-diamine (Sigma-Aldrich, 14509) and Atto647N-NHS (Sigma-Aldrich, 18373). 100 mM HEPES-KOH pH = 8.3, 1 wt% PEG20k-diamine, 1.26 g/l Atto647N-NHS dissolved in DMSO (initial concentration: 10 g/l) were mixed with Milli-Q water and incubated for 1.5 h. Unreacted dyes were excluded by Zeba Spin Desalting Column (Thermo Scientific, 89882) according to the manufacturer’s instruction. The product was diluted to 0.1 wt% by Milli-Q water and stored at 4 °C.
Preparation of BG-Dex
Benzyl-guanine conjugated Dextran was prepared from Amino Dextran 500k (Invitrogen, D7144) and BG-GLA-NHS (NEB, S9151S). 50 mM HEPES-KOH pH = 8.3, 1 wt% Amino Dextran, 0.16 mM BG-GLA-NHS freshly dissolved in DMSO (initial concentration: 10 g/l) were mixed with Milli-Q water and incubated for 1.5 h. Unreacted BG-GLA-NHS was washed out with Milli-Q water by Amicon Ultra Centrifugal Filter, 100 kDa MWCO repeatedly for four times. Finally, Milli-Q water was added until the volume reached two-thirds of the initial volume to make the conjugated Dextran concentration 1.5 wt% and stored at 4 °C.
Labeling lacO DNA, T7RNAP, and ribosomes with FL (fluorescein)
FL-labeled lacO DNA was prepared by PCR labeling with FL-dUTP (Roche, 11373242910). PCR enzyme and 10x PCR buffer are from TaKaRa Taq Hot Start Version (Takara, R007A). The PCR mixture was composed of 1x PCR buffer, 0.05 U/µL of PCR enzyme, 0.1 mM each of dATP, dCTP, dGTP, 0.05 mM of dTTP, 0.05 mM of FL-dUTP, 0.3 µM each of forward and reverse primers, and 20 pg/µl of template DNA. The amplicon was designed to contain a lacO sequence. The details about sequences of primers and the amplicon can be found in supplementary information. The PCR product was purified by FastGene Gel/PCR Extraction Kit (NIPPON Genetics, FG-91202) according to the manufacturer’s instructions. The degree of labeling was calculated to be 356 dyes per dsDNA.
FL-labeled SZ2-T7RNAP and T7RNAP were prepared from purified SZ2-T7RNAP, T7RNAP (mentioned above), and FL-NHS (Thermo Scientific, 46410). 10 g/l SZ2-T7RNAP or T7RNAP, 0.7 g/l FL-NHS freshly dissolved in DMSO (initial concentration: 10 g/l) were mixed with labeling buffer (50 mM HEPES-KOH pH = 8.3, 500 mM NaCl dissolved in Milli-Q water). The mixture was incubated at 4 °C for 2 h. Unreacted dyes were excluded by exchanging the buffer to stock buffer (20 mM HEPES-KOH pH = 7.6, 100 mM Potassium acetate, 7 mM 2-mercaptoethanol dissolved in Milli-Q water) by Zeba Spin Desalting Column (Thermo Scientific, 89882) according to the manufacturer’s instruction. The degrees of labeling were calculated to be 0.35 dye per SZ2-T7RNAP and 0.24 dye per T7RNAP.
FL-labeled ribosomes were prepared from ribosome solution (Solution III of PURE frex2.0, GeneFrontier) and FL-NHS (Thermo Scientific, 46410). 4.0 µM ribosomes, 0.03 g/l FL-NHS freshly dissolved in DMSO (initial concentration: 10 g/l) were mixed with labeling buffer (50 mM HEPES-KOH pH = 8.3, 10 mM Magnesium acetate, 100 mM KCl dissolved in Milli-Q water). The mixture was incubated at 4 °C for 2 h. Unreacted dyes were excluded by exchanging the buffer to stock buffer (40 mM HEPES-KOH pH = 7.6, 10 mM Magnesium acetate, 30 mM KCl, 7 mM 2-mercaptoethanol dissolved in Milli-Q water) by Zeba Spin Desalting Column (Thermo Scientific, 89882) according to the manufacturer’s instruction. The degree of labeling was calculated to be 0.3 dye per ribosome.
Preparation of 384-well microscopy plates
384-well glass bottom microplate (Corning, 4581) was added with 25 µL per well of 5% Cell Cleaning Solution (SANSYO, T-A-28), incubated for 4 h at 37 °C and rinsed copiously with Milli-Q water. Silica was etched with 25 µL per well of 8 N KOH for 1 h at room temperature and rinsed copiously with Milli-Q water, followed by ethanol. Silanization was conducted overnight by adding 25 µL per well of 2% Dichlorodimethylsilane (Sigma, 440272) dissolved in a solvent of 95% ethanol, 4.99% Milli-Q water, and 0.01% acetic acid, and rinsed copiously with ethanol. The plate was sealed with an adhesive PCR foil. Just before use, silanized wells were opened by cutting the foil, washed by pipetting 20 µL per well of Milli-Q water, and 20 µL per well of 5% Pluronic F-127 was added, and incubated for 5 min. Then, the remaining solution was sucked up and washed again by pipetting 50 µL per well of Milli-Q water. Sample solutions were immediately added to the vacant wells.
Microscopy and image analysis
All images were acquired by TCS-SP8 X (Leica Microsystems) confocal laser scanning microscope equipped with white-light laser lines and HyD detectors. Acquired images were cropped and/or merged with Fiji and analyzed using custom Python codes available here: https://github.com/tomohara97/droplet-in-droplet. Microscopy configurations can be found in Supplementary Data 2.
Sample preparations for microscopy observations
For the observation of the droplet-in-droplet structure with four channels (IDP, Dex, PEG, colloidal emulsifiers), the components were as follows: IDP (3.2 µM), Dex 550k (0.1 wt%, Sigma 31392), TRITC-Dex (0.01 wt%), mPEG2k-BLG (2.4 mg/ml), Atto647N-PEG (0.001 wt%), SYPRO orange in DMSO (10x, Supelco S5692), PURE frex 2.0 Solution 1 (0.54x), PURE frex 2.0 Solution 2 ΔT7RNAP (1x), PURE frex 2.0 Solution 3 (1x), and PEG 35k (6 wt%, Sigma 81310). Unless otherwise noted, concentrations are given in parentheses as final concentrations.
For other observations, samples contained the following components unless otherwise noted: IDP (3.2 µM), SZ2-LacR (1 µM), PEG 35k (6 wt%), PURE frex 2.0 Solution 1 (0.54x), PURE frex 2.0 Solution 2 ΔT7RNAP (1x), PURE frex 2.0 Solution 3 (1x), mPEG2k-BLG (2.4 mg/ml), Atto647N-PEG (0.001 wt%), Dex 550k (0.1 wt%), SZ2-T7RNAP (20 nM). Some components were mixed beforehand as a master mix, which typically included PURE components (Solution 1–3), mPEG2k-BLG, Atto647N-PEG, Dex 550k, and SZ2-T7RNAP. All PURE frex 2.0 components were purchased from GeneFrontier, and PURE frex 2.0 Solution 2 ΔT7RNAP was custom-ordered (PFC-Z2601). Additional specific information for each observation is specified below.
To observe and compare FL-labeled T7RNAP (Fig. 2b), not every experimental condition included SZ2-T7RNAP and SZ2-LacR. Instead, one of the following was added for comparison: 1 µM of labeled T7RNAP, 1 µM of unlabeled T7RNAP, and an optically equivalent concentration of FL, or Milli-Q water.
To observe and compare the interaction between FL-labeled lacO DNA and SZ2-LacR (Fig. 2d), 0.1 nM of FL-labeled lacO DNA was added with or without SZ2-LacR (1 µM).
To observe transcription activities (Fig. 2f), 0.5 µM of molecular beacon (MB) was added with or without linear template DNA. This template DNA encodes a GFP gene and a binding site for the MB under the control of the T7 promoter, preceded by two repeats of the lacO sequence. The design of the molecular beacon is identical to Tabuchi et al. 40. It is a 2′-O-methyl-RNA molecular beacon (IDT), with its 5’ end conjugated to ROX and its 3’ end to BHQ-2. The sequence is CCUGUACGAUGCCAGCAGG to detect CCUGCUGGCAUCGUACAGG in mRNA.
To observe FL-labeled ribosomes (Fig. 3b), 0.1 µM of labeled ribosomes or an optically equivalent concentration of FL was added.
To observe the expression of SNAP-GFP and SNAP-RFP (Figs. 3d and 4b), template DNA at a concentration of 0.1 nM was supplemented with 0.015 wt% benzyl-guanine conjugated Dextran (BG-Dex). To verify genotype-phenotype correspondence, three distinct droplet-in-droplet structures were prepared by varying the DNA added: (1) SNAP-GFP coding DNA, (2) SNAP-RFP coding DNA, and (3) a premixed solution containing equal parts of (1) and (2). Each mixture was vigorously agitated to ensure uniformity. Subsequently, equal volumes of the mixtures from (1) and (2) were combined gently in a tube and then carefully transferred to a 384-well plate without any agitation.
To evaluate the effect of Dex-PEG interface stabilization facilitated by PEG-BLG (Supplementary Fig. 1), the following components were pre-mixed as a master mix: Tris-HCl at pH 7.5 (14 mM), Dextran 550k (1.95 wt%), TRITC-Dextran 500k (0.05%, Sigma 52194), and Thioflavin T (20 µM, Wako 202-01002). Following distribution of this master mix, PEG-BLG colloids, or BLG colloids (1 g/L), were introduced along with PEG 35k (8 wt%).
For examining the partitioning of SNAP-GFP into the Dex phase mediated by BG-Dex (Supplementary Fig. 6), the following components were included: IDP (3.2 µM), SZ2-LacR (1 µM), PEG 35k (6 wt%), PURE frex 2.0 Solution 1 (0.54x), PURE frex 2.0 Solution 2 ΔT7RNAP (1x), PURE frex 2.0 Solution 3 (1x), mPEG2k-BLG (2.4 mg/ml), Atto647N-PEG (0.001 wt%), Dex 550k (0.1 wt%), and SZ2-T7RNAP (20 nM). A master mix was prepared in advance, comprising PURE components (Solution 1–3), mPEG2k-BLG, Atto647N-PEG, Dex 550k, and SZ2-T7RNAP. Depending on the conditions, 0.015 wt% of BG-Dex and template DNA (0.1 nM) were added.
All samples were vigorously agitated in the tube unless otherwise specified and loaded onto a 384-well microscopy plate. The plate was mildly centrifuged at 700 rpm (100 × g) for 1 min to maximize the number of droplets to be observed near the glass surface at the bottom. Wells with samples were sealed by adding 10 µL of mineral oil (Sigma-Aldrich, M5904) and subjected to microscopy observation.
Calibrating fluorescein’s fluorescence intensities in polymer-rich solutions
Fluorescein (FL) fluorescence intensities were compared across Dex-rich, PEG-rich, IDP-rich, and polymer-free solutions (Supplementary Fig. 2). For Dex and PEG measurements, samples contained 20 mM HEPES-KOH (pH 7.5), FL at specified concentrations, and either Dex, PEG, or MilliQ water (control). IDP samples consisted of 30 mM HEPES-KOH (pH 7.5), 300 mM potassium glutamate, 330 mM ATP (adjusted to neutral pH with NaOH), and specified concentrations of both IDP and FL. Concentrations are given in parentheses as final concentrations.
Real-time fluorescence measurement by qPCR machine
For real-time fluorescence measurement of transcription and translation in reconstituted IDP-Dex systems (Supplementary Fig. 5c, d), sampled mixtures were prepared in triplicate, added to 0.2 ml 8-Tube PCR Strips without Caps (Bio-Rad, TLS0851) and sealed with 0.2 ml Flat PCR Tube 8-Cap Strips (Bio-Rad, TCS0803). Tubes were set in CFX Opus 96 Real-Time PCR Instrument (Bio-Rad, 12011319J1). Measurement was conducted in two channels (red “ROX” channel for molecular beacon representing transcription and green “FAM” channel for GFP expression representing translation), 10 min intervals, and 30 °C (isothermal). The samples are composed of SZ2-T7RNAP (20 nM), molecular beacon (0.5 µM), SZ2-LacR (1 µM), IDP (genetically fused with SZ1 and BFP, 3.2 µM), PURE frex 2.0 Solution 1 (0.54x), PURE frex 2.0 Solution 2 ΔT7RNAP (1x), PURE frex 2.0 Solution 3 (1x), Dex 550k (2 wt%), Recombinant RNAse Inhibitor (NEB M0314, 2 U/µL), MilliQ water, and additive (DNA, RNA or MilliQ). Concentrations are given in parentheses as final concentrations.
RT-qPCR
RNA levels were quantified by RT-qPCR in parallel with real-time fluorescence measurements of transcription and translation activity (Supplementary Fig. 5e). The ReverTra Ace qPCR RT Kit and THUNDERBIRD Next SYBR qPCR Mix (TOYOBO) were used for reverse transcription and quantitative PCR, respectively, following manufacturer’s protocols. All reactions were performed on a CFX Opus 96 Real-Time PCR Instrument (Bio-Rad, 12011319J1). RNA concentrations were determined using standard curves generated from in vitro transcribed RNA. Both IDP + Dex mixtures and Dex-only supernatants were analyzed at 0, 300, and 600 min, with sample compositions matching those used in real-time fluorescence measurements. All conditions were measured in triplicate.
Calibration of GFP level
GFP expression levels were quantified using a standard curve generated from known concentrations of purified mNeonGreen protein spiked into control samples (Supplementary Fig. 5f). Measurements were performed using a CFX Opus 96 Real-Time PCR Instrument (Bio-Rad, 12011319J1). The compositions matched those used in real-time fluorescence measurements, with additives replaced by purified GFP at various concentrations (measured in triplicate). This GFP was purified as a recombinant protein with a regular E. coli expression and His-tag affinity column purification.
Endpoint fluorescence measurement by a plate reader
For endpoint fluorescence measurement after real-time imaging (Supplementary Fig. 6), the 384-well plate modified as above mentioned was transferred to SpectraMax iD3 (Molecular Devices), and wells were read for each fluorescence with the following settings: excitation at 500 nm, detection at 540 nm, read from the bottom, temperature control OFF (25.5 °C).
Statistics and reproducibility
For the comparison of droplet sizes (Fig. 1d) and fluorescence intensities between IDP and Dex phases (Figs. 2c, e, 3c), the grouped data were visualized using boxenplots generated with the Seaborn library in Python. In a boxenplot, the central line represents the median, while the two innermost boxes extend to the quartiles (25th and 75th percentiles). The next narrower boxes display the 12.5th and 87.5th percentiles (1/8), followed by the 6.25th and 93.75th percentiles (1/16), with additional boxes generated based on sample size. Statistical significance was assessed using two-tailed Welch’s t-tests. All statistical values (sample size, t-statistic, p-value, Cohen’s d, confidence intervals, and degrees of freedom) are documented in Supplementary Data 3. Cohen’s d was calculated by dividing the difference in means by a combined measure of both groups’ variability (pooled variance).
No statistical analysis was carried out. For droplet observation, at least 500 Dex droplets were acquired, which ensures sufficient statistics. No data were excluded from the analyses. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All plotted data generated in this study are provided in the Supplementary Information/Source Data file. The microscopy image data used in this study are available in Figshare (https://doi.org/10.6084/m9.figshare.27917592.v1)41. Source data are provided with this paper.
Code availability
Custom Python codes for detecting droplet-in-droplet structures and setting both outer and inner droplets as ROIs for fluorescence measurement are available at: https://github.com/tomohara97/droplet-in-droplet42.
References
Shimizu, Y. et al. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 19, 751–755 (2001).
Van Nies, P. et al. Self-replication of DNA by its encoded proteins in liposome-based synthetic cells. Nat. Commun. 9, 1–12 (2018).
Sakatani, Y., Yomo, T. & Ichihashi, N. Self-replication of circular DNA by a self-encoded DNA polymerase through rolling-circle replication and recombination. Sci. Rep. 8, 13089 (2018).
Ueno, H. et al. Amplification of over 100 kbp DNA from single template molecules in femtoliter droplets. ACS Synth. Biol. 10, 2179–2186 (2021).
Libicher, K., Hornberger, R., Heymann, M. & Mutschler, H. In vitro self-replication and multicistronic expression of large synthetic genomes. Nat. Commun. 11, 904 (2020).
Schwille, P. Division in synthetic cells. Emerg. Top. Life Sci. 3, 551–558 (2019).
Spoelstra, W. K., Deshpande, S. & Dekker, C. Tailoring the appearance: what will synthetic cells look like? Curr. Opin. Biotechnol. 51, 47–56 (2018).
Hyman, T. & Brangwynne, C. In retrospect: the origin of life. Nature 491, 524–525 (2012).
Li, M., Huang, X., Tang, T.-Y. D. & Mann, S. Synthetic cellularity based on non-lipid micro-compartments and protocell models. Curr. Opin. Chem. Biol. 22, 1–11 (2014).
Martin, N. Dynamic synthetic cells based on liquid-liquid phase separation. Chembiochem 20, 2553–2568 (2019).
Keating, C. D. Aqueous phase separation as a possible route to compartmentalization of biological molecules. Acc. Chem. Res. 45, 2114–2124 (2012).
Crowe, C. D. & Keating, C. D. Liquid-liquid phase separation in artificial cells. Interface Focus 8, 0032 (2018). 2018.
Nakatani, N. et al. Specific spatial localization of actin and DNA in a water/water microdroplet: self-emergence of a cell-like structure. Chembiochem 19, 1370–1374 (2018).
Strulson, C. A., Molden, R. C., Keating, C. D. & Bevilacqua, P. C. RNA catalysis through compartmentalization. Nat. Chem. 4, 941–946 (2012).
Mizuuchi, R. & Ichihashi, N. Translation-coupled RNA replication and parasitic replicators in membrane-free compartments. Chem. Commun. 56, 13453–13456 (2020).
Minagawa, Y., Nakata, S., Date, M., Ii, Y. & Noji, H. On-chip enrichment system for digital bioassay based on aqueous two-phase system. ACS Nano 17, 212–220 (2023).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Simmel, F. C. Synthetic organelles. Emerg. Top. Life Sci. 3, 587–595 (2019).
Allen, M. E. et al. Biomimetic behaviors in hydrogel artificial cells through embedded organelles. Proc. Natl Acad. Sci. USA 120, e2307772120 (2023).
Deng, N.-N. & Huck, W. T. S. Microfluidic formation of monodisperse coacervate organelles in liposomes. Angew. Chem. Int. Ed. Engl. 56, 9736–9740 (2017).
Peters, R. J. R. W. et al. Cascade reactions in multicompartmentalized polymersomes. Angew. Chem. Int. Ed. Engl. 53, 146–150 (2014).
Aufinger, L. & Simmel, F. C. Artificial gel-based organelles for spatial organization of cell-free gene expression reactions. Angew. Chem. Int. Ed. Engl. 57, 17245–17248 (2018).
Niederholtmeyer, H., Chaggan, C. & Devaraj, N. K. Communication and quorum sensing in non-living mimics of eukaryotic cells. Nat. Commun. 9, 5027 (2018).
Deng, N.-N., Yelleswarapu, M., Zheng, L. & Huck, W. T. S. Microfluidic assembly of monodisperse vesosomes as artificial cell models. J. Am. Chem. Soc. 139, 587–590 (2017).
Schuster, B. S. et al. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat. Commun. 9, 2985 (2018).
Elbaum-Garfinkle, S. et al. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl Acad. Sci. USA 112, 7189–7194 (2015).
Binks, B. P. Colloidal particles at a range of fluid-fluid interfaces. Langmuir 33, 6947–6963 (2017).
Balakrishnan, G., Nicolai, T., Benyahia, L. & Durand, D. Particles trapped at the droplet interface in water-in-water emulsions. Langmuir 28, 5921–5926 (2012).
Perro, A. et al. Building micro-capsules using water-in-water emulsion droplets as templates. J. Colloid Interface Sci. 613, 681–696 (2022).
Gonzalez-Jordan, A., Nicolai, T. & Benyahia, L. Influence of the protein particle morphology and partitioning on the behavior of particle-stabilized water-in-water emulsions. Langmuir 32, 7189–7197 (2016).
Dewey, D. C., Strulson, C. A., Cacace, D. N., Bevilacqua, P. C. & Keating, C. D. Bioreactor droplets from liposome-stabilized all-aqueous emulsions. Nat. Commun. 5, 1–9 (2014).
Thompson, K. E., Bashor, C. J., Lim, W. A. & Keating, A. E. SYNZIP protein interaction toolbox: in vitro and in vivo specifications of heterospecific coiled-coil interaction domains. ACS Synth. Biol. 1, 118–129 (2012).
Wei, M.-T. et al. Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat. Chem. 9, 1118–1125 (2017).
Bindels, D. S. et al. mScarlet: a bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 14, 53–56 (2017).
Takakuwa, H. et al. Shell protein composition specified by the lncRNA NEAT1 domains dictates the formation of paraspeckles as distinct membraneless organelles. Nat. Cell Biol. 25, 1664–1675 (2023).
Welles, R. M. et al. Determinants that enable disordered protein assembly into discrete condensed phases. Nat. Chem. 16, 1062–1072 (2024).
Doerr, A. et al. Modelling cell-free RNA and protein synthesis with minimal systems. Phys. Biol. 16, 025001 (2019).
Li, J., Gu, L., Aach, J. & Church, G. M. Improved cell-free RNA and protein synthesis system. PLoS ONE 9, e106232 (2014).
Seo, K. & Ichihashi, N. Investigation of compatibility between DNA replication, transcription, and translation for in vitro central dogma. ACS Synth. Biol. https://doi.org/10.1021/acssynbio.3c00130 (2023).
Tabuchi, T. & Yokobayashi, Y. High-throughput screening of cell-free riboswitches by fluorescence-activated droplet sorting. Nucleic Acids Res. 50, 3535–3550 (2022).
Tomohara, K., Minagawa, Y. & Noji, H. Source data and raw data (Tomohara et al., 2024). figshare https://doi.org/10.6084/M9.FIGSHARE.27917592.V1 (2024).
Tomohara, K., Minagawa, Y. & Noji, H. Custom Python Codes for Analysing Droplet-in-Droplet Structures. Zenodo. https://doi.org/10.5281/zenodo.14243623 (2024).
Acknowledgements
We thank members of the Noji lab for valuable discussions and critical assessment of the work. This work was supported in part by JST GteX (JPMJGX23B1) to H.N., JST CREST (JPMJCR19S4), to H.N., JST ASPIRE (JPMJAP24B5) to H.N., and Grants-in-Aid for Scientific Research (S) (JP19H05624) to H.N. from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
K.T., Y.M., and H.N. designed the research. K.T. performed experiments and analyses. K.T. and H.N. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Job Boekhoven, and the other, anonymous, reviewer (s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tomohara, K., Minagawa, Y. & Noji, H. Artificial cells with all-aqueous droplet-in-droplet structures for spatially separated transcription and translation. Nat Commun 16, 627 (2025). https://doi.org/10.1038/s41467-024-55366-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55366-9