Abstract
Ovarian function declines significantly as females enter middle-age, but the mechanisms underlying this decline remain unclear. Here, we utilize whole-organ imaging to observe a notable decrease in ovarian blood vessel (oBV) density and angiogenesis intensity of middle-aged mice. This leads to a diminished blood supply to the ovaries, resulting in inadequate development and maturation of ovarian follicles. Utilizing genetic-modified mouse models, we demonstrate that granulosa cell secreted VEGFA governs ovarian angiogenesis, but the physiological decline in oBV is not attributed to VEGFA insufficiency. Instead, through single-cell sequencing, we identify the aging of the ovarian vascular endothelium as the primary factor contributing to oBV decline. Consequently, the administration of salidroside, a natural compound that is functional to reverse oBV aging and promote ovarian angiogenesis, significantly enhances ovarian blood supply and improve fertility in older females. Our findings highlight that enhancing oBV function is a promising strategy to boost fertility in females.
Similar content being viewed by others
Introduction
With the development of technology, the average life expectancy of women continues to increase, exceeding 80 years in developed countries1,2. However, women’s reproductive aging occurs at around 35 years of age when their bodies are still robust in terms of other physiological functions apart from reproduction3,4. This not only leads to fertility issues but also triggers various health problems in women. The core reason for female reproductive aging lies in the significant decline in ovarian function during middle age, especially the irreversible decrease in the number of ovarian follicles as age advances5. Interestingly, ovarian follicles, as the basic functional units of female reproduction, still exist in large numbers in the ovaries of middle-aged females, and maintaining normal endocrine and reproductive functions requires only a few to several follicles at antral stage for most species. However, the contradiction between this follicle quantity and reproductive aging remains unexplained.
Folliculogenesis is a complex and orderly process that begins with the activation of primordial follicles6 and culminates in ovulation7, regulated by autocrine, paracrine, and endocrine factors8,9. It is only when follicles reach the gonadotropin-dependent stage that they gain the ability to produce sex hormones and support oocyte maturation10. Therefore, the number and quality of gonadotropin-dependent follicles in the ovaries have a significant impact on female reproductive functions throughout all life stages. It is noteworthy that in clinical observations, the responsiveness of follicles to gonadotropins diminishes significantly with increasing female age11,12. Hence, older females require larger doses of gonadotropins to stimulate the growth and ovulation of antral follicles in IVF treatment11,13. The underlying reason for the declining responsiveness of growth follicles in aging ovaries to hormones and other endocrine signals remains unknown.
During the advanced stages of follicle development, follicles establish a dense vascular network in the theca layer through active angiogenesis14,15,16, enabling them to receive a rich supply of endocrine modulators and vital nutrients for sustained growth. Previous studies have shown that the introduction of VEGFA into the ovary effectively promotes follicle development17,18,19, while angiogenesis inhibitors suppress follicle growth and the formation of antral follicles in vivo20,21. These findings highlight the crucial role of angiogenesis and remodeling of ovarian blood vessels in adulthood in regulating the efficient development of follicles and maintaining female fertility. However, due to technological limitations, it is currently unknown whether there are changes in the vascular angiogenesis and remodeling capacity of the ovaries with aging and the role of vascular changes in ovarian aging has also not been reported.
Salidroside (2-[4-hydroxyphenyl] ethyl β-D-glucopyranoside) is a primary active compound derived from Rhodiola rosea L22. It has been reported functional to overcome ischemia in various organs by stimulating angiogenesis23 and reducing oxidative stress24. In skin repair, Salidroside has been found to boost the expression of VEGFA, promoting blood vessel formation25. Furthermore, a previous study also suggested that Salidroside treatment alleviated endothelial oxidative stress by promoting superoxide dismutase activity and increasing catalase and glutathione peroxidase levels while inhibiting the intracellular generation of reactive oxygen species26. This suggests that salidroside has potential as a therapeutic agent for enhancing endothelial cell function and promoting angiogenesis through alleviating oxidative stress. However, whether salidroside can benefit ovarian angiogenesis and the fertility function of aging ovaries remains unknown.
In this study, we employed endogenous dual-fluorescent vascular reporter mouse models along with a tissue-scale 3D high-resolution imaging system to examine the developmental patterns of ovarian blood vessels in relation to aging. Our results highlighted a reduction in ovarian angiogenesis and remodeling, resulting in insufficient follicle development and ovarian aging. Through the use of genetically modified animal models and single-cell transcriptomic analysis, we established that the aging of ovarian vascular endothelium, rather than a decrease in follicular developmental potential, plays a key role in ovarian aging. Building on these mechanistic finds, we investigated the potential of enhancing ovarian angiogenesis to enhance fertility in aging females. Our in vivo experiments provided evidence that the angiogenesis stimulator Salidroside effectively improved the fertility of aged mice. In conclusion, our research provides a comprehensive understanding of the physiological mechanisms involved in ovarian aging and emphasizes the potent of promoting ovarian angiogenesis as a promising strategy to boost fertility in aging females.
Results
Ovarian specific vascular decline leaded insufficient blood supply in the middle-aged ovaries
In adult life, ovaries continuously undergo angiogenesis to reconstruct the vascular network supporting follicle development. To investigate changes in the ovarian vascular network during age-related reproductive decline, we established a 3D whole-mount vascular imaging system to capture ovarian vascular development at different ages. In short, we conducted whole-mount imaging of transparent ovaries (Figure S1) from the Tek-CreERT2;mTmG mouse model, where all blood endothelium expresses membrane-GFP (Fig. 1a), to reconstruct 3D images of the ovarian blood vessel network with subcellular resolution (Fig. 1b). Analysis of these images allowed us to evaluate the overall profiles of ovarian blood vessels (Figs. 1b, Supplementary movies 1–3) at different ages and simultaneously identify the intensity of angiogenesis by quantifying the number of tip cells (Fig. 1b, arrowheads), which are the marker cells of angiogenesis.
a Schematic representation illustrating the methodology employed for achieving high-resolution 3D imaging of ovarian blood vessels. b Images of 3D ovarian blood vessels in adult Tek-CreERT2;mTmG mice, showing the reconstructed images are flexible to analyze the ovarian blood vessels from whole organ level to the subcellular level. Whole-mount, single-slice (white frame), Follicle (blue frame), Tip cells (yellow frame, arrowheads) (c) High-resolution 3D images of ovarian blood vessels at different ages. Showing a notable decrease of blood vessel density (left) and a reduction in the number of tip cells (right, arrows) with reproductive aging. d Quantifying the number of tip cells showed a notable decline of angiogenesis with reproductive aging (4 M: n = 7 mice, 8 M: n = 6 mice, 12 M: n = 7 mice). e Quantifying the density of blood vessels revealed a substantial decrease with reproductive aging (4 M: n = 3 mice, 8 M: n = 4 mice, 12 M: n = 5 mice). f Detecting the age-associated blood vessel changes in ovary, heart, liver, lung, and kidney, showing vascular density at 4 months, 8 months, and 12 months (green: PODXL). g Quantification of relative vascular density in organs indicating no significant changes in the heart, liver, lung, and kidney, but a marked decline in ovaries since middle age (Ovary: 4 M: n = 3 mice, 8 M: n = 4 mice, 12 M: n = 4 mice, Heart: n = 4 mice, Liver: n = 4 mice, Lung: n = 4 mice, Kidney: n = 4 mice). h Illustration of the methodology employed to assess blood vessel permeability following Evans blue injection (upper) and the distribution of Evans blue (arrowheads) in ears (middle) and ovaries (bottom) subsequent to tail vein injection at 4, 8, and 12 months. i Quantification of Evans blue signal demonstrating a significant reduction in vascular permeability in ovaries with reproductive aging (n = 3 mice). Data are presented as the means ± SD. Data were analyzed by 2-tailed unpaired Student’s t-test without adjustments; *** P < 0.001, ** P < 0.01, * P < 0.05, n.s. (not significant) P ≥ 0.05. Scale bars: 10 μm (c), 50 μm (f), 500 μm (h). Source data are provided as a Source Data file including exact P values.
Using the whole-mount vascular imaging system, we selected 4-month-old, 8-month-old, and 12-month-old females to represent the stages of reproductive activity, reproductive decline, and reproductive aging for ovarian vascular whole-tissue imaging. Overall, we observed a rich vascular network (Fig. 1c, left) in the ovaries at all ages. High-resolution analysis revealed the presence of tip cells (Fig. 1c, right, arrows) in the ovaries at all ages, indicating ongoing angiogenesis in aging ovaries. However, quantitative analysis indicated a significant decrease in the density of tip cells in the stroma region of ovaries with age (Fig. 1d). This decline in tip cell density corresponded to a noticeable reduction in overall ovarian vascular density from 4 months to 12 months (Fig. 1c, left, e). Further analysis showed that the decrease in ovarian vascular density occurred in both the cortical region (Figures S2a, b), where follicles are stored, and the medullary region, where follicle growth takes place. Additionally, when we analyzed the density of blood vessels in other non-reproductive organs including the heart, liver, lung, and kidney (Fig. 1f, g), we found that the decline in blood vessel density in the ovaries was a tissue-specific feature from 4 months to 12 months. This suggests that the age-related decline in ovarian vascular density might be related to reduced fertility in females.
As an endocrine gland, the efficiency of blood supply directly decides the function of ovaries. Due to the significant decrease in ovarian vascular density with age, we further evaluated the blood supply status of various tissues at different ages by performing in vivo injections of Evans Blue (EB) dye. Following the vein injections, the control tissue ear, displayed adequate blood perfusion within 15 min, with no significant statistical variances observed between the 4-month-old, 8-month-old, and 12-month-old tissues (Figs. 1h and S3). However, the efficiency of the blue dye entering into the 8-month-old and 12-month-old ovaries notably decreased compared to that in the 4-month-old ovaries (Fig. 1h, i), showing a substantial reduction in ovarian blood supply associated with aging.
Blood vessel decline leaded an insufficient in vivo follicle development in middle-aged females
In the ovaries, the most active angiogenesis occurs on the ovarian follicles20, which are the functional units to decide fecundity of females. Therefore, we next focused on the changes of angiogenesis and blood supply on ovarian follicles with aging. By analyzing the 3D ovarian vascular imaging, we found that the density of tip cells (Fig. 2a, arrows, Supplementary Movies 4–6) on ovarian follicles dramatically decreased with age from 4 months ((8.47 ± 2.16)×103/mm3) to 12 months ((1.57 ± 0.67) × 103/mm3) (Fig. 2b). High-resolution imaging of tip cell subcellular structures showed a significant reduction in both the length and number of filopodia per tip cell (Figures S4a, b and c), indicating a reduced intensity of angiogenesis on follicles with age. Consistent with the decline in tip cells, live imaging tracing of the growth of follicle vascular endothelium by in vitro culturing Tek-CreERT2;mTmG follicles demonstrated that a significantly retardation of follicle vascular growth in 8 and 12 months ovaries (Fig. 2c, d), but the average length of each endothelial cell on follicles increased with age (Figures S4d, e), suggesting that an insufficient follicle vascular network construction in aged ovaries. Additionally, the follicular vascular became progressively thinner from 4 months to 12 months (Figure S4f). Interestingly, although a clearly retardation of follicle vascular growth occurred in the middle-aged ovaries, the in vitro tracing showed that the developmental progress of follicles from all age groups is similar, with an approximately 40% increase in diameter within 24 h (Fig. 2c, e). Moreover, q-PCR detections demonstrated that the expressions of Fshr, the key receptor to respond gonadotropin for boosting follicle development, dramatically enhanced in the granulosa cells (GCs) of aged ovaries compared to that in young ovaries (Figure S5), confirming follicles in aged ovaries retain the potential for efficient development. This finding suggests that the decline in ovarian blood vessels is one of the primary causes of ovarian follicle developmental retardation and fertility decline with aging.
a Age-related changes in tip cells (arrows) on growing follicles during reproductive aging. b Quantification of the tip cells on growing follicles at different ages, showing a significantly reduced angiogenesis with age in the ovaries (4 M: n = 7 mice, 8 M: n = 10 mice, 12 M: n = 7 mice). c Live imaging tracking the growth of blood vessels on follicles at 4, 8, and 12 months in vitro culture. d Statistical analysis of the relative growth rate of follicle blood vessels over 24 h in vitro culture, showing a significant decrease at 8 and 12 months compared to 4 months (4 M: n = 8 follicles from 3 mice, 8 M: n = 11 follicles from 3mice, 12 M: n = 10 follicles from 3 mice). e Relative growth rate of follicle diameter over 24 h in vitro culture, demonstrating consistent growth ability of follicles across different ages (4 M: n = 8 follicles from 3 mice, 8 M: n = 11 follicles from 3 mice, 12 M: n = 10 follicles from 3 mice). f Evans blue signal (left) and quantification of EB intensity (right) in the follicles at different ages, revealing a significant decrease in dye penetration in aging follicles (4 M: n = 21 follicles from 3 mice, 8 M: n = 9 follicles from 3 mice, 12 M: n = 15 follicles from 3 mice). g BrdU staining detection of proliferative granulosa cells in antral follicles at different ages (red: BrdU, blue: Hoechst). h Quantification of BrdU+ GCs indicating a significant decreased GC proliferation in antral follicles with age (n = 8 follicles from 3 mice). i BrdU staining detection of proliferative GCs after 24 h of PMSG treatment (red: BrdU, blue: Hoechst) at different ages (left). j Quantification of BrdU+ GCs showing significantly decreased responses of PMSG in follicle development at 8 months and 12 months compared to that at 4 months (n = 4 mice). Data are presented as the means ± SD. Data were analyzed by 2-tailed unpaired Student’s t-test without adjustments; *** P < 0.001, ** P < 0.01, n.s. P ≥ 0.05. Scale bars: 20 μm (a), 100 μm (c and f), 50 μm (g and i). Source data are provided as a Source Data file including exact P values.
Owing of the in vivo follicle development is strictly dependent on the blood transported upstream hormones, we next tested whether the insufficient follicle angiogenesis affected the follicle blood supply and development in the middle-aged females. We performed in vivo EB injection experiments and found that a significant decreased dye signal entered into the follicles at 8 months and 12 months ovaries compared to that at 4 months ovaries (Fig. 2f). In consistent to the declined blood supply, we found that the proliferating ratio of GCs in antral follicles, which are strictly dependent on upstream hormone stimulation, was significantly decreased at 8 months and 12 months ovaries (Fig. 2g, h). Moreover, even we supplied extra gonadotropin (PMSG), only small increases of GCs proliferating ratio were observed in the follicles at 8 months (7.8 ± 0.5%) and 12 months (4.6 ± 0.6%), which was in sharp contrast to the increase of GCs proliferation in the follicle at 4 months (20.5 ± 0.9%) ovaries (Fig. 2i, j). These results demonstrated that reduced ovarian angiogenesis and blood supply dramatic decrease the growth efficiency of follicles with age, which might be the reason of fertility decline in the middle-aged females.
Follicle granulosa cell secreted VEGFA governed the adult ovarian angiogenesis
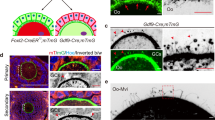
To explore the mechanisms underlying the decline in ovarian angiogenesis associated with aging, we investigated the maintaining signals for active angiogenesis in adult ovaries, focusing on the expression of VEGFA, a crucial factor in supporting angiogenesis27. In-situ hybridization staining revealed that Vegfa was primarily expressed in follicular GCs (Fig. 3a, black arrows) in the ovaries. Subsequently, we crossed GCs-specific Foxl2-Cre mice with Vegfaflox/flox (nocre) mice to create a GCs-Cre;Vegfafl/fl (vko) mouse model (Fig. 3b) that specifically deleted Vegfa in ovarian GCs. RT-PCR analysis confirmed the complete deletion of Vegfa in GCs of vko ovaries (Fig. 3c).
a In situ hybridization demonstrating granulosa cells (arrows) as the primary cells of VEGFA secretion in ovaries. Three experiments were repeated independently with similar results. b Schematic representation of the strategy of Vegfa deletion in GCs. Exon 3-7 deletion via Foxl2-Cre-mediated recombination in granulosa cells within GC;Vegfafl/fl (vko) ovaries. c Validation of Vegfa deletion in GCs of vko females. Three experiments were repeated independently with similar results. d Histological analysis showing a significant suppression of ovarian and follicle development in the vko females compared to nocre ovaries at 23 dpp and 6 months. No ovulated follicles and many empty follicle-like structures (arrows) were observed in vko ovaries at 6 months. e Follicle counting results in nocre ovaries at 23 dpp and 6 months, showing a dramatically decreased number of growing follicles at 23 dpp (left) and a failure of ovulated follicle formation at 6 months (right) in vko ovaries (23dpp: n = 4 mice, 6 M: n = 3 mice). f Immunostaining of endothelium revealing the failure of follicle blood vessel formation (arrows) in vko ovaries (green: PODXL, gray: Hoechst). g Quantification of relative vascular density in vko ovaries at both 23 dpp and 6 months (n = 3 mice). h Histological analysis of ovaries before and after PMSG treatment, showing a failure of PMSG response with no markedly changes of in both ovarian size and follicle developmental profiles in vko ovaries after PMSG treatment. i Maximum follicle diameter in 23 dpp ovaries before and after PMSG treatment, showing a significantly increase of follicle diameter in nocre ovaries, but the size of largest follicles had no changes in vko ovaries (n = 10 follicles from 3 mice). Data are presented as the means ± SD. Data were analyzed by 2-tailed unpaired Student’s t-test without adjustments; *** P < 0.001, ** P < 0.01, n.s. P ≥ 0.05. Scale bars: 200 μm (a), 100 μm (d and f), 500 μm (h). Source data are provided as a Source Data file including exact P values.
Histological analysis demonstrated that the absence of GCs-secreted VEGFA significantly interfered with ovarian development (Fig. 3d). Although a normal ovarian reserve was established at 5 days postpartum (dpp) (Figures S6a, b), the mutant females were completely infertile. Histological analysis showed that a notable suppression of follicle development (Fig. 3d, arrowheads) and a significantly decreased number of growing follicles were observed at 23 dpp (Fig. 3e, left, nocre vs. vko). In the adult vko ovaries, there were no healthy ovulated follicles and many empty follicle-like structures (Fig. 3d, arrows, 3e, right) were observed, leading to infertility in vko females. These findings revealed that GCs-secreted VEGFA is essential for the maintenance of normal follicle development, especially the development of growing follicles.
We then proceeded to examine the formation of the ovarian vascular network in vko females. Through immunostaining of the vascular endothelium in the ovaries, we observed, as follicle development progressed, a significant decrease in vascular density (Fig. 3f, g) was noted in vko ovaries, and only a few blood vessels remained in the vko ovaries at both 23 dpp and 6 months (Fig. 3f, g). This is in sharp contrast to the high density of blood vessels in the ovaries of nocre females at the same ages. Furthermore, we observed a complete failure of follicle vascular construction, as all growing follicles lacked their surrounding blood vessels (Fig. 3f, arrows) in the vko ovaries. Additionally, the lack of a follicular vascular network resulted in a complete failure of follicular response to gonadotropin, preventing the follicles from developing to the antral stage (Fig. 3h, i) even after extensive PMSG treatment. These findings highlight the role of GCs-secreted VEGFA in governing active angiogenesis and blood vessel reconstruction in adult ovaries, and GCs-VEGFA stimulated follicle vascular network construction is essential for the gonadotropin induced follicle maturation.
Aging of endothelium rather than insufficient VEGF secretion caused the ovarian blood vessel decline in the middle-aged females
Due to the crucial role of GCs-secreted VEGFA in maintaining angiogenesis in adult ovaries, we next determined whether the decrease in VEGFA secretion led to the age-related decline in ovarian blood vessels. Unexpectedly, quantitative analysis revealed that the expression of VEGFA was not reduced in GCs with aging. On the contrary, a significant increase in VEGFA at both the mRNA level (Fig. 4a, b) and protein level (Fig. 4c) was observed in the ovaries of 8-month-old and 12-month-old ovaries compared to those at 4 months. This finding demonstrated that GCs secret sufficient VEGFA in middle-aged females, and the decline in ovarian blood vessels is not caused by a lack of stimulators. We therefore changed our research focus to the aging related changes of ovarian vascular endothelial cells (oVEs), which are the contributors of ovarian blood vessels.
a In situ hybridization results showing the expressions of Vegfa in ovaries at 4 months and 12 months, highlighting the granulosa cell (GCs) as the primary cells secreting VEGFA. b Relative Vegfa expression levels in GCs demonstrating increased Vegfa expressions with age (n = 3 mice). c Western blot detections revealing a significant increase in VEGFA expression in ovaries with age from 4 months to 12 months (n = 3 mice). d UMAP plot illustrating that young (2 months, red plots) and middle-aged (10 months, blue plots) ovarian vascular endothelial cells (oVEs) share similar cell populations, including capillary (C0), artery (C1), vein (C2), and Ada+ endothelium(C4), with proliferating endothelium (C3) only present in young oVEs. e UMAP plot (left) displaying the cellular atlas of young (green frame) and middle-aged oVEs (blue frame), emphasizing changes in cell type proportions between the two age groups (right). f Immunostaining results (left) showing the ratio of PODXL+BrdU+ endothelium in follicles (upper, arrows) and livers (bottom, arrowheads) (green: PODXL, red: BrdU). Quantification of the PODXL+BrdU+ endothelium ratio (right) revealing a significant decrease in oVEs with reproductive aging (4 M: n = 5 mice, 8 M: n = 3 mice, 12 M: n = 5 mice). g Relative telomere length detection indicating a significant decrease in oVEs during reproductive aging (n = 3 mice). h Gene Ontology analysis of differential genes in capillaries (upper), arteries (middle) and veins (bottom) of young and middle-aged oVEs. i Violin plot displaying angiogenesis-related genes including End1 and Tspo significantly decreasing and Flt1 significantly increasing in middle-aged oVEs, indicating reduced angiogenesis with reproductive aging (upper); oxidative stress-related genes including Gpx1, Gsr and Prdx5 significantly decreasing in middle-aged oVEs, suggesting increased oxidative stress with reproductive aging (bottom). Data shown as median, interquartile range (IQR), and 1.5x IQR (Young: n = 1992 cells, Middle-age: n = 488 cells). Data are presented as the means ± SD. Data were analyzed by 2-tailed unpaired Student’s t-test without adjustments (b, c, f, g) and Wilcoxon rank sum test with adjustments (i); *** P < 0.001, ** P < 0.01, * P < 0.05. Scale bars: 200 μm (a), 25 μm (f). Source data are provided as a Source Data file including exact P values.
To identify the cell atlas and gene expressing changes in oVEs, we isolated Tek positive cells from young (2 months) and middle-aged (10 months) ovaries (Figure S7) and performed 10× Genomics single cell RNA-seq analysis. A total of 1992 cells in young ovaries and 488 cells in middle-aged ovaries expressing an average of 3838 genes were retained for analysis after stringent quality control. Although the limited sample may have contributed to the potential omission of certain rare cell types, power calculations using SCOPIT confirmed that our dataset has sufficient power to detect significant differences and perform sub-clustering analysis28. Then, the highly variable genes were selected to perform a principal-component analysis (PCA) and KNN clustering, and the Tek positive cells were separated to 5 clusters (C0 to C4) (Fig. 4d) based on their gene expressing characteristics (Figures S8a, b). On the basis of expression levels of feature genes, cluster 0 (52.30% in young ovaries and 44.47% in middle-aged ovaries) was identified to be the capillary (Fig. 4e), which highly expressed Rgcc (Regulator of cell cycle)29, Vwa1 (Von willebrand factor a domain containing protein 1)30 and Emcn (Endomucin)31 related-marker genes (Figures S8a, b). As the functional components in blood vessel network to nutrient tissues, we found that the ratio of ovarian capillary was dramatically decreased in middle-aged ovaries compared to that in young ovaries (Fig. 4e). This result confirmed our finding that a reduced blood supply in the middle-aged ovaries (Fig. 1h, i). Clusters 1 and 2 were termed stable blood vessels including arteries (C1, 11.90% in young ovaries and 14.50% in middle-aged ovaries) and veins (C2, 7.88% in young ovaries and 7.38% in middle-aged ovaries) (Fig. 4e). In these 2 clusters, the high expression of genes including Bmx (BMX non-receptor tyrosine kinase)29,32 and Gja4 (Gap junction alpha-4, Connexin-37)33 in C1 and Selp (Silk-elastinlike protein)34 and Bmp4 (Bone morphogenetic protein 4)35 in C2 (Figures S8a, b) suggested that they constructed the stable blood vessels in ovaries. Cluster 3 (C3, 6.58% in young ovaries) was termed proliferating oVE highly expressing Ccna2 (Cellular communication network factor 2)36, Top2a (topoisomerase (DNA) II alpha)37 and Mki6734 (Figs. 4e, Figure S8a, b). Notably, the middle-aged ovaries lacked C3 showing that the proliferating capability of oVE was dramatically reduced, which is in consistent to our functional observations that insufficient angiogenesis in the middle-aged ovaries (Fig. 4e). Cluster 4 (C4, 21.34% in young ovaries and 33.65% in middle-aged ovaries) consisted of endothelial cells with high expression of genes including Ada (Adenosine deaminase)38 and Itga2 (Integrin subunit alpha 2)39 were significantly increased with aging implying the blood vessel aging (Figs. 4e, Figure S8a, b).
To verify the findings from scRNA-seq, we examined the proliferation of oVEs by conducting KI67 staining on tissues collected from females of different ages. Upon comparing with the control tissues, we noted that while only a few liver vascular endothelial cells (lVEs) were actively proliferating (Fig. 4f), a consistent proliferation rate of lVEs was evident in the liver between 4 months and 12 months (Figure S9a). In contrast, oVEs displayed sustained active proliferation compared to lVEs as the subjects aged, but a noticeable decline in the proliferation rate (Fig. 4f) was observed in the ovaries as aging progressed. Additionally, telomere length detection revealed a significant decrease in telomere length in oVEs but not in lVEs with aging (Fig. 4g, Figure S9b). This data suggested that the continuous angiogenesis might lead to shortened telomeres in oVEs, resulting in a declined proliferating capability of these cells and insufficient ovarian blood network in middle-aged females.
To further explore the gene expression changes associated with aging in oVEs, we compared the transcriptomic profiles of oVEs in young and middle-aged ovaries and conducted Gene Ontology (GO) analysis. Our findings indicated that the differentially expressed genes (DEGs) in the primary components of oVEs (C0 to C2) were significantly enriched in the term “regulation of angiogenesis” (Fig. 4h). Within this category, a set of genes that stimulate angiogenesis and endothelial proliferation, including Edn1 (Endothelin 1)40 and Tspo (translocator protein)41 were notably downregulated, while Flt142, which hinders angiogenesis, was markedly upregulated in the oVEs of middle-aged ovaries (Fig. 4i, upper). Moreover, focusing on VEGFR-related downstream signaling pathways43, we found significantly reduced expression of Rac1, Mapk3, and Akt1 in aged endothelial cells compared to younger cells (Figure S10). Additionally, “cellular response to oxidative stress” and “response to oxidative stress” were also enriched in oVEs, with the mRNA expression levels of oxidative stress-related genes such as Gpx1, Gsr, and Prdx544 significantly decreased in the middle-aged ovaries (Fig. 4i, bottom). To validate this finding, we cultured follicles from 4-month-old Tek-CreERT2;mTmG females and induced the oxidative stress by adding H2O2 to culture (Figure S11a). The results demonstrated that H2O2 treatment markedly inhibited angiogenesis on the follicles, while having a slightly suppressive effect on follicle growth (Figures S11b, c). Collectively, single-cell sequencing analysis and functional assays indicated that oxidative stress-related endothelial aging is a key factor contributing to the diminished angiogenesis and blood supply in middle-aged ovaries.
Salidroside supplementation improved aged female fertility through enhancing ovarian vascular function
Our findings revealed that the aging of oVEs with reduced angiogenesis led to inadequate blood supply in middle-aged ovaries. Therefore, promoting angiogenesis appears to be a promising strategy for enhancing the fertility of aged females. To explore this further, we included three natural components, Salidroside (Sal)26, Notoginsenoside Ft1 (Ft1)45 and Deoxyshikonin (Dosk)46, known for their angiogenesis-promoting properties in in vitro studies, to assess their potential in enhancing ovarian angiogenesis. To evaluate the efficacy of these components in stimulating angiogenesis in aged ovaries, we isolated follicles from Tek-CreERT2;mTmG females at 12 months old and conducted live-cell imaging to monitor the growth of both follicles and the surrounding GFP-positive blood vessels (Fig. 5a). Our results demonstrated that both Sal and Ft1 effectively improved the growth of follicle blood vessels without affecting follicle development (Fig. 5a, b). Furthermore, statistical analysis revealed that Sal was the most effective stimulant for promoting ovarian vascular growth, enhancing the growth of aged follicle blood vessels to a level comparable to that seen in young follicles (Fig. 5b). Mechanistically, we found that Sal supplementary markedly reversed the H2O2 induced suppression of angiogenesis on the follicles (Figures S11a, b and c), suggesting Sal mitigates oxidative stress accumulation in oVEs, thereby promoting angiogenesis.
a Live-cell imaging analysis tracking the growth of blood vessels of Tek-CreERT2;mTmG follicles from 12-month-old females with Sal, Ft1, Dosk or vehicle. b Relative growth rate of vascular area in follicles during 24 h of in vitro culture, revealing Sal as the most significant component for increasing blood vessel growth in cultured follicles (Control: n = 10 follicles from 3 mice, Sal: n = 8 follicles from 3 mice, Ft1: n = 9 follicles from 3 mice, Dosk: n = 8 follicles from 3 mice). c Sal treatment strategy in 12-month-old females. After 7 days of Sal (300 mg/kg BW, once a day) or vehicle treatment, females were evaluated for their reproductive ability or the angiogenesis intensity. d Tip cells on growing follicles after 7 days of Sal or vehicle treatment (left), quantifying the number of tip cells (right) showing a significant increase in tip cells on growing follicles after 7 days of Sal treatment (n = 5 mice). e Whole-mount reconstruction of ovarian blood vessels (left) after 7 days of Sal or vehicle treatment at 12 months and quantification analysis (right) showing a slight elevation in ovarian vessel density after Sal treatment compared to the control (n = 5 mice). f Intensity of Evans blue (arrowheads) in ovaries (left) of 12-month-old females after Sal or vehicle treatment. Quantification of the EB signal (right) indicating Sal treatment significantly increased the blood supply to the ovaries (n = 4 mice). g EB signals in follicles from the females with or without Sal treatment (left), and quantitation of EB abundance in follicles showing Sal treatment significantly enhanced the blood supply to the follicles (right, Control: n = 8 follicles from 3 mice, Sal: n = 7 follicles from 3 mice). Data are presented as the means ± SD. Data were analyzed by 2-tailed unpaired Student’s t-test without adjustments; *** P < 0.001, ** P < 0.01, * P < 0.05. Scale bars: 100 μm (a and g), 10 μm (d), 500 μm (f). Source data are provided as a Source Data file including exact P values.
Subsequently, we assessed the in vivo effectiveness of Sal in enhancing ovarian vascular functions by administering Sal (intraperitoneal injection, 300 mg/kg body weight, once daily) to 12-month-old Tek-CreERT2;mTmG female mice for 7 consecutive days (Fig. 5c). Following the treatment, we harvested the ovaries to conduct whole-mount imaging and observed a marked increase in tip cell density ((5.71 ± 0.49) × 103/mm3) in the oVEs within the follicles post-Sal treatment, in comparison to the control ovaries ((1.37 ± 0.85) × 103/mm3) (Fig. 5d). Meanwhile, a slight elevation in ovarian blood vessel density was observed in the Sal-treated ovaries (Fig. 5e). Furthermore, upon EB injection, a notable increase in EB signal was detected in both ovaries and follicles of the Sal-treated females compared to the control group (Fig. 5f, g), suggesting that the administration of Sal enhanced the ovarian vascular function and blood supply to aged ovaries.
Aligned with the improved blood supply to the ovaries, we observed a higher number of growing follicles, particularly secondary and preantral follicles (Figure S12), in the Sal-treated ovaries (166.0 ± 32.66) compared to those in the control ovaries (50.8 ± 15.45) at 12 months of age (Fig. 6a). The superovulation experiment revealed that the growing follicles in Sal-treated females displayed increased sensitivity to PMSG stimulation, leading to a significantly higher proliferation ratio of follicle granulosa cells (Fig. 6b) and an increased average diameter of ovulated follicles compared to controls (Figure S13a, b). This resulted in a marked increase in the number of ovulated oocytes (5.90 ± 3.54) compared to the control group (1.70 ± 2.26) (Fig. 6c). Additionally, assessments of chromosome aneuploidy and spindle morphology indicated that Sal treatment improved the quality of ovulated oocytes (Figure S14a-d). In our in vitro fertilization studies, we noted an increase in both the ratio of 2-cell embryos and blastocysts in the ovulated oocytes from the Sal group compared to those in the control ovaries (Fig. 6d, e), indicating that Sal treatment not only enhances the quantity but also the quality of oocytes. Finally, we evaluated the impact of Sal on the natural pregnancy and birth rates in females, and a significant increase in both pregnancy rate (Sal vs. Control: 53.33% vs. 13.33%, n = 15), and litter size (Sal vs. Control: 2.13 ± 0.31 vs. 0.71 ± 0.62, n = 3 groups, five females per group) were observed in females after treated with 7 days of Sal compared to those in control (Fig. 6f). Furthermore, safety evaluations indicated that Sal treatment did not affect the vascular network in other major organs (Figure S15a, b), and long-term monitoring of treated females showed no increased risk of tumor development in aged mice (Figure S15c). These findings demonstrated that Sal is a safe agent for reversing ovarian aging and enhancing fertility in aged females.
a Ovarian morphology with or without Sal treatment at 12 months, exhibiting an increase in the number of growing follicles (arrows) in the Sal group compared to the control (n = 5 mice). b Immunostaining detection of proliferating granulosa cells (red: BrdU, blue: Hoechst) in Sal and vehicle-treated ovaries at 12 months post-PMSG treatment. Statistical analysis revealing a significantly increase in GC proliferating ratio after Sal treatment compared to the control (n = 5 mice). c A significantly higher number of ovulated oocytes was retrieved from Sal-treated ovaries compared to controls after superovulation (n = 8 mice). d Representative images of 2-cells (arrowheads) and blastocysts (arrows) derived from in vitro fertilized oocytes from Sal-treated and control mice. e The ratio of 2-cell embryos (left) and blastocysts (right) significantly increased in Sal-treated mice compared to controls (Control: n = 4 mice, Sal: n = 8 mice). f Fertility test demonstrating a significant increase in litter size (left) after Sal treatment (n = 3 groups, 5 females per group) and general appearance of Sal-treated and control females at 12 months, displaying increased number of healthy pups with Sal-treated mothers (right). g The graphic model illustrates how the decline of ovarian angiogenesis contributes to ovarian aging. In young ovaries, active ovarian angiogenesis results in the development of high-density blood vessels that provide ample nutrition and hormones for follicle growth, thereby ensuring a high fertility efficiency (left). Conversely, reduced angiogenesis in middle-aged ovaries leads to insufficient nutrition and hormone supply, resulting in decreased fertility (middle). Efficiently supplying Sal reverses these age-related negative effects on fertility (right). Data are presented as the means ± SD. Data were analyzed by 2-tailed unpaired Student’s t-test without adjustments; *** P < 0.001, ** P < 0.01, * P < 0.05. Scale bars: 200 μm (a), 100 μm (b, c and d). Source data are provided as a Source Data file including exact P values.
In summary, our study highlights a notable decrease in angiogenesis and vascular aging in aging ovaries, resulting in reduced ovarian blood supply and insufficient follicle development, leading to ovarian aging and decreased reproductive capacity in middle-aged females (Fig. 6g, left and middle). The efficacy of Sal supplementation in reversing the aging of ovarian blood vessels and enhancing ovarian angiogenesis proves effective in improving the fertility and reproductive quality of aging females (Fig. 6g, right). This underscores its potential as a promising new strategy for addressing reproductive aging in females.
Discussion
Women’s ovaries are the first organs to undergo aging47,48, resulting in a significant decline in fertility during early middle age and impacting various aspects of health. Early fertility decline poses challenges for modern women in balancing the ideal childbearing age with career advancement, leading to increased life stresses. Middle-aged women also face health issues related to ovarian aging, such as bone loss, heart problems, and hormonal imbalances, affecting their overall well-being49. While ovarian aging presents numerous challenges, the understanding of its underlying physiological mechanisms is limited, hampering the development of effective interventions in clinical settings. Traditionally, the reduction in the number of follicles, basic functional units for female reproduction, is believed to be the primary cause of ovarian aging in women5,50. However, it is known that a substantial number of follicles persist in the ovaries of women before menopause. Research indicates that at 35 years old, when fertility decline begins, ovaries contain over 25,000 follicles, and nearly lost fertility by age 45, thousands of follicles remain51,52. Despite the ample follicle reserves exceeding fertility requirements, the reason why a significant number of follicles fail to sustain fertility remains unclear to date.
Our research indicates that the aging of oVEs plays a crucial role in triggering the decline of ovarian function in middle-aged females. As an endocrine organ, the ovary relies on a supply of hormones and nutrients through the circulation to maintain its functions. A distinguishing developmental feature of ovaries, setting them apart from other organs, is the ongoing active angiogenesis that occurs in adulthood, continually reshaping their internal vasculature to meet the demands of follicle development. Our previous study demonstrated that the newly formed oVEs are transient structures that emerge during follicle growth and are eliminated when follicle function ceases20. Consequently, with the continuous recruitment of follicles over the years, the oVEs undergo vigorous and persistent proliferation, remodeling, and elimination, potentially leading to premature aging of oVEs in midlife. This hypothesis is supported by the significant telomere shortening and a notable increase in oxidative stress levels observed in the oVEs of middle-aged females, indicating that oVEs aging contributes to the decline in ovarian function during middle age. Thus, our finding indicates that the aging of oVEs, occurring earlier than in the vascular endothelium of non-reproductive organs, contributes to the premature physiological aging of the ovaries in middle age.
In the clinical practice of assisted reproduction, it has been widely documented that with advancing patient age, there is a noticeable increase in the required dosage of follicle-stimulating medications to achieve optimal follicular growth outcomes11. Simultaneously, there is a continuous elevation in circulating gonadotropin levels beyond middle age under normal physiological condition53, suggesting a potential decline in the developmental capacity of granulosa cells within the follicles. However, our studies in mice did not show significant variations in the developmental potential of follicles cultured in vitro at different ages. This implies that follicles in aged ovaries still possess robust developmental potential for contributing fertility. Noteworthy are our findings indicating increased expressions of Vegfa and Fshr in granulosa cells of aged ovaries, implying that follicles in middle-aged ovaries may be under a condition of insufficient gonadotropin and oxygen supply. This observation confirmed the notion that insufficient blood supply is a primary factor leading to decreased follicular developmental capabilities post-middle age. Indeed, we found that supplementing with Sal to enhance angiogenesis and blood supply in aged ovaries significantly improved follicular response to gonadotropin stimulation. Moreover, both oocyte quality and fertility were enhanced with the promotion of ovarian vascular development in aged females. Therefore, our results strongly suggest that the aging of the ovarian vascular endothelium is a crucial, potentially primary, factor in the substantial decline of ovarian function during middle age. Furthermore, enhancing ovarian angiogenesis through Sal or other stimulants appears to be an effective approach to boost fertility in aging females.
In the past decade, numerous potential drugs have been developed to combat ovarian aging, focusing on enhancing the quality of oocytes in aged ovaries. By reducing reactive oxygen species in aged oocytes, several drugs like NMN54,55, spermidine56 and metformin57,58 have been found to efficiently improve oocyte quality in aged ovaries. Furthermore, through transcriptomic analysis, Liu et al., discovered a correlation between the mevalonate (MVA) pathway in GCs and decreased oocyte quality in aged females. Supplementing with the MVA isoprenoid geranylgeraniol has also shown efficiency in reducing meiotic defects and aneuploidy in aged mice59. These drugs have demonstrated a reduction in oxidative stress levels in oocytes, leading to a decrease in chromosome abnormalities during oocyte maturation, as well as an enhancement in oocyte developmental capacity, ultimately improving the fertility of aging animals. Importantly, these agents targeting ovarian aging reach the ovarian follicles through oVEs. Our current study shows that enhancing ovarian angiogenesis and vascular remodeling through the supply of Salidroside significantly improves the stimulatory effectiveness of gonadotropins on follicular development. Therefore, our proposed approach of enhancing oVEs functions in aged ovaries should be beneficial for all other anti-ovarian aging medications, and further research is required to figure out the optimal combinations in clinical practice.
Overall, our research demonstrates that vascular aging in the ovaries is a significant factor leading to ovarian aging and decreased fertility in females in middle age. These results offer valuable perspectives for a deeper and more thorough comprehension of ovarian development and the preservation of female fertility. Additionally, our research introduces a hopeful approach to enhance fertility in older women in clinical settings by promoting the regeneration of ovarian blood vessels to enhance the quality of follicle development and ultimately restore fertility. The discovery of Sal also provides a promising avenue for combating female reproductive aging in the future.
Methods
Ethics statement
All animal experiments were conducted in accordance with the guidelines and regulations set forth by the Institutional Animal Care and Use Committee (IACUC) of China Agricultural University (approval number: AW13012202-3-1). The study adhered to the ARRIVE guidelines for the ethical reporting of animal research.
Animals
The CD1 mice were purchased from the Laboratory Animal Center of the Institute of Genetics (Beijing, China). The Tek-CreERT2 mice were purchased from National Applied Research Laboratories (Taiwan, China). The mTmG (007576, Jackson Laboratory) mice were generated as previously reported. The Tek-CreERT2;mTmG mice were generated by Tek-CreERT2 mice crossing with mTmG mice60. Tamoxifen (75648, Sigma-Aldrich) was resuspended in 95% (v/v) ethanol (100 mg/mL) and then diluted in corn oil (C8267, Sigma-Aldrich) to a final concentration of 20 mg/mL. To label the vascular endothelial cells in the ovaries of Tek-CreERT2;mTmG females, Tamoxifen, at a dosage of 40 mg/kg.BW, was intraperitoneally injected for one time in 4, 8 and 12 month-females 7 days before sacrificed.
The Vegfafl/fl mice were purchased from Gempharmatech Co., Ltd. The Foxl2-Cre knock-in mice61 were obtained from Dr. Fei Gao, the Institute of Zoology, Chinese Academy of Sciences. The Foxl2-Cre mice were crossed with Vegfafl/fl mice to generate Foxl2-Cre; Vegfafl/fl mice.
All mice were housed in animal facilities under 16/8-hour light/dark cycles at 26 °C and humidity of 40–70% with access to chow and water at libitum.
High-resolution 3D tissue imaging
The tissue clearing method was conducted following the previously described protocol62 with modifications. Generally, a stock N-methylacetamide (M26305, Sigma-Aldrich) was prepared by diluting melted N-methylacetamide to 40% (v/v) concentration in phosphate-buffered saline (PBS). This stock solution was then utilized to dissolve Histodenz (D2158, Sigma-Aldrich) to 86% (w/v) concentration. Subsequently, Triton X-100 (at 0.1% v/v) and 1-thioglycerol (M1753, Sigma-Aldrich) (at 0.5% v/v) were incorporated into the Histodenz solution to form the final clearing solution.
To image the ovarian vascular network, the ovaries at various ages were harvested and rinsed with PBS containing 0.2% Triton X-100 and 1-thioglycerol (0.5%) in darkness for 24 h at room temperature to eliminate blood cells. The tissues were then placed in the clearing solution (1:50 v/v) and left to incubate in darkness at room temperature on a rotor for 72 h.
Subsequently, the cleared ovaries were embedded in a 35-mm dish with 14-mm glass bottom (D35-14-1-N, Cellvis) filled with fresh clearing solution. Confocal imaging was conducted using an inverted Leica (DMi8) and Andor Dragonfly spinning-disc confocal microscope, along with a scientific complementary metal-oxide semi-conductor (sCMOS) camera (Andor Zyla 4.2). Imaging was performed with either a 20 × 0.8 numerical aperture (NA) 650 μm working distance or a 40 × 1.3 NA 250 μm working distance objective. A pixel density of 2048 × 2048 was used, and Z-step set at 3 μm for 650 μm (20× objective) or 0.8 μm for 250 μm (40× objective). The 488-nm (mG) and 568-nm mTomato (mT) lines of the Andor Integrated Laser Engine (ILE) system with a spinning-disc confocal scan head (Andor Dragonfly 500) were employed for imaged. Image acquisition was performed using Fusion 2.1 software (available at https://andor.oxinst.com/products/dragonfly#fusion). Post-acquisition, images were processed using ImageJ (http://rsbweb.nih.gov/ij/) for the projection of all z stacks and merging of color channels. To enhance the visibility of filopodia structures of the tip cells, the mG (488 nm) channel was inverted to black and white by ImageJ software.
Quantification of the number of tip cells and the vascular density
To conduct quantitative analysis of tip cells and vascular density in the reconstructed 3D ovarian imaging, the images were scanned using the 3D View and Slice modes in Imaris software (https://imaris.oxinst.com/). Specifically, the whole-mount imaging was segmented to optical stacks (50 μm) in the z-axis. Filopodia identification involved manually scanning the follicles or randomly selected optical stacks focusing on GFP-labeled blood vessels using the filament-tracing function in Imaris. For tip cell quantification in the ovarian stromal region, the number of tip cells was counted in randomly chosen regions of ovaries devoid of follicles (volume: 500 μm × 500 μm × 50 μm, with 5 to 10 points per ovary). The average number of tip cells was calculated by analyzing the ovaries form at least three animals. Tip cell density in the tissues was computed by dividing the number of tip cell by the tissue volume. Ovarian vascular volume and total ovarian volume were calculated using the surface function in Imaris software to derive the ovarian vascular density.
Histological analysis and immunofluorescence staining
The ovaries, hearts, livers, lungs and kidneys were fixed in 4% (w/v) paraformaldehyde (SC-281692, Santa Cruz) for 24 h and subsequently embedded in paraffin (39601095, Leica) following dehydration. Subsequently, all tissues were serially sectioned into 8 μm slices using a microtome (RM2245, Leica).
To count the follicle number, tissue sections were stained with hematoxylin (SC-24973A, Santa Cruz). Primordial follicles were counted in every fifth section and then multiplied by five to determine the total number of primordial follicles in each ovary. To quantify the number of growing follicles, all sections from one ovary were meticulously examined under the microscope. Follicles were counted only when oocytes with clear nuclei were identified, and the developing stage of the follicles was determined based on their diameter and the number of granulosa cell layers. The maximum diameters of the follicles were measured using the analyze-measure function in ImageJ.
Immunofluorescence was performed following previously established protocols63. Briefly, after deparaffinization and gradual hydration, the sections were put into 0.01% sodium citrate buffer (pH 6.0) and subjected to microwave antigen retrieval for 16 mins. Subsequently, the sections were blocked with 10% donkey serum (Jackson ImmunoResearch) at room temperature for 1 hour, followed by overnight incubation with primary antibodies at 4 °C. The sections were then thoroughly washed in PBS, incubated with the secondary antibody at room temperature for 1 hour, and counterstained with Hoechst33342 (B2261, Sigma-Aldrich) for 40 sec.
The primary antibodies used in immunofluorescence were as follows: PODXL antibody (goat, 1:200 dilution; AF1556, R&D Systems), BrdU antibody (sheep, 1:200 dilution; Ab1893, Abcam) and KI67 antibody (rabbit, 1:200 dilution; Ab15580, Abcam). The second antibodies were as follows: donkey anti-goat Alexa Fluor 488 (1:100 dilution; A11055, Invitrogen) and donkey anti-rabbit Alexa Fluor 555 (1:100 dilution; A31572, Invitrogen). Images were captured using a Nikon Eclipse Ti digital fluorescence microscope. Image data analysis was performed using the software ImageJ, and image merging was carried out using the color-merge channels function in ImageJ.
Follicle isolation and culture
Ovaries were harvested from Tek-CreERT2;mTmG mice aged 4, 8 and 12 months. Secondary follicles (average diameter: 314.7 ± 34.4 μm) were isolated by tearing ovaries with syringe needle under a stereomicroscope (Stemi 305, Zeiss) with sterile conditions in pre-cooled PBS (10 mM, pH 7.4). To evaluate the follicle angiogenesis and follicle growth rates, the follicles were cultured on a Millicell culture insert (Millipore, Merck) in Minimum Eagle Medium alpha (MEMα) (12000-014, Invitrogen) supplemented with 1% ITS (insulin-transferrin-sodium selenite medium) (I3146, Invitrogen), NaHCO3 (2.1 mg/mL; Sigma-Aldrich), 5% fetal bovine serum (GIBCO, Life Technologies), FSH (1:4000), and penicillin-streptomycin (100 IU/mL; 15140122, Invitrogen)64. The cultured follicles were imaged at 37 °C, 5% CO2 using a living cell workstation (Okolab). Specifically, images were captured with an Andor Dragonfly 502 spinning-disc confocal microscope equipped with a 10× 0.32 NA, a sCMOS camera (Andor Zyla 4.2), and 488-nm (mG) and 568-nm (mT) lines of the Andor ILE system with a spinning-disc confocal scan head (Andor Dragonfly 500). Blood vessels were acquired using Z-step mode with the following settings: laser 488-nm around 10 to 20%, laser 568-nm around 15 to 25%, exposure time 100 to 200 ms, and Z-step of 5 μm using Fusion 2.1 software (https://andor.oxinst.com/products/dragonfly#fusion). The time-lapse imaging was conducted every hour for 24 h continuously to monitor changes of follicle blood vessels.
To evaluate the effect of natural components on angiogenesis in 12-month follicles, Sal (50 μM, HY-N0109, MedChemExpress) was dissolved in PBS, Ft1 (50 μM, HY-N0910, MedChemExpress) and Dosk (50 μM, HY-N2187, MedChemExpress) was dissolved in DMSO. These compounds were then added to the follicle culture medium. To evaluate the effect of H2O2 and Sal on angiogenesis in follicles, H2O2(0.8 mM), and Sal (50 μM, HY-N0109, MedChemExpress) were added to the follicle culture medium. Blood vessel areas were quantified using the threshold function in ImageJ, while follicle diameters were measured using analyze-measure function in ImageJ.
Evans blue injection
Evans blue was dissolved in PBS and administered via intravenous injection to females at a dosage of 0.05 mg/kg.BW. Ovaries, ears, and follicles were harvested 15 mins post-injection. Images of ovaries and ears were captured by fluorescence stereo microscope, Leica (MZ10 F, DFC7000T), while images of follicles were obtained using a Nikon Eclipse Ti digital fluorescence microscope. The images were analyzed and quantified by ImageJ software.
Granulosa cells proliferating rate analysis
To assess the proliferative rate of granulosa cells (GCs), female subjects were exposed to either PMSG (500 IU/kg body weight, intraperitoneal injection) for 12 h or left untreated. Following this, all subjects received an injection of 50 mg/kg body weight BrdU. Ovaries were collected 3 h after the BrdU injection for proliferative staining and evaluation. Using ImageJ software, the number of BrdU+ GCs and total GCs in the largest cross-section of antral follicles were quantified to calculate the ratio of BrdU+ GCs.
In situ hybridization
In situ hybridization was performed as previously described protocols65. Gene-specific primers were designed using Beacon Designer 8 software with the following criteria: optimal fragment length of 200-300 bp, best annealing temperature of 60 °C, and a GC content of 55%. T7 RNA polymerase promoter sequence (5′-AATTAACCCTCACTAAAGGG-3′) was added to the sense primer (5′-ACAGAACAGTCCTTAATCCA-3′), and T3 RNA polymerase promoter sequence (5′-TAATACGACTCACTATAGGG-3′) was added to the antisense primer (5′-CCAGTGAAGACACCAATAAC-3′). Oligos were then ordered. Ovary cDNA samples were used for standard PCR with the primers to obtain more than 400 ng of the product. The PCR reaction was run on a 1% agarose gel, and the band corresponding to the expected size amplicon was excised. DNA was purified from the gel using a gel extraction kit (DP219-02, TIANGEN), and the DNA concentration was quantified using a nanodrop. The DNA concentration needed to be higher than 20 ng/mL for the next step. A mouse-specific Vegfa cRNA probe was labeled with digoxigenin using the DIG Northern Starter Kit (12039672910, Roche, Switzerland). T7 RNA polymerase was used to generate the sense probe of Vegfa, and T3 RNA polymerase was used to generate the antisense probe of Vegfa. Ovarian cryosections of 10 μm were hybridized with the Vegfa antisense probe to detect Vegfa mRNA localization, with the sense probe serving as a negative control. After hybridization with cRNA probes for 24 h at 65 °C, anti-digoxigenin-AP (75 mU/mL) was used to bind the DIG-label at 4 °C overnight. The slices were then washed with MABT (maleic acid with 10%v/v Tween-20) solution for 1 hour before incubating with NBT/BCIP solution to react with the AP for color development. Positive signals appeared as purple in the cytoplasm of the cells. After the color development, the slices were washed with PBS and stained with Nuclear Fast Red solution for 10 mins to visualize the cell nuclei. The images were analyzed under a microscope (DM500, Leica).
Gene expression analysis
To detect the expressions of Vegfa and Fshr, granulosa cells from females aged 4, 8 and 12 months were isolated. To validate the deletion efficiency of Vegfa, granulosa cells from vko and nocre females at 3 months were isolated. Total RNA was isolated using the RNeasy Micro Kit (74004, Qiagen). cDNA samples were generated using the PrimeScript™RT reagent Kit with gDNA Eraser (RR047Q, Takara). The relative mRNA levels were quantified and normalized to the Gapdh levels in the same samples. Applied LightCycler® 96 Real-Time PCR System (05815916001, Roche) was used to analyze the expression levels. The following primers were used for gene detections: Vegfa: (forward: 5′-ACAGAACAGTCCTTAATCCA-3′, reverse: 5′-CCAGTGAAGACACCAATAAC-3′), Fshr: (forward: 5′- AGGTACAGCTCTGCCATGCT-3′, reverse: 5′- GTACGAGGAGGGCCATAACA-3′), Gapdh: (forward: 5′-AGGTCGGTGTGAACGGATTTG-3′, reverse:5′- TGTAGACCATGTAGTTGAGGTCA-3′). Deletion efficiency of Vegfa: (forward: 5′-GGCTGCTGTAACGATGAA-3′, reverse: 5′-ATCTGCTGTGCTGTAGGA-3′).
Western blot
The ovaries in different groups were collected and then lysed in WIP lysis solution (Cell signaling technology, United States). Separated the prepared protein samples by electrophoresis with 10% SDS-PAGE and transferred the protein to polyvinylidene fluoride (PVDF) membranes (IPVH00010, Millipore, United States). Then, blocked the membranes by using 5% nonfat-dry milk for 1 hour at 37 °C and incubated the membranes at 4 °C overnight with the primary antibodies to be tested. The used primary antibody in the western blot was VEGFA (Ab214424, rabbit, 1:1000, Abcam). After overnight incubating, the membrane was washed with tris-buffered saline with 0.05% tween (TBST) for 30 mins and then processed to incubate with the secondary antibody at room temperature for 1 h (1:5000, Beyotime). The level of TUBULIN β (AP0064, rabbit, 1:1000, Bioworld) was used as an internal control. The membrane was washed with TBST for 30 mins. The protein on membrane was captured by the Super Signal detection system (Prod 34080, ThermoFisher Scientific, United States). The images were then quantified by ImageJ software.
Single-cell dissociation and fluorescence-activated cell sorting
The method for single-cell dissociation was adapted from a previously published method66. Ovaries or livers from Tek-CreERT2;mTmG female aged 2 or 10 months were collected and cut into small pieces. The tissue fragments were then treated with 1 mg/mL Collagenase Type IV (V900893, Sigma- Aldrich) and 5 IU/mL RNase-free DNase I (EN0523, Thermo Fisher Scientific) at 37 °C for a total of 60 mins (20 mins×3), followed by neutralization in DMEM-F12 medium (11320-033, Gibco) with 10% (vol/vol) FBS (Gibco). After digestion, the mixture was passed through a 40 μm cell strainer (352340, Corning) to eliminate any cell aggregations, and the cells were centrifuged at 300 g for 5 mins at 4°C. The supernatant was removed, and cell pellet was resuspended in 1% BSA (V900933, Sigma- Aldrich). The single cell suspension was stained with 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific) to remove dead cells. Flow sorting was carried out using a FACS Aria II (BD Biosciences). Viable Tek+ oVEs were sorted into collecting medium (0.05% BSA). Data were analyzed using FlowJo software (https://www.bdbiosciences.com/zh-cn/products/software/flowjo-v10-software#).
RNA-sequencing and primary sequencing analysis
For Single-Cell RNA-sequencing, FACS sorted Tek+ oVEs were detected by LUNA (LUNA-FL TM, Logos Biosystems, Republic of Korea) to measure cell viability and cell conn-centration after AO/PI Cell Viability Kit staining (Logos Biosystems). Subsequently, the cells were processed following 10x Genomics protocol67. Briefly, the cellular suspensions were loaded onto a Chromium Controller instrument (10x Genomics, USA) to create single-cell gel bead-in-emulsions (GEMs). GEM-reverse transcriptions (GEM-RTs) were carried out in a Veriti 96-well thermal cycler (Thermo Fisher Scientific). Following reverse transcription, the GEMs were harvested and the cDNAs were amplified and purified using the SPRI select Reagent Kit (Beckman Colter, USA). Libraries were prepared using the Chromium Single-Cell 3′ Library Kit version 3.1 (10x Genomics, Dual Index) for processes such as enzymatic fragmentation, end-repair, A-tailing, adapter ligation, ligation cleanup, sample index PCR, and PCR cleanup. Sequencing libraries were generated using NEB-Next® UltraTM RNA Library Prep Kit for Illumina® (NEB, USA), and the library preparations were sequenced on an Illumina Novaseq platform by the ANOROAD (Beijing, China).
Single-cell RNA-seq data processing
Barcode processing and gene counting were performed using Cell Ranger (v6.0.0) count pipeline with default parameters. Briefly, reads after trimming were mapped to the mm10 mouse genome (https://10xgenomics.com/) and the genome annotation (gencode vM23) with STAR packaged in the Cell Ranger, and then aligned reads were filtered for identifying cell barcodes and unique molecular identifiers (UMIs). As a result, we sequenced 12,471 barcodes, on average, a median of 4714 UMIs, a sequencing depth of 42,587 reads, median genes 1969 per cell and 8038 barcodes, on average, median of 4604 UMIs, a sequencing depth of 42,498 reads, median genes 1592 per cell were got for young and middle-aged mouse ovaries, respectively. The valid cell barcodes and UMIs were used for identifying cell clusters.
Cluster and DEG analysis in single cell sequencing analysis
The R package Seurat (v4.3.0)68 was used to identify cell clusters and markers. Firstly, cells were filtered by three criteria: the number of detected genes was greater than 200 and less than 7000, the number of Count was less than 40,000 and the percentage of mitochondrial genes was greater than 0% and less than 6%. After the filter, 2480 (1992 from the young sample and 488 from the middle-aged sample) cells were retained. Then, data normalization and scale were performed in order using functions NormalizeData and ScaleData. Briefly, for each cell, the UMI counts of each gene were divided by the total UMI counts for that cell, multiplied by 10,000, and after natural-log transformed, the normalized data were scaled and centered. Then scaled data were performed dimensionality reduction using function RunPCA based on PCA. Using a permutation test method based on null distribution (function Jackstraw), we selected the top 20 PCA dims to cluster cells using the function FindNeighbors and FindClusters based on the shared nearest neighbor (SNN) algorithm. To visualization the cell clusters, function RunUMAP was performed. Then, for each cluster, genes with an average log2-transformed difference greater than 0.25, a P value less than 0.01, and expressed in at least 25% cells were identified as cluster markers using function FindAllMarkers based on the Wilcoxon rank sum test. These cluster markers (Figure S8 and Supplementary Data 1 in Supporting Information) were compared with widely accepted markers to help to determine the cell identities of each cluster.
GO enrichment analysis
The differentially expressed genes (DEGs) between young and middle-aged cell clusters, used for GO analysis, were provided in Supplementary Data 2. The R package clusterProfiler (v4.6.2) was used to perform GO enrichment analysis. Multiple hypothesis test correction method was set to “BH”, the P value and q value cutoff were set to 0.05. The results of GO enrichment analysis were attached in Supplementary Data 3 and representative GO terms were show in corresponding figures.
Telomere measurement by quantitative real-time PCR
Ovarian and liver endothelial cells were obtained from Tek-CreERT2;mTmG mice as mentioned above. DNA was extracted from oVEs and lVEs using TIANamp Micro DNA kit (DP316, TIANGEN) and quantified. Average telomere length was measured from total genomic DNA using a real-time PCR assay as previously described69. PCR reactions were performed on LightCycler® 96 Real-Time PCR System (05815916001, Roche), using telomeric primers, single copy reference primers for the control gene in the relative mouse telomere length quantification qPCR Assay Kit (M8908, ScienCell). The telomere signal was normalized to the signal from the single copy gene to generate a T/S ratio indicative of relative telomere length.
Salidroside treatment
Salidroside (HY-N0109, MedChemExpress) was dissolved in PBS and was intraperitoneally injected to the 12-month females with a dosage of 300 mg/kg.BW every day for 7 days. The control mice were injected with the same volume of PBS in the same way.
For the experiment of angiogenesis detection, Sal was intraperitoneally into 12-month-old Tek-CreERT2;mTmG females for 7 days and then the ovaries were applied in high-resolution 3D analysis to calculate the tip cell number and vascular density. For the experiment of blood supply function and fertility detection, Sal was intraperitoneally into 12-month-old CD1 females for 7 days and then the treated females were injected with Evans blue to evaluate the blood supply in ovaries and follicles, sacrificed for histological analysis and IVF or mated with adult fertile males to monitor fertility.
Oocyte collection
After 7 days of Salidroside treatment, females were intraperitoneally injected PMSG (500 IU/kg) for superovulation. After 46-48 h, human chorionic gonadotrophin (500 IU/kg) was intraperitoneally injected. The cumulus oocyte complexes (COCs) were collected from oviducts at 16 h after injection. To obtain oocytes, the cumulus cells were separated by pipetting in EmbryoMax M2 medium with hyaluronidase (MR-051-F, Millipore, USA). The number of ovulated oocytes was counted.
Chromosome spreading and kinetochore staining
After ovulation, the cumulus cells were removed by treating the oocytes with hyaluronidase. The zona pellucida (ZP) was subsequently removed by exposure to Tyrode’s buffer (EMD Millipore Corp, MR-004-D). ZP-free oocytes were fixed on glass slides in a solution containing 1% paraformaldehyde and 0.15% Triton X-100, air dried, and incubated overnight at 4°C with CREST antibody (1:50, antibodiesinc, ABI-15-234). This was followed by 1-hour incubation with Alexa Fluor 647-conjugated secondary antibody. Chromosomes were counterstained with Hoechst 33342 (1:100, Beyotime, C1022), and images were captured using a Nikon Eclipse Ti digital fluorescence microscope.
Spindle staining
Oocytes were fixed in a 4% paraformaldehyde solution for 15 min, followed by treatment with 0.1% Triton X-100 for 15 min. Subsequently, the oocytes were transferred to 3%BSA for 20 min, then incubated overnight at 4°C with α-Tubulin (1:100, proteintech, CL647-66031) and counterstained with Hoechst 33342 (1:50, Beyotime, C1022) for 1 min. Fluorescence images were captured using a Nikon Eclipse Ti microscope.
In vitro fertilization
In vitro fertilization assay was performed as previously described protocols70. The COCs in HTF medium (M1135, Nanjing Aibei biotechnology) were co-incubated with capacitated epididymal sperm of wildtype male mice in an incubator with 37 °C and 5% CO2 for 6 h. Zygotes were washed and transferred to KSOM medium (M1435, Nanjing Aibei biotechnology) for culturing to later stages and the embryos at different stages were acquired by Nikon Eclipse Ti digital fluorescence microscope, Leica (DMi8).
Animal fertility testing
Mating trials began immediately after the Salidroside treatment immediately. Healthy fertile males aged 8 to 10 weeks of age were chosen for the mating study. Two female mice were housed in a cage with one male for a period of 10 days to cover 2 entire estrus cycles, ensuring a sufficient duration for successful mating. After the 10-day breeding, the females were separated from male, and the pregnant rate along with the number of offspring delivered per female were recorded.
Statistical analysis
All statistical analyzes were conducted with GraphPad Prism software (version 8.0.1). All experiments were repeated more than three times and the data were presented as means ± SD of each experiment. Two-tailed Student’s t test was used to determine the significance of difference between two groups and the data were considered statistically significant at P < 0.05. P is indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and n.s. (not significant, P ≥ 0.05). All mice were randomly assigned to different experimental groups.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in National Genomics Data Center (GSA: CRA016139) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa. The scRNA-seq data analysis was performed using scripts in R (version 4.2.3). Source data are provided with this paper.
References
Timonin, S. et al. Disparities in length of life across developed countries: measuring and decomposing changes over time within and between country groups. Popul Health Metr. 14, 29 (2016).
Adams, O. Life expectancy in Canada-an overview. Health Rep. 2, 361–376 (1990).
Lehallier, B. et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 25, 1843–1850 (2019).
Li, J. et al. Determining a multimodal aging clock in a cohort of Chinese women. Med. 4, 825–848.e813 (2023).
te Velde, E. R. & Pearson, P. L. The variability of female reproductive ageing. Hum. Reprod. Update 8, 141–154 (2002).
Zhang, H. et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr. Biol. 24, 2501–2508 (2014).
Wang, Y. et al. Polycomb subunit Pcgf2 mediates ovulation and fertility through transcriptional regulation progesterone receptor. Front Cell Dev. Biol. 10, 1010601 (2022).
Kim, S. Y., Xu, J., Mayerhofer, A. & Bishop, C. V. Editorial: Small molecules and peptides in paracrine/autocrine regulation of ovarian folliculogenesis. Front Endocrinol. (Lausanne) 14, 1168701 (2023).
Canipari, R., Cellini, V. & Cecconi, S. The ovary feels fine when paracrine and autocrine networks cooperate with gonadotropins in the regulation of folliculogenesis. Curr. Pharm. Des. 18, 245–255 (2012).
Lee, E. B., Chakravarthi, V. P., Wolfe, M. W. & Rumi, M. A. K. ERβ Regulation of gonadotropin responses during folliculogenesis. Int. J. Mol. Sci. 22, 10348(2021).
La Marca, A. et al. Development of a nomogram based on markers of ovarian reserve for the individualisation of the follicle-stimulating hormone starting dose in in vitro fertilisation cycles. Bjog 119, 1171–1179 (2012).
Djuwantono, T., Tjahyadi, D. & Ferdian, M. W. Nomogram of follicle stimulating hormone starting dose based on antral follicle count, third day follicle stimulating hormone levels, and age in Hasan Sadikin Hospital Bandung. Adv. Reprod. Sci. 04, 74–85 (2016).
Hanevik, H. I. & Hessen, D. O. IVF and human evolution. Hum. Reprod. Update 28, 457–479 (2022).
Knight, P. G., Satchell, L. & Glister, C. Intra-ovarian roles of activins and inhibins. Mol. Cell Endocrinol. 359, 53–65 (2012).
Tamanini, C. & De Ambrogi, M. Angiogenesis in developing follicle and corpus luteum. Reprod. Domest. Anim. 39, 206–216 (2004).
Martelli, A. et al. Spatio-temporal analysis of vascular endothelial growth factor expression and blood vessel remodelling in pig ovarian follicles during the periovulatory period. J. Mol. Endocrinol. 36, 107–119 (2006).
Shimizu, T. et al. Induction of follicular development by direct single injection of vascular endothelial growth factor gene fragments into the ovary of miniature gilts. Biol. Reprod. 69, 1388–1393 (2003).
Shimizu, T. et al. Follicular microvasculature and angiogenic factors in the ovaries of domestic animals. J. Reprod. Dev. 49, 181–192 (2003).
Lu, X. et al. Stimulation of ovarian follicle growth after AMPK inhibition. Reproduction 153, 683–694 (2017).
Xu, X. et al. Imaging and tracing the pattern of adult ovarian angiogenesis implies a strategy against female reproductive aging. Sci. Adv. 8, eabi8683 (2022).
Feng, Y. et al. CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions. Sci. Rep. 7, 44810 (2017).
Torrens-Spence, M. P., Pluskal, T., Li, F. S., Carballo, V. & Weng, J. K. Complete pathway elucidation and heterologous reconstitution of rhodiola salidroside biosynthesis. Mol. Plant 11, 205–217 (2018).
Wang, Y., Han, J., Luo, L., Kasim, V. & Wu, S. Salidroside facilitates therapeutic angiogenesis in diabetic hindlimb ischemia by inhibiting ferroptosis. Biomed. Pharmacother. 159, 114245 (2023).
Han, J., Luo, L., Wang, Y., Wu, S. & Kasim, V. Therapeutic potential and molecular mechanisms of salidroside in ischemic diseases. Front Pharm. 13, 974775 (2022).
Deheng, C. et al. Salidroside promotes random skin flap survival in rats by enhancing angiogenesis and inhibiting apoptosis. J. Reconstr. Microsurg 32, 580–586 (2016).
Liu, C. et al. Discovery of salidroside-derivated glycoside analogues as novel angiogenesis agents to treat diabetic hind limb ischemia. J. Med Chem. 65, 135–162 (2022).
Melincovici, C. S. et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 59, 455–467 (2018).
Davis, A., Gao, R. & Navin, N. E. SCOPIT: sample size calculations for single-cell sequencing experiments. BMC Bioinformatics 20, 566 (2019).
Schupp, J. C. et al. Integrated single-cell atlas of endothelial cells of the human lung. Circulation 144, 286–302 (2021).
Wakabayashi, T. & Naito, H. Cellular heterogeneity and stem cells of vascular endothelial cells in blood vessel formation and homeostasis: Insights from single-cell RNA sequencing. Front Cell Dev. Biol. 11, 1146399 (2023).
Shen, J. et al. EGFL6 regulates angiogenesis and osteogenesis in distraction osteogenesis via Wnt/β-catenin signaling. Stem Cell Res Ther. 12, 415 (2021).
Huo, R. et al. Somatic GJA4 mutation in intracranial extra-axial cavernous hemangiomas. Stroke Vasc. Neurol. 8, 453–462 (2023).
Kumar, T. et al. A spatially resolved single-cell genomic atlas of the adult human breast. Nature 620, 181–191 (2023).
Rohlenova, K. et al. Single-cell RNA sequencing maps endothelial metabolic plasticity in pathological angiogenesis. Cell Metab. 31, 862–877.e814 (2020).
Kim, S. H. L. et al. Ectopic transient overexpression of OCT-4 facilitates BMP4-induced osteogenic transdifferentiation of human umbilical vein endothelial cells. J. Tissue Eng. 11, 2041731420909208 (2020).
Xu, Z. et al. Autophagic degradation of CCN2 (cellular communication network factor 2) causes cardiotoxicity of sunitinib. Autophagy 18, 1152–1173 (2022).
Crouch, E. E. et al. Ensembles of endothelial and mural cells promote angiogenesis in prenatal human brain. Cell 185, 3753–3769.e3718 (2022).
Lu, J. et al. Inflammation and endothelial function-related gene polymorphisms are associated with carotid atherosclerosis-A study of community population in Southwest China. Brain Behav. 13, e3045 (2023).
Kutryb-Zajac, B. et al. Inhibition of LPS-stimulated ecto-adenosine deaminase attenuates endothelial cell activation. J. Mol. Cell Cardiol. 128, 62–76 (2019).
Zhu, Y. et al. ARHGEF2/EDN1 pathway participates in ER stress-related drug resistance of hepatocellular carcinoma by promoting angiogenesis and malignant proliferation. Cell Death Dis. 13, 652 (2022).
Jiang, H., Li, F., Cai, L. & Chen, Q. Role of the TSPO-NOX4 axis in angiogenesis in glioblastoma. Front Pharm. 13, 1001588 (2022).
Xu, F. et al. Intrauterine inflammation damages placental angiogenesis via Wnt5a-Flt1 activation. Inflammation 42, 818–825 (2019).
Pérez-Gutiérrez, L. & Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 24, 816–834 (2023).
Wang, S. et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell 180, 585–600 (2020).
Shen, K. et al. Notoginsenoside Ft1 promotes angiogenesis via HIF-1α mediated VEGF secretion and the regulation of PI3K/AKT and Raf/MEK/ERK signaling pathways. Biochem Pharm. 84, 784–792 (2012).
Park, J. Y. et al. Beneficial effects of deoxyshikonin on delayed wound healing in diabetic mice. Int. J. Mol. Sci. 19, 3660 (2018).
Khan, S. S., Singer, B. D. & Vaughan, D. E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 16, 624–633 (2017).
Niikura, Y., Niikura, T. & Tilly, J. L. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany NY) 1, 971–978 (2009).
Zhou, C. et al. Single-cell atlas of human ovaries reveals the role of the pyroptotic macrophage in ovarian aging. Adv. Sci. (Weinh.) 11, e2305175 (2024).
Wang, X., Wang, L. & Xiang, W. Mechanisms of ovarian aging in women: a review. J. Ovarian Res. 16, 67 (2023).
Broekmans, F. J., Soules, M. R. & Fauser, B. C. Ovarian aging: mechanisms and clinical consequences. Endocr. Rev. 30, 465–493 (2009).
Richardson, M. C., Guo, M., Fauser, B. C. & Macklon, N. S. Environmental and developmental origins of ovarian reserve. Hum. Reprod. Update 20, 353–369 (2014).
Zhu, D., Li, X., Macrae, V. E., Simoncini, T. & Fu, X. Extragonadal effects of follicle-stimulating hormone on osteoporosis and cardiovascular disease in women during menopausal transition. Trends Endocrinol. Metab. 29, 571–580 (2018).
Miao, Y. et al. Nicotinamide mononucleotide restores the meiotic competency of porcine oocytes exposed to ethylene glycol butyl ether. Front Cell Dev. Biol. 9, 628580 (2021).
Miao, Y., Cui, Z., Gao, Q., Rui, R. & Xiong, B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep. 32, 107987 (2020).
Zhang, Y. et al. Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging. Nat. Aging 3, 1372–1386 (2023).
Landry, D. A. et al. Metformin prevents age-associated ovarian fibrosis by modulating the immune landscape in female mice. Sci. Adv. 8, eabq1475 (2022).
Qin, X. et al. Metformin prevents murine ovarian aging. Aging (Albany NY) 11, 3785–3794 (2019).
Liu, C. et al. Granulosa cell mevalonate pathway abnormalities contribute to oocyte meiotic defects and aneuploidy. Nat. Aging 3, 670–687 (2023).
Zheng, W. et al. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum. Mol. Genet 23, 920–928 (2014).
Cen, C. et al. Inactivation of Wt1 causes pre-granulosa cell to steroidogenic cell transformation and defect of ovary development†. Biol. Reprod. 103, 60–69 (2020).
Li, W., Germain, R. N. & Gerner, M. Y. Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (C(e)3D). Proc. Natl Acad. Sci. USA 114, E7321–E7330 (2017).
Feng, L. et al. ADAM10-Notch signaling governs the recruitment of ovarian pregranulosa cells and controls folliculogenesis in mice. J. Cell Sci. 129, 2202–2212 (2016).
Green, L. J. & Shikanov, A. In vitro culture methods of preantral follicles. Theriogenology 86, 229–238 (2016).
Wang, H. et al. Fatty acid amide hydrolase deficiency limits early pregnancy events. J. Clin. Invest 116, 2122–2131 (2006).
Zhang, H. et al. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc. Natl Acad. Sci. USA 109, 12580–12585 (2012).
Mostovoy, Y. et al. A hybrid approach for de novo human genome sequence assembly and phasing. Nat. Methods 13, 587–590 (2016).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
Cawthon, R. M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, e47 (2002).
Zhang, H. et al. Male fertility in Mus musculus requires the activity of TRYX5 in sperm migration into the oviduct. J. Cell Physiol. 235, 6058–6072 (2020).
Acknowledgements
The authors are grateful to Dr. Longzhong Jia (School of Life Sciences, Tsinghua University) and Wenji Wang (School of Life Sciences, Taizhou University), Dr. Da Mi (School of Life Sciences, Tsinghua University) for scRNA-seq technology support. This study was supported by the National Key Research and Development Program of China to H.Z. (2022YFC2703803), the Key Program of National Natural Science Foundation of China to H.Z. (82230051), the Innovative Project of State Key Laboratory of Animal Biotech Breeding to H.Z. (2023SKLAB1-6) and the 2115 Talent Development Program of China Agricultural University to H.Z. (1021-00109035).
Author information
Authors and Affiliations
Contributions
L.M., G.W., Y.Z., and H.Z. designed the research; L.M., G.W., X.Y., H.T., L.L., K.G., Y.B., and X.H. performed the experiments; L.M., G.W., X.Y., J.L., L.L., X.X., Y.Z., and H.Z. analyzed the data; L.M., R.Y., and H.Z. draw the graphic model. L.M., G.W., and H.Z. wrote the paper. All authors have seen and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Communications thanks Farners Amargant i Riera, who co-reviewed with Sara Pietroforte; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mu, L., Wang, G., Yang, X. et al. Physiological premature aging of ovarian blood vessels leads to decline in fertility in middle-aged mice. Nat Commun 16, 72 (2025). https://doi.org/10.1038/s41467-024-55509-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55509-y
This article is cited by
-
Combining network pharmacology and experimental validation to demonstrate that salidroside alleviates acute lung injury by inhibiting ferroptosis via the MAPK/GPX4 pathway
Respiratory Research (2026)
-
Hyperbaric oxygen therapy improves oocyte yield and embryo quality in poor ovarian responders: a pre-post cohort study
Reproductive Biology and Endocrinology (2025)
-
Roles of exosomes in microenvironment-related premature ovarian insufficiency: mechanisms and therapeutic intervention
Reproductive Biology and Endocrinology (2025)
-
Mast cell tryptase-PAR2 axis promotes ovarian fibrosis through RNF152-mediated stabilization of Bcl-xL.
Journal of Ovarian Research (2025)
-
Ovarian vascular aging: a hidden driver of mid-age female fertility decline
npj Aging (2025)