Abstract
Photodynamic immunotherapy (PIT) has emerged as a promising approach for efficient eradication of primary tumors and inhibition of tumor metastasis. However, most of photosensitizers (PSs) for PIT exhibit notable oxygen dependence. Herein, a concept emphasizing on transition from molecular PSs into semiconductor-like photocatalysts is proposed, which converts the PSs from type II photoreaction to efficient type I photoreaction. Detailed mechanism studies reveal that the nanostructured phthalocyanine aggregate (NanoNMe) generates radical ion pairs through a photoinduced symmetry breaking charge separation process, achieving charge separation through a self-substrate approach and leading to exceptional photocatalytic charge transfer activity. Additionally, a reformed phthalocyanine aggregate (NanoNMO) is fabricated to improve the stability in physiological environments. NanoNMO showcases significant photocytotoxicities under both normoxic and hypoxic conditions and exhibits remarkable tumor targeting ability. Notably, the NanoNMO-based photodynamic therapy and PD-1 checkpoint inhibitor-based immunotherapy synergistically triggers the infiltration of cytotoxic T lymphocytes into the tumor sites of female mice, leading to the effective inhibition of breast tumor growth.
Similar content being viewed by others
Introduction
Cancer is a devastating disease, with one of the most fatal aspects lying in the metastasis of cancer cells1,2,3,4,5. In recent years, photodynamic immunotherapy (PIT), which combines photodynamic therapy (PDT) with tumor immunotherapy, especially immune checkpoint blocking therapy, has emerged as a prominent hotspot in the field of tumor therapy6,7,8,9,10. However, the current research is still in its early stages. Most of photosensitizers (PSs) used for PIT mainly inhibit tumor through the oxygen-dependent type II photoreaction3,11,12,13. The hypoxic tumor microenvironment (TME) limits the therapeutic effect of this type of PDT, which limits the synergistic effect of PIT accordingly6,14. Moreover, the hypoxic TME promotes cancer cell metastasis and tumor immunosuppression15,16, while the consumption of oxygen during type II PDT further exacerbates tumor hypoxia, thereby impeding the effectiveness of PIT against tumor metastasis17,18. Consequently, a crucial aspect for advancing the clinical application of PIT lies in the development of PSs that exhibit less oxygen dependency.
Type I PSs have been proved to possess the advantage of less oxygen dependency19,20,21. However, most of the existing type I PSs primarily rely on various substrates as electron transfer agents to enhance electron transfer efficiency, which impose stringent requirements on the type and concentration of substrates, as well as their redox potential and binding affinity with PSs22,23,24,25. Therefore, the research on type I PSs remains relatively limited. Our prior research has discovered that a phenoxy-linked polyamine mono-substituted zinc (II) phthalocyanine (Pc) can self-assemble into nanodots in water and generate reactive oxygen species (ROS) through type I photoreaction26. Nevertheless, it is urgent to delve deeply into the quantitative structure activity relationship (QSAR) between the structural characteristics of zinc (II) Pc and its type I photoreaction. Additionally, the internal mechanisms underlying the efficient type I photoreaction of the nanodots need to be elucidated as well.
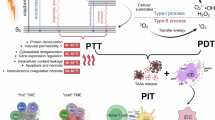
In this work, a series of tetrasubstituted zinc (II) Pc aggregates and the Pc monomers are designed and fabricated to investigate the QSAR between the electronic characteristics of the substituents and the ROS generations of the Pc aggregates and Pc monomers. The obtained results reveal that the Pc aggregates substituted with electron donor group (such as NanoNMe) exhibit a significant type I photoactivity. Detailed mechanism (Fig. 1a) studies reveal that NanoNMe undergo a photoinduced symmetry breaking charge separation (SBCS) process, achieving charge separation and formating Pc•+ and Pc•− ion pairs through a self-substrate approach. The products of SBCS exhibit similarities to electron-hole pairs27,28, enabling to consider NanoNMe as a semiconductor to analyze its charge transfer properties. Band analysis reveal that NanoNMe possess the capability to reduce O2 to superoxide anion (O2•−) and exhibit remarkable photocatalytic charge transfer activity, making NanoNMe a semiconductor-like photocatalyst to facilitate efficient charge transfer. Additionally, a reformed Pc aggregate (NanoNMO) is fabricated to improve the stability in physiological environments by incorporating octaethylene glycol into the Pc structure. Both in vitro and in vivo experiments demonstrate significant photocytotoxicities of NanoNMO. Notably, NanoNMO based type I PDT trigger a systemic anti-tumor immune response and promote dendritic cell (DC) maturation. NanoNMO and αPD-1 synergistically enhance T lymphocyte infiltration, achieving the greatest suppression of bilateral tumors (Fig. 1b). This work introduces a concept transforming molecular PSs into semiconductor-like photocatalysts, providing a perspective to design less oxygen dependent PSs.
a Schematic illustration of the mechanism of molecular PSs and the charge separation and photocatalytic mechanism of semiconductor-like photocatalysts. b Schematic illustration of the PIT synergistic process of NanoNMO and PD-1 antibody. Created in BioRender54.
Results
Preparation of Pc aggregates and Pc monomers and their ROS generations
Zinc (II) phthalocyanine is a functional dye with notable advantages, including strong absorption within the phototherapeutic window (650–850 nm), high ROS yield, and tunable photochemical properties29,30. These attributes render it a promising PS for PDT. However, most of the reported zinc (II) Pcs are oxygen dependent type II PSs31,32,33. Besides, the hydrophobic plane makes Pcs prone to aggregate, leading to the quenching of photoactivity34,35,36. Our prior work has demonstrated a zinc (II) Pc nanodot capable of generating ROS through type I mechanism26. To investigate the QSAR between the structural characteristics of zinc (II) Pc and its type I photoreaction, ten tetrasubstituted zinc (II) Pcs (Fig. 2a) were meticulously designed and synthesized herein. The structural modification involved replacing the para position of the phenoxy group connected to the Pc with various electron donor/acceptor substituents (Fig. 2b). Specifically, the Pcs were named by the abbreviation of their substituents successively as NMe, NH, OMe, Bu, H, Cl, OCF, COP, CN and NO. The detailed synthesis and characterization of these Pcs are provided in the supporting information (Figs. S2–S21).
a The structure of tetrasubstituted zinc (II) Pcs and b quantitative electron properties of the substituent group (σρ) on zinc (II) Pcs. The σρ value is a substituent constant derived from the Hammett equation55. The σρ value of electron donor substituents are negative, while the σρ value of electron accept substituents are positive. The absolute value of the σρ indicates the electron donating or accepting ability of substituent. c Schematic illustration of the fabrication of NanoPcs and MonoPcs. Created in BioRender56. d Relative ROS generations of NanoPcs and MonoPcs. e Relative O2•− generations of NanoPcs and MonoPcs. f Relative •OH generations of NanoPcs and MonoPcs. (g) Relative 1O2 generations of NanoPcs and MonoPcs. n = 3 independent samples with similar results. Data are presented as mean values ± standard deviation (SD). The relative ROS, O2•−, •OH, and 1O2 generations of NanoPcs or MonoPcs were determined comparing them with the reference samples (MB). The probe slope of MB was considered as 1 (marked as dashed line), and the slope ratios were served as the ordinate.
Pc aggregates (NanoPcs) with a size range of 200 ± 50 nm and Pc monomers (MonoPcs) were fabricated in H2O through different methods (Fig. 2c). Specifically, NanoPcs were obtained by diluting a tetrahydrofuran (THF) solution of the Pc into H2O, with or without the application of ultrasound. For NMe, NH, and OMe, NanoPcs were prepared by diluting the THF solution of the Pc into H2O under ultrasound conditions. For other structures, NanoPcs were prepared by diluting the THF solution of the Pc into H2O directly. The detailed preparation steps are described in the Methods section. The dynamic light scattering (DLS) results (Fig. S22) revealed that all of NanoPcs exhibited uniform dispersion in H2O, with particle sizes ranging of 200 ± 50 nm. Transmission electron microscope (TEM) images of NanoPcs (Fig. S23) demonstrated that their morphology resembled of regular spherical shapes, with a diameter of approximate 200 nm, which was consistent with the DLS results. The steady-state absorption spectra (Fig. S24) revealed that the absorption spectra of NanoPcs exhibited broadening and decreased intensity compared to the absorption features of the Pc monomers in N, N-dimethylformamide (DMF). Additionally, the steady-state fluorescence emission of NanoPcs (Fig. S25) attenuated significantly compared to that of Pc monomers in DMF. These findings indicated the significantly changed light absorption and fluorescence emission properties of NanoPcs compared to Pc monomers. Besides, MonoPcs were obtained by mixing a THF solution of Pc with surfactant Cremophor EL (CEL) in advance, followed by H2O dilution. DLS results (Fig. S26a) and TEM images (Fig. S26b) demonstrated that all of MonoPcs possessed particle sizes near 10 nm, with no observable nanostructure, indicating the uniform disperation of MonoPcs in H2O. Moreover, the steady-state absorption and fluorescence emission revealed that the absorption and fluorescence emission of MonoPcs exhibited similar features with those of Pc monomers in DMF, suggesting that the CEL-regulated MonoPcs could exist as monomers in H2O.
The ROS efficiencies for both NanoPcs and MonoPcs were initially assessed to conduct a QSAR analysis. The ROS generations were evaluated using the dihydrodichlorofluorescein (DCFH) probe (Figs. S27, S28), whose fluorescence at 525 nm can be produced by various ROS including O2•−, hydroxyl radical (•OH) and singlet oxygen (1O2). To enable a quantative comparison, the relative ROS generations of NanoPcs and MonoPcs were determined comparing them with the reference samples (Fig. 2d). For NanoPcs, an equal concentration of methylene blue (MB) was used as the reference sample, while for MonoPcs, a mixture of CEL and MB was used as the reference sample. The observations indicate that as the value of σρ decreases, both NanoPcs and MonoPcs exhibit higher ROS generation. Moreover, when the σρ value is more than 0, NanoPcs demonstrate lower ROS generation than MonoPcs, and when the σρ value is less than 0, NanoPcs demonstrate higher ROS generation than MonoPcs, surpassing that of MB significantly.
To assess the specific contributions, the O2•− generation was evaluated using dihydrohexidine (DHE) probe (Figs. S29, S30), the •OH generation was evaluated using aminophenyl fluorescein (APF) probe (Figs. S31, S32), and the 1O2 generation was evaluated using singlet oxygen sensing green (SOSG) probe (Figs. S33, S34). The O2•− generation results were presented in Fig. 2e. The observations indicate that as the value of σρ decreases, NanoPcs display higher O2•− generation. Moreover, when the σρ value is more than 0, NanoPcs demonstrate lower O2•− generation than MonoPcs, and when the σρ value is less than 0, NanoPcs demonstrate significantly higher O2•− generation than MonoPcs. The O2•− generation of NanoPcs can reach up to 15-fold higher than that of MB at most.
The •OH generation results were depicted in Fig. 2f. The observations indicate that as the value of σρ increases, both NanoPcs and MonoPcs demonstrate decreased •OH generations. Notably, NanoPcs demonstrate lower •OH generation compared to MonoPcs. Moreover, the •OH generations of both NanoPcs and MonoPcs are relatively inefficient, which are either comparable or lower than that of MB. The 1O2 generation results were depicted in Fig. 2g. The observations indicate that as the value of σρ increases, MonoPcs demonstrate a mild decrease in 1O2 generation. Notably, NanoPcs demonstrate lower 1O2 generation than MonoPcs. Moreover, both NanoPcs and MonoPcs display low level of 1O2 generations, which are consistently lower than that of MB. To sum up, all the QSAR results demonstrate that both NanoPcs and MonoPcs exhibit higher ROS generation when the σρ value is less than 0. Notably, the generations of both ROS and O2•− by NanoPcs (σρ < 0) demonstrate very high level, making them excellent ROS and O2•− generators.
Investigation on intersystem crossing process of MonoPcs
Generally, the generation of ROS is closely related to the intersystem crossing (ISC) process of PSs21,37. In order to gain insights into the ROS generation mechanism of MonoPcs, the ISC process was analyzed. Initially, time-dependent density functional theory (TD-DFT) calculations were conducted to analyze the energy levels of excited state involved in the ISC process. The results revealed that as the σρ value increased, the energy gap (∆EST) between singlet state (S1) and triplet state (T1) of Pcs gradually widened (Fig. 3a), indicating an increased energy interval of the ISC process. The increased ∆EST are unfavorable for the ROS generations, which explain the observed trend where, as the σρ values increased, the ROS generations of MonoPcs decreased.
a The calculated S1 and T1 energy levels of Pcs. Ground-state geometries optimizations and TD-DFT calculations were carried out at B3LYP50/6-31 G* method. All calculations were carried out using the Gaussian 16 package21. The polarizable continuum model51 with default parameters was used to implicitly consider solvation effects of H2O. 2D pseudo-color fs-TA spectra of b NMe and c CN obtained with time delays from 0 to 3080 ps (NMe: 694 nm, 47 μJ; CN: 685 nm, 77 μJ) and plots at different pump-probe delay times. The upward arrow symbolizes the population of triplet states while the downward arrow represents the depopulation of singlet states. The presence of an isosbestic point (marked with a black solid lines) indicates the appearance of both singlet and triplet species, which represents the occurrence of ISC. d The excitation energy data calculated by B3LYP/6-31 G* and the ISC parameters obtained from the fs-TA results of NMe and CN.
Furthermore, the excited state dynamics of MonoPcs were investigated using femtosecond transient absorption (fs-TA) spectroscopy37. To conduct specific analysis, NMe and CN were chosen as representative examples. As depicted in Fig. 3b, c, the contour maps illustrated two-dimensional color-coded fs-TA spectra of NMe and CN upon excitation at 694 and 685 nm, respectively, providing insights into the evolution of excited states. The negative absorption was attributed to the ground state bleaching (outlined with white dotted lines). The positive absorption was attributed to the excited state absorption (ESA, outlined with blue and red dotted lines), where the blue dotted lines indicated the ESA of singlet state, and the red dotted line indicated the ESA of triplet state. To distinguish singlet ESA from triplet ESA, fs-TA plots at different delay times were extracted from the contour map and presented at the bottom of the contour map.
For NMe, after a 90 fs timeframe following excitation, a series of ESA grew in intensity with prolonged delay time within the 500–750 nm range. Among these absorptions, the dynamic decay trace at 510 nm exhibited a decay lifetime of 3.18 ns, which was consistent with the fluorescence lifetime (3.98 ns) determined by time-correlated single photon counting (Fig. S35a). Based on these evidences, the ESA around 510 nm was assigned to singlet ESA. Over time, the ESA near 560 nm exhibited a continuously growing peak after 500 ps with no attenuation trend observed within the maximum range of 3.08 ns. Furthermore, the fs-TA plots revealed the occurrence of an obvious isosbestic point between the ESA near 560 nm and the ESA near 510 nm, indicating the occurrence of a singlet-to-triplet ISC process. Eventually, the hysteresis and prolonged lifetime of the ESA near 560 nm was attributed to triplet ESA. Likewise, for CN, a series of ESA grew in intensity within the 500–750 nm range as well. The dynamic decay trace at 510 nm aligned with the fluorescence lifetime (Fig. S35b). Moreover, an isosbestic point was observed between the ESA near 560 nm and the ESA near 510 nm, indicating the occurrence of ISC process and triplet state.
Afterwards, the parameters related to ISC were calculated based on the singlet and triplet ESA (Fig. 3d). The occurrence times of ISC (tISC) were estimated by determining the populated time of triplet state, which were found to be approximately 500 ps and 566 ps for NMe and CN, respectively. The rates of ISC (kISC) were calculated as 0.20 and 0.16 (×1010 s-1, 1/tISC) for NMe and CN, respectively. Subsequently, the efficiencies of ISC (ΦISC) were estimated according to the previous studies37,38 (ΦISC = [1/t(T1, rise)]/[1/t(S1, decay)]) as 16.4% and 8.6% for NMe and CN, respectively. The excited state dynamics results were consistent with the theoretical calculations, indicating that as the σρ value decreased, the ΦISC of Pcs increased, result in a higher generation of ROS.
Self-substrate photoinduced electron transfer
Unlike MonoPcs, NanoPcs generate ROS through type I photoreaction involved electron transfer. The crucial step in this mechanism is the intermolecular photoinduced electron transfer (PET) of NanoPcs39,40. Accordingly, the mechanism of the ROS generation by NanoPcs is proposed. As shown in Fig. 4a, upon photon excitation, partial molecules of NanoPcs are excited, resulting in the formation of an energy asymmetric local excited state (Pc* + Pc). The local excited state is unstable and promptly undergoes SBCS. As a result, a pair of free radical ions is formed (Pc•+ + Pc•−).
a Schematic illustration of the electron transfer mechanism of NanoPcs. fs-TA spectra of b NMe in THF and c NanoNMe in H2O upon excitation by 694 nm pulses. * represents excited state species, ▴ represents Pc•−. d The dynamic decay trace and lifetimes of excited states and Pc•− species of NanoNMe within a 3 ns range. e fs-TA spectra of NanoCN in H2O at different time delays upon excitation by 685 nm pulses. f Difference between the oxidation and reduction potentials (shown as e(Eox-Ered)) and the calculated excited energy (E*) of Pcs. “∆G” is displayed in the form of color bars. The redox potentials of Pcs were measured through cyclic voltammetry in DMF containing 0.1 M (n-Bu)4N+PF6–, using glassy carbon as the working electrode, Ag/AgCl as the reference electrode, Pt wire as the counter electrode, with a scan rate of 100 mV·s-1. Ferrocene was used as an external reference. The excited energy of Pcs was calculated by B3LYP/6-31 G* method.
To analyze the intermolecular SBCS, the excited state photophysical processes of NMe and NanoNMe were analyzed using fs-TA. The results revealed a significant difference in the photophysical process between NMe and NanoNMe. As shown in Fig. 4b and Fig. S36, the ESA of NMe in the 450–600 nm range exhibited uniformly decreased vibration peaks within 500 ps, indicating the absence of intermediate species during excited state decay process of NMe. For NanoNMe (Fig. 4c), the ESA transformed into mixed species features in the 450–600 nm range within 1 ps, suggesting the generation of intermediate species. Specifically, the ESA at 450–550 nm showed uniformly decreased vibration peaks, with an absorption range consistent with the ESA of NMe, indicating the formation of excited states. Furthermore, after approximately 0.58 ps, different species features appeared near 600 nm, indicating the generation of other species. The SBCS has been extensively reported in various organic molecular aggregates, including perylenediimide (PDI) and its derivatives41,42. The occurrences of SBCS (τSBCS) for PDI derivatives ranges from sub-ps to hundreds-ps and are closely related to molecular structure, distance, and solvent polarity43. Based on the mechanism and the reported results, the ESA near 600 nm was attributed to the absorption signal of the Pc•− species resulting from SBCS. The dynamic decay trace (Fig. 4d) indicated that Pc•− gradually formed after the decay of the excited state, yet it exhibited a longer lifetime compared to the excited state, demonstrating a distinct kinetic property. Conversely, no excited state or intermediate species was detected in the fs-TA analysis of NanoCN (Fig. 4e).
To investigate the feasibility of a photoinduced SBCS, the Gibbs free energy (∆G) was calculated. Typically, the condition for SBCS process to be possible is that ∆G is negative44. Therefore, the cyclic voltammetry was employed to determine the redox potentials of all the Pcs (Fig. S37, Table S2). The ∆G of Pcs was calculated based on the Rehm-Weller equation45 (Eq. 1) (Fig. 4f).
Where e represents the elementary charge; Eox is the potential for one-electron oxidation of Pcs, and Ered is the potential for one-electron reduction of Pcs; E* is the excitation energy obtained from TD-DFT. The last term accounts for the coulombic interactions between two ions produced at a distance d and screened by the solvent with a static dielectric constant εs. The results indicated that for substituents with σρ < 0, the ∆G of Pcs was negative, suggesting that Pcs with σp < 0 were capable of undergoing photoinduced SBCS. However, as the σp value increased, the ∆G of Pcs increased, indicating a gradual decrease in charge separation ability of Pcs. Consequently, the electron transfer mechanism of NanoPcs can be described as follows: the local excited state of NanoPcs undergoes a SBCS process, resulting in the generation of Pc•+ and Pc•− free radical species, thereby achieving charge separation through a way of self-substrate.
Semiconductor-like photocatalysis for O2 •− generation
The free radical ion pairs of SBCS (Pc•+ and Pc•−) in NanoPcs can exhibit spatial overlap and extend from localized orbitals to delocalized orbitals27,28, which can be described as free electron-hole pairs within a semiconductor (band theory) framework (Fig. 5a). Therefore, the redox properties of NanoPcs were investigated through band theory46 to analyze the generations of O2•− and •OH. The reduction of O2 depends on the conduction band (CB) potential of NanoNMe. Consequently, the flat band potential of NanoNMe was determined using the Mott-Schottky plots (Fig. 5b). The results revealed that the flat band potential of NanoNMe was −0.47 V, which was approximately equal to the CB potential and less negative than the reduction potential of O2 (−0.33 V), indicating that NanoNMe could photoreduce O2 to generate O2•−. Additionally, the oxidation of H2O depends on the valence band (VB) potential of NanoNMe. The VB potential of NanoNMe was determined by ultraviolet photoelectron spectroscopy (UPS) valence band spectra (Fig. 5c). The edge of the valence band maximum energy (Ef) for NanoNMe was found to be 0.18 eV, and the work function (Φ) was calculated to be 5.47 eV. The VB potential of NanoNMe (vs NHE) was concluded to be 1.21 V, which is less than the oxidation potential of H2O (1.99 V), indicating that NanoNMe cannot photooxidize H2O to generate •OH. Further experiments have shown that NanoNMe could photooxidize dyes to degrade (Fig. S38). Additionally, the optical bandgap energy determined from absorption spectra (Eg = 1.69 eV, Fig. S39) matched closely with the calculated bandgap (1.68 eV, Fig. 5d), providing convincing evidence of the redox capability of NanoNMe.
a Schematic illustration of the molecular orbital diagram of Pcs and the band diagram of NanoPcs. The excited Pcs transfer energy to O2, generating 1O2. The excited NanoPcs transfer electron to O2, generating O2•−. b Mott-Schottky plots of NanoNMe under dark conditions (100 Hz). The lyophilized NanoNMe adhered ITO glass was served as the working electrode, Ag/AgCl as the reference electrode, and platinum wire as the counter electrode, with 0.2 M Na2SO4 as the supporting electrolyte. c UPS valence band spectrum of NanoNMe. The work function (Φ) was calculated through the formula Φ = hν - (cutoff - Ef), where the excitation energy of the He light source was 21.22 eV. The VB potential was calculated through the formula EVB (NHE) = Ef + Φ - 4.44. d Band diagram of NanoNMe, including CB, VB potentials and the band gap. e Representative photocurrent response of NanoNMe and NanoCN on an ITO glass electrode with the interval of 20 s. f Electrochemical impedance spectroscopy of NanoNMe and NanoCN. Ag/AgCl served as the reference electrode, platinum wire as the counter electrode, and 0.2 M Na2SO4 as the supporting electrolyte with a bias of −0.2 V, white light, 180 mW·cm-2. g Changes in conductivity of NanoNMe, NanoCN, and NMe (all at 1 mg·mL-1) over time with the interval of 30 s, white light, 180 mW·cm-2. n = 3 independent samples with similar results. Data are presented as mean values ± SD.
Furthermore, the charge transfer abilities of NanoNMe were further investigated through transient photocurrent response and charge transfer resistance, with NanoCN used as a comparison. Interestingly, NanoNMe exhibited a significantly enhanced photocurrent under light illumination (Fig. 5e), while the photocurrent of NanoCN was negligible. Electrochemical impedance spectroscopy (Fig. 5f) revealed that the charge transfer resistance of NanoNMe was significantly smaller than that of NanoCN. Additionally, the charge flow abilities in the aqueous solution were studied by analyzing the conductivity changes (Fig. 5g). Over the course of illumination, the conductivity of NanoNMe showed a significant enhancement compared to NMe or NanoCN. These results indicate that NanoNMe exhibits a semiconductor-like photocatalytic activity, leading to a significant enhancement of charge transfer under illumination. Based on these findings, the present study proposes a design strategy for Type I PSs by converting molecular PSs to semiconductor-like photocatalysts, offering insights for addressing PDT in hypoxic tumors.
Fabrication of physiologically stable NanoPcs and their in vitro phototherapeutic efficacy
Motivated by the efficient photocatalytic generation of O2•− by NanoNMe, we further investigated the potential of NanoNMe for biomedical applications. The stability is a crucial requirement for biomedical application of NanoPcs. Therefore, we initially examined the stability of NanoNMe in physiological conditions. In a phosphate-buffered saline (PBS) solution containing fetal bovine serum (FBS), the particle size of NanoNMe gradually increased over time (Fig. S40a), suggesting an unstable nanostructure of NanoNMe. Additionally, the absorption spectra of NanoNMe (Fig. S40b, c) demonstrated a gradual decreased intensity in PBS containing FBS over time, indicating the decreased light-harvesting properties. Conversely, an increment in fluorescence intensity was observed for NanoNMe as the concentration of FBS increased (Fig. S40d, e), indicating the gradually restored fluorescence emission of NanoNMe under the influence of FBS. The significant changes in nanostructure and optical activity collectively demonstrated the poor stability of NanoNMe in a physiological environment. To facilitate the stable dispersion of NanoPc in physiological environments, an amphiphilic Pc (NMO) was designed by replacing one substituent of NMe with octaethylene glycol based on our previous experience32,47. The detailed synthesis and characterization of NMO are presented in the Supporting Information (Figs. S41, S42).
The nanostructured aggregate of NMO (NanoNMO) was prepared through a nano precipitation method (Fig. 6a). The detailed fabrication steps of NanoNMO are provided in the Methods section. DLS and TEM results exhibited that the average size of NanoNMO was approximately 110 nm (Fig. 6b), exhibiting a morphology resembling regular spherical shapes. As expected, NanoNMO exhibited improved stability in physiological environments compared to NanoNMe. During the initial 12 h period upon the addition of FBS, the size distribution of NanoNMO remained relatively unchanged, along with consistent absorption and fluorescence emission (Fig. S40f–j). Afterwards, the size of NanoNMO started to increase, accompanied by an intensified fluorescence emission, indicating the instability of NanoNMO after 24 h. Additionally, the ROS generations of NanoNMO were further evaluated with NMO, Chlorin e6 (Ce6), and MB serving as control (Fig. S43). The results demonstrated that NanoNMO exhibited robust ROS generations, higher than that of NMO and Ce6, and approximately 1.8-fold higher than that of MB (Fig. 6c). Specifically, the O2•− generation of NanoNMO was approximately 7.9-fold higher than that of MB (Fig. 6d), while no significant generation of •OH and 1O2 was observed (Fig. S44). These results indicate that NanoNMO is an efficient O2•− generator.
a Structure of NMO and schematic illustration of the fabrication of NanoNMO. Created in BioRender. Liu, H. (2024) BioRender.com/m21y350. b Diameter size distribution and morphology of NanoNMO (5 μM) measured by DLS and TEM. c Relative ROS generations and d relative O2•− generation of NanoNMO. n = 3 independent samples. Data are presented as mean values ± SD. The relative ROS and O2•− generations of NanoNMO, NMO and Ce6 were determined comparing them with the reference samples (MB). The probe slope of MB was considered as 1, and the slope ratios were served as the ordinate. e CLSM images of intracellular DCFH fluorescence and f quantitative fluorescence emission of 4T1 cells incubated with Ce6 (***p = 0.0004), NMO (***p = 0.0008), and NanoNMO (ns = 0.4060) under both normoxic and hypoxic conditions. g CLSM images of intracellular DHE fluorescence and h quantitative fluorescence emission of 4T1 cells incubated with Ce6 (***p = 0.0007), NMO (***p = 0.0003), and NanoNMO (*p = 0.0342) under both normoxic and hypoxic conditions. Cells were irradiated with 685 nm laser for 5 min (15 mW·cm-2). F.I., Fluorescence intensity. Cytotoxic effects of Ce6, NMO, and NanoNMO on 4T1 cells under i normoxic and j hypoxic conditions in dark or with 685 nm laser irradiation (30 min, 15 mW·cm-2). k Calcein-AM/PI costaining images detected by CLSM, with Calcein-AM staining displayed in the green channel and PI staining in the red channel (scale bar = 100 μm). n = 3 biologically independent experiments for cell samples with similar results. Data were expressed as mean values ± SD, *p < 0.05, ***p < 0.001, NS. not significant, determined by two-tailed Student’s t test.
Further investigation was conducted to assess the in vitro PDT activity of NanoNMO. Initially, the intracellular ROS generations in mouse breast cancer cells (4T1) were evaluated using confocal laser scanning microscopy (CLSM) under both normoxic and hypoxic conditions. As shown in Fig. S45, the fluorescence intensities of the oxygen stress indicator (ROS-ID) under both normoxic and hypoxic conditions were significantly higher than the negative control and comparable to the positive control, indicating the successful establishment of normoxic and hypoxic conditions. Subsequently, the intracellular ROS generation ability was assessed using DCFH as a fluorescent indicator. As depicted in Fig. 6e, negligible fluorescence was observed in cells without laser irradiation. However, after 685 nm laser irradiation, significant fluorescence was observed in the Ce6, NMO, and NanoNMO treated cells under normoxic conditions. Notably, NanoNMO group exhibited the highest fluorescence emission (Fig. 6f), indicating its superior ROS production ability. However, under hypoxic conditions, only NanoNMO group displayed bright fluorescence signals comparable to those observed under normoxic conditions, suggesting that NanoNMO could effectively generate ROS in hypoxic conditions as well. Additionally, the intracellular O2•− generation capacity was assessed using DHE as a fluorescent indicator. As shown in Fig. 6g, minimal fluorescence was observed in cells without laser irradiation. After 685 nm laser irradiation, neither Ce6 nor NMO displayed significant fluorescence signals under normoxic or hypoxic conditions, suggesting limited intracellular O2•− generation. In contrast, NanoNMO displayed robust fluorescence signals under both normoxic or hypoxic conditions (Fig. 6h), indicating efficient O2•− generation of NanoNMO under both normoxic or hypoxic conditions.
Next, the in vitro phototherapeutic effects of Ce6, NMO, and NanoNMO were evaluated through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. As shown in Fig. 6i and j, no obvious toxicity was observed for Ce6, NMO, and NanoNMO under dark conditions. Under normoxic conditions, Ce6, NMO, and NanoNMO exhibited significant concentration-dependent cytotoxicities following 685 nm laser irradiation (30 min, 15 mW·cm-2), with 50% inhibitory concentrations (IC50) of 2.35, 0.40, and 0.31 μM, respectively. However, under hypoxic conditions, the photocytotoxicity of Ce6 and NMO decreased significantly, leading to an increase in IC50 to 5.74 and 2.60 μM, respectively. In contrast, NanoNMO maintained a potent photocytotoxicity, with an IC50 of 0.41 μM. The live/dead cell staining assay (Fig. 6k, S46) also demonstrated the effective cell damage induced by NanoNMO under both normoxic and hypoxic conditions. These findings suggest that O2 concentration barely affect the photocytotoxicity of NanoNMO.
In vivo biodistribution and phototherapeutic efficacy of NanoNMO
To explore the biodistribution, Balb/c mice bearing a 4T1 tumor were administered with an intravenous injection of NanoNMO (200 μM, 100 μL), employing NMO (200 μM, 100 μL) as a control. Fluorescence and photoacoustic dual-modal imaging was used to evaluate the in vivo biodistribution. As shown in Fig. 7a, after intravenous injection, a significent fluorescence signal of NMO rapidly disseminated throughout the body and diminished gradually over time, with no significant tumor accumulation observed. Conversely, faint fluorescence signal was only observed at the tumor site within 24 h after injection of NanoNMO. Moreover, photoacoustic imaging (Fig. 7b) revealed that NanoNMO predominantly exhibited photoacoustic signals at the tumor site after intravenous injection and reached a maximum at 8 h (Fig. 7c), indicating the superior tumor targeting ability of NanoNMO. Notably, at 24 h post intravenous injection, the fluorescence signal of NanoNMO at the tumor site significantly increased, whereas the photoacoustic signal noticeably diminished. Since the photoacoustic signal of NanoNMO in H2O containing 10% FBS decreased significantly compared to that in pure water (Fig. S47), we speculated that the tumor accumulated NanoNMO may gradually disassemble, leading to enhanced fluorescence and reduced photoacoustic signals after 24 h. As a control, mice injected with NMO exhibited low systemic photoacoustic signals, indicating negligible tumor accumulation. To evaluate the distribution of NMO and NanoNMO in different organs, mice were sacrificed 36 h post injection and the fluorescence intensities of the tumor and major organs including heart, liver, spleen, lung, kidney and skin were assessed. As shown in Fig. 7d and e, the fluorescence signal of NanoNMO at the tumor site was significantly higher than that of other organs, registering 5.2-fold higher signal than that in the liver. In contrast, the fluorescence signal of NMO at the tumor site was only 0.8-fold than that of the liver, revealing an significant tumor targeting capability of NanoNMO. Consequently, NanoNMO can remain stable in vivo for 12 h, thereby facilitating the effective implementation of PDT.
a In vivo fluorescence imaging (excited at 640 nm, ×107 ps−1·cm−1·sr−1·μW−1·cm2, white ellipses representing the tumor area) and b in vivo photoacoustic imaging (excited at 690 and 700 nm, yellow ellipses representing the tumor area) of 4T1 tumor-bearing mice before and after intravenous injection of NMO or NanoNMO. c Quantitative fluorescence and photoacoustic intensities of the tumor sites after intravenous injection of NanoNMO. F.I., fluorescence intensity; PA.I., photoacoustic intensity. d Ex vivo fluorescence imaging of 4T1 tumor bearing mice injected with NMO and NanoNMO (×107 ps−1·cm−1·sr−1·μW−1·cm2) and e quantitative fluorescence intensities. H., heart; Li., liver; Sp., spleen; Lu., lung; K., kidney; T., tumor; Sk., skin. n = 3 biologically independent experiments for mice with similar results. Data were expressed as mean values ± SD. f Tumor growth plots of 4T1 tumor bearing mice following various treatments (***p < 0.0001). g Average body weight changes of mice after indicated treatments. n = 5 biologically independent experiments for mice. Data were expressed as mean values ± SD, ***p < 0.001, determined by two-tailed Student’s t test.
Subsequently, the in vivo phototherapeutic efficacy of NanoNMO was further conducted on 4T1 tumor-bearing mice. Once the volume of tumor reached 100 mm3, the mice were divided into four groups (five mice per group): (1) treated with saline (control); (2) treated with saline followed by laser irradiation (685 nm, 100 mW·cm-2) 8 h after injection; (3) treated with NanoNMO (200 μM, 100 μL); (4) treated with NanoNMO (200 μM, 100 μL) followed by laser irradiation (685 nm, 100 mW·cm-2) 8 h after injection. As depicted in Fig. 7f, all the control groups (including Saline, Saline+Laser, and NanoNMO only) exhibited significant level of tumor growth. In contrast, the tumor growth in NanoNMO+laser group was inhibited obviously, with a tumor inhibition rate of 96 ± 4% on the 14th day after treatment. Additionally, the phototherapeutic efficacy of NanoNMO+laser group was obviously enhanced than that of the conventional PSs (Fig. S48). These results suggest that NanoNMO possesses exceptional in vivo phototherapeutic efficacy. Furthermore, the biosafety of NanoNMO was assessed in vivo. The examination of blood routine indexes (Fig. S49) and liver and kidney function indexes (Fig. S50) showed no noticeable differences between the saline-treated and NanoNMO+Laser-treated mice. H&E staining results of major organs from NanoNMO+Laser-treated mice revealed minimal damage or injury (Fig. S51). Moreover, the body weight of the mice remained consistent and exhibited natural growth throughout the treatment period (Fig. 7g). Finally, NanoNMO exhibited virtually no hemolytic effect (Fig. S52 and Table S3). Overall, these findings indicate that NanoNMO exhibits excellent biocompatibility and biosafety throughout the course of treatments.
In vivo PIT efficiency of NanoNMO in combination with PD-1 antibody
To investigate the potential of type I PSs-mediated PDT to induce an anti-tumor immune response, the level of immune cell cytokines in the plasma was studied on NanoNMO injected 4T1 tumor-bearing mice. The mice were divided into four groups (five mice per group): (1) treated with saline (control); (2) treated with saline followed by laser irradiation (685 nm, 100 mW·cm-2) 8 h after injection; (3) treated with NanoNMO (200 μM, 100 μL); (4) treated with NanoNMO (200 μM, 100 μL) followed by laser irradiation (685 nm, 100 mW·cm-2) 8 h after injection. As shown in Fig. 8a–c, the expression of immunogenic substances (calreticulin (CRT), adenosine triphosphate (ATP), high-mobility group box 1 protein (HMGB-1)) of the NanoNMO+Laser group was significantly higher than those observed in the control groups (including Saline, Saline+Laser, and NanoNMO only), indicating a significant enhancement of the tumor immunogenic cell death (ICD) effect induced by PDT. Additionally, the level of interleukin-2 (IL-2), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ) (Fig. 8d–g) in NanoNMO+laser group showed a significant increase compared to those observed in the control group. The increased secretion of toxic cytokines suggests the occurrence of acute inflammation, which is crucial mechanism in inducing tumor-specific immunity through PDT. These results suggest that NanoNMO-mediated PDT can induce the tumor ICD effect and toxic cytokines in the host, leading to a systemic immune response.
Biochemical analysis of a CRT (***p < 0.0001), b ATP (***p < 0.0001), c HMGB-1 (***p < 0.0001), d IL-2 (***p < 0.0001), e IL-6 (***p < 0.0001), f TNF-α (***p < 0.0001) and g IFN-γ (***p < 0.0001) in the serum of mice 24 h after different treatments. n = 5 biologically independent experiments for mice. h Schematic outline of the establishment of a 4T1 bilateral tumor model and the treatment steps and procedures for different treatments. Created in BioRender. Liu, H. (2024) BioRender.com/w77z183. PDT treatment was performed 8 h after intravenous injection of NanoNMO with a 685 nm laser (100 mW·cm−2, 5 min), followed by intravenous injection of αPD-1 (2.5 mg·kg-1) on the first and third days, respectively. Tumor volume was continuously monitored until day 14, after which mice were sacrificed for further biochemical studies. Tumor growth plots of i primary tumors (Group 1:***p < 0.0001, Group 5:***p < 0.0001, *p = 0.0268) and j distant tumors (***p < 0.0001, **p = 0.0045) in 4T1 tumor-bearing mice following the indicated treatments. Average weights of k primary tumors (***p < 0.0001, **p = 0.0046, *p = 0.0164) and l distant tumors (***p < 0.0001, **p = 0.0020) following different treatments. m Average body weight changes of mice after different treatments. n Representative tumor images of primary tumors and distant tumors of mice after 14 d of indicated treatments. n = 5 biologically independent experiments for mice. The quantitative data of the population of DC maturation (CD11c+ CD80+ CD86+) in o primary tumors (Group 6: ***p < 0.0001, Group 4: ***p = 0.0046, **p = 0.0080), p distant tumors (Group 6: ***p < 0.0001, Group 5: ***p < 0.0001, Group 4: ***p < 0.0001, **p = 0.0096) and q lymph nodes (Group 6: ***p < 0.0001, Group 5: ***p < 0.0001, Group 5: **p = 0.0038, Group 1–3: **p = 0.0018) of mice after the indicated treatments. The quantitative data of CD4+ T lymphocytes in r primary tumors (***p < 0.0001), s distant tumors (***p < 0.0001) and t spleen (***p < 0.0001) of mice after the indicated treatments. The quantitative data of CD8+ T lymphocytes in u primary tumors (***p < 0.0001, **p = 0.0011), v distant tumors (***p < 0.0001) and w spleen (***p < 0.0001) of mice after the indicated treatments. n = 5 biologically independent experiments for mice. x Schematic illustration of the PIT synergistic process of NanoNMO and αPD-1. Created in BioRender. Liu, H. (2024) BioRender.com/r19s666. Data were expressed as mean values ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, determined by two-tailed Student’s t test.
Further investigation was conducted to explore the synergistic anti-tumor potential of NanoNMO-mediated PDT in combination with immune checkpoint inhibitors (ICIs). A 4T1 bilateral tumor model of Balb/c mouse was established by injecting 4T1 cells into the right flank of the mice (primary tumor), followed by injecting 4T1 cells into the left flank of mice (distant tumor) after 6 d (Fig. 8h). Once the volume of the distant tumor reached 100 mm3 (where as the volume of the primary tumor was larger than 100 mm3), the mice were divided into six groups (five mice per group) to monitor tumor growth following different treatments. Group 1: treated with saline (control); Group 2: treated with saline followed by laser irradiation (685 nm, 100 mW·cm-2) 8 h after injection; Group 3: treated with NanoNMO (200 μM, 100 μL); Group 4: treated with NanoNMO (200 μM, 100 μL) followed by laser irradiation (685 nm, 100 mW·cm-2) on the primary tumor 8 h after injection; Group 5: treated with αPD-1 twice (2.5 mg·kg-1) on day 1 and day 3, respectively; Group 6: treated with NanoNMO (200 μM, 100 μL) followed by laser irradiation (685 nm, 100 mW·cm-2) on the primary tumor 8 h after injection, and then treated with αPD-1 twice (2.5 mg·kg-1) on day 1 and day 3, respectively. The distant tumor received no laser irradiation. The tumor volume of both tumors and the weight of mice were monitored throughout the process.
As shown in Fig. 8i, the control groups (including Saline and Saline+Laser) exhibited rapid growth of the primary tumor. In addition, neither NanoNMO group nor αPD-1 group exhibited significant tumor inhibition on the primary tumor. In contrast, both the NanoNMO+Laser group and the NanoNMO+Laser+αPD-1 group demonstrated a significant suppression of primary tumor growth. Notably, the NanoNMO+Laser+αPD-1 group demonstrated the highest treatment efficacy, with a tumor inhibition rate of 82 ± 3% compared to Group 1 after 14 days of treatment, surpassing that of the NanoNMO+Laser group (73 ± 2%). These results indicate an enhanced inhibitory effect on the primary tumor through the synergistic therapy. Additionally, as shown in Fig. 8j, the control groups of the distant tumor (Saline, Saline+Laser, and NanoNMO) exhibited a rapid increase. Moreover, the NanoNMO+Laser group showed minimal distant tumor inhibition as well, suggesting that the immune response triggered by PDT was inferior and unable to suppress the growth of the distant tumor. The αPD-1 group showed a slight distant tumor inhibitory effect, which was attributed to the mild anti-tumor immune response induced by αPD-1. In comparison, the NanoNMO+Laser+αPD-1 group demonstrated a significant distant tumor inhibitory effect, achieving a tumor inhibition rate of 71 ± 2% after 14 days of treatment. These results indicate that the combination of NanoNMO and αPD-1 could significantly enhance the systemic anti-tumor immune response, thereby suppressing the growth of the distant tumor effectively.
After 14 days of treatment, the average weight of the primary tumors in the NanoNMO+Laser+αPD-1 group was 0.15 g (Fig. 8k), which was 4.5-fold lower than that of Saline group (0.68 g), 4.3-fold lower than that of Saline+Laser group (0.64 g), 5.0-fold lower than that of NanoNMO group (0.75 g), 3.4-fold lower than that of αPD-1 group (0.51 g) and 2.2-fold lower than that of NanoNMO+Laser group (0.33 g). Furthermore, the average weight of the distant tumors in the NanoNMO+Laser+αPD-1 group (0.01 g) was almost diminished (Fig. 8l), which was 27-fold lower than that of Saline group (0.27 g), 21-fold lower than that of Saline+Laser group (0.21 g), 21-fold lower than that of NanoNMO group (0.21 g), 15-fold lower than that of NanoNMO+Laser group (0.15 g), and 5-fold lower than that of αPD-1 group (0.05 g). The total results demonstrate that the synergistic treatment combining NanoNMO-mediated PDT and αPD-1 not only improves the inhibition effect on primary tumors but also triggers a significient systemic anti-tumor immune response to suppress distant tumors. The excellent synergistic anti-tumor efficacy of the NanoNMO+Laser+αPD-1 group was further confirmed by ex vivo photos of the tumor tissues (Fig. 8n). Notably, the body weight of mice displayed a natural growth during the treatment period (Fig. 8m), indicating the excellent biocompatibility of all treatments.
Inspired by the remarkable synergistic anti-tumor effect of NanoNMO and PD-1 antibodies, we proceeded to investigate the anti-tumor immune response evoked by NanoNMO-mediated PDT and its synergistic mechanism with PD-1 antibody by monitoring the key immune cells through flow cytometry. A 4T1 bilateral tumor model of Balb/c mouse was established as outlined in Fig. 8h and the mice were divided into six groups as mentioned above. Firstly, the maturation of DCs was investigated by collecting DCs from the primary tumors, distant tumors, and lymph nodes of bilateral 4T1 tumor-bearing mice 7 d post treatment and staining the sample with anti-CD11c, anti-CD80, and anti-CD86 antibodies. As shown in Fig. 8o–q and Fig. S54, the frequency of CD86+ DCs in the NanoNMO+Laser+αPD-1 group (primary tumor: 11.6%, distant tumor: 5.8%, lymph node: 12.2%) increased significantly compared to those observed in the Saline group (primary tumor: 1.4%, distant tumor: 2.7%, lymph node: 3.8%). These levels were also higher than those observed in the NanoNMO+Laser group (primary tumor: 7.8%, distant tumor: 5.4%, lymph node: 8.6%) and the αPD-1 group (primary tumor: 4.0%, distant tumor: 3.7%, lymph node: 5.3%). These findings indicate that the synergistic treatment of NanoNMO+Laser and αPD-1 can significantly enhance the maturation of DCs (CD80+ CD86+ cells) and effectively trigger the activation of T lymphocytes. Furthermore, the frequency of CD86+ DCs of the NanoNMO+Laser group was significantly higher than those observed in the αPD-1 group. According to the reported mechanism48, the immunogenic substances and tumor-associated antigens (TAAs) generated by PDT-induced ICD can promote the maturation of DCs. The mature DCs further promote T lymphocytes for activation. Therefore, the improved frequency of CD86+ DCs in the NanoNMO+laser group highlights the significant role of NanoNMO-mediated ICD effect on T cell activation during synergistic therapy.
Furthermore, the infiltration of cytotoxic T lymphocytes (CTLs) was investigated by collecting lymphocytes from the primary tumors, distant tumors, and spleens of bilateral 4T1 tumor-bearing mice 7 d post treatment and staining the sample with anti-CD3, anti-CD4, and anti-CD8 antibodies. As shown in Fig. 8r–t and Fig. S55, the frequency of CD4+ T cells of the NanoNMO+Laser+αPD-1 group (primary tumor: 8.2%, distant tumor: 7.9%, spleen: 12.8%) increased significantly compared to those observed in the Saline group (primary tumor: 1.9%, distant tumor: 3.7%, spleen: 7.8%). These levels were also higher than those observed in the NanoNMO+Laser group (primary tumor: 3.9%, distant tumor: 3.8%, spleen: 8.8%) and the αPD-1 group (primary tumor: 4.2%, distant tumor: 4.9%, spleen: 10.0%). These findings indicate that the synergistic treatment of NanoNMO and αPD-1 promotes the infiltration of CD4+ T cells in tumor sites significantly, resulting in an effective anti-tumor immune response. Moreover, the frequency of CD8+ T cells (Fig. 8u–w and Fig. S56) of the NanoNMO+Laser+αPD-1 group (primary tumor: 14.5%, distant tumor: 8.9%, spleen: 5.8%) exhibited a significant increase compared to those observed in the Saline group (primary tumor: 1.9%, distant tumor: 3.6%, spleen: 2.1%). Notably, these values surpassed those observed in the NanoNMO+Laser group (primary tumor: 6.7%, distant tumor: 4.3%, spleen: 4.1%) and the αPD-1 group (primary tumor: 10.1%, distant tumor: 6.8%, spleen: 4.2%), demonstrating significant improvement in immune response. These results are consistent with the anti-tumor activity studies, indicating the improved infiltration of CTLs in the NanoNMO+Laser+αPD-1 group. In summary, NanoNMO-mediated type I PDT not only effectively inhibits the primary tumor but also significantly triggers the body’s anti-tumor immune response to promote the maturation of DCs. Ultimately, the synergistic therapy of NanoNMO-mediated type I PDT with αPD-1 effectively improves CTL tumor infiltration, thereby achieving effective anti-tumor efficacy for bilateral tumors (Fig. 8x).
Discussion
This work presents a concept that converts molecular PSs into semiconductor-like photocatalysts, facilitating the shift of PSs from the traditional type II photoreaction to highly efficient type I photoreaction. We offer an explanation of why the self-assembled PSs can significantly stimulate electron transfer activity from two perspectives. On the one hand, the understanding of the electron excitation mechanism of PS aggregates undergoes a transformation from a molecular PS model to a semiconductor photocatalyst model. On this basis, the electron transfer and redox capabilities of PS aggregates are successfully analyzed through band theory. On the other hand, the efficient self-substrate SBCS mechanism has been employed to elucidate the ROS generations of PS aggregates. This self-substrate photoinduced charge separation approach not only minimizes photon energy loss28,42 but also eliminates the dependence on external substrates. Compared with previous works22,23,24,25,46, the self-substrate design for Type I PS does not require consideration on the type and concentration of substrates, as well as their redox potential and binding affinity with PSs. This design strategy offers a more convenient and efficient pathway for constructing Type I PSs.
To facilitate biomedical applications, an amphiphilic Pc molecular structure was designed to prepare a relatively stable Pc aggregate (NanoNMO) under physiological conditions. The results of in vitro studies demonstrated the excellent photocytotoxicity of NanoNMO under both normoxic and hypoxic conditions. The results of in vivo experiments revealed the remarkable tumor-targeting ability and phototherapeutic efficacy of NanoNMO. Notably, NanoNMO-mediated type I PDT triggered the systemic anti-tumor immune response, promoting DC maturation to activate T lymphocytes. The synergistic therapy of type I PDT with immunotherapy ultimately resulted in the most effective tumor-infiltrating CTLs and achieved the greatest suppression of bilateral tumors. This contribution pioneers the investigation of combining highly efficient PSs with ICIs under hypoxic conditions, providing a reference for the design of PSs combined with immunotherapy to effectively inhibit the growth and metastasis of hypoxic tumors, garnering significant implications.
Methods
Ethical statement and confirmation
The protocols for animal experiments in this study undergo rigorous review and approval by the Institutional Animal Care and Use Committees of Fuzhou University (2023-SG-001), and are conducted in strict accordance with the principles outlined by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All animal testing is carried out in full compliance with applicable local and national ethical regulations.
Materials
Reactions were conducted under a nitrogen atmosphere to minimize side reactions. Methylene blue (MB, >98.0%) was brought from TCI, Shanghai, China. 4-trifluoromethoxyphenol ( ≥ 98.0%), Cremophor EL (CEL, >99.0%), p-hydroxybenzophenone ( ≥ 98.0%), 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, ≥97%) and chlorin e6 (Ce6, >98.0%) were brought from Alfa, Shanghai, China. Dihydroethidium (DHE, ≥95.0%), ctDNA ( ≥ 95.0%), aminophenyl fluorescein (APF, ≥95.0%) were purchased from Sigma-Aldrich China. Singlet oxygen sensor green (SOSG) and ROS-ID™ hypoxia/oxidative stress detection kit were purchased from Enzo Life Sciences Inc. Fetal bovine serum (FBS) was bought from HyClone, Shanghai, China. 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT, ≥97.5%) was bought from Sigma, Shanghai, China. Calcein-AM/PI cell flow cytometry kit and Annexia V-FITC/PI cell flow cytometry kit were obtained from Solarbio, Beijing, China. The CRT, ATP, HMGB-1, IL-2, IL-6, TNF-α and IFN-γ ELISA kits were obtained from Shanghai Enzyme Biotechnology Co., Ltd. αPD-1 antibodies (Catalog No: AF1021, clone No: RMP1-14) were purchased from BioX cell Co., Ltd. USA. The anti-CD11c-FITC (Catalog No: 117305; clone No: N418), anti-CD80-APC (Catalog No: 104713; clone No: 16-10A1), anti-CD86-PE (Catalog No: 159203; clone No: A17199A), anti-CD45-FITC (Catalog No: E-AB-F1136C; clone No: 30-F11), anti-CD8-PerCP-Cy5.5 (Catalog No: E-AB-F1104E; clone No: 53-6.7), anti-CD4-APC (Catalog No: E-AB-F1097f; clone No: GK1.5) and anti-CD3-PE (Catalog No: E-AB-F1103B; clone No: 145-2C11) were purchased from BD Biosciences, USA.
Fabrication of phthalocyanine aggregates (NanoPcs) and phthalocyanine monomers (MonoPcs)
Fabrication of NanoPcs
NanoPcs were prepared using two different methods. Method 1, a 2 mM solution of Pc in THF was directly added to 1995 μL of water. The mixture underwent 6 pumping cycles at a constant speed using a 1000 μL pipette gun. Method 2, the THF solution of Pc (2 mM) was added to 1995 μL of water under ultrasonic conditions and kept under ultrasonic action for 10 s without any other actions. The final concentration of NanoPcs was 5 μM. Specifically, NanoNMe, NanoNH, NanoOMe, and NanoNMO were fabricated using Method 2, while the other NanoPcs were fabricated using Method 1.
Fabrication of MonoPcs
MonoPcs were prepared using the following method: A 2 mM solution of Pc in THF was initially mixed with 20 μL of CEL. The resulting mixture was further diluted with 1975 μL of water and underwent 6 pumping cycles at a constant speed using a 1000 μL pipette gun. The final concentration of MonoPcs was 5 μM, with the final proportion of CEL being 1% (v:v).
Measurement of ROS properties
ROS detection
The ROS generation efficiencies of photosensitizers were evaluated by the specific capture agent DCFH. Photosensitizers (5 μM) were dissolved with DCFH (10 μM) in a water solution. The mixture was then irradiated with > 610 nm light (5 mW·cm-2). After various irradiation times, fluorescence intensity (excited at 488 nm) of the mixture was recorded. The fluorescence intensities at 525 nm of different irradiation times were recorded to obtain the slope of fluorescence emission. The relative ROS generations of NanoPcs or MonoPcs were determined by comparing them with the reference samples (MB). The probe slope of MB was considered as 1, and the slope ratios were served as the ordinate.
O2 •− detection
The O2•− generation efficiencies of photosensitizers were evaluated by the specific capture agent DHE and ctDNA. Photosensitizers (5 μM) were dissolved with DHE (50 μM) and ctDNA (250 μg·mL-1) in a water solution. The mixture was than irradiated with > 610 nm light (5 mW·cm-2). After different irradiation times, fluorescence intensity (excited at 510 nm) of the mixture was recorded. The fluorescence intensities at 599 nm of different irradiation time were recorded to obtain the slope of fluorescence emission. The relative O2•− generations of NanoPcs or MonoPcs were determined comparing them with the reference samples (MB). The probe slope of MB was considered as 1, and the slope ratios were served as the ordinate.
•OH detection
The •OH generation efficiencies of photosensitizers were evaluated by the specific capture agent APF. Photosensitizers (5 μM) were dissolved with APF (10 μM) in a water solution. The mixture was than irradiated with > 610 nm light (5 mW·cm-2). After different irradiation times, fluorescence intensity (excited at 490 nm) of the mixture was recorded. The fluorescence intensities at 515 nm of different irradiation time were recorded to obtain the slope of fluorescence emission. The relative •OH generations of NanoPcs or MonoPcs were determined by comparing them with the reference samples (MB). The probe slope of MB was considered as 1, and the slope ratios were served as the ordinate.
1O2 detection
The 1O2 generation efficiencies of photosensitizers were evaluated by the specific capture agent SOSG. Photosensitizers (5 μM) were dissolved with SOSG (5 μM) in a water solution. The mixture was than irradiated with > 610 nm light (5 mW·cm-2). After different irradiation times, fluorescence intensity (excited at 488 nm) of the mixture was recorded. The fluorescence intensities at 520 nm of different irradiation time were recorded to obtain the slope of fluorescence emission. The relative 1O2 generations of NanoPcs or MonoPcs were determined comparing them with the reference samples (MB). The probe slope of MB was considered as 1, and the slope ratios were served as the ordinate.
Measurement of fs-TA
The ultrafast fs-TA measurements were conducted under ambient conditions using a fs pump-probe system in conjunction with an amplified laser setup49. The setup included a mode-locked Ti: sapphire seed laser (Spectra Physics, Maitai) coupled with a regenerative amplifier (Spitfire Pro, Spectra Physics) and a high-powered laser (3 mJ, ~35 fs, Empower, Spectra Physics) for pumping and amplification. The amplified 800 nm output was divided into two parts, with the majority of the beam ( ~ 85%) passing through an optical parametric amplifier (TOPAS prime, Spectra Physics) to generate pump pulses with tunable wavelengths. The remaining portion of the beam was used to generate the white light continuum probe and reference pulses (400–600 nm) after traversing an optical delay line and passing through photosensitizer solutions. In this setup, the probe beam passed through the sample, while the reference beam went straight to the reference spectrometer. To obtain fs-TA spectra with and without the pump pulses, a chopper modulating the pump pulse was used. An optical fiber, connected to a multichannel spectrometer equipped with a CMOS sensor, captured the changes in intensity of the probe/reference beam caused by the pump pulses. The optical delay line, with a maximum delay of approximately 3 ns, was adjusted during the measurements. The acquired spectral profiles were subsequently processed using Surface Xplorer software.
Measurement of electrochemical properties
Cyclic voltammetry measurement
Cyclic voltammetry experiment was performed using a three-electrode system, where the working electrode was a glassy carbon electrode, the counter electrode was a Pt wire electrode, and the reference electrode was an Ag/AgCl electrode. Measurements were carried out in DMF containing 0.1 M (n-Bu)4N+PF6–. The scan rate used was 100 mV·s-1. Ferrocene was employed as an external reference. The feasibility of photoinduced symmetry-breaking charge separation (SBCS) can be estimated by evaluating the Gibbs-free energy (∆G). The calculation of ∆G can be accomplished using the Rehm-Weller equation (Eq. 1).
Mott−Schottky (MS) analysis
Mott-Schottky analysis was performed using a three-electrode system, where the working electrode consisted of an indium tin oxide (ITO) glass electrode adhered with lyophilized NanoNMO or NanoCN, the counter electrode was a Pt wire electrode, and the reference electrode was an Ag/AgCl electrode. The measurements were carried out in an aqueous solution of 0.2 M Na2SO4.
Transient photocurrent and electrochemical impedance spectroscopy measurement
The transient photocurrent and electrochemical impedance spectroscopy measurements were performed using a three-electrode system, where the working electrode consisted of an ITO glass electrode adhered with lyophilized NanoNMO or NanoCN, the counter electrode was a Pt wire electrode, and the reference electrode was an Ag/AgCl electrode. The measurements were carried out in an aqueous solution of 0.2 M Na2SO4. For the transient photocurrent measurements, the working electrode was irradiated with a white light (180 mW·cm-2), with an interval of 20 s between each irradiation. For the electrochemical impedance spectroscopy measurements, the working electrode was consistently irradiated with a white light (180 mW·cm-2).
Conductivity measurement
The conductivity measurement was performed in aqueous solutions containing NMe, NanoNMe, and NanoCN at a concentration of 1 mg·mL-1, respectively. As a control, water was used as the reference solution. The solution was irradiated with a white light (180 mW·cm-2), and the conductivity values were recorded with an interval of 30 s.
Measurement of redox properties
Valence band (VB) measurement
The UPS measurement was performed using the He excitation energy (hν = 21.22 eV). The value of VB was determined by fitting a straight line into the leading edge of the spectra. The VB (vs NHE) of NanoNMe can be calculated according to the Eq. 2.
Where ENHE is the VB potential of NanoNMe; Ef is the binding energy of Fermi edge (Ef); Φ is the work function. Φ can be calculated according to Φ = hν - (cutoff - Ef), where the value of cutoff was obtained as 15.93 eV. Φ was calculated as 5.47 eV. The ENHE of NanoNMe was calculated as 1.21 eV (vs NHE).
Computational details
Ground-state geometries optimizations and time-dependent density functional theory (TD-DFT) calculations were carried out at B3LYP50/6-31 G* method. All calculations were carried out using the Gaussian 16 Rev.A.03 package. The polarizable continuum model51 with default parameters was used to implicitly consider solvation effects of water solution.
In vitro study
Cell Culture
The 4T1 cells were grown in DMEM at 37 °C in a humidified 5% CO2 atmosphere, with the medium supplemented with 10% FBS, Bispecific Antibody (0.02 units·mL-1), and Amphotericin (0.25 μg·mL-1).
Measurement of the intracellular ROS production52
Approximately 5 × 104 4T1 cells suspension in DMEM cell culture medium (0.4 mL) were inoculated on confocal dishes. To construct the in vitro normoxic and hypoxic conditions, a part of 4T1 cells was cultured in an incubator chamber at 37 °C with a humidified, 21% O2, 5% CO2, and 74% N2 atmosphere for 12 h. Another 4T1 cells were cultured in an incubator chamber at 37 °C with a humidified, 2% O2, 5% CO2, and 93% N2 atmosphere for 12 h. After the removal of the medium, the cells were incubated in fresh medium containing Ce6 or NMO or NanoNMO (4 μM) in darkness for 1.5 h. Subsequently, DCFH-DA was added at a final concentration of 10 µM and incubated for 30 min. The cells were then washed three times with PBS, replenished with 500 μL of culture medium, and utilized a 685 nm laser (15 mW·cm-2) for 5 min. Upon removing the culture medium, the cells underwent a PBS rinse before imaging via a LEICA TCS SPE confocal microscope. The probe was excited at 488 nm and monitored at 500–600 nm. The images were then digitized and analyzed using SPE ROI Fluorescence Statistics software. The average intracellular fluorescence intensities were recorded for a total of 50 cells in each sample.
Measurement of the intracellular O2 •- production52
Approximately 5 × 104 4T1 cells suspension in DMEM cell culture medium (0.4 mL) were inoculated on confocal dishes. 4T1 cells were divided into two parts to culture under both normoxic and hypoxic conditions for 12 h. After the removal of the medium, the cells were incubated in a fresh medium containing Ce6 or NMO or NanoNMO (4 μM) in darkness for 1.5 h. DHE was added at a final concentration of 10 µM and incubated for 30 min. The cells were then washed three times with PBS, replenished with 500 μL of culture medium, and utilized a 685 nm laser (15 mW·cm-2) for 5 min. Upon removing the culture medium, the cells underwent a PBS rinse before imaging via a LEICA TCS SPE confocal microscope. The probe was excited at 532 nm and monitored at 540–650 nm. The images were then digitized and analyzed by SPE ROI Fluorescence Statistics software. The average intracellular fluorescence intensities were recorded for a total of 50 cells in each sample.
In vitro photodynamic activity assay
4T1 cells (about 1 × 104 cells per well) were cultured in 96-well plates and divided into two parts to culture under both normoxic and hypoxic conditions for 12 h at 37 °C in a humidified 5 % CO2 atmosphere. The previous medium was aspirated and discarded, and 100 μL per well of Ce6 or NMO or NanoNMO with the specified concentration gradient was subsequently added. After 2 h of cultivation and removal of the culture medium, the cells were washed with PBS and replenished with 100 μL of culture medium. Subsequently, the cells were exposed to ambient temperature illumination for 0.5 h. The light source utilized a 685 nm laser (30 min, 15 mW·cm-2), and the total fluence was 27 J·cm-2. Cell viability was determined by the MTT assay. Following illumination, the cells were incubated overnight at 37 °C with 5% CO2. A 40 μL MTT solution in PBS (2.5 mg mL-1) was added to each well, and the cells were subsequently incubated for 4 h under the same conditions. After removing the medium, 150 μL of DMSO was added to each well. The 96-well plate was gently agitated on a Tecan M200Pro microplate reader at room temperature for 20 s prior to measuring the absorbance at 490 nm. The cell viability was then detected by the Eq. 3:
Where Ai is the absorbance of the ith data (i = 1, 2, …, n), Ācontrol is the average absorbance of the control wells, in which the Pc is absent, and n (≥3) is the number of the data points.
Live/Dead cell co-staining
4T1 cells (about 1 × 104 cells per well) were cultured in 96-wellplates and devided two parts to culture under both normoxic and hypoxic conditions for 12 h at 37 °C in a humidified 5 % CO2 atmosphere. The previous medium was aspirated and discarded, and 100 μL per well of NMO or NanoNMO were added at concentrations of 0.4 or 0.8 μM, respectively. After 2 h of cultivation and removal of the culture medium, the cells were washed with PBS and replenished with 100 μL of culture medium. Subsequently, the cells were exposed to ambient temperature illumination for 0.5 h. The light source utilized a 685 nm laser (30 min, 15 mW·cm-2), the total fluence was 27 J·cm-2. After the aforementioned treatments, 4T1 cells were additionally stained using the Calcein-AM/PI Double Stain Kit52 following the instructions provided in the manual. The excitation wavelength was 488 nm, and the emission wavelength was set to 505–545 nm for the green channel and 600–700 nm for the red channel.
In vivo study
Mouse models
Mouse breast cancer (4T1) cells (catalog No: CRL-2539) were acquired from the China Center for Type Culture Collection (CCTCC, Shanghai, China), and the Balb/c mice aged 5–6 weeks old were procured from Wushi Animal Co. Ltd. (FuZhou, China). Sex and gender was considered in the study design. The research findings in this work apply to female vertebrates. Mice were kept in a fully equipped animal facility with temperatures controlled between 20–25 °C and humidity levels maintained at 40–60%. Mice were exposed to a consistent 12-hour dark/light cycle. The maximum tumor size allowed by the ethics committee or institutional review board was 2000 mm3, and we ensured this limit was not exceeded in any experimental group. To initiate a unilateral tumor model, approximately 5 × 106 4T1 cells in 200 μL DMEM culture medium (Figs. 7a–g, 8a–g, S49-S52) or 200 μL Matrigel (Fig. S48) were subcutaneously inoculated on the flank of mice weighing 18–20 g. To initiate a bilateral tumor model, approximately 5 × 106 4T1 cells in 200 μL DMEM culture medium were subcutaneously inoculated on the right flank of mice weighing 18–20 g. After 6 days, approximately 5 × 106 4T1 cells in 200 μL of DMEM culture medium were injected into the left flank of the mice (Figs. 8i–w, S53–S55).
Fluorescence imaging
The mice received an intravenous injection of either NMO or NanoNMO (200 μM, 100 μL) in water. In vivo fluorescence images of the mice were performed on a IVIS Lumina III imaging system. Both NMO and NanoNMO were excited at 640 nm and monitored at 660–750 nm. After the in vivo imaging studies, the mice were euthanized 36 h after the injection. The heart, liver, spleen, lung, kidney, tumor and skin were extracted and imaged. Data was processed through the IVIS system. Three mice were utilized for each experimental group.
Photoacoustic imaging
The mice received an intravenous injection of either NMO or NanoNMO (200 μM, 100 μL) in water. In vivo photoacoustic images of the mice were performed on a photoacoustic computerized tomography scanner (MSOT 256-TF, Germany). Data was processed through the iThera Medical system. Three mice were utilized for each experimental group.
In vivo PDT effect assay
To evaluate the in vivo PDT effects of NanoNMO, the mice received an intravenous injection of either NMO or NanoNMO (200 μM, 100 μL) in water. 8 h post injection, the mice underwent irradiation at the tumor site using a 685 nm laser (100 mW·cm-2) for a duration of 5 min. As a control, blank mice were treated with saline only (100 μL). To evaluate the therapeutic efficacy, the tumor size was measured using a digital caliper every alternate day. Daily photographs were taken for a duration of 14 d. Tumor volumes were calculated as 0.5 × length × width × width.
In vivo cytokine release
The tumor-bearing mice received an intravenous injection of NanoNMO (200 μM, 100 μL) in water. 8 h post injection, the mice underwent irradiation at the tumor site using a 685 nm laser (100 mW·cm-2) for a duration of 5 min. The blood was collected 12 h after PDT and the levels of serum CRT, ATP, HMGB-1, IL-2, IL-6, TNF-α, and IFN-γ were determined by enzyme-linked immunosorbent assay (ELISA) to evaluate the acute inflammation induced by the treatment8,53.
In vivo photodynamic immunotherapy (PIT) effect assay
To evaluate the in vivo PIT effects of NanoNMO, the mice received an intravenous injection of NanoNMO (200 μM, 100 μL) in water first. 8 h post injection, the mice underwent irradiation at the tumor site using a 685 nm laser (100 mW·cm-2) for a duration of 5 min. Then αPD-1 was intravenously injected (2.5 mg·kg-1) at 24 h and 72 h after injection, respectively. To evaluate the therapeutic efficacy, the tumor size was measured using a digital caliper every alternate day. Daily photographs were taken for a duration of 14 d. Tumor volumes were calculated using the formula 0.5 × length × width × width.
Flow cytometry assay for immune response
All mice were euthanized on the 7th day post treatment, and the bilateral tumors, inguinal lymph nodes and spleens were excised. The tumors, lymph nodes and spleens were subsequently digested using a DMEM mixture that included 0.2% collagenase IV, 0.1% hyaluronidase, and 0.002% DNase I at 37 °C for 1 h, followed by mechanical grinding using the rubber end of a syringe. The resulting cell suspension was sieved through cell strainers and rinsed with PBS. The single-cell suspension were then incubated with anti-CD11c-FITC, anti-CD80-APC, and anti-CD86-PE (frequency evaluation of CD80+ CD86+ DCs) or anti-CD45-FITC, anti-CD3-PE, anti-CD4-PerCP-Cy5.5, and anti-CD8-APC (frequency evaluation of CD4+ CD8+ T lymphocytes), followed by shaking for 30 min at 4 °C8. After centrifugation at 300 x g for 5 min, the supernatant was discarded, and the cell suspension was diluted with sterilized PBS. Cell acquisition was performed using a NovoCyte Flow Cytometer (ACEA Biosciences, USA), and data analysis was conducted using the FlowJo 10.8.1 software.
Statistical information
Statistical comparisons were made using two-tailed Student’s t test (between two groups). All statistical analysis was performed using SPSS 30.0.0.0(172). p < 0.05 was considered to be statistically different; p < 0.01 was considered to be a significant statistical difference; p < 0.001 was considered to be an extremely substantial statistical difference. Sample sizes for cell and animal experiments were selected based on typical sample sizes in the field. No statistical method was used to predetermine sample size, no data were excluded from the analyses. The experiments were not randomized, and the investigators were not blinded to group allocation during experiments or outcome assessment. Quantitative data were presented as mean values ± standard deviation (SD).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that all data generated in this study are provided in the Article, Supplementary Information or Source Data file. Source data are provided with this paper. The full image dataset is available from the corresponding author upon request. Source data are provided with this paper.
References
Felsher, D. W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 3, 375–380 (2003).
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4, 437–447 (2004).
Li, X., Lovell, J. F., Yoon, J. & Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674 (2020).
Chen, H., Zhang, W., Zhu, G., Xie, J. & Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2, 17024–17042 (2017).
Dolmans, D., Fukumura, D. & Jain, R. K. Photodynamic therapy for cancer. Nat. Rev. Cancer 3, 380–387 (2003).
Sang, W., Zhang, Z., Dai, Y. & Chen, X. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem. Soc. Rev. 48, 3771–3810 (2019).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
He, C. et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 7, 12499–12510 (2016).
Castano, A. P., Mroz, P. & Hamblin, M. R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 6, 535–545 (2006).
Ouyang, B. et al. The dose threshold for nanoparticle tumour delivery. Nat. Mater. 19, 1362–1371 (2020).
Singh, S. et al. Glycosylated porphyrins, phthalocyanines, and other porphyrinoids for diagnostics and therapeutics. Chem. Rev. 115, 10261–10306 (2015).
Du, J. et al. Enhanced photodynamic therapy for overcoming tumor hypoxia: From microenvironment regulation to photosensitizer innovation. Coord. Chem. Rev. 427, 213604 (2021).
Yuan, B. et al. A self-degradable supramolecular photosensitizer with high photodynamic therapeutic efficiency and improved safety. Angew. Chem. Int. Ed. 60, 706–710 (2021).
Li, X., Kwon, N., Guo, T., Liu, Z. & Yoon, J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew. Chem. Int. Ed. 57, 11522–11531 (2018).
Rankin, E. B. & Giaccia, A. J. Hypoxic control of metastasis. Science 352, 175–180 (2016).
Chouaib, S., Noman, M. Z., Kosmatopoulos, K. & Curran, M. A. Hypoxic stress: Obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 36, 439–445 (2017).
Kang, X. et al. A photo-triggered self-accelerated nanoplatform for multifunctional image-guided combination cancer immunotherapy. Nat. Commun. 14, 5216–5233 (2023).
Zhang, P., Cui, Y. & Wang, Y. Designing temporal- and spatial-control multifunctional nanoformulations for synergistic photodynamic-enhanced tumour immunotherapy. Nano Today 49, 101816–101835 (2023).
Fan, W., Huang, P. & Chen, X. Overcoming the achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 45, 6488–6519 (2016).
Pham, T. C., Nguyen, V.-N., Choi, Y., Lee, S. & Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 121, 13454–13619 (2021).
Teng, K. X. et al. BODIPY-based photodynamic agents for exclusively generating superoxide radical over singlet oxygen. Angew. Chem. Int. Ed. 60, 19912–19920 (2021).
Chen, W. et al. Integration of TADF photosensitizer as “electron pump” and BSA as “electron reservoir” for boosting type I photodynamic therapy. J. Am. Chem. Soc. 145, 8130–8140 (2023).
Teng, K.-X., Niu, L.-Y., Xie, N. & Yang, Q.-Z. Supramolecular photodynamic agents for simultaneous oxidation of NADH and generation of superoxide radical. Nat. Commun. 13, 6179 (2022).
An, J. et al. An unexpected strategy to alleviate hypoxia limitation of photodynamic therapy by biotinylation of photosensitizers. Nat. Commun. 13, 2225 (2022).
Teng, K.-X., Niu, L.-Y. & Yang, Q.-Z. A host-guest strategy for converting the photodynamic agents from a singlet oxygen generator to a superoxide radical generator. Chem. Sci. 13, 5951–5956 (2022).
Li, X., Lee, D., Huang, J. D. & Yoon, J. Phthalocyanine-assembled nanodots as photosensitizers for highly efficient type I photoreactions in photodynamic therapy. Angew. Chem. Int. Ed. 57, 9885–9890 (2018).
Köhler, A. et al. Charge separation in localized and delocalized electronic states in polymeric semiconductors. Nature 392, 903–906 (1998).
Bradley, J. M. et al. Harvesting electrons and holes from photodriven symmetry-breaking charge separation within a perylenediimide photosynthetic model dimer. PNAS 120, e2313575120 (2023).
Li, X. et al. A tumor-pH-responsive supramolecular photosensitizer for activatable photodynamic therapy with minimal in vivo skin phototoxicity. Theranostics 7, 2746–2756 (2017).
Li, X., Kolemen, S., Yoon, J. & Akkaya, E. U. Activatable photosensitizers: Agents for selective photodynamic therapy. Adv. Funct. Mater. 27, 1604053 (2017).
Lo, P.-C. et al. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 49, 1041–1056 (2020).
Li, X. et al. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 379, 147–160 (2019).
Ishii, K. Functional singlet oxygen generators based on phthalocyanines. Coord. Chem. Rev. 256, 1556–1568 (2012).
Manzano, H. et al. Unprecedented J-aggregated dyes in pure organic solvents. Adv. Funct. Mater. 26, 2756–2769 (2016).
Li, X. et al. New application of phthalocyanine molecules: From photodynamic therapy to photothermal therapy by means of structural regulation rather than formation of aggregates. Chem. Sci. 9, 2098–2104 (2018).
Liu, H., Li, X.-Y., Li, X. & Huang, J.-D. Nanostructured self-assemblies of photosensitive dyes: Green and efficient theranostic approaches. Green. Chem. Eng. 4, 399–416 (2023).
Hu, W. et al. Stimuli-responsive reversible switching of intersystem crossing in pure organic material for smart photodynamic therapy. Angew. Chem. Int. Ed. 58, 11105–11111 (2019).
Zhang, G., Palmer, G. M., Dewhirst, M. W. & Fraser, C. L. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat. Mater. 8, 747–751 (2009).
Gai, P. et al. Solar-powered organic semiconductor-bacteria biohybrids for CO2 reduction into acetic acid. Angew. Chem. Int. Ed. 59, 7224–7229 (2020).
Liu, D. et al. Long-lived charge-transfer state induced by spin-orbit charge transfer intersystem crossing (SOCT-ISC) in a compact spiro electron donor/acceptor dyad. Angew. Chem. Int. Ed. 59, 11591–11599 (2020).
Markovic, V., Villamaina, D., Barabanov, I., Lawson Daku, L. M. & Vauthey, E. Photoinduced symmetry‐breaking charge separation: The direction of the charge transfer. Angew. Chem. Int. Ed. 50, 7596–7598 (2011).
Lin, C., Kim, T., Schultz, J. D., Young, R. M. & Wasielewski, M. R. Accelerating symmetry-breaking charge separation in a perylenediimide trimer through a vibronically coherent dimer intermediate. Nat. Chem. 14, 786–793 (2022).
Zhao, X. et al. Near-infrared self-assembled hydroxyl radical generator based on photoinduced cascade electron transfer for hypoxic tumor phototherapy. Adv. Mater. 35, 2305163 (2023).
Vauthey, E. Photoinduced symmetry-breaking charge separation. ChemPhysChem 13, 2001–2011 (2012).
Weller, A. Photoinduced electron transfer in solution: Exciplex and radical ion pair formation free enthalpies and their solvent dependence. Phys. Chem. 133, 93–98 (1982).
Teng, K.-X., Niu, L.-Y. & Yang, Q.-Z. Supramolecular photosensitizer enables oxygen-independent generation of hydroxyl radicals for photodynamic therapy. J. Am. Chem. Soc. 145, 4081–4087 (2023).
Li, X. et al. Nanostructured phthalocyanine assemblies with protein-driven switchable photoactivities for biophotonic imaging and therapy. J. Am. Chem. Soc. 139, 10880–10886 (2017).
Guo, Y. et al. Copackaging photosensitizer and PD-L1 sirna in a nucleic acid nanogel for synergistic cancer photoimmunotherapy. Sci. Adv. 8, 2941–2954 (2022).
Zou, S. et al. From nonluminescent to blue-emitting Cs4PbBr6 nanocrystals: Tailoring the insulator bandgap of 0d perovskite through Sn cation doping. Adv. Mater. 31, 1900606 (2019).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Luzhkov, V. B., Volokhov, V. M. & Pokatovich, G. A. Molecular modelling and quantum mechanical calculations of the hydration free energy of buckminsterfullerene. Chem. Phys. Lett. 676, 95–98 (2017).
Zhao, Y. Y. et al. Nanostructured phthalocyanine assemblies with efficient synergistic effect of type I photoreaction and photothermal action to overcome tumor hypoxia in photodynamic therapy. J. Am. Chem. Soc. 143, 13980–13989 (2021).
Zheng, B.-Y. et al. Phthalocyanine-based photosensitizers combined with anti-PD-L1 for highly efficient photodynamic immunotherapy. Dyes Pigm 185, 108907–108917 (2021).
Liu, H. BioRender.com/s58b425. (2024).
Hansch, C., Leo, A. & Taft, R. W. A survey of hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Liu, H. BioRender.com/g04i339. (2024).
Acknowledgements
The authors thank the National Natural Science Foundation of China (Grant Nos. 22078066, X, L; Grant Nos. T2322004, X, L; Grant Nos. 22178065, J. -D, H).
Author information
Authors and Affiliations
Contributions
H. L.: Performed the synthesis and the in vivo test, analyzed the data, and prepared the manuscript draft; Z. L.: Performed the in vitro test and analyzed the data; X. Z.: Performed the in vivo test and analyzed the data; Y. X.: Assisted the in vivo test; G. T.: Assisted the in vitro test; Z. W.: Performed the computational calculation; Y.-Y. Z.: Assisted the in vivo test; M.-R. K.: Assisted the synthesis; B.-Y. Z.: Assisted the biological test; S. H., Assisted the computational calculation; J.-D. H.: Conceptualization of the Semiconductor-like photocatalysts, design of the project, supervision, review & editing, funding acquisition; X. L.: Conceptualization, design of the project and experiments, analysis of data, writing, funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Mingle Li, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, H., Li, Z., Zhang, X. et al. Phthalocyanine aggregates as semiconductor-like photocatalysts for hypoxic-tumor photodynamic immunotherapy. Nat Commun 16, 326 (2025). https://doi.org/10.1038/s41467-024-55575-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-024-55575-2