Abstract
Flexible perovskite solar cells (F-PSCs) are appealing for their flexibility and high power-to-weight ratios. However, the fragile grain boundaries (GBs) in perovskite films can lead to stress and strain cracks under bending conditions, limiting the performance and stability of F-PSCs. Herein, we show that the perovskite film can facilely achieve in situ bifacial capping via introducing 4-(methoxy)benzylamine hydrobromide (MeOBABr) as the precursor additive. The spontaneously formed MeOBABr capping layers flatten the grain boundary grooves (GBGs), enable the release of the mechanical stress at the GBs during bending, rendering enhanced film robustness. They also contribute to the reduction of the residual strain and the passivation of the surface defects of the perovskite film. Besides, the molecular polarity of MeOBABr can result in surface band bending of the perovskite that favors the interfacial charge extraction. The corresponding inverted F-PSCs based on nickel oxide (NiOx)/poly(triaryl amine) (PTAA) hole transport bilayer reach a 23.7% power conversion efficiency (PCE) (22.9% certified) under AM 1.5 G illumination and a 42.46% PCE under 1000 lux indoor light illumination. Meanwhile, a robust bending durability of the device is also achieved.
Similar content being viewed by others
Introduction
The low-temperature processability of perovskite solar cells (PSCs) enables their fabrication on flexible substrates1,2. With the advantages of light-weight and mechanical durability, the flexible PSCs (F-PSCs) have better prospects for application in building integrated photovoltaics, portable and wearable electronic devices than the rigid ones3,4,5. Besides, they could be exceptionally appealing for indoor photovoltaic applications in the field of the Internet of Things (IoT)6. Therefore, continuous efforts have been made to develop high-performance F-PSCs, leading to a remarkable increase in power conversion efficiencies (PCEs) in the past two years7,8,9,10.
In addition to efficiency, the mechanic stability or bending stability (in general case), is another key figure-of-merit of F-PSCs. Ideally, the F-PSCs are expected to withstand thousands of bending cycles throughout their entire service life11,12. The overall mechanical stability of F-PSCs is closely related to the functional layers (i.e. flexible substrate, perovskite, and charge transport layers) and the interfaces of the device13. Among them, the perovskite layer has been intensely researched for its fundamental role in determining the performance of F-PSCs. The perovskite films used in PSCs are predominantly polycrystalline featuring the ubiquitous distribution of grain boundaries (GBs) across the film and resulting in inevitable risk for the long-term stability of F-PSCs14,15,16. On one hand, the GBs are the most vulnerable regions of the perovskite film, which could easily undergo stress and strain cracks under bending condition, leading to significant decrease in efficiency17,18,19,20. On the other hand, the cracks create direct pathways for moisture intrusion and ion migration, thus accelerating the degradation of F-PSCs21.
In general, two types of strategies have been commonly used to enhance the mechanical stability of F-PSCs: one approach involves improving the contact between the perovskite and the charge transport layers (CTLs) by either introducing functional interlayers or using novel CTLs22,23,24,25,26, the other strategy involves healing of the GBs by incorporating elastomers or crosslinkable polymers into the perovskite films11,18,19,27. The latter is a straightforward and effective way to release the stress at the GBs under bending condition. In this scenario, the integrity of the perovskite film could be interrupted to some extent, as these materials tend to aggregate across the entire GB between individual grains and they are typically insulating28,29. Therefore, the types and the corresponding addition amounts of these materials should be carefully scrutinized, otherwise, the device performance could be compromised.
Here, we report a method that can be facilely applied to achieve collective roles of the two strategies mentioned above. Specifically, an organic ammonium salt 4-(methoxy)benzylamine hydrobromide (MeOBABr) is introduced into the perovskite precursor solution, which renders spontaneously formed MeOBABr capping layers on both the top and bottom surfaces of the perovskite film. As a result, the residual strain in the perovskite film is reduced with the presence of MeOBABr soft buffer at the buried interface. When bending the perovskite film, the mechanical stress at the GBs could be considerably released. We attribute it mainly to the flattening of the grain boundary grooves (GBGs) as they are stress-concentrated regions under bending state. Besides, the capping layer can also passivate the surface defects of the perovskite film to reduce non-radiative recombination losses. Moreover, the surface potential of the resultant perovskite film is altered, resulting in favorable surface band bending for interfacial charge transfer. The inverted F-PSCs based on NiOx/PTAA hole transport bilayer achieved a 23.7% PCE (22.9% certified). Such an efficiency is among the best results reported for inverted F-PSCs. Notably, a 42.46% PCE was obtained with 1000 lux indoor light illumination, which could be the highest one for F-PSCs to date. The devices also demonstrated excellent operational and mechanical stability, which maintained 95% of the initial PCE after more than 800 hours of maximum power point (MPP) tracking, and retained over 80% of the initial efficiency after 10000 convex or concave bending cycles at a small curvature radius of 3 mm.
Results
MeOBABr modulated growth of perovskite film
Figure 1a presents the molecular structure and the calculated electrostatic potential (ESP) of MeOBABr. It can be seen that the negative charges accumulate dominantly on the bromide side, which might be correlated to the electron-pushing effect of the methoxymethyl group. The ESP analysis indicates that the MeOBABr could have strong ionic interaction with Pb2+ ions. We then calculated the binding energy between MeOBABr and Pb2+ ion and found it was higher than that of the commonly used solvents such as dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) (Supplementary Fig. 1), which means the colloidal status of the perovskite precursor solution could be easily changed with the presence of MeOBABr. As indicated by the dynamic light scattering (DLS) measurements (Supplementary Fig. 2), the size of the colloidal clusters in the precursor solution was increased after MeOBABr addition, which is beneficial for reducing the nucleus density and promoting the homogeneous crystal growth of the perovskite film (Supplementary Fig. 3 and Supplementary Fig. 4)30,31,32,33.
a Molecular structure and calculated electrostatic potential profile (ESP) of MeOBABr. b, c Top-view (b) and cross-section (c) SEM images of the control perovskite films. d, e Top-view (d) and cross-section (e) SEM images of the target perovskite films. f XRD patterns of the control and target perovskite films. g, h XPS spectra of Pb (g) and I (h) elements for the target and control perovskite films.
As compared in the surface scanning electron microscopy (SEM) images (Fig. 1b, c), the perovskite film without MeOBABr addition (control) is composed of crystals with an average grain size of ~200 nm and the grain size distribution is non-uniform. While the one with MeOBABr addition (0.5 mol%, the amount was preliminarily optimized, see Supplementary Note 1) is composed of larger crystals (Avg. ~300 nm) with more uniform size distribution (see the statistical grain size analysis in Supplementary Fig. 5). Besides, the MeOBABr-modified perovskite film (target) exhibits more even surface and better contact with the substrate than the control one (Fig. 1d, e and Supplementary Fig. 6).
As a type of bulky organic ammonium halide, MeOBABr is possible to form two-dimensional (2D) perovskite with PbI2 (Supplementary Fig. 7). However, we didn’t observe this case when it is used as an additive, since both the control and target perovskite films show identical X-ray diffraction (XRD) patterns (Fig. 1f) and absorbance spectra (Supplementary Fig. 8). While the target film should preserve higher crystallinity than the control one, regarding to XRD intensities of the main (001), (002) peaks and the SEM morphological results. Since the MeOBABr molecules are not incorporated into the perovskite lattice, they are most likely to accumulate at the grain boundaries and on the surface of the perovskite film (this issue will be discussed later). Along with the presence of MeOBABr, we observed the downshifts of the binding energies of the Pb 4 f and I 3 d peaks in their X-ray photoelectron spectroscopy (XPS) spectra (Fig. 1g, h) and this can be explained by the electron-donating role of MeOBABr when it interacts with the perovskite29,34. The chemical interaction between the MeOBABr and perovskite is expected to passivate the defects at the grain boundaries and on the film surface11,22.
In-situ bifacial passivation and effects on perovskite film
Then, we performed transmission electron microscopy (TEM) observation to characterize the microstructure of the target perovskite. As displayed in Fig. 2a, there are amorphous layers with thicknesses of several to tens of nanometers found at the grain boundaries between perovskite crystals, which should be assigned to the accumulated MeOBABr. In addition, the high-resolution TEM (HR-TEM) image of an individual perovskite crystal clearly shows the coverage of MeOBABr thin layer and proves that there is no 2D crystal structure formed at the perovskite/MeOBABr interface (Fig. 2b). Based on these results, we can further exclude the formation of new perovskite phase and confirm the MeOBABr molecules are expelled outside the perovskite crystals. With the help of time-of-flight secondary ion mass spectroscopy (TOF-SIMS), we managed to further determine the spatial distribution of MeOBABr in the perovskite film. Instead of a uniform distribution across the entire film thickness as one may expect, the MeOBABr can be only found on the bottom and top surfaces of the perovskite film (Fig. 2c) and this is further proved by the observation of thin amorphous layers on both surfaces of the perovskite film through focused ion beam (FIB) TEM cross-sectional characterization (Supplementary Fig. 9). For better understanding, we have illustrated microstructure of the target perovskite film in Fig. 2d.
a HR-TEM images of the target perovskite film. b Magnified HR-TEM images of the target perovskite film. Domain 1 and Domain 2 represent the crystalline and amorphous region, respectively. c ToF-SIMS depth profile of the target perovskite film deposited on ITO/NiOx/PTAA substrate. d Schematic illustration of microstructure of target perovskite film with spontaneously formed MeOBABr capping layers on both the top and bottom surfaces. e-h DFT simulations of a MeOBABr ligand on the perovskite top surface for the head (e) and double-end (f) connections, as well as on the perovskite bottom surface with a PTAA interface for the (g) head and (h) double-end connections. i Microstrain values calculated by ε function from GIXRD patterns of control and target perovskite films. ε = (dstrained (001) - dnon-strained (001))/dnon-strained (001). j Steady-state PL spectra of the control and target perovskite films. k TRPL decay curves of the control and target perovskite films. l Recorded TRMC traces of the control and target perovskite films.
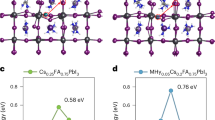
According to a recent study35, the MeOBA cation is likely to adsorb on the perovskite surface through single N-I bonding (head connection) or through both N-I and O-Pb bondings (double-end connection). We then performed density functional theory (DFT) calculations to illustrate the possible connection modes of MeOBABr on both top and bottom surfaces. Our results show that the adsorption energy of double-end connection is slightly higher than that of head connection on top surface (Fig. 2e, f), indicating a probability of co-existence of them. While at the bottom surface (with presence of PTAA, Fig. 2g, h), the adsorption energy of the double-end connection is about 25.6% higher than that of the head connection, considering the steric effect at the bottom (due to the limited space), the double-end mode could be more favorable.
As proposed in the literature36, materials with large molecular size are easy to be expelled outside the perovskite film and this could be the possible reason for the absence of them inside the perovskite (along the grain boundaries). However, we find this explanation is not always valid. Taking the analogue of MeOBABr, 4-(Trifluoromethoxy)benzylamine hydrobromide (F-MeOBABr) for example, its impacts on the crystallization and morphology of the perovskite film were similar to MeOBABr, while a continuous distribution of F-MeOBABr was found through the entire perovskite film (Supplementary Fig. 10) and its molecular size is even larger than MeOBABr. To understand this phenomenon, we also calculated the bonding between F-MeOBABr and the perovskite and found the corresponding adsorption energy is almost 3 times higher than that of MeOBABr (Supplementary Fig. 11), which means the F-MeOBABr could bond to the perovskite more tightly. Besides, the fluorinated group in F-MeOBABr can induce extra hydrogen bonding interaction with the perovskite37. And these should account for the continuous distribution of F-MeOMBABr. Our finding is expected to guide the future design or selection of functional materials with controlled spatial distribution in perovskite film.
We found the interesting distribution of MeOBABr could bring several advantages to the target perovskite film. On one hand, the presence of MeOBABr layer at the buried interface helps reduce the residual strain in perovskite due to its buffering role (Fig. 2i and Supplementary Fig. 12)38,39. On the other hand, referring to the interaction between MeOBABr and perovskite, the MeOBABr capping layer is anticipated to passivate the defects at the grain boundaries and on the surface of the perovskite film, such as the uncoordinated Pb2+ and I- vacancies40. As evidenced by the photoluminescence (PL), time-resolved PL (TRPL), and time-resolved microwave conductivity (TRMC) measurements (Fig. 2j-l), the stronger PL intensity, longer carrier lifetime, and higher carrier mobility were found for the target film than the control one, indicating the well-reduced defect density of the former38.
Besides, the surface potential (SP) of the perovskite film was found to be changed with the presence of polar MeOBABr capping layer (its molecular dipole moment was calculated to be 10.58 Debye). As presented in Supplementary Fig. 13, the Kelvin probe force microscope (KPFM) measurements revealed a larger average contact potential difference (CPD) at the top surface of the target film than that of the control, indicating a smaller work function of the former and this is further confirmed by the ultraviolet photoelectron (UPS) characterizations (Supplementary Fig. 14)14,41. These results indicate a downward surface band bending of the target film, which is reasonable according to the connection modes of MeOBABr shown above. As on the bottom surface, the abundance of head connection could result in total dipole pointing away from the perovskite (see illustration in Supplementary Fig. 15), which subsequently leads to reduced work function (or downward band bending) of the surface layer35,42. Such a band bending is beneficial for the charge extraction at the perovskite/ETL interface43. On the other hand, this type of band bending is supposed to be unfavorable at the bottom perovskite/HTL interface. Unfortunately, we failed to quantify the band change of the bottom surface through KPFM due to the unavoidable contamination of the perovskite film peeled off from the substrate (see Supplementary Fig. 16). However, the MeOBABr-induced surface bending should be less pronounced on the bottom surface due to the lack of vertical dipole as the double-end connection prevails. Therefore, the general role of the bifacial MeOBABr capping should be positive for interfacial charge transfer, as supported by the following characterizations of the real PSCs.
Photovoltaic property and mechanical stability of perovskite film
To illustrate the different photovoltaic performance of the two perovskite films, we first fabricated PSCs on rigid glass substrates with an inverted device structure shown in Supplementary Fig. 17a, b. In accordance with the improved perovskite film quality, the target PSC delivered a higher 24.65% PCE than that (22%) of the control device (Supplementary Fig. 17c, d). The improved efficiency is mainly ascribed to the increased open-circuit voltage (VOC) and fill factor (FF), which result from the suppressed recombination loss (Supplementary Fig. 18), owing to the effective defect passivation and energetically favorable surface band bending of the target film44.
Prior to fabricating F-PSCs, the mechanical stability of control and target perovskite films were examined via cyclic bending tests on flexible ITO/PET substrates. Figure 3 compares the surface morphologies of the two films after 10000 bending cycles with a curvature radius of 5 mm, from which cracks with widths up to hundreds of nanometers were found on both top and bottom surfaces of the control film after convex and concave bending, respectively. In contrast, the target film was just gently cracked and the cracks were even invisible in the low-magnification SEM image, indicating the greatly enhanced bending durability.
a Top-view SEM images of the top and bottom surfaces of control perovskite film after 10000 convex bending cycles at a curvature radius of 5 mm. b,c Schematic diagram of the control (b) and target (c) perovskite films in convex and concave bending conditions. The difference at GBs during bending indicates the possible working mechanism of MeOBABr capping. d Top-view SEM images of the top and bottom surfaces of target perovskite film after 10000 concave bending cycles at a curvature radius of 5 mm.
To understand the reasons behind the observed results, we performed nanoindentation measurements to determine the mechanical properties of the two perovskite films27. From the load-displacement characteristics (Fig. 4a), the film hardness (H) and effective elastic modulus (Eeff) could be obtained. Clearly, both smaller H and Eeff values were recorded for the target film (Fig. 4b, c), indicating its better flexibility than the control film. To confirm these results, we further conducted the peak force quantitative nanomechanical atomic force microscopy (PFQNM-AFM) measurements, which also revealed considerably reduced average Young’s modulus of the target film (Fig. 4d, e).
a-c Loading-unloading curves (a), instrument hardness (b), and instrument elastic moduli (c) of control and target perovskite films. d, e PFQNM images of control (d) and target (e) perovskite films. f, g 3D stress distribution diagram obtained from microstructure simulation of control (g) and target (h) perovskite films at a curvature radius of 5 mm. h, i 2D cross-sectional stress distribution diagram obtained from microstructure simulation of control (i) and target (j) perovskite films at a curvature radius of 5 mm.
In previous studies, improved mechanical stability of the perovskite film is generally achieved through the fixing of the grain boundaries across the entire film thickness7,24,45,46. Obviously, it is not the case in our work and we suppose the flattening of the GBGs could be the key reason for the excellent mechanical stability observed for the target perovskite film. To verify such a hypothesis, we carried out finite element analysis (FEA) to simulate the stress distributions in the perovskite films with normal and flattened CBGs under bending status (see methods in Supplementary Note 2, 3). According to the 3D simulation (Fig. 4f, g and Supplementary Fig. 19), the maximum stress within the target film is uniformly distributed along the bending end, while that within the control film is found at the grain boundaries in the region of the bending end (Supplementary Fig. 20). The 2D simulation further highlights the maximum stress is actually located at the GBG due to induced stress concentration (Fig. 4h, i). Both 3D and 2D simulations show significantly higher values of the maximum stress within control film, indicating its higher risk of cracking from the grain boundary under bending condition47. These results are consistent with our experimental observations (Fig. 3) and therefore strongly support our hypothesis. It should be noted that the difference between the 2D and 3D simulation results for the same sample can be attributed to the simplification of no stress/strain from the adjacent region in 2D simulations.
Photovoltaic performance and stability of F-PSCs
Subsequently, inverted F-PSCs were fabricated (Fig. 5a) with both control and target perovskite absorbing layers and Supplementary Fig. 21 shows the statistical photovoltaic performance (20 devices) of these two types of solar cells, where the target F-PSCs showed an obvious higher average PCE (23.22%) than the control devices (20.14%). Similar to the case of rigid PSCs, the improvement in PCE is also mainly due to the increased VOC and FF. Figure 5b presents the current density-voltage (J-V) curves of the best-performed control and target F-PSCs, the corresponding external quantum efficiency (EQE) spectra and the stabilized power output (SPO) curves are given in Supplementary Fig. 22. Negligible J-V hysteresis was observed for both devices and a best 23.72% PCE was determined for the target F-PSC, together with a high VOC of 1.17 V and a high FF of 0.846 (the detailed photovoltaic parameters of both devices are listed in Supplementary Table 1). Such an efficiency is one of the highest values reported for inverted F-PSCs, especially among those employing similar HTLs (Fig. 5c and Supplementary Table 2). The most efficient target F-PSCs have been sent out for certification and a 22.9% PCE was certified (Supplementary Fig. 23). Additionally, we would like to mention that a higher PCE over 24% could be obtained if the crystal size of the perovskite film was increased, while the corresponding mechanical stability of the device was compromised (see Supplementary Note 4). Moreover, our device has proved a potential for upscaling, as an over 20% PCE was obtained with a large active area of 1.2 cm2 (Fig. 5d and Supplementary Table 3). Notably, the MeOBABr capping layers could also be individually introduced at the bottom and the top of the perovskite film, which also improved the device performance to some extent, but still lagged behind the target PSC (Supplementary Fig. 24). It might be due to the rinse of the bottom MeOBABr layer during the deposition of the perovskite film, which highlights the advantage of the spontaneous capping strategy.
a Illustrated structure of F-PSCs. b The reverse and forward scanned J-V curves of the best-performed control and F-PSCs. c PCE evolution of p-i-n F-PSCs with different HTLs in recent years. d The representative J-V curves of the large-area (1.2 cm2) target device, inset shows a photograph of the device. e The MPP tracking of the unencapsulated control and target devices under continuous white light illumination (100 mW cm-2) in the atmospheric environment (30%). f Normalized PCE of the control and target devices measured after 1000 bending cycles with various bending radii. g Bending stability of F-PSCs with convex bending cycles at a curvature radius of 5 mm. h Bending stability of F-PSCs with concave bending cycles at a curvature radius of 5 mm.
The multifunctional bifacial MeOBABr capping not only leads to improved photo-conversion efficiency, but also results in prolonged shelf, thermal, and ultraviolet (UV) stability of the F-PSCs (Supplementary Fig. 25). Notably, the target F-PSC has demonstrated a T80 lifetime over 1000 h under UV irradiation, which is longer than all of the results reported previously (Supplementary Table 4), indicating promising prospect for outdoor applications. Such excellent UV stability can be attributed to the synergy of the improved perovskite film quality and the effective UV screen ability of the NiOx/PTAA bilayer48. Moreover, the unencapsulated target F-PSC was able to maintain over 95% of the initial PCE after 800 h maximum power point tracking (MPPT) under one sun illumination in air, while the control device lost over 50% of its efficiency during the same operation time (Fig. 5e). The prolonged operational stability of the target device is benefited from the flattened GBGs and surface passivation of the perovskite film20,35,49.
We further carried out cyclic bending tests on the completed devices. Owing to the robust mechanical stability of the MeOBABr modified perovskite film as identified above, the target F-PSC readily exhibited better bending stability than the control device at different curvature radiuses, especially at the small one (Fig. 5f). Importantly, the target F-PSCs were able to maintain 86% and 82% of the initial PCEs after 10000 convex and concave bending cycles, respectively. In contrast, the control F-PSCs only retained 68% and 57% of the PCEs after the same bending tests (Fig. 5g, h). It is noted that these tests were initially conducted without device encapsulation, in order to exclude the unintentional influences of the sealing materials. We then re-performed the bending stability tests with device encapsulation and the results are presented in Supplementary Fig. 26. It can be found that the encapsulated F-PSCs showed improved bending stability than the unencapsulated ones for the prevention of the chemical and physical damages from the outside45 and the target device was still better than the control one. The target device even preserved over 80% of the PCE after 10000 convex or concave bending cycles at a smaller curvature radius of 3 mm. The excellent bending durability of the F-PSCs achieved in this work is comparable to the best results reported in literatures (Supplementary Table 5), which highlights the effectiveness of our GBG-flattening strategy.
Indoor photovoltaic performance of F-PSCs
Finally, in consideration of the increasing demands of low-cost indoor photovoltaic devices for IoT applications (Fig. 6a) and the corresponding advantages of F-PSCs, we also characterized the photovoltaic performance of our devices under different indoor light intensities (Fig. 6b and Supplementary Fig. 27). Remarkably, we obtained a best-performed 42.46% PCE at 1000 lux illumination (Fig. 6c-e and Supplementary Table 6), which is so far, the record indoor efficiency reported for F-PSCs regardless of the device structure (Fig. 6f and Supplementary Table 7). Such an impressive indoor performance should also be attributed to the growth of high-quality, defect-less perovskite films facilitated by MeOBABr modulation. Notably, the perfect match between the EQE response of the F-PSCs and the indoor light spectrum (Fig. 6d) could also be the reason for the excellent photovoltaic performance. This leverages the shortcomings of the low transmittance of the flexible substrates in the short-wave region50, indicating that the F-PSCs are ideally suitable for indoor photovoltaics.
a Schematic illustration of various IoT devices powered by indoor PV. b The spectra of 3000 K LED light at 1000 lux, 500 lux, and 200 lux illuminances. c The J-V curves of F-PSCs measured under different indoor light illumination. d The EQE spectra and the integrated JSC of target flexible devices under 1000 lux. e Histograms showing the target flexible device efficiencies of 20 cells under 1000 lux, 500 lux and 200 lux. f PCE evolution of F-PSCs under indoor light illumination in recent years.
Discussion
In summary, we proposed a feasible strategy to facilitate the access to efficient and mechanically robust F-PSCs. The introduction of MeOBABr as a precursor additive was observed to trigger the subsequent in situ bifacial capping of the perovskite film, resulting in more flattened GBGs in the film. Specially, we have proven the significant importance of GBGs flattening on strengthening the mechanical robustness of the perovskite film, as it could greatly reduce the stress at the GBs under bending condition. Besides, the presence of MeOBABr capping layers on both sides displayed multifunctional roles in reducing the interfacial residual strain, suppressing non-radiative recombination losses, and promoting interfacial charge transports. By employing the NiOx/PTAA bilayer HTL, the fabricated F-PSCs achieved a certified PCE of 22.9%. Moreover, a PCE of 42.46% was obtained under 1000 lux indoor light illumination, potentially the highest reported for F-PSCs to date. The resulted devices also demonstrated excellent operational and mechanical stability. This study offers an approach to promote the development of high-performance F-PSCs and highlights the bright prospect for the future deployment of light-weight solar power.
Methods
Materials
DMF (99.9%), DMSO (99.9%) and chlorobenzene (CB, 99.9%) were purchased from Sigma-Aldrich. Isopropyl alcohol (IPA, 99.7%), anhydrous ethanol (99.5%), toluene, 4-(Methoxy)benzylamine, 4-(Trifluoromethoxy) benzylamine, hydrobromic acid, petroleum ether, Nickel nitrate hexahydrate (Ni(NO3)2·6H2O), Lead iodide (PbI2, ultra-dry, 99.999%) and Bathocuproine (BCP, 96%) were obtained from Alfa Aesar. Tetrabutylammonium hydroxide (TBAOH) was purchased from Innochem. Flexible ITO/PET, ITO/glass, formamidinium iodide (FAI), cesium iodide (CsI), and phenyl-C61-butyric acid methyl ester (PC61BM, 99.9%) were bought from Advanced Election Technology. Lead bromide (PbBr2, 99.999%), methylammonium bromide (MABr), and PTAA were purchased from Xi’an Yuri Solar Co., Ltd. All starting materials were obtained from commercial suppliers without further purification.
Synthesis of NiOx nanoparticles (NPs)
The Ni(NO3)2·6H2O (50 mmol) was dissolved in 50 mL deionized water. Subsequently, 50 mL TBAOH was added dropwisely under vigorous stirring. The resulting precipitates were collected by centrifugation and washed with deionized water 4 times to remove residual TBAOH. The washed precipitates were vacuum-dried at 100 °C for 12 h, followed by sintering at 270 °C for 2 h to obtain NiOx NPs.
Synthesis of 4-(methoxy)benzylamine hydrobromide (MeOBABr)
4-(Methoxy)benzylamine (0.69 g, 5 mmol), and hydrobromic acid (0.4 g, 5 mmol), were dissolved in 20 mL of anhydrous ethanol. The resulting solution was degassed with a nitrogen stream and the mixture was stirred at 0°C for 2 h under a nitrogen atmosphere. After the removal solvent, the crude product was washed with petroleum ether several times to afford a white solid. Then this white solid was dissolved in ethanol and petroleum ether and recrystallized to obtain white flake crystals (0.89 g, 81.2%).
Synthesis of 4-(Trifluoromethoxy)benzylamine hydrobromide (F-MeOBABr)
4-(Trifluoromethoxy) benzylamine (0.95 g, 5 mmol), and hydrobromic acid (0.4 g, 5 mmol), were dissolved in 20 mL of anhydrous ethanol. The resulting solution was degassed with a nitrogen stream before and the mixture was stirred at 0°C for 2 h under a nitrogen atmosphere. After the removal solvent, the crude product was washed with petroleum ether several times to afford a white solid. Then this white solid was dissolved in ethanol and petroleum ether and recrystallized to obtain white flake crystals (1.12 g, 83%).
Device fabrication
ITO/PET and ITO/glass were ultrasonically cleaned with deionized water, ethanol, isopropyl alcohol, and ethanol for 10 min, and then dried by flowing nitrogen (N2) and treated with ultraviolet ozone for 10 min. To fabricate flexible devices, The NiOx NPs were dispersed in deionized water and IPA (4:1) to prepare 5 mg/mL NiOx solution and filtered with a 0.45 µm nylon filter. The NiOx solution was spin-coated on the ITO/PET substrates at 4000 rpm for 30 s and annealed on a hotplate at 100 °C for 30 min in air. PTAA (1.5 mg/mL in Toluene) was coated on NiOx at 6000 rpm for 40 s and then annealed at 100 °C for 10 min. For deposition of perovskite layer, The (1.4 M) perovskite precursor solution was constructed by mixing PbI2, PbBr2, FAI, MABr, and CsI in a mixed solvent (DMF/DMSO = 4/1) according to a formula of Cs0.05(FA0.95MA0.05)0.95Pb(I0.95Br0.05)3. A trace amount of benzylamine hydrobromide (0.5 mol% MeOBABr and 0.2 mol% F-MeOBABr) was added into the perovskite precursor solution. The precursor solution was dripped onto HTL-coated substrates and spin-coated at 5000 rpm for 30 s, 150 μL CB as anti-solvent was dripped on the film at 18 s from the start and then annealed at 100 °C for 30 min. After that, the PC61BM (1.5 mg/mL in CB) was coated on the perovskite surface at 5000 rpm for 30 s and annealed at 100°C for 10 min, then the BCP solution (0.5 mg/mL in IPA) was spin-coated at 4000 rpm for the 30 s on the PCBM layer. Finally, 200 nm thickness of Ag electrode was deposited by thermal evaporation as an electrode using a shadow mask. The preparation method of rigid PSCs is the same as that of flexible PSCs, except that ITO/glass was used as the substrate.
Preparation of (MeOBA)2PbI2Br2 film
PbI2 and MeOBABr (1/2 by molar, 1.0 M) precursor in DMF and DMSO (4:1 by molar) was deposited on the glass substrate at 5000 rpm for 30 s and then annealed at 100°C for 30 min to get (MeOBA)2PbI2Br2 film.
Material and device characterization
The XRD and Grazing Incidence X-ray Diffraction (GIXRD) analysis were obtained with Smartlab 9 kW X-ray diffractometer and with Cu Kα (λ = 1.5406 Å) radiation source. To better understand the morphological changes at the buried interface, we peeled off the perovskite films with UV curable glue and examined the morphology of the exposed bottom surface. The morphology of samples was acquired by field-emission scanning electron microscope (FE-SEM, Hitachi SU8010). The XPS and UPS characterizations were performed on a Thermo Fisher ESCALAB 250XI (USA), with -5 V bias for UPS measurement. The UV-Vis absorption spectra were obtained from a UV-2600 spectrophotometer (Shimadzu, Japan). Measurements were made on a Zetasizer Nano ZSP (Malvern Instruments, Malvern UK) using a detection angle of 173° at a temperature of 25 °C. The Nano ZSP uses a 10 mW He-Ne laser operating at a wavelength of 633 nm. The perovskite TEM sample was prepared by directly spin-coating the diluted perovskite precursor solution onto the copper grid, according to a method we have previously used51. TEM lift-out samples were prepared via FIB polishing using a Tescan AMBER FIB-SEM instrument. The TEM and HRTEM images were taken on JEM-2100 (HR), JEOL Co.Ltd. The time-of-flight secondary ion mass spectrometry (ToF-SIMS) characterization was performed under a TOFSIMS5 (IONTOF GmbH, Germany) instrument. The atomic force microscopy (AFM, Bruker Dimension ICON) analysis was used to measure the surface roughness of perovskite films and instrumented KPFM and PFQNM of perovskite films. The PL and TRPL spectra were obtained by DW-PLE03 (Fluo Time 300) with a pulsed excitation laser of 485 nm. The TRMC was investigated using Keysight FieldFox spectrum analyzer (N9915A) with a nanosecond OPO laser (LS-2145-OPO-PC, Lotis-tii). The indentation stress-strain curves of perovskite films were measured using a Hysitron TI 950 TriboIndenter with a triangular Berkovich tip. The electrochemical impedance spectra (EIS), transient photo-current (TPC) decay, transient photovoltage (TPV), and Capacitor-voltage (C-2-V) were recorded by electrochemical workstation (Zahner, Germany) with a white light LED supplied 80 mW cm-2 light intensity to excite the perovskite solar cells. Electroluminescent spectra (EL) measurement was gained from an external quantum efficiency (EQE) system (Enli Technology Co. Ltd.) The maximum-power-point-tracking (MPPT) stability test was conducted by irradiating the perovskite solar cells under white light (100 mW cm-2). The current density-voltage (J-V) curves of the perovskite solar cells were obtained by a Keithley 2400 source under AM 1.5 G simulated irradiation (100 mW cm-2) from a solar simulator (Newport 94023 A) and then obtain the indoor J-V curve by altering the illuminance to 1000, 500 and 200 lux using the illuminance meter (ENLITECH) with fluorescent lamps (ILS-30).
Calculation details
First-principles calculations were performed with the projector augmented plane-wave method and the generalized gradient approximation by Perdew, Burke, and Ernzerhof as the exchange-correlation potential52. The long-range van der Waals interaction was described by the DFT-D3 approach53. To calculate the surface adsorption, FAPbI3 supercells were cut along the crystalline plane (001). A vacuum layer of 15 Å was created perpendicular to the revealed perovskite surface to avoid potential interactions between periodic cells. The Brillouin zone integration was sampled with a 2 × 2 × 2 k-point grid with an energy cutoff of 400 eV. The convergence criteria of energy and force during structure relaxation were 1×10-4 and 0.05 eV/Å, respectively. Multiwfn54 and VESTA55 were utilized for model preparation and visualization.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this study are available in the article and supplementary information. Source data are provided in this paper. Source data are provided with this paper.
References
Song, F. et al. Mechanical durability and flexibility in perovskite photovoltaics: advancements and applications. Adv. Mater. 36, 2312041 (2024).
Liu, J. et al. Evolutionary manufacturing approaches for advancing flexible perovskite solar cells. Joule 8, 944–969 (2024).
Tai, Q. & Yan, F. Emerging semitransparent solar cells: materials and device design. Adv. Mater. 29, 1700192 (2017).
Tang, G. & Yan, F. Recent progress of flexible perovskite solar cells. Nano Today 39, 101155 (2021).
Liu, J. et al. A bioinspired flexible neuromuscular system based thermal-annealing-free perovskite with passivation. Nat. Commun. 13, 7427 (2022).
Liu, S. et al. Perovskite smart windows: the light manipulator in energy‐efficient buildings. Adv. Mater. 36, 202306423 (2023).
Xie, L. et al. Molecular dipole engineering-assisted strain release for mechanically robust flexible perovskite solar cells. Energy Environ. Sci. 16, 5423–5433 (2023).
Xu, W. et al. Multifunctional entinostat enhances the mechanical robustness and efficiency of flexible perovskite solar cells and minimodules. Nat. Photonics 18, 379–387 (2024).
Liu, J., Ye, T., Yu, D., Liu, S. & Yang, D. Recoverable flexible perovskite solar cells for next‐generation portable power sources. Angew. Chem. Int. Ed. 62, 202307225 (2023).
Tong, X. et al. Large orientation angle buried substrate enables efficient flexible perovskite solar cells and modules. Adv. Mater. 36, 2407032 (2024).
Kang, Y. et al. Ionogel-perovskite matrix enabling highly efficient and stable flexible solar cells towards fully-R2R fabrication. Energy Environ. Sci. 15, 3439–3448 (2022).
Meng, X. et al. Bio-inspired vertebral design for scalable and flexible perovskite solar cells. Nat. Commun. 11, 3016 (2020).
Zhang, J., Zhang, W., Cheng, H.-M. & Silva, S. R. P. Critical review of recent progress of flexible perovskite solar cells. Mater. Today 39, 66–88 (2020).
Wang, Y. et al. Utilizing electrostatic dynamic bonds in zwitterion elastomer for self-curing of flexible perovskite solar cells. Joule 8, 1–22 (2024).
Chen, C. H. et al. Full‐dimensional grain boundary stress release for flexible perovskite indoor photovoltaics. Adv. Mater. 34, 202200320 (2022).
Han, B. et al. Rational design of ferroelectric 2D perovskite for improving the efficiency of flexible perovskite solar cells over 23%. Angew. Chem. Int. Ed. 62, 202217526 (2023).
Li, X. et al. Progress and challenges toward effective flexible perovskite solar cells. Nano-Micro Lett. 15, 206 (2023).
Lu, Z. et al. Grain‐slip derived network topology to remarkable strength–toughness combination of perovskite film for flexible solar cells. Adv. Energy Mater. 12, 2202298 (2022).
Chen, Z. et al. Perovskite grain-boundary manipulation using room-temperature dynamic self-healing “Ligaments” for developing highly stable flexible perovskite solar cells with 23.8% efficiency. Adv. Mater. 35, 2300513 (2023).
Hao, M. & Zhou, Y. Grain-boundary grooves in perovskite solar cells. Joule 8, 913–921 (2024).
Hu, X. et al. Cementitious grain-boundary passivation for flexible perovskite solar cells with superior environmental stability and mechanical robustness. Sci. Bull. 66, 527–535 (2021).
Gao, D. et al. Highly efficient flexible perovskite solar cells through Pentylammonium Acetate modification with certified efficiency of 23.35%. Adv. Mater. 35, 2206387 (2023).
Gong, C. et al. An enhanced couette flow printing strategy to recover efficiency losses by area and substrate differences in perovskite solar cells. Energy Environ. Sci. 15, 4313–4322 (2022).
Dong, Q. et al. Flexible perovskite solar cells with simultaneously improved efficiency, operational stability, and mechanical reliability. Joule 5, 1587–1601 (2021).
Jiang, N. et al. Transfer-imprinting-assisted growth of 2D/3D perovskite heterojunction for efficient and stable flexible inverted perovskite solar cells. Nano Lett. 23, 6116–6123 (2023).
Liu, H. et al. Semi-planar non-fullerene molecules enhance the durability of flexible perovskite solar cells. Adv. Sci. 9, e2105739 (2022).
Han, T. H. et al. Spontaneous hybrid cross‐linked network induced by multifunctional copolymer toward mechanically resilient perovskite solar cells. Adv. Funct. Mater. 32, 2207142 (2022).
Meng, X. et al. A biomimetic self‐shield interface for flexible perovskite solar cells with negligible lead leakage. Adv. Funct. Mater. 31, 2106460 (2021).
Xie, L. et al. A deformable additive on defects passivation and phase segregation inhibition enables the efficiency of inverted perovskite solar cells over 24%. Adv. Mater. 35, 2302752 (2023).
Wu, X. et al. Realizing 23.9% flexible perovskite solar cells via alleviating the residual strain induced by delayed heat transfer. ACS Energy Lett. 8, 3750–3759 (2023).
Zhu, P. et al. Aqueous synthesis of perovskite precursors for highly efficient perovskite solar cells. Science 383, 524–531 (2024).
McMeekin, D. P. et al. Crystallization kinetics and morphology control of formamidinium–cesium mixed‐cation lead mixed‐halide perovskite via tunability of the colloidal precursor solution. Adv. Mater. 29, 1607039 (2017).
Wu, Y. et al. In situ crosslinking-assisted perovskite grain growth for mechanically robust flexible perovskite solar cells with 23.4% efficiency. Joule 7, 398–415 (2023).
Fei, C. et al. Lead-chelating hole-transport layers for efficient and stable perovskite minimodules. Science 380, 823–829 (2023).
Kong, L. et al. Fabrication of red-emitting perovskite LEDs by stabilizing their octahedral structure. Nature 631, 73–79 (2024).
Zheng, X. et al. Co-deposition of hole-selective contact and absorber for improving the processability of perovskite solar cells. Nat. Energy 8, 462–472 (2023).
Chen, H. et al. Quantum-size-tuned heterostructures enable efficient and stable inverted perovskite solar cells. Nat. Photonics 16, 352–358 (2022).
Zhou, Y. et al. Defect-less formamidinium Sn–Pb perovskite grown on a fluorinated substrate with top-down crystallization control for efficient and stable photovoltaics. Energy Environ. Sci. 17, 2845–2855 (2024).
Zhou, Y. et al. Manipulation of the buried interface for robust Formamidinium-based Sn-Pb perovskite solar cells with NiOx hole-transport layers. Angew. Chem. Int. Ed. 62, 202300759 (2023).
Chen, B., Rudd, P. N., Yang, S., Yuan, Y. & Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 48, 3842–3867 (2019).
Yan, Y. et al. Polarity and moisture induced trans-grain-boundaries 2D/3D coupling structure for flexible perovskite solar cells with high mechanical reliability and efficiency. Energy Environ. Sci. 15, 5168–5180 (2022).
Canil, L. et al. Tuning halide perovskite energy levels. Energy Environ. Sci. 14, 1429–1438 (2021).
Zhu, H. et al. In situ energetics modulation enables high-efficiency and stable inverted perovskite solar cells. Nat. Photonics https://doi.org/10.1038/s41566-024-01542-8 (2024).
Guo, T. et al. Designed p-type graphene quantum dots to heal interface charge transfer in Sn-Pb perovskite solar cells. Nano Energy 98, 107298 (2022).
Lee, G. et al. Ultra-flexible perovskite solar cells with crumpling durability: toward a wearable power source. Energy Environ. Sci. 12, 3182–3191 (2019).
Liu, S. et al. Effective Passivation with Size‐Matched Alkyldiammonium Iodide for High‐Performance Inverted Perovskite Solar Cells. Adv. Funct. Mater. 32, 2205009 (2022).
Xue, T. et al. A shape memory scaffold for body temperature self‐repairing wearable perovskite solar cells with efficiency exceeding 21%. InfoMat 4, e12358 (2022).
Cui, X. et al. NiOx nanocrystals with tunable size and energy levels for efficient and UV stable perovskite solar cells. Adv. Funct. Mater. 32, 2203049 (2022).
Hao, M. et al. Flattening grain‐boundary grooves for perovskite solar cells with high optomechanical reliability. Adv. Mater. 35, 2211155 (2023).
Tian, R., Zhou, S., Meng, Y., Liu, C. & Ge, Z. Material and device design of flexible perovskite solar cells for next‐generation power. Adv. Mater. 36, 2311473 (2024).
Tai, Q. et al. Antioxidant grain passivation for air-stable tin-based perovskite solar cells. Angew. Chem. Int. Ed. 58, 806–810 (2019).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Lee, K., Murray, É. D., Kong, L., Lundqvist, B. I. & Langreth, D. C. Higher-accuracy van der Waals density functional. Phys. Rev. B 82, 081101 (2010).
Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 161, 082503 (2024).
Momma, K. & Izumi, F. VESTA 3for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Acknowledgements
We acknowledge the support from National Natural Science Foundation of China (No. 61974106, No. 52103229), Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515110632). The authors thank Dr Xiaodong Zhou, Dr Ye Ma and Dr Ying Zhang from the Core Facility of Wuhan University for their assistance with AFM, KPFM, PFQNM measurements and data analysis. The authors thank Shiyanjia Lab for the support of 3D Schematic diagram.
Author information
Authors and Affiliations
Contributions
Conceptualization: Q.T., H.M. and J.J. Supervision: Q.T., H.M., Z.H., Z.C. and S.L. Device fabrication: J.J. and Z.Z. Device characterization and data analysis: J.J., Z.Z., Y.M., Y.Z., J.S., S.W., X.C., T.G. D.Z., G.T., Q.L., J.L. and X.L. Finite element analysis simulation: Z.C. Writing - original draft: Q.T. and J.J. Writing - discussion, review, editing and finalization: Q.T., J.J., H.M., Z.H. and Z.C. All authors were involved in the discussion of the results and provided feedback on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shangfeng Yang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, J., Zhu, Z., Ming, Y. et al. Spontaneous bifacial capping of perovskite film for efficient and mechanically stable flexible solar cell. Nat Commun 16, 90 (2025). https://doi.org/10.1038/s41467-024-55652-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-55652-6
This article is cited by
-
Engineered Radiative Cooling Systems for Thermal-Regulating and Energy-Saving Applications
Nano-Micro Letters (2026)