Abstract
In this study, we introduce a highly effective non-metallic iodine single-atom catalyst (SAC), referred to as I-NC, which is strategically confined within a nitrogen-doped carbon (NC) scaffold. This configuration features a distinctive C-I coordination that optimizes the electronic structure of the nitrogen-adjacent carbon sites. As a result, this arrangement enhances electron transfer from peroxymonosulfate (PMS) to the active sites, particularly the electron-deficient carbon. This electron transfer is followed by a deprotonation process that generates the peroxymonosulfate radical (SO5•−). Subsequently, the SO5•− radical undergoes a disproportionation reaction, leading to the production of singlet oxygen (1O2). Furthermore, the energy barrier for the rate-limiting step of SO5•− generation in I-NC is significantly lower at 1.45 eV, compared to 1.65 eV in the NC scaffold. This reduction in energy barrier effectively overcomes kinetic obstacles, thereby facilitating an enhanced generation of 1O2. Consequently, the I-NC catalyst exhibits remarkable catalytic efficiency and unmatched reactivity for PMS activation. This leads to a significantly accelerated degradation of pollutants, evidenced by a relatively high observed kinetic rate constant (kobs ~ 0.436 min−1) compared to other metallic SACs. This study offers valuable insights into the rational design of effective non-metallic SACs, showcasing their promising potential for Fenton-like reactions in water treatment applications.
Similar content being viewed by others
Introduction
Given the capacity to produce highly reactive oxygen species (ROS) over a wide pH range, PMS-based advanced oxidation processes (PMS-AOPs) featuring easy handling, storage, and transport of oxidant have emerged as promising technologies for water decontamination1,2,3. The activation of PMS typically commences with the adsorption of a PMS molecule onto a catalyst’s surface4,5, which subsequently catalyzes the formation of various reactive species, including radicals (e.g., hydroxyl radicals, sulfate radicals, and superoxide radicals)6,7 and nonradicals (e.g., 1O2 and high-valent metal-oxo species)8,9,10,11. However, the efficacy of radical species is compromised by the interference of anions and organic matter presenting in waters, which largely reduces PMS utilization and may lead to the formation of undesirable halide byproducts12,13. In contrast, nonradical pathways offer selective and robust oxidation mechanisms9,14,15, especially in the case of 1O2 that allows for selective degradation of contaminants due to its specificity, longevity, and resistance to matrix components16.
Leveraging the benefits of SACs, such as maximal atomic efficiency, defined active sites, tunable electronic structures, and heightened activity, they have gained great attention in various domains, including electrocatalysis, chemical synthesis, and environmental remediation17,18,19,20. Notably, the critical role of SACs in elucidating the structure-activity relationship at an atomic level underscores the potential in PMS-AOPs9,21,22. Distinguished by the nature of the active sites, metallic SACs (M-SACs) have shown promise in transforming PMS to 1O2 for micro-pollutant degradation23,24. However, the high production costs, complexity in synthesis, and risk of secondary contamination25,26,27 of metallic SACs encourage us to design the non-metallic SACs (NM-SACs) for water treatment, which are economically and environmentally more viable.
At present, the investigation regarding NM-SACs is nascent, primarily due to the difficulties in ascertaining active sites and elucidating catalytic pathways. Yet, pioneering works on NM-SACs unveil their potential in modulating electronic structures for the enhancement of Fenton-like reactivity. Yang et al. developed a NM-SAC composed of high-density, isolated phosphorus atoms anchored at the edge of graphene as a robust electrocatalyst for the CO2 reduction28. Nevertheless, because of the limited ability to increase the catalysts’ intrinsic activity, phosphorus atom-doped catalysts are less used in PMS-AOPs29. Generally, there are several non-metallic elements in this category, including fluorine, chlorine, bromine and iodine (I), that can perform as effective candidates for PMS-AOPs. Among them, the I atom possesses several notable advantages. Primarily, the atomic structure of I is clearly distinct from that of carbon, and the incorporation of I can effectively modulate the electronic structure of the neighboring carbon matrix. Subsequently, the addition of I can enhance the conductivity of the support, thereby facilitating efficient electron transfer between the catalyst and PMS30. Moreover, the incorporation of I atom will lead to more defects due to its larger atomic radius than carbon, thereby exposing more active sites and enhancing the mass transfer of PMS molecules effectively31. Finally, the larger atomic mass of I atom allows for the improved identification of its active sites at the atomic scale using advanced techniques such as aberration-corrected high-angle annular dark-field scanning transmission electron microscopy and X-ray absorption fine spectroscopy, thereby facilitating a more thorough establishment of the structure-activity relationship of the catalyst32. Due to its unique properties, I emerges as a strong candidate for enhancing the Fenton-like activity of carbon-based materials.
This study presents a potent atomically dispersed I-doped nitrogen-carbon catalyst (referred to as I-NC), synthesized through chemical vapor deposition, which demonstrates effectiveness in activating PMS for water treatment applications. Notably, the incorporation of I significantly enhances the electronic structure of the carbon-based materials, thereby improving the conversion efficiency of PMS at the active electron-deficient carbon sites and boosting its Fenton-like catalytic performance. The predominant presence of 1O2 species in oxidation reactions, validated through comprehensive characterizations and theoretical analyses, highlights the catalyst’s underlying mechanism and efficacy. The impressive performance and remarkable durability of I-NC position it as a leading option in single-atom catalysis for practical water treatment, setting a benchmark for catalyst design and application in PMS-AOPs.
Results and discussion
Fabrication of the I-NC catalyst and structure characterization
The I-NC catalyst with atomically dispersed I was synthesized using a chemical vapor deposition (CVD) technique. Initially, ZIF-8 frameworks, formed by the assembly of 2-methylimidazole and zinc nitrate, were subjected to pyrolysis in an inert atmosphere to produce nitrogen-doped carbon (NC) substrates. The successful synthesis of these substrates was confirmed through X-ray diffraction (XRD) patterns, as illustrated in Supplementary Fig. 1. Subsequently, the NC substrate placed in a tube furnace to initiate the CVD process, utilizing NH4I as both an etchant and an I source (Fig. 1a). Specifically, a porcelain boat containing NC was situated centrally within the tube furnace, with NH4I positioned at the upstream end to ensure exposure to the evolving gas flow. The annealing was conducted in an inert environment, leading to the decomposition of NH4I and the release of ammonia (NH3), hydrogen iodide (HI), and iodine vapor (I2), which interact with the NC. This interaction promotes the formation of I-NC single-atom catalysts with carbon-iodine (C-I) moieties, thereby augmenting the intrinsic activity32.
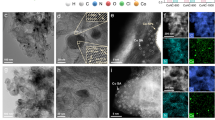
a Schematic illustration of the preparation of atomically dispersed I-doped nitrogen-carbon catalyst (denoted as I-NC). The yellow sphere represents the largest van der Waals sphere that would fit in the cavity. b, c A high-resolution high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of I-NC. Yellow circles indicate single iodine atoms. d 3D atom-overlapping Gaussian-function fitting map of a region in inset (c). e Energy-dispersive X-ray spectroscopy (EDS) elemental mappings (C, N, I, and Zn) of I-NC.
As depicted in Supplementary Fig. 2, the scanning electron microscopy analysis reveals that the I-NC catalysts largely retain the original rhombic dodecahedral shape characteristic of ZIF-8, with an average particle size ranging between 300 and 500 nm and a consistent size distribution. Transmission electron microscopy (TEM) images, shown in Supplementary Fig. 3a, b, indicate that the surface of I-NC appears notably rougher compared to NC. This observation suggests that the NH3 and HI gases released during NH4I decomposition can uniformly etch the carbon matrix to promote the anchor of I single-atom. High-resolution TEM (HRTEM) investigations (Supplementary Fig. 3) confirm the absence of discernible crystal lattices, indicating that no large nanoparticles are presented in the I-NC framework. To delineate the atomic-level dispersion of I within I-NC, aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) was employed. As shown in Fig. 1b, c, the I atoms exhibited a brighter contrast due to their higher atomic number compared to Zn atoms and are uniformly scattered across the I-NC surface. Furthermore, using the advanced three-dimensional (3D) atom-overlapping Gaussian-function fitting mapping technique, the area marked by a purple square in Fig. 1c can be reconstructed into Fig. 1d, where the isolated non-metallic I atoms are clearly distinguished within the matrix of the I-NC catalyst. Complementary energy-dispersive X-ray spectroscopy (EDS) mapping, showcased in Fig. 1e, corroborates the homogeneous distribution of I and nitrogen (N) across the carbon substrate. Collectively, these findings affirm the successful introduction and atomic dispersion of I on the N-doped carbon substrates.
The X-ray powder diffraction (XRD) pattern of I-NC (Supplementary Fig. 4) displays two pronounced peaks at the 2θ of approximately 21.6° and 44.2°. These peaks are assigned to the (002) and (101) planes of graphitic carbon, respectively, and show a high degree of similarity to the corresponding peaks in NC. This resemblance suggests that the fundamental graphitic structure is preserved in I-NC. To further probe the microstructural attributes of NC and I-NC, Raman spectroscopy was conducted and the results are presented in Supplementary Fig. 5. We identify two distinct peaks at roughly 1349 and 1598 cm−1, which are attributed to the D-band and G-band of carbon-based materials, respectively. Notably, the intensity ratio of the D-band to G-band (ID/IG) for I-NC registers a marginal increase compared to NC. This increment is indicative of a reduced degree of graphitization, aligning with the observations of etching on the carbon surface revealed through TEM analysis. Collectively, these spectroscopic insights corroborate the structural modifications induced by NH4I treatment and provide a microscopic understanding of the altered properties of I-NC.
The electron paramagnetic resonance (EPR) spectrum for I-NC, shown in Fig. 2a, displays a pronounced peak signal at a g-value of 2.005, indicative of enhanced carbon vacancies within the NC bulk structure33. These vacancy defects are expected to enhance the mass transfer of reactant molecules and promote further I atoms incorporation. Further pore structure and surface area analysis were conducted by measuring nitrogen adsorption-desorption isotherms via Brunauer-Emmett-Teller (BET) methodology (Supplementary Fig. 6). I-NC exhibits a typical type-I isotherm, suggesting a microporous-dominant structure, in alignment with the pore size distribution outcomes. Notably, I-NC features significantly improvement in BET surface area (1373 m2 g−1) and pore volume (0.59 cm3 g−1) when compared with NC (913 m2 g−1 and 0.40 cm3 g−1). These enhancements presumably optimize the exposure of catalytic sites and promote the diffusion of reactant molecules (e.g., ROS, CIP).
a The electron paramagnetic resonance (EPR) spectra of NC and I-NC. b High-resolution X-ray photoelectron spectroscopy (XPS) N 1s spectra of NC and I-NC. c High-resolution XPS I 3d spectra of I-NC. The relevant XPS fitting parameters of N 1s and I 3d5/2 are listed in Supplementary Table 3. d Normalized I K-edge X-ray absorption near edge structure (XANES) spectra of Iodine, Iohexol, and I-NC. e I K-edge Fourier-transformed extended X-ray absorption fine structure (EXAFS) spectra for Iodine, Iohexol, and I-NC. Purple region denotes the characterized peaks of I-C both in NC and I-NC. f I K-edge EXAFS fitting analysis for I-NC. g–i Wavelet transform EXAFS plots of iodine, iohexol, and I-NC.
The compositional and electronic structures of NC and I-NC were thoroughly investigated using X-ray photoelectron spectroscopy (XPS), providing insights into the surface elemental compositions and chemical states. The XPS survey spectra (Supplementary Fig. 7) revealed comparable levels of carbon (C), N, and oxygen (O) across both catalysts. High-resolution N 1s spectra for NC and I-NC could be deconvolved into four distinct peaks corresponding to various N configurations: pyridinic N (at ~398.4 eV), pyrrolic N (at ~399.5 eV), graphitic N (at ~400.9 eV), and oxidized N species (~403.5 eV), as displayed in Fig. 2b, and Supplementary Fig. 8 and summarized in Supplementary Table 2 and Table 3. A notable enhancement in the graphitic N content was observed for I-NC, registering at 48.0%, an increase from NC at 42.9%. This predominance of graphitic N in I-NC, especially when compared to other nitrogen species, underscores its potential role in enhancing Fenton-like catalytic activities. Furthermore, the I-NC spectrum features a new peak at approximately 620 eV, attributing to I element. The detailed analysis of the I 3d5/2 region in I-NC’s XPS spectrum (Fig. 2c) reveals deconvolution into two peaks at 618.58 and 620.46 eV, corresponding to ionic (C-I+-C) and covalent (C-I) iodine species, respectively. This observation supports the integration of C-I bonding configurations, corroborated by a specific peak at 285.6 eV in the XPS C 1s spectrum (Supplementary Fig. 9), affirming the successful introduction and chemical interaction of I within the I-NC structure.
The electronic structure and atomic configuration of I-NC were further elucidated using I K-edge X-ray absorption fine spectroscopy (XAFS). In Fig. 2d, the X-ray absorption near edge structure (XANES) profiles of I-NC and references reveal a notable resemblance in the absorption edge of I within I-NC to that observed for iohexol with model C-I bonds (Supplementary Fig. 10). This similarity indicates that the oxidation state of I in I-NC is between elemental iodine and iohexol, suggesting a mixed valence of I. Further insights were obtained through the Fourier-transformed extended X-ray absorption fine structure (EXAFS) analysis, detailed in Fig. 2e, f and Supplementary Fig. 11. These results highlight the absence of distinct peaks corresponding to I–I bonding at 2.71 Å, affirming the premise that I atoms are integrated within the I-NC matrix as isolated entities. This isolation supports the assertion of single-atom coordination, aligning with the anticipated enhancement of catalytic activity and providing a cogent explanation for the observed physicochemical properties of I-NC. Additional examination of the XAFS data for I-NC, particularly the peak observed at 2.0 Å, corroborates the presence of C-I coordination, which is akin to that identified in iohexol. An additional discernible peak in the I-NC spectrum can be ascribed to the C-I and Zn-I backscattering pathways. The detection of Zn-I interactions suggests potential direct coordination between I and Zn within the NC structure or the formation of zinc iodide (ZnI2) as a consequence of iodine vapor etching, a hypothesis supported by the absence of a washing step post-etching. However, it should be noted that the Zn-I feature is not predominant, which aligns with the inference that I within the material predominantly exists in a positively charged state, more evidences were provided later. Further substantiation is provided by the EXAFS wavelet transform (WT) analysis depicted in Fig. 2g–i, affirming the nonexistence of I–I bonding within I-NC. This finding is congruent with observations from HAADF-STEM investigations. Moreover, EXAFS fitting results suggest the mean coordination number around C-I in I-NC mirrors observed in iohexol, reinforcing the atomically dispersed nature of I sites within the catalyst (Supplementary Fig. 12 and Table 4).
Catalytic Performance and Identification of ROS
The Fenton-like catalytic performance of I-NC was evaluated by activating PMS for the degradation of ciprofloxacin (CIP). Initially, an examination of the impact of various operating parameters was carried out, illustrated in Supplementary Figs. 13–15. Specifically, the optimal concentration of PMS was determined to be 1 mM while the catalyst dosage was screened at 0.04 g·L−1. The most effective secondary annealing temperature in the CVD process for fabricating I-NC was found to be 850 °C. The results presented in Fig. 3a demonstrate that CIP was nearly completely degraded by the I-NC/PMS system within 10 min. In contrast, only about 35% of CIP degradation was observed with the NC/PMS system. This significant difference underscores the superior Fenton-like catalytic activity of the I-NC system. The liquid chromatography-mass spectrometry (LC-MS) chromatograms of CIP before and after the reaction in the I-NC/PMS system further confirmed that CIP was virtually eliminated within 10 min (Supplementary Fig. 16). In addition, PMS alone only degraded less than 10% of CIP, and around 40% of CIP was removed by I-NC through adsorption, indicating that catalytic oxidation played a major role in the rapid elimination of CIP. Notably, the leaching of 83.2 µg L−1 of iodine ions from the I-NC/PMS system contributed to about 6% degradation rate of CIP, indicating that the leached iodine ions had a minimal impact on the degradation process (Supplementary Fig. 17).
a The degradation of ciprofloxacin (CIP) in different catalysts-activated PMS systems. b Comparison of I-NC and reported catalysts in a Fenton-like reaction. The relevant references are listed in Supplementary Table 5. c EPR spectra using 5,5-dimethyl−1-pyrroline-N-oxide (DMPO) and 2,2,6,6-tetramethylpiperidine (TEMP) as trapping agents. d Linear sweep voltammetry (LSV) curves of NC and I-NC under different conditions. e In situ Raman spectra of PMS, I-NC and their reaction. The cyan, purple and yellow regions represent the characteristic peaks of SO42−, SO3(HSO5−) and PMS*, respectively. f The 1O2 yield in different catalyst/PMS/CIP systems. g Degradation of multiple pollutants in the I-NC/PMS system. The name and chemical structure of relevant micropollutants are listed in Supplementary Fig. 33. h The oxidation mechanism of 1O2 for multiple micropollutants. The blue and yellow surfaces represent positive isosurface and negative isosurface, respectively. white, Grey, blue, red, yellow, and cyan spheres represent H, C, N, O, S, and F atoms, respectively. Conditions: [catalyst] = 0.04 g L−1, [PMS] = 1 mM, [pollutants]0 = 20 mg L−1, [TEMP] = [DMPO] = 50 mM (if any). Error bars denote standard deviation of the experiments performed in triplicate. Source data are provided as a Source Data file.
CIP degradation can be well described by pseudo-first-order kinetics. The kinetic constant (kobs) of I-NC/PMS (0.436 min−1) was 10.1 times higher than that of NC/PMS (0.043 min−1), and 11.4 ~ 48.4 times greater than those of commercial carbonaceous materials. Notably, the kobs of I-NC outperformed most of the state-of-the-art catalysts with effective PMS activation on CIP degradation (Supplementary Fig. 18). Additionally, given that kobs may vary with reaction conditions and the type of organic substances, a normalized kinetic model (k-value) was employed. This model adjusts the kobs by dividing it by the concentration of PMS and the catalyst dosage, and multiplying by the concentration of the pollutant, to uniformly assess the removal rates across different heterogeneous Fenton-like systems34. As illustrated in Fig. 3b and summarized in Supplementary Table 5, the k-value for I-NC (217.75 min−1·M−1) surpassed those of all compared metal-free catalysts (0.23 to 3.48 min−1·M−1) in terms of PMS activation for CIP degradation and was higher than most leading-edge metallic catalysts, for example, 14.35 min−1·M−1 for Co-N3O1.
The results underscored that I-NC significantly outperforms NC in Fenton-like catalytic activity, highlighting the effectiveness of the NH4I etching method. As depicted in Supplementary Fig. 19a, a direct relationship is evident between the Fenton-like catalytic activity and factors such as surface area, pore volume, and the ID/IG ratio. This correlation suggests that NH4I etching successfully modified the pore structure of NC, which enhanced the number of active sites and improved their accessibility. Remarkably, a 6.8-fold enhancement in the specific surface area normalized kobs value (k per surface area) was still attained, indicating a more advantageous intrinsic catalytic activity of I-NC (Supplementary Fig. 19b). Intriguingly, the degradation characteristics of I-NC did not align with the variations in Zn concentration, as illustrated in Supplementary Fig. 20. This indicates that Zn plays a negligible role in the effectiveness of the I-NC/PMS system. Additionally, the secondary calcination of NC in an inert atmosphere without NH4I, referred to as NC-blank, did not significantly enhance CIP degradation performance. This indicates that the secondary calcination process alone does not substantially affect the intrinsic active sites of NC, as supported by the data in Supplementary Fig. 21. Previous studies35,36 have shown that carbon-based materials can undergo effective etching to introduce defects through NH3 heat treatment. Consequently, urea was chosen as the NH3 source for treating NC. The experimental results revealed that the CIP degradation efficiency in the NC-urea/PMS system exhibited only a slight improvement over that of NC alone. Moreover, despite the addition of extra iodine ions, minimal improvement was observed in the NC-urea and iodine ions/PMS system, further demonstrating the limited influence of dissolved iodine ions in PMS-mediated catalytic reactions (Supplementary Fig. 21). This finding highlights the critical role of iodine single-atom in enhancing PMS activation and facilitating the degradation of CIP.
Various reactive species, including •OH, SO4•−, O2•−, and 1O2, have been identified in carbon-based catalyst/PMS processes11,37,38. To investigate the specific reactive species generated during PMS activation with NC and I-NC, scavenging experiments were conducted using methanol (MeOH) and tert-butyl alcohol (TBA) as radical scavengers. Specifically, MeOH and TBA were employed as radical scavengers of •OH and/or SO4•−39. The presence of overdosed MeOH and TBA scarcely hindered CIP degradation (Supplementary Fig. 22), suggesting that •OH and SO4•− were likely not the primary active species in the NC/PMS and I-NC/PMS. As MeOH and TBA cannot quench surface-bound radicals, dimethyl sulfoxide (DMSO) and NaF were employed to explore the contribution of radicals on the surface of I-NC40,41. As illustrated in Supplementary Fig. 23, DMSO and NaF had a limiting effect on the degradation of CIP in the I-NC/PMS system, suggesting that surface-bound radicals contribute little to the degradation of CIP. Additionally, the introduction of chloroform (CHCl3), with a high-rate constant for reacting with O2•− of 3.0 × 1010 M−1 s−1 42,43, caused only a slight decrease in CIP degradation. This result indicates that O2•− also did not play a significant role in degrading CIP. Additionally, nitroblue tetrazolium dichloride (NBT) was employed as a probe for O2•−, as shown in Supplementary Fig. 24, the negligible influence of NBT suggested the minimal involvement of O2•− in the I-NC/PMS system. In stark contrast, the introduction of furfuryl alcohol, with a known rate constant with 1O2 of 1.2 × 108 M−1 s−1 44, significantly impeded the degradation of CIP. This remarkable inhibition points to a critical role of 1O2 in the degradation process, aligning with the observed selective oxidation behavior towards various micropollutants in the I-NC/PMS. As depicted in Supplementary Fig. 25, the similar quenching effects observed in both NC and I-NC when using L-histidine, NaN3 and 2,2,6,6-tetramethylpiperidine (TEMP) as quenchers suggest that these catalysts share a common mechanism for PMS activation and micropollutant degradation. This further emphasizes the dominance of 1O2 in these reactions45,46.
Considering that carbon-based materials can act as electronic mediators to facilitate electron transfer from pollutants to PMS, thereby oxidizing pollutants with the independence of ROS. To validate the contribution of electron transfer to CIP degradation, a series of experiments including electrochemical measurement, premixing experiment, and PMS decomposition experiment were conducted. As shown in Supplementary Fig. 26, the open circuit potential (OCP) of glassy carbon electrodes coated with NC or I-NC increased immediately after the addition of PMS, indicating that the active PMS complexes formed on the catalyst surface can elevate the potential8,47. Nevertheless, there was no discernible drop in potential with the addition of CIP, suggesting that the catalyst-mediated electron transfer process for pollutants to the catalyst-PMS complexes is negligible48,49. Furthermore, to further verify the existence of the catalyst-mediated electron transfer process, the catalyst and PMS were mixed in the blank solution, and the CIP-containing solution was added at 0, 3, 6, and 9 min subsequently. As depicted in Supplementary Fig. 27, we observed an extended premixing time resulted in a more significant decrease in CIP degradation efficiency, with only 55% of CIP being degraded with kobs of 0.062 min−1 after a 9-min premixing period in the I-NC/PMS system. This outcome further suggests that the electron transfer process might not play a significant role in CIP degradation under these conditions. Additionally, existing studies indicate that the decomposition of PMS often depends on organic substrates acting as electron donors in the electron transfer mechanism50. However, when CIP was introduced as a potential electron donor, there was no observed acceleration in PMS consumption, as illustrated in Supplementary Fig. 28. This lack of impact indicates that CIP does not facilitate the PMS decomposition via electron transfer, contrasting with the expected behavior if electron transfer were a predominant degradation pathway in the I-NC/PMS.
The referenced studies provide evidence that catalyst-mediated electron transfer processes are not significant contributors to the I-NC/PMS activity. To reinforce this conclusion, the research utilized 9,10-diphenylanthracene (DPA) as a specific probe to detect the presence of 1O2 in the system24. DPA interacts with 1O2, undergoing oxidation to 9,10-diphenylanthracene dioxide (DPAO2), which is accompanied by a decrease in the absorption peak intensity around 380 nm. The observed reduction in this peak, as shown in Supplementary Fig. 29, confirms the generation and involvement of 1O2 in the CIP degradation process. Additionally, the study employed 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) and TEMP as trapping agents to probe for other reactive species, specifically •OH and SO4•−, and 1O2, respectively. The utilization of these trapping agents in Fig. 3c aimed to differentiate the reactive species involved and further ascertain the dominant role of 1O2 in the degradation mechanism, unlike the involvement of other radical species in I-NC/PMS. Furthermore, the EPR experiments revealed significant insights into the reactive species responsible for CIP degradation within the I-NC/PMS. The EPR spectra clearly showed a distinctive peak attributed to 5,5-dimethylpyrroline-N-oxide (DMPOX), indicating that DMPO undergoes deep oxidation, potentially caused by the rapid production of hydroxyl radicals, high-valent metal-oxo species, or 1O251. Given the exclusion of free radicals as the dominant active species and the catalyst’s multivalent metal-free nature, we can speculate that 1O2 is responsible for the formation of DMPOX. Moreover, the experiment utilizing TEMP as a trapping agent revealed a distinct signal for 1O2-TEMP adducts. This signal was significantly more intense in the I-NC/PMS system compared to the NC/PMS system, reinforcing the idea that 1O2 plays a crucial role in the catalytic degradation of CIP by I-NC. The presence of this 1O2-TEMP adduct provides strong evidence that 1O2 is a dominant oxidative species in the I-NC/PMS system, effectively participating in the degradation process. The accumulation of experimental evidence indicates that 1O2 plays a pivotal role in the I-NC/PMS and NC/PMS, with I-NC exhibiting superior catalytic performance compared to NC/PMS. The significant reduction in the intensity of the 1O2-TEMP adduct peak upon introducing CIP into the I-NC/PMS system reinforces the conclusion that 1O2 is instrumental in degrading CIP (Supplementary Fig. 30). Additionally, the results from the CHCl3 quenching experiment and the NBT probe, along with the TEMP-trapping EPR experiment, support the conclusion that 1O2 in the system does not stem from O2•−. The observation that gas aeration with argon (Ar) and oxygen (O2) does not influence CIP elimination (Supplementary Fig. 31), suggesting that dissolved oxygen is not a precursor to the 1O2 generated in these reactions. Hence, the derived inference is that 1O2 is produced through the interaction between PMS molecules and the catalyst’s active sites.
The reaction process characterization of the Fenton-like system
Considering PMS’s dual role as both an oxidant and reductant, the electron transfer pathways between PMS and the catalyst are essential for the generation of 1O2. When electrons are transferred from PMS to the catalyst, this process triggers the dissociation of the O-H, resulting in the formation of the SO5•− (Eq. 1). This radical subsequently leads to the generation of 1O2 through disproportionation reaction (Eq. 2)8. Conversely, when electrons are transferred from the catalyst to PMS, this initiates the cleavage of the O-O bond to produce *OH, subsequently dissociated into *O, which then combine with another intermediate to ultimately generate 1O252. To discriminate the electron transfer process between I-NC and PMS, electrochemical tests were carried out. In linear sweep voltammetry analyses (Fig. 3d), the current density of both the I-NC electrode and NC electrode increased significantly in PMS presence, with the increment of the former being much greater than that of the latter, showcasing an enhanced electron transfer from PMS to I-NC compare to NC53,54. Moreover, the current observed in the system increased significantly following the addition of CIP, indicating a favorable reaction among PMS, CIP, and the catalysts. Additionally, the OCP of glassy carbon electrode coated with NC and I-NC catalysts exhibited an immediate increase upon the addition of PMS. Notably, the increase in OCP for the I-NC catalyst was 0.54 V, which was substantially greater than the 0.45 V increase observed with the NC catalyst (Supplementary Fig. 26). This difference further supports the notion of effective electron transfer from PMS to the active sites on the catalysts55.
To further investigate the electron transfer process between I-NC and PMS, the Raman spectroscopy was conducted (Fig. 3e). The spectra revealed three distinct peaks in the PMS solution ([H-OI-OII-SO3]−1, located at 880, 980, and 1059 cm−1), corresponding to the stretching vibrations of O-O, SO42−, and SO3, respectively. Notably, the peaks associated with the O-O and SO3 peaks showed a marked decrease upon the addition of I-NC over time, indicating a rapid conversion of PMS to SO42− following activation by I-NC. In addition, the adsorption of PMS on catalysts is crucial for its activation. The attachment of PMS molecules to the catalyst surface can lead to the formation of a metastable complexes, denoted as *PMS. This process was evidenced by the appearance of new peaks at 1110 and 1145 cm−1 in the Raman spectra, suggesting the presence of the *PMS complex8. Notably, the characteristic vibration of SO3 appeared significant blue shift (1052 cm−1). The blue shift typically derives from the decrease in electron density, suggesting the donation of electrons from PMS to catalysts56. Subsequently, PMS is converted into the SO5•−, which then leads to the generation of 1O2. These findings suggest that the electron transfer from PMS to the active sites occurs first, followed by deprotonation to form SO5•−. This radical then undergoes a disproportionation process, resulting in the production of 1O2. Furthermore, the incorporation of I optimizes the local electronic configuration of NC, facilitating the electron transfer from PMS to catalysts and thereby enhancing the forming of 1O2.
Moreover, changes in pH were monitored before and after reaction process, as depicted in Supplementary Fig. 32. The gradual decline in the solution’s pH value reaffirms the efficient generation of 1O2 coupled with proton release (Eq. 3). This observation further rules out the predominant role of free radical pathway that produces •OH maintaining pH or generates SO4•− in conjunction with an increasing pH. Notably, we can calculate the theoretical value of 1O2 generation using Eq. 3 and the observed variations in pH. The 1O2 generated by I-NC activated PMS was found to be 0.22 mM, which is 2.59 times higher than that generated by NC (Fig. 3f). This result further indicates that the addition of I atoms effectively modifies the electronic structure of the material, enhancing its inherent catalytic activity and improving the efficiency of 1O2 generation efficiency.
1O2 serves as an electrophilic agent, readily participating in electrophilic addition toward electron-rich pollutants. Consequently, the PMS activation system catalyzed by I-NC can selectively eliminate a wide range of pollutants. In particular, the degradation rates of electron-rich compounds such as CIP, carbamazepine (CBZ), phenol (Ph), bisphenol A (BPA), 4-chlorophenol (4-CP) and tetracycline (TC) exceeded 90% within a 10-min duration, with the exception of sulfamethoxazole (SMX), which achieved approximately 73.5%. Conversely, electron-deficient containments, including nitrobenzene (NB), benzoic acid (BA) and chloramphenicol (CPL) exhibited significantly lower degradation efficiencies, all falling below 20% (Fig. 3g). This selective reactivity highlights the effectiveness of the I-NC activated PMS system in targeting specific types of pollutants, as illustrated in Supplementary Fig. 33. The difference of 1O2 oxidation ability toward different selected organics were further explored through the perspective of the charge transfer pathway, as depicted in Fig. 3h. A smaller energy gap between lowest unoccupied molecular orbital (LUMO) of 1O2 and highest occupied molecular orbital (HOMO) of the pollutants represents that electrons migration from the pollutants to 1O2 can be more readily initiated. This facilitates and accelerates the oxidation process targeted at the specific pollutants. Thus, the efficiency of 1O2 in oxidizing various organic compounds is closely linked to the energetic compatibility of their molecular orbitals57. It was clearly observed that the HOMO of electron-rich contaminants such as CIP (−4.72 eV), TC (−4.86 eV), BPA (−4.91 eV), CBZ (−5.25 eV), Ph (−5.27 eV), 4-CP (−5.32) and SMX (−5.49 eV) were slightly lower than the LUMO of 1O2 (−4.50 eV). This energy alignment indicates that 1O2 can readily withdraw electrons from HOMO of these electron-rich contaminants, facilitating their degradation. Conversely, the LUMO of 1O2 was substantially higher than the HOMO energy levels of CPL (−6.22 eV), BA (−6.33 eV) and NB (−6.59 eV). This substantial energy difference demonstrates that it is challenging for 1O2 to perform oxidation reactions on these electron-deficient compounds, further highlighting the selective reactivity of 1O2 based on the electronic structure of the pollutants. In summary, CIP undergoes a rapid degrading effectiveness in the I-NC/PMS system compared to the 10 representative pollutants examined, due to a narrower gap between LUMO of 1O2 and HOMO of CIP, facilitating more efficient electron transfer and subsequent oxidation. The degradation intermediates of CIP were identified by LC-MS as shown in Supplementary Fig. 34. Specifically, the m/z = 332 peak corresponding to CIP appeared at a retention time of 7.5 min. Additionally, six degradation intermediates were identified, which together suggest a possible degradation pathway for CIP, as shown in Supplementary Fig. 35. This pathway provides insights into the transformation of CIP in the I-NC/PMS system, highlighting the complexity and effectiveness of the degradation process.
Theoretical study of the catalytic mechanism
As validated by previous findings58,59, the different types of N species exhibit different activation mechanisms in non-metal active site-mediated PMS activation process, and graphitic N-doped carbonaceous catalysts are favorable for PMS activation to generate 1O2. Here we found a higher content of graphitic N compared to pyridinic and pyrrolic N in the catalysts, associating this with the dominance of 1O2 as the active species in both NC/PMS and I-NC/PMS. This suggests a crucial role of graphitic N in the PMS activation process dominated by non-radical pathway. To further substantiate this conjecture, computational models of various carbon forms, including pristine graphene and N-doped configurations (graphitic N, pyridinic N, and pyrrolic N), were created, and their optimal geometric configurations are presented in Supplementary Fig. 36. DFT calculations indicated that the adsorption energy (Ead) of PMS on graphitic N sites is significantly more negative (−1.60 eV) than on pristine graphene (−1.11 eV), pyridinic N (−0.80 eV), or pyrrolic N (−0.33 eV). This finding underscores the superior electron density and reactivity of graphitic N sites, establishing them as preferential for PMS interaction, as depicted in Fig. 4a37,60. As a result, the graphitic N-doped carbon structure was chosen as the theoretical model for understanding and optimizing the NC catalyst’s activity.
a The adsorption energy of PMS on pristine graphene, pyridinic N, pyrrolic N and graphitic N. b Schematic diagram of I-NC configuration. c Charge density distribution images of N-doped carbon models with different configurations of graphitic N, I-NC. d The adsorption energy of PMS on N sites and C sites of graphitic N, graphitic N-D, and I-NC models. e Proposed reaction process for PMS oxidation to 1O2 on I-NC catalysts. f Potential energy profiles of the reaction pathway for generating the 1O2 species. Graphitic N-D model represent graphitic N with defect. Grey, blue, purple, red, yellow, and white spheres represent C, N, I, O, S, and H atoms, respectively. g Diagrammatic representation of the CIP degradation mechanism in the I-NC/PMS system. The purple region and dashed area in Fig. 4g denote I-NC’s active site and I-NC-PMS complex, respectively. Source data are provided as a Source Data file.
We further performed the DFT calculations to elucidate the foundation of the I-NC excellent performance, which aimed to explore the impact of I doping on the electronic structures of the carbon framework, as well as its influence on the catalytic characteristics relevant to PMS activation. Drawing on structural characterization, feasible models for graphitic N with defect (graphitic N-D) and I-NC with defect were constructed. The optimal geometrical configurations of these models are depicted in Fig. 4b and Supplementary Fig. 37a, providing a visual representation of the molecular structures that facilitate an understanding of the enhanced catalytic mechanism. Furthermore, the charge density distribution images (Fig. 4c and Supplementary Fig. 37b) reveal that the introduction of defects and the doping with I substantially reconfigure the electronic structure of the carbon framework. This modification is made to significantly influence the activation of PMS. Recent studies37,61,62 have highlighted that carbon atoms with electronic deficiency can serve as active sites for the activation of PMS, leading to the formation of the SO5•− radical. This step is a crucial rate-limited step and pivotal for the generation of 1O2. Thus, the adsorption of PMS on carbon sites with the lowest electron density (Supplementary Fig. 38) and on nitrogen sites with the highest electron density were analyzed. The results indicated that the Ead at carbon sites on the catalysts is more negative compared to those on nitrogen sites (Fig. 4d). This suggests that PMS is more likely to adsorb onto electron-deficient carbon atoms which is in accordance with previous experimental results on the electron transfer process between the catalyst and PMS. Moreover, the Ead value for PMS adsorption on I-NC (−1.88 eV) is more negative than on graphitic N-D (−1.76 eV) and graphitic N (−1.66 eV). This confirmed that I doping can led to localized charge distributions, break the symmetry of surface electron distribution, create an electron-deficient region, further enhance the electron transfer of PMS to electron-deficient carbon sites, and consequently increase the generation of 1O2 and enhance the Fenton-like catalytic activity.
Therefore, the process of PMS oxidation to generate 1O2 triggered by I-NC was proposed, as depicted in Fig. 4e. Initially, the PMS molecule preferentially adsorbs onto the sites with electron-deficient carbon, leading to the formation of *HSO5−. Following this, the adsorbed PMS undergoes activation and deprotonation, followed by the dissociation of the O-H bond to generate *SO5•−. The final step involves the disproportionation of *SO5•−, which ultimately enables the production of 1O2. Furthermore, in order to validate this view, the energy barriers associated with the pathways for the generation of 1O2 in graphitic N/PMS, graphitic N-D/PMS, and I-NC/PMS were calculated (Fig. 4f). Notably, the PMS is first represented by the negatively charged HSO5−, and therefore, the charge was considered a background parameter, with no additional charge added when constructing the model containing either HSO5 or SO555,63,64. Briefly, PMS adsorbs onto electron-deficient carbon sites, forming *HSO5 and releasing energy in the process. The I-NC released more energy (1.840 eV) compared to the graphitic N (1.593 eV) and graphitic N-D (1.747 eV), indicating that the adsorption of PMS onto I-NC is more likely to occur in an exothermic and spontaneous manner. Furthermore, the formation of the intermediate transition state of *SO5 (step II) represents the step with the highest energy barrier. This step is considered the rate-determining step for the systems involving graphitic N, graphitic N-D, and I-NC alike. In addition, the observed lower energy barrier for the I-NC (1.45 eV) compared to graphitic N (1.65 eV) and graphitic N-D (1.54 eV) suggested that I doping facilitates a pathway with a relatively low energy barrier for both the adsorption and deprotonation of PMS during the generation of 1O2. To summarize, the incorporation of I into defect sites effectively modulates the electronic structure of carbon sites adjacent to graphitic N. This modulation leads to a lower energy barrier for the generation of 1O2 and enhances the kinetics of the Fenton-like reaction. Consequently, the I-NC configuration demonstrates significantly higher intrinsic Fenton-like catalytic activity compared to the NC (Fig. 4g).
Potential practicability of I-NC
The practical application of catalysts is crucial for addressing the issue of water pollution. However, transitioning Fenton-like catalysts from laboratory settings to industrial plants is challenging due to the presence of complex inorganic ions and organic components in natural water bodies. These substances may compete with the target pollutants for consuming ROS. Notably, Fig. 5a illustrates that the removal efficiency of CIP by I-NC/PMS was only marginally affected by typical anions (i.e., NO3−, Cl−, and SO42−), cations (i.e., K+, Ca2+, and Mg2+) and DOM (i.e., HA). This observation suggests that the 1O2-mediated degradation system exhibits a high resistance to the influence of inorganic ions and electron-deficient pollutants. Furthermore, the I-NC/PMS demonstrated excellent performance across a broad initial pH range (from 3.1 to 10.9), achieving more than 95% removal of CIP (Fig. 5a and Supplementary Fig. 39). This result confirms that both the I-NC and the 1O2 active species possess a strong tolerance to variations in the solution pH. The rapid kinetics (~82% elimination within 30 s) observed in the removal of CIP at a pH of 10.9 can be attributed to the activation of PMS under highly alkaline conditions (i.e., pH greater than 10.0)65. The viability of the I-NC/PMS was further evaluated across various water matrices, with water quality data provided in Supplementary Table 6. The process demonstrated superior removal rates of CIP in different types of natural waters. Achieving about 97% removal in tap water, groundwater, lake water, and river water. Additionally, it showed significant effectiveness in real wastewater, with approximately 91% removal achieved in hospital wastewater. This highlights the improvement of resistance to counteract common anions frequently found in water. Furthermore, the system maintained high decontamination activity during five consecutive running cycles (Fig. 5b), although a slight performance decline occurred during prolonged operation due to catalyst passivation by the oxidation intermediates. As illustrated in Supplementary Fig. 40. a continuous-flow reactor was subsequently developed to showcase the significant potential for the practical application of the I-NC in long-term operation (flow rate of 20 mL h−1). Impressively, I-NC demonstrated impressive capability in the continuous treatment of CIP over a period of 30 days of consecutive operation. Specifically, the removal efficiency of CIP was maintained at approximately 100%, as shown in Fig. 5c. Notably, due to the potent catalytic activity of I-NC and the abundant graphite N adsorption sites, a remarkable mineralization efficiency was achieved in the removal of CIP, with over 90% TOC removal within the first 20 days. The observed decrease in efficiency, with more than 51% TOC removal in the subsequent 10 days, could be attributed to the inactivation of some active sites by the CIP intermediates. Furthermore, we conducted a comprehensive analysis of the catalysts before and after five cyclic tests, including XPS spectra and HAADF-STEM characterizations. As shown in Supplementary Figs. 41 and 42, the XPS spectra of I-NC catalyst exhibited no significant changes before and after use. Additionally, the HAADF-STEM and associated EDS mapping confirmed that no aggregation occurred for either N or I sites, further demonstrating the excellent structural stability of I-NC catalyst. In summary, the I-NC’s powerful activity and durability underscore its vast potential for real-world environmental remediation applications.
a The removal efficiency of I-NC activated PMS systems in the presence of different ions, different humic acid (HA) concentrations, different initial pH, and different natural waters. Conditions: [catalyst] = 0.04 g L−1, [PMS] = 1 mM, [CIP]0 = 20 mg L−1, [K+] = [Mg2+] = [Ca2+] = [Cl–] = [NO3–] = [SO42–] = [PO43–] = 5 mM (if any). Each data point in Fig. 5a is independent of the other. b CIP abatement in five consecutive cycles in the I-NC/PMS system. c CIP concentration and TOC in the effluent from a continuous-flow reactor consisting of the I-NC-filled column, conditions: catalyst dosage = 200 mg, [PMS]0 = 1 mM, [CIP]0 = 10 mg L−1, flow rate = 20 mL h−1. Error bars denote standard deviation of the experiments performed in triplicate. Source data are provided as a Source Data file.
In summary, the CVD process with NH4I etching was developed to synthesize a distinctive well-dispersed I single-atom anchored onto N-doped carbon substrates. DFT calculations revealed that the electronic structure of the graphitic N adjacent to carbon could be optimized by the construction of C-I coordination in the non-metallic I single-atom. This unique coordination configuration facilitates the electron transfer from PMS to active sites and reduces the energy barrier for the formation of 1O2, thereby enhancing the Fenton-like activity of I-NC catalyst. Moreover, the I-NC catalyst exhibits great Fenton-like oxidation efficiency in terms of high oxidation selectivity and anti-interference capability for the degradation of micropollutants. Column experiments have demonstrated the great potential of I-NC for real-world applications, showcasing its ability to continuously and effectively remove CIP and TOC over a 30-day period. Our work opens an avenue for the design and synthesis of non-metallic single atom-doped carbon-based catalysts for highly efficient Fenton-like reactions.
Methods
Preparation of catalysts
The specific chemicals and materials utilized are detailed in Supplementary Note 1 in the Supporting Information. The synthesis procedures for ZIF-8 and NC are elaborated in Supplementary Note 2. For preparing I-NC, the following methodology was employed: NC underwent a secondary annealing process wherein a porcelain boat containing NC was positioned at the center of a tube furnace. This setup was heated to 850 °C and maintained for 1 h under an argon (Ar) atmosphere. Concurrently, another porcelain boat containing ammonium iodide (NH4I), serving both as an etchant and an I source, was placed at the tube furnace’s upstream end, where the temperature was approximately 550 °C. Following the secondary annealing and subsequent cooling phases, the I-NC product was obtained and required no further processing. In the case of the NC-blank control sample, its preparation mirrored that of I-NC with the exception that NH4I was omitted during the secondary annealing phase. Similarly, the NC-urea control sample was prepared using a protocol akin to that for I-NC, except that NH4I was substituted with urea during the secondary annealing step. Additionally, the preparations of the I-NC-1000 and I-NC-acid control samples were identical to that of the I-NC sample. The NC synthesis was accomplished by altering the calcination temperature (i.e., annealing at 1000 °C) and acid washing, respectively.
Degradation experiments
To evaluate catalytic efficiency, batch experiments were conducted using 100 mL conical flasks, each containing 50 mL of CIP solution (20 mg L−1). The pH levels of the suspensions were meticulously adjusted to range from 3.1 to 10.9 utilizing 0.1 mol L−1 NaOH and HCl solutions. For the degradation process, 0.04 g L−1 of the I-NC catalyst was introduced into the CIP solution, and subsequently activated by the addition of PMS (1 mM) under consistent mechanical stirring at 400 rpm. At predetermined time intervals, approximately 0.5 mL samples were extracted, promptly stabilized with roughly 1 mL of methanol solution, and subsequently filtered through a 0.45 μm water-compatible membrane filter. The filtered samples were analyzed using high-performance liquid chromatography (model LC-20 AT, Shimadzu). Tap water samples were obtained from our laboratory. Groundwater samples were obtained from Baihe Spring, Yuelu Mountain. Lake water samples were taken from the Taozi Lake. Hospital wastewater samples were taken from a hospital in changsha, China. TOC in the water samples was measured on an Elementar liquiTOC II Total Organic Carbon analyzer. Anion concentration in water samples is detected by anion chromatography (Dionex Integrion HPIC, USA).
Data availability
The data supporting the findings of the study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Wu, Q.-Y., Yang, Z.-W., Wang, Z.-W. & Wang, W.-L. Oxygen doping of cobalt-single-atom coordination enhances peroxymonosulfate activation and high-valent cobalt–oxo species formation. Proc. Natl. Acad. Sci. USA 120, e2219923120 (2023).
Wang, C. et al. Metal–organic frameworks and their derived materials: emerging catalysts for a sulfate radicals-based advanced oxidation process in water purification. Small 15, 1900744 (2019).
Guo, Z.-Y. et al. Crystallinity engineering for overcoming the activity–stability tradeoff of spinel oxide in Fenton-like catalysis. Proc. Natl. Acad. Sci. USA 120, e2220608120 (2023).
Zhang, D. et al. Dynamic active-site induced by host-guest interactions boost the Fenton-like reaction for organic wastewater treatment. Nat. Commun. 14, 3538 (2023).
Miao, J. et al. Spin-state-dependent peroxymonosulfate activation of single-atom M–N moieties via a radical-free pathway. ACS Catal. 11, 9569–9577 (2021).
Zhou, Q. et al. Generating dual-active species by triple-atom sites through peroxymonosulfate activation for treating micropollutants in complex water. Proc. Natl. Acad. Sci. USA 120, e2300085120 (2023).
Yu, X. et al. A green edge-hosted zinc single-site heterogeneous catalyst for superior Fenton-like activity. Proc. Natl. Acad. Sci. 120, e2221228120 (2023).
Huang, B. et al. Modulating electronic structure engineering of atomically dispersed cobalt catalyst in Fenton-like reaction for efficient degradation of organic pollutants. Environ. Sci. Technol. 57, 14071–14081 (2023).
Yang, M. et al. Unveiling the origins of selective oxidation in single-atom catalysis via Co–N4–C intensified radical and nonradical pathways. Environ. Sci. Technol. 56, 11635–11645 (2022).
Song, J. et al. Asymmetrically coordinated CoB1N3 moieties for selective generation of high-valence Co-Oxo species via coupled electron–proton transfer in Fenton-like reactions. Adv. Mater. 35, 2209552 (2023).
Shao, P. et al. Revisiting the graphitized nanodiamond-mediated activation of peroxymonosulfate: singlet oxygenation versus electron transfer. Environ. Sci. Technol. 55, 16078–16087 (2021).
Yang, Z. et al. Toward selective oxidation of contaminants in aqueous systems. Environ. Sci. Technol. 55, 14494–14514 (2021).
Qian, K. et al. Single-atom Fe catalyst outperforms its homogeneous counterpart for activating peroxymonosulfate to achieve effective degradation of organic contaminants. Environ. Sci. Technol. 55, 7034–7043 (2021).
Yao, Y. et al. Rational regulation of Co–N–C coordination for high-efficiency generation of 1O2 toward Nearly 100% selective degradation of organic pollutants. Environ. Sci. Technol. 56, 8833–8843 (2022).
Xu, P. et al. CuFe2O4/diatomite actuates peroxymonosulfate activation process: mechanism for active species transformation and pesticide degradation. Water Res. 235, 119843 (2023).
Yang, P., Long, Y., Huang, W. & Liu, D. Single-atom copper embedded in two-dimensional MXene toward peroxymonosulfate activation to generate singlet oxygen with nearly 100% selectivity for enhanced Fenton-like reactions. Appl. Catal. B: Environ. 324, 122245 (2023).
Wu, Z.-Y. et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 12, 2870 (2021).
Li, J. et al. Metal–organic framework-derived graphene mesh: a robust scaffold for highly exposed Fe–N4 Active sites toward an excellent oxygen reduction catalyst in acid media. J. Am. Chem. Soc. 144, 9280–9291 (2022).
Mehmood, A. et al. High loading of single atomic iron sites in Fe–NC oxygen reduction catalysts for proton exchange membrane fuel cells. Nat. Catal. 5, 311–323 (2022).
Zhou, X. et al. Identification of Fenton-like active Cu sites by heteroatom modulation of electronic density. Proc. Natl. Acad. Sci. USA 119, e2119492119 (2022).
Liu, D., He, Q., Ding, S. & Song, L. Structural regulation and support coupling effect of single-atom catalysts for heterogeneous catalysis. Adv. Energy Mater. 10, 2001482 (2020).
Shan J. et al. Metal-metal interactions in correlated single-atom catalysts. Sci. Adv. 8, eabo0762 (2022).
Tang, W. et al. Ru single atom catalyst with dual reaction sites for efficient Fenton-like degradation of organic contaminants. Appl. Catal. B: Environ. 320, 121952 (2023).
Gao, Y. et al. Activity trends and mechanisms in peroxymonosulfate-assisted catalytic production of singlet oxygen over atomic Metal-N-C catalysts. Angew. Chem. Int. Ed. 60, 22513–22521 (2021).
Gao, J. et al. Cobalt single-atom catalysts for domino reductive amination and amidation of levulinic acid and related molecules to N-heterocycles. Chem. Catal. 2, 178–194 (2022).
Cao, D. et al. Volcano-type relationship between oxidation states and catalytic activity of single-atom catalysts towards hydrogen evolution. Nat. Commun. 13, 5843 (2022).
Shang, Y., Xu, X., Gao, B., Wang, S. & Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 50, 5281–5322 (2021).
Bin Yang, H. et al. Identification of non-metal single atomic phosphorus active sites for the CO2 reduction reaction. EES Catal. 1, 774–783 (2023).
Duan, X., Indrawirawan, S., Sun, H. & Wang, S. Effects of nitrogen-, boron-, and phosphorus-doping or codoping on metal-free graphene catalysis. Catal. Today 249, 184–191 (2015).
Singh, K. P., Song, M. Y. & Yu, J.-S. Iodine-treated heteroatom-doped carbon: conductivity driven electrocatalytic activity. J. Mater. Chem. A 2, 18115–18124 (2014).
Jeon, I.-Y. et al. Facile, scalable synthesis of edge-halogenated graphene nanoplatelets as efficient metal-free eletrocatalysts for oxygen reduction reaction. Sci. Rep. 3, 1810 (2013).
Liu, J. et al. Iodine-doping-induced electronic structure tuning of atomic cobalt for enhanced hydrogen evolution electrocatalysis. ACS Nano 15, 18125–18134 (2021).
Tian, H. et al. High durability of Fe–N–C single-atom catalysts with carbon vacancies toward the oxygen reduction reaction in alkaline media. Adv. Mater. 35, 2210714 (2023).
Wang, B. et al. A site distance effect induced by reactant molecule matchup in single‐atom catalysts for fenton‐like reactions. Angew. Chem. 134, e202207268 (2022).
Dong, Y. et al. Ammonia thermal treatment toward topological defects in porous carbon for enhanced carbon dioxide electroreduction. Adv. Mater. 32, 2001300 (2020).
Liu, S. et al. Atomically dispersed iron sites with a nitrogen–carbon coating as highly active and durable oxygen reduction catalysts for fuel cells. Nat. Energy 7, 652–663 (2022).
Gao, Y., Chen, Z., Zhu, Y., Li, T. & Hu, C. New insights into the generation of singlet oxygen in the metal-free peroxymonosulfate activation process: important role of electron-deficient carbon atoms. Environ. Sci. Technol. 54, 1232–1241 (2020).
Sun, B. et al. Polyaniline: a new metal-free catalyst for peroxymonosulfate activation with highly efficient and durable removal of organic pollutants. Environ. Sci. Technol. 53, 9771–9780 (2019).
Li, F., Lu, Z., Li, T., Zhang, P. & Hu, C. Origin of the excellent activity and selectivity of a single-atom copper catalyst with unsaturated Cu-N2 Sites via peroxydisulfate activation: Cu(III) as a dominant oxidizing species. Environ. Sci. Technol. 56, 8765–8775 (2022).
Guo, C. et al. Ball-milled layer double hydroxide as persulfate activator for efficient degradation of organic: alkaline sites-triggered non-radical mechanism. J. Hazard. Mater. 461, 132219 (2024).
Li, M. et al. Single cobalt atoms anchored on Ti3C2Tx with dual reaction sites for efficient adsorption–degradation of antibiotic resistance genes. Proc. Natl. Acad. Sci. USA 120, e2305705120 (2023).
Wu, Z. et al. Facilely tuning the first-shell coordination microenvironment in iron single-atom for Fenton-like chemistry toward highly efficient Wastewater purification. Environ. Sci. Technol. 57, 14046–14057 (2023).
Pan, Y., Su, H., Zhu, Y., Vafaei Molamahmood, H. & Long, M. CaO2 based Fenton-like reaction at neutral pH: accelerated reduction of ferric species and production of superoxide radicals. Water Res. 145, 731–740 (2018).
Bokare, A. D. & Choi, W. Singlet-oxygen generation in alkaline periodate solution. Environ. Sci. Technol. 49, 14392–14400 (2015).
Lei, Y. et al. Assessing the use of probes and quenchers for understanding the reactive species in advanced oxidation processes. Environ. Sci. Technol. 57, 5433–5444 (2023).
Chen, F. et al. Single-atom iron anchored tubular g-C3N4 catalysts for ultrafast Fenton-like reaction: roles of high-valency iron-oxo species and organic radicals. Adv. Mater. 34, 2202891 (2022).
Li, S. et al. Performance enhancement and mechanism of electroenhanced peroxymonosulfate activation by single-atom Fe catalyst modified electrodes. Proc. Natl. Acad. Sci. USA 121, e2404965121 (2024).
Meng, Y. et al. Nanoconfinement steers nonradical pathway transition in single atom Fenton-like catalysis for improving oxidant utilization. Nat. Commun. 15, 5314 (2024).
Wu, Z. et al. Long-range interactions driving neighboring Fe–N4 sites in Fenton-like reactions for sustainable water decontamination. Nat. Commun. 15, 7775 (2024).
Yun, E.-T., Lee, J. H., Kim, J., Park, H.-D. & Lee, J. Identifying the nonradical mechanism in the peroxymonosulfate activation process: singlet oxygenation versus mediated electron transfer. Environ. Sci. Technol. 52, 7032–7042 (2018).
Li, H., Yuan, N., Qian, J. & Pan, B. Mn2O3 as an Electron shuttle between peroxymonosulfate and organic pollutants: the dominant role of surface reactive Mn(IV) species. Environ. Sci. Technol. 56, 4498–4506 (2022).
Wang, Z. et al. Cobalt single atoms anchored on oxygen-doped tubular carbon nitride for efficient peroxymonosulfate activation: simultaneous coordination structure and morphology modulation. Angew. Chem. Int. Ed. 61, e202202338 (2022).
Xu P. et al. A nanoconfined FeCo2O4-embedded ceramic membrane regulates electron transfer in peroxymonosulfate activation to selectively generate singlet oxygen for water decontamination. Environ. Sci. Technol. 58, 17464–17474 (2024).
Jiang, X. et al. Precise coordination of high-loading Fe single atoms with sulfur boosts selective generation of nonradicals. Proc. Natl. Acad. Sci. USA 121, e2309102121 (2024).
Gu, C.-H. et al. Tuning electronic structure of metal-free dual-site catalyst enables exclusive singlet oxygen production and in-situ utilization. Nat. Commun. 15, 5771 (2024).
Mo, F. et al. The optimized Fenton-like activity of Fe single-atom sites by Fe atomic clusters–mediated electronic configuration modulation. Proc. Natl. Acad. Sci. USA 120, e2300281120 (2023).
Lan, M.-Y. et al. Multi-channel electron transfer induced by polyvanadate in metal-organic framework for boosted peroxymonosulfate activation. Nat. Commun. 15, 7208 (2024).
Liu, S. et al. Carbonized polyaniline activated peroxymonosulfate (PMS) for phenol degradation: role of PMS adsorption and singlet oxygen generation. Appl. Catal. B: Environ. 286, 119921 (2021).
Zhang, J. et al. Melamine-cyanurate supramolecule induced graphitic N-rich graphene for singlet oxygen-dominated peroxymonosulfate activation to efficiently degrade organic pollutants. Sep. Purif. Technol. 265, 118474 (2021).
Qin, J. et al. Rational design of efficient metal-free catalysts for peroxymonosulfate activation: selective degradation of organic contaminants via a dual nonradical reaction pathway. J. Hazard. Mater. 398, 122808 (2020).
Liang, X. et al. Coordination number dependent catalytic activity of single-atom cobalt catalysts for Fenton-like reaction. Adv. Funct. Mater. 32, 2203001 (2022).
Zhang, X. et al. Metal-free catalysts with local fluorination regulation for peroxymonosulfate activation: nearly 100 % singlet oxygen production for selective degradation of aqueous organic pollutants. Chem. Eng. J. 480, 148026 (2024).
Wu, Z. et al. Active center size-dependent Fenton-like chemistry for sustainable water decontamination. Environ. Sci. Technol. 57, 21416–21427 (2023).
Zhen, J. et al. M−N3 configuration on boron nitride boosts singlet oxygen generation via peroxymonosulfate activation for selective oxidation. Angew. Chem. Int. Ed. 63, e202402669 (2024).
Hu, P. et al. Selective degradation of organic pollutants using an efficient metal-free catalyst derived from carbonized polypyrrole via peroxymonosulfate activation. Environ. Sci. Technol. 51, 11288 (2017).
Acknowledgements
The work was supported by the National Natural Science Foundation of China (22176124, J.L.), and the Research Start-up Funding from Shanghai Jiao Tong University (WH220416002, J.L.). The authors also thank the help from Ceshigo Research Service (www.ceshigo.com) for the theoretical calculation. The views and ideas expressed herein are solely those of the authors and do not represent the ideas of the funding agencies in any form.
Author information
Authors and Affiliations
Contributions
J.P. and J.Luo designed the research. J.P., J.Liu, K.F., and Y.F. synthesized the materials and carried out the experiments. J.P., K.Y., and J.Luo contributed to the interpretation of the results. J.P. wrote the manuscript. K.Y., S.L., D.Y., M.X., and J.Luo revised the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xiaoqiang An, Lejuan Cai, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pei, J., Liu, J., Fu, K. et al. Non-metallic iodine single-atom catalysts with optimized electronic structures for efficient Fenton-like reactions. Nat Commun 16, 800 (2025). https://doi.org/10.1038/s41467-025-56246-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56246-6