Abstract
Pancreatic islets are densely packed cellular aggregates containing various hormonal cell types essential for blood glucose regulation. Interactions among these cells markedly affect the glucoregulatory functions of islets along with the surrounding niche and pancreatic tissue-specific geometrical organization. However, stem cell (SC)-derived islets generated in vitro often lack the three-dimensional extracellular microenvironment and peri-vasculature, which leads to the immaturity of SC-derived islets, reducing their ability to detect glucose fluctuations and insulin release. Here, we bioengineer the in vivo-like pancreatic niches by optimizing the combination of pancreatic tissue-specific extracellular matrix and basement membrane proteins and utilizing bioprinting-based geometrical guidance to recreate the spatial pattern of islet peripheries. The bioprinted islet-specific niche promotes coordinated interactions between islets and vasculature, supporting structural and functional features resembling native islets. Our strategy not only improves SC-derived islet functionality but also offers significant potential for advancing research on islet development, maturation, and diabetic disease modeling, with future implications for translational applications.
Similar content being viewed by others
Introduction
Pancreatic islets are micro-organs characterized by densely packed cellular aggregates containing various endocrine hormonal cells, including insulin-producing β cells, glucagon-producing α cells, and somatostatin-producing δ cells. These islets are surrounded by a complex network of extracellular matrix (ECM) and extensively branched vasculature, which play a pivotal role in orchestrating the insulin secretion machinery to maintain blood glucose homeostasis1,2,3. The importance of coordination between cell-ECM interactions and cell-cell adhesion during the process of islet development and maturation has been highlighted4,5. Disrupting integrin-mediated ECM signaling and junctional proteins, which are responsible for the linkage of islets with ECM and niche cells, results in a drastic disturbance of islet morphology and vasculature architecture, and decrease in the functional measures of glucose metabolism. These findings provide evidence of the immature metabolic characteristics of in vitro-produced stem cell (SC)-derived islets, which lack a three-dimensional (3D) ECM environment and perivascular niches. Despite recent advances in multistage differentiation protocols6,7,8, SC-derived islets often remain functionally immature9,10. Recent studies have shown that differentiated SC-derived islets attain a more mature state when transplanted in vivo, indicating that SC-derived islets lack essential microenvironmental factors present in the in vivo setting, which are required for continuous maturation11,12.
To address the challenges in islet engineering, recent efforts have been made to create microenvironments that provide in vivo-like extracellular cues with the aim of facilitating further maturation of SC-derived islets. The pancreatic ECM encompasses a diverse spectrum of proteins, offering both physiological signals and mechanical protection to the islets13. Among the various ECM components, basement membrane (BM) proteins constitute specialized ECM surrounding islets and occupy only approximately 2% of the entire ECM proportion in the human pancreatic proteome14. Despite their small percentage, they play a critical role in supporting the viability and functionality of islets by providing anchorage points to stabilize the ECM-binding affinity of endocrine cells and integrin signaling for robust glucose responsiveness15,16,17,18. BM proteins have been used as ECM-derived components to generate an in vitro peri-islet niche for SC-derived islets15,19. However, most studies with BM proteins have primarily focused on coating tissue culture plates or islet surfaces with low concentrations ranging from 10 to 200 µg/mL. To reproduce more pancreatic-mimetic 3D microenvironments, researchers have introduced decellularized pancreatic tissue-derived ECM (pdECM) as a potential biomaterial with the capacity to preserve the biochemical composition of native pancreatic tissues20,21,22,23,24,25,26. SC-derived islets cultured in pdECM enhance the gene expression levels of key hormones for glucose metabolism with tissue-specific cues, compared to islets cultured in other types of conventionally applied ECM-derived materials such as collagen I23 and Matrigel26. Particularly, the higher secretory performance of encapsulated islets in pdECM compared to islets cultured in lung and bladder dECM has been demonstrated, highlighting pancreatic tissue-specific factors in terms of the intrinsic functionality of islets20. However, it is worth noting that decellularization tends to disrupt BM proteins because of the harsh removal of cellular components27. Despite the importance of native pancreatic ECM for islet function, no bespoke pancreatic niche has been developed for SC-derived islets, considering the combined influence of pdECM and BM proteins.
Vascular networks are also key features uniquely presented in the peri-islet niche. They are intricately connected to the islets, serving the vital roles of providing nutritional support and optimizing glucose sensing to fulfill the metabolic demands of islets1,3,28,29,30. In the process of pancreatic organogenesis, the expression of vascular endothelial growth factor from β cells is essential to encourage islet vascularization for close coupling with systemic blood circulation, contributing to their role as an endocrine organ3. To recapitulate the in vivo developmental niche, several studies have explored the incorporation of endothelial cells (ECs) at the definitive endoderm stage31 or end stage of differentiation5,32 to promote the maturation of SC-derived islets. However, these studies primarily addressed the effects of co-culturing ECs and SC-derived islet cells in a 2D environment. This approach has limitations in reproducing the 3D architectural organization of islets and their perivasculature, which is crucial for mimicking the physiological conditions of native islets. Recently, a 3D vascularized SC-derived islet model was introduced that was reconstructed by physically mixing islet cells and ECs after the dissociation of SC-derived islets into single cells33. However, this approach failed to establish the formation of the intra-islet vasculature, a key feature of the anatomy of native islets1,2, owing to the migration of ECs out of the re-aggregated SC-derived islets. This limitation could potentially impair the efficient delivery of metabolites via cell-cell contact, which is a crucial requirement for the dynamic insulin release from SC-derived islets. An engineered microvascular mesh that covers encapsulated islets in 3D has shown the possibility of effective revascularization and anastomosis leading to enhanced metabolic functions of the islets after transplantation34. However, the limited cellular contact of ECs and islets within the vascular mesh, under settings that are completely different from in vivo vascular niches, may account for the lack of functional data in vitro. Employing ready-made micro-vessels isolated from the adipose tissue fragments to SC-derived pancreatic progenitor (PP) cells also accelerates the formation of connections between the vasculature and intra-islet vasculature in vivo, compared to PP cells combined with single EC35. This approach improved islet graft survival by reducing apoptosis and hypoxia, underscoring the importance of establishing a structurally stable and dense vascular network with lumen integrity for the effective engraftment of SC-derived islets. Despite the advantages of rapid vascularization in vivo, the application of isolated micro-vessels poses challenges related to scalability and reproducibility and may not fully replicate the intricate perivascular niche required for optimal islet maturation in controlled in vitro settings. Extensive vascular networks, which are fully integrated with islet cells, provide a beneficial set of molecules, including hepatic-, fibroblast-, and connective tissue growth factors, which create a favorable pericellular niche for islet survival and function36,37,38,39. In terms of factor delivery, a combination of the vessel network growth modes (e.g., angiogenesis and arteriogenesis) that drive vessel network expansion can effectively orchestrate these factors within the islet niche40. Therefore, establishing an islet-specific perivascular niche is essential to facilitate crosstalk between SC-derived islets and ECs and enhance the timely transport of insulin to the peripheral circulation.
With respect to recapitulating the 3D hierarchy of a target tissue, bioprinting technology is gaining popularity because of its ability to faithfully replicate complex structures41,42. Most studies that apply bioprinting technology to fabricate pancreatic constructs have introduced the extrusion of islet-laden hydrogels as a lattice type without considering printing designs related to islet architecture23,43,44. The SC-derived islets used in these studies underwent an aggregation process using a mold system23 or spinner flasks for suspension culture44, which required laborious procedures and more than 24 h to generate islet-like clusters. A recent study facilitated the bioprinting of high-density SC-derived islet cells in an aggregate form within pdECM, achieving an extrusion rate of 0.3 s per islet, harnessing the benefits of bioprinting technologies for rapid fabrication and high structural reproducibility24. This study established a bioprinting method for SC-derived islet-like aggregates that displayed tightly assembled structures and significantly higher insulin gene expression than randomly dispersed islet cells. Nevertheless, the printed islet-like aggregates did not show significant differences in insulin secretion under low- and high-glucose conditions, indicating their inability to achieve the proper glucose responsiveness required to regulate glucose metabolism, as revealed by the glucose-stimulated insulin secretion (GSIS) test.
In this study, we propose bioprinting-based bespoke islet-specific niches to promote the glucoregulatory functions of SC-derived islets by recapitulating in vivo-like functional coordination of the islets, ECM, and vasculature. Our approach includes replicating the microenvironment specific to islets by refining the blend of pdECM and BM proteins, exerting significant extracellular effects on enhancing further maturation of insulin-secreting β cell lineage. Additionally, we employ bioprinting-based geometric guidance to reconstruct the spatial arrangement of islet peripheries, fostering synchronized interactions between SC-derived islets and the vasculature. The bioprinted human islet-like cellular aggregates-vasculatures (HICA-V) within the engineered peri-islet niche suggest optimal customization, which employs biochemical and biophysical cues simultaneously for boosting maturation, thereby overcoming the constraints related to the immaturity of SC-derived islets. These synergistic biomanufacturing approaches lead to remarkable enhancements in the functional output encompassing both healthy and inflammatory conditions, closely recapitulating native islet physiology. This perspective opens promising avenues for mechanistic studies on how environmental factors influence islet development, maturation, and the modeling of diabetic diseases, enhancing the potential for translating diabetic therapeutics into clinical applications. Furthermore, our insights into engineering bespoke niches provide an adaptable concept for functional transplantable constructs with the goal of treating diabetes, which involves a biochemically defined matrix and hierarchically organized cells in a peri-islet-specific manner.
Results
Islet niche-specific biochemical complexity induces maturation of SC-derived β cells
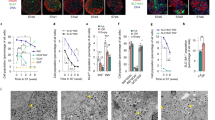
To establish a realistic peri-islet niche that faithfully replicates the biochemical complexity of native pancreatic tissues, we conducted proteomic analysis of the major ECM proteins in pdECM using the matrisome database. We also compared the composition of pdECM with Collagen I (Col), a widely used ECM-derived biomaterial obtained from porcine employed as a supplement and adjuvant to enhance islet function in transplantable applications45,46,47. The pdECM was prepared by decellularizing porcine pancreatic tissues and subsequently analyzed for compositional compatibility (Fig. 1a, b). Compositional ratio of whole protein content revealed that 74.8% and 81.1% of the matrisome proteins were present in pdECM and Col, respectively (Fig. 1c). There were 59 ECM proteins exclusively identified in pdECM, and a minority of proteins detected in pdECM (5 of 64 ≈ 7.8%) overlapped with the proteins identified in Col (Fig. 1e). Furthermore, a more detailed classification based on the subtypes of matrisome proteins showed the presence of abundant pancreatic-specific components preserved in pdECM, which were distinct from the Col proteome components (Fig. 1d). In total matrisome proteins, collagenous proteins, glycoproteins, and proteoglycans accounted for 58.2%, 24.4%, and 15.5% of pdECM, respectively. The most abundant elements in pdECM were collagens, which play crucial roles in maintaining the structural organization of matrix and tissue homeostasis45,48. Collagen VI had the highest LFQ values, followed by collagen I, VIII, V, XII, and III (Fig. 1k). Notably, collagen VI is the most abundant protein in the human pancreas, and collagen I, III, V, and VI found in pdECM are also present in the peripheral matrix of native islets49,50. Glycoproteins including dermatopontin (DPT) and nidogen-1 (NID1) are extensively distributed in the non-collagenous region of ECM and contribute to the formation of a delicate niche essential for SC lineage-specific differentiation51,52. Proteoglycans in pdECM such as mimecan (OGN), asporin (ASPN), lumican (LUM), biglycan (BGN), and proline/arginine-rich end leucine-rich repeat protein (PRELP) modulate multiple signaling pathways associated with cell differentiation, growth, and repair mechanisms53. In contrast, the majority of matrisome proteins in Col were collagen (collagen I and III), constituting approximately 96% of the total matrisome (Fig. 1d). This variety of glycoproteins and proteoglycans in pdECM more closely reflects the pancreatic microenvironment compared to Col, which possesses only 4.2% glycoproteins, with proteoglycans being undetectable (Fig. 1d, l). Gene ontology biological process (GOBP) analysis of pdECM proteins indicated that these proteins were highly involved in cellular and metabolic processes, which are important for the biological activity and cell signaling of multicellular islets (Fig. 1j). Furthermore, GOBP enrichment analysis revealed that the proteins found exclusively in pdECM were involved not only in ECM organization but also in multicellular organismal process and response to stress. In contrast, those identified solely in Col were predominantly associated with epithelial cell differentiation and epithelium development (Fig. 1h, i). Notably, the GO term “response to stress” encompasses a process that results in changes in cellular activity, including secretion and enzyme production, in response to disturbances in homeostasis54,55. This finding indicates that pdECM contains specific components related to pancreatic function that are absent in Col. The extracellular region was identified as the dominant cellular component of pdECM, with the molecular functions of its proteins primarily involved binding, catalytic activity, and structural molecular activity (Fig. 1f, g). Interestingly, GO terms such as “metabolic process” and “catalytic activity” were identified as pdECM-specific categories, suggesting that pdECM may support the glucoregulatory functions of encapsulated islets (Fig. 1f, j).
a Optical images of native and decellularized pancreatic tissues. b Schematic illustration of development process of PINE bioink. Created in BioRender. Kim, M. (2024) https://BioRender.com/n16r790. The percentages of (c) matrisome proteins out of total proteins and (d) subtypes of matrisome proteins in Col and pdECM. e Venn diagram showing the numbers of total proteins identified in Col and pdECM. f GO molecular function analysis and (g) cellular component analysis of overlapped proteins in Col and pdECM. Top 7 enriched GOBP terms of proteins with the lowest P values identified only in (h) Col and (i) pdECM. j GO biological process analysis of overlapped proteins in Col and pdECM. Matrisome abundance of (k) collagens and (l) glycoproteins and proteoglycans in pdECM. m Function-based categories of top 10 matrisome proteins in pdECM. Source data are provided as a Source Data file.
To further assess the variations in pdECM, we categorized the top 10 matrisome proteins in pdECM using function-based analysis14. Structural ECM was the dominant category whereas the BM category, including laminin and collagen IV, which are representative components of the peri-islet niche, was not prominent in the pdECM (Fig. 1j). The decellularization process tends to harshly disrupt the BM structure, particularly sodium dodecyl sulfate (SDS)-based decellularization, which results in a severe reduction in BM proteins27. In a previous study, we used SDS for the decellularization of pancreatic tissue to remove cellular components23. The ECM composition of SDS-treated pdECM showed a 99.4% abundance of collagen I, representing a significant loss of glycoproteins and proteoglycans (Supplementary Fig. 1). We subsequently optimized the decellularization protocol by replacing of SDS to Triton-X 100 as a milder detergent to better retain diverse pancreatic tissue-specific ECM proteins22. Despite our efforts to minimize the detrimental impact on preserving ECM proteins by changing the main reagents for decellularization, a portion of BM proteins was deficient compared to other ECM proteins (Fig. 1j).
To resolve this issue, we developed a peri-islet niche-like (PINE) bioink by optimizing combinatorial biochemical cues from pdECM and BM proteins, reflecting the composition of the native human pancreatic proteome14 (Fig. 1b). Laminin and collagen IV, representative BM proteins, were combined in a 1:1 ratio and supplemented to the pdECM to match the compositional ratio to the BM level in the human pancreas, reinforcing the intrinsic pancreatic ECM microenvironment. To demonstrate the maturation capacity of SC-derived islet cells by niche cues, we differentiated human embryonic stem cells (hESC) into insulin-producing β cells using a standardized protocol7, and the stage 6 cells were applied according to the specific purpose of each experiment (Fig. 2a). A golden hue was observed in stage 6 cells cultured on a 2D planar surface, while some of these cells were aggregated into clusters using suspension culture for further experiments (Fig. 2b). Using gene expression analysis of representative markers at each stage, we confirmed that the SC-derived cells were properly differentiated (Fig. 2c). The definitive endodermal marker, FOXA2, exhibited a high expression level in the early stages of differentiation. Key transcription factors of β cells, PDX1 and NKX6.1, showed the highest expression level at stage 4, indicating effective induction of β cell lineage. Ultimately, the expression of INS, GCG, and SST, hormones secreted by β, α, and δ cells, respectively, significantly increased in the final stage of differentiation compared to that of hESC. Furthermore, we characterized immunostained stage 6 cells using a panel of pancreatic islet markers and measured the percentage of cells co-expressing PP markers and hormonal markers (Figs. 2d, S2). The presence of PDX1- and NKX6.1-positive cells indicates that the stage 6 cells, using this differentiation protocol, produced a β cell lineage6. However, many C-peptide-positive cells also expressed other pancreatic hormones, such as GCG and SST, which are characteristic of polyhormonal cells that do not resemble the mature β cell phenotype9. To explore β cell populations and the surrounding niches of SC-derived and native islets, we compared the expression levels of INS, laminin, and collagen IV (Fig. 2e). Our analysis clearly revealed that compared to native islets, SC-derived islets had lower INS expression and lacked proper BM proteins at the periphery. These results strongly suggest implementing a peri-islet niche for SC-derived islets in vitro.
a Schematic illustration of differentiation process and subsequent workflow for hESC-derived islet cells. Created in BioRender. Kim, M. (2024) https://BioRender.com/h44f786. b Brightfield images of stage 6 cells in 2D culture (top) and suspension culture (bottom) (scale bars, 500 µm). c Quantitative real time polymerase chain reaction (qPCR) analysis of endodermal and pancreatic genes at each differentiation stage. Forkhead box protein A2 (FOXA2), Pancreatic and duodenal homeobox 1 (PDX1), NK6 homeobox 1 (NKX6.1), Insulin (INS), Glucagon (GCG), Somatostatin (SST) (n = 3 samples for FOXA2, PDX1, NKX6.1, INS, n = 4 samples for GCG, SST, mean ± SD). Statistical significance was analyzed using one-way ANOVA with Dunnett’s multiple comparisons test: NS, not significant; PESC, S1 = 1.5 × 10−5, PESC, S2 = 2.2 × 10−6, PESC, S3 = 0.0131 (FOXA2); PESC, S2 = 0.9999, PESC, S3 = 0.5314, PESC, S4 = 0.1543 (PDX1); PESC, S2 = 0.9999, PESC, S3 = 0.9999, PESC, S4 = 3.3 × 10−5 (NKX6.1); PESC, S4 = 0.9999, PESC, S5 = 0.0743, PESC, S6 = 0.0023 (INS); PESC, S4 = 1, PESC, S5 = 0.9968, PESC, S6 = 0.1548 (GCG); PESC, S4 = 1, PESC, S5 = 0.1287, PESC, S6 = 7.1 × 10−5 (GCG) d Immunostaining for PDX1, NKX6.1, C-peptide, Chromogranin A (CHGA), GCG, SST of differentiated cells (scale bars, 50 µm). Three independent experiments were conducted to produce three different samples, and imaging was independently repeated with similar results. e Immunostaining for laminin (LAM), INS, collagen IV (COL IV) of human islets and SC-derived islets (scale bars, 100 µm). Three independent experiments were conducted to produce three different samples, and imaging was independently repeated with similar results. Source data are provided as a Source Data file.
After evaluating cellularity and peri-cellular niche of SC-derived islets, we investigated optimal concentrations of supplemented BM proteins ranging from 0.02 mg/mL to 0.4 mg/mL by measurement of the expression levels of β cell-specific genes after encapsulation of SC-derived islet cells (Fig. S3a). The highest levels of PDX1 and CHGA were observed at 0.2 mg/mL, suggesting the appropriate PINE bioink condition for supporting the maturation of SC-derived β cells. Indeed, laminin and collagen IV at 0.2 mg/mL increased the assembled BM network structures surrounding the islets in vitro, enhancing insulin secretion under high glucose stimulus15. To evaluate the effect of PINE bioink on the functionality of SC-derived islet cells, we investigated the expression levels of representative hormones and glucose responsiveness of SC-derived islet cells varying on encapsulating biomaterials at day 5 after encapsulation (Fig. 3a–f). The presence of various hormonal cells in each bioink was confirmed by the expression of α cell marker GCG, β cell markers INS and CHGA, and δ cell marker SST (Fig. 3a). SC-derived β cells cultured in the PINE bioink showed a significantly higher number of INS- and CHGA-positive cells than those cultured in Col and pdECM bioinks, and the degree of GCG- and SST-positive cells was similar in all groups (Fig. 3b). The results of qPCR analysis demonstrated that PINE bioink generated β cell-favorable microenvironments, showing a correlation with the fluorescence intensity profiles (Fig. 3c). Furthermore, the PINE bioink group exhibited a higher quantity of secreted insulin than other groups on day 5, as observed in the GSIS test, indicating that the recruitment of additional BM proteins with PINE bioink to recreate bespoke islet niche provided potent extracellular cues for the maturation of β cell lineage (Fig. 3d). To further assess the impact of the microenvironment on enhancing the metabolic activity of SC-derived β cells, we analyzed the calcium (Ca2+) intensity profiles of encapsulated cells in response to low glucose and high glucose with exendin-4, a secretagogue that amplifies glucose-induced insulin secretion by facilitating Ca2+ influx (Figs 3e, f, S3 c, d). Under high glucose conditions with exendin-4, the Ca2+ signals from 50 individual SC-derived β cells encapsulated in the PINE bioink exhibited significantly pronounced responses compared to the Col group, suggesting that the PINE bioink effectively imparted essential niche factors that enhance secretory responsiveness to glucose and secretagogue stimulation. In contrast, under low glucose conditions, the Ca2+ signals were comparable between the PINE and Col groups, indicating that the basal activity of β cells was not affected by the additional BM proteins. Notably, encapsulated SC-derived islets stably maintained INS expression until day 14, confirming that the PINE bioink provides a supportive microenvironment conducive to the long-term maturation and functional stability of SC-derived islets. Taken together, these results demonstrate that the bespoke islet niche facilitates the proper maturation of SC-derived islets over an extended period, which promotes sustained insulin production and glucose responsiveness (Fig. S3b).
a Immunostaining for INS, CHGA, GCG, and SST of encapsulated cells in each bioink (scale bars, 50 µm). Three independent experiments were conducted to produce three different samples, and imaging was independently repeated with similar results. b Quantification of hormone-positive cells in each group (n = 5 samples, mean ± SD). Statistical significance was analyzed using two-way ANOVA with Dunnett’s multiple comparisons test: NS, not significant; PCol, pdECM = 0.5972, PCol, PINE = 5.3 × 10−6 (INS + ); PCol, pdECM = 0.0057, PCol, PINE = 1.8 × 10−6 (CHGA + ); PCol, pdECM = 0.2146, PCol, PINE = 0.8875 (GCG + ); PCol, pdECM = 0.5692, PCol, PINE = 0.4759 (SST + ). c Comparison of relative gene expression levels of hormones in each group (n = 5 samples, mean ± SD). Statistical significance was analyzed using one-way ANOVA with Dunnett’s multiple comparisons test: NS, not significant; PCol, pdECM = 1.9 × 10−5, PCol, PINE = 6.2 × 10−10 (INS); PCol, pdECM = 0.3119, PCol, PINE = 0.0573 (CHGA); PCol, pdECM = 0.2620, PCol, PINE = 0.2428 (GCG); PCol, pdECM = 0.3377, PCol, PINE = 0.3028 (SST). d GSIS of encapsulated cells in each bioink (n = 7 samples, mean ± SD). Statistical significance was analyzed using two-way ANOVA with Dunnett’s multiple comparisons test: NS, not significant; P2, 20 = 7.6 × 10−9 (Col); P2, 20 = 2.6 × 10−11 (pdECM); P2, 20 = 3.7 × 10−13 (PINE); PCol, pdECM = 0.0896, PCol, PINE = 0.0003 (20 mM). e Still images of Ca2+ signals of encapsulated SC-derived islet cells in each bioink exposed to high glucose (20 mM) with exendin-4 (10 nM) (top, left) (scale bars, 100 µm). Mean and average traces of Ca2+ signals (top, right) (n = 50 cells). Heatmaps of Ca2+ signals of cells from each bioink (n = 50 cells). High Ca2+ signal (red), low Ca2+ signal (violet). f Quantification of Ca2+ signal peak height of individual SC-derived islet cells (n = 50 cells, mean ± SD) in each encapsulated condition. Dots denote data points of individual cells. Statistical significance was analyzed using ANOVA with Dunnett’s multiple comparisons test: NS, not significant; PCol, pdECM = 0.2702, PCol, PINE = 3.3 × 10−5. Source data are provided as a Source Data file.
Bioprinting of HICA-V using PINE bioink facilitates the formation of robust islet-vasculature-ECM network
To achieve in vivo-like cellular organization within the 3D islet niche, we applied bioprinting technology, specifically the in-bath printing technique, to construct a tightly integrated 3D network of islets, vasculature, and ECM (Fig. 4a). For a successful in-bath printing process, the bioink used as the bath material should exhibit Bingham plastic properties. It behaves like a solid under low shear stress but flows like a viscous fluid under high shear stress56, which is essential to ensure extrusion and stable retention of the cellular structure at a predetermined location within the bath suspension57. We evaluated the rheological properties of Col, pdECM and PINE bioinks to verify that the bioink could provide physical confinement to the printed cells (Fig. 4b–e). Both the pdECM and PINE bioinks exhibited shear-thinning viscosity within the established shear rate range, whereas Col retained a fluid-dominant property, indicating its instability in adequately maintaining the structure owing to its low viscosity (Fig. 4b, e). To further investigate the effect of cells on rheological behavior, additional measurements were conducted with PINE bioink-containing cells at the same density used in the bioprinting process. The inclusion of cells did not significantly affect the shear-thinning behavior of the bioink, confirming that the rheological properties of the bioink are not primarily controlled by the encapsulated cells, assuming the same bioink concentration conditions are maintained. Shear recovery analysis of the PINE bioink demonstrated a greater recovery of the storage modulus when exposed to cyclic periods of low and high strain compared to that seen with the pdECM bioink, showing self-healing behavior (Fig. 4c, d). Taken together, we expected that the PINE bioink would serve as a suitable material for the in-bath printing technique as well as a functional niche for printed cells, which was confirmed by performing the printing process (Fig. 4f).
a Schematic illustration of printing process of islet-specific cellular organization using SC-derived islet cells and EC. Created in BioRender. Kim, M. (2025) https://BioRender.com/n16r790. Self-healing properties of (b) Col, (c) pdECM, and (d) PINE bioinks exposed to cyclical periods of low (1 %) and high (300 %) strain. e Shear thinning behavior of each bioink. f Positional placement and movement of nozzles during in bath-printing process of HICA-V. g Live/dead staining for HICAs and vasculatures according to the pneumatic pressure. Three independent experiments were conducted to produce three different samples, and imaging was independently repeated with similar results. h Live/dead staining for optimized printing condition of HICA-V at day 1 and day 4 (left) and structural visualization of 3D rendered HICA-V (right). Three independent experiments were conducted to produce three different samples, and imaging was independently repeated with similar results. i Immunostaining for F-actin, CD31, and DAPI of HICA-V at each timepoint. Three independent experiments were conducted to produce three different samples, and imaging was independently repeated with similar results. All scale bars = 100 µm. Source data are provided as a Source Data file.
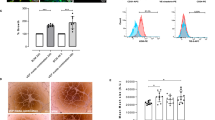
To demonstrate the ability of developing physiologically relevant 3D cellular environments within the engineered niche, HICA-V was fabricated using PINE bioink and in-bath printing approach. Initially, a polyethylene vinyl acetate (PEVA)-based polymeric frame was printed, and the PINE bioink-based bath suspension was loaded inside the frame. Subsequently, the EC-laden PINE bioink and SC-derived islet cells and EC-laden PINE bioink were extruded in a linear and aggregate pattern within the bath suspension, respectively with the aim of single-step fabrication of HICA-V (Fig. 4f). Before patterning HICA-V with the desired geometries, we examined the effects of the presence of ECs on the metabolic maturity of SC-derived islet cells (Fig. S4a, b). Co-culture of ECs and SC-derived islet cells in a 1:5 ratio significantly improved the gene expression levels of metabolic maturation markers including GLUT1 and GCK, confirming that the recapitulating cellular communication aspects of the peri-islet niche mediate the maturation of SC-derived islet cells5,32.
The structural design of the vasculature was inspired by the microscopic vascular anatomy of the pancreatic tissue, in which most islets receive a single vessel and dense vascular networks branch throughout the interior of the islets2,58. To recapitulate the spatial relationship between islets and vasculatures, ECs were linearly printed as vessel-like structures, and HICA was printed at the close proximal region (~50 µm), encouraging coordinated insulin secretion at junctions between printed structures59,60. In this process, we incorporated SC-derived islet cells with ECs for HICA to induce the formation of intra-islet vasculature (Fig. 4f–i). We anticipated that placing HICA and V close to each other would influence the gradual self-assembly of their structures, facilitating the formation of well-organized cellular networks59. HICA-V successfully mimicked the realistic size and morphologies of native islets and perivasculature in a precise and reproducible manner under optimized printing conditions (Fig. 4g, Supplementary Fig. 4d). The diameters of HICA and V were in the range of 254.1 ± 9.5 ~ 461.6 ± 27.3 µm and 220.9 ± 24.5 ~ 356.2 ± 20.1 µm, respectively, achieving controlled size variations under varying pneumatic pressures (Fig. 4g, h). Opting for the 5 kPa condition ensured the creation of uniformly sized HICA (<300 µm in diameter) known as the functional size of islets. Simultaneously, the 0.7 kPa condition was chosen to enable the printing of a larger peri-islet vasculature than intra-islet capillaries2,61. The printed HICA-V exhibited a viability exceeding 94%, indicating that the printing process did not impair the viability of HICA-V (Figs. 4g, S4 c, e). The initial size of the HICA, controlled by the nozzle size (30 G) and extrusion time (0.8 s), was consistent across the experiments, demonstrating robust reproducibility under the specified pneumatic pressure conditions during printing. However, as cellular reorganization occurred over time, the diameter of the printed constructs decreased due to compaction. This reduction in size is primarily attributed to the high density of SC-derived islet cells, leading to overall cellular contraction (Fig. 4h, i)28,62. Red fluorescent protein (RFP)-labeled human umbilical vein endothelial cells (HUVECs) were used to observe the vascular density of each printed HICA and V (Fig. 4h, Supplementary Fig. 4f). RFP-HUVECs were randomly distributed in the HICA and were highly localized in the printed straight line of V, showing stable deposition of cells in accordance with the programmed pattern. We confirmed that the printed HICA-V successfully implemented the spatial arrangement of native islets and perivasculature via 3D rendered image (Fig. 4h). Remarkably, HICA-V generated robust vascular networks that formed sprouting capillaries, following bioprinting-based structural guidance over time (Fig. 4i).
A bioprinted HICA-V recapitulates the in vivo-like functional coordination of native islets and their perivasculature
The close relationship among islets, their vasculature, and the ECM is known to have a profound impact on gap junction-mediated cellular communication, which is critical for synchronized insulin release4,63. To demonstrate the potential of HICA-V to replicate the physiologically relevant functions of native islets with bioprinting-assisted paracrine signaling, we compared three groups, all maintained at the same cell density and in the same 3D environment within PINE bioink: (i) a randomly dispersed group, composed of single SC-derived islet cells and single ECs within PINE; (ii) a suspension-cultured group, consisting of SC-derived islet aggregates, prepared through suspension culture and combined with single ECs within PINE; and (iii) printed HICA-V, which incorporates single SC-derived islet cells and single ECs, precisely patterned into an islet-specific geometry within PINE using the bioprinting process. These groups were evaluated based on vascular and insulin expression and insulin secretory functions (Fig. 5a, c, f, g, Supplementary Fig. S5b). In particular, to further analyze gene expression levels of cell adhesion molecules and secreted factors, which are related to bidirectional communication between β cells and ECs within the islets, we compared three groups and used isolated human islets as controls (Fig. 5d, e). To distinguish ECs from V and visualize cellular contact between printed HICA and V, we mixed RFP-HUVECs with SC-derived islet cells. V exhibited a hollow lumen-like morphology on Day 4, and ECs from HICA sprouted inside V, integrating the two structures (Fig. 5b). Self-aggregation of HICA and maintenance of the peri-islet vasculature-specific patterns via reinforced cell-cell contact were observed, demonstrating the possibility of natural formation of junctions between printed cells for the coordinated secretory activity of HICA-V (Fig. 5c). Moreover, localized insulin expression was observed in suspension-cultured SC-derived islet aggregates and HICA, as opposed to randomly scattered SC-derived islet cells. Notably, insulin expression increased over time in both HICA and suspension-cultured aggregates, highlighting the importance of maintaining structural cues to support β cell functionality. However, the randomly dispersed group and suspension-cultured group exhibited insufficient connections between single ECs, demonstrating that ECs without structural guidance were unable to form a dense and functional vascular network. These findings suggest that while structural aggregation enhances insulin secretion capacity, bioprinting-based geometrical organization and integration with peri-islet vasculature are essential for further developing intra-islet vascular networks, optimizing vascular interactions, and maximizing insulin production.
a Schematic illustration for cellular interactions and junctions between β cell and EC (left) and experimental groups (right). Created in BioRender. Kim, M. (2025) https://BioRender.com/w23l782. b Immunostaining for CD31 in HICA-V at day 4 (scale bar, 100 µm). Three independent experiments were conducted to produce three different samples, and imaging was independently repeated with similar results. c Immunostaining for INS and CD31 of each group at day 1 (top) and 4 (bottom) (scale bars, 100 µm). Three independent experiments were conducted to produce three different samples, and imaging was independently repeated with similar results. d, e qPCR analysis to compare gene expression in human islets (H islets), randomly dispersed (Random), suspension-cultured (Suspension), and HICA-V group (n = 3 samples, mean ± SD). Statistical significance was analyzed using one-way ANOVA with Dunnett’s multiple comparisons test: NS, not significant; PH islets, Random = 0.6444, PH islets, Suspension = 0.0003, PH islets, HICA-V = 0.0157, PSuspension, HICA-V = 0.0256 (E-CAD); PH islets, Random = 0.9812, PH islets, Suspension = 0.0291, PH islets, HICA-V = 0.0003, PSuspension, HICA-V = 0.0152 (CX36); PH islets, Random = 1, PH islets, Suspension = 0.0494, PH islets, HICA-V = 3.1 × 10−5, PSuspension, HICA-V = 0.0004 (VE-CAD); PH islets, Random = 0.8357, PH islets, Suspension = 0.0242, PH islets, HICA-V = 0.0042, PSuspension, HICA-V = 0.4829 (CX43); PH islets, Random = 0.9787, PH islets, Suspension = 0.7645, PH islets, HICA-V = 0.0169, PSuspension, HICA-V = 0.0547 (INS); PH islets, Random = 0.9498, PH islets, Suspension = 0.0210, PH islets, HICA-V = 3.6 × 10−5, PSuspension, HICA-V = 0.0009 (FGF). f Comparative GSIS (n = 4 samples, mean ± SD). Statistical significance was analyzed using two-way ANOVA with Sidak’s multiple comparisons test: NS, not significant; P2, 20 = 0.2758 (Random); P2, 20 = 0.0003 (Suspension); P2, 20 = 6.7 × 10−6 (HICA-V) and Dunnett’s multiple comparisons test; PRandom, Suspension = 8.7 × 10−5, PRandom, HICA-V = 3.4 × 10−7 (20 mM). g Stimulation index (n = 4 samples, mean ± SD). Statistical significance was analyzed using one-way ANOVA with Dunnett’s multiple comparisons test: NS, not significant; PRandom, Suspension = 0.9947, PRandom, HICA-V = 0.4633. Source data are provided as a Source Data file.
We analyzed the gene expression levels of cadherin and connexin complexes in each group, including the human islets group, to confirm that the bioprinting-assisted structural cues participated in the formation of enriched junctions between cells (Fig. 5d). The expression levels of CX36, CX43, and VE-CAD in HICA-V were significantly higher than those in the human islets, random, and suspension group, except for E-CAD, whose expression level was similar to that of the suspension group, implying that geometrical cues contributed to enhanced gap junction connectivity of HICA-V. CX36 junctions, the predominant connexin in islets, serve as key micro-anatomical bases that support proper glucose-stimulated insulin release and Ca2+ release64,65. Heterotypic gap junctions composed of CX43 and CX36, which are predominantly expressed in the vasculature and β cells, respectively, have been identified within native islets, suggesting that HICA-V may facilitate effective physical contact between ECs and SC-derived islet cells66. In concert with the expression of connexins, which mediate contact-dependent communication, we expected that high expression of VE-CAD in HICA-V would support structural integrity to promote insulin secretion1,35. Specifically, E-CAD mediates homotypic cell-cell interaction between β cells; α cells also express E-CAD for heterotypic interaction with β cells in native islets, establishing the islet architecture48,67. The robustness of physical contact-mediated cellular interactions was determined by evaluating the gene expression levels of secreted proteins in each group (Fig. 5e). Compared with other groups, the HICA-V group, with abundant perivascular coverage, exhibited significantly higher expression levels of insulin and fibroblast growth factor (FGF), which play crucial roles in the interaction between β cells and ECs. Insulin supports the upregulation of endothelial nitric oxide synthase in ECs in a paracrine manner, enhancing intra-islet circulation68. Additionally, FGF, an endothelium-derived factor, promotes β cell survival and proliferation69. Compared with those in the other groups, the lower gene expression levels of connexins, cadherins, insulin, and FGF in isolated human islets likely result from the disruption of the native microenvironment, loss of peri-vascular support, and physical and enzymatic stress during the isolation process. Together, these factors may contribute to impaired cell-cell communication and diminish the metabolic function of β cells and ECs in human islets70. The suspension group exhibited higher gene expression levels of junctional proteins and secreted factors compared to the random group, aligning with findings from the immunofluorescent imaging results and suggesting that cellular aggregation through suspension culture can partially improve cell-cell interactions and functionality. However, the gene expression levels of CX36, VE-CAD, and FGF were significantly higher in the HICA-V group than in the suspension group, highlighting the critical role of bioprinting-assisted peri-islet vascular network formation in establishing robust cellular communication. Using the GSIS test, we further observed that junction engagement improved insulin release from SC-derived β cells on day 4 (Fig. 5f, g). The suspension group exhibited higher insulin secretion under high glucose conditions compared to the random group, emphasizing the role of cellular aggregation in supporting glucose metabolism. The printed HICA-V group demonstrated significantly higher insulin secretion under low and high glucose conditions compared to the other groups. Specifically, the HICA-V exhibited a 3.63-fold increase in insulin release under low glucose conditions and a 3.82-fold increase under high glucose conditions relative to the random group, reflecting an overall enhancement in insulin secretion capacity. Interestingly, while the GSIS index remained comparable across all groups, the substantial increase in both basal and stimulated insulin secretion in the HICA-V group suggests that simultaneously providing islet-specific geometrical cues with microenvironmental cues synergistically enhances β cell functionality by promoting insulin release under both conditions. Collectively, these findings strongly indicate that a bioprinted HICA-V can boost the coordination of cell-cell adhesion and the ECM, which is responsible for the reflecting critical physiology in native islets (e.g., paracrine signaling between islet cells, islet-vasculature interactions, and glucose responsiveness).
We investigated how HICA-V recapitulates physiological responses to hyperglycemic conditions to assess its potential as a native islet niche mimicry for type 2 diabetes. This study aimed to provide an in vivo-like pathophysiological rationale for HICA-V by evaluating its ability as an islet niche-specific analog to demonstrate the pharmacological effects of antidiabetic drug combinations targeting the islets and its peripheral cells, as revealed via in vivo and clinical studies71,72. Elevated blood glucose levels have been suggested to contribute to the occurrence of endothelial disruption in the peri-islet niche and microvascular complications73,74,75. In this study, HICA-V was exposed to high glucose concentrations in the culture medium to induce hyperglycemic-like conditions (Fig. 6a). To test the reproducibility of the therapeutic effects observed in the in vivo study, we selected empagliflozin (Empa), an antidiabetic drug known to ameliorate endothelial dysfunction in islets, in combination with metformin (Met), a first-line glucose-lowering agent for patients with diabetes71. We characterized the physio-mimicry of HICA-V under four conditions, normal for a positive control, Met-/Empa- for a negative control (representing the non-drug-treated groups in high-glucose conditions), Met + /Empa- for metformin-only-treated groups, and Met + /Empa+ for the combination drug-treated groups (Fig. 6b). All analyses were performed on day 8 to demonstrate the pharmacological effects after 3 days of drug treatment. Gene expression of IL-6 and VCAM-1, indicative of inflammatory cytokines and endothelial dysfunction, respectively, was the highest in the Met-/Empa- group because of glucotoxicity, and the Met + /Empa+ group revealed a significantly lower level of each gene than the Met-/Empa- group (Fig. 6c). Both immunofluorescence images and quantitative intensity profiles of Met + /Empa+ showed that CD31 was highly expressed similar to that in the normal group, indicating functional restoration of the perivasculature in HICA-V (Fig. 6f, g). The expression of INS in the Met + /Empa+ and Met + /Empa- groups was significantly higher than that in the Met-/Empa- group. Although glucose responsiveness of the Met-/Empa- group was maintained at an appropriate level even after 6 days of high-glucose treatment, the GSIS index demonstrated that combinatorial drug treatments with Empa had the most potent effects on enhancing insulin release in a hyperglycemic environment (Fig. 6d, e). Taken together, this comprehensive investigation illustrates that printed HICA-V mimics in vivo pathophysiological phenomena and drug responses under disease conditions, suggesting the potential of HICA-V as a valuable model for studying islet physiology and evaluating the pharmacological effects of antidiabetic drugs.
a Schematic illustration of inducing hyperglycemic-like condition and anti-diabetic drug treatments using printed HICA-V. b Timepoints for high glucose and drug treatments. c qPCR analysis of Met-/Empa-, Met + /Empa-, Met + /Empa + , and normal group (n = 3 samples, mean ± SD). Interleukin-6 (IL-6), Vascular cell adhesion molecule-1 (VCAM-1). Statistical significance was analyzed using one-way ANOVA with Dunnett’s multiple comparisons test: NS, not significant; PMet-/Empa-, Met+/Empa- = 0.0512, PMet-/Empa-, Met+/Empa+ = 0.0354, PMet-/Empa-, Normal = 0.1248 (IL-6); PMet-/Empa-, Met+/Empa- = 0.0362, PMet-/Empa-, Met+/Empa+ = 0.0142, PMet-/Empa-, Normal = 0.0411 (VCAM-1). d GSIS test (n = 4 samples, mean ± SD). Statistical significance was analyzed using two-way ANOVA with Sidak’s multiple comparisons test: NS, not significant; P2, 20 = 0.0135 (Met-/Empa-); P2, 20 = 0.0006 (Met + /Empa-); P2, 20 = 2.6 × 10−6 (Met + /Empa + ); P2, 20 = 0.0024 (Normal). e Stimulation index of each group (n = 4 samples, mean ± SD). Statistical significance was analyzed using one-way ANOVA with Dunnett’s multiple comparisons test: NS, not significant; PMet-/Empa-, Met+/Empa- = 0.7234, PMet-/Empa-, Met+/Empa+ = 0.0269, PMet-/Empa-, Normal = 0.3277. f Immunostaining for CD31 and INS of each group at day 8 (scale bars, 100 µm). Three independent experiments were conducted to produce three different samples, and imaging was independently repeated with similar results. g Immunofluorescence intensity profiles of each condition (n = 3 samples, mean ± SD). Statistical significance was analyzed using one-way ANOVA with Dunnett’s multiple comparisons test: NS, not significant; PMet-/Empa-, Met+/Empa- = 1.1 × 10−5, PMet-/Empa-, Met+/Empa+ = 7.4 × 10−11, PMet-/Empa-, Normal = 1.2 × 10−11 (CD31); PMet-/Empa-, Met+/Empa- = 5.3 × 10−6, PMet-/Empa-, Met+/Empa+ = 4.4 × 10−6, PMet-/Empa-, Normal = 0.0001 (INS). Source data are provided as a Source Data file.
Discussion
In this study, we proposed a synergistic approach that combines the development of a pancreatic ECM-based bespoke islet niche with the application of bioprinting technology to achieve the functional coordination among SC-derived islets, ECM, and vascular networks. The HICA-V developed using the PINE bioink aimed to emulate the structural and functional characteristics of native pancreatic islets within the complex niche, facilitating the elicitation of in vivo-like physiological responses of SC-derived islets and vascular networks under both healthy and inflammatory conditions.
We successfully optimized the in vitro pancreatic microenvironment to recreate the intrinsic and complex composition of native pancreatic tissue, considering the inevitable reduction in BM proteins during the decellularization process. The maturation of SC-derived islets is highly dependent on ECM environment that modulate autocrine and paracrine signaling pathways. Laminin activates integrin-mediated signaling pathways, including FAK and downstream signaling cascades such as PI3K/AKT76. This activation promotes insulin gene expression by enhancing the autocrine release of IGF-1 and facilitating paracrine signaling through factors like FGF45. Similarly, collagen IV regulates the autocrine release of TGF-β1, which is critical for β cell differentiation, and stabilizes the activity of paracrine growth factors such as VEGF and FGF, thereby facilitating crosstalk between β cell and islet niche cells45. Indeed, the orchestration of pancreatic-specific proteins in pdECM along with additional laminin and collagen IV, exerts significant microenvironmental effects by enhancing the expression levels of representative β cell-associated genes and markers (i.e., INS and CHGA), as well as the glucose responsiveness and Ca2+ influx of encapsulated SC-derived islet cells, compared to those of collagen and pdECM bioink. In addition to the functional analysis of PINE bioink, we evaluated whether the core ECM components remained stable throughout the bioprinting and culture processes (Supplementary Fig. 4g). We compared the total collagen and glycosaminoglycan (GAG) contents between the cell-free PINE bioink and the PINE bioink encapsulating SC-derived islet cells and ECs under printed conditions. The results indicated no significant differences in collagen or GAG levels between the two conditions, suggesting that the essential ECM components remained intact following encapsulation and in vitro culture. Our findings indicate that the printing process and subsequent cell culture did not significantly alter the protein composition of the PINE bioink. This highlights its ability to provide a stable and supportive environment for the maturation of SC-derived islets. Furthermore, the PINE bioink supplemented with BM proteins enabled substantial improvement in spatial fidelity as a supportive bath material for in-bath bioprinting technique. BM proteins in native tissues have fibrous network structures that behave like physical barriers, and their modulus measurements range from 1 to 4.1 MPa, with values varying depending on the tissue location77. In the future, additional in-depth studies ascertaining the effects of BM proteins on the mechanical properties would highlight the advantages of adding BM proteins. Furthermore, while our study demonstrates key features of maturing islets, we acknowledge the need for further validation to comprehensively evaluate functional maturity. Specifically, markers such as MAFA and UCN3, which are critical indicators of β cell maturation, and real-time measuring of insulin secretion dynamics in response to varying glucose concentrations should be assessed in future studies to provide a more thorough understanding of the system’s ability to support functionality.
Utilizing bioprinting technology, particularly the in-bath bioprinting technique, we implemented a strategic arrangement of HICA-V to reproduce 3D pancreatic tissue-specific geometry. Islets are highly vascularized, receiving 10% of the blood flow of pancreas, although they make up only 1-2% of the total pancreatic tissue mass and intra-islet vasculatures allow sensitive detection of glucose levels and rapid supply of secreted insulin from β cells78. Furthermore, native islets possess enriched signaling and secretory elements at junctional edges within highly organized aggregate structures60. Considering the unique cellular organization of islets and peri-vasculatures, we hypothesized that the close proximal condition (~50 µm) of HICA and V could induce active crosstalk via gradual structural fusion and up-regulation of the combined secretion of growth factors from both ECs and SC-derived islets. Closely patterned HICA and V in the PINE bioink bath exhibited more compact structures over time, facilitated by the formation of tight cell-cell and cell-ECM networks within the printing path. Simultaneously, their sprouting behavior from the ECs in each structure extended toward each other, creating robust vascular networks. In contrast, the distal condition failed to achieve physical contact between HICA and V, probably leading to limited cellular communication for coordinating insulin secretion (Supplementary Fig. 5a). Geometrically guided HICA-V allowed for higher gene expression levels of junctions compared to randomly dispersed group and suspension-cultured group, thereby considerably enhancing the glucoregulatory functions of SC-derived islets. While our study demonstrated robust insulin secretion and gene expression, we recognize the limitations of imaging-based viability assessments in dense 3D constructs. Future studies incorporating quantitative approaches, such as lactate dehydrogenase release assays, apoptosis markers, or DNA-based cell quantification, could provide a more precise evaluation of cell survival and selective death, allowing for the development of more refined and optimized bioprinted models. Our study focused on proposing fully integrated SC-derived islets and vascular networks that mimic islet-specific cellular architectures within the peri-islet niche-like microenvironment. These findings offer valuable mechanistic insights into the intricate phenomena within the native islet niche, such as cell-cell communication networks and dynamic niche signals, which cannot be determined from isolated islets alone. To further elucidate the effects of bioprinting on the maturation of multicellular islets, future studies could benefit from the integration of single-cell RNA sequencing (scRNA seq)62,79. This approach could be utilized to profile the transcriptional landscape of individual cells within bioprinted islets and peri-vasculature, offering insights into how bioprinting configurations influence the cellular composition, differentiation states, and maturation of SC-derived islets. Importantly, scRNA-seq could clarify the specific contributions of vascular and islet components to observed changes in gene expression, addressing the inherent limitations of qPCR, which reflects only the overall gene expression levels of the entire constructs. Furthermore, it could reveal how ECM-based biochemical cues influence each cellular component in HICA-V and their multicellular interactions, providing a deeper understanding of the bioprinted microenvironment. Comparing the scRNA-seq profiles of HICA-V across various printing conditions and time points would help identify optimal parameters for achieving complete β cell maturation.
Further studies should incorporate a more intricate pancreatic cellular organization. In terms of the cell-intrinsic ability, the transcriptomic profile of human pancreatic microvascular endothelial cells (hPanc Endothelial) was compared with that of HUVECs80. Principal component analysis of the HUVECs transcriptomes revealed a profile similar to that of hPanc Endothelial, indicating the suitability of HUVECs for mimicking pancreatic ECs and forming vascular niches. However, to ensure clinical translatability of pancreatic models encompassing ECs, pancreatic tissue-specific endothelial cell heterogeneity must be considered81. Furthermore, in the native islet niche, pericytes cover 40% of the microvasculature and are crucial in regulating local blood flow to ensure proper insulin release into the circulation82. Although our study did not specifically investigate the presence of pericytes within the vasculature of our bioprinted HICA-V model or their effect on the hormonal output of HICA-V, further studies are needed to elucidate the detailed composition of the islet vasculature and its metabolic impact. Moreover, nerve cells play a significant role in regulating pancreatic hormone secretion, thereby generating neuronal signals to suppress insulin secretion and counteract hypoglycemia83. Specifically, most sympathetic axons that penetrate islets, interact with contractile smooth muscle cells of vessels within the islets, and regulate local circulation, influencing hormone secretion84,85. Using more complex engineered HICA-V with pancreatic innervation could pave the way for therapeutic approaches that consider the connection between local blood flow control via innervation and subsequent insulin release. In this study, we verified whether HICA-V could simulate the response to diabetes, particularly hyperglycemia, which is deeply associated with endocrine tissue, and conducted drug testing to validate whether we could accurately reproduce physiologically relevant phenomena. To study deeper pathophysiological phenomena in the peri-islet niche, combining both endocrine and exocrine niches will provide valuable insights into the interaction between islet cells and acinar and ductal cells, which is also crucial in diabetes research.
Although there is a significant need to gain a deeper understanding of the coordinated interactions between islets and the vasculature, particularly in the context of hyperglycemia, and metabolic disorders such as diabetes, only a few relevant studies have been conducted to develop islet-vasculature models21,86,87. Indeed, diabetes is accompanied by inflammation, characterized by elevated expression levels of pro-inflammatory cytokines, along with blood vessel pathologies, such as reduced capillary densities and upregulation of vascular cell adhesion molecules88,89,90. These conditions result in impaired functional coordination between the islet and vasculature, leading to deterioration in the glucose responsiveness of islets36. Previous antidiabetic drug testing platforms based on 3D culture systems, such as microfluidic devices and encapsulation systems, have focused primarily on β cell functions while often overlooking the complex interactions between islets and their peripheral niche67,91,92. We expected that HICA-V would represent the inevitable functional relationship between islets and the vasculature, which should be considered for implementing realistic diabetic conditions, in both healthy and dysfunctional phenotypes. In this study, HICA-V reproduced pathophysiologically relevant responses, such as the upregulation of inflammation and vascular adhesion proteins under hyperglycemic conditions and restoration of insulin secretory functions after antidiabetic drug treatment71,72,93,94,95. Furthermore, pharmacological effects of the combination of Empa and Met, which concurrently target ECs and β cells, were convincingly demonstrated using HICA-V. These findings suggest that HICA-V further enables the evaluation of pharmacological interventions targeting both β cells and ECs, thus allowing the investigation of the islet-specific niche in the context of diabetic therapeutics.
Future research could explore the adaptation of HICA-V for transplantation applications by refining its vascular components to create transplantable, fully matured SC-derived islet constructs96,97,98. Recent advancements emphasize the importance of developing vascular systems with capillaries spaced within diffusion limits, branching from larger vessels capable of withstanding pressure and connecting with the host’s blood supply35,40. By leveraging bioprinting technologies, HICA-V could be designed with sophisticated vascular patterns that promote efficient perfusion, which is essential for long-term graft function and survival99,100. This approach could create a functional prevascularized niche, facilitating the integration of bioprinted vasculature with the host’s circulatory system to ensure adequate support for islets post-transplantation. Furthermore, achieving perfusion in HICA-V would enable the study of metabolite dynamics, including insulin transport, within the intra-islet vasculature and peri-vasculature. By using fluorescent dyes to track these metabolites, we would able to visualize and quantify their movement across vascular networks, offering valuable insights into nutrient exchange, hormone distribution, and paracrine signaling. These studies would enhance our understanding of islet physiology, providing mechanistic insights that cannot be obtained from static models or isolated islets while also positioning HICA-V as a valuable platform for both therapeutic transplantation and disease modeling, with significant potential for clinical applications.
Overall, the bioprinted HICA-V using the PINE bioink successfully achieved crucial features resembling mature native islets as follows: (1) the structural aspects of highly clustered islet morphogenesis and tight connection with the vascular networks that induce timely insulin secretion with enriched signaling molecules; (2) functional properties such as stable glucose responsiveness and high expression levels of metabolic markers; and (3) physio-mimetic responses spanning from healthy to diabetic states, emphasizing the importance of implementing a bespoke niche to test various drug combinations. Our engineering approach, involving bioprinting-based bespoke niches with advanced islet-specific cues, offers promising avenues for mechanistic studies on how environmental factors influence islet development, maturation, and modeling of diabetic diseases and enhances the potential for translating diabetic therapeutics into clinical applications.
Methods
Ethical statement
This work was approved by the Human Stem Cell Research Oversight Committee at Pohang University of Science and Technology (PIRB-2020-E043) and the Asan Medical Center Institutional Review Board (2022-0251-029, 2022-0251-036, 2022-0251-037) with appropriate consent and conditions.
Preparation of bioink
To prepare Col hydrogel, collagen I (PC-001, DALIM TISSEN) powder was dissolved using 0.1 M hydrochloric acid (H1758, Sigma Aldrich) and neutralized with 10 N NaOH (S2018, Biosesang). The pdECM was prepared following previously published methods22,24. Briefly, the porcine pancreas was chopped into 1 mm-thick pieces using grater. These sliced tissues were washed with distilled water for 12 h. Then, the pancreatic tissues underwent treatment with a 1% Triton X-100 solution (T1020, Biosesang) for 84 h. Subsequently, tissues were treated with a 2-propanol (000I0348, Samchun Chemicals, Republic of Korea.) for 2 h, followed by phosphate-buffered saline (PBS) for 12 h. For sterilization, the tissues were treated with 0.1% peracetic acid in 4% ethanol for 2 h. After 6 h washing with PBS, the resulting decellularized tissues were lyophilized and stored at −80 °C. The lyophilized pdECM underwent digestion to transform it into a hydrogel by dissolution in 0.5 M acetic acid and 10% (w/w) pepsin for 5 days at 330 rpm. The pdECM hydrogel was neutralized with 10 N NaOH and 10X PBS, and the volume was adjusted with 1X PBS. To prepare 10 mg/mL PINE hydrogel, laminin (354259, Corning) and type IV collagen (C5533, Sigma Aldrich) at a concentration of 0.1 mg/mL, respectively, were supplemented in 9.8 mg/mL pdECM. For PINE bioink preparation, hESC-derived islet cells and ECs were combined in a 5:1 ratio at 1 × 108 cells/mL for printing HICA. Subsequently, ECs were encapsulated at a concentration of 4 × 107 cells/mL to print vasculatures. The final concentration of PINE bioink, mixed with the resulting cell suspensions, was 5 mg/mL.
Proteomic analysis
ECM compositions of pdECM and Col were identified using high-resolution liquid chromatography-mass spectrometry-based proteomic analysis. Initially, a buffer containing 8 M urea and 50 mM Tris (pH 8.0) was used to denature the proteins in the pdECM and Col. Following reduction and alkylation, the solution was diluted with 50 mM Tris to achieve a final concentration of 1 M urea. The ECM proteins were then digested into peptides using a trypsin/Lys-C protease mixture, with an enzyme-to-substrate ratio of 1:50, and the samples were incubated at 37 °C for 16 h. Digestion cessation involved the addition of 0.4% trifluoroacetate, and C18 macro spin columns (Harvard Apparatus, Holliston, MA) were employed for sample desalting. The resulting peptides were lyophilized, reconstituted in 100 µL of 0.1% formic acid, and subjected to quantification using the Q Exactive Plus Hybrid Quadrupole-Orbitrap mass spectrometer coupled with a Nanoacquity UPLC (Waters, Manchester, UK). Elution occurred through a trap column, and ionization was facilitated by an NSI system linked with a fused-silica column filled with C18 particles of 2 µm, operating at an electric potential of 2.0 kV. In the MS/MS process, the maximal ion injection time and dynamic exclusion time were configured as 60 ms and 30 s, respectively. Subsequently, Proteome Discoverer version 2.2. (Thermo Fisher Scientific) was employed to identify the proteomic composition of the pdECM and Col from the resulting files. A Matrisome analysis was conducted by matching the human matrisome database with each identified gene101. GO analysis was carried out using the PANTHER classification system102 and the DAVID program (https://david.ncifcrf.gov/).
Cell culture and differentiation
The H1 human embryonic stem cell line (20-W0403, WiCell institute) was utilized for differentiation of pancreatic β cells following a protocol derived from published methods6,7. H1 hESC were cultured in TeSR-E8 medium (ST05990, STEMCELL Technologies) on Matrigel-coated (356231, Corning) plates at 37 °C for stemness maintenance. The cells were fed with fresh medium every day from stage 1 to stage 5 for differentiation. To induce pancreatic β cell differentiation, hESC were seeded at a concentration of 4.9 × 106 cells/well in a 6-well cell culture plate with TeSR-E8 medium supplemented with 10 µM Y-27632 (1254, Tocris Bioscience). After 24-h, the medium was replaced with Base (BE) 1 medium supplemented with 100 ng/mL Activin A (338-AC, R&D systems) and 3 µM CHIR99021 (13122, Cayman Chemical) for 24 h (day 1). On day 2, the cells were cultured with BE1 medium supplemented with 100 ng/mL Activin A. On day 5, the medium was changed to BE2 medium supplemented with 50 ng/mL KGF (AF-100-19, Peprotech). On day 7, the medium was replaced to BE3 medium supplemented with 50 ng/mL KGF, 0.2 µM TPPB (5343, Tocris Bioscience), 0.25 µM SANT1 (S4572, Sigma Aldrich), 2 µM retinoic acid (R2625, Sigma Aldrich), and 0.2 µM LDN (04-1974, Stemgent). On day 9, the cells were cultured with BE3 medium supplemented with 50 ng/mL KGF, 0.2 µM TPPB, 0.25 µM SANT1, 0.2 µM retinoic acid, and 0.2 µM LDN. On day 13, the medium was changed to stage 5 medium supplemented with 1 µM XXI (15579, Cayman Chemical), 10 µM Alk 5 inhibitor II (ALX-270-445, Enzo Life Sciences), 1 µM T3 (T6397, Sigma Aldrich), 0.25 µM SANT1, 0.2 µM retinoic acid, 1 µM Latrunculin A (10010630, Cayman Chemical). After 24 h, the medium was replaced with the same composition without Latrunculin A. On day 20, the cells were fed with ESFM, and the medium was changed every alternate day. On day 27, the differentiated cells were dissociated for encapsulation. The media composition for differentiation is detailed in Supplementary Table 1. For co-culturing with the vascular cells, HUVECs (C-12200, Promocell) and RFP-HUVECs (cAP-0001RFP, Angio-Proteomie) were maintained in EGM-2 medium (C-22011, Promocell) on a 0.1% gelatin-coated tissue culture dish. HUVECs were co-cultured with the differentiated islet cells using a mixed medium of CMRL medium (11530037, Thermo Fisher Scientific) and EGM-2 in a 1:1 ratio. Human pancreatic islets from deceased nondiabetic donors were obtained from the Asan Medical Center (Supplementary Table 3).
Rheological analysis
For evaluating the rheological properties of the 5 mg/mL Col, pdECM, and PINE hydrogel, a rheometer (Discovery HR-2, TA Instruments) was utilized. Firstly, viscosity was assessed through a shear rate sweep ranging from 0.1 to 1000 s−1 at 4 °C, aligning with the printing temperature. Secondly, the shear recovery behavior of each hydrogel was explored by altering shear strain to 1% and 300%, with the temperature held constant at 15 °C.
Fabrication of HICA-V using PINE bioink
We utilized a micro extrusion-based 3D bioprinting system (3DXPrinter, T&R Biofab) for the printing of PEVA polymeric frame and HICA-V. PEVA was printed as polymeric frame to sustain 3D structure of HICA-V. PEVA was heated in a printing barrel to 120 °C and then extruded through a 25 N nozzle, using 500 kPa of pneumatic pressure. The PINE bioink without cells was filled within the PEVA frame as a bath, subsequently, ECs-laden PINE bioink, at a cell density of 4 × 106 cells per ml, was extruded in a linear pattern to print vasculature through a 27 G nozzle at 0.5 kPa pneumatic pressure within the bath. For HICA printing, islet cells and ECs-laden PINE bioink, at a cell density of 107 cells per ml, was extruded in an aggregate shape through a 30 G nozzle at 5 kPa pneumatic pressure within the bath. Following this, the bioprinted HICA-V was incubated at 37 °C for 1 h for thermal crosslinking. The crosslinked HICA-V was supplied with CMRL/EGM2 medium supplemented with 50 ng/mL VEGF (100-20, Peprotech), and the PEVA frame was removed in this process.
Immunostaining
All samples for immunofluorescence staining were fixed in 4% paraformaldehyde (CBPF-90004-1000, Chembio) for 30 min at room temperature (RT), followed by washing with 1X PBS. The resulting samples were permeabilized with 0.1% Triton X-100 for 20 min, and blocked with 5% normal goat serum (50062Z, Gibco) for 3 h at RT. The samples underwent incubation with primary antibodies for 16 h at 4 °C. To illustrate the cellular composition and arrangement, the following specific markers were employed. Rabbit anti-insulin antibody (1:100, 3014S, Cell Signaling Technology), Rabbit anti-C-peptide antibody (1:100, 4593S, Cell Signaling Technology), Mouse anti-NKX6.1 antibody (1:100, F55A12, Developmental Studies Hybridoma Bank), Guinea pig anti-PDX1 antibody (1:100, ab47308, Abcam), Mouse anti-chromogranin A antibody (1:100, MA5-13096, Invitrogen), Mouse anti-glucagon antibody (1:100, SC-514592, Santa Cruz Biotechnology), Mouse anti-somatostatin antibody (1:100, SC-55565, Santa Cruz Biotechnology), Mouse anti-CD31 antibody (1:50, ab9498, Abcam) were used to stain SC-derived hormonal cells or endothelial cells. After rinsing with 1X PBS, the primary antibodies were identified through a 2 h incubation with Goat anti-Mouse Alexa Fluor 488-(1:200, A11029, Invitrogen) and Goat anti-Rabbit Alexa Fluor 594-(1:200, A11037, Invitrogen) conjugated secondary antibodies at RT. The resulting samples were washed with 1X PBS. A mounting medium containing DAPI (1:1000, H1500, Vector Lab) was applied for nucleus staining and simultaneous sample mounting. Cell imaging was conducted using both a confocal microscope (LEICA DMi 8, Leica Microsystems, Germany) and a fluorescent confocal laser scanning microscope (Nikon Ti Eclipse, Nikon, Japan). The obtained images were subsequently processed using ImageJ software (National Institute of Health, USA), and each differentiation marker, including transcriptional factors and pancreatic hormones, was normalized to the DAPI count for quantification of the fraction of cells expressing the indicated markers.
Cell viability test
The viability of HICA-V was assessed using a LIVE/DEAD Viability/Cytotoxicity Kit (L3224, Thermo Fisher Scientific). Briefly, a combination of calcein-AM (2 μL) and ethidium homodimer-1 (2 μL) was diluted with 1X PBS to create a staining solution (1 mL), and this solution was applied to samples, followed by incubation at 37 °C for 30 min. The fluorescence was detected and captured using the NIS-Elements Advanced Research software on a fluorescent confocal laser scanning microscope (Nikon Ti Eclipse; Nikon, Japan).
Quantitative reverse transcription-polymerase chain reaction analysis
The total mRNA from each sample was extracted using TRIzol (9109, Takara) and quantified with a NanoDrop™ Lite Spectrophotometer (Thermo Fisher Scientific). Reverse transcription was carried out using a complementary DNA synthesis kit (18091050, Thermo Fisher Scientific) following the provided protocol. Comparative analysis of gene expression was conducted using SYBR Green on a StepOnePlus Real-Time PCR system (Applied Biosystems). The primer sequences, derived from published gene sequences (NCBI and PubMed), are detailed in Supplementary Table 2. Data analysis was performed using StepOne software v2.3, with expression levels of each target gene normalized to that of the housekeeping gene (GAPDH) and calculated using the 2−ΔΔCT method.
Glucose-stimulated insulin secretion test
To evaluate the functionality of SC-derived β cells encapsulated in each bioink, GSIS assay was conducted. Prior to experimentation, the samples underwent washing twice with KRB buffer composed of 128 mM NaCl (S3014, Sigma Aldrich), 5 mM KCl (P9541, Sigma Aldrich), 2.7 mM CaCl22H2O (C7902, Sigma Aldrich), 1.2 mM MgSO4 (M7506, Sigma Aldrich) 1 mM Na2HPO4 (S3264, Sigma Aldrich), 1.2 mM KH2PO4 (P9791, Sigma Aldrich), 5 mM NaHCO3 (S5761, Sigma Aldrich), and 10 mM HEPES (15630080, Gibco). The samples were initially incubated in KRB containing 2 mM glucose (G7528, Sigma Aldrich) at 37 °C for 2 h. Then, this solution was discarded, and the samples were washed twice with fresh KRB, followed by replacement with 2 mM glucose KRB buffer. After 1 h, the supernatant was collected, and the samples were washed with fresh KRB. Subsequently, 20 mM glucose KRB buffer was introduced for the next 1 h, and the supernatant was collected again. Insulin secreted by SC-derived β cells were quantified using Ultrasensitive Human Insulin ELISA kit (80-INSHUU-E01.1, ALPCO) following the manufacturer’s instructions.
Calcium imaging
SC-derived islet cells encapsulated in bioink were stained with Fluo-4, AM (F14201; Thermo Fisher Scientific) to visualize calcium signal. After washing twice with KRB buffer, 10 μM Fluo-4, AM diluted in Krebs buffer was treated for 20 min. Afterwards, KRB buffer contained 20 mM glucose and 10 nM of Exendin-4 (E7144, Sigma Aldrich) was treated and incubated for 20 min at 37 °C. The calcium signal was recorded at 30 frames per second using an ORCA-Flash4.0 V3 Digital CMOS camera (C13440-20CU, Hamamatsu, Japan). Individual Ca2+ traces of encapsulated cells were quantified and generated using custom-made MATLAB software.
Anti-diabetic drug testing
The bioprinted HICA-V was cultured for 2 days to achieve stabilization. To assess the effectiveness of anti-diabetic drugs, it was exposed to CMRL/EGM2 media supplemented with 30 mM glucose (high glucose) for 3 days to induce a hyperglycemic-like condition. Following this, treatment with 20 µM Metformin (PHR1084, Sigma Aldrich) and 0.8 µM Empagliflozin (17375, Cayman Chemical) was administered in the high glucose culture medium for an additional 3 days.
Biochemical analysis
The GAG content in PINE bioinks, both with and without cells, was assessed using a GAG quantification kit (B3000, Biocolor) following the manufacturer’s protocol. To GAG extraction, 10 mg of the lyophilized samples were digested with papain solution (P4762, Sigma Aldrich) at 60 °C for 16 h. After centrifugation, the pellets were resuspended in deionized water. The test samples and reference standards were then incubated with a dye reagent for 30 min. Following another centrifugation step, dissociation reagent was added to the samples, and the absorbance was measured at 656 nm using a microplate reader (Thermo Fisher Scientific, USA). The collagen content was quantified using a hydroxyproline colorimetric assay kit (K555, BioVision) according to the manufacturer’s instructions. 10 mg of lyophilized PINE bioink was homogenized in deionized water, and the samples and standards were hydrolyzed with ~12 M hydrochloric acid (H1758, Sigma Aldrich) at 120 °C for 3 h. After cooling to RT, Chloramine T was added and allowed to react for 10 min. The mixture was then treated with dimethyl methylene blue reagent and incubated at 60 °C for 90 min. The absorbance was measured at 560 nm using a microplate reader (Thermo Fisher Scientific, USA).
Statistical analysis and reproducibility
For each experiment, a minimum of n = 3 independent samples was used for every experimental group. No data were excluded from the analyses. All assays were conducted in at least three independent replicates, with consistent results across all repetitions. Samples were randomly selected when multiple samples were used for experiments. All quantitative data are presented as means ± SD. The n numbers specified in the figure legends, representing biological replicates. Statistical analysis utilized GraphPad Prism 9 software, employing unpaired two-sided t-tests or ANOVA, followed by Dunnett’s multiple comparisons test, Tukey’s multiple comparisons test, or Sidak’s multiple comparisons test. Significance levels are denoted by one asterisk for P-values < 0.05, two asterisks for P-values < 0.01, three asterisks for P-values < 0.001, and four asterisks for P-values < 0.0001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD047892. The protein samples were identified by MS/MS data of peptides against the Sus scrofa (pig) Uniprot database. Proteins identified in col and pdECM were compared with the datasets in Matrisome DB and analyzed with PANTHER classification system and DAVID program [https://david.ncifcrf.gov/]. All data generated during this study are available within the article and its supplementary information. Source data are provided with this paper.
Code availability
The g-codes for HICA-V printing have been provided in the Supplementary Information file.
Change history
05 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41467-025-57524-z
References
Patel, S. N., Mathews, C. E., Chandler, R. & Stabler, C. L. The foundation for engineering a pancreatic islet niche. Front Endocrinol. 13, 1–20 (2022).
Fowler, J. L. et al. Three-dimensional analysis of the human pancreas. Endocrinology 159, 1393–1400 (2018).
Burganova, G., Bridges, C., Thorn, P. & Landsman, L. The role of vascular cells in pancreatic beta-cell function. Front Endocrinol. 12, 1–9 (2021).
Tixi, W. et al. Coordination between ECM and cell-cell adhesion regulates the development of islet aggregation, architecture, and functional maturation. Elife 12, e90006 (2023).
Jaramillo, M., Mathew, S., Mamiya, H., Goh, S. K. & Banerjee, I. Endothelial cells mediate islet-specific maturation of human embryonic stem cell-derived pancreatic progenitor cells. Tissue Eng. Part A 21, 14–25 (2015).
Hogrebe, N. J., Augsornworawat, P., Maxwell, K. G., Velazco-Cruz, L. & Millman, J. R. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 38, 460–470 (2020).
Hogrebe, N. J., Maxwell, K. G., Augsornworawat, P. & Millman, J. R. Generation of insulin-producing pancreatic β cells from multiple human stem cell lines. Nat. Protoc. 16, 4109–4143 (2021).
Rezania, A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133 (2014).
Velazco-Cruz, L. et al. Acquisition of dynamic function in human stem cell-derived β cells. Stem Cell Rep. 12, 351–365 (2019).
Chmielowiec, J. et al. Human pancreatic microenvironment promotes β-cell differentiation via non-canonical WNT5A/JNK and BMP signaling. Nat. Commun. 13, 1952 (2022).
Augsornworawat, P., Maxwell, K. G., Velazco-Cruz, L. & Millman, J. R. Single-cell transcriptome profiling reveals β cell maturation in stem cell-derived islets after transplantation. Cell Rep. 32, 108067 (2020).
Balboa, D. et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat. Biotechnol. 40, 1042–1055 (2022).
Kim, B. S., Das, S., Jang, J. & Cho, D. W. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem. Rev. 120, 10608–10661 (2020).
Asthana, A. et al. Comprehensive characterization of the human pancreatic proteome for bioengineering applications. Biomaterials 270, 120613 (2021).
Santini-González, J. et al. In vitro generation of peri-islet basement membrane-like structures. Biomaterials 273, 120808 (2021).
Llacua, L. A., de Haan, B. J. & de Vos, P. Laminin and collagen IV inclusion in immunoisolating microcapsules reduces cytokine-mediated cell death in human pancreatic islets. J. Tissue Eng. Regen. Med 12, 460–467 (2018).
Pal, V., Wang, Y., Regeenes, R., Kilkenny, D. M. & Rocheleau, J. V. Laminin matrix regulates beta-cell FGFR5 expression to enhance glucose-stimulated metabolism. Sci. Rep. 12, 1–15 (2022).
Brandhorst, D. et al. Basement membrane proteins improve human islet survival in hypoxia: Implications for islet inflammation. Acta Biomater. 137, 92–102 (2022).
Yang, K. et al. A 3D culture platform enables development of zinc-binding prodrugs for targeted proliferation of β cells. Sci. Adv. 6, 1–12 (2020).
Jiang, K. et al. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials 198, 37–48 (2019).
Kim, J. J. et al. Pathophysiological reconstruction of a tissue-specific multiple-organ on-a-chip for type 2 diabetes emulation using 3D cell printing. Adv. Funct. Mater. 33, 2213649 (2023).
Kim, J. et al. Pancreatic tissue-derived extracellular matrix bioink for printing 3D cell-laden pancreatic tissue constructs. J. Vis. Exp. 2019, 3–9 (2019).
Kim, J. et al. 3D cell printing of islet-laden pancreatic tissue-derived extracellular matrix bioink constructs for enhancing pancreatic functions. J. Mater. Chem. B 7, 1773–1781 (2019).
Hwang, D. G. et al. A 3D bioprinted hybrid encapsulation system for delivery of human pluripotent stem cell-derived pancreatic islet-like aggregates. Biofabrication 14, (2022).
Sackett, S. D. et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 8, 1–16 (2018).
Bi, H., Ye, K. & Jin, S. Proteomic analysis of decellularized pancreatic matrix identifies collagen V as a critical regulator for islet organogenesis from human pluripotent stem cells. Biomaterials 233, 119673 (2020).
Gaetani, R. et al. Evaluation of different decellularization protocols on the generation of pancreas-derived hydrogels. Tissue Eng. Part C. Methods 24, 697–708 (2018).
Takahashi, Y., Sekine, K., Kin, T., Takebe, T. & Taniguchi, H. Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation. Cell Rep. 23, 1620–1629 (2018).
Forbes, S. et al. Human umbilical cord perivascular cells improve human pancreatic islet transplant function by increasing vascularization. Sci. Transl. Med 12, eaan5907 (2020).
Brissova, M. et al. Human islets have fewer blood vessels than mouse islets and the density of islet vascular structures is increased in type 2 diabetes. J. Histochem. Cytochem. 63, 637–645 (2015).
Talavera-Adame, D. et al. Effective endothelial cell and human pluripotent stem cell interactions generate functional insulin-producing beta cells. Diabetologia 59, 2378–2386 (2016).
Augsornworawat, P., Velazco-Cruz, L., Song, J. & Millman, J. R. A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells. Acta Biomater. 97, 272–280 (2019).
Nguyen-Ngoc, K.-V. et al. Engineered vasculature induces functional maturation of pluripotent stem cell-derived islet organoids. https://doi.org/10.1101/2022.10.28.513298.
Song, W. et al. Engineering transferrable microvascular meshes for subcutaneous islet transplantation. Nat. Commun. 10, 4602 (2019).
Aghazadeh, Y. et al. Microvessels support engraftment and functionality of human islets and hESC-derived pancreatic progenitors in diabetes models. Cell Stem Cell 28, 1936–1949.e8 (2021).
Hogan, M. F. & Hull, R. L. The islet endothelial cell: a novel contributor to beta cell secretory dysfunction in diabetes. Diabetologia 60, 952–959 (2017).
Staels, W., Heremans, Y., Heimberg, H. & De Leu, N. VEGF-A and blood vessels: a beta cell perspective. Diabetologia 62, 1961–1968 (2019).
Pepper, A. R. et al. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol. 33, 518–523 (2015).
Vlahos, A. E., Cober, N. & Sefton, M. V. Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets. Proc. Natl Acad. Sci. USA 114, 9337–9342 (2017).
Bowers, D. T., Song, W., Wang, L. H. & Ma, M. Engineering the vasculature for islet transplantation. Acta Biomater. 95, 131–151 (2019).
Ravnic, D. J., Leberfinger, A. N. & Ozbolat, I. T. Bioprinting and cellular therapies for type 1 diabetes. Trends Biotechnol. 35, 1025–1034 (2017).
Jo, Y., Hwang, D. G., Kim, M., Yong, U. & Jang, J. Bioprinting-assisted tissue assembly to generate organ substitutes at scale. Trends Biotechnol. 41, 93–105 (2023).
Liu, X. et al. Development of a coaxial 3D printing platform for biofabrication of implantable islet-containing constructs. Adv. Health. Mater. 8, e1801181 (2019).
Song, J. & Millman, J. R. Economic 3D-printing approach for transplantation of human stem cell-derived β-like cells. Biofabrication 9, 015002 (2017).
Llacua, L. A., Faas, M. M. & de Vos, P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia 61, 1261–1272 (2018).
Vlahos, A. E. et al. Endothelialized collagen based pseudo-islets enables tuneable subcutaneous diabetes therapy. Biomaterials 232, 119710 (2020).
Seo, H., Son, J. & Park, J. K. Controlled 3D co-culture of beta cells and endothelial cells in a micropatterned collagen sheet for reproducible construction of an improved pancreatic pseudo-tissue. APL Bioeng. 4, 046103 (2020).
Wieland, F. C., van Blitterswijk, C. A., van Apeldoorn, A. & LaPointe, V. L. S. The functional importance of the cellular and extracellular composition of the Islets of Langerhans. J. Immunol. Regen. Med. 13, 100048 (2021).
Llacua, L. A., Hoek, A., de Haan, B. J. & de Vos, P. Collagen type VI interaction improves human islet survival in immunoisolating microcapsules for treatment of diabetes. Islets 10, 60–68 (2018).
Hughes, S. J. et al. Comparison of the collagen VI content within the islet-exocrine interface of the head, body, and tail regions of the human pancreas. in Transplantation Proceedings 37, 3444–3445 (2005).
Kim, T. et al. Dermatopontin in skeletal muscle extracellular matrix regulates myogenesis. Cells 8, 332 (2019).
Zhou, S., Chen, S., Pei, Y. A. & Pei, M. Nidogen: a matrix protein with potential roles in musculoskeletal tissue regeneration. Genes Dis. 9, 598–609 (2022).
Low, S. W. Y., Connor, T. B., Kassem, I. S., Costakos, D. M. & Chaurasia, S. S. Small leucine-rich proteoglycans (Slrps) in the retina. Int. J. Mol. Sci. 22, 7293 (2021).
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221 (2022).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Kim, B. S. et al. Construction of tissue-level cancer-vascular model with high-precision position control via in situ 3d cell printing. Small Methods 5, 2100072 (2021).
Ahn, M., Cho, W. W., Kim, B. S. & Cho, D. W. Engineering densely packed adipose tissue via environmentally controlled in-bath 3D bioprinting. Adv. Funct. Mater. 32, 2200203 (2022).
El-Gohary, Y. & Gittes, G. Structure of Islets and Vascular Relationship to the Exocrine Pancreas. https://doi.org/10.3998/panc.2017.10.
Ayan, B. et al. Aspiration-assisted freeform bioprinting of pre-fabricated tissue spheroids in a yield-stress gel. Commun. Phys. 3, 1–14 (2020).
Geron, E., Boura-Halfon, S., Schejter, E. D. & Shilo, B. Z. The edges of pancreatic islet β cells constitute adhesive and signaling microdomains. Cell Rep. 10, 317–325 (2015).
Dybala, M. P. et al. Integrated pancreatic blood flow: bidirectional microcirculation between endocrine and exocrine pancreas. Diabetes 69, 1439–1450 (2020).
Skylar-Scott, M. A. et al. Orthogonally induced differentiation of stem cells for the programmatic patterning of vascularized organoids and bioprinted tissues. Nat. Biomed. Eng. 6, 449–462 (2022).
Benninger, R. K. P., Zhang, M., Steven Head, W., Satin, L. S. & Piston, D. W. Gap junction coupling and calcium waves in the pancreatic islet. Biophys. J. 95, 5048–5061 (2008).
Speier, S., Gjinovci, A., Charollais, A., Meda, P. & Rupnik, M. Cx36-mediated coupling reduces β-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes 56, 1078–1086 (2007).
Johnston, N. R. et al. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab. 24, 389–401 (2016).
Farnsworth, N. L. & Benninger, R. K. P. New insights into the role of connexins in pancreatic islet function and diabetes. FEBS Lett. 588, 1278–1287 (2014).
Jun, Y. et al. In vivo–mimicking microfluidic perfusion culture of pancreatic islet spheroids. Sci. Adv. 5, eaax4520 (2019).
Kuboki, K. et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo—a specific vascular action of insulin. Circulation 101, 676–681 (2000).
Kolodziejski, P. A. et al. FGF-1 modulates pancreatic β-cell functions/metabolism: an in vitro study. Gen. Comp. Endocrinol. 294, 113498 (2020).
Doherty, D. T., Khambalia, H. A., van Dellen, D., Jennings, R. E. & Piper Hanley, K. Unlocking the post-transplant microenvironment for successful islet function and survival. Front. Endocrinol. 14, 1–9 (2023).
Hogan, M. F. et al. SGLT2-i improves markers of islet endothelial cell function in db/db diabetic mice. J. Endocrinol. 248, 95–106 (2021).
Mone, P. et al. SGLT2 inhibition via empagliflozin improves endothelial function and reduces mitochondrial oxidative stress: insights from frail hypertensive and diabetic patients. Hypertension 79, 1633–1643 (2022).
Stumvoll, M., Goldstein, B. J. & Van Haeften, T. W. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365, 1333–1346 (2005).
Beckman, J. A. & Creager, M. A. Vascular complications of diabetes. Circ. Res 118, 1771–1785 (2016).
Almaça, J., Caicedo, A. & Landsman, L. Beta cell dysfunction in diabetes: the islet microenvironment as an unusual suspect. Diabetologia 63, 2076–2085 (2020).
Krishnamurthy, M. et al. Integrin α3, but not β1, regulates islet cell survival and function via PI3K/Akt signaling pathways. Endocrinology 152, 424–435 (2011).
Jain, P., Rauer, S. B., Möller, M. & Singh, S. Mimicking the natural basement membrane for advanced tissue engineering. Biomacromolecules 23, 3081–3103 (2022).
Peiris, H., Bonder, C. S., Coates, P. T. H., Keating, D. J. & Jessup, C. F. The β-Cell/EC axis: How do islet cells talk to each other? Diabetes 63, 3 LP–11 (2014).
Lawlor, K. T. et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater. 20, 260–271 (2021).
Yoshihara, E. et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 586, 606–611 (2020).
Urbanczyk, M., Zbinden, A. & Schenke-Layland, K. Organ-specific endothelial cell heterogenicity and its impact on regenerative medicine and biomedical engineering applications. Adv. Drug Deliv. Rev. 186, 114323 (2022).
Tamayo, A. et al. Pericyte control of blood flow in intraocular islet grafts impacts glucose homeostasis in mice. Diabetes 71, 1679–1693 (2022).
Alvarsson, A. et al. A 3D atlas of the dynamic and regional variation of pancreatic innervation in diabetes. Sci. Adv. 6, 1–18 (2020).
Campbell-Thompson, M. et al. Islet sympathetic innervation and islet neuropathology in patients with type 1 diabetes. Sci. Rep. 11, 1–17 (2021).
Rodriguez-Diaz, R. et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 14, 45–54 (2011).
Hugh, R. et al. A vascularized 3D model of the human pancreatic islet for ex vivo study of immune cell-islet interaction. https://doi.org/10.1101/2021.12.21.473744.
Kim, M. & Jang, J. Construction of 3D hierarchical tissue platforms for modeling diabetes. APL Bioengineering 5, 041506 (2021).
Tan, Y. et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat. Rev. Cardiol. 17, 585–607 (2020).
Drawnel, F. M. et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 9, 810–820 (2014).
Wang, L. et al. Mesenchymal stem cells ameliorate β cell dysfunction of human type 2 diabetic islets by reversing β cell dedifferentiation. EBioMedicine 51, 102615 (2020).
Goswami, I., de Klerk, E., Carnese, P., Hebrok, M. & Healy, K. E. Multiplexed microfluidic platform for stem-cell derived pancreatic islet β cells. Lab Chip 22, 4430–4442 (2022).
Wang, X. et al. Construction of engineered 3D islet micro-tissue using porcine decellularized ECM for the treatment of diabetes. Biomater. Sci. 11, 5517–5532 (2023).
Wimmer, R. A. et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510 (2019).
Cole, J. B. & Florez, J. C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0278-5 (2020).
Tsakmaki, A., Fonseca Pedro, P. & Bewick, G. A. Diabetes through a 3D lens: organoid models. Diabetologia 63, 1093–1102 (2020).
Laurent, J. et al. Convergence of microengineering and cellular self-organization towards functional tissue manufacturing. Nat. Biomed. Eng. 1, 939–956 (2017).
Shapiro, A. M. J., Pokrywczynska, M. & Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 13, 268–277 (2017).
Grattoni, A. et al. Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead. Nat. Rev. Endocrinol. https://doi.org/10.1038/s41574-024-01029-0 (2024).
Szklanny, A. A. et al. 3D bioprinting of engineered tissue flaps with hierarchical vessel networks (VesselNet) for direct host-to-implant perfusion. Adv. Mater. 33, e2102661 (2021).
Gao, G., Park, J. Y., Kim, B. S., Jang, J. & Cho, D. W. Coaxial cell printing of freestanding, perfusable, and functional in vitro vascular models for recapitulation of native vascular endothelium pathophysiology. Adv. Health. Mater. 7, 1–12 (2018).
Shao, X., Taha, I. N., Clauser, K. R., Gao, Y. (Tom) & Naba, A. MatrisomeDB: the ECM-protein knowledge database. Nucleic Acids Res. 48, D1136–D1144 (2020).
Mi, H., Muruganujan, A., Casagrande, J. T. & Thomas, P. D. Large-scale gene function analysis with the panther classification system. Nat. Protoc. 8, 1551–1566 (2013).
Acknowledgements
We sincerely thank Profs. Jiwon Jang (Pohang University of Science and Technology, Republic of Korea) and Kisuk Yang (Incheon National University, Republic of Korea) for critical comments on the manuscript. This work was supported by the National Research Foundation of South Korea (NRF) grant funded by the Ministry of Science and ICT (No. 2021R1A2C2004981, received by Jinah. J.), Korean Fund for Regenerative Medicine funded by Ministry of Science and ICT, and Ministry of Health and Welfare (21A0104L1, Republic of Korea, received by Jinah. J.), and the Alchemist Project 1415180884 (20012378, Development of Meta Soft Organ Module Manufacturing Technology without Immunity Rejection and Module Assembly Robot System, received by Jinah. J.) funded By the Ministry of Trade, Industry & Energy(MOTIE, Korea).
Author information
Authors and Affiliations
Contributions
M.K. and Jinah.J. conceived the concept of the study. M.K. performed the experiments, interpreted data, prepared figures, and wrote the manuscript. S.C. assisted with the execution of experiments, D.G.H. contributed to initiating the essential technological development, I.K.S., S.C.K., and Jiwon.J. provided conceptual advice. Jinah.J. supervised the study, acquired funding, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.K. and J.J. are co-inventors on a patent application (Korean Patent 10-2023-0009798, pending) related to the PINE bioink. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Corinne Hoesli who co-reviewed with Ahmed Mahmoud and Praveen Pedabaliyarasimhuni; Malgorzata Borowiak and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, M., Cho, S., Hwang, D.G. et al. Bioprinting of bespoke islet-specific niches to promote maturation of stem cell-derived islets. Nat Commun 16, 1430 (2025). https://doi.org/10.1038/s41467-025-56665-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56665-5
This article is cited by
-
Advancements in photobiomodulation for generating functional beta cells from adipose derived stem cells in 3D culture: a comprehensive review
Stem Cell Research & Therapy (2025)