Abstract
RNA degradation using ribonuclease targeting chimeras (RiboTACs) is a promising approach for cancer therapy. However, potential off-target degradation is a serious issue. Here, a RiboTAC is designed for tumor microenvironment triggered activation. The tumor microenvironment activated RiboTAC (TaRiboTAC) incorporates two pre-miR-21 binders, a near-infrared fluorophore IR780, an RGD targeting peptide and a phenylboronic acid caged ribonuclease recruiter. The caged ribonuclease recruiter is embedded in the molecule and exposed in acidic pH, the phenylboronic acid cage is removed by H2O2 making the TaRiboTAC responsive to the acidic and high H2O2 levels in the tumor microenvironment. It is shown the TaRiboTAC targets tumor tissue and degrades pre-miR-21. The degradation of pre-miR-21 by TaRiboTACs significantly increases the radiotherapeutic susceptibility of cancer cells achieving efficient suppression of human lung adenocarcinoma A549 tumors in living mice.
Similar content being viewed by others
Introduction
Ribonucleic acids (RNAs) including mRNAs, miRNAs, and long non-coding RNAs play crucial roles in many biological processes, such as gene regulation1,2,3, protein synthesis4,5, and enzymatic catalysis6,7,8. RNA interference (RNAi) that selectively silences the expression of target genes has been recognized as a promising treatment modality for cancer and other diseases9,10,11,12,13,14,15. Antisense oligonucleotides and small interfering RNAs (siRNAs) are commonly utilized to silence the expression or function of target RNAs in conventional RNAi therapy16,17,18,19,20. However, their clinical promises are greatly hindered by concerns such as poor cellular uptake, low delivery efficiency, potential system toxicity, and susceptibility of RNA to serum ribonucleases (RNases). Therefore, it is highly desirable to explore a technology that can specifically degrade the target RNAs with high efficiency and low biotoxicity for precise cancer treatment.
To date, numerous small molecules that selectively bind to target RNAs and suppress their expression or function have been reported for disease treatment owing to high stability, good biocompatibility, and fast metabolism21,22,23,24,25,26. For instance, Disney and co-workers pioneered the ribonuclease targeting chimeras (RiboTACs) that consists of an RNA-binding small molecule and a ribonuclease recruiter for selective degradation of target RNAs by recruiting the RNase L without activation of the immune system27,28,29,30,31. The emergence of this RiboTACs technology as an RNAi, which overcomes the undruggability of traditional RNA targets, has recently drawn tremendous attention and opened up an avenue for accurate therapy of various diseases32,33,34. Nevertheless, the potential systemic biotoxicity from undesired off-target RNA degradation of RiboTACs may pose a significant constraint on clinical translation. Therefore, it is highly meaningful to develop advanced RiboTACs with spatiotemporal control of RNA degradation while alleviating the toxicity concern of conventional RiboTACs.
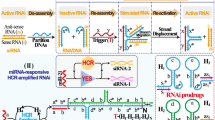
Malignant tumors are well known to have a special tumor microenvironment (TME) which possesses many special biological characteristics including weak acidity, high levels of reactive oxygen species (ROS), over-expressed proteases, etc.35,36,37,38,39,40,41,42,43,44. Leveraging these unique features of TME, developing stimuli-responsive molecules has been proven to be a promising strategy to improve the accuracy of tumor diagnosis and therapy45,46,47,48,49,50. As the traditional RiboTACs typically consist of an RNA binder and an RNases L recruiter in which one hydroxyl group plays a pivotal role in promoting RNases L dimerization for target RNA degradation (Fig. 1a), the caging and controllable decaging of this hydroxyl group might spatiotemporally activate the recruitment of RNase L achieving precise RNA degradation.
In this study, we present a TME-activated RiboTACs (TaRiboTACs) approach to selectively regulate ribonuclease activity and degrade target miRNAs by caging the RNase L recruiter’s hydroxyl group with phenylboronic acid (PBA), which is cleavable by hydrogen peroxide (H2O2) (Fig. 1b). Firstly, we design and synthesize RIBOTAC21-BA, a pre-miR-21 degrader composed of two pre-miR-21 binders, a H2O2-activatable RNase L recruiter, a tumor-targeting peptide (cRGD), and a near-infrared fluorophore (IR780) (Supplementary Fig. 1 and Fig. 2a). The degrader spontaneously assembles into nanoparticles in aqueous buffer, initially quenching fluorescence and maintaining an inactive RNase L recruitment function. Upon accumulation at the tumor site, low pH and high H2O2 levels in the TME activate RNase L recruitment and fluorescence, leading to targeted degradation of pre-miR-21 (Fig. 2b). Notably, pre-miR-21 degradation enhances cancer cell radiosensitivity and effectively suppresses human lung adenocarcinoma A549 tumors in vivo. This work introduces a pH/H2O2 dual-responsive RiboTAC for precise cancer treatment.
Results
Synthesis and characterization of pH/H2O2 dual-responsive pre-miR-21 degrader
A pH and H2O2 dual-responsive pre-miR-21 degrader RIBOTAC21-BA was synthesized as illuminated in Supplementary Fig. 1. A pre-mir-21 binder 5 and a RNases L recruiter 7 were first synthesized according to previously reported methods51,52,53. The phenol group of 7 was caged by a phenylboronic acid through the classical substituted reaction to give inactivated recruiter 8. Meanwhile, a cyclic peptide cRGD NIR dye IR780 was conjugated to NIR dye IR780 through the nucleophilic substituted reaction between thiol and chlorine group to give compound 10 which subsequently reacted with two molecules of pre-mir-21 binder 8 via the Cu(I)-catalyzed alkyne-azide cycloaddition reaction to afford compound 11. Next, DBCO-NHS and caged RNases L recruiter 8 were sequentially reacted with 11 to yield the desired degrader RIBOTAC21-BA with a total yield of 80% after HPLC purification. For better comparison, two controls RIBOTAC-BA and RIBOTAC21 were also synthesized. RIBOTAC-BA can recruit RNases L but lacks the RNA targeting ability, while RIBOTAC21 has an “always-on” ability to recruit RNases L but without H2O2-responsiveness. All intermediates and degraderes were eventually characterized by NMR and mass spectrometry (MS) (Supplementary Figs. 2–38).
The optical property of the resulting probes was next investigated
As shown in Fig. 3a, both RIBOTAC21-BA and RIBOTAC-BA in PBS buffer display obvious characteristic absorption of IR780 dye in the NIR region with a slight redshift, their fluorescence was apparently quenched and the intensity is significantly lower than that of IR780 (pH = 7.4) (Fig. 3b). To study the cause of fluorescence quenching, we then performed the morphological analysis for these compounds in aqueous solution. Dynamic light scattering (DLS) measurement indicates both RIBOTAC21-BA and RIBOTAC-BA are present as nanoparticles with diameters of 98.7 nm and 963.9 nm, respectively. The particle size of RIBOTAC21-BA was determined by transmission electron microscopy (TEM) to be 96.2 ± 13.3 nm (Fig. 3c), demonstrating that our degrader can spontaneously aggregate into nanoparticles in aqueous solution owing to the amphiphilic feature. The particle formation was further verified by determining the absorption spectra of RIBOTAC21-BA in PBS buffer with different volume fractions of DMSO solvent. With the increasing fraction of DMSO, the maximum absorbance gradually blue-shifted from 820 to 800 nm (Supplementary Fig. 39), again supporting the fact that the degraders form nanoaggregates in an aqueous solution. Collectively, these results strongly demonstrate that our degrader possesses a low fluorescent background and has a great potential for bioimaging applications. The real-time DLS results indicate that RIBOTAC21-BA has excellent stability in PBS buffer over 7 days (Supplementary Fig. 40).
a Absorption and b fluorescence (FL) emission spectra of IR780, RIBOTAC-BA, and RIBOTAC21-BA at a concentration of 16 μM in PBS buffer (pH = 7.4). c Size of RIBOTAC21-BA nanoparticles in PBS buffer (pH = 7.4). d TEM images and DLS of RIBOTAC21-BA in PBS buffers with different pH values (3 times the experiment was repeated with similar results). e Absorption and f FL intensity of RIBOTAC21-BA (5 μM) in pH PBS buffers with different pH values. g Structures (shown in stick mode) of simulated RIBOTAC21-BA and RIBOTAC21-BA (H+), in which the numbers of atoms C (gray), H (gray), N (blue), O (red), and S (yellow) were labeled. Nonpolar H atoms were omitted for clarity. Hydrogen bond (green dashed lines), π–π interaction (red dashed lines), O–π interaction (black dashed lines). h Molecular configuration transformation of the probe in solid-state particles after protonation in acidic conditions according to theoretical calculations (The red shaded is the RNases L recruiter, the green shaded part is Pre-mir-21 binder). Source data are provided as a Source Data file.
Study of pH-responsive molecular configuration transformation
We next examined the responsiveness of RIBOTAC21-BA toward the pH variation of buffers. The DLS results clearly show the particle sizes of RIBOTAC21-BA dramatically decrease from 98.7 nm to 22 nm as the pH of solutions drops from 7.4 to 5.5, and TEM results also show a similar trend of particle size change (Fig. 3d). Meanwhile, the absorbance maximum of the corresponding solutions at 817 nm was gradually increased with the decrease of pH (Fig. 3e), and the corresponding fluorescence intensities at 825 nm were progressively enhanced (Fig. 3f). However, no apparent change in particle size and optical property were observed for control RIBOTAC-BA that does not include pre-miR-21 binder (Supplementary Fig. 41). We speculate that it may be attributed to the disassembly of RIBOTAC21-BA aggregates in an acidic condition caused by the protonation of pre-miR-21 binders. The possible aggregating structure of RIBOTAC21-BA was also simulated by calculation. Figure 3g shows that the molecules mainly form aggregates through the intermolecular π–π stacking via fluorophore IR780. The interlayer distances of RIBOTAC21-BA in neutral and acidic conditions are 3.65 and 5.1 Å, respectively, indicating the molecular packing becomes loose after protonation. More notably, it was found that both pre-miR-21 binders and RNases L recruiters stretched out from the inside of aggregates and were exposed to the outside (Fig. 3h), which is highly favorable for pre-miR-21 targeting as well as RNases L recruitment. Therefore, this pH-responsive feature endows great potential for improving the accuracy of RiboTACs-based cancer treatment.
Tumor-targeting ability of RIBOTAC21-BA
Inspired by the above exciting findings, we next investigated the tumor-targeting ability and responsiveness of RIBOTAC21-BA in living cells. Both lung cancer cells A549 and normal cells HEK293 were treated with an equal amount of RIBOTAC21-BA (5 μM) followed by real-time confocal imaging. The results in Fig. 4a show that the fluorescence signals in both groups of cells gradually increased over time, but the fluorescence intensity of A549 cells is significantly higher than that of BEAS-2B cells all the time (Fig. 4b), which should be attributed to the specific binding of cRGD to the over-expressed integrin αvβ3 on the membrane of cancer cells. To study the pH responsiveness of our degraders in living cells, different groups of A549 cells were treated with RIBOTAC21-BA in a medium with various pH values (5.5, 6.5, and 7.4) for 4 h (Supplementary Fig. 42). Comfocal microscopy imaging shows the fluorescence intensity of the cells in a culture medium of pH 5.5 is the strongest and approximately 3.4 times higher than that of the cells in a neutral medium (Fig. 4c, d). However, the fluorescence signals of RIBOTAC-BA are quite low all the time no matter in a neutral or acidic medium (Supplementary Figs. 43 and 44). To investigate whether the pH of the culture medium affects the uptake of probes in living cells, A549 cells and BEAS-2B cells were cultured in RIBOTAC21-BA (5 μM) containing medium for 4 h, and then cultured in medium (pH = 7.4 or 5.5) for further 30 min followed by confocal fluorescence imaging. No significant fluorescence difference was observed for the cells in different pH of cell medium (Supplementary Fig. 45), revealing that the pH of the culture medium does not affect the targeting ability of the probes in living cells. Besides, RIBOTAC21-BA and RIBOTAC-BA were subcutaneously injected into muscle or tumors, respectively. Their fluorescence was then real-time monitored by the IVIS system. In contrast, the fluorescence intensity of RIBOTAC21-BA in tumors remained the highest all the time (Supplementary Fig. 46). These results again support that the disassembly of RIBOTAC21-BA aggregates occurs in acidic conditions leading to enhanced fluorescence signals, which endows RIBOTAC21-BA great potential for tumor imaging application. Thus, we next examined its cellular uptake in A549 cancer cell spheroids to assess the tissue penetration capability (Fig. 4e). As shown in Fig. 4f, the entire cell spheroid with the treatment of RIBOTAC21-BA emits intensive red fluorescence after 12 h incubation, whereas fluorescence was only detected at the edge of tumor spheroid that was subjected to RIBOTAC-BA, implying RIBOTAC21-BA has deep tissue penetration capability. Further, we investigated the in vivo imaging performance of RIBOTAC21-BA for tumors in living systems. Real-time fluorescence imaging of subcutaneous A549 tumor-bearing mice after intravenous injection of RIBOTAC-BA or RIBOTAC21-BA apparently indicates RIBOTAC21-BA has a great promise for in vivo tumor imaging compared to RIBOTAC-BA (Fig. 4g, h), which is also supported by ex vivo fluorescence imaging of major organs at 16 h post-injection (Fig. 4i). Together, these results highly demonstrate that this acidic pH-responsive disassembly approach significantly facilitates the accumulation and penetration of RIBOTAC21-BA in tumors.
a Real-time FL images of A549 and BEAS-2B cells treated with 5 μM of RIBOTAC21-BA (scale bar = 50 μm). b Quantitative FL intensity of the images in (a). n = 4 independent samples. c Real-time FL images and d the corresponding FL intensities of A549 cells after incubating with RIBOTAC21-BA (5 μM) in culture medium with different pH for 4 h (scale bar = 50 μm). n = 4 independent samples. e Schematic illustration of acidification-induced disassembly for enhanced permeability. f Real-time FL images of A549 tumor spheres receiving 5 μM of RIBOTAC-BA or RIBOTAC21-BA (scale bar = 100 μm). g Real-time FL images of A549 tumor-bearing mice after i.v. injection of RIBOTAC-BA and RIBOTAC21-BA (10 mg/kg), respectively, through the tail vein. h Quantitative FL intensities of g. n = 3 independent samples. i FL images and the corresponding quantitative intensities of isolated organs at 16 h in (g). n = 3 independent samples. Data denote the mean ± standard deviation. Two-sided unpaired t-test. Source data are provided as a Source Data file. Figure 4e created in BioRender. Zhang (2025) https://BioRender.com/x11q131.
In vitro study of pH/H2O2 dual-responsive degradation of pre-miR-21
In light of the fact that the hydroxyl group of RNase L recruiter was caged by PBA, we next investigated the H2O2-responsiveness of our degrades to assess the accuracy and controllability of RNA degradation. The decaging process of RIBOTAC21-BA (t = 28.2 min) in the presence of H2O2 (100 μM) was monitored by HPLC. Figure 5a shows the PBA groups were progressively cleaved by H2O2 with the increase of incubation time from 0 to 20 min, and almost completely removed to afford active RIBOTAC21 (t = 28.7 min). Besides, RIBOTAC21-BA displays excellent selectivity for H2O2 (Fig. 5b). To validate the H2O2-mediated ribonuclease recruitment performance of RIBOTAC21-BA, its oligomerization ability to RNase L before and after treatment of H2O2 was evaluated. As shown in Fig. 5c, almost no oligomerization was observed for RNase L in the presence of RIBOTAC21-BA (40 μM) without H2O2, while great oligomerization of RNase L was induced by RIBOTAC21-BA in the presence of H2O2 (100 μM). Next, we further compared the oligomerization performance of caged and non-caged RIBOTAC21. As expected, the oligomerization of caged RIBOTAC21-BA only occurred in the presence of both RNase L and H2O2, but non-caged RIBOTAC21 could be induced to form oligomerization by RNase L no matter with or without H2O2 (Supplementary Fig. 47), indicating that H2O2 does not affect the activity of RNase L. These results again demonstrate that H2O2 is able to activate the capability of RIBOTAC21-BA to recruit RNase L. Moreover, the oligomerization degree gradually enhanced with the increasing concentration of RIBOTAC21-BA used. When RIBOTAC21-BA (40 μM) was treated to H2O2 (100 μM), the ratio of oligomers/monomers was 3.9 times that of the one without H2O2 (Fig. 5d). We also assessed the capacity of RIBOTAC21-BA to degrade pri-miR-21 in aqueous solution. As shown in Fig. 5e, apparent degradation of pre-miR-21 underwent in the simultaneous presence of RIBOTAC21-BA and H2O2 and the degradation rate was gradually enhanced with the increase of the concentration of RIBOTAC21-BA. However, almost no pri-miR-21 degradation was determined for the control assays without either RIBOTAC21-BA or H2O2 (Fig. 5f), implying that our degrader can recruit RNase L and degrade the pre-miR-21 efficiently under the regulation of H2O2. Interestingly, if the pH value of the assays was changed from neutral to acidic, the amount of degraded pri-miR-21 was significantly increased (Fig. 5g, h). For instance, we adjusted the pH value of the assays containing RNase L (60 nM), RIBOTAC21-BA (40 μM), and H2O2 (50 μM) from 7.4 to 5.5, it was found that the amount of degraded Pre-miR-21 would be increased by 2.4-folds (Fig. 5i). To demonstrate the specificity of RIBOTAC21-BA to Pre-miR-21, a competition experiment containing RIBOTAC21-BA and Pre-miR-21 binder (compound 11) was also conducted. The degradation of Pre-miR-21 was significantly inhibited if the assay was pre-treated to Pre-miR-21 binder compared to the ones without treatment, suggesting that the degradation of Pre-miR-21 occurs at the same binding site. Besides, for other control RNAs, no obvious degradation was observed (Supplementary Fig. 48), demonstrating that RIBOTAC21-BA has good specificity to Pre-miR-21. Collectively, these results strongly demonstrate this delicate acidic pH/H2O2 dual-activated RiboTACs strategy can potentially accurately and efficiently degrade target miRNA for precision disease treatment.
a Real-time HPLC profiling of RIBOTAC21-BA aqueous solutions in the presence of H2O2 (100 μM). b HPLC profiles of RIBOTAC21-BA in the presence of various species (1 mM), e.g. Na+, NaHS, GSH, SO42-, NO3-, NOO-, and H2O2. Representative PAGE gel images (c) and quantitative analysis (d) of the RNase L oligomerization induced by RIBOTAC21-BA or RIBOTAC21-BA + H2O2. n = 3 independent samples. Representative SDS-PAGE gel images (e) and quantitative analysis (f) of the degradation of pre-miR-21 under different treatments (the commercial pre-miR-21 was labeled by Cy3). n = 3 independent samples. g Diagram of the acidic pH/H2O2 dual-activated RiboTACs concept. Representative SDS-PAGE gel images (h) and (i) quantitative analysis of the pre-miR-21 degradation under different treatments. n = 3 independent samples. Data denote the mean ± standard deviation. Two-sided unpaired t-test. Source data are provided as a Source Data file.
Study on pre-miR-21 degradation in cancer cells
Since miR-21 plays a vital role in the growth, proliferation, and metastasis of malignant tumors, numerous studies have undoubtedly proven that the downregulation of pre-miR-21 can induce serious cancer cell apoptosis and necrosis (Fig. 6a)54,55,56. To investigate whether our degraders can downregulate the endogenous miR-21 in cancer cells, the cytotoxicity of RIBOTAC21-BA, RIBOTAC21, and RIBOTAC-BA to BEAS-2B cells was first studied through MTT assays. Figure 6b and Supplementary Fig. 49 show that both RIBOTAC21-BA and RIBOTAC-BA exhibit negligible cytotoxicity to BEAS-2B cells indicative of great biocompatibility, while RIBOTAC21 has significant cytotoxicity to both BEAS-2B and A549 cells, which should be attributed to the feature of “always-on” RNA degradation thereby inhibiting the cell proliferation. Intriguingly, significant cell growth inhibition was determined for A549 cells that were treated with RIBOTAC21-BA. If catalase (CAT), a core antioxidant enzyme that can catalyze the decomposition of H2O2, was pre-added into the cells, the cell death was greatly suppressed (Fig. 6c). We speculate this should be due to the fact that high level of H2O2 in cancer cells activates the recruitment of RNase L initiating pre-miR-21 degradation. Next, we extracted and amplified miR-21-5p from the above cells receiving different treatments for gel electrophoresis analysis. Figure 6d, e shows that the degradation of miR-21-5p in A549 cells gradually enhanced with the increasing amounts of RIBOTAC21-BA used, whereas no miR-21-5p degradation was observed in BEAS-2B cells regardless of how much RIBOTAC21-BA is used (Supplementary Fig. 50a). However, both A549 and BEAS-2B cells treated with RIBOTAC21 showed apparent miR-21-5p degradation (Supplementary Fig. 50b). As programmed cell death 4 (PDCD4) is well known to suppress neoplastic transformation, cell proliferation, and metastasis, it can be typically downregulated by miR-21 in cancer cells57,58,59. We next monitored the expression of the downstream protein PDCD4 in A549 and BEAS-2B cells after the treatments of different concentrations of RIBOTAC21-BA or BEAS-2B cells for 48 h through western blotting analysis. It was consistently found that the expression of PDCD4 in A549 cells was gradually enhanced with the increase of RIBOTAC21-BA concentrations, but remained unchanged in BEAS-2B cells (Fig. 6f, g). However, the expressions of PDCD4 both in A549 and BEAS-2B cells could be upregulated by RIBOTAC21 (Fig. 6h, i). Additionally, we also monitored the expression levels of miR-21 and PDCD4 in mouse subcutaneous A549 tumors. Compared to the control group, the miR-21 expression in the tumors of RIBOTAC21-BA exhibited a 42% reduction accompanied by a significant increase of PDCD4 expression (Supplementary Fig. 51). These results strongly demonstrate that the tumor suppression by RIBOTAC21-BA and X-ray is mainly attributed to the regulation of miR-21 and PDCD4. Together, these exciting findings strongly demonstrate that our RIBOTAC21-BA degrader possesses great tumor specificity and excellent biocompatibility, and can be potentially applied for precise tumor treatment. Numerous studies have proven that the elevation of protein PDCD4 can enhance the radiosensitivity of cancer cells60,61. Encouraged by the above exciting findings, we further assessed the radiosensitization potential of our degraders in cancer cells. The radiotherapeutic efficacy of RIBOTAC21-BA on A549 cells was first evaluated via clonogenic assays (Fig. 6j). Figure 6k, l indicates that the cells treated with RIBOTAC21-BA have the lowest survival rate under various doses of X-ray irradiation with an SER of 1.30, while the SER value of control RIBOTAC-BA is only 1.03. Furthermore, the γ-H2AX immunofluorescence staining images given in Fig. 6m clearly show that obvious γ-H2AX foci, appearing as green fluorescent spots, were determined in the cells receiving both RIBOTAC21-BA and X-ray (4 Gy), but weak green fluorescence was detected in other control groups of cells (Fig. 6n). These results highly suggest that our RIBOTAC21-BA can remarkably improve the radiation susceptibility of lung cancer cells and has a great potential for in vivo tumor radiotherapy.
a The mechanism of TME-activated degradation of pre-miR-21. b Cytotoxicity of RIBOTAC21-BA and RIBOTAC-BA toward BEAS-2B detected by CCK8 assays. n = 6 independent samples. c Cytotoxicity of RIBOTAC21-BA, RIBOTAC21, and RIBOTAC21-BA + CAT (catalase) toward A549 cells detected by CCK8 assays. n = 6 independent samples. Degradation of miR-21-5p both in A549 and BEAS-2B cells treated with different concentrations of RIBOTAC21-BA determined by agarose gel (d) and the corresponding quantitative analysis (e). n = 3 independent samples. Expression of PDCD4 both in A549 and BEAS-2B cells treated with different concentrations of RIBOTAC21-BA determined by WB (f) and the corresponding quantitative analysis (g). n = 3 independent samples. Expression of PDCD4 both in A549 and BEAS-2B cells treated with different concentrations of RIBOTAC21 determined by WB (h) and the corresponding quantitative analysis (i). n = 3 independent samples. j Colony formation of A549 cells with different treatments (PBS, 50 nM RIBOTAC-BA, or 50 nM RIBOTAC21-BA) followed by X-ray irradiation (0, 2, 4, or 6 Gy). k Surviving fractions and l SER calculation of A549 cells with various treatments by colony forming assays. m Ionizing radiation biomarker γ-H2AX detection, A549 cells were treated with PBS, RIBOTAC-BA (50 nM), or RIBOTAC21-BA (50 nM) followed by 4 Gy of X-ray irradiation. n The corresponding quantitative analysis of m (SER represents sensitivity enhance ratio. D0 (Gy) parameter represents the dose required for survival to drop to 37% before exposure. Box plots show 25–75% percentiles, minimal and maximal values (whiskers), median (line), and mean (cross). n = 4 independent samples). Data denote the mean ± standard deviation. Two-sided unpaired t-test. Source data are provided as a Source Data file. Figure 6a created in BioRender. Zhang (2025) https://BioRender.com/c46k585.
TaRiboTACs-mediated radiotherapy of lung tumors
Inspired by the above excellent radiosensitization capability of RIBOTAC21-BA, we further evaluated its radiotherapeutic efficacy for lung tumor treatment. Four groups (n = 6 per group) of Luc-A549 subcutaneous tumor-bearing nude mice were randomly treated with different combinations: PBS, PBS + X-ray, and RIBOTAC21-BA were set as control groups, RIBOTAC21-BA + X-ray was the experimental group in which the RIBOTAC21-BA (10 mg/kg) was intravenously injected into the mice via the tail vein followed by 4 Gy of X-ray irradiation at 24 h post-treatment. The tumor sizes and body weights were recorded every 2 days throughout the 18-day treatment period. As shown in Fig. 7a, b, the tumor growths in all three control groups were significantly faster than the ones of the RIBOTAC21-BA + X-ray group whose tumor suppression rate is up to 74.3% on day 18 compared to the PBS group (Fig. 7c, d). No single death over 18 days was found in the RIBOTAC21-BA + X-ray group indicative of a high survival rate of mice (Fig. 7e). Meanwhile, noticeable tumor suppression was also observed for the mice receiving RIBOTAC21-BA but without X-ray irradiation, implying that the degradation of intratumoral miR-21 can effectively inhibit the tumor growth and improve the susceptibility of tumors to X-ray. Moreover, the tumor tissue slices administered after three days were stained by hematoxylin and eosin (HE), Ki67, and TUNEL for immunohistochemistry. Severe nuclear pyknosis and necrosis were apparently determined in both RIBOTAC21-BA + X-ray and RIBOTAC21-BA groups compared to PBS and PBS + X-ray groups. The Ki67 and TUNEL immunofluorescence results further revealed the poorest proliferation and the most severe apoptosis of the tumors in RIBOTAC21-BA + X-ray group (Fig. 7f). The HE staining of major organs post-treatment indicates that no obvious liver metastasis and other organ damage were determined especially in group RIBOTAC21-BA + X-ray (Supplementary Fig. 52), and all groups of mice did not have apparent body weight loss (Supplementary Fig. 53), again proving the degrader RIBOTAC21-BA has great biocompatibility and low cytotoxicity. Collectively, our pH/H2O2 dual-responsive RIBOTAC21-BA can selectively downregulate the intratumoral miR-21 together with X-ray irradiation to achieve accurate and efficient treatment of lung tumors in vivo.
a Monitoring of the subcutaneous A549-Luc tumor growth in different groups of mice receiving various treatments. b Tumor growth profiles of the mice in each treatment group. c Tumor volumes of the mice in each treatment group. d Tumor photograph of each group at the end of treatment. e Survival rate of the mice for each treatment group. f H&E, tunnel, and ki67 staining images of tumor slices 2 days after indicated treatments (yellow arrows mark areas of necrosis). Data denote the mean ± standard deviation. Two-sided unpaired t-test. Source data are provided as a Source Data file.
Discussion
Selective and efficient downregulation of target RNA with minimal side effects is a huge challenge in RNA interference therapy. Although conventional RIBOTACs show great promise in disease treatment by recruiting RNase L to degrade target RNAs, there are still some problems that need to be solved, e.g. low cellular uptake efficiency, poor targeting specificity, and potential system toxicity. To this end, we herein report a TME-activated robotic RiboTACs strategy to realize accurate regulation of ribonuclease activity and efficient degradation of target miRNA for precision tumor treatment. Firstly, a smart pre-miR-21 degrader RIBOTAC21-BA that consists of two pre-miR-21 binders, an H2O2-activatable ribonuclease recruiter, a tumor-targeted peptide cRGD, and a NIR dye IR780, was rationally designed and synthesized. Secondly, both theoretical calculation and experimental data indicate that RIBOTAC21-BA remains inert to target RNA in normal physiological conditions, while its activity to recruit RNase L is activated under the combined action of acidic pH and H2O2. It was found that RIBOTAC21-BA molecules initially formed stable aggregates in a neutral buffer and both pre-miR-21 binder/recruiter were embedded internally. After protonation of pre-miR-21 binders in acidic conditions, disassembly of RIBOTAC21-BA aggregates occurred spontaneously leading to the exposure of both pre-miR-21 binders and RNases L recruiters that can be specifically decaged by H2O2, which potentially enables selective degradation of intratumoral pre-miR-21 while reducing systemic toxicity. Since the downregulation of miR-21 is highly pivotal for cancer treatment owing to its key role in promoting tumor growth and resistance to apoptosis. Our studies indicate that RIBOTAC21-BA can be specifically internalized into A549 cancer cells and efficiently knock down the endogenous pre-miR-21 but having less effect on normal BEAS-2B cells, showing excellent cancer specificity and biological safety. Moreover, in vivo, studies again demonstrate that RIBOTAC21-BA can specifically accumulate at the tumor site and selectively silence the tumor-associated miR-21 through our TaRiboTACs approach in which the capability of RIBOTAC21-BA to recruit RNase L is specifically activated by high level of H2O2 in TME. More notably, the downregulation of pre-miR-21 by TaRiboTACs greatly reduces the radiation resistance of cancer cells together with X-ray irradiation ultimately realizing efficient suppression of human lung A549 tumor in living mice. In conclusion, we have developed a kind of RiboTACs technology with specific TME responsiveness, namely TaRiboTACs, for in vivo tumor treatment. Taking advantage of TME-activated ribonuclease recruitment, selective and efficient downregulation of tumor-associated pre-miR-21 was achieved by utilizing RIBOTAC21-BA degrader while remarkably enhancing the radiosensitivity of cancer cells for effective radiotherapy of malignant tumors. Therefore, we believe that these delicate TaRiboTACs demonstrated herein would be potentially extended to treat other types of diseases and may offer a new insight for precise gene therapy.
Methods
Materials
Peptide HS-RGD-NH2 was ordered from GL Biochem (Shanghai, China). 2,3,3-Trimethylindolenine, IR-775 were purchased from Saan Chemical Technology (Shanghai). 5-Iodo-1-pentyne was bought from Bide Pharmatech Co., Ltd. DBCO-NHS was obtained from Shanghai Shaoyuan Co., Ltd. RNases L was bought from Hubei Aipu Di Biological Engineering Co., Ltd. RNase L antibody was bought from Shanghai Abways Biotechnology Co., Ltd. Tris-HCl, ATP, and catalase were obtained from Shanghai Yuanye Bio-Technology Co., Ltd. Dimethyl suberimidate was bought from TCI (Shanghai). PAGE gel rapid preparation kit was purchased from Shandong Sparkjade Biotechnology Co., Ltd. SanPrep Column microRNA Extraction Kit, miRNA First Strand cDNA Synthesis (Tailing Reaction), MicroRNAs qPCR Kit (SYBR Green Method), RNase-free ddH2O Pre-mir-21-cy3 (cy3-5′-UAGCUUAUCAGACUGAUGUUGACUGUUGAAUCUCAUGGCAACACCAGUCGAUG GGCUGU-3′), miR-501-cy3 (cy3-5′- GUAAUCCUUUGUCCCUGGGUGAGAGUGCUUUCUGAAUGCAAUGCACCCGGGCAAGGAUUCUC-3′), miR-4761-cy3 (cy3-5′- GGACAAGGUGUGCAUGCCUGACCCGUUGUCAGACCUGGAAAAAGGGCCGGCUGUGGGCAGGGAGGGCAUGCGCACUUUGUCC-3′), Pre-mir-21BP-cy3 (cy3-5′- UAGCUUAUCAGACUGAUGUUGACUGUUGAAUCUCAAUGGUCAACACCAGUCGAUGGGCUGU-3′) and miR-21-5p F (CGCGTAGCTTATCAGACTGATGTTGA) were bought from Sangon Biotech (Shanghai) Co., Ltd. PDCD4 polyclonal antibody was bought from Proteintech Group, Inc. Urea-PAGE Gel Preparation Kit (RNA only), CCK8, Hoechst 33342, FITC-labeled goat anti-rabbit IgG (H + L) were purchased from Beyotime (Shanghai, China). Anti-γ-H2AX rabbit monoclonal antibody was purchased from Abcam (Cambridge, MA, USA). All commercially available reagents and solvents were used as received unless otherwise specified. Water was supplied by Milli-Q Plus System.
Instrumentation
1H NMR spectra were carried out on 600 MHz (150 MHz for 13C NMR) Agilent NMR spectrometer with DMSO-d6 as the solvent and tetramethylsilane (TMS) as the internal standard. Mass spectra were tested using an Agilent 1200/6220. UV-Vis spectra were measured with a UV spectrometer (PerkinElmer LAMBDA 750). Fluorescence (FL) spectra were measured on an Edinburgh FLS980 spectrophotometer. FL images were taken on an IVIS spectrum imaging system (PerkinElmer). The confocal images were tested using a confocal microscope (FV1200, Olympus). The X-ray irradiator is RS-2000-PRO made by Radsource Technologies Co., LTD. Gel electrophoresis was acquired with BIO-RAD 1645050 and images were recorded on Gel Doc EZ Imager (BIO-RAD). RNA amplification was performed using a professional thermocycler. Life technologies ViiA 7 was used for mRNA quantification.
Synthesis of compound 1
(4-(4-methylpiperazin-1-yl) phenyl) boronic acid (127.6 mg), 5-chloro-2-nitroaniline (119.7 mg), pd (pph3)4, and K2CO3 (276.4 mg) were dissolved in 2 mL DMF under the protection of nitrogen, heated at 100 °C, separated and purified by spinning dry solvent silica gel column after reaction for 3 h. The product (162.8 mg) was obtained with a yield of 90%1.H NMR (600 MHz, DMSO-d6) δ 7.96 (d, J = 9.0 Hz, 1H), 7.52 (d, J = 8.9 Hz, 2H), 7.22 (d, J = 1.9 Hz, 1H), 7.02 (d, J = 8.9 Hz, 2H), 6.89 (dd, J = 9.1, 1.9 Hz, 1H), 3.24–3.20 (m, 4H), 2.49–2.47 (m, 4H), 2.23 (s, 3H)13.C NMR (151 MHz, DMSO-d6) δ 151.73, 147.13, 147.06, 129.23, 127.93, 126.55, 115.49, 114.84, 114.26, 54.78, 47.63, 46.04. Calcd. for C17H20N4O2 312.1586, found ESI-MS: m/z 312.92.
Synthesis of compound 2
Compound 1 (100 mg) and Pd/C (10 mg) were dissolved in 15 mL ethanol, charged with nitrogen to discharge the air, then injected with hydrogen for 24 h, spun dry the solvent, and purified by HPLC to obtain compound 2 (36 mg, yield 90%)1.H NMR (600 MHz, DMSO-d6) δ 7.44 (d, J = 8.8 Hz, 2H), 7.19 (d, J = 1.5 Hz, 1H), 7.05 (t, J = 5.9 Hz, 3H), 7.01 (d, J = 8.2 Hz, 1H), 3.87 (s, 2H), 3.50 (s, 2H), 3.16 (s, 2H), 2.99 (s, 2H), 2.85 (s, 4H), 2.63–2.16 (m, 3H)13.C NMR (151 MHz, DMSO-d6) δ 159.03, 158.82, 148.98, 131.53, 127.10, 121.20, 117.22, 116.68, 52.60, 45.91, 42.52. Calcd. for C17H22N4 281.1766 (M-H+), found ESI-MS: m/z 281.11.
Synthesis of compound 3
p-hydroxybenzaldehyde (2 g), ethyl 4-(4-formylphenoxy) butyrate (3.8 g) and potassium carbonate (2.7 g) were reacted in anhydrous DMF at room temperature for 16 h followed the purification by silica gel column to obtain compound 3 with a yield of 69% (2.7 g)1.H NMR (600 MHz, DMSO-d6) δ 9.84 (s, 1H), 7.89–7.76 (m, 2H), 7.08 (d, J = 8.7 Hz, 2H), 4.10–4.02 (m, 4H), 2.44 (t, J = 7.3 Hz, 2H), 1.98 (p, J = 6.8 Hz, 2H), 1.15 (t, J = 7.1 Hz, 3H)13.C NMR (151 MHz, DMSO-d6) δ 191.63, 172.85, 163.86, 132.22, 130.07, 115.29, 67.51, 60.32, 30.47, 24.47, 14.49. Calcd. for C13H16O4 259.0946 (M-Na+), found ESI-MS: m/z 259.378.
Synthesis of compound 4
Compound 2 (50 mg), compound 3 (50.2 mg), and Na2S2O5 (40.4 mg) were dissolved in a mixture of water (30 mL), and ethanol (30 mL). After reflux overnight, the resulting solid was added to tetrahydrofuran aqueous solution containing excessive KOH (THF: H2O = 3:1) and reflux overnight. The crude products were purified by silica gel column and gave 40.8 mg of compound 4 with a yield of 49%1.H NMR (600 MHz, CD3OD) δ 8.55 (s, 1H), 8.11 (d, J = 8.8 Hz, 1H), 7.90–7.17 (m, 6H), 7.07–6.74 (m, 4H), 3.99 (dt, J = 24.2, 10.3 Hz, 3H), 3.33–3.08 (m, 4H), 2.62-ff2.22 (m, 8H), 2.07 (dd, J = 32.9, 25.9 Hz, 3H)13.C NMR (151 MHz, CD3OD) δ 180.68, 169.13, 159.99, 149.58, 142.96, 141.97, 134.26, 133.85, 130.40, 127.95, 127.17, 119.69, 116.31, 114.91, 114.28, 112.02, 67.63, 54.50, 48.69, 44.72, 34.04, 26.06. Calcd. for C28H30N4O3 471.2396 (M + H+), found ESI-MS: m/z 471.43.
Synthesis of compound 5
Compound 4 (186 mg), azide propylamine (109.9 mg), EDC (90.56 mg), and NHS (54.55 mg) were dissolved in anhydrous DMF and stirred for 8 h. The crude products were purified by silica gel column and compound 5 (174 mg) was obtained with a yield of 80%1.H NMR (600 MHz, DMSO-d6) δ 10.08 (s, 1H), 8.18 (d, J = 8.8 Hz, 2H), 7.94 (d, J = 8.1 Hz, 2H), 7.78 (d, J = 8.5 Hz, 1H), 7.71 (d, J = 9.3 Hz, 1H), 7.65 (d, J = 8.7 Hz, 2H), 7.25 (d, J = 8.9 Hz, 2H), 7.13 (d, J = 8.8 Hz, 2H), 4.11 (t, J = 6.4 Hz, 2H), 3.32 (t, J = 6.8 Hz, 2H), 3.10 (dd, J = 12.6, 6.6 Hz, 2H), 2.87 (s, 7H), 2.71 (s, 4H), 2.26 (t, J = 7.4 Hz, 2H), 2.00–1.92 (m, 2H), 1.67–1.57 (m, 2H)13.C NMR (151 MHz, DMSO-d6) δ 171.90, 162.73, 162.52, 158.92, 158.70, 150.27, 149.42, 137.64, 131.59, 129.99, 128.19, 124.19, 116.76, 115.98, 114.74, 111.05, 68.05, 52.59, 48.87, 45.83, 42.54, 36.29, 36.22, 31.20, 28.87, 25.09. Calcd. for C31H36N8O2 553.3039 (M + H+), found ESI-MS: m/z 553.58.
Synthesis of compound 6
3,4-dihydroxybenzaldehyde (69 mg), Bromo-PEG3-azide (141 mg), and K2CO3 (69 mg) were dissolved in 2 mL of DMF and reacted at 50 °C for 8 h. The crude products were purified by silica gel column and compound 6 (125.43 mg) was obtained with a yield of 74%1.H NMR (600 MHz, DMSO-d6) δ 9.75 (s, 1H), 9.55 (s, 1H), 7.36 (dd, J = 8.2, 2.0 Hz, 1H), 7.25 (d, J = 2.0 Hz, 1H), 7.11 (d, J = 8.3 Hz, 1H), 4.21–4.16 (m, 2H), 3.79–3.76 (m, 2H), 3.59–3.56 (m, 4H), 3.53 (dd, J = 6.5, 1.8 Hz, 6H), 3.37–3.34 (m, 2H)13.C NMR (151 MHz, DMSO-d6) δ 191.88, 153.02, 147.59, 130.34, 124.72, 114.07, 113.15, 70.40, 70.28, 70.25, 70.12, 69.67, 69.20, 68.52, 50.42. Calcd. for C15H21N3O6 340.1509 (M + H+), found ESI-MS: m/z 340.38.
Synthesis of compound 7
Compound 6 (41 mg) and ethyl 2-(phenylamino)-4-oxo-4,5-dihydrothiophene-3-carboxylate (35 mg) were dissolved in 2 mL of EtOH (containing 12 μL piperidine) and reacted at 50 °C for 10 h. The crude products were purified by silica gel column and compound 7 (58 mg) was afforded with a yield of 83%1.H NMR (600 MHz, DMSO-d6) δ 9.40 (s, 1H), 7.93 (s, 1H), 7.56–7.33 (m, 6H), 7.05–6.90 (m, 2H), 4.29–4.19 (m, 2H), 4.15–4.04 (m, 2H), 3.56 (d, J = 4.8 Hz, 2H), 3.53–3.51 (m, 4H), 3.39–3.32 (m, 2H), 2.87 (s, 2H), 2.71 (s, 2H), 1.35–1.12 (m, 5H)13.C NMR (151 MHz, DMSO-d6) δ 181.37, 165.30, 162.73, 149.12, 147.48, 130.23, 130.04, 129.92, 128.46, 128.24, 126.83, 125.93, 125.61, 123.44, 116.31, 114.22, 70.36, 70.24, 70.11, 69.67, 69.28, 68.40, 59.89, 59.71, 50.42, 44.16, 14.86. Calcd. for C28H32N4O8S 585.2019 (M + H+), found ESI-MS: m/z 585.47.
Synthesis of compound 8
Compound 7 (100 mg), 4-bromomethylphenylboronic acid pinacol ester (50.7 mg), and K2CO3 (28.4 mg) were dissolved in 2 Ml of DMF and reacted at 50°C for 8 h. The crude products were purified by HPLC and compound 8 (38.1 mg) was obtained with a yield of 31%1.H NMR (600 MHz, DMSO-d6) δ 11.24 (s, 1H), 8.01 (s, 1H), 7.93 (s, 1H), 7.77 (dd, J = 41.3, 7.8 Hz, 2H), 7.52 (dd, J = 5.2, 2.4 Hz, 4H), 7.34 (d, J = 7.7 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.20 (d, J = 13.2 Hz, 1H), 7.07 (d, J = 10.3 Hz, 2H), 5.11 (d, J = 17.3 Hz, 2H), 4.26 (dd, J = 12.7, 5.6 Hz, 2H), 3.76–3.72 (m, 2H), 3.58 (dd, J = 9.4, 4.0 Hz, 2H), 3.56–3.52 (m, 2H), 3.37–3.31 (m, 2H), 2.87 (s, 4H), 2.71 (s, 4H), 1.31–1.17 (m, 5H)13.C NMR (151 MHz, DMSO-d6) δ 181.27, 175.69, 165.27, 162.72, 157.61, 150.57, 148.32, 148.26, 139.04, 137.96, 134.64, 133.97, 129.99, 129.84, 128.62, 126.92, 126.74, 126.07, 125.94, 125.57, 125.48, 116.61, 116.42, 115.55, 114.23, 70.45, 70.10, 69.66, 69.21, 68.66, 59.95, 50.41, 50.37, 36.21, 31.20, 14.85. Calcd. for C35H39BN4O10S 719.2558 (M + H+), found ESI-MS: m/z 719.44.
Synthesis of compound 9
5-Iodo-1-pentyne (3.8 g), potassium iodide (6.6 g), and 2,3,3-trimethyl-3H-indole (1.6 g) were dissolved and stirred in 1,2-dichlorobenzene at 90 °C for 24 h. The resulting residue was diluted with dichloromethane (DCM) and precipitated with ethyl ether. Pure compound 9 were obtained (2.35 g, yield = 95%)1.H NMR (600 MHz, CDCl3) δ 7.88–7.78 (m, 1H), 7.59–7.54 (m, 2H), 7.26 (s, 1H), 4.88 (t, J = 7.5 Hz, 2H), 3.21 (s, 3H), 2.50 (td, J = 6.5, 2.3 Hz, 2H), 2.31–2.06 (m, 3H), 1.66 (s, 6H)13.C NMR (151 MHz, CDCl3) δ 181.27, 175.69, 165.27, 162.72, 157.61, 150.57, 148.32, 148.26, 139.04, 137.96, 134.64, 133.97, 129.99, 129.84, 128.62, 126.92, 126.74, 126.07, 125.94, 125.57, 125.48, 116.61, 116.42, 115.55, 114.23, 70.45, 70.10, 69.66, 69.21, 68.66, 59.95, 50.41, 50.37, 36.21, 31.20, 14.85. Calcd. for C16H20N+ 226.3425, found ESI-MS: m/z 226.35.
Synthesis of compound IR780
Compound 9 (353 mg) and (E)-2-chloro-3-(hydroxymethylene) cyclohex-1-ene-1-carbaldehyde (86 mg) were dissolved in anhydrous ethanol and stirred at 80 °C for 12 h. The resulting residue was diluted with 1 mL of methanol and precipitated with ethyl ether. Pure IR780 were obtained (597 mg, yield = 65%)1.H NMR (600 MHz, DMSO-d6) δ 8.26 (d, J = 14.1 Hz, 2H), 7.62 (d, J = 7.4 Hz, 2H), 7.48–7.39 (m, 4H), 7.28 (td, J = 7.3, 1.3 Hz, 2H), 6.35 (d, J = 14.2 Hz, 2H), 4.25 (t, J = 7.3 Hz, 4H), 2.97 (d, J = 2.5 Hz, 1H), 2.69 (t, J = 6.0 Hz, 4H), 2.49–2.48 (m, 2H), 2.37–2.28 (m, 4H), 1.96–1.87 (m, 4H), 1.87–1.81 (m, 2H), 1.66 (s, 11H)13.C NMR (151 MHz, DMSO-d6) δ 172.87, 148.61, 143.62, 142.45, 141.52, 129.06, 126.81, 125.67, 123.01, 111.75, 101.98, 83.98, 72.71, 49.50, 43.27, 40.37, 40.23, 40.10, 39.96, 39.82, 39.68, 39.54, 27.93, 26.38, 20.77, 15.75. Calcd. for C40H44ClN2+ 587.3188, found ESI-MS: m/z 587.581.
Synthesis of compound 10
IR780 (58 mg), 86.7 mg HS-RGD-NH2 (58 mg), and 1.5 equivalent of triethylamine were dissolved in 2 mL of DMF and then reacted at RT for 8 h. After the reaction, the solvent was spun dry, the solid mixture was dissolved in anhydrous ethanol, and then purified by HPLC to give compound 10 (76.8 mg, yield = 70%). Calcd. for C61H80N11O7S+ 1110.5957, found ESI-MS: m/z 1110.4243.
Synthesis of compound 11
Compound 10 (55 mg) and compound 5 (65.6 mg) were dissolved in 2 mL of mixing solvent (DMF: H2O = 1:1). After stirring of 10 min, an aqueous mixture of CuSO₄·5H₂O (29.7 mg) and sodium ascorbate (23.5 mg) were added. After 5 h of reaction, the solvent was spun dry, the solid mixture was dissolved in anhydrous ethanol, and then purified by HPLC to obtain compound 11 (88 mg, yield = 80%). Calcd. for C123H152N27O11S+ 2216.1913, found ESI-MS: m/z 2216.1900.
Synthesis of compound RIBOTAC21-BA
Compound 11 (50 mg) and DBCO-NHS (9.1 mg) were dissolved and stirred in 1 mL of anhydrous DMF at room temperature, anhydrous triethylamine was then added after 10 min, and then the solvent was spun dry for 2 h. The solid was dissolved into 1.8 mL of acetonitrile containing 1‰ trifluoroacetic acid followed by the addition of compound 8 (19.4 mg) and 200 μL of ultra-pure water. After 2 h reaction, the crude products were purified by HPLC to give RIBOTAC21-BA (58 mg, yield = 80%). Calcd. for C177H209BN32O23S26+ 537.9290 (M/6), found ESI-MS: m/z 537.9312.
Synthesis of compound 12
IR-775 (53.9 mg), HS-RGD-NH2 (88.6 mg), and 1.5 equivalent of triethylamine were dissolved in 2 mL of DMF and reacted at RT. After 8 h reaction, the solvent was spun dry and the solid mixture was dissolved in anhydrous ethanol followed by purification by HPLC to afford compound 12 (74.3 mg, yield = 70%). Calcd. for C57H80N11O7S+ 1062.5957, found ESI-MS: m/z 1062.5982.
Synthesis of compound RIBOTAC-BA
Compound 12 (106.2 mg) and DBCO-NHS (49 mg) were dissolved and stirred in 1 mL of anhydrous DMF at room temperature. After 10 min, anhydrous triethylamine was added and the solvent was spun dry for 2 h. Then the solid was dissolved into 1.8 mL of acetonitrile containing 1‰ trifluoroacetic acid followed by the addition of compound 8 (107.7 mg) and 200 μL of ultra-pure water. After a 2 h reaction, the crude products were then purified by HPLC to obtain RIBOTAC-BA (130.2 mg, yield = 63%). Calcd. for C111H132BN16O19S2+ 2067.9384, found ESI-MS: m/z 2067.9392.
Synthesis of compound RIBOTAC21
Compound 11 (75 mg) and DBCO-NHS (16.5 mg) were dissolved and stirred in 1 mL of anhydrous DMF at room temperature. After 10 min, anhydrous triethylamine was added and the solvent was then spun dry for 2 h. The solid was dissolved into 1.8 mL of acetonitrile containing 1‰ trifluoroacetic acid followed by the addition of compound 7 (19.7 mg) and 200 μL of ultra-pure water. After 2 h reaction, the crude products were then purified by HPLC to give RIBOTAC21 (84 mg, yield = 80%). Calcd. for C170H199N32O21S222+ 1544.2437 (M/2), found ESI-MS: m/z 1544.7360.
Optical property of probes
The stock solution (16 mM) of probe RIBOTAC21-BA and control probe RIBOTAC-BA was prepared in DMSO. Stock solution (1 μL) was added into 2 mL of PBS buffer (pH = 7.4), and their absorption and fluorescence spectra were measured after ultrasound.
In vitro pH-responsiveness study
The stock solution of RIBOTAC21-BA (16 mM, 1 μL) was added into the PBS buffer (pH = 5.5, 6.0, 6.5, 7.0, and 7.4), and the absorption and fluorescence spectra of each sample were subsequently collected. Hydration particle size (DLS) and TEM images (pH = 5.5, 6.5, 7.4) were also measured. The stock solution of RIBOTAC-BA (16 mM, 1 μL) was added into PBS buffers (pH = 5.5 or 7.4), and the absorption and fluorescence spectra, hydration particle size (DLS), and TEM images were subsequently determined.
Cell culture
Human lung cancer cells A549 and human normal lung bronchial epithelial cells BEAS-2B were purchased from Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China). A549 and BEAS-2B cells were cultured with RPMI 1640 and DMEM, respectively, at 37 °C in a humidified atmosphere (5% CO2). All medium was supplemented with 10% fetal bovine serum (Hyclone Inc.) and 1% penicillin-streptomycin (Beyotime Inc.).
Animals and tumor models
All animal experiments were conducted according to the Guidelines for the Care and Use of Laboratory Animals of Soochow University and were approved by the Animal Ethics Committee of the Soochow University Laboratory Animal Center (Suzhou, China). In the subcutaneous tumor model, tumor weight was restricted to under 10% of normal body weight or maximum diameter of 13 mm (approximately 1098.5 mm³ in volume). Six-week-old Balbc-Nu male mice, weighing 14–16 g, were obtained from GemPharmatech Co., Ltd. The mice were kept in standard conditions with a temperature of 25 ± 3 °C, relative humidity of 60% ± 10%, and a 12-h light/dark cycle. Tumors were induced by injecting 3 × 10⁶ A549-Luc cells suspended in 50 μL of PBS into the back of each mouse. Tumor dimensions were measured using calipers, and volumes were calculated with the formula (length × width²)/2. Throughout all experiments, tumor volumes were maintained within the limits set by the Animal Ethics Committee of Soochow University (Ethics approval number: SUDA20241225A05).
In vivo pH-responsiveness study
A549 and BEAS-2B cells were cultured in confocal dishes for 24 h, and then incubated with RIBOTAC21-BA (16 μM) followed by confocal microscope imaging at various time points (0, 1, 2, 4, and 8 h). A549 cells were cultured in confocal dishes for 24 h, and the medium was then changed by a slightly acidic culture medium for 0.5 h incubation. The fluorescence images were taken by confocal microscope after 4 h incubation of RIBOTAC21-BA (16 μM). A549 cells were cultured in confocal dishes for 24 h, and then incubated with RIBOTAC-BA (16 μM) for 0, 1, 2, and 4 h. The group of 4 h was then treated with 1640 medium for 0.5 h followed by confocal microscope imaging. A mouse model of a unilateral tumor on the hind limb of A549 was constructed, the first group was injected with 50 μL of RIBOTAC21-BA (16 μM) in the muscle of the left hind limb and in the subcutaneous tumor of the right hind limb. The second group was injected with 50 μL of RIBOTAC-BA (16 μM) in the muscle of the left hind limb and in the subcutaneous tumor of the right hind limb. Fluorescence images were collected at 10 min, 2 h, and 4 h after injection of the probes by the IVIS Spectrum system.
In vitro H2O2-responsiveness study
The stock solution (16 mM) of probe RIBOTAC21-BA was prepared in DMSO. The mixture of the probes (16 μM) and H2O2 (100 μM) was incubated at 37 °C for 1, 5, 10, and 20 min in PBS buffer (pH = 7.4). The decaging of RIBOTAC21-BA by H2O2 was measured by HPLC.
Selectivity of RIBOTAC21-BA toward H2O2
The mixture of RIBOTAC21-BA (16 μM) and 1 mM of Na+, NaHS GSH, SO42-, NO3-, NOO-, H2O2, respectively, in PBS buffer (pH = 7.4) were incubated at 37 °C for 1 h. The decaging of RIBOTAC21-BA by H2O2 was evaluated by HPLC.
In vitro RNase L oligomerization study
A solution of RNase L (3 µM) was incubated with various concentrations of RIBOTAC21-BA (0, 20, 40 µM) in Tris-HCl buffer (pH 7.4) containing MgCl2 (10 mM), β-mercaptoethanol (7 mM), ATP (50 µM), KCl (100 mM), H2O2 (0 or 100 µM) at 25 °C for 5 min. 1 µL of dimethyl suberimidate (44 mM) was added and then these samples were incubated at 25 °C for 2 h. These samples were diluted with denatured reducin protein loading buffer and heated at 95 °C for 15 min. Finally, the samples were isolated by PAGE gel and imaged by BIO-RAD.
In vitro degradation of pre-mir-21
Pre-mir-21-Cy3 was heated to 65 °C and then cooled down to room temperature slowly. Then a solution of pre-mir-21-Cy3 (1 µM) and RNase L (3 µM) was incubated with RIBOTAC21-BA (0, 20 or 40 µM) in Tris-HCl buffer (pH 7.4, 6.5 or 5.5) containing MgCl2 (10 mM), β-mercaptoethanol (7 mM) and ATP (50 µM), KCl (100 mM), H2O2 (0 or 100 µM) at 25 °C for 12 h. These samples were diluted with 6× RNA loading and heated at 70 °C for 10 min. Finally, the samples were isolated by SDS-PAGE gel and imaged by BIO-RAD.
Downregulation and quantitative real-time PCR (RT-qPCR) of miR-21-5p
A549 and BEAS-2B cells were separately cultured in six-well plates for 24 h followed by the addition of RIBOTAC21-BA or RIBOTAC21 for 48 h incubation. The mRNA was then extracted from the cells with SanPrep Column microRNA Extraction Kit and amplified by miRNA First Strand cDNA Synthesis (Tailing Reaction) for quantification with MicroRNAs qPCR Kit (SYBR Green Method). The amplified RNA miR-21-5p was also quantified by agarose gel electrophoresis. All procedures are strictly in accordance with the kit instructions.
Studying the cytotoxicity of all probes
A549 and BEAS-2B cells were separately seeded into 96 well plates at a density of 1 × 103 cells per well and grown for 24 h. And then the cells were incubated with different concentrations (0, 0.5, 5, 50, 500 nM) of RIBOTAC21-BA, RIBOTAC-BA, or RIBOTAC21 for 48 h. The relative cell viabilities were finally determined by the standard CCK8 assay.
Clonogenic assays
A549 cells are cultured at a density of 200, 400, 600, or 800 cells/well in 6-well plates for 24 h. Then the cells were treated with PBS, RIBOTAC-BA (50 nM), or RIBOTAC21-BA (50 nM) for 48 h. After X-ray (0, 2, 4, and 6 Gy) irradiation, the cells were cultured for a further 9 days with fresh medium replacement every other day. The cells were then fixed with 4% paraformaldehyde for 20 min, cleaned with PBS, and then stained with 1×Giemsa staining solution for 15 min. The cloning results were then photographed and recorded.
DNA damage
The assessment of DNA double-stranded breaks was accomplished through the γ-H2AX staining test. A549 cells were seeded into glass bottom dishes at a density of 1 × 105 cells/well and then cultured for 24 h. Subsequently, the cells were subjected to RIBOTAC21-BA (50 nM) and cultured for a further 48 h followed by exposure of different doses (0, 4 Gy) of X-ray radiation. The cells were then maintained in culture for an additional 24 h. Following fixation with 4% PFA, permeabilization with a 1% Triton-100 solution ensued. The cells were then sequentially incubated in a BSA-blocking buffer at 25 °C for 1 h followed by γ-H2AX rabbit monoclonal antibody overnight at 4 °C. Subsequent steps involved staining with FITC-labeled goat anti-rabbit IgG (H + L) for 1 h and Hoechst 33342 for 15 mins. Critically, three PBS washes were intercalated between each experimental step. Finally, cellular observations were conducted using confocal laser scanning microscopy (CLSM).
Radiotherapy of A549 tumors
In a randomized grouping, mice bearing A549 xenograft tumors (five mice per group) were subject to intravenous administration of either PBS buffer (200 μL) or RIBOTAC21-BA (10 mg/kg) via the tail vein. Subsequently, after a 24-h interval, the tumors underwent X-ray irradiation at a dose of 6 Gy. Tumor dimensions were gauged bi-daily, culminating in the sacrifice of the mice on the 18th day. H&E staining was applied to analyze tissue slices from the heart, lung, spleen, liver, kidney, and tumor, while TUNEL and Ki67 immunofluorescence staining specifically assessed the tumor slices.
Data analysis
Average area intensities in cells were given by a fluorescence microscope (FV1200, Olympus). Average area intensities in mice were given by the Living Image software of PerkinElmer IVIS. All data were analyzed and calculated with Microsoft Excel 2019 software (Microsoft), and the statistical differences were analyzed by a two-tailed student’s test. All statistical data were presented as means ± SD. All statistical graphs and fluorescent spectra were performed using Origin 2020.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the results in this study are available within the Article, its Supplementary Information, and Source Data File. Source data is available for Figs. 3, 4, 5, 6, 7 and Supplementary Figs. 39, 40, 41, 42, 44, 45, 47, 48, 49, 50, 51, 53, 55, and 56 in the associated source data file. Source data are provided with this paper.
References
Schimmel, P. The emerging complexity of the tRNA world: mammalian tRNAs beyond protein synthesis. Nat. Rev. Mol. Cell Bio. 19, 45–58 (2018).
Sharp, P. A. The centrality of RNA. Cell 136, 577–580 (2009).
Ying, S. et al. tRF-Gln-CTG-026 ameliorates liver injury by alleviating global protein synthesis. Sig. Transduct. Target. Ther. 8, 144 (2023).
Zhang, Y., Sun, C., Wang, C., Jankovic, K. E. & Dong, Y. Lipids and lipid derivatives for RNA delivery. Chem. Rev. 121, 12181–12277 (2021).
Milicevic, N., Jenner, L., Myasnikov, A., Yusupov, M. & Yusupova, G. mRNA reading frame maintenance during eukaryotic ribosome translocation. Nature 625, 393–400 (2023).
Piwecka, M. et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 357, eaam8526 (2017).
Scheitl, C. P. M., Ghaem Maghami, M., Lenz, A.-K. & Höbartner, C. Site-specific RNA methylation by a methyltransferase ribozyme. Nature 587, 663–667 (2020).
Janzen, E., Blanco, C., Peng, H., Kenchel, J. & Chen, I. A. Promiscuous ribozymes and their proposed role in prebiotic evolution. Chem. Rev. 120, 4879–4897 (2020).
Pecot, C. V., Calin, G. A., Coleman, R. L., Lopez-Berestein, G. & Sood, A. K. RNA interference in the clinic: challenges and future directions. Nat. Rev. Cancer 11, 59–67 (2011).
Petrocca, F. & Lieberman, J. Promise and challenge of RNA interference-based therapy for cancer. J. Clin. Oncol. 29, 747–754 (2011).
Borkhardt, A. Blocking oncogenes in malignant cells by RNA interference-new hope for a highly specific cancer treatment? Cancer Cell 2, 167–168 (2002).
Xia, H. et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat. Med. 10, 816–820 (2004).
Czech, M. P., Aouadi, M. & Tesz, G. J. RNAi-based therapeutic strategies for metabolic disease. Nat. Rev. Endocrinol. 7, 473–484 (2011).
Balwani, M. et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N. Engl. J. Med. 382, 2289–2301 (2020).
Scherer, L. J. & Rossi, J. J. Approaches for the sequence-specific knockdown of mRNA. Nat. Biotechnol. 21, 1457–1465 (2003).
Nikan, M. et al. Targeted delivery of antisense oligonucleotides using neurotensin peptides. J. Med. Chem. 63, 8471–8484 (2020).
Knerr, L. et al. Glucagon like peptide 1 receptor agonists for targeted delivery of antisense oligonucleotides to pancreatic beta cell. J. Am. Chem. Soc. 143, 3416–3429 (2021).
Zimmermann, T. S. et al. RNAi-mediated gene silencing in non-human primates. Nature 441, 111–114 (2006).
Shahrudin, S. & Ding, S.-W. Boosting stem cell immunity to viruses. Science 373, 160–161 (2021).
Tang, Q. & Khvorova, A. RNAi-based drug design: considerations and future directions. Nat. Rev. Drug Discov. 23, 341–364 (2024).
Warner, K. D., Hajdin, C. E. & Weeks, K. M. Principles for targeting RNA with drug-like small molecules. Nat. Rev. Drug Discov. 17, 547–558 (2018).
Costales, M. G. et al. A designed small molecule inhibitor of a non-coding RNA sensitizes HER2 negative cancers to herceptin. J. Am. Chem. Soc. 141, 2960–2974 (2019).
Haniff, H. S. et al. Design of a small molecule that stimulates vascular endothelial growth factor A enabled by screening RNA fold–small molecule interactions. Nat. Chem. 12, 952–961 (2020).
Ye, S. et al. Red light-initiated cross-linking of NIR probes to cytoplasmic RNA: an innovative strategy for prolonged imaging and unexpected tumor suppression. J. Am. Chem. Soc. 142, 21502–21512 (2020).
Wojtczak, B. A. et al. 5′-Phosphorothiolate dinucleotide cap analogues: reagents for messenger RNA modification and potent small-molecular inhibitors of decapping enzymes. J. Am. Chem. Soc. 140, 5987–5999 (2018).
Falese, J. P., Donlic, A. & Hargrove, A. E. Targeting RNA with small molecules: from fundamental principles towards the clinic. Chem. Soc. Rev. 50, 2224–2243 (2021).
Tong, Y. et al. Programming inactive RNA-binding small molecules into bioactive degraders. Nature 618, 169–179 (2023).
Suresh, B. M. et al. A general fragment-based approach to identify and optimize bioactive ligands targeting RNA. Proc. Natl Acad. Sci. USA 117, 33197–33203 (2020).
Childs-Disney, J. L. et al. Targeting RNA structures with small molecules. Nat. Rev. Drug Discov. 21, 736–762 (2022).
Suresh, B. M. et al. Altering the cleaving effector in chimeric molecules that target RNA enhances cellular selectivity. ACS Chem. Biol. 18, 2385–2393 (2023).
Zhang, P. et al. Reprogramming of protein-targeted small-molecule medicines to RNA by ribonuclease recruitment. J. Am. Chem. Soc. 143, 13044–13055 (2021).
Zhang, Y., Wang, L., Wang, F., Chu, X. & Jiang, J.-H. G-Quadruplex mRNAs silencing with inducible ribonuclease targeting chimera for precision tumor therapy. J. Am. Chem. Soc. 146, 15815–15824 (2024).
Haniff, H. S. et al. Targeting the SARS-CoV-2 RNA genome with small molecule binders and ribonuclease targeting chimera (RIBOTAC) degraders. ACS Cent. Sci. 6, 1713–1721 (2020).
Fang, Y. et al. Aptamer‐RIBOTAC strategy enabling tumor‐specific targeted degradation of microRNA for precise cancer therapy. Small Methods https://doi.org/10.1002/smtd.202400349 (2024).
Mahoney, K. M. & Freeman, G. J. Acidity changes immunology: a new VISTA pathway. Nat. Immunol. 21, 13–16 (2020).
Feng, Q. et al. Severely polarized extracellular acidity around tumour cells. Nat. Bio. Eng. 8, 787–799 (2024).
Liu, M. et al. A transistor-like pH-sensitive nanodetergent for selective cancer therapy. Nat. Nanotechnol. 17, 541–551 (2022).
Wang, Y. et al. A DNA robotic switch with regulated autonomous display of cytotoxic ligand nanopatterns. Nat. Nanotechnol. https://doi.org/10.1038/s41565-024-01676-4 (2024).
Cheung, E. C. & Vousden, K. H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 22, 280–297 (2022).
Reczek, C. R. & Chandel, N. S. ROS promotes cancer cell survival through calcium signaling. Cancer Cell 33, 949–951 (2018).
Tan, X. et al. Transformable nanosensitizer with tumor microenvironment‐activated sonodynamic process and calcium release for enhanced cancer immunotherapy. Angew. Chem. Int. Edit. 60, 14051–14059 (2021).
Fujita, K. & Urano, Y. Activity-based fluorescence diagnostics for cancer. Chem. Rev. 124, 4021–4078 (2024).
Fang, J. et al. Alkaline phosphatase-controllable and red light-activated RNA modification approach for precise tumor suppression. J. Am. Chem. Soc. 144, 23061–23072 (2022).
Yin, L. et al. Quantitatively visualizing tumor-related protease activity in vivo using a ratiometric photoacoustic probe. J. Am. Chem. Soc. 141, 3265–3273 (2019).
Zhang, Y. et al. A hydrogen sulphide-responsive and depleting nanoplatform for cancer photodynamic therapy. Nat. Commun. 13, 1685 (2022).
Hyer, M. L. et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat. Med. 24, 186–193 (2018).
Weng, J. et al. Controlled in situ self-assembly of biotinylated trans-cyclooctene nanoparticles for orthogonal dual-pretargeted near-Infrared fluorescence and magnetic resonance imaging. J. Am. Chem. Soc. 146, 13163–13175 (2024).
Cui, L. et al. Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat. Biotechnol. 39, 357–367 (2021).
Choi, J. et al. Light‐triggered PROTAC nanoassemblies for photodynamic IDO proteolysis in cancer immunotherapy. Adv. Mater. https://doi.org/10.1002/adma.202405475 (2024).
An, K. et al. Stimuli‐responsive PROTACs for controlled protein degradation. Angew. Chem. Int. Edit. 62, e202306824 (2023).
Costales, M. G. et al. Small-molecule targeted recruitment of a nuclease to cleave an oncogenic RNA in a mouse model of metastatic cancer. Proc. Natl Acad. Sci. USA 117, 2406–2411 (2020).
Disney, M. D. Targeting RNA with small molecules to capture opportunities at the intersection of chemistry, biology, and medicine. J. Am. Chem. Soc. 141, 6776–6790 (2019).
Velagapudi, S. P. et al. Defining RNA–small molecule affinity landscapes enables design of a small molecule inhibitor of an oncogenic noncoding RNA. ACS Cent. Sci. 3, 205–216 (2017).
Shi, Z. et al. AC1MMYR2, an inhibitor of Dicer-mediated biogenesis of oncomir miR-21, reverses epithelial–mesenchymal transition and suppresses tumor growth and progression. Cancer Res 73, 5519–5531 (2013).
Chen, Y. et al. Targeted inhibition of oncogenic miR-21 maturation with designed RNA-binding proteins. Nat. Chem. Biol. 12, 717–723 (2016).
Zhu, S. et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 18, 350–359 (2008).
Matsuhashi, S., Manirujjaman, M., Hamajima, H. & Ozaki, I. Control mechanisms of the tumor suppressor PDCD4: expression and functions. Int. J. Mol. Sci. 20, 2304 (2019).
Vikhreva, P. N., Shepelev, M. V. & Korobko, I. V. mTOR-dependent transcriptional repression of Pdcd4 tumor suppressor in lung cancer cells. BBA Gene Regul. Mech. 1839, 43–49 (2014).
Wang, Q. & Yang, H. S. The role of Pdcd4 in tumour suppression and protein translation. Biol. Cell 110, 169–177 (2018).
Li, C. et al. SKP2 promotes breast cancer tumorigenesis and radiation tolerance through PDCD4 ubiquitination. J. Exp. Clin. Cancer Res. 38, 76 (2019).
Guo, P. et al. Upregulation of miR-96 promotes radioresistance in glioblastoma cells via targeting PDCD4. Int. J. Oncol. 53, 1591–1600 (2018).
Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (T2325019 and 22077092, received by H.S.), the Basic Research Program of Jiangsu (BK20243030, received by H.S.), the special project of “Technological Innovation” project of CNNC Medical Industry Co. Ltd (ZHYLYB2021001, received by Y.F.), Four “Batches” Innovation Project of Invigorating Medical through Science and Technology of Shanxi Province (2022XM19, received by H.S.), the Open Project Program of the State Key Laboratory of Radiation Medicine and Protection (GZK12024016, GZK12023050, and GZK12024013, received by H.S.), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (received by H.S.).
Author information
Authors and Affiliations
Contributions
H. Shi conceived and designed the experiments. Y Zhang, Q.L., J.Z., Z.L., Z.Z., X.R., Y.G., Y.C., M.L., Y.F., Z.H., and Y.F. performed the experiments. H. Shi analyzed the data and supervised the project. Y Zhang and H. Shi wrote the manuscript and all authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Zhu, J., Qiu, L. et al. Stimulus-activated ribonuclease targeting chimeras for tumor microenvironment activated cancer therapy. Nat Commun 16, 1288 (2025). https://doi.org/10.1038/s41467-025-56691-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56691-3
This article is cited by
-
MSCs-EXOs containing miR-21a-5p promoted cell proliferation in the rat retinal Müller cell rMC-1 via targeting LATS1
Stem Cell Research & Therapy (2025)