Abstract
Liver metastasis (LM) poses a significant challenge in cancer treatment, with limited available therapeutic options and poor prognosis. Understanding the dynamics of tumor microenvironment (TME) and immune interactions is crucial for developing effective treatments. We find that WNT11 promoted CD8+ T-cell exclusion and suppression, which was correlated with poor prognosis in LM. Mechanistically, WNT11-overexpressing tumor cells directly reduce CD8+ T-cell recruitment and activity by decreasing CXCL10 and CCL4 expression through CAMKII-mediated β-catenin/AFF3 downregulation. WNT11-overexpressing tumor cells promote immunosuppressive macrophage polarization by inducing IL17D expression via the CAMKII/NF-κB pathway, which result in CD8+ T-cell suppression. Moreover, CAMKII inhibition increases the efficacy of anti-PD-1 therapy in mouse model of LM. Serum expression of WNT11 is identified as a potential minimally invasive biomarker in the management of colorectal cancer-LM with immunotherapy. Our findings highlight WNT11/CAMKII axis as a critical regulator of the TME and a promising target for immunotherapy in patients with LM.
Similar content being viewed by others
Introduction
Tumor metastasis accounts for over 90% of deaths related to solid tumors and presents a significant challenge in the effective treatment of malignant tumors. The liver is the primary site of tumor metastasis, with liver metastasis (LM) most commonly originating from colorectal cancer, followed by pancreatic cancer, melanoma, lung cancer, and breast cancer1. Surgical resection stands out as an effective treatment option for resectable liver metastases. Nonetheless, it is uncommon for a patient to be eligible for surgical resection, as they typically present with liver metastases originating from colorectal cancer or a neuroendocrine tumor2. The limited availability of current therapeutic strategies, combined with their restricted applicability and limited responsiveness, underscore the urgent need to explore novel targets and approaches for treating liver metastases3.
The interaction among tumor cells, resident cells, and immune cells in the hepatic microenvironment is crucial for the progression of LM3. The exclusion of CD8+ T-cells is a primary characteristic of the TME in most liver metastases, playing a crucial driving role in its progression. It has been revealed that LM triggers systemic T-cell exclusion mediated by suppressive macrophages, which results in resistance to immunotherapy. Interestingly, targeted radiotherapy to liver metastases followed by immunotherapy effectively restores immune cell function in a mouse model of colorectal cancer-liver metastasis (CRC-LM)4. The use of PD-1 inhibitors alone or in combination with CTLA-4 inhibitors has been demonstrated to exhibit good efficacy in patients with high microsatellite instability or mismatch repair-deficient metastatic colorectal cancer5. After a minimum follow-up of 3 years, two phase III clinical trials showed that nivolumab, an anti-PD-1 therapy, significantly improved overall survival (17% vs. 8%) and progression-free survival (10% vs. 1%) in non-small cell lung cancer patients with liver metastases compared to docetaxel6. However, owing to immune-excluded TME, most liver metastases, whether originating from CRC or others, exhibited resistance to immunotherapy. Therefore, uncovering the mechanisms underlying the remodeling of the immune-excluded TME holds promise for providing novel diagnostic and therapeutic strategies for patients with unresectable LM.
Recently, single-cell technologies, particularly single-cell transcriptome sequencing and mass cytometry, have emerged as revolutionary tools for deciphering the intricacies of the TME. By employing single-cell transcriptomics and spatial transcriptomics, the immune landscape and spatial dynamic changes were uncovered within liver metastases originating from CRC, which revealed the presence of MRC1+CCL18+ M2-like macrophages that play a crucial role in reshaping the microenvironment of liver metastases7. It is well established that both primary tumor cells and host organs exhibit specific roles during the remodeling of the microenvironment in liver metastases8. In addition, several teams have conducted small-scale single-cell transcriptomic analyses of the microenvironment of LM, including gallbladder cancer, pancreatic cancer, breast cancer, and melanoma9,10,11,12. In the above studies, certain cell types with similar markers and functions were found in LM from different primary cancers, such as SPP1+ macrophages, CCL18+ macrophages, and GZMK+CD8+ T-cells. Classification of an immune microenvironment in liver metastases was determined using mass cytometry13. This study identified SLC2A1 as a potential key molecular mediator in the remodeling of the immune-tolerant microenvironment in liver metastases13. Interestingly, SLC2A1 was significantly overexpressed in LM compared to both hepatocellular carcinoma and the corresponding primary tumors13. This suggests that novel diagnostic and therapeutic strategies tailored to different sources of liver metastases may offer opportunities for patients with LM.
The WNT signaling pathway plays a critical role in tumor progression across several types of cancer. In addition to its function in regulating the malignant characteristics of tumor cells, it also plays a significant role in remodeling the tumor microenvironment14. It was reported that activation of the WNT/β-catenin signaling pathway is closely associated with T-cell exclusion in various human solid tumors15. Prior studies have elucidated its involvement in promoting T-cell exclusion, recruiting polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), and facilitating dendritic cell (DC) tolerization, thus hampering anti-tumor immunity. The WNT signaling pathway contributes to T-cell exclusion from the TME while enhancing the recruitment of PMN-MDSCs, further suppressing immune responses16. In addition, the WNT signaling pathway promotes DC tolerization, reducing the efficacy of anti-tumor immunity17. Given these insights, targeting the WNT pathway could represent a promising strategy for overcoming resistance to immunotherapies, as WNT pathway inhibitors have shown potential to enhance immune responses in the tumor microenvironment18. Understanding the complex interplay between WNT signaling and immune regulation is essential for developing effective therapeutic strategies for liver metastases.
In this work, we explored the mechanism underlying immune-exclusion TME remodeling of liver metastases and its implications for therapeutic intervention. We identify WNT11 and AFF3 as potential key players closely associated with T-cell exclusion in liver metastases by analyzing published RNA-seq data13. WNT11 was an essential member of the non-canonical WNT signaling pathway, which played critical roles in various tumor biological processes, including cell proliferation, migration, and invasion19,20,21. AFF3, a transcription factor directly targeted by β-catenin22, played a significant role in the progression of various cancers23,24. However, the roles and mechanisms of WNT11 and AFF3 in tumor immune regulation have not been extensively explored. By elucidating the regulatory mechanisms, we discover that CAMKII, a downstream molecule of WNT11, negatively regulates the expression of CXCL10 and CCL4 by inhibiting β-catenin/AFF3 axis, while positively modulating the expression of IL17D via classical NF-κB pathway. Moreover, downregulation of CXCL10 and CCL4 directly prevents the infiltration and activation of CD8+ T-cells, whereas upregulation of IL17D promotes the polarization of CD206+ macrophages, which suppresses CD8+ T-cells. Importantly, in an LM mouse model, the combination of KN93 (a CAMKII inhibitor) and anti-PD-1 significantly suppressed the progression of liver metastases compared with the control group or monotherapy. These discoveries hold promise for understanding the mechanism of immune escape and the development of biomarkers predicting the effectiveness of ICB therapy in LM.
Results
WNT11 is associated with CD8+ T-cell exclusion and a poorer prognosis in liver metastases
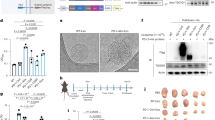
It has been reported that CD8+ T-cell exclusion is a common phenomenon in the immune landscape of liver metastases3. Interestingly, we noticed that the Wnt signaling pathway was closely correlated with reduced CD8+ T-cell infiltration in a published liver metastases cohort13 (LM cohort, 86 cases of liver metastases from LCLM cohort, HRA001142) (Fig. 1a). This cohort included patients with liver metastases that originated from colorectal cancer, pancreatic cancer, upper gastrointestinal tumors, and gallbladder cancer amongst others13. We identified the Wnt signaling pathway-associated genes from KEGG canonical and noncanonical Wnt signaling. Then, the relationship between the Wnt signaling pathway-associated genes and patient prognosis was evaluated using univariate Cox regression analysis and Kaplan-Meier survival analysis in the LM cohort. These results showed that WNT11 was predictive of a poorer prognosis while AFF3 was predictive of a better prognosis (Fig. 1b–d). FZD4 was not a prognostic-related molecule in the KM survival analysis (Supplementary Fig. 1a). Furthermore, the expression of WNT11 and AFF3 were negatively correlated with each other in the LM cohort (Fig. 1e). Based on WNT11 and AFF3 expression, patients in the LM cohort were categorized into four groups: WNT11-high/AFF3-high, WNT11-high/AFF3-low, WNT11-low/AFF3-high, and WNT11-low/AFF3-high groups. (Fig. 1f). Moreover, by analyzing the expression of CD3D, CD3E, CD8A, and CD4 among these four groups, we found that the WNT11-low/AFF3-high group exhibited the highest level of CD8A, CD3D, and CD3E, but not CD4. This indicated a higher degree of CD8+ T-cell infiltration in the WNT11-low/AFF3-high group (Fig. 1g, h and Supplementary Fig. 1b, c). Interestingly, compared to the WNT11-low/AFF3-high group, the other three groups showed a relatively lower proportion of CRC and PDAC (Fig. 1f). Therefore, the role of WNT11/AFF3 in regulating CD8+ T-cell exclusion and liver metastasis was further explored based on CRC and PDAC liver metastases.
a The correlation between the GSVA score of the KEGG WNT Signaling Pathway gene set and the expression of CD3D and CD8A in the LM cohort (n = 86). b The association between genes in the WNT signaling pathway and the clinical prognosis in the LM cohort. c, d Overall survival according to AFF3 and WNT11 expression in the LM cohort. e Pearson correlation analysis of AFF3 levels with WNT11 levels in the LM cohort. f Liver metastases in the LM cohort were divided into four groups based on the expression of AFF3 and WNT11. The plot shows the proportion of cancer types in the different groups. g, h The expression of CD3D and CD8A in the above four groups. i, j AFF3 and WNT11 IHC staining of primary tumors and matched liver metastases in the FUSCC_CRC_cohort (n = 71) and the SGH_PDAC_cohort (n = 50). k–n Tissue microarray analysis of AFF3 and WNT11 expression in primary tumors and matched liver metastases from the FUSCC_CRC_cohort (k, l n = 71) and SGH_PDAC_cohort (m, n n = 50). o–r Overall survival based on AFF3 and WNT11 expression in the FUSCC_CRC_cohort (o, p) and SGH_PDAC_cohort (q, r). s, t Pearson correlation of AFF3 with WNT11 expression in the FUSCC_CRC_cohort (s) and SGH_PDAC_cohort (t). a, e, s, t P-values and r-values were determined using Pearson correlation (two-tailed) without adjustment. b P-values were determined using univariate proportional hazard analysis without adjustment. c, d, o–r P-values were determined using a log-rank test. g, h P-values were determined using a one-way ANOVA followed by a post hoc Tukey’s multiple comparison test. k–n P-values were determined using a Wilcoxon rank-sum test (two-tailed). g, h, k–n In the box plots, the bounds of the box span from 25% to 75% percentile, the line represents the median, and the whiskers show the minima and maxima of data points. Source data are provided as a Source Data file.
We then verified the potential involvement of WNT11 and AFF3 in liver metastases using IHC staining in a colorectal cancer tissue microarray chip (FUSCC_CRC_cohort, 71 cases) and a pancreatic cancer tissue microarray chip (SGH_PDAC_cohort, 50 cases), both consisting of primary tumor and paired liver metastasis (Supplementary Data 1). We found that liver metastases had higher WNT11 and lower AFF3 expression compared with the primary tumor in both the CRC and PDAC cohorts (Fig. 1i–n). In addition, WNT11 expression was significantly negatively associated with AFF3 expression in the CRC and PDAC liver metastases (Fig. 1o, p). We further verified the association between clinical outcomes and the expression levels of WNT11 and AFF3 in the FUSCC CRC cohort and SGH PDAC cohort, and the results were consistent with those from the LM cohort (Fig. 1q–t). To determine the relationship between WNT11 and AFF3, we constructed stable WNT11 and AFF3-knockdown MC38 and Panc02 cell lines. We examined the mRNA and protein expression levels of WNT11 and AFF3 and only found reduced expression of AFF3 in WNT11-knockdown MC38 and Panc02 cells (Supplementary Fig. 1d–k), which suggested that AFF3 was downstream of WNT11, and was regulated by WNT11 at the transcriptional level. Overall, we found that a WNT11-AFF3 axis may be closely associated with T-cell exclusion and a poor prognosis in patients with LM.
Knockdown of WNT11 in cancer cells impairs tumor growth and improves survival via the adaptive immune system in immunocompetent mice
To further explore the regulatory roles of WNT11 in immune evasion of liver metastases, we used shRNAs to study the functional roles of WNT11 in cancer cells. The results showed that the knockdown of WNT11 had no discernable effect on the in vitro proliferation of Panc02 or MC38 cancer cells compared with the respective controls (Supplementary Fig. 2a–d). Furthermore, Transwell assays indicated that the knockdown of WNT11 did no effect in vitro migration and invasion of MC38 and Panc02 cells (Supplementary Fig. 2e–h). To investigate the functional effects of WNT11 knockdown on tumor phenotypes in vivo, we inoculated both scramble and shWNT11 Panc02 and MC38 cells into syngeneic and immunodeficient mice. We performed intrasplenic injections to establish a model of liver metastases and subsequently conducted tumor growth and survival analyses. Differences in phenotypes were observed in immunodeficient versus immunocompetent mice bearing experimental liver metastases. The scramble and WNT11 knockdown MC38 and Panc02 cells experimental liver metastases models had similar survival outcomes in the immunodeficient NCG mice (Fig. 2a, c). However, the survival time was markedly prolonged in the WNT11 knockdown group compared with the scramble group in immunocompetent mice (Fig. 2b, d). WNT11 knockdown impaired metastatic tumor burden in immunocompetent C57BL/6 mice (Fig. 2e–j). Collectively, the data indicated that WNT11 knockdown resulted in slower tumor growth in immunocompetent mice compared to immunodeficient mice, highlighting the adaptive immune system’s role in WNT11-driven LM formation.
a–d Overall survival of immunodeficient NCG or immunocompetent C57BL/6 mice bearing liver metastases seeded with MC38 and Panc02 scramble or shWnt11 cancer cells. n = 6 mice per group. e Gross appearance of MC38 scramble and shWnt11 liver metastases. f, g The liver weight and the number of metastases per liver in the MC38 LM mouse model. h Gross appearance of Panc02 scramble and shWnt11 liver metastases. i, j The liver weight and the number of metastases per liver in the Panc02 LM mouse model. k–n Comparison of CD8+ T-cell infiltration in the MC38 (k, l) and Panc02 (m, n) LM mouse model between the scramble and shWnt11 groups. o–r FACS analysis to determine the frequency of GZMB + CD8 + T-cells relative to the total CD8 + T-cell population in MC38 (o, p) and Panc02 (q, r) liver metastases. s–v FACS analysis to determine the frequency of TNFα + CD8 + T-cells relative to the total CD8 + T-cell population in MC38 (s–t) and Panc02 (u, v) liver metastases. a–d Statistics of survival by Kaplan-Meier test (two-tailed). f, g, i, j, l, n, p, r, t, v. Data are presented as the mean ± SD (n = 5 biological replicates). P-values were calculated using a Student’s t test (two-tailed). Source data are provided as a Source Data file.
To assess whether WNT11 knockdown could induce an adaptive immune response, we analyzed scramble and WNT11 knockdown tumors for the presence of CD8+ T-cells. Compared to the scramble group, the shWnt11 liver metastases exhibited increased infiltration of CD8+ T-cells (Fig. 2k–n). Furthermore, fluorescence-activated cell sorting (FACS) analysis also confirmed a higher proportion of CD8+ T-cells were present in shWnt11 liver metastases (Supplementary Fig. 3a–d). The tumor-infiltrating CD8+ T-cells from the shWnt11 liver metastases exhibited increased expression of the cytotoxic T lymphocyte markers GZMB (Fig. 2o–r) and TNFα (Fig. 2s–v). Thus, we speculated that liver metastasis with WNT11 knockdown may promote a response to anti-PD-1 therapy. Compared with the scramble liver metastases treated with anti-PD-1 antibody, WNT11 knockdown significantly augmented the response to anti-PD-1 antibody treatment (Supplementary Fig. 3e, f). We also validated the role of WNT11 in vivo using the CT26 and KPC liver metastasis models, where WNT11 knockdown led to a reduced liver tumor burden (Supplementary Fig. 4a, b). Collectively, the above data show that shWnt11 induced infiltration of CD8+ T-cells, reduced liver metastatic tumor burden, and promoted the response to anti-PD-1 therapy, highlighting the immunostimulatory effects of the TME induced by cancer cells lacking WNT11.
CD8+ T-cells are increased and CD206+ macrophages are decreased in the microenvironment of MC38 liver metastases following WNT11 knockdown
After confirming that inflammation by CD8+ T-cells against tumors with WNT11 knockdown in cancer cells significantly hindered tumor growth, we explored the cellular and molecular mechanisms underlying the observed immunosuppression in scramble tumors and the induction of adaptive immunity in shWnt11 tumors. MC38 scramble and shWnt11 liver metastatic tumors were analyzed using mass cytometry (CyTOF) (Supplementary Fig. 5a). The data revealed 20 distinct cell clusters and showed significant differences in the cellular composition between scramble and shWnt11 liver metastatic tumors (Fig. 3a and Supplementary Fig. 5b). We observed that several lymphocyte clusters, including C16 (I-A/I-E+CD8+ T-cells), C05 (CD4+ T-cells), C13 (CD44- B cells), and C12 (I-A/I-E- NK cells), increased in shWnt11 liver metastases (Fig. 3b). We also found that C02, a CD206+ macrophage cluster with the highest abundance, significantly decreased in shWnt11 liver metastases (Fig. 3b). C16 exhibited negative PD-1 and positive I-A/I-E expression compared to the other CD8+ T-cell cluster (C01) (Fig. 3e and Supplementary Fig. 5b). CD206+ macrophages were reported to suppress the function of CD8+ T-cells, suggesting that WNT11 may also indirectly regulate CD8+ T-cells through CD206+ macrophages. Interestingly, we further found lower CD206 expression of the C02 cluster and higher I-A/I-E and CD24 expression of the C16 cluster in the shWnt11 liver metastases, which indicated the improvement of the liver metastatic microenvironment following WNT11 knockdown25,26,27,28 (Fig. 3c–e). Next, we verified the altered clusters in tissue sections of MC38 liver metastases by multiplex immunohistochemistry (mIHC). The findings revealed no differences in the frequencies of CD44- B cells, I-A/I-E- NK cells, and CD4+ T-cells between scramble and shWNT11 MC38 liver metastases (Supplementary Fig. 5c). Moreover, we observed an increase in the I-A/I-E+CD8+ T-cell counts and a reduction in the CD206+ macrophage counts in shWnt11 MC38 liver metastases (Fig. 3f–i). We further confirmed that CD206 expression (macrophages) decreased in shWnt11 MC38 liver metastases (Fig. 3j). Furthermore, a similar change was observed with the I-A/I-E+CD8+ T-cells in the shWnt11 MC38 liver metastases as shown by flow cytometry analysis (Fig. 3k, l). CD163 has been reported as a marker of suppressive macrophages29. Notably, CD206+ macrophages also express CD163, and CD163+CD206+ macrophage counts decreased sharply in the shWNT11 MC38 liver metastases as evidenced by flow cytometry (Fig. 3m, n). Taken together, our results indicated that shWnt11 might improve the immune microenvironment of liver metastases by directly and indirectly regulating CD8+ T-cells.
a t-SNE plot illustrating the distribution of all CD45 + immune cells from liver metastases of MC38 cells with scramble control or WNT11 knockdown. b Proportion of differential cell clusters between MC38 scramble and shWnt11 liver metastases. c t-SNE plot of all CD45+ cells colored by the expression levels of CD206 in the scramble and shWnt11 group. d Comparison of CD206 expression of C02 between the scramble and shWnt11 group. e Comparison of I-A/I-E and CD24 expression of C16 between the scramble and shWnt11 group. f–i mIHC staining was used to verify the differences of I-A/I-E+CD8+ T-cells (f, g) and CD206+ macrophages (h, i) in MC38 liver metastases between the scramble and shWnt11 group. j CD206 expression of macrophages in MC38 liver metastases between the scramble and shWnt11 group detected using mIHC. k, l FACS analysis to determine the frequency of I-A/I-E + CD8 + T-cells relative to the total CD45 + T-cell population in MC38 liver metastases. m, n FACS analysis to determine the frequency of CD206 + F4/80 + macrophages relative to the total macrophages in MC38 liver metastases. b, g–j, l, n. P-values were calculated using a Student’s t test (two-tailed). Data are presented as the mean ± SD (n = 5 biological replicates). d, e P-values were calculated using a Wilcoxon rank-sum test (two-tailed). Source data are provided as a Source Data file.
WNT11 knockdown in tumor cells stimulates CD8+ T-cell proliferation and CD8+ T-cell-mediated tumor cell killing via AFF3
We sought to further assess the hypothesis that scramble and shWnt11 cancer cells had direct and differential interactions with CD8+ T-cells. As enrichment of CD8+ T-cells was higher in liver metastases with WNT11 knockdown, we examined the proliferation and apoptosis of CD8+ T-cells using Ki67 and TUNEL staining within MC38 and Panc02 liver metastases. The results showed increased proliferation and decreased apoptosis of CD8+ T-cells in both MC38 and Panc02 liver metastases with WNT11 knockdown (Fig. 4a–d), in line with results in CT26 and KPC liver metastases (Supplementary Fig. 6a–f). Next, a culture assay was established involving ex vivo activated and tumor antigen nonspecific splenic T-cells combined with conditioned media from tumor cells with different treatments. T-cell proliferation without the confounding complexity of T-cell–mediated killing of the cancer cells was assessed by FACS. The parental MC38 and Panc02 cells and the cells with scramble, shWNT11, and double knockdown of WNT11 and AFF3 (shWNT11-shAFF3) were assessed in coculture with CD8+ T-cells. Compared with T-cells cultured alone, the parental cells and scramble cancer cells were potently inhibitory for CD8+ T-cell proliferation (Fig. 4e, f and Supplementary Fig. 7a, b). By contrast, the shWNT11 cells only modestly inhibited the proliferation of CD8+ T-cells (Fig. 4e, f and Supplementary Fig. 7a, b). In addition, shWNT11-shAFF3 cells exhibited similar inhibitory action towards CD8+ T-cell proliferation with scramble cancer cells (Fig. 4e, f and Supplementary Fig. 7a, b), which established AFF3 as a regulator involved in counteracting the direct immunosuppressive effect of cancer cells on CD8+ T-cells. Notably, WNT11 did not affect the MHC-I expression of tumor cells and the viability of CD8 + T-cells (Supplementary Fig. 6g–i). Furthermore, we assessed antigen-specific killing by CD8+ T-cells in a classical CTL assay, involving OVA-specific OT1 CD8+ T-cells (isolated from OT1 transgenic mice) cocultured with scramble, shWNT11 and shWNT11-shAFF3 MC38-OVA or Panc02-OVA cancer cells. Knockdown of WNT11 promoted sensitivity to T-cell killing at all ratios; AFF3 knockdown reversed the effect of WNT11 knockdown (Fig. 4g, h and Supplementary Fig. 6k).
a–d Flow cytometry analysis of the proliferation and apoptosis of CD8+ T-cells in MC38 (a, b) and Panc02 (c, d) liver metastases between scramble and shWnt11 group. e, f FACS analysis of CD8+ T-cell proliferation (CFSE low) in cocultures with MC38 (e) or Panc02 (f) scramble and shWnt11 cancer cells. g. Schematic representation of an in vitro T-cell killing assay. OVA-expressing tumor cells were cocultured in different ratios with CD8+ T-cells isolated from OVA-specific T-cell receptor transgenic (OT1) mice that had been preactivated using an OVA peptide and IL-2 plus IL-7 treatment. h Titration assay involving coculture at different effector-to-target ratios assessing the sensitivity to cytotoxic T-cell killing of MC38-OVA scramble, shWnt11, and shWnt11-shAff3 cells. i Alterations in AFF3 and β-catenin protein levels in MC38 cells following treatment with WNT11 and inhibitors of CAMKII, JNK, and PKC as indicated. j Knockdown of β-catenin abrogated the AFF3-induced increase in expression following Wnt11 knockdown. k, l Knockdown of WNT11 increased the nuclear translocation of β-catenin. m FACS analysis of CD8+ T-cell proliferation (CFSE low) in cultured with conditioned media derived from MC38 cells treated with WNT11 and CAMKII, JNK, and PKC inhibitors as indicated. n qPCR analysis of the expression of Camk2b in MC38 cells following CAMKII knockdown. o Aff3 mRNA levels were detected using qPCR in MC38 cells following CAMKII knockdown and WNT11 treatment as indicated. p FACS analysis of CD8+ T-cell proliferation (CFSE low) in cultured with conditioned media derived from MC38 cells with CAMKII knockdown and WNT11 treatment as indicated. q Titration assay involving coculture at different effector-to-target ratios assessing the sensitivity to cytotoxic T-cell killing of MC38-OVA scramble and shCamk2b cells with or without WNT11 treatment. b, d P-values were calculated using a Student’s t test (two-tailed), n = 5 biological replicates. e, f, m–p P-values were calculated using a one-way ANOVA followed by a Tukey’s multiple comparison test, n = 3 biological replicates. h, q P-values were calculated using a two-way ANOVA, n = 3 biological replicates. Data are presented as the mean ± SD. Source data are provided as a Source Data file.
It was reported that WNT11 primarily activates non-canonical Wnt pathways, including the WNT/Ca2+ pathway and the Planar Cell Polarity (PCP) pathway20. We examined the alterations of several key kinases involved in a non-canonical Wnt pathway, including CAMKII, PKC, and JNK30. We inhibited their activities and assessed the changes in AFF3 expression. The results indicated that exogenous WNT11 could suppress AFF3 expression, while only inhibition of CAMKII could block this effect (Fig. 4i). Previous studies have reported that AFF3 is an important target of β-catenin22. Following WNT11 treatment, we observed a substantial reduction in β-catenin protein levels, which were subsequently restored upon inhibition of CAMKII. Interestingly, CAMKII was reported as a negative regulator of β-catenin via directly phosphorylating β-catenin31. Our investigations revealed that inhibition of CAMKII could restore the downregulation of β-catenin induced by WNT11 in MC38 cells (Fig. 4i). We additionally verified increased expression of β-catenin in MC38 cells following WNT11 knockdown. Knockdown of β-catenin prevented the upregulation of AFF3 induced by WNT11 knockdown (Fig. 4j). Furthermore, we confirmed that knockdown of WNT11 promoted the nuclear translocation of β-catenin via immunofluorescence (Fig. 4k, l). ChIP-qPCR analysis using an anti-β-catenin antibody was performed in MC38 cells. A total of eight pairs of primers that covered the Aff3 promoter – 1 to – 2000 region were used. The results showed that β-catenin bound to the middle of the promoter region (–1335 to –1502) in MC38 cells (Supplementary Fig. 6j).
Then, MC38 cells, after treatment with WNT11, JNK inhibitor, CAMKII inhibitor, or PKC inhibitor as indicated, were used for the coculture systems to assess the proliferation of activated antigen-nonspecific T-cells. Interestingly, we found that conditioned media from MC38 with CAMKII inhibition substantially restored CD8+ T-cell proliferation inhibited by WNT11-treated MC38 conditioned media (Fig. 4m and Supplementary Fig. 7c). Therefore, we constructed stable CAMKII knockdown cells (targeting the Camk2b subunit) MC38 cells (Fig. 4n) and verified the expression of AFF3. The results indicated that the knockdown of CAMKII markedly increased the expression of AFF3 in MC38 cells, regardless of WNT11 treatment (Fig. 4o). Then, conditioned media from scramble and shCamk2b MC38 cells treated with vehicle or WNT11 was used for coculture with tumor antigen nonspecific splenic T-cells. The results revealed that WNT11-treated MC38 cells inhibited the proliferation of T-cells, which was restored by the knockdown of CAMKII (Fig. 4p and Supplementary Fig. 7e). Furthermore, an antigen-specific killing assay demonstrated that WNT11-treated cancer cells were resistant to T-cell killing, and the knockdown of CAMKII increased sensitivity to T-cell killing at all ratios (Fig. 4q). These results were also validated in Panc02 cells (Supplementary Figs. 6l–p, 7d and f). Thus, shWnt11 cancer cells are markedly less inhibitory toward T-cell proliferation, resulting from modulation of CAMKII, and somewhat more susceptible to CD8+ T-cell killing. The results establish a direct mechanistic link between the shWnt11 cancer cells and the adaptive immune response in shWnt11 tumors.
WNT11 knockdown in cancer cells promotes T-cell activation and consequent antitumor immunity through CXCL10/CXCR3 and CCL4/CCR5
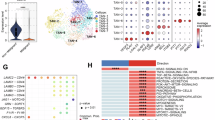
To explore the potential mechanism by which shWnt11 promoted T-cell activation and consequent antitumor immunity, we assessed the expression of differential secretory genes among WNT11-high/AFF3-high, WNT11-high/AFF3-low, WNT11-high/AFF3-low, WNT11-low/AFF3-low groups in the LM cohort. As shown in Supplementary Figs. 8a, 14 differential secretory genes were identified among these four groups. Among them, CXCL10, CXCL13, and CCL4 exhibited a positive correlation with T-cell markers, and IL17D showed a negative correlation with T-cell markers (Supplementary Fig. 8b). Further in vitro analysis confirmed increased mRNA expression and secretion of Cxcl10 and Ccl4 in shWnt11 MC38 and Panc02 cells, with this increase being abrogated in the shAff3 cells (Fig. 5a–e). This highlights Cxcl10 and Ccl4 as potential targets of the WNT11/AFF3 axis. CXCR3 and CCR5 are receptors for CXCL10 and CCL4, respectively32,33. Interestingly, previous studies have shown that CXCR3 and CCR5 participate in T-cell proliferation and activation34. To determine whether Cxcl10 and Ccl4 mediate WNT11-regulated T-cell activation, we conducted in vitro coculture experiments using inhibitors of CXCR3 and CCR5. Our results indicated that inhibitors targeting CXCR3 and CCR5 could partially impede CD8+ T-cell proliferation induced by conditioned media derived from shWnt11 cancer cells when used individually (Fig. 5f–h). Notably, combined CXCR3 and CCR5 inhibition showed a more potent suppression of T-cell proliferation compared with inhibition of either receptor alone, which indicated that shWnt11 cancer cells potentially promoted T-cell activity via CXCL10/CXCR3 and CCL4/CCR5 (Fig. 5f–h).
a The expression of cytokines was confirmed using qPCR in MC38 and Panc02 scramble, shWnt11, and shWnt11-shAff3 cells. b–e ELISA was used to measure the secretion of CXCL10 and CCL4 in the supernatants of MC38 and Panc02 scramble, shWnt11, and shWnt11-shAff3 cells. f–h FACS analysis of CD8+ T-cell proliferation (CFSE low) in cocultures with MC38 and Panc02 scramble and shWnt11 cells, while CXCR3 and CCR5 were inhibited by AMG487 and maraviroc, respectively, in the coculture system. i–l Cxcl10 and Ccl4 expression were detected using qPCR in MC38 and Panc02 cells with CAMKII knockdown or double knockdown of CAMKII and AFF3. m–o Quantification and representative images of liver weight, number of metastases, and IHC staining of CD8a in an LM mouse model established using MC38 cells treated with a combination of a CXCR3 inhibitor (AMG487) and CCR5 inhibitor (maraviroc). p–r Quantification and representative images of liver weight, number of metastases, and IHC staining of CD8a in an LM mouse model established using Panc02 cells treated with a combination of AMG487 and maraviroc. b–e, g–m, p P-values were calculated using a one-way ANOVA followed by a post hoc Tukey’s multiple comparison test, data are presented as the mean ± SD (n = 3 biological replicates). Source data are provided as a Source Data file.
To confirm whether AFF3, as a transcriptional activator, could directly bind to the Cxcl10 and Ccl4 promoters, ChIP-qPCR analysis using an anti-Flag antibody was performed in Flag-AFF3-overexpressing MC38 cells. Eight pairs of primers that covered the Cxcl10 and Ccl4 promoter regions from − 1 to − 2000 bp were used. The results showed that AFF3 bound to the promoter-proximal element (− 1 to − 209 bp) and middle region (− 725 to − 908 bp) of Cxcl10 and the proximal (− 249 to − 1 bp) and middle (− 1085 to − 841 bp) regions of Ccl4 promoter in MC38 cells (Supplementary Fig. 8c, d). Next, we assessed the expression of Cxcl10 and Ccl4 in MC38 and Panc02 cells treated individually with inhibitors targeting CAMKII, JNK, and PKC, respectively. We found that only inhibition of CAMKII induced Cxcl10 and Ccl4 expression (Supplementary Fig. 5e–j). Meanwhile, the knockdown of CAMKII also significantly promoted Cxcl10 and Ccl4 expression in MC38 and Panc02 cells, which could be prevented by AFF3 knockdown (Fig. 5i– l).
Furthermore, we also assessed the functional importance of CXCL10 and CCL4 for CD8+ T-cell-dependent tumor immunity by pharmacologically inhibiting their receptors. Then, we found that a combination of AMG487 and maraviroc significantly decreased the recruitment of CD8+ T-cells and restored the impaired growth of both MC38 and Panc02 shWnt11 liver metastases (Fig. 5m–r). Similar phenotypic changes in tumor growth also were observed in CT26 and KPC shWnt11 liver metastases (Supplementary Fig. 8k–p). Taken together, these findings indicated that the chemokine signaling pathways mediated by the CCR5 and CXCR3 receptors exhibited functional complementarity, necessitating their combined activity for robust CD8+ T-cell activation and subsequent antitumor immune response.
WNT11 promotes polarization of CD206+ macrophages through an IL17D-CD93 axis in liver metastases
As the above CyTOF assay identified that increased CD206+ macrophage counts may indirectly inhibit CD8+ T-cells in high WNT11 liver metastases, we further verified and found upregulated CD86+CD80+ macrophage (M1) and downregulated CD206+CD163+ macrophage (M2) counts in MC38 and Panc02 shWnt11 liver metastases compared to scramble LMs (Fig. 6a–d). The experiments were repeated in CT26 and KPC LMs, and the same results were observed (Supplementary Fig. 9a–d) To explore the molecular mechanism by which WNT11-overexpressing cancer cells regulated macrophage polarization, we subsequently validated the effects of the cytokines regulated by WNT11 (CXCL10, CCL4, and IL17D), on the polarization of macrophages. The results indicated that apart from CXCL10 promoting M1 macrophage polarization, neither CXCL10 nor CCL4 induced M2 macrophage polarization, and CCL4 had no effect on M1 polarization (Supplementary Fig. 10a–d). Interestingly, IL17D significantly promoted M2 polarization of macrophages whilst having no effect on M1 polarization (Fig. 6e, f and Supplementary Fig. 10e, f). Then, we generated stable MC38 scramble and shIl17d cells, followed by treatment with vehicle or WNT11 to collect conditioned media. Subsequent co-culture experiments with bone marrow-derived macrophages (BMDMs) demonstrated that cancer cells with IL17D expression knocked down significantly attenuated Mrc1, Cd163, and Arg1 expression in BMDMs (Fig. 6g, h and Supplementary Fig. 10i). Furthermore, IL17RA or CD93 (receptors of IL17D) expression was knocked down in BMDMs (Supplementary Fig. 10g, h), which were then cultured with conditioned media from MC38 cells with or without WNT11 treatment. It was found that knockdown of CD93 in BMDMs could significantly reduce the CD206+ macrophage count (Fig. 6i, j). Furthermore, we constructed a CD93 knockout mouse (Cd93ΔMAC) model and isolated the BMDMs (Cd93ΔMAC BMDMs), which were used for culture with the conditioned media from WNT11-treated MC38 cells. The results showed that CD93 knockout could suppress CD206 expression of BMDMs (Fig. 6k, l). Cd93ΔMAC and the wild-type littermates were subsequently used to construct MC38 liver metastases; we found that Cd93ΔMAC mice exhibited reduced liver metastatic burden and fewer infiltrating CD206+ macrophages within the tumors (Fig. 6m, n). Similar results also were observed in Panc02, CT26, and KPC liver metastases (Supplementary Fig. 11a–n). We further confirmed that WNT11 could significantly increase Il17d expression in both MC38 and Panc02 cells (Supplementary Fig. 12a, b). To investigate the mechanism by which IL17D expression was induced by WNT11, we first detected the expression of Il17d in MC38 and Panc02 cells with CAMKII, JNK, or PKC inhibition. The results showed that only CAMKII inhibition could prevent the upregulation of Il17d induced by WNT11 (Supplementary Fig. 12c, d). However, the knockdown of β-catenin did not alter the expression of Il17d (Supplementary Fig. 12e, f), which suggested the presence of another mechanism involved in WNT11-CAMKII regulated Il17d expression. NFAT and NF-κB have been reported to mediate CAMKII-regulated transcriptional regulation35,36. Thus, we further examined Il17d expression in tumor cells with Nfatc1 or Rela (gene coding NF-κB p65) expression knocked down. Rela knockdown prevented the transcription and secretion of Il17d (Fig. 6o, p and Supplementary Fig. 12g–n). In addition, we also observed that WNT11 induced an increase in the phosphorylation of IKKα, decreased IκBα expression, and subsequently increased Il17d expression in MC38 cells, while WNT11 knockdown had the inverse effect (Fig. 6q). More importantly, CAMKII inhibition could partly reverse the roles of the WNT11-induced increase in the nuclear translocation of NF-κB p65 (Fig. 6r).
a, b FACS analysis confirmed the infiltration of CD86+ CD80+ macrophages and CD206+CD163+ macrophages in MC38 scramble and shWNT11 liver metastases. c, d FACS analysis confirmed the infiltration of CD86+ CD80+ macrophages and CD206+CD163+ macrophages in Panc02 scramble and shWNT11 liver metastases. e, f The effects of IL17D on M2 polarization (Mrc1, Arg1) of macrophages. g, h Conditioned media from MC38 scramble and shIl17d cells treated with WNT11 was used to culture BMDMs. The expression of M2 markers (Mrc1, Cd163) was detected using qPCR. i, j BMDMs transfected with siRNA targeting Il17ra or Cd93 were cultured with conditioned medium from MC38 cells treated with vehicle or WNT11. Flow cytometry analysis was used to detect CD206 expression in the BMDMs treated with the conditioned medium. k, l Cd93f/f, and Cd93ΔMAC BMDMs were cultured with conditioned medium from MC38 cells treated with vehicle or WNT11. m, n Representative images and quantification of liver weight, number of metastases, and mIHC analysis of CD206+ macrophage infiltration in the mouse LM model established using MC38 cells in Cd93f/f and Cd93ΔMAC mice. o, p mRNA levels and secretion of IL17D were detected in MC38 cells following Rela knockdown. q Western blotting was used to detect alterations in IL17D, NF-κB p65, p-IKKα, and IκBα protein expression levels in MC38 cells following WNT11 knockdown or WNT11 treatment. r NF-κB p65 distribution was examined in nuclear and cytoplasmic extracts from MC38 cells treated with vehicle, WNT11, and WNT11/CAMKII-IN−1 treatments. b, d, m, n P-values were calculated using a Student’s t test (two-tailed), n = 5 biological replicates. e–h, j, l, o, p P-values were calculated using a one-way ANOVA followed by Tukey’s multiple comparison test, n = 3 biological replicates. Data are presented as the mean ± SD. Source data are provided as a Source Data file.
Together, these findings identified the distinct immune profiles resulting from the presence or absence of WNT11 expression in tumor cells. Cancer cells expressing WNT11 triggered the development and polarization of CD206+ macrophages and the reduced CD8+ T-cell activity and infiltration by promoting Il17d and inhibiting Cxcl10 and Ccl4 expression, respectively, leading to an immunosuppressive microenvironment characterized by the exclusion and suppression of CD8+ T-cells and increased infiltration of CD206+ macrophages.
CAMKII antagonist increases the efficacy of anti-PD-1 therapy in WNT11-expressing tumor-bearing mice
Based on the above findings, we confirmed that WNT11 could regulate both CD8+ T-cells and CD206+ macrophages through CAMKII-mediated cytokine expression in liver metastases. We thus investigated whether inhibiting CAMKII could suppress the interaction between tumor cells and immune cells, thus enhancing anti-PD-1 immunotherapy in liver metastases. The molecule KN93 is the most widely used specific inhibitor for studying the in vivo functions of CAMKII37. We constructed mouse LM models with MC38 and KPC cells followed by single treatment or combination treatment with KN93 and anti-PD-1. Combination therapy resulted in a more significant decrease in LM burden compared with the control and monotherapies (Fig. 7a–h). In addition, as indicated by the mIHC analyses, mice treated with combination therapy had a greater count of I-A/I-E+CD8+ T-cells and reduced CD206+ macrophage infiltration in the liver metastases (Fig. 7i, j), suggesting that CAMKII antagonist treatment shifted liver metastases toward immunologically “hot” tumors with a relatively higher response to immunotherapy. ELISA and qPCR analysis showed that KN93 significantly induced the expression of CXCL10 and CCL4 and reduced the expression of IL17D (Fig. 7k, l and Supplementary Fig. 13a, b). As WNT11 can be secreted into the blood, we then examined whether the serum levels of WNT11 could be used to predict the response to immunotherapy in LM. The concentration of WNT11 in serum was markedly lower in CRC-LM patients who responded to anti-PD-1 therapy than in non-responders (Fig. 7m, n and Supplementary Data 2). We also found that CRC-LM patients responsive to immunotherapy had relatively higher serum CXCL10 and CCL4 levels and lower IL17D levels than non-responders (Fig. 7o–q). These results show that serum WNT11 level may serve as a potential minimally invasive biomarker in the management of CRC-LM patients being treated with immunotherapy. Taken together, our findings identified the regulatory role of WNT11 in reprogramming the TME immune landscape in LM through regulating cytokine expression. On one hand, WNT11 inhibited the expression of CXCL10 and CCL4 through the CAMKII/β-catenin/AFF3 axis, leading to the exclusion of CD8+ T-cells. On the other hand, WNT11 promoted the infiltration of CD206+ macrophages via the CAMKII/NF-κB/IL-17D axis, resulting in the suppression of CD8+ T-cells (FiG. 7j). Therefore, this study highlighted a critical target, CAMKII, that could be pharmacologically targeted to sensitize patients with an LM to immunotherapy, particularly for patients with high serum WNT11 expression.
a, b Anti-PD−1 and CAMKII antagonist KN93 treatment in mice bearing MC38 liver metastases. Tumor progression was monitored post-tumor cell inoculation using live animal bioluminescent imaging. c, d Quantification of the liver weight and number of metastases in the mouse LM model established using MC38 cells in each group at the endpoint. e, f Anti-PD-1 and CAMKII antagonist (KN93) treatment in mice bearing KPC liver metastases. Tumor progression was monitored post-tumor cell inoculation using live animal bioluminescent imaging. g, h Quantification of the liver weight and number of metastases in the mouse LM model established using KPC cells in each group at the endpoint. i, j mIHC staining of I-A/I-E, CD8, CD206, and F4/80 in the sections of MC38 (i) and KPC (j) liver metastases in each group. k, l The mRNA expression levels of CXCL10, CCL4, and IL17D in tissues of MC38 (k) and KPC (l) liver metastases in each group. m Representative abdominal MRI images of responder and non-responder to immunotherapy before and after treatment. n–q ELISA analysis of WNT11, CXCL10, CCL4, and IL17D levels in serum samples from responder (n = 10) and non-responder (n = 10) mice to immunotherapy. r Schematic representation of the WNT11-mediated immunosuppressive mechanism in liver metastases. b–d, f–h, k, l P-values were calculated using a one-way ANOVA followed by a post hoc Tukey’s multiple comparison test, data are presented as the mean ± SD, n = 5 biological replicates. n–q P-values were calculated using a Student’s t test(two-tailed), in the box plots, bounds of the box spans from 25% to 75% percentile, the line represents the median, and the whiskers show minima and maxima of data points. Source data are provided as a Source Data file.
Disscusion
Liver metastases often exhibit features of a TME with CD8+ T-cell exclusion38. Recently, an immune landscape study of liver metastases revealed that a CD8+ T-cell-excluded microenvironment was commonly observed in a high proportion of patients with liver metastases13. Suppressive macrophages are one of the most important mechanisms for CD8+ T-cell exclusion in liver metastases by inhibiting systemic T-cells, leading to resistance to immunotherapy4. However, liver metastases from various origins retain certain TME characteristics linked to cancer types8. This suggests that tumor cells themselves may remain pivotal in orchestrating the remodeling of the immune microenvironment. In this study, we observed a significant correlation between CD8+ T-cell exclusion and the expression of WNT11. Interestingly, both CRC and PDAC liver metastases exhibited higher WNT11 expression. We further confirmed a poor prognosis of liver metastases when WNT11 expression was higher using clinical cohorts. In addition, the mouse model and mass cytometry analysis strongly suggested a potential association between WNT11 and CD8+ T-cell exclusion and suppression.
WNT11 was found to be an essential member of the non-canonical WNT signaling pathway that played critical roles in various tumor biological processes, including cell proliferation, migration, and invasion19,20,21. However, the roles and mechanisms of WNT11 in tumor immune regulation have not been extensively explored. Notably, the WNT signaling pathway has been reported to play a significant role in remodeling the TME [15]. It was reported that activation of the WNT/β-catenin signaling pathway is closely linked to T-cell exclusion in various human solid tumors [16]. Prior studies also elucidated its role in promoting T-cell exclusion, recruiting PMN-MDSCs, and facilitating DC tolerization, thus undermining anti-tumor immunity16,17. Therefore, understanding the mechanistic interplay between WNT11 and immune regulation is essential for developing effective therapeutic strategies for liver metastases.
To elucidate the molecular mechanism by which WNT11 regulates CD8+ T-cell exclusion and suppression, CyTOF and mIHC were used to assess alterations of immune cells. Our findings revealed not only changes in CD8+ T-cells but also significant alterations in CD206+ macrophages. These results suggest that WNT11 may exert both indirect and direct effects on the immune microenvironment of LM. Coculture experiments with CD8+ T-cells demonstrated that the WNT11/CAMKII/β-catenin/AFF3 axis within tumor cells played a key role in modulating infiltration and activation of CD8+ T-cells by regulating the expression of CXCL10 and CCL4. Additional experiments highlighted the significance of CAMKII as a key mediator in the function of WNT11. By inhibiting CXCR3 and CCR5, receptors of CXCL10 and CCL4, the stimulatory effects of shWNT11 on CD8+ T-cell proliferation and activity could be entirely prevented. These observations demonstrated that WNT11-expressing tumor cells directly interacted with CD8+ T-cells, inhibiting their recruitment and activation via a CAMKII-mediated negative regulatory mechanism. In addition, suppressive macrophages also played an important role in the formation of a microenvironment where CD8+T-cells are excluded. In this study, we found that IL17D derived from tumor cells facilitated the M2 polarization of macrophages via the CD93 receptor. Moreover, the mechanism by which WNT11 regulated IL17D expression was mediated by CAMKII and NF-κB positive regulatory mechanisms.
In our study, CAMKII was identified as a key molecule involved in mediating TME remodeling induced by WNT11. It has been reported that CAMKII is a target molecule for the management of cardiovascular and neurological diseases39. In recent years, several inhibitors targeting CAMKII have been assessed in clinical trials40. KN93 is the most widely used specific inhibitor for studying the in vivo functions of CAMKII37. Therefore, we used KN93 to explore the role of blocking the WNT11 signaling pathway in immunotherapy-resistance. The results showed that KN93 and anti-PD-1 therapy combined could significantly inhibit high WNT11 LM. This provides us with a novel molecular mechanism underlying the exclusion and suppression of CD8+ T-cell-microenvironment remodeling in liver metastases and highlights potential targets for addressing the immune microenvironment remodeling, thereby reducing resistance to immunotherapy resistance in liver LM. However, this study is limited by the use of syngeneic non-orthotopic/non-autochthonous tumor models, which may not fully replicate the tumor-immune interactions in human cancers. Future studies employing orthotopic or genetically engineered models are needed to confirm and expand upon our findings.
Overall, our findings highlight the critical role of WNT11 in shaping the immune landscape of liver metastases and provide a rationale for targeting this pathway as a therapeutic intervention to enhance antitumor immunity and improve clinical outcomes in patients with liver metastases. More importantly, our study also identifies potential biomarkers, such as serum WNT11 levels, which may aid in predicting the response to immunotherapy in patients with an LM. Of course, further investigation involving a larger patient population is essential to confirm the clinical significance of WNT11 levels in plasma and its potential as a biomarker for liver metastasis. In summary, understanding the complex interplay between tumor cells and the immune microenvironment in liver metastases holds promise in the development of innovative diagnostic tools and therapeutic approaches. Through leveraging cutting-edge technologies and interdisciplinary collaborations, we may unlock novel avenues for managing liver metastases and improving patient outcomes in the era of precision medicine.
Methods
Ethics statement
All patient samples were obtained with the approval of the Hospitals’ Research Ethics Committee of Fudan University Shanghai Cancer Center (approval no. 050432-4-2108) with written informed consent provided by all participants. The animal experiments were performed following the institutional guidelines and approved by the Ethics Committee of Fudan University Shanghai Cancer Center (FUSCC-IACUC-S2023-0388). The maximal tumor size permitted by the ethics committee is 2000 mm3, and the maximal tumor size was not exceeded in this study.
Tissue microarray and patients
The colorectal cancer liver metastases tissue microarray chip (FUSCC_CRC_cohort), consisting of 71 pairs of CRC primary tumor tissues and matched liver metastases, was collected from Fudan University Shanghai Cancer Center (Shanghai, China) between August 2015 and January 2019. Follow-up data were summarized in March 2020. The pancreatic cancer liver metastases tissue microarray chip (SGH_PDAC_cohort), consisting of 50 pairs of PDAC primary tumor tissues and matched liver metastases, was collected from Shanghai Jiaotong University, School of Medicine, Shanghai General Hospital (Shanghai, China) between May 2010 and June 2019. Follow-up data were summarized in March 2020 (Supplementary Data 1). The primary endpoint was overall survival, calculated from the date of LM resection to the date of death or the last follow-up. The TMA chips were primarily utilized for IHC. 20 colorectal cancer-liver metastasis patients with immune checkpoint blockade were collected as CRC-ICB cohort from Fudan University Shanghai Cancer Center (Shanghai, China), consisting of 10 responder and 10 non-responder samples. The response was assessed after 12 weeks of treatment initiation (Supplementary Data 2). All patient samples were obtained with the approval of the Hospitals’ Research Ethics Committee of Fudan University Shanghai Cancer Center (approval no. 050432-4-2108) with written informed consent provided by all participants. Consent to publish clinical information potentially identifying individuals was obtained. All participants provided written informed consent to publish information that identifies individuals. Due to the limited samples available, sex and gender were not considered in the study design.
Mice and mouse LM model
The mice used in this study (C57BL/6, BALB/c, Cd93ΔMAC, and OT-1 transgenic mice) were obtained from GemPharmatech and maintained under specific pathogen-free conditions. Cd93ΔMAC and OT-1 transgenic mice with a genetic background of C57BL/6 were used for the LM mouse model. To avoid the influences of sex and estrogen, male mice only were used in the study. Mice were maintained under the following housing conditions: ambient temperature 22 °C, humidity control 50%, 12 h light/12 h dark cycle. The animal experiments were performed following the institutional guidelines and approved by the Ethics Committee of Fudan University Shanghai Cancer Center (FUSCC-IACUC-S2023-0388). The maximal tumor size permitted by the ethics committee is 2000 mm3, and the maximal tumor size was not exceeded in this study. Male OT-1 transgenic mice aged 4–6 weeks old were used for OT-1 T cell isolation. Male mice (C57BL/6, BALB/c, and Cd93ΔMAC) aged 6-8 weeks old were used to establish an LM model via splenic injection. A total of 5 × 105 Panc02 cells, 1 × 106 MC38 cells, 1 × 106 CT26 cells, or 5 × 105 KPC cells transfected with the indicated lentivirus construct, were resuspended in 50 μl PBS and then injected into the spleen, followed by splenectomy. For survival experiments, there were a total of 6 mice per group. For drug intervention, 7 days after construction of the model, mice with an MC38 LM were randomly divided into 4 groups (5 mice per group). Mice were injected intraperitoneally with 100 μg anti-PD-1 antibody (Bioxcell, cat. no. #BE0146) or 1 mg/kg KN93 (MedChemExpress, cat. no. HY-15465), a combination of both, or IgG and vehicle control twice a week for 3 weeks.
Generation of stable shRNA knockdown and AFF3 overexpression cells
To generate stable shRNA knockdown cell lines, lentiviruses carrying gene-specific shRNAs (Supplementary Table 5) were produced in 293 T cells using the PLKO.1 lentivirus packaging system. Each gene was targeted using two distinct shRNA sequences. The Flag-AFF3-overexpressing MC38 cells were established using the pLJM1 lentiviral vector system. After 48 hours of lentivirus production, the media containing lentivirus was harvested, and cell debris were removed via centrifugation. The lentivirus, supplemented with polybrene (8 μg/ml, Santa Cruz Biotechnology, Inc.), was immediately added to cells cultured in a 6-well dish. A total of 3 days post-transduction, cells were transferred to new plates and subjected to puromycin selection (10 μg/ml; MilliporeSigma) until single-cell colonies emerged, which were subsequently analyzed by RT-qPCR or western blotting to validate successful knockdown or overexpression.
Mass cytometry and analyses
Single-cell suspensions from mouse liver tumors were immunostained with specific antibodies against surface markers (Supplementary Table 1) to identify various cell types41. Antibodies were sourced from Fluidigm. The manufacturer’s protocol was followed for barcoding and staining: First, the collected cells were washed with PBS Buffer and stained with 0.5 μM cisplatin (Fluidigm, cat. no. 201064) for 2 min. Then, the cells were fixed at room temperature for 10 min with Fix I buffer (Fluidigm, cat. no. 201065). For pelleting, the fixed cells were washed with Barcode Perm buffer (Fluidigm, cat. no. 201067) and centrifuged for 5 min at 800 × g. The fixed cells were then barcoded using a Cell-ID 20-Plex Pd Barcoding Kit (Fluidigm, cat. no. 201060) according to the manufacturer’s instructions. At room temperature, ten barcoded samples were pooled for 30 min for antibody cocktail staining (dilution 1:100). The cells were incubated overnight at 4 °C in Cell-ID Intercalator-Ir (Fluidigm, cat. no. 201192 A) solution following staining. Then, the samples were washed twice with ddH2O, filtered into 30 μm strained cap tubes, and diluted in ddH2O containing 10% EQ Four Element Calibration Beads (Fluidigm, cat. no. 201078).
CyTOF data (Supplementary Table 2) were acquired using a Helios Mass Cytometer (v7.0, Fluidigm), which was calibrated before each analysis following the manufacturer’s guidelines. Following the manufacturer’s instructions, FCS files were randomized and normalized using the signal from the EQ beads. Subsequently, the files were concatenated, debarcoded, and randomized. The gating of live lymphocytes was performed using Cytobank (v9.4)42. Clustering analysis was conducted using the t-distributed stochastic neighbor embedding (t-SNE) and PhenoGraph algorithms in the R package Cytofkit (1.4.10) with default settings (seed = 42, t-SNE perplexity = 30, nearest neighbors K = 30, Euclidean distance). Diffusion maps were generated using the Cytofkit ShinyApp interactive interface43. Other analysis was performed using R-script (v3.6.3) and ggplot2 (3.4.0).
ChIP-qPCR
MC38 cells were fixed in 1.5% formaldehyde in PBS for 10 min at room temperature and quenched using glycine. Following cell lysis, chromatin was fragmented into 100–500 bp fragments using a Bioruptor Sonicator (Diagenode), and protein-DNA complexes were immunoprecipitated using 5 μg Flag antibody (Cell Signaling Technology, Inc. cat. no. #14793), 5 μg of anti-β-catenin antibody (Abcam, cat. no. ab227499), or 5 μg of anti-IgG antibody (Cell Signaling Technology, cat. no. #3900), conjugated with Dynabeads Protein G (Invitrogen; Thermo Fisher Scientific, Inc., cat. no. 10003D), on a rotator at 4 °C overnight. After washing, crosslink reversal, and DNA purification, equal amounts of immunoprecipitated DNA (using the Flag antibody or IgG control) and input DNA were used as templates for qPCR. The sequences of the primers used are listed in Supplementary Table 3. Microsoft Excel (v2019) and Graphpad (8.0.1) were used for data analysis.
Western blotting
The lysates were resolved using SDS-PAGE and subsequent transfer to nitrocellulose membranes. To prevent nonspecific binding, the membranes were blocked with 5% BSA in TBST solution for 1 h at room temperature. Following blocking, the membranes were incubated with a primary antibody overnight at 4 °C, followed by three washes with TBST. After 1 h of incubation with the secondary mouse antibody (IRDye 680, cat. no. 926-68070) pr secondary rabbit antibody (IRDye 800, cat. no. 926-32211), several additional wash steps were performed. Signals were visualized using an Odyssey scanner (Odyssey Application Software v3.0). The primary antibodies used are stated in Supplementary Table 4.
IHC
Briefly, 4 μm-thick formalin-fixed paraffin-embedded sections were used for IHC analysis. Following 1 h of baking at 60 °C, tissue sections underwent deparaffinization using xylene and subsequent rehydration using a series of decreasing ethanol solutions. For IHC staining, endogenous peroxidases were deactivated using 3% hydrogen peroxide for 20 min, followed by rinsing with ddH2O. Antigen retrieval was performed in a pressure cooker using 0.01 mol/L citrate buffer solution (pH 6.0). Blocking was performed using 1% BSA solution at 37 °C for 30 min. The sections were then incubated overnight at 4 °C with primary antibodies, followed by a 30-minute incubation at room temperature with secondary peroxidase-conjugated antibodies. Staining was visualized using a DAB peroxidase substrate kit. Subsequently, the IHC-stained sections underwent dehydration, and coverslips were placed on top. Imaging was acquired using a Leica Aperio AT2 microscope, and the positivity of staining was quantified for comparison using Imagescope (v12.1). Information regarding the antibodies used in this process can be found in Supplementary Table 4.
mIHC
mIHC was performed following the manufacturer’s instructions. Mouse liver tumor sections were stained with specific dyes: Opal520 for CD206 and Opal570 for F4/80 to detect CD206+ macrophages; Opal620 for I-A/I-E and Opal690 for CD8A to identify I-A-I-E+CD8+ T-cells; Opal520 for CD44 and Opal620 for CD19 to identify CD44- B cells; Opal570 for I-A/I-E and Opal690 for NK1.1 to identify I-A/I-E- NK cells; Opal620 for CD4 to label CD4+ T-cells; and DAPI as a nuclear counterstain. Following serial dewaxing and rehydration steps, slides were fixed in 10% neutral buffered formaldehyde for 20 min. Heat-induced antigen retrieval was achieved by immersing slides in citrate antigen retrieval solution and heating them in a microwave oven for 30 min. Subsequently, slides were rinsed with ddH2O and TBST, then blocked with Blocking Buffer before incubation with antibodies. The Opal 6-Plex Detection Kit (Akoya Biosciences, cat. no. NEL821001KT) was used according to the manufacturer’s protocol, and the stained slides were scanned using a Pathology Imaging System Microscope (Vectra v3.0). Information regarding the antibodies used in this process can be found in Supplementary Table 4. Quantification was performed using Qupath (0.2.3).
RT-qPCR
Total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Inc., cat. no. 15596026). The extracted RNA was used for cDNA synthesis using an AdvanceFast cDNA Synthesis Kit according to the manufacturer’s protocol (Shanghai Yeasen Biotechnology Co., Ltd., cat. no. 11150ES). qPCR was performed using SYBR Green (Roche Diagnostics GmbH, cat. no. 04707516001) and the LightCycler 480 real-time PCR system (software, v1.5.0 SP4), according to the manufacturer’s protocol. Gene expression levels were normalized to β-actin using the 2−ΔΔCt method. ChIP-qPCR using Flag antibody was normalized to the input fraction. The sequences of the primers used are listed in Supplementary Table 3. Microsoft Excel (v2019) and Graphpad (8.0.1) were used for data analysis.
ELISA
ELISA kits for human CXCL10 (cat. no. 900-T39) and human CCL4 (cat. no. 88-7034-22) were obtained from Thermo Fisher Scientific, Inc., while the mouse CXCL10 (cat. no. DY466-05), mouse CCL4 (cat. no. MMB00), and mouse IL17D ELISA kits (cat. no. DY410) were obtained from R&D Systems, Inc. ELISA kits for human WNT11 (cat. no. LS-F26557-1) and human IL17D (cat. no. LS-F35817-1) were purchased from LSbio. ELISA was conducted following the respective manufacturer’s instructions. Absorbance was measured using a BioTek™ Synergy™ HTX Multi-Mode Microplate Reader (BioTek’s Gen5 software, v2.0) at 450 nm. Microsoft Excel (v2019) and Graphpad (8.0.1) was used for data analysis.
Isolation of BMDMs and culture with conditioned media
BMDMs were prepared as previously outlined44. Briefly, bone marrow cells were harvested by flushing the femurs and tibiae of 6–8-week-old wild-type C57BL/6 mice or CD93KO mice. Red blood cells were lysed using ACK lysis buffer, and the cells were then cultured in BMDM culture medium (complete 1640 medium supplemented with macrophage colony-stimulating factor (M-CSF, 10 ng/ml, R&D Systems, Inc., cat. no. 416-ML-010)). On day 6, non-adherent cells were discarded, and the culture medium was replaced. On day 7, M2 macrophages were stimulated with IL-4 (10 ng/ml, R&D Systems, Inc., cat. no. 404-ML-010) for 2 days, while M1 macrophages were stimulated using LPS (10 ng/ml, Beyotime Institute of Biotechnology, cat. no. S1732) and IFN-γ (5 ng/ml, R&D Systems, Inc., cat. no. 485-MI) for 2 days.
For a culture with tumor cell-conditioned media, the culture media of tumor cells treated with WNT11 was replaced with normal media after treatment and cultured for 6 h to obtain the conditioned medium. Subsequently, the medium of BMDMs was substituted with the conditioned medium on day 6. Then, qPCR or flow cytometry analyses were performed after 2 days.
Cell culture and treatment
MC38 (CRL-2639) and CT26 (CRL-2638) were obtained from ATCC. Panc02 was a gift from Yu Xianjun (Fudan University Shanghai Cancer Center, the National Infrastructure of Cell Line Resource, 1101MOU-PUMC000446). KPC (a mouse pancreatic cancer cell line with Kras and Tp53 mutation) was a gift from Wang Peng (Fudan University Shanghai Cancer Center, Kerafast, EUP012-FP). MC38 and CT26 cells were cultured in 1640 medium supplemented with 10% FBS and a combination of penicillin and streptomycin. Panc02 and KPC cells were cultured in DMEM medium supplemented with 10% FBS and a combination of penicillin and streptomycin. Cells were cultured in an incubator at 37 ˚C supplied with 5% CO2 air. The cells were treated with WNT11 (20 ng/ml, cat. no. CSB-YP026131MO, Cusabio) and inhibitors including JNK-IN-8 (JNK, 25 nM, MedChemExpress, cat. no. HY-13319), CAMKII-IN-1 (CAMKII, 100 nM, MedChemExpress, cat. no. HY-18271) and Go 6983 (PKC, 100 nM, MedChemExpress, cat. no. HY-13689), as indicated in the figures. Cells were harvested at the indicated time points, and protein and RNA were isolated.
Immunofluorescence
For immunofluorescence analysis, 3 × 105 tumor cells were seeded into 12-well plates containing coverslips. Following cell attachment, cells were fixed using 4% paraformaldehyde. Subsequently, permeabilization was achieved by incubating the cells with 0.5% Triton X-100 for 30 min, facilitating antibody penetration. To prevent non-specific binding, cells were then treated with a blocking solution consisting of 5% BSA for 1 h. Primary antibodies (listed in Supplementary Table 4) were appropriately diluted in the blocking solution, applied to the cells, and incubated at 4 °C overnight. Following primary antibody incubation, thorough washing with PBS was performed to remove unbound antibodies. Subsequently, cells were incubated with the corresponding secondary antibodies for 1 h at room temperature. Cell nuclei were counterstained using Hoechst 33342 (MilliporeSigma, cat. no. #B2883). It is worth noting that all subsequent procedures, including secondary antibody incubation and staining, were performed in the dark to prevent photobleaching. Utilizing a single-photon epifluorescence microscope, images of the stained tumor cells were captured. The antibodies used in this immunofluorescence assay included β-catenin (Abcam, 1:200, cat. no. ab68183). The images were obtained using LAS X (v5.2.2).
Transient knockdown using siRNA
MC38 and Panc02 were initially seeded at a density of 1 × 105 cells per well in a 6-well plate. BMDMs were seeded at a density of 5 × 105 cells per well in a 12-well plate. The following day, transfection was performed using 10 mΜ siRNA, facilitated by Lipofectamine® 3000, and conducted in OptiMEM Reduced Serum Media (Thermo Fisher Scientific, Inc., cat. no. 13778075). After transfection, the cells were used in various bioassays, including gene expression detection in the tumor cells and macrophage coculture assays, with assessments conducted 48 h post-transfection. Negative Control (MilliporeSigma, cat. nos. SIC001 and SIC002) were employed as the negative control constructs. The siRNA targeting sequences are listed in Supplementary Table 5.
In vitro T-cell coculture assays
CD8 T-cells were isolated from a spleen cell suspension using the EasySep Mouse CD8 T-cell isolation kits (StemCell Technologies, Inc., cat. no. 19853), following the manufacturer’s instructions. Subsequently, the isolated cells were stained with CFSE (BioLegend, Inc., cat. no. 422701) at a concentration of 1 mM for 6 min and then resuspended in RPMI media supplemented with 10% FBS, 1% penicillin and streptomycin, non-essential amino acids (NEAA, Thermo Fisher Scientific, Inc., cat. no. 11140050), and β-mercaptoethanol (Thermo Fisher Scientific, Inc., cat. no. 21985023). The activated T-cells were then stimulated with CD3/CD28 Dynabeads (Thermo Fisher Scientific, Inc., cat. no. 11456D) and plated at a density of 2 × 105 cells per well in a 96-well plate. Cancer cells were subsequently added to the culture at a 1:1 ratio with the T-cells.
In vitro T-cell killing assay
Tumor cells with different treatments as indicated, each at a density of 3 × 103 cells, were seeded into individual wells of a 96-well plate and incubated for 48 h. OT1 CD8+ T-cells were isolated from the splenocytes of OVA-specific T-cell receptor transgenic OT−1 mice. Subsequently, they were activated using an OVA peptide along with IL−2 and IL-7 (each at a concentration of 10 ng/ml; BioLegend, Inc., cat. nos. 575406 and 577806) for a duration of 5 days. Upon completion of the activation process, 3 × 103 preactivated OT1 CD8 + T-cells were added to the cancer cell cultures at various ratios, with the inclusion of IL-2 (10 ng/ml) to sustain their activity. After 24 h of coculture, viable cancer cells were enumerated using flow cytometry to assess cell viability and quantify the effects of T-cell cytotoxicity. FlowJo (v10.10) and Graphpad (8.0.1) was used for data analysis.
Migration and invasion assay
In the cell migration assay, 5 × 103 cells suspended in 50 µl of basal medium were seeded into the upper chamber of a 96-well Boyden chamber (Corning, Inc., cat. no. 3374). In the cell invasion assay, Matrigel (BD Biosciences, Inc., cat. no. 356255) was diluted with serum-free DMEM (1:8) and used to coat the upper chamber of the Transwell insert. The insert was incubated 37 °C for 4 h to allow for polymerization of the Matrigel. Subsequently, 2 × 104 tumor cells were added to the upper chamber for the invasion assay. For both invasion and migration assays, 200 µl of complete medium was added to the bottom well. After incubation for 16 h (migration assay) or 24 h (invasion assay), non-migratory cells present on the upper membrane surface of the inserts were carefully removed using a cotton swab to remove cells that had not migrated, and the remaining cells that had migrated or invaded were fixed using 4% paraformaldehyde. Subsequently, cells were stained with crystal violet, and the number of cells that had migrated or invaded were counted in 3 fields of view using a light microscope at a magnification of 100x. ImageJ (1.43) and Graphpad (8.0.1) were used for data analysis.
Colony formation assay
To evaluate colony formation potential capacity, colony formation assays were used. Scramble and shWNT11 tumor cells (5 × 103) were seeded in triplicate into 6-well plates. Following a 7-day incubation period, the cells were carefully washed twice with PBS to remove non-adherent cells. Subsequently, the cells were fixed with methanol for 10 min. After fixation, the cells were stained with a 0.1% crystal violet solution for an additional 10 min to visualize the formed colonies. The stained colonies were then analyzed to quantify the clonogenic ability of the treated cells. This assay provides valuable information regarding the long-term proliferative potential and survival capacity of the cells following treatment. ImageJ (1.43) and Graphpad (8.0.1) were used for data analysis.
Flow cytometry
To acquire single-cell suspensions, harvested tumors were initially cut into small fragments. Subsequently, these fragments were subjected to digestion using a mixture consisting of DNase I (144 µ/mL, Roche Diagnostics GmbH, cat. no. 11284932001), Dispase II (0.85 µ/mL, Roche GmbH, cat. no. 04942078001), and Collagenase A (0.33 µ/mL, Roche Diagnostics GmbH, cat. no. 10103578001). Following digestion, the tissue suspension was filtered through a 70 mm cell strainer to remove any undigested tissue fragments and obtain a homogeneous single-cell suspension. T-cells were isolated using EasySep Mouse CD8+ T-cell Isolation kit (Stemcell Technologies, Inc., cat. no. 19853). Lymphocytes were isolated using EasySep™ Mouse CD45 Positive Selection kit (Stemcell Technologies, Inc., cat. no. 18945). Macrophages were isolated using EasySep™ Mouse F4/80 Positive Selection Kit (Stemcell Technologies, Inc., cat. no. 100-0659). Isolated CD45 + cells were stained with CD45 (PE, Invitrogen; Thermo Fisher Scientific, Inc., cat. no. 12-0451-82, 1:100), CD3 (APC, BioLegend, Inc., cat. no. 100235, 1:100), CD8 (Alexa Fluor 488, R&D Systems Inc., cat. no. FAB116G-025, 1:20) or CD8 (Alexa Fluor 488, R&D Systems, Inc., cat. no. FAB116G-025, 1:20) and I-A/I-E (APC, BioLegend, Inc., cat. no. 107613, 1:75). For proliferation and apoptosis analysis of CD8 + T-cells, isolated CD8 + T-cells fixed in the dark at 4 °C for 30 min. After fixation and permeabilization, a TUNEL Assay Kit (594 nm, Cell Signaling Technology, Inc., cat. no. #48513) was used for identifying apoptotic cells before staining of CD8 (Alexa Fluor 488, R&D Systems Inc., cat. no. FAB116G-025, 1:20) and Ki67 (Alexa Fluor 647, BD Pharmingen, cat. no. 561126, 1:20). Isolated CD8 + T-cells were stained with CD8 (Alexa Fluor 488, R&D Systems, Inc., cat. no. FAB116G-025, 1:20), and intracellular markers TNFα (Alexa Fluor 647, Systems, Inc., cat. no. IC4101R, 1:50), or GZMB (Alexa Fluor 700, BioLegend, Inc., cat. no. 396425, 1:20) to identify cytotoxic CD8 + T-cells. Isolated F4/80+ macrophages were stained with F4/80 (APC, Invitrogen; Thermo Fisher Scientific, Inc., cat. no. 17-4801-82, 1:10), CD80 (PE, Invitrogen; Thermo Fisher Scientific, Inc., cat. no. 12-0801-82, 1:20) and CD86 (FITC, BioLegend, Inc., 105109, 1:50), or CD206 (PE, BioLegend, cat. no. 141705, 1:20) and CD163 (FITC, Invitrogen; Thermo Fisher Scientific, Inc., cat. no. 11−1631-82, 1:200), or CD206 alone (PE, BioLegend, cat. no. 141705, 1:20). A CFSE dilution assay was performed using the CellTrace CFSE Cell Proliferation kit (Thermo Fisher Scientific, Inc., cat. no. C34554), according to the manufacturer’s protocol. The results were then acquired using a BD Fortessa flow cytometer (BD FACSDiva™ Software v8.0). Gating strategies can be found in Supplementary Fig. 14. FlowJo (v10.10) and Graphpad (8.0.1) were used for data analysis.
Statistical analysis
Statistical details of experiments and analyses can be found in the figure legends and the main text. The Pearson correlation test was used to examine the correlation between the quantitative variables. Kaplan-Meier curves and log-rank tests were used to compare overall survival. Differences between the two groups were compared using a Wilcoxon rank-sum test or Student’s t test as indicated. Comparisons between multiple groups were performed using a one-way ANOVA followed by Tukey’s multiple comparison test. Exact P-values are reported unless smaller than 0.0001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Raw CyToF FCS data are available at Mendeley Data (accession https://doi.org/10.17632/6p38bdctr2.1). The remaining data are available within the Article. Source data are provided in this paper.
Code availability
No new code was generated in this study.
References
Tsilimigras, D. I. et al. Liver metastases. Nat. Rev. Dis. Prim. 7, 1–23 (2021).
Petrowsky, H. et al. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat. Rev. Gastroenterol. Hepatol. 17, 755–772 (2020).
Ciner, A. T., Jones, K., Muschel, R. J. & Brodt, P. The unique immune microenvironment of liver metastases: Challenges and opportunities. Semin. Cancer Biol. 71, 143–156 (2021).
Yu, J. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 27, 152–164 (2021).
Kasi, P. M. et al. Circulating tumor DNA (ctDNA) serial analysis during progression on PD−1 blockade and later CTLA-4 rescue in patients with mismatch repair deficient metastatic colorectal cancer. J. Immunother. Cancer 10, e003312 (2022).
Vokes, E. E. et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann. Oncol. 29, 959–965 (2018).
Wu, Y. et al. Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discov. 12, 134–153 (2022).
Liu, Y. et al. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell 40, 424–437.e5 (2022).
Che, L.-H. et al. A single-cell atlas of liver metastases of colorectal cancer reveals reprogramming of the tumor microenvironment in response to preoperative chemotherapy. Cell Discov. 7, 1–21 (2021).
Lin, W. et al. Intra- and intertumoral heterogeneity of liver metastases in a patient with uveal melanoma revealed by single-cell RNA sequencing. Cold Spring Harb. Mol. Case Stud. 7, a006111 (2021).
Zou, Y. et al. The single-cell landscape of intratumoral heterogeneity and the immunosuppressive microenvironment in liver and brain metastases of breast cancer. Adv. Sci. 10, e2203699 (2023).
Zhang, Q. et al. Liver metastasis modulate responses of suppressive macrophages and exhausted T cells to immunotherapy revealed by single cell sequencing. Adv. Genet. 3, 2200002 (2022).
Yang, S. et al. Integrated multi-omics landscape of liver metastases. Gastroenterology 164, 407–423.e17 (2023).
Zhan, T., Rindtorff, N. & Boutros, M. Wnt signaling in cancer. Oncogene 36, 1461–1473 (2017).
Luke, J. J., Bao, R., Sweis, R. F., Spranger, S. & Gajewski, T. F. WNT/β-catenin Pathway activation correlates with immune exclusion across human cancers. Clin. Cancer Res. 25, 3074–3083 (2019).
Theivanthiran, B. et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti–PD−1 immunotherapy. J. Clin. Investig. 130, 2570 (2020).
Zhao, F. et al. Paracrine Wnt5a-β-catenin signaling triggers a metabolic program that grives dendritic cell tolerization. Immunity 48, 147 (2018).
DeVito, N. C. et al. Pharmacological Wnt ligand inhibition overcomes key tumor-mediated resistance pathways to anti-PD-1 immunotherapy. Cell Rep. 35, 109071 (2021).
Toyama, T., Lee, H. C., Koga, H., Wands, J. R. & Kim, M. Noncanonical Wnt11 inhibits hepatocellular carcinoma cell proliferation and migration. Mol. Cancer Res. 8, 254–265 (2010).
Pandur, P., Läsche, M., Eisenberg, L. M. & Kühl, M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418, 636–641 (2002).
Menck, K. et al. WNT11/ROR2 signaling is associated with tumor invasion and poor survival in breast cancer. J. Exp. Clin. Cancer Res. 40, 395 (2021).
Lefèvre, L. et al. Combined transcriptome studies identify AFF3 as a mediator of the oncogenic effects of β-catenin in adrenocortical carcinoma. Oncogenesis 4, e161 (2015).
Zhang, Y., Li, L., Han, Q. & Wen, L. The differential expression of AFF3 in cervical cancer and its correlation with clinicopathological features and prognosis. J. Obstet. Gynaecol. 44, 2333784 (2024).
Zeng, Y., Zhang, X., Li, F., Wang, Y. & Wei, M. AFF3 is a novel prognostic biomarker and a potential target for immunotherapy in gastric cancer. J. Clin. Lab. Anal. 36, e24437 (2022).
Di Rosa, F. et al. HLA class II molecules transduce accessory signals affecting the CD3 but not the interleukin−2 activation pathway in T blasts. Hum. Immunol. 38, 251–260 (1993).
Holling, T. M., Schooten, E. & van Den Elsen, P. J. Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men. Hum. Immunol. 65, 282–290 (2004).
Li, O., Zheng, P. & Liu, Y. CD24 Expression on T cells is required for optimal T cell proliferation in lymphopenic host. J. Exp. Med 200, 1083–1089 (2004).
Naik, S. H. et al. Cutting edge: Generation of splenic CD8+ and CD8− dendritic cell equivalents in fms-like tyrosine kinase 3 ligand bone marrow cultures1. J. Immunol. 174, 6592–6597 (2005).
Rőszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015, 816460 (2015).
Chae, W.-J. & Bothwell, A. L. M. Canonical and non-canonical Wnt signaling in immune cells. Trends Immunol. 39, 830–847 (2018).
Flentke, G. R., Garic, A., Hernandez, M. & Smith, S. M. CaMKII represses transcriptionally active β-catenin to mediate acute ethanol neurodegeneration and can phosphorylate β-catenin. J. Neurochem. 128, 523–535 (2014).
Pandey, V. et al. CXCL10/CXCR3 signaling contributes to an inflammatory microenvironment and its blockade enhances progression of murine pancreatic precancerous lesions. ELife 10, e60646 (2021).
Mukaida, N., Sasaki, S.-I. & Baba, T. CCL4 Signaling in the tumor microenvironment. Adv. Exp. Med. Biol. 1231, 23–32 (2020).
Zeng, Q. et al. Aberrant hyperexpression of the RNA binding protein FMRP in tumors mediates immune evasion. Science 378, eabl7207 (2022).
Ishiguro, K. et al. Ca2+/Calmodulin-dependent protein kinase II is a modulator of CARMA1-mediated NF-κB activation. Mol. Cell Biol. 26, 5497–5508 (2006).
Hogan, P. G., Chen, L., Nardone, J. & Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232 (2003).
Wong, M. H. et al. The KN-93 molecule inhibits calcium/calmodulin-dependent protein kinase II (CaMKII) activity by binding to Ca2+/CaM. J. Mol. Biol. 431, 1440–1459 (2019).
Milette, S., Sicklick, Jk., S., Lowy., A. M. & Brodt, p. Molecular pathways: Targeting the microenvironment of liver metastases. Clin. Cancer Res. 23, 6390–6399 (2017).
Reyes Gaido, O. E. et al. An improved reporter identifies ruxolitinib as a potent and cardioprotective CaMKII inhibitor. Sci. Transl. Med. 15, eabq7839 (2023).
Raedler, L. A. Jakafi (Ruxolitinib): First FDA-approved medication for the treatment of patients with polycythemia Vera. Am. Health Drug Benefits 8, 75–79 (2015).
Gadalla, R., Boukhaled, G. M., Brooks, D. G. & Wang, B. X. Mass cytometry immunostaining protocol for multiplexing clinical samples. STAR Protoc. 3, 101643 (2022).
Chen, T. J. & Kotecha, N. Cytobank: providing an analytics platform for community cytometry data analysis and collaboration. Curr. Top. Microbiol. Immunol. 377, 127–157 (2014).
Chen, H. et al. Cytofkit: A bioconductor package for an integrated mass cytometry data analysis pipeline. PLOS Comput. Biol. 12, e1005112 (2016).
Toda, G., Yamauchi, T., Kadowaki, T. & Ueki, K. Preparation and culture of bone marrow-derived macrophages from mice for functional analysis. STAR Protoc. 2, 100246 (2021).
Acknowledgements
This research was supported by the National Nature Science Foundation of China (82370656, 82372643), Shanghai Rising-Star Program (22QA1401800), and Natural Science Foundation of Shanghai (23ZR1450900). Thanks for the kind help from Prof. Ping Wei (Cancer Institute, Fudan University Shanghai Cancer Center).
Author information
Authors and Affiliations
Contributions
Study concept and design: S.L.Z.; acquisition of data: W.L.J., B.J.G., H.C.S., and Y.S.M.; analysis and interpretation of data: S.L.Z.; drafting of the manuscript: W.L.J., B.J.G., H.C.S., and Y.S.M.; critical revision of the manuscript for important intellectual content: S.L.Z.; statistical analysis: W.L.J., B.J.G., H.C.S., and Y.S.M.; obtained funding: W.L.J., D.W.L., and S.L.Z.; administrative, technical, or material support: S.J.C., R.W., X.X.L., P.L., D.W.L., and S.L.Z.; study supervision: S.J.C., D.W.L., and S.L.Z. All authors read and approved the final manuscript. All authors met the authorship inclusion criteria for Nature Portfolio journals.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ainhoa Mielgo, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, W., Guan, B., Sun, H. et al. WNT11 Promotes immune evasion and resistance to Anti-PD-1 therapy in liver metastasis. Nat Commun 16, 1429 (2025). https://doi.org/10.1038/s41467-025-56714-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56714-z
This article is cited by
-
Emerging nano-immunotherapeutic strategies achieve metastatic colorectal cancer precision therapy
Journal of Nanobiotechnology (2026)
-
The “Cold Tumor” to “Hot Tumor” transformation strategy for triple-negative breast cancer: from mechanism to clinical translation
Molecular and Cellular Biochemistry (2026)
-
The hepatic macrophage: a key regulator of liver metastatic tumor microenvironment through cell crosstalk
Journal of Translational Medicine (2025)
-
LINC02888 promotes HGSOC progression and immune evasion via PPIB-mediated stabilization of LAPTM5 mRNA and inhibition of RIG-I-like receptor signaling
Journal of Translational Medicine (2025)
-
The relationship between galectin-1 expression and the efficacy of anti-PD-1 therapy for non-small cell lung cancer patients
Scientific Reports (2025)