Abstract
Minimizing the energy loss, particularly the non-radiative energy loss (ΔEnr), without sacrificing the charge collection efficiency, is the key to further improve the photovoltaic performance of organic solar cells (OSCs). Herein, we proposed an asymmetric molecular design strategy, via developing alkyl/thienyl hybrid side chain based asymmetric small molecule acceptors (SMAs) BTP-C11-TBO and BTP-BO-TBO, to manipulate the intermolecular interactions to realize enhanced luminescence efficiency and reduced energy loss. Theoretical and experimental results indicate that compared to the three symmetric SMAs BTP-DC11, BTP-DTBO and BTP-DBO, the asymmetric SMAs BTP-C11-TBO and BTP-BO-TBO exhibit repressed electron-vibration coupling and reduced ΔEnr. Moreover, the asymmetric nature of BTP-BO-TBO allows the formation of multiple D:A interfacial conformations and interfacial energies, which have made the charge-transfer state energies closer to that of the strongly absorbing (and emitting) local-exciton state, thus gaining the low ΔEnr while maintaining efficient exciton dissociation. Consequently, the PM6:BTP-BO-TBO-based OSCs achieve a higher power conversion efficiency of 19.76%, with a high open circuit voltage of 0.913 V and an efficient fill factor of 81.17%, profiting from the more improved and balanced charge mobility and longer carrier lifetime. This work provides molecular design ideas to suppress nonradiative decay and paves the way to obtain high-performance OSCs.
Similar content being viewed by others
Introduction
Organic solar cells (OSCs) have distinct merits such as lightness, thinness, flexibility, translucency, and colorization1,2,3,4,5, which can meet special applications in multiple scenarios such as the Internet of Things, smart buildings, indoor photovoltaics, and wearable devices6,7,8,9, and have become one of the most promising green energy technologies. Thanks to advances in organic semiconductor photovoltaic materials and device processing techniques, particularly the swift advancement of A-DA’D-A type small-molecule acceptors (SMAs)10,11,12, the power conversion efficiency (PCE) has exceeded 19% currently for a single junction OSC13,14,15. However, compared to the more efficient inorganic materials-based photovoltaic technologies, e.g. the devices based on crystalline silicon, GaAs, or perovskite, there is still considerable room for further improvement in the photovoltaic performance of OSCs due to the relatively higher energy loss (Eloss) and lower fill factor (FF).
Generally, minimizing the non-radiative voltage loss (ΔVnr) is essential for enhancing the open-circuit voltage (VOC) and hence the photovoltaic properties of OSCs. Further innovation and development of organic semiconductor materials have been recognized as the key to solving this issue. Gao et al. demonstrated that a low energy offset between donor and acceptor molecular states and high photoluminescence yield of the low bandgap material are the two key factors for minimizing the voltage losses (Vloss) of OSCs16,17. Recent studies have demonstrated that introducing luminescent moieties such as fused ring groups (thiophene, benzene, etc.) to enhance the rigidity of molecular structures and/or the extent of electron delocalization could be an effective method for achieving highly luminescent donor and acceptor molecules, as well as optimal photovoltaic performance18,19. In addition, the film morphology is closely related to the charge transport properties, and thus the FF and overall efficiency of OSCs. Synergistically improving the luminescence efficiencies of materials and optimizing the morphological structure of the active layer have become the key to further improve the photovoltaic properties but with great challenges. Therefore, manipulating the intermolecular interactions between donor molecules, acceptor molecules, and/or donor–acceptor molecules to control the intermolecular coupling properties becomes a more viable strategy to realize suppressed Eloss and improved FF.
Inspired by their specific electronic structure and physical properties, modulating the chemical structures of A-DA’D-A type SMAs have been extensively utilized by regulating the three fundamental building blocks, including the central fused ring core unit (DA’D), electron-withdrawing end unit (A), and solubilizing side chains. Owing to the special electron delocalization characteristics, the singlet exciton (SE) excited state of Y-series acceptors could be transferred into the intra-moiety excited (i-EX) state (or namely delocalized singlet exciton (DSE)), which is beneficial for charge generation and decreased non-radiative energy loss20,21. Modifications of the side chains, such as the variation of length, topology (linear or branched), branching points, and dimension are the most frequently employed approaches for precisely modulating the solubility, molecular crystallization, and stacking behavior of SMAs22,23,24. For instance, Yan et al. discovered that different positions of alkyl-chain-branching can alter the molecular stacking of SMAs, optimizing phase separation and exciton dissociation25. It has been well established that the molecular stacking behavior can be regulated with improved structural order and charge transport in thin films by replacing the linear n-undecyl chain on Y6 with branched 2-butyloctyl, delivering significantly promoted open-circuit voltage (VOC) and fill factor (FF)26. Additionally, breaking the symmetry of the alkyl chains is also an effective way to boost photovoltaic performance20,27. For example, Yang and coworkers developed the hybrid cycloalkyl-alkyl chain-based symmetric/asymmetric acceptors Y-C10ch/A-C10ch, and the PM6:A-C10ch device based on asymmetric molecules had less energy loss28. Development of the acceptors with asymmetric side chains, and an in-depth understanding of the intrinsic properties of their molecules, such as molecular packing, electron coupling, and charge transport properties, especially the electron-vibrational coupling, which describes the deformations of the molecular geometries in the course of the electron-transfer process and reflects the interactions between electrons and intramolecular vibrations. Reducing the electron-vibrational coupling has been demonstrated as an effective strategy to suppress the non-radiative recombination, thus leading to suppressed non-radiative energy loss29. Therefore, a comprehensive investigation of the molecular performance of side-chain asymmetric acceptors in combination with detailed density functional theory (DFT) horizontal calculations and single-crystal structures is essential for a deeper understanding of the energy loss and charge transport mechanisms of their devices.
To investigate deeply the effects of SMAs with symmetric or asymmetric alkyl-thienyl chains on the device performance from the perspectives of photophysical properties, morphological features, charge dynamic behaviors, etc., two SMAs with asymmetric alkyl/thienyl outer side chains (BTP-C11-TBO and BTP-BO-TBO, respectively) were synthesized in this study and compared in detail with symmetric SMAs (BTP-DC11, BTP-DBO and BTP-DTBO). In comparison to the symmetrical SMAs, the side-chain asymmetrical structure of BTP-C11-TBO and BTP-BO-TBO presented slightly broader optical bandgaps with elevated LUMO energy levels. The molecular packing results examined by GIWAXS indicate that the asymmetric alkyl/thienyl outer side chains endow the acceptors with a more planar skeleton and promote a denser network packing than the corresponding symmetric SMAs, resulting in the higher domain purity in active layers. Furthermore, the asymmetric alkyl/thienyl outer side chains can subtly modulate the D/A interfacial energetics and inhibit molecular vibration, thereby expediting exciton dissociation and mitigating energetic disorder, resulting in enhanced charge generation efficiency and reduced charge recombination. Consequently, these factors contribute to the remarkable photovoltaic performance of the OSCs based on PM6:BTP-BO-TBO and PM6:BTP-C11-TBO, with the maximum PCEs of 19.76% and 18.51%, respectively. Overall, our research not only demonstrated that the asymmetric alkyl/thienyl outer side chain strategy is a feasible approach for reducing non-radiative energy loss, improving charge generation, suppressing charge recombination, and thus boosting the efficiency of OSCs but also provided an instructive guideline for the further development of emerging organic semiconductor materials and organic optoelectronics.
Results
Physical properties of the acceptors
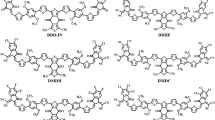
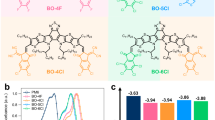
Molecular structures of the symmetrical SMAs BTP-DC11, BTP-DBO, and BTP-DTBO and the asymmetric SMAs BTP-C11-TBO and BTP-BO-TBO were shown in Fig. 1a, and their synthetic details are displayed in Supplementary Fig. 1 in Supplementary Information (SI). The ultraviolet-visible (UV-vis) absorption spectra in chloroform (CF) solution state as well as in solid film state are displayed in Fig. 1b, and the related optical parameters are listed in Table 1. In CF solution, the five SMAs exhibit nearly identical absorption spectra, with the coincident maximum absorption wavelength (λmax) located at about 733 nm, and their absorption coefficients are measured to be in the range of 2.34~2.61×105 M cm−1 (Supplementary Fig. 2 and Supplementary Table 1), indicating that the side chain structures of SMAs exert minimal influence on the intramolecular charge-transfer absorption. In film state, both symmetric and asymmetric SMAs exhibit a similar trend, manifesting notable redshifts when compared with their corresponding solution absorptions (94 nm for BTP-DC11, 77 nm for BTP-DTBO, 68 nm for BTP-DBO, 89 nm for BTP-C11-TBO,73 nm for BTP-BO-TBO, respectively). In addition, the asymmetric SMAs of BTP-C11-TBO (1.02 × 105 cm−1) and BTP-BO-TBO (1.07 × 105 cm−1) present higher absorption coefficients than those of the symmetric SMAs (BTP-DC11, BTP-DTBO, and BTP-DBO), suggesting the distinct aggregation behavior of these five SMAs in the solid state. Meanwhile, according to their absorption edge onsets (the intersection of the tangent line of the absorption peak edge and the vertical axis Y = 0), the optical bandgaps of BTP-DC11, BTP-DTBO, and BTP-DBO, BTP-C11-TBO, and BTP-BO-TBO are estimated to be 1.359, 1.348, 1.379, 1.336, and 1.375 eV, respectively. In both solutions and films, the stokes shifts of BTP-BO-TBO are smaller than that of other SMAs, suggesting that the excited-state relaxation in BTP-BO-TBO is smaller, which is beneficial for the associated voltage losses. This is in agreement with the reduced reorganization energy for the transition between the ground state and the first excited state (S1). In addition, compared with the solutions, the films exhibit relatively larger stokes shifts (Supplementary Fig. 3). This is presumably due to the fact that there exists an energy disorder for the S1 state in the films, and the excitons on the molecules with higher S1 energy can transfer to the molecules with lower S1 energy to emit photons30. Generally speaking, the higher the PL fluorescence quantum yield (PLQY), the smaller the non-radiative energy loss, and the results in Supplementary Table 2 show that BTP-C11-TBO and BTP-BO-TBO have the highest PLQY in both solution and thin film, which also fully confirms the above results31.
The electronic energy levels of five acceptors were measured by electrochemical cyclic voltammetry (CV)32 (Supplementary Fig. 4). The HOMO/LUMO energy values of BTP-DC11, BTP-DTBO and BTP-DBO, BTP-C11-TBO, and BTP-BO-TBO are calculated to be −5.84/−4.06 eV, −5.67/−3.99 eV, −5.78/−3.94 eV, −5.75/−4.04 eV, and −5.69/−3.91 eV, respectively (Fig. 1c). From the symmetric SMAs BTP-DC11, BTP-DTBO and BTP-DBO to the asymmetric SMAs BTP-C11-TBO and BTP-BO-TBO, the slightly elevated LUMO level should be attributed to the weak electron-donating properties of thiophene unit, which may contribute to a larger VOC in resulting OSCs. The density functional theory (DFT) calculations were performed at the B3LYP/6-31 G (d,p) level to study the molecular geometry and the frontier molecular orbital energy levels of symmetric SMAs (BTP-DBO, BTP-DTBO, and BTP-DBO) and asymmetric SMAs (BTP-C11-TBO and BTP-BO-TBO). As is demonstrated in Table 1 and Supplementary Fig. 5, the calculated HOMO/LUMO electronic energy levels are −5.88/−3.77 eV for BTP-DC11, −5.88/−3.74 eV for BTP-DTBO, −5.91/−3.79 eV for BTP-DBO, −5.88/−3.76 eV for BTP-C11-TBO, and −5.90/−3.77 eV for BTP-BO-TBO, respectively. Besides, as shown in Supplementary Figs. 6 and 7, the asymmetric structure appears to induce a partial weakening of the molecular configuration in comparison to the symmetrical structure. This results in maintaining dihedral angles and molecular dipole moments within a range intermediate between those of BTP-DC11, BTP-DTBO, and BTP-DBO molecules for both BTP-C11-TBO and BTP-BO-TBO.
Due to the close relationship between the energy loss mechanism and charge transport properties, the impact of asymmetric alkyl/thienyl side chain structure on the electronic structure properties of the acceptors was thoroughly investigated through DFT calculations, the source data are provided in Supplementary Data 1–5. Unlike the charge transport with band transport in inorganic semiconductors such as crystalline silicon, charge transport in thin films composed of organic molecules is generally assumed to occur through a hopping mechanism. Assuming a thermoneutral transport in the thin films, the rate of this charge hopping \({k}_{{ct}}\) is given by the Marcus rate equation33:
where the rate depends on the reorganization energy λ and the electron coupling between adjacent molecules V, whereas the charge recombination energy λ dominates. The non-radiative recombination is highly related to electron-vibration coupling (i.e., reorganization energy λ)34. According to the above theory, the smaller the reorganization energy, the smaller the driving force required for exciton dissociation, and the faster the rate of charge transfer and transport. Therefore, the reorganization energy plays an important role in the photoelectric conversion and Eloss of OSCs35.
The reorganization energies between different electronic states in photoelectric conversions are shown in Fig. 2a. The reorganization energy for the S0 to S1 transition (λS0→S1) is associated with the geometric relaxation of the S1 state after light absorption, while the reorganization energy of the S1 to S0 transition (λS1→S0) is relevant for the non-radiative exciton decay from the excited state (S1) back to the ground state (S0), and the sum of the two is the reorganization energy for exciton diffusion (λEET, exciton energy transfer). The process of exciton dissociation into CT states has two pathways: one is the dissociation of donor exciton by electron transfer, and the other is the dissociation of acceptor exciton by hole transfer, which are responsible for the reorganization energies for the transitions from S0 to anion (λS0→Anion) and from S1 to anion (λS1→Anion), and both are related to the reorganization energy for exciton dissociation (λED). The non-radiative charge recombination (λCR) hinges on the recombination energy of the anion→S0 transition (λanion→S0)30.
a Schematic diagram of the related transitions among the S0, S1, and the anionic state during the photoelectric conversion processes. b The corresponding reorganization energies of BTP-DC11, BTP-DTBO, BTP-DBO, BTP-C11-TBO, and BTP-BO-TBO acceptors at the level of ωB97XD/6-31G (d, p). c Contributions of each vibrational mode to the reorganization energy for the S1→S0 and anion→S0 transitions of five SMAs, and the displacement vectors for the vibrational normal modes marked by squares (at around 1170 cm−1), triangle (at around 1320 cm−1) and circles (1495 cm−1) are inserted. The height of displacement vectors stands for the magnitude of vibrational strength.
As shown in Fig. 2b, significantly reduced reorganization energies (below 0.2 eV) between the S0 and S1 transitions were obtained for both the symmetric SMAs system and the asymmetric SMAs system, which indicates that exciton decay is inhibited. The reorganization energies between S0 and S1 to the anion transition in the BTP-C11-TBO and BTP-BO-TBO molecular structures were slightly higher than those of BTP-DC11 and BTP-DBO, and significantly lower than that of BTP-DTBO. It is noteworthy that during exciton dissociation to the CT state, the reorganization energy (λS1→anion <0.1 eV) of the hole-transfer exciton dissociation for all acceptors is very small due to the geometric similarity of the S1 state and the anionic state, which helps to reduce the Vloss during exciton dissociation. However, the recombination energy of the exciton dissociated by electron transfer (λS0→anion) is slightly higher and greater than 0.1 eV. In the process from CT state to charge-separated (CS) state, whether the CT state decays into the ground state or the partially separated free carriers recombine back to the ground state, both processes generally cause energy loss. Interestingly, the reorganization energies for the transitions between the anion and S0 states are all below 0.15 eV for both asymmetric acceptors (BTP-C11-TBO and BTP-BO-TBO) and symmetric acceptors (BTP-DC11, BTP-DTBO, and BTP-DBO), which is conducive to reducing non-radiative recombination loss and accelerating electron transport.
The reorganization energy may be further decomposed into the contribution of each vibrational mode in the acceptor, as shown in Fig. 2c. In the transition from S1 to S0, all the five acceptor molecules have a dominant vibrational mode and correspond to the side chain moiety with a frequency of 1615 cm−1, which should be ascribed to the alkyl side chains. Among them, both the undecyl side chain in BTP-DC11 and the 2-butyloctyl side chain in BTP-DBO exhibit relatively strong vibrations (about 22 meV). Interestingly, the vibration intensity is significantly reduced in the SMAs with the thiophene side chain, indicating that the introduction of a two-dimensional thiophene side chain can effectively inhibit the vibration of the backbone chain. The vibration of the asymmetric acceptors (BTP-C11-TBO and BTP-BO-TBO) with both the alkyl chain and thiophene side chain display the lower vibrations below 20 meV, thereby promoting the reduction of exciton decay and facilitating exciton transfer. Similarly, there are three dominant vibrational modes from anion to S0 state, in addition to the tensile vibration of the C-C bond and C-H located at 1320 cm−1, the linear alkyl side chain (BTP-DC11) has strong vibration at 1170 cm−1, and the characteristic vibration frequency of branched alkyl side chain (BTP-DBO) is 1495 cm−1, while the two vibrations are significantly suppressed in the thiophene alkyl side chain substituted SMA (BTP-DTBO). More interestingly, all three vibrational modes are significantly inhibited in both the asymmetric acceptor molecules (BTP-C11-TBO and BTP-BO-TBO), aiming to reduce non-radiative recombination. Ultimately, these results further suggest that asymmetric structures can reduce Eloss by inhibiting C-C bond stretching, thereby improving the efficiency of OSCs.
To gain an in-depth understanding of the exciton dissociation and recombination dynamics, the BTP-DC11, BTP-DTBO, BTP-DBO, BTP-C11-TBO, and BTP-BO-TBO-based systems were investigated by femtosecond transient absorption (TAS) spectroscopy, as shown in Fig. 3. The 2D transient absorption spectra and the corresponding transient absorption spectra of these blend films at different decay times are displayed in Supplementary Figs. 8 and 9 in the Supplementary Information, respectively. A pump wavelength of 800 nm was adopted to selectively excite the acceptors of the D:A blends. Figure 3a, b compare the transient dynamics of the ground-state-bleach (GSB) signal of donor probing at 633 nm and excited-state absorption (ESA) signal of acceptor probing at 990 nm in blend films, respectively. This donor GSB feature is an indication of ultrafast hole transfer from the SMA exciton to PM6, producing the CT state at the donor–acceptor interface. For the acceptor ESA signal, in comparison with the symmetrical acceptor-based blends, the BTP-C11-TBO and BTP-BO-TBO-based blends show a relatively higher intensity of ESA signal centered at 900 nm after photoexcitation. Subsequently, as the ESA peak gradually decreased, a red-shifted ESA signal on a long timescale from 20 to 1000 ps could be observed, which should be assigned to the polaron states. The stronger and prolonged polaron states of the BTP-C11-TBO and BTP-BO-TBO-based blend films indicated the lower bimolecular recombination probability in the corresponding devices. In addition, for the GSB signal of the donor component, the kinetic curve of the BTP-C11-TBO-based blend reached the maximum value at the beginning and then rapidly decreased, suggesting the fast exciton separation and diffusion, which is conducive to obtaining a higher short-circuit current density (JSC). Interestingly, BTP-BO-TBO-based blend increases slowly to the maximum value and then decays slowly, demonstrating the longer exciton diffusion lifetime and CT state lifetime, which also indicates a significant reduction of charge recombination in the blend (Fig. 3c). In other words, the charge separation and recombination are limited by exciton diffusion, benefiting in the higher FF for PM6:BTP-BO-TBO-based devices. Furthermore, to get an in-depth understanding of the exciton lifetimes of these acceptor materials, transient fluorescence measurements were tested to obtain the exciton lifetime of the pristine films. By fitting the attenuation curve with a double exponential (as shown in Supplementary Fig. 10 and Supplementary Table 3 in the Supplementary Information), it can be found that all the acceptors showed similar t1 (50 ps) and t2 (300 ps) values, suggesting the similar exciton lifetime of these thin acceptor films. Besides, considering that the exciton lifetime is also closely related to the molecular packing features, we suspect that the BTP-BO-TBO component in the PM6:BTP-BO-TBO blend possesses a longer exciton diffusion length, thus resulting in a longer exciton diffusion lifetime.
According to the classical Marcus electron transfer model36, the exciton dissociation rate constants (τ1) follow the formula:
where 1/τ1 refers to the hole-transfer rate; kB is the Boltzmann constant; T is the temperature; V is the electronic coupling between initial and final states; ΔG is the change in free energy. By fitting the hole-transfer kinetics with a bi-exponential function, the BTP-DC11, BTP-DTBO, BTP-DBO, BTP-C11-TBO, and BTP-BO-TBO-based blends exhibited fast τ1 values of 0.51, 0.50, 0.48, 0.57, and 0.49 ps, respectively, and slow τ2 values of 2.5, 15, 5.2, 3.8, and 12 ps, respectively (Supplementary Fig. 11 and Supplementary Table 4). Typically, the slower τ2 is thought to represent the time required for exciton diffusion toward the interface prior to dissociation, while the ultrafast τ1 is thought to represent the time needed for acceptor exciton dissociation at the D/A interface. Results reveal that the introduction of the thienyl outer side chains marginally decreased the exciton transfer rate at the D:A interface, whereas it significantly prolonged a portion of the exciton diffusion-mediated transfer process, which may be related to the existence of larger pure acceptor domains and a smaller mixed-phase37. Therefore, to evaluate the differences in domain purity among these blend films, the surface energies (SEs) and corresponding Flory-Huggins interaction parameter (χ) values of these materials were calculated by contact angle measurements, as shown in Supplementary Fig. 12 and Supplementary Table 5. The closer the SE means the better the miscibility between the donor and acceptor38. According to the empirical formula:
where K is a constant, γD/γA represents the SE of the donor/acceptor, and the χ values of the donor PM6 and five SMAs blends were calculated to be 0.28 K, 0.64 K, 0.49 K, 0.62 K, and 0.74 K, respectively. Among them, the χ of PM6: BTP-BO-TBO blend film presented the highest value, indicating the higher domain purity of the BTP-BO-TBO-based blend film. As mentioned above, the asymmetric molecular geometry can promote exciton diffusion charge transfer while inhibiting charge recombination, thereby reducing the non-radiative voltage loss39,40.
Recent studies demonstrated that enhancing the hybridization of the LE and CT states is conducive to suppressing the non-radiative recombination, thereby reducing the non-radiative voltage loss (\({\varDelta V}_{{nr}}\)) of the OSC devices41. As the strength of the hybridization of LE and CT states is closely related to the energy offset between LE and CT states (\({\varDelta E}_{{LE}-{CT}}\)). The smaller \({\varDelta E}_{{LE}-{CT}}\) indicates the stronger hybridization of LE and CT states. Therefore, a combination of theoretical and experimental approaches was applied to accurately evaluate the effects of asymmetric molecular structures on the hybridization of LE and CT states. Generally, the low-energy shift of the blended system reduces the driving force for exciton dissociation to charge-transfer (CT) states, which is unfavorable for the effective dissociation of exciton17. Thus, the driving force (ΔELE-CT) for exciton dissociation can be considered as the energy difference between the CT states and locally excited (LE) states (Fig. 4a)42,43. Gaussian fitting for the highly sensitive EQE and EL in the charge-transfer absorption region was performed to acquire ECT according to the Marcus equations, as follows44:
where \({f}_{j}\) refers to the transfer integral of electronic coupling between the CT and LE states, and \({\lambda }_{j}\) represents the reorganization energy. As a result, the ECT values were 1.42, 1.38, and 1.38 eV for BTP-DBO-, BTP-DTBO- and BTP-BO-TBO-based devices, respectively45, and calculated ΔELE-CT values of the PM6:BTP-BO-TBO-based was slightly smaller than PM6:BTP-DBO and PM6:BTP-DTBO-based devices (Fig. 4b-d). Depressed ΔELE-CT contributes to exciton transfer from the CT state back to the LE state, improves the luminescence efficiency, and reduces the non-radiative recombination46.
a Energy level diagram of CT and LE states, and correlation ΔELE-CT of the binary system. b–d Gaussian fits of sEQE and EL curves for devices based on PM6:BTP-DBO, PM6:BTP-DTBO, and PM6:BTP-BO-TBO. e, f Diagrams of energy differences. g–i The complexes of PM6:BTP-DBO, PM6:BTP-DTBO, and PM6:BTP-BO-TBO and the related ELE, ECT, and ΔELE-CT.
To further gain a clear understanding of the effects of the acceptor side chain structures on the nature of their CT states, TD-DFT calculations on the D: A complexes of two symmetric acceptor-based blends (PM6:BTP-DBO and PM6:BTP-DTBO) and one asymmetric acceptor-based blend (PM6:BTP-BO-TBO) were performed to characterize the nature of their excited states, as shown in Fig. 4e, f, the source data are provided in Supplementary Data 6–13. For the two symmetric acceptors (BTP-DBO and BTP-DTBO), two types of complexes could be obtained depending on whether the side chain group is close to the BDT or BDD unit of PM6. Besides, it could be obtained that, the conformations of BTP-DBO-based complexes differed from those of BTP-DTBO-based complexes, suggesting the significant effects of side chain structures of acceptors on the D: A intermolecular interactions. In the case of the asymmetric acceptor BTP-BO-TBO, four different types of complexes were generated depending on whether the alkyl side chain or thiophene side chain of the acceptor is close to the BDT or BDD moiety of PM6.
As seen from Fig. 4g–i, despite the symmetric molecular structures, the two complexes (complex A and complex B) based on BTP-DBO or BTP-DTBO present different LE and CT state energies. As a consequence, both BTP-DBO and BTP-DTBO-based complexes exhibit two different ΔELE-CT values (0.242 eV and 0.327 eV for PM6:BTP-DBO, 0.265 eV and 0.259 eV for PM6:BTP-DTBO). While for the PM6:BTP-BO-TBO-based complexes (complex A1 and complex A2) with the side chain close to the BDT unit, the ΔELE-CT values are similar to the corresponding complexes of BTP-DBO (complex A) and BTP-DTBO (complex A), with a smaller ΔELE-CT of 0.228 eV. Similarly, for the complexes (complex B1 and complex B2) with the side chain close to the BDD unit, a smaller ΔELE-CT value of 0.214 eV could also be obtained, in comparison with the corresponding parts of BTP-DBO (complex B) and BTP-DTBO (complex B).
The ΔELE-CT value is recognized as a factor in determining the degree of LE-CT electronic hybridization and the magnitude of the charge recombination processes46. Calculations indicate that the ΔELE-CT values of the PM6:BTP-BO-TBO-based complexes are smaller than those of symmetric acceptors and expected to have a stronger hybridization between the CT and LE states, which contributes to the observation of smaller ΔEnr in PM6:BTP-BO-TBO-based devices17. In addition, it should be noted that, despite the slightly smaller ΔELE-CT values, the asymmetric acceptor-based complexes also possess similar ΔELE-CT values with the symmetric acceptor-based, indicating the sufficient driving force for exciton dissociation. In brief, these features highlight that the dual nature of the interfacial structural and electronic properties, thanks to the asymmetric design of BTP-BO-TBO, may be the basis for rapid exciton dissociation and small non-radiative voltage loss in blends36,47.
Considering the energy migration between the donor and acceptor molecular states coupled with the hybridization of the CT exciton formed after the initial charge transfer with the emitted LE state, results in slow charge separation, as observed in transient absorption spectra, and also minimizes non-radiative relaxation to the ground state. Therefore, it is helpful in the above study low ΔELE-CT OSC systems show small non-radiative recombination and high VOC. Next, we will investigate a series of device characterizations.
Photovoltaic characteristics and energy loss
To investigate the effect of asymmetric side chains on the photovoltaic performance of OSCs, we fabricated the conventional binary device structure with the ITO /PEDOT: PSS /PM6:SMAs /PDINN/Ag, where polymer PM6 was selected as the donor due to its complementary absorption and energy level matching with five acceptors. Details of device preparation are shown in the “Method” section, and the optimal J–V curve and external quantum efficiency (EQEs) spectrum are shown in Fig. 5a, b, and Supplementary Tables 6 and 7.
a J–V curves of the optimal OSCs. b EQE curves of the corresponding devices. c Dependences of VOC and JSC on Plight. d Jph versus Veff curves of the OSCs. e Photo-CELIV characteristics of OSCs f Hole and electron mobilities of the optimized devices. g EQEEL curves of the optimal OSCs h Statistical diagram of Eloss of OSCs.
As summarized in Table 2, the incorporation of the branched alkyl side chain and thiophene-based side chain led to the gradual increase in the VOC values, that is, the VOC of PM6:BTP-BO-TBO (0.913 V) is significantly higher than that of PM6:BTP-C11-TBO (0.856 V) and PM6:BTP-DTBO (0.881 V). Notably, the devices based on asymmetric acceptors BTP-BO-TBO and BTP-C11-TBO realize satisfactory PCEs of 19.76% and 18.51%, respectively, greater than those devices based on symmetric acceptors, particularly the OSC of PM6:BTP-BO-TBO exhibits a splendid FF of 81.17% and a decent JSC of 26.67 mA cm−2. The enhanced JSC and FF values in the BTP-BO-TBO-based device might arise from the favorable molecular stacking and better charge transport capability of the PM6:BTP-BO-TBO blend active layer. To our knowledge, the impressive PCE of 19.76% and FF of 81.17% are among the highest values for binary OSCs based on asymmetric acceptors. As depicted in Fig. 5b, almost all the five optimal devices exhibit high EQE values (exceeding 80%), suggesting efficient internal carrier conversion (or charge transfer/collection). It is important to note that the PM6:BTP-C11-TBO-based device has a broader EQE response relative to the PM6:BO-TBO-based device, obtaining an integrated JSC of 26.71 mA cm−2, which should be expected to derive from the narrower bandgap of BTP-C11-TBO. The EQE profile of the PM6:BTP-BO-TBO-based device exhibits a slight blue shift versus the device based on PM6:BTP-C11-TBO, leading to a slightly lower JSC in the devices. The operational stability of the two asymmetric acceptor molecules (PM6:BTP-C11-TBO and PM6:BTP-BO-TBO) based OSCs have been measured. Both of them retained above 95% initial efficiency after 600 s maximum power point tracking under continuous AM 1.5 G illumination. In addition, after storage in a nitrogen-filled glovebox for 1600 h, both BTP-C11-TBO and PM6:BTP-BO-TBO-based OSCs devices retained more than 80% of their initial PCEs. Furthermore, upon continuous white LED irradiation for 250 h, both of the devices maintained more than 90% of their initial PCEs. These results demonstrate the preferable device stability of BTP-C11-TBO and PM6:BTP-BO-TBO-based OSCs (as shown in Supplementary Figs. 13 and 14 in the Supplementary Information).
The relationship between light intensity (Plight) and VOC as well as JSC was investigated to elucidate the dynamics of charge recombination. Focusing on the relationship of VOC vs ln Plight, the slopes of the devices BTP-DC11-, BTP-DTBO-, BTP-DBO-, and BTP-C11-TBO- and BTP-BO-TBO-based are calculated to be 1.11, 1.12, 1.14, 1.07 and 1.02 kT/q, respectively (Fig. 5c and Supplementary Fig. 15a), demonstrating the minimal trap-assisted recombination for PM6:BTP-BO-TBO device26. Figure 5c and Supplementary Fig. 15b present the dependence of JSC and Plight following the relation of JSC ∝ Plighta, where the slope (a) of all devices is close to 1, meaning that bimolecular recombination losses are negligible for the five devices48,49. Meanwhile, the exciton dissociation probability (Pdiss) can be obtained by investigating the relation between the effective voltage (Veff) and the saturated photocurrent density (Jph) in the five devices (Fig. 5d). The Pdiss values of five devices are 85.1% of PM6:BTP-DC11, 87.1% of PM6:BTP-DTBO, 90.2% of PM6:BTP-DBO, 93.1% of PM6:BTP-C11-TBO and 95.8% of PM6:BTP-BO-TBO, respectively. The PM6:BTP-BO-TBO-based OSCs manifest the optimal exciton dissociation ability, which could be responsible for its higher FF.
To shed more light on the asymmetric side chain influence on charge mobility, we looked into the electron mobility (μe) and the hole mobility (μh) of the blend active layers in detail by the space-charge limited current (SCLC) measurements. As exhibited in Supplementary Table 8, the highest μe of 3.47 × 10−4 cm2 V−1 s−1 and μh of 3.73 × 10−4 cm2 V−1 s−1 are achieved for the PM6:BTP-BO-TBO blend due to the significantly enhanced molecular packing (Fig. 5f and Supplementary Fig. 16). Furthermore, the μe/μh ratios of devices for BTP-DC11-, BTP-DTBO-, BTP-DBO-, BTP-C11-TBO and BTP-BO-TBO-based are 0.70, 0.77 0.83, 0.89, and 0.93, respectively. Moreover, we further evaluated the charge mobility via photo-CELIV measurements (Fig. 5e), where the PM6:BTP-BO-TBO-based device presents the highest charge mobilities, consistent with the results of the SCLC method. The higher charge mobilities and more balanced μe/μh in the PM6:BTP-BO-TBO device are responsible for the better FF of its OSCs.
To investigate the cause of VOC enhancement in devices based on asymmetric acceptor molecules, we carried out Eloss analysis by utilizing highly sensitive EQE (sEQE) and electroluminescence (EL). The overall Eloss of OSCs resulted from three aspects: the charge recombination generated by unavoidable black body radiation (ΔE1), radiative recombination loss from below-gap absorption (ΔE2), and non-radiative recombination loss (ΔE3)50,51. Detailed Eloss analyses are presented in Supplementary Fig. 17 and Table 3. The charge-transfer state energy (ECT) of devices based on BTP-DC11, BTP-DTBO, BTP-DBO, BTP-C11-TBO, and BTP-BO-TBO were 1.34, 1.38, 1.42, 1.36, and 1.38 eV, respectively, accounting for slightly different energetic differences between the singlet excited states and the charge-transfer states (ΔECT) (Supplementary Fig. 18 and Supplementary Table 9)52,53. Moreover, the electroluminescence quantum efficiencies (EQEEL) of the devices are shown in Fig. 5g, and the EQEEL values of the five devices are measured to be 1.84 × 10−4 for PM6:BTP-DC11, 1.22 × 10−4 for PM6:BTP-DTBO, 2.27 × 10−4 for PM6:BTP-DBO, 2.51 × 10−4 for PM6:BTP-C11-TBO, and 4.75 × 10−4 for PM6:BTP-BO-TBO, respectively, among which the EQEEL of the PM6:BTP-BO-TBO device is higher than those of the other devices. According to the equation:
The ΔEnr of these devices could be obtained, as presented in Table 3. Ultimately, the devices constructed by PM6: BTP-BO-TBO achieved the lowest ΔEnr of 0.198 eV and the smaller Eloss values of 0.485 eV (see Fig. 5h). Subsequently, we calculated the Urbach energy (EU) by fitting the sEQE spectra beyond the bandgap edge with a linear equation (see Supplementary Fig. 19)54. The EU values of BTP-C11-TBO- and BTP-BO-TBO-based devices were 23.27 and 22.39 meV, respectively, which were lower than those of BTP-DC11-, BTP-DTBO-, and BTP-DBO-based devices (23.43, 24.37, and 25.26 meV, respectively), revealing the PM6:BTP-BO-TBO blend film has a lower energetic disorder. Overall, the alkyl/thienyl asymmetric side chain is an effective strategy to reduce the total Eloss and increase the VOC values without sacrificing photocurrent.
Film morphology
The microscopic morphology of the acceptor materials and the active layer films of the OSCs play a crucial role in their photoelectric conversion efficiency. To acquire a profound understanding of the influence of various side chains on the film morphology of the active layers, atomic force microscopy (AFM), transmission electron microscopy (TEM), and scanning transmission electron microscopy (STEM) were conducted on these PM6:SMAs blend films. As depicted in Fig. 6a, compared with the blends based on BTP-DC11 and BTP-DBO, the root-mean-square roughness (RMS) of the blends based on BTP-C11-TBO (0.903 nm) and BTP-BO-TBO (0.868 nm) decreased after the introduction of thiophene side chains, especially that of PM6:BTP-BO-TBO, showing a more uniform and smoother surface characteristics. Moreover, the TEM images of PM6:BTP-BO-TBO showed a more dense and uniform black-and-white distribution compared with other blend films (Fig. 6b and Supplementary Fig. 20). It is worth noting too that the STEM images of PM6:BTP-BO-TBO exhibited a clearer and more pronounced phase separation structure characteristics of the fibrous 3D network showed in Supplementary Fig. 21. Additionally, it is interesting to note that the STEM images of PM6:C11-TBO and PM6:BO-TBO are relatively consistent with those of PM6:BTP-DC11 and PM6:BTP-DBO, respectively, that is that PM6:C11-TBO and PM6:BTP-DC11 show cluster-like aggregation, while PM6:BO-TBO and PM6:DBO mainly exhibit fibrous-like aggregation, indicating that the morphology regulation of donor–acceptor blends based on side-chain asymmetric acceptor is mainly affected by aliphatic alkyl chains while the thiophene alkyl chain had little effect. The above results manifest that modifying the side chains of SMAs is conducive to achieving the preferable phase separation morphology of blend films, promoting charge transport and improving FF, thereby optimizing photovoltaic performance.
To fully understand the difference in the stacking behavior of SMAs with side-chain symmetric and asymmetric, we first explored the single-crystal structure55,56 (see Supplementary Figs. 22–24 and Supplementary Table 10), the crystallographic data are provided in Supplementary Data 14–16. Compared with BTP-DBO, BTP-DTBO and BTP-BO-TBO both have four different stacking modes in a single unit cell, providing a richer transport channel for charge transfer. Meanwhile, the single-crystal structure showed that tMode 2: A/A and Mode 3:D-A/A-D in BTP-BO-TBO have a shorter π–π distance than BTP-DTBO (Supplementary Fig. 25), which indicates that the introduction of asymmetric side chains can promote formation tighter π–π packing. Subsequently, we calculated electronic coupling (| J |) based on a single-crystal structure to estimate the charge transfer among SMAs57. As shown in Supplementary Fig. 26, taking 1 as a reference molecule, the adjacent molecules named 2, 3, 4, etc., respectively, the electron-coupled pairs formed are represented by 1–2, 1–3, 1–4…, etc. The BTP-DBO, BTP-DTBO, and BTP-BO-TBO single crystals all possess 4 nearest neighbors and 4 electron-coupled pairs, and the detailed | J | are summarized in Supplementary Table 11. The relative order of the overall | J | values of the three molecules is as follows: | J |(BTP-DTBO)>| J |(BTP-BO-TBO)> | J | (BTP-DBO). Significantly, however, the | J |max of BTP-BO-TBO is located 1–2 dimer (Mode 1:D-A/A-D), rather than the 1–4 dimer (Mode 3:D-A/A-D) in BTP-DTBO and BTP-DBO single crystals. The former has a larger stacking area between molecules, which is more conducive to charge transfer58.
The grazing-incidence wide-angle X-ray scattering (GIWAXS) was utilized to probe the molecular packing and crystallinity behaviors. As exhibited in Supplementary Figs. 27 and 28 and Supplementary Table 12, the five SMAs neat films all exhibit obvious (100) lamellar stacking peaks in the in-plane (IP) direction and ordered π–π stacking (010) peaks in the out-of-plane (OOP) direction, and manifest a significant face-on stacking orientation. The diffraction signals of the alkyl/thienyl asymmetric side-chain acceptors BTP-C11-TBO and BTP-BO-TBO were more similar to those of the symmetric alkyl side-chains acceptors BTP-DC11 and BTP-DBO, respectively, indicating that the thienyl side-chain diffraction signals of the asymmetric SMAs BTP-C11-TBO and BTP-BO-TBO were not prominent. A more interesting thing is that the one-dimensional diffraction patterns of BTP-DBO and BTP-BO-TBO acceptors molecules show two connected scattering in the (010) peaks in the OOP direction, indicating that the strong crystallinity of their molecules leads to periodic diffraction peaks in the direction of π–π accumulation59. Nevertheless, the asymmetric acceptor BTP-BO-TBO exhibits a smaller π–π packing d-spacing than the symmetric molecules (BTP-DBO and BTP-DTBO), (BTP-DC11: d-spacing = 3.713 Å, CCL = 12.68 Å; BTP-DTBO: d-spacing = 3.891 Å, CCL = 15.56 Å; BTP-DBO: d-spacing = 3.556 Å, CCL = 29.67 Å; BTP-C11-TBO: d-spacing = 3.758 Å, CCL = 14.74 Å; BTP-BO-TBO: d-spacing = 3.534 Å, CCL = 28.21 Å), indicating that the introduction of the alkyl/thienyl asymmetric side chains could lead to more tightly ordered molecular packing of the BTP-BO-TBO.
Supplementary Figs. 27 and 28 show the 2D diffraction and corresponding 1D linecuts pattern of PM6 neat film, respectively. There is a strong (100) scattering peak (qr = 0.322 Å−1) along the IP direction while (010) relatively weak π–π stacking diffraction peak (qz = 1.639 Å−1) in the OOP direction, the whole showing a weak face-on packing orientation. Blended PM6 with these five SMAs, the face-on molecular orientation of all the blends gain remarkably strengthened with (100) peak qr at ca. 0.33 Å−1 and (010) peak qz at ca. 1.66 Å−1(see Fig. 6c and Supplementary Table 13). Significantly, PM6:BTP-C11-TBO and PM6:BTP-BO-TBO exhibit similar diffraction signals to PM6:BTP-DC11 and PM6:BTP-DBO, respectively, which aligns closely with the results of neat SMAs, suggesting that the alkyl/thienyl asymmetric side chain substituted SMAs possess the similar molecular properties (including molecular stacking behavior and/or miscibility), with their corresponding alkyl-substituted symmetric SMAs. In addition, the blend film of PM6:BTP-BO-TBO was more tightly packed versus PM6:BTP-C11-TBO in the (010) region (BTP-C11-TBO: d-spacing = 3.820 Å, CCL = 18.08 Å; BTP-BO-TBO: d-spacing = 3.760 Å, CCL = 18.87 Å), indicating the maintainable chain-extended feature and comparatively large crystalline domain of BTP-BO-TBO, which facilitates charge transport within the molecular backbone, thus increasing electron mobility. Meanwhile, in order to in-depth inquiring the reasons for BTP-BO-TBO-based device enhanced FF, we also conducted the small-angle X-ray scattering (GISAXS) to study the phase segregation of the five blends. Figure 6d presents the 2D patterns and Supplementary Fig. 29 displays the IP direction intensity profiles fitted by a fractal-like network model and the Debye–Anderson–Brumberger (DAB) model is employed for quantization of the size of the amorphous intermixing region60. Although the average domain sizes (2Rg) of the BTP-C11-TBO- and BTP-BO-TBO-based blends are within the proper range for effective exciton dissociation (<30 nm), the larger intermixing domain size (26 nm) of BTP-BO-TBO-based films is conducive to gaining higher domain purity, thereby improving charge transport, inhibiting charge recombination, and obtaining higher FF values61 (the data are summarized in Supplementary Table 14).
Discussion
In summary, we have demonstrated that the alkyl/thienyl asymmetric side chain strategy offers an effective method to suppress exciton-vibration coupling and reduce the offset between the CT and LE states, thus realizing inhibited non-radiative loss without sacrificing the charge collection efficiency. The two asymmetric SMAs (BTP-C11-TBO and BTP-BO-TBO) with alkyl/thienyl asymmetric outer side chains demonstrated fine-tuned molecular packing and blend morphology. With the introduction of the thienyl side chain, BTP-C11-TBO and BTP-BO-TBO neat thin films exhibit slightly larger optical bandgaps, elevated LUMO energy levels, and facilitated electron mobility. Liken to BTP-DC11 with a straight alkyl chain, the alkyl/thienyl asymmetric SMAs exhibit enhanced crystallization, contributing to the tighter π–π packing distances along the conjugated backbone. As a consequence, benefiting from the specific electron-vibration coupling properties, packing characteristics, and electronic structure features caused by the alkyl/thienyl asymmetric side chains, the OSCs based on PM6:BTP-BO-TBO delivered the lowest non-radiative energy loss of 0.198 eV with efficient exciton dissociation and charge collection efficiency, thus contributing to its high VOC and JSC, as well as the champion PCE of 19.76%, which is one of the highest values for binary OSCs. This work highlights the role of alkyl/thienyl asymmetric side chain strategy in reducing the non-radiative recombination energy, realizing efficient charge generation, suppressing charge recombination, and thus improving the photovoltaic performance of OSCs.
Methods
Materials
The detailed synthesis process of BTP-C11-TBO and BTP-BO-TBO and the corresponding structural characteristics were described in the Supplementary information. Other materials are provided by commercial suppliers: PM6 (Solarmer Energy Inc.), Pd (PPh3)4, and 1-chloronaphthalene (J& K Chemical Co.), and these reagents and solvents purchased are used directly without further purification.
Molecular structure characterization
1H NMR and 13C NMR spectra were recorded using Bruker AVANCE NEO 600 MHz spectrometer in d-chloroform solution. Chemical shifts were reported as δ values (ppm) with tetramethyl silane (TMS) as reference (Supplementary Figs. 30–45). High-resolution matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) was performed on the Shimadzu spectrometer. (Supplementary Figs. 46–53).
Device fabrication
The OSCs with ITO/PEDOT: PSS/ PM6:SMAs/PDINN/Ag structure were fabricated by consisting of the following five steps: (1) Pre-cleaning ITO-coated glass in turn with detergent, deionized water, acetone and isopropyl alcohol in an ultrasonic bath, then drying and treating ultraviolet ozone generator for 15 min. (2) Spinning coated PEDOT: PSS at 4000 rpm for 30 s and then annealing at 150 °C for 20 min. (3) Spinning coated the active layer (PM6:SMAs = 1.1.2; 16 mg/mL(CF); 0.5% (v/v) 1-CN), then annealing at 100 °C for 10 min in the glovebox. (4) Spinning coated the electron transport material PDINN. (5) Vaporizing 100 nm electrode (Ag) in a thermal evaporation chamber with a vacuum of ~1 × 10−4 Pa, obtained the OSCs with an effective area of 0.06 mm2.
J–V and EQE measurements
The current density–voltage (J–V) curve of OSCs was measured by a Keithley 2450 Source Measure Unit and an AAA class solar simulator (Model, Newport 94023 A) with 450 W xenon lamp under AM 1.5 G illumination. External quantum efficiency (EQE) was measured by the solar cell spectral response measurement system QE-R3011 (Taiwan Enli Technology Co., Ltd.).
FTPS-EQE and EQEEL
Fourier-transform photocurrent spectroscopy external quantum efficiency (FTPS-EQE) and external electroluminescence quantum efficiency (EQEEL) of the optimized devices were measured by PECT-600 and ELCT3010 (Enlitech), respectively.
The calculation processes of E loss
The Eloss is mainly composed of the following three components, the calculation formula is as follows:
1. Radiative recombination above the bandgap (ΔE1) (5)
2. Radiative recombination blew the bandgap (ΔE2)
3. Non-radiative recombination loss (ΔE3)
where Eg, \({V}_{{OC}}^{{SQ}}\), k, T, q, \({\varnothing }_{{BB}}\), and \({V}_{{oc}}^{{rad}}\) refer to energy bandgap, Shockley-Queisser (SQ) VOC limit, the Boltzmann constant, the temperature, the elementary charge, the black body spectrum, and radiative recombination VOC limit, respectively.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available within the published article, Supplementary Information, Source Data Files, and Supplementary Data files. Additional data are available from the corresponding author on request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2405808; 2405811; 2405812. Source data are provided with this paper.
References
Ye, Q. et al. Ductile oligomeric acceptor‐modified flexible organic solar cells show excellent mechanical robustness and near 18% efficiency. Adv. Mater. 35, 2305562 (2023).
Kini, G. P., Jeon, S. J. & Moon, D. K. Latest progress on photoabsorbent materials for multifunctional semitransparent organic solar cells. Adv. Funct. Mater. 31, 2007931 (2021).
Li, Y., Xu, G., Cui, C. & Li, Y. Flexible and semitransparent organic solar cells. Adv. Energy Mater. 8, 2007931 (2017).
Zhang, Y. F. et al. Optically enhanced semitransparent organic solar cells with light utilization efficiency surpassing 5.5%. Adv. Energy Mater. 14, 2400970 (2024).
Li, S. et al. Achieving record‐high stretchability and mechanical stability in organic photovoltaic blends with a dilute‐absorber strategy. Adv. Mater. 36, 2307278 (2023).
Li, H. et al. Anti‐fatigue tandem organic photovoltaics for indoor illumination. Adv. Mater. 36, 2311476 (2024).
Wang, Z. et al. Self-sustaining personal all-day thermoregulatory clothing using only sunlight. Science 382, 1291–1296 (2023).
Wang, D. et al. High‐performance semitransparent organic solar cells with excellent infrared reflection and see‐through functions. Adv. Mater. 32, 2001621 (2020).
Park, J. S. et al. Material design and device fabrication strategies for stretchable organic solar cells. Adv. Mater. 34, 2201623 (2022).
Lin, Y. et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 27, 1170–1174 (2015).
Yuan, J. et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule 3, 1140–1151 (2019).
Tian, L., Liu, C. & Huang, F. Recent progress in side chain engineering of Y-series non-fullerene molecule and polymer acceptors. Sci. China Chem. 67, 788–805 (2023).
Fu, J. et al. 19.31% binary organic solar cell and low non-radiative recombination enabled by non-monotonic intermediate state transition. Nat. Commun. 14, 1760 (2023).
Gao, W. et al. 3D crystal framework regulation enables se‐functionalized small molecule acceptors achieve over 19% efficiency. Adv. Energy Mater. 14, 2304477 (2024).
Chen, H. et al. Heterogeneous nucleating agent for high‐boiling‐point nonhalogenated solvent‐processed organic solar cells and modules. Adv. Energy Mater. 36, 2402350 (2024).
Chen, X.-K. et al. A unified description of non-radiative voltage losses in organic solar cells. Nat. Energy 6, 799–806 (2021).
Qian, D. et al. Design rules for minimizing voltage losses in high-efficiency organic solar cells. Nat. Mater. 17, 703–709 (2018).
Chai, G. et al. Fine-tuning of side-chain orientations on nonfullerene acceptors enables organic solar cells with 17.7% efficiency. Energy Environ. Sci. 14, 3469–3479 (2021).
Zhang, J. et al. Alkyl‐chain branching of non‐fullerene acceptors flanking conjugated side groups toward highly efficient organic solar cells. Adv. Energy Mater. 11, 2102596 (2021).
Huang, J. et al. On the role of asymmetric molecular geometry in high-performance organic solar cells. Nat. Commun. 15, 3287 (2024).
Chen, Z. et al. Ultrafast energy transfer from polymer donors facilitating spectral uniform photocurrent generation and low energy loss in high-efficiency nonfullerene organic solar cells. Energy Environ. Sci. 16, 3373–3380 (2023).
Wu, X. et al. Introducing a phenyl end group in the inner side chains of A‐DA’D‐A acceptors enables high‐efficiency organic solar cells processed with nonhalogenated solvent. Adv. Mater. 35, 2302946 (2023).
Kong, X. et al. 18.55% efficiency polymer solar cells based on a small molecule acceptor with alkylthienyl outer side chains and a low-cost polymer donor PTQ10. CCS Chem 5, 841–850 (2023).
Kong, X. et al. The effect of alkyl substitution position of thienyl outer side chains on photovoltaic performance of A–DA′D–A type acceptors. Energy Environ. Sci. 15, 2011–2020 (2022).
Jiang, K. et al. Alkyl chain tuning of small molecule acceptors for efficient organic solar cells. Joule 3, 3020–3033 (2019).
Li, C. et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 6, 605–613 (2021).
Fan, Q. et al. Unidirectional sidechain engineering to construct dual‐asymmetric acceptors for 19.23 % efficiency organic solar cells with low energy loss and efficient charge transfer. Angew. Chem. Int. Ed. 62, e202308307 (2023).
Xiao, C. et al. Hybrid cycloalkyl‐alkyl chain‐based symmetric/asymmetric acceptors with optimized crystal packing and interfacial exciton properties for efficient organic solar cells. Adv. Sci. 10, 2402350 (2023).
Benduhn, J. et al. Intrinsic non-radiative voltage losses in fullerene-based organic solar cells. Nat. Energy 2, 17053 (2017).
Shi, Y. et al. Small reorganization energy acceptors enable low energy losses in non-fullerene organic solar cells. Nat. Commun. 13, 3256 (2022).
Jiang, Y. et al. Suppressing electron-phonon coupling in organic photovoltaics for high-efficiency power conversion. Nat. Commun. 14, 5079 (2023).
Li, X. & Li, Y. Measurement of electronic energy levels of conjugated polymers and organic molecules. Acta Polym. Sin. 53, 995–1004 (2022).
Brückner, C. & Engels, B. A theoretical description of charge reorganization energies in molecular organic P‐type semiconductors. J. Comput. Chem. 37, 1335–1344 (2016).
Brückner, C. et al. Structure–property relationships for exciton and charge reorganization energies of dipolar organic semiconductors: a combined valence bond self-consistent field and time-dependent Hartree-Fock and DFT study of merocyanine dyes. J. Phys. Chem. C. 119, 17602–17611 (2015).
Shi, Y. et al. Small energetic disorder enables ultralow energy losses in non‐fullerene organic solar cells. Adv. Energy Mater. 13, 2300458 (2023).
He, C. et al. Asymmetric electron acceptor enables highly luminescent organic solar cells with certified efficiency over 18%. Nat. Commun. 13, 2598 (2022).
Qin, L. et al. Triplet scceptors with a D‐A structure and twisted conformation for efficient organic solar cells. Angew. Chem. Int. Ed. 59, 15043–15049 (2020).
Zhou, H. et al. Over 18.2% efficiency of layer-by-layer all-polymer solar cells enabled by homoleptic iridium(III) carbene complex as solid additive. Sci. Bull. 69, 2862–2869 (2024).
Chen, Z. et al. Triplet exciton formation for non-radiative voltage loss in high-efficiency nonfullerene organic solar cells. Joule 5, 1832–1844 (2021).
Jiang, K. et al. Suppressed recombination loss in organic photovoltaics adopting a planar-mixed heterojunction architecture. Nat. Energy 7, 1076–1086 (2022).
Qian, D. et al. Correlating the hybridization of local‐exciton and charge‐transfer states with charge generation in organic solar cells. Adv. Energy Mater. 13, 2301026 (2023).
Zhong, Y. et al. Sub-picosecond charge-transfer at near-zero driving force in polymer:non-fullerene acceptor blends and bilayers. Nat. Commun. 11, 833 (2020).
Zheng, Z., Tummala, N. R., Wang, T., Coropceanu, V. & Brédas, J. L. Charge‐transfer states at organic-organic interfaces: impact of static and dynamic disorders. Adv. Energy Mater. 9, 1803926 (2019).
Zheng, X. et al. Suppressing trap density and energy loss via skeleton asymmetry strategy enables highly efficient all-small-molecule organic solar cells. Chem. Eng. J. 475, 145520 (2023).
Xia, H. et al. Novel oligomer enables green solvent processed 17.5% ternary organic solar cells: synergistic energy loss reduction and morphology fine‐tuning. Adv. Mater. 34, 2107659 (2022).
Dela Peña, T. A. et al. Understanding the charge transfer state and energy loss trade-offs in non-fullerene-based organic solar cells. ACS Energy Lett 6, 3408–3416 (2021).
Menke, S. M. et al. Limits for recombination in a low energy loss organic heterojunction. ACS Nano 10, 10736–10744 (2016).
Zhang, Y. et al. Achieving 19.4% organic solar cell via an in situ formation of p-i-n structure with built-in interpenetrating network. Joule 8, 509–526 (2024).
Liang, W. et al. Oligothiophene additive‐assisted morphology control and recombination suppression enable high‐performance organic solar cells. Adva. Energy Mater. 14, 2303661 (2024).
Chen, H. et al. Lowing the energy loss of organic solar cells by molecular packing engineering via multiple molecular conjugation extension. Sci. China Chem. 65, 1362–1373 (2022).
Tian, H. et al. Over 19.2% efficiency of layer-by-layer organic photovoltaics enabled by a highly crystalline material as an energy donor and nucleating agent. Energy Environ. Sci. 17, 5173–5182 (2024).
Liu, S. et al. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat. Photonics 14, 300–305 (2020).
Coropceanu, V., Chen, X.-K., Wang, T., Zheng, Z. & Brédas, J.-L. Charge-transfer electronic states in organic solar cells. Nat. Rev. Mater. 4, 689–707 (2019).
Zhu, L. et al. Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology. Nat. Mater. 21, 656–663 (2022).
Gong, Y. et al. C-shaped ortho-benzodipyrrole-based acceptors with different electronic effects of top substituents for as-cast green-solvent processed high-performance organic solar cells. Energy Environ. Sci. 17, 6844–6855 (2024).
Yang, C. et al. Optimized crystal framework by asymmetric core isomerization in selenium‐substituted acceptor for efficient binary organic solar cells. Angew. Chem. Int. Ed. 135, e202313016 (2023).
Luo, Z. et al. Asymmetric side-chain substitution enables a 3D network acceptor with hydrogen bond assisted crystal packing and enhanced electronic coupling for efficient organic solar cells. Energy Environ. Sci. 15, 4601–4611 (2022).
Zhu, L. et al. Small exciton binding energies enabling direct charge photogeneration towards low‐driving‐force organic solar cells. Angew. Chem. Int. Ed. 60, 15348–15353 (2021).
Xia, X. et al. Uncovering the crystalline packing advantages of asymmetric Y‐series acceptors for efficient additive‐insensitive and intrinsically stable organic solar cells. Adv. Energy Mater. 14, 2303785 (2024).
Mai, J. et al. Hidden structure ordering along backbone of fused‐ring electron acceptors enhanced by ternary bulk heterojunction. Adv. Mater. 30, 1802888 (2018).
Yu, H. et al. A vinylene‐linker‐based polymer acceptor featuring a coplanar and rigid molecular conformation enables high‐performance all‐polymer solar cells with over 17% efficiency. Adv. Mater. 34, 2200361 (2022).
Acknowledgements
This work was supported by the Advanced Talents Incubation Program of the Hebei University (No. 521100224204 (J.G.)), the National Natural Science Foundation of China (Nos. 52403224 (J.G.), 52103235 (B.Q.), 52203248 (X.L.) and 52103243 (J.Z.)), the Beijing Nova Program (20240484597 (X.L.)) and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB 0520102 (L.M.)). A portion of this work is based on the data obtained at BSRF-1W1A. The authors gratefully acknowledge the cooperation of the beamline scientists at the BSRF-1W1A beamline.
Author information
Authors and Affiliations
Contributions
J.G. and B.Q. conceived the study. J.G synthesized and characterized acceptor materials and B.Q. performed device optimization and performance characterization. S.Q. carried out DFT calculation and data analysis. J.Z. conducted the TA measurements and data analysis. C.Z. carried out Eloss measurements. H.Z. and Y.L. (Yuechen Li). conducted in UV-vis and CV characterization. X.X. performed GISAXS measurements. Y.G. participated in the synthesis of acceptors. T. L., Y. Z., G. H., and Y. Y. analyzed the single-crystal data. All authors contributed to discussions and commented on the manuscript. Y.L. (Yongfang Li), L.M., and J.C. directed the project. The manuscript was prepared, revised, and finalized by J.G., X.L., B.Q., and Y.L. (Yongfang Li).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, J., Qin, S., Zhang, J. et al. Asymmetric small-molecule acceptor enables suppressed electron-vibration coupling and minimized driving force for organic solar cells. Nat Commun 16, 1503 (2025). https://doi.org/10.1038/s41467-025-56799-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56799-6