Abstract
Apart from the classic features, it is almost unknown whether there exist other new pathological features during pre-metastatic niche formation in hepatocellular carcinoma (HCC). Our previous works have highlighted the contribution of increased matrix stiffness to lung pre-metastatic niche formation and metastasis in HCC. However, whether increased matrix stiffness influences glucose metabolism and supply of lung pre-metastatic niche remains largely unclear. Here we uncover the underlying mechanism by which matrix stiffness-tuned exosomal miRNAs as the major contributor modulate glucose enrichment during lung pre-metastatic niche formation through decreasing the glucose uptake and consumption of lung fibroblasts and increasing angiogenesis and vascular permeability. Our findings suggest that glucose enrichment, a new characteristic of the lung pre-metastatic niche triggered by matrix stiffness-tuned exosomal miRNAs, is essential for the colonization and survival of metastatic tumor cells, as well as subsequent metastatic foci growth.
Similar content being viewed by others
Introduction
The proposal of the pre-metastatic niche theory not only effectively integrates three classic tumor metastasis theories (seed and soil hypothesis, circulatory theory, and the cascade theory of metastatic spread), but also creatively generates a direct association between primary tumor and the distant metastatic lesion via tumor-released soluble factors1,2. Since pre-metastatic niche is beneficial for the adhesion and colonization of circulating tumor cells (CTCs) as well as the growth of metastatic foci3, its formation is generally regarded as an important speed-limiting step for the final realization of a metastatic lesion. Soluble factors derived from primary tumors such as LGALS3, VEGFA, PDGF, LOXL2, LOX, etc. have been well characterized to trigger the formation of pre-metastatic niches at distant metastatic organs by educating stromal cells, recruiting bone marrow-derived cells (BMDCs), and influencing inflammatory/immune cells4,5,6,7,8. Besides that, tumor-released microvesicles and their contents also present a prominent induction ability in the formation of distant pre-metastatic niches. Exosomes, a crucial subset of extracellular vesicles, can be transferred from tumor cells into the recipient cells at the distant target organ through systemic circulation to achieve cross-organ intercellular communication9. HCC cells-secreted exosomes educate lung fibroblasts to produce pro-inflammatory factors and contribute to cancer-associated fibroblast activation during lung pre-metastatic niche formation10. Exosomal miR-21 from breast cancer cells accelerates the activation of osteoclasts by changing PDCD4 protein levels and promotes bone pre-metastatic niche formation and bone metastasis occurrence11. Exosomal miR-934 from colorectal cancer cells induces the polarization of M2 macrophages, and the polarized macrophages secrete CXCL13 to boost liver pre-metastatic niche formation and facilitate liver metastasis of colorectal cancer12. These findings suggest that exosomes, as the “messengers” from primary tumors, possess the strong ability to educate resident cells and immune cells at distant target organs, thereby assisting in pre-metastatic niche formation. To date, the identified common pathological features of pre-metastatic niches include BMDCs recruitment, matrix remodeling, inflammation, immunosuppression, organotropism, angiogenesis, and vascular permeability, which are often used to define the formation of the pre-metastatic niche2. However, apart from the above features, it is almost unknown whether there exist other new pathological features during pre-metastatic niche formation.
Sufficient nutrients are considered essential for cancer initiation. Glycogen accumulation occurs in early lesions and small tumors in the liver but disappears in the middle and late stages of tumors13, it suggests the importance of glucose enrichment for tumor occurrence and survival. Glucose, glutamine and fatty acids are also likely to aid cancer cells in colonizing the secondary sites and improving the occurrence of distant metastasis14,15. The pre-metastatic niche as the remodeled favorable “soil” at the distant metastasis site belongs to an ectopic colonization and survival place for tumor cells, which means that colonization and survival of CTCs in the pre-metastatic niche are very similar to the early stage of cancer initiation. Thus, nutrient enrichment in the pre-metastatic niche seems to be equally important for the colonization and survival of tumor cells. A literature describes that exosomal miR-122 from breast cancer cells suppresses glucose uptake of stromal cells in the lung and brain pre-metastatic niches16, and another report reveals that IL-1β from breast cancer cells and the recruited BMDCs decreases the triglyceride decomposition of lung fibroblasts to create a high-triglyceride microenvironment in the lung pre-metastasis niche17, indicating that cancer cell-released exosomes and soluble factors may influence glucose and lipid metabolism in the lung pre-metastatic niche. Additionally, the resident cells in the pre-metastatic niche are natural competitors in glucose uptake and consumption for ectopic adhered or colonized tumor cells. Accordingly, a sufficient glucose supply in the pre-metastatic niche may support the survival and outgrowth of adhered tumor cells, subsequently determine the successful realization of metastatic foci. Consistently, angiogenesis and vascular permeability, as the characteristics of the pre-metastatic niche, also prominently increase and improve glucose supply and aggregation18,19. Overall, we assume that there exists a glucose-enriched environment during the formation of the pre-metastatic niche, and glucose enrichment may be a new characteristic of the pre-metastatic niche.
The effects of biochemical stimuli within microenvironment on tumor invasion and metastasis have been extensively studied during the last two decades20,21, but the roles of biomechanical stimuli in tumor pre-metastatic niche formation are still largely undefined, partially due to a lack of ideal stiffness-related experiment models in vitro and in vivo. Being a shared biomechanical characteristic of solid tumors, increased matrix stiffness has become an important regulatory factor in tumor progression and metastasis in recent years22,23,24. HCC is a highly aggressive and metastatic malignancy with a poor prognosis, and the lung is its most frequent extrahepatic metastasis site25. Existing clinical studies have suggested that increased matrix stiffness is positively correlated with an unfavorable prognosis of HCC26,27, and liver stiffness has been taken as a biomarker to predict HCC progress and its outcomes26,27. By developing new stiffness-related experiment models, different mechanisms by which increased matrix stiffness promotes HCC invasion and metastasis have been elucidated successively, including increasing HCC angiogenesis28,29,30, triggering epithelial-mesenchymal transition31, inducing invadopodia formation32, enhancing stemness characteristics33, modulating lipid metabolism34, driving chemotherapy resistance35, and intensifying exosome secretion36, etc. And yet, the contribution of matrix stiffness to the formation and glucose metabolic reprogramming of lung pre-metastatic niche is barely reported in HCC. We previously discovered that the secreted LOXL2 from HCC cells and polarized M2 macrophages under high stiffness stimulation facilitated lung pre-metastatic niche formation by educating lung fibroblasts and increasing BMDCs recruitment, highlighting an association between matrix stiffness and pre-metastatic niche formation in HCC37,38,39. However, little is known about whether and how increased matrix stiffness as an initiating contributor influences glucose metabolism and supply in the pre-metastatic niche. In this study, we explore whether increased matrix stiffness-tuned exosomal contents modulate distant glucose metabolism and supply to create a glucose-enriched environment in the pre-metastatic niche before the arrival of metastatic HCC cells.
Different from other studies on the roles of matrix stiffness in primary tumor, this study mainly focuses on its effects on the pathological changes at distant metastatic site (lung). It not only discloses a new regulatory mechanism by which matrix stiffness-tuned exosomal miRNAs modulate glucose enrichment during lung pre-metastatic niche formation through decreasing glucose uptake and consumption of lung fibroblasts and increasing angiogenesis and vascular permeability, but also proposes that glucose enrichment as a new characteristic of the lung pre-metastatic niche, is essential for colonization and survival of metastatic tumor cells, as well as subsequent metastatic foci growth.
Results
Conditioned medium derived from HCC cells grown on the high-stiffness substrate accelerates the formation of the lung pre-metastatic niche
Referring to an induction method of pre-metastatic niche animal models reported previously by our group37, we developed another tumor-free mouse models with lung pre-metastatic niches to evaluate the contribution of conditioned medium from HCC cells under high stiffness stimulation to lung pre-metastatic niche formation. As depicted in a schematic diagram of an animal experiment in Supplementary Fig. 1a and Fig. 1a, we respectively collected the conditioned medium (CM) from Hepa1-6 cells grown on 6 kPa and 16 kPa substrates (named as L-CM and H-CM) and then injected them into the mouse body (C57BL/6) through the tail vein every other day to induce tumor-free mouse models with lung pre-metastatic niches. The injected L-CM and H-CM represented the soluble factors released by HCC tumors with a normal liver and cirrhotic liver background, respectively. Considering that BMDCs recruitment was one of the most prominent characteristics of the lung pre-metastatic niche2, we assessed the recruitment levels of CD11b+CD45+ BMDCs in fresh lung tissues at different induction time points by flow cytometry. As the induction days prolonged, the numbers of CD11b+CD45+ BMDCs were all increased in both the L-CM and H-CM groups. Simultaneously, compared with those in the L-CM group, the recruitment levels of CD11b+CD45+ BMDCs on days 18, 22, and 26 in the H-CM group all exhibited enhancement, and day 26 was the day with the greatest difference in BMDCs recruitment (Fig. 1b, c). The above findings indicate that H-CM may possess a strong induction ability in the formation of the lung pre-metastatic niche in HCC. Additionally, they enable us to preliminarily determine the induction time point of day 26 as the day for the successful formation of tumor-free mouse models with lung pre-metastatic niches. We further analyzed the expressions of pre-metastatic niche-related genes (Fn, Mmp9, S100a8, S100a9, and Bv8) in fresh lung tissues of these animal models on day 26, and found that the expression levels of pre-metastatic niche-related genes in the H-CM group were all prominently higher than those in the L-CM group (Fig. 1d), in accordance with the results of BMDCs recruitment in the H-CM group. Consistently, we also observed obvious increases in the expression of fibronectin (FN) (Fig. 1e) and the proportion of myeloid-derived suppressor cells (MDSCs) (Fig. 1f), as well as a significant decrease in the proportion of effector CD8+ T cells in the H-CM group (Fig. 1g), confirming that H-CM indeed facilitates the formation of the lung pre-metastatic niche. Besides that, we also examined the molecular markers for vascular density and permeability in lung tissues on day 26, and the results showed that CD31 expression was apparently increased but VE-cadherin coverage within CD31+ areas was significantly reduced in the H-CM group (Fig. 1h, i), indicating that H-CM has a stronger ability to promote angiogenesis and damage vascular integrity. Based on the significant differences between the two groups in BMDCs recruitment, matrix remodeling (FN and MMP9), immunosuppression (MDSCs and CD8+ T cells), angiogenesis and vascular permeability (BV8, CD31, and VE-cadherin), and inflammation (S100A8 and S100A9), we concluded that H-CM prominently accelerated the formation of the lung pre-metastatic niche as compared with L-CM.
a Schematic illustration of tumor-free mouse models with lung pre-metastatic niches induced by Hepa1-6-L/H-CM, created in BioRender. Zhao, Y. (2025) https://BioRender.com/d32b589. b, c Flow cytometry analysis (b) and quantification (c) of CD11b+CD45+ bone marrow-derived cells (BMDCs) in lung tissues (n = 2 mice per group on days 6, 14, 18, and 22; n = 4 mice per group on day 26). d qRT-PCR analysis of pre-metastatic niche-related genes in lung tissues on day 26 (n = 4 mice per group). Data were normalized to β-actin. e IHC staining of fibronectin in lung tissues on day 26 (n = 4 mice per group). f, g Percentage of CD11b+Gr-1+ myeloid-derived suppressor cells (MDSCs) (f) and CD8+ T cells (g) in lung tissues on day 26 (n = 4 mice per group). h IHC staining of CD31 in lung tissues on day 26 (n = 4 mice per group). Scale bars: black, 200 μm; red, 50 μm (e, h). i IF images for CD31 and VE-cadherin in lung tissues on day 26 (n = 4 mice per group). Scale bars: 20 μm. j Western blot analysis of fibronectin and MMP9 in lung fibroblasts treated with MHCC97H/Hep3B-L/H-CM grown on lung stiffness substrates. k Adherent HCC cells on lung fibroblast monolayer treated with MHCC97H/Hep3B-L/H-CM grown on lung stiffness substrates (n = 6 biological replicates). Scale bars: 200 μm. l Western blot analysis of ZO-1, VE-cadherin and VEGFR2 in HUVECs treated with MHCC97H/Hep3B-L/H-CM. The samples derive from the same experiment but different gels for ZO-1 and VE-cadherin, and another for VEGFR2 and β-actin were processed in parallel. m Permeability of HUVEC monolayer treated with MHCC97H-L/H-CM to FITC-dextran (n = 3 biological replicates). No monolayer cells, no HUVECs on the upper chamber. Representative images are presented from indicated biologically independent experiments (b, e–i, k). Representative blot (20 μg protein per group) was shown from 3 biologically independent experiments (j, l), and β-actin was used to normalize protein quantification. Data are presented as mean ± SD, and P values were calculated using two-tailed unpaired Student’s t-test (c–m). L low-stiffness substrates, H high-stiffness substrates, CM conditioned medium, OD498 optical density (OD) measured at 498 nm. Source data are provided as a Source Data file.
In addition to the in vivo findings mentioned above, we subsequently constructed a gel substrate in vitro mirroring the stiffness of lung tissue in a pathological state (926.18 Pa, lung stiffness substrate) as described previously37 to cultivate the resident cells, and then employed H-CM to intervene these cells for simulating a lung pre-metastatic niche environment in vivo. We used L-CM and H-CM from human HCC cells cultured on 6 kPa and 16 kPa stiffness substrates separately to treat lung fibroblasts grown on lung stiffness substrates, and discovered that H-CM intervention significantly upregulated the protein levels of FN and MMP9 in lung fibroblasts compared with L-CM intervention (Fig. 1j), in agreement with the results of the animal experiment (Fig. 1d). qPCR and ELISA analysis also showed that H-CM intervention obviously promoted the gene and protein expression of FN and MMP9 in lung fibroblasts (Supplementary Fig. 1b, c). Meanwhile, we observed that there was a significant increase in the numbers of adherent HCC cells on the surface of lung fibroblast monolayer treated with H-CM (Fig. 1k). These results strongly supported the prominent roles of H-CM in lung matrix remodeling by influencing lung fibroblasts and creating a suitable soil for tumor cell adhesion. On the other hand, we also evaluated the effects of H-CM on the expression of VEGFR2, ZO-1, and VE-cadherin in vascular endothelial cells (HUVECs), and the results revealed that H-CM remarkably improved VEGFR2 expression but suppressed ZO-1 and VE-cadherin expressions (Fig. 1l), implying that H-CM has a stronger induction ability in promoting angiogenesis and injuring vascular integrity, which was consistent with the findings in animal models (Fig. 1h, i). Additionally, the treated HUVEC monolayer with H-CM also showed an increase in vascular permeability to FITC-dextran (Fig. 1m), validating that H-CM can obviously increase vascular permeability by educating vascular endothelial cells. Totally, the evidence in vivo and in vitro sufficiently suggests that the released conditioned medium from HCC cells under high stiffness stimulation significantly facilitates the formation of the lung pre-metastatic niche.
Glucose enrichment occurs during H-CM-induced lung pre-metastatic niche
The resident cells (fibroblasts, vascular endothelial cells, etc.) in the metastatic target organ, educated by tumor-derived soluble factors, are often involved in remodeling a favorable ‘soil’ environment for circulating tumor cell colonization and survival4,5,6,7,8. Our previous studies also suggest that the secreted LOXL2 from HCC cells under high stiffness stimulation can educate lung fibroblasts and recruit BMDCs to promote lung pre-metastatic niche formation37,38. Glucose as the principal nutrient mainly meets energy needs for proliferation and survival of tumor cells, and reprogrammed glucose metabolism is also regarded as a typical characteristic of cancer18. The lung pre-metastatic niche belongs to an ectopic survival site for HCC cells. Glucose enrichment in the pre-metastatic niche may be crucial for supporting ectopic colonization, survival and growth of disseminated tumor cells. Thereby, in addition to classic pathological changes such as BMDCs recruitment, matrix remodeling, immune suppression, inflammation, angiogenesis, and vascular permeability, we speculate that glucose metabolic reprogramming may also occur during the formation of a pre-metastatic niche. To investigate whether the released conditioned medium from HCC cells under high stiffness stimulation modulates the glucose metabolism of the resident cells in the lung pre-metastatic niche, we constructed the same experiment system mentioned above mirroring the lung pre-metastatic niche environment to assess the expression changes of glucose transporters and glycolytic enzymes in the resident stroma cells. The results demonstrated that H-CM intervention significantly depressed the expressions of GLUT1, PFKP, PKM2, and HK2 in lung fibroblasts, but had little effect on SGLT2 expression (Fig. 2a), meaning that levels of glucose uptake and glucose metabolism in lung fibroblasts were all seriously weakened. Consistently, the 2-NBDG uptake and glucose consumption assay also displayed an obvious decrease in glucose uptake and consumption in lung fibroblasts treated with H-CM (Fig. 2b, c). Subsequently, we performed an untargeted metabolomic analysis to further clarify the effect of H-CM on metabolic alternations in lung fibroblasts, and found that H-CM intervention resulted in a significant decrease in the content of intracellular glycolytic metabolites including glucose 6-phosphate, fructose-6-phosphate, 2-phospho-D-glyceric acid, and phosphoenol pyruvate, and these differential metabolites were also enriched in the central carbon metabolism pathway (Fig. 2d and Supplementary Fig. 1d). The above data all support that H-CM intervention effectively attenuates glucose uptake and consumption in lung fibroblasts and thereby creates a glucose-enriched microenvironment. To further validate this finding, we continued to examine the expression changes of glucose transporters and glycolytic enzymes in fresh lung tissues of tumor-free mouse models with lung pre-metastatic niches. Our results also revealed that except for Slc5a2 (gene name for SGLT2), the expression levels of glycolytic enzymes (Pfkp, Pkm, and Hk2) and the glucose transporter (Slc2a1) were all significantly diminished in the H-CM group (Fig. 2e). Simultaneously, low expression of GLUT1 and increased glycogen content at lung tissue level (Fig. 2f and Supplementary Fig. 1e) also supported a prominent decrease in glucose metabolism levels and an increase in glucose accumulation in the H-CM group. Besides, an improvement in vascular permeability and angiogenesis in the H-CM-induced lung pre-metastatic niche by educating vascular endothelial cells (Fig. 1l, m) also indicated an increase in glucose supply and aggregation in the lung pre-metastatic niche. All these data suggest that there exists a new feature of glucose enrichment during lung pre-metastatic niche formation induced by H-CM.
a–c Western blot analysis of glucose transporters and glycolytic enzymes (a), 2-NBDG uptake (b), and glucose consumption (c) in lung fibroblasts treated with MHCC97H/Hep3B-L/H-CM grown on lung stiffness substrates (a, b n = 3 biological replicates; c n = 4 biological replicates). a The samples derive from the same experiment but different gels for GLUT1 and PFKP, another for SGLT2 and β-actin, and another for PKM2 and HK2 were processed in parallel. d Untargeted metabolomic analysis of differential intracellular glycolytic metabolites in lung fibroblasts treated with MHCC97H-L/H-CM grown on lung stiffness substrates (n = 6 biological replicates). e qRT-PCR analysis of glucose transporters and glycolytic enzymes in pre-metastatic lung tissues (n = 4 mice per group). Data were normalized to β-actin. f IHC staining of GLUT1 in pre-metastatic lung tissues (n = 4 mice per group). Scale bars: black, 200 μm; red, 50 μm. g Adherent HCC cells on lung fibroblast monolayer treated with MHCC97H-L-CM, H-CM, or L-CM + HG grown on lung stiffness substrate (n = 6 biological replicates). h Adherent HCC cells pre-treated with function-blocking antibody of integrin β1 on lung fibroblast monolayer grown on lung stiffness substrates (n = 6 biological replicates). Scale bars: 200 μm (g, h). i, j Flow cytometry analysis of CD11b+Gr-1+ MDSCs (i) in differentiated bone marrow cells (BMCs) treated with NG, HG, or HG + 2-DG and their p-mTOR (S2448) expression (j) (n = 3 biological replicates). k-m Western blot analysis of glucose transporters and glycolytic enzymes (k), 2-NBDG uptake (l) and glucose consumption (m) in lung fibroblasts treated with MHCC97H/Hep3B-H-CM-Exo-free grown on lung stiffness substrates (k, l n = 3 biological replicates; m n = 4 biological replicates). k The samples derive from the same experiment but different gels for GLUT1, another for PFKP and β-actin, and another for PKM2 and HK2 were processed in parallel. Representative images are presented from indicated biologically independent experiments (b, f–j, l). Representative blot (20 μg protein per group) was shown from 3 biologically independent experiments (a, k), and β-actin was used to normalize protein quantification. Data are presented as mean ± SD, and P values were calculated using two-tailed unpaired Student’s t-test (a–f, k–m) or one-way ANOVA (g–j). L low-stiffness substrates; H high-stiffness substrates; CM conditioned medium, MFI mean fluorescence intensity, NG normal glucose concentration, 5.5 mM, HG high glucose concentration, 25 mM, Ab antibody, Exo exosome. Source data are provided as a Source Data file.
For identifying glucose enrichment as a new characteristic of the lung pre-metastatic niche, it is indispensable to clarify its pathological significance. Based on the results that glucose enrichment is beneficial for improving HCC cell adhesion ability (Fig. 2g), and MDSCs accumulation may be partially attributed to the increase in MDSC differentiation (Fig. 1f), we reasonably speculated that glucose enrichment in the pre-metastatic niche might influence adhesion molecules expression to promote HCC cell adhesion, or enhance surrounding immunosuppressive strength to favor their survival and metastasis. We focused on an adhesion molecule, integrin β1, that can bind to fibronectin produced by stromal cells40, and explored the potential mechanism of high glucose upregulating integrin β1 expression. The results showed that the expression level of integrin β1 was significantly elevated in HCC cells exposed to high glucose condition (Supplementary Fig. 2a), indicating that there is an association between high glucose and integrin β1 expression. AMPK, a key regulator in cellular energy sensor, has been reported to be negatively regulated by high glucose in cancer cells, myoblasts and podocytes41,42,43. Given that AMPKα could degrade SNX17, a regulator of endosomal recycling implicated in integrin β1 recycling back to the plasma membrane44,45,46, we further speculated that upregulation of integrin β1 by high glucose might be achieved by regulating AMPKα/SNX17 signaling. We analyzed the activity and expression of AMPKα and SNX17 under high glucose condition in HCC cells, and discovered that high glucose inhibited AMPKα phosphorylation but elevated SNX17 expression (Supplementary Fig. 2a). Furthermore, A-769662, an agonist of AMPK activity, significantly reversed the effects of high glucose on SNX17 and integrin β1 upregulation (Supplementary Fig. 2a). We next analyzed the cell surface level of integrin β1 in HCC cells using membrane protein extraction and immunofluorescence staining, and discovered that high glucose significantly upregulated the cell surface level of integrin β1, but activation of AMPK reversed this effect (Supplementary Fig. 2a, b), indicating an increase in integrin β1 recycling back to the plasma membrane under high glucose stimulation. To further determine whether high glucose-induced integrin β1 upregulation participated in HCC cell adhesion to the lung pre-metastatic niche, we used the AMPK agonist (A-769662) or function-blocking antibody of integrin β1 (integrin β1 Ab) to treat HCC cells for observing their adhesion changes. The results revealed that activation of AMPK or blockage of integrin β1 could obviously suppress HCC cell adhesion on lung fibroblast monolayer (Fig. 2h and Supplementary Fig. 2c). Accordingly, glucose enrichment in the lung pre-metastatic niche can effectively upregulate the cell surface level of integrin β1 in HCC cells by modulating AMPKα/SNX17 pathway, thereby promoting tumor cell adhesion to the pre-metastatic niche. Apart from cell adhesion properties, the number of MDSCs in lung pre-metastatic niches is also crucial for the colonization and survival of circulating tumor cells in foreign tissues47. In tumor-free mouse models with lung pre-metastatic niches, we validated the significant role of H-CM in MDSCs accumulation in the lung pre-metastatic niche (Fig. 1f). Considering that MDSCs accumulation may be partially attributed to the increase in MDSC differentiation, we continued to clarify whether glucose enrichment influenced MDSCs differentiation. In the presence of IL-6 and GM-CSF, high glucose remarkably promoted bone marrow cells (BMCs) differentiating into MDSCs, whereas glycolysis inhibitor (2-DG) intervention significantly attenuated its effect on MDSCs differentiation (Fig. 2i). In previous reports, activation of mTOR pathway may participate in the effects of glucose accumulation on MDSCs differentiation and function48,49. Here, we also observed an increase in mTOR phosphorylation level under high glucose stimulation in MDSCs, but 2-DG intervention effectively inhibited this effect (Fig. 2j). Similarly, rapamycin, an inhibitor of mTOR activity, also significantly reduced MDSCs differentiation under high glucose stimulation (Supplementary Fig. 2d, e), confirming the promoting role of glucose enrichment in MDSCs differentiation. Together, glucose enrichment in the pre-metastatic niche remarkably upregulated integrin β1 expression via AMPKα/SNX17 pathway to promote HCC cell adhesion to the niche, also increased MDSCs differentiation to strengthen the immunosuppressive microenvironment for subsequent colonization and survival, which highlights that glucose enrichment is a new feature of the lung pre-metastatic niche.
Tumor-derived exosomes as the major contributor modulate glucose enrichment during H-CM-induced lung pre-metastatic niche
Primary tumor-released soluble factors generally contain secreted proteins, extracellular vesicle (EVs), and other molecular elements2,9. Exosomes as the secreted microvesicles can be transferred from tumor cells into the recipient cells to achieve intercellular information exchange9. Considering that glucose metabolic reprogramming occurs within cells, tumor cell-derived exosomes are likely to play a leading role in regulating glucose enrichment during the formation of the lung pre-metastatic niche. We prepared exosome-free-H-CM (H-CM-Exo-free) from H-CM by differential ultracentrifugation and confirmed its quality by analyzing exosomes markers including TSG101, CD63, Hsp70 and ALIX (Supplementary Fig. 2f). Then we respectively utilized H-CM-Exo-free and H-CM to treat lung fibroblasts grown on lung stiffness substrates, and the results showed that H-CM-Exo-free intervention markedly reversed the effects of H-CM intervention on glycolytic enzymes and glucose transporter (Fig. 2k), as well as glucose uptake and consumption (Fig. 2l, m). These data illustrate that exosomes indeed serve as the major contributor to regulate glucose enrichment during H-CM-induced lung pre-metastatic niche.
To further verify the dominant role of exosomes in regulating glucose enrichment during H-CM-induced lung pre-metastatic niche, we employed differential ultracentrifugation method to purify exosomes from L-CM and H-CM and named them as L-Exo and H-Exo, respectively. The purified exosomes exhibited the typical cup-shaped structure (Fig. 3a), and their mean sizes ranged from 100 to 150 nm (Supplementary Fig. 2g), which conform to the typical phenotypic characteristics of exosomes. Besides, their molecule markers, TSG101, CD63, Hsp70, and ALIX, were all detected while negative molecule markers such as Albumin and Cytochrome C were absent (Fig. 3b), further confirming that the quality of purified exosomes meets the characteristics of exosomes and can be used for downstream experiments. We subsequently applied a membrane green fluorescent probe (DIO) to label L-Exo or H-Exo to determine the recipient cells, and found that both recipient cells (lung fibroblasts and vascular endothelial cells) had intracellular green spots after incubation with DIO-labeled exosomes (Fig. 3c and Supplementary Fig. 2h), indicating that L-Exo and H-Exo from HCC cells can be successfully transferred into lung fibroblasts and vascular endothelial cells. Since the secreted exosomes from tumor cells mobilize BMDCs into the secondary organ sites to trigger pre-metastatic niche formation1,2, we utilized Millicell hanging cell culture inserts to construct a co-culture system in vitro (Fig. 3d) simulating the pre-metastatic niche environment for clarifying exosomes’ role in pre-metastatic niche formation. Specifically, BMCs were cultured in the lower chamber in the presence of IL-6 and GM-CSF for 48 h, and then they were co-cultured with L-Exo- or H-Exo-treated lung fibroblasts grown on lung stiffness substrates in the upper chamber for another 48 h (Supplementary Fig. 2i). We observed that compared with L-Exo intervention, H-Exo intervention significantly improved the expressions of FN and MMP9 in lung fibroblasts, while co-culturing with BMCs further strengthened these effects (Fig. 3d). Simultaneously, there was a significant increase in BMCs differentiating into MDSCs when they were co-cultured with H-Exo-treated lung fibroblasts (Fig. 3e). The results of H-Exo intervention are basically the same as those of H-CM intervention in vivo, indicating that H-Exo, similar to H-CM, also facilitates lung pre-metastatic niche formation. Using L-Exo or H-Exo to treat lung fibroblasts grown on lung stiffness substrate, we proceeded to examine glycolysis-related protein expression and glucose uptake ability of lung fibroblasts for validating their regulatory roles in glucose metabolism. The results revealed that H-Exo intervention prominently decreased gene and protein expression levels of glucose transporter (GLUT1) and glycolytic enzymes (PFKP, PKM2, and HK2) in lung fibroblasts (Supplementary Fig. 3a–c). Furthermore, H-Exo treatment also weakened their glucose uptake and consumption abilities (Supplementary Fig. 3d, e), in line with the results of H-CM intervention (Fig. 2a–c), confirming the regulatory role of exosomes in glucose metabolism. Simultaneously, we discovered an increase in the number of adherent HCC cells on the surface of lung fibroblast monolayer incubated with H-Exo (Supplementary Fig. 3f), indicating that H-Exo creates the favorable “soil” environment for CTCs adhesion and colonization by remodeling matrix and promoting glucose enrichment. Additionally, compared with the control cells treated with L-Exo, HUVECs treated with H-Exo displayed higher expression of VEGFR2 and lower expressions of ZO-1 and VE-cadherin (Supplementary Fig. 3g). Moreover, H-Exo treatment facilitated tube formation of HUVECs (Supplementary Fig. 3h) and promoted HUVEC monolayer permeability to FITC-dextran (Supplementary Fig. 3i), implying that H-Exo intervention increases glucose supply and aggregation in the lung pre-metastatic niche by changing vascular permeability and angiogenesis.
a Transmission electron microscope (TEM) images of exosomes purified from MHCC97H/Hep3B-L/H-CM (n = 3 biological replicates). Scale bars: 50 μm. b Molecule markers of exosomes detected by western blot (n = 3 biological replicates). The samples derive from the same experiment but different gels for TSG101, CD63, and Hsp70, another for ALIX, and another for Albumin and Cytochrome C were processed in parallel. c Internalization of DIO-labeled exosomes (green) by lung fibroblasts and vascular endothelial cells (n = 3 biological replicates). Scale bars: 5 μm. d Schematic illustration of a co-culture system in vitro simulating pre-metastatic niche environment (upper panel) and western blot analysis of fibronectin and MMP9 in lung fibroblasts treated with MHCC97H-L/H-Exo in this system (lower panel). Upper panel was created in BioRender. Zhao, Y. (2025) https://BioRender.com/k02k700. e Flow cytometry analysis of CD11b+Gr-1+ MDSCs in differentiated bone marrow cells (BMCs) co-cultured with L/H-Exo-treated lung fibroblasts (n = 3 biological replicates). f Schematic illustration of tumor-free mouse models with lung pre-metastatic niches induced by Hepa1-6-L/H-Exo, created in BioRender. Zhao, Y. (2025) https://BioRender.com/k94x898. g Flow cytometry analysis of CD11b+CD45+ BMDCs in the pre-metastatic lung tissues on day 26 (n = 5 mice per group). h Levels of glucose in the cell-free interstitial fraction fluid from the pre-metastatic lung tissues on day 26 (n = 5 mice per group). i Multiplex immunofluorescence of TSG101, CD31, and VE-cadherin in the pre-metastatic lung tissues on day 26 (n = 5 mice per group). Scale bars: 20 μm. j Bioluminescence imaging (BLI) of lung metastasis lesions in lung tissues on day 54 and day 61 (n = 5 mice per group). k HE staining and quantification of lung metastasis lesions in lung tissues on day 61 (n = 5 mice per group). Scale bars: red, 2 mm; blue, 500 μm; black, 100 μm. Representative images are presented from indicated biologically independent experiments (a–c, e, g, i–k). Representative blot (20 μg protein per group) was shown from 3 biologically independent experiments (d), and β-actin was used to normalize protein quantification. Data are presented as mean ± SD, and P values were calculated using one-way ANOVA (d) or two-tailed unpaired Student’s t-test (e, g–i, k). L low-stiffness substrates, H high-stiffness substrates, Exo exosome. Source data are provided as a Source Data file.
To further validate the contribution of H-Exo to lung pre-metastatic niche formation, especially glucose enrichment, in vivo, we developed H-Exo-induced tumor-free mouse models with lung pre-metastatic niches. We isolated exosomes from Hepa1-6-L-CM or H-CM and then confirmed their quality by analysis of the exosome molecule markers (Supplementary Fig. 3j). Similar with the method described in Fig. 1a, we respectively injected L-Exo and H-Exo into the bodies of C57BL/6 mice through the tail vein every other day to induce lung pre-metastatic niche formation (Fig. 3f). On the 26th day of exosomes induction, we collected fresh lung tissues for subsequent analysis. Same as H-CM induction, H-Exo induction also significantly facilitated lung pre-metastatic niche formation including increasing CD11b+CD45+ BMDCs recruitment, inducing matrix remodeling, and promoting angiogenesis and inflammation in lung tissues (Fig. 3g and Supplementary Fig. 3k). Simultaneously, the expression levels of glycolytic enzymes (Pfkp, Pkm, and Hk2) and the glucose transporter (Slc2a1) in lung tissues were also suppressed by H-Exo induction (Supplementary Fig. 3l). We isolated and collected the interstitial fraction fluid from dissociated lung tissues and detected the levels of glucose in the cell-free interstitial fraction fluid. The results showed that interstitial glucose content in lung tissues from mice induced by H-Exo was higher than those from mice induced by L-Exo (Fig. 3h), indicating the formation of glucose enrichment in H-Exo-induced lung pre-metastatic niches. Additionally, multiplex immunofluorescence analysis of exosomes marker TSG101, fibroblasts marker S100A4 (FSP-1), and glucose transporter GLUT1 displayed that lung fibroblasts received exosomes and their glucose uptake (GLUT1 expression) was obviously decreased in exosomes-concentrated regions in H-Exo-induced group (Supplementary Fig. 3m). Furthermore, H-Exo induction also increased vascular permeability by inhibiting the expression of VE-cadherin in CD31-positive vascular endothelial cells (Fig. 3i), suggesting that H-Exo increases glucose supply and aggregation. Certainly, H-Exo induction also promoted the accumulation of MDSCs in the pre-metastatic lung tissues, accompanied by increased mTOR activity (Supplementary Fig. 3n), simultaneously reduced the infiltration of CD8+ T cells (Supplementary Fig. 3o), further indicating the role of glucose enrichment in strengthening immunosuppression during lung pre-metastatic niche formation. We continued to intravenously inject Hepa1-6-Luc cells into the body of established animal models (Fig. 3f), and discovered that H-Exo-induced glucose-enriched lung pre-metastatic niche significantly facilitated HCC cell colonization and lung metastasis in vivo (Fig. 3j, k), validating the promoting role of glucose-enriched lung pre-metastatic niche for colonization and survival of metastatic tumor cells, as well as subsequent metastatic foci growth. Together, these findings suggest that exosomes not only mediate matrix stiffness-induced lung pre-metastatic niche formation, but also serve as the major contributor to result in glucose enrichment during the formation of lung pre-metastatic niche.
Matrix stiffness-tuned exosomal let-7d-5p promotes glucose enrichment during lung pre-metastatic niche formation
Due to its high regulatory efficacy in gene expression50, miRNAs in exosome are often considered as an important mediator for intercellular crosstalk. We further determined what miRNAs in the matrix stiffness-tuned exosomes contribute to glucose enrichment during the formation of the lung pre-metastatic niche. We performed small RNA sequencing to screen differentially expressed miRNAs between MHCC97H-L-Exo and MHCC97H-H-Exo. Taking the criteria of |FC| > 1.5 (FC: fold change) and P < 0.05 as the threshold, we obtained a total of 15 known differentially expressed miRNAs including 9 upregulated miRNAs and 6 downregulated miRNAs in MHCC97-H-Exo (Fig. 4a), and 15 novel unknown differentially expressed miRNAs (Supplementary Fig. 4a). We employed miRNA qRT-PCR to randomly validate the expressions of some differential miRNAs (let-7d-5p, miR-365a-5p, miR-194-5p, miR-125a-5p), and these miRNAs all showed significant upregulations in MHCC97H-H-Exo (Fig. 4b), suggesting the reliability of the sequencing results. While in Hep3B-H-Exo, except for miR-194-5p, these miRNAs also exhibited overexpression (Fig. 4b). Besides, we observed a similar expression trend in these miRNAs at the intracellular expression level (Supplementary Fig. 4b), indicating that there exists a consistency in the expression trends of these miRNAs between extracellular exosomes and inside the cells. So, we respectively transfected 9 upregulated miRNA mimics into lung fibroblasts to determine the contribution of different miRNAs to glucose enrichment. Analysis of glycolysis-related genes demonstrated that let-7d-5p mimic had the most significant inhibitory effects on the expression of SLC2A1, PFKP, PKM, and HK2, compared with other miRNA mimics (Fig. 4c), suggesting that let-7d-5p in H-Exo may be the most crucial miRNA in regulating the glucose metabolism of lung fibroblasts. On the other hand, we applied either lentivirus-mediated overexpression or RNAi technology to construct lung fibroblasts with let-7d-5p overexpression or knockdown (Supplementary Fig. 4c), and then cultured them on lung stiffness substrates mirroring the lung pre-metastatic niche in vitro for analyzing the expressions of glycolysis-related proteins. The results manifested that let-7d-5p overexpression significantly suppressed the expressions of GLUT1, PFKP, and HK2, whereas let-7d-5p knockdown obviously improved their expressions (Fig. 4d). Intriguingly, in lung fibroblasts with let-7d-5p overexpression, we observed a decrease in PKM at the mRNA level (Fig. 4c), but little change at its protein level (Fig. 4d). Besides, we also assessed the influence of let-7d-5p on glucose uptake and found that let-7d-5p overexpression obviously reduced the glucose uptake of lung fibroblasts, while let-7d-5p knockdown elevated their glucose uptake (Fig. 4e), validating the positive contribution of let-7d-5p upregulation to glucose enrichment during lung pre-metastatic niche formation.
a Heatmap of the known differentially expressed miRNAs between MHCC97H-L-Exo and MHCC97H-H-Exo (n = 3 biological replicates). b miRNA qRT-PCR analysis of let-7d-5p, miR-365a-5p, miR-194-5p, and miR-125a-5p in MHCC97H/Hep3B-L/H-Exo (n = 3 biological replicates). Data were normalized to U6. c qRT-PCR analysis of glycolysis-related genes in lung fibroblasts transfected with indicated miRNA mimics or mimic NC (n = 4 biological replicates). Data were normalized to β-actin. d Western blot analysis of glucose transporters and glycolytic enzymes in lung fibroblasts with let-7d-5p overexpression or knockdown (n = 3 biological replicates). The samples derive from the same experiment but different gels for GLUT1 and PFKP, and another for PKM2, HK2, and β-actin were processed in parallel. e 2-NBDG uptake in lung fibroblasts with let-7d-5p overexpression or knockdown (n = 3 biological replicates). f Flow cytometry analysis of CD11b+CD45+ BMDCs recruitment in the pre-metastatic niche lung tissues induced by Hepa1-6-Mock/Exo or Hepa1-6-let-7d-5p-OE/Exo (n = 4 mice per group). g 2-NBDG uptake in lung fibroblasts (CD45-CD31-CD140a+ cells) in the pre-metastatic niche lung tissues induced by Hepa1-6-Mock/Exo or Hepa1-6-let-7d-5p-OE/Exo (n = 4 mice per group). h IHC staining of GLUT1, PFKP, and HK2 expressions in the pre-metastatic lung tissues induced by Hepa1-6-Mock/Exo or Hepa1-6-let-7d-5p-OE/Exo (n = 4 mice per group). Scale bars: black, 200 μm; red, 50 μm. i HE staining and quantification of lung metastasis lesions in lung tissues on day 61 (n = 5 mice per group). Scale bars: red, 2 mm; blue, 500 μm; black, 100 μm. Representative images are presented from indicated biologically independent experiments (e–i). Representative blot (20 μg protein per group) was shown from 3 biologically independent experiments (d), and β-actin was used to normalize protein quantification. Data are presented as mean ± SD, and P values were calculated using two-tailed unpaired Student’s t-test (b, c, f–i) or one-way ANOVA (d, e). L low-stiffness substrates, H high-stiffness substrates, MFI mean fluorescence intensity, Exo exosome, WT wild type, OE overexpression. Source data are provided as a Source Data file.
To testify the contribution of exosomal let-7d-5p to glucose enrichment in vivo, we administrated tail vein injection of let-7d-5p-OE/Exo to develop tumor-free mouse models with lung pre-metastatic niches. We used lentivirus-mediated overexpression technology to construct Hepa1-6 cells with let-7d-5p overexpression (Hepa1-6-let-7d-5p-OE) (Supplementary Fig. 4d), and then collected their conditioned media for extracting their exosomes. We subsequently determined that the quality of purified exosomes meets the characteristics of exosomes (Supplementary Fig. 4e, f). More importantly, the expression level of let-7d-5p in extracellular exosomes derived from Hepa1-6-let-7d-5p-OE was remarkably enhanced compared to the controls (Supplementary Fig. 4d), suggesting that let-7d-5p-OE/Exo is qualified as the mediator to induce the pre-metastatic niche animal model. Similar as the methods described in Fig. 1a and Fig. 3f, we injected let-7d-5p-OE/Exo or Mock/Exo into the bodies of C57BL/6 mice through the tail vein every other day to induce lung pre-metastatic niche formation. We observed a significant increase in the proportion of CD11b+CD45+ BMDCs in fresh lung tissues of let-7d-5p-OE/Exo-induced mice (Fig. 4f) on the 26th day of exosomes induction, highlighting an important role of exosomal let-7d-5p in facilitating lung pre-metastatic niche formation. Additionally, we examined glucose metabolism in lung fibroblasts (CD45-CD31-CD140a+) of exosomes-induced lung pre-metastatic niche animal models, and discovered that in comparison to those in the Mock/Exo group, lung fibroblasts in the let-7d-5p-OE/Exo group had a significant decrease in 2-NBDG absorption (Fig. 4g). Moreover, let-7d-5p-OE/Exo administration obviously suppressed GLUT1, PFKP, and HK2 expressions (Fig. 4h) and elevated the glycogen content (Supplementary Fig. 4g) in lung tissues of exosomes-induced pre-metastatic niche animal models, confirming the prominent role of exosomal let-7d-5p in glucose enrichment during lung pre-metastatic niche formation.
We continued to measure the proportion of MDSCs and CD8+ T cells in fresh lung tissues of exosomes-induced lung pre-metastatic niche animal models. We observed an obvious increase in the proportion of MDSCs and a significant decrease in the proportion of CD8+ T cells in the let-7d-5p-OE/Exo group (Supplementary Fig. 4h, i), indirectly reflecting that MDSCs accumulation in the lung pre-metastatic niche is partially attributed to glucose enrichment, in agreement with the finding in vitro that high glucose promoted MDSCs differentiation (Fig. 2i). So, glucose enrichment is likely to participate in strengthening immunosuppressive microenvironment in the lung pre-metastatic niche. To clarify the effect of let-7d-5p-OE/Exo-induced lung pre-metastatic niche animal models on HCC metastasis, we intravenously injected HCC cells into the lung pre-metastatic niche animal models and detected the occurrence of lung metastasis in the two groups on the 61th day (35 days after HCC cells injection). The results showed that there were significant increases in the number and size of lung metastases in the let-7d-5p-OE/Exo group (Fig. 4i and Supplementary Fig. 4j, k), validating that the glucose-enriched lung pre-metastatic niche induced by exosomal let-7d-5p as a favorable microenvironment is beneficial for the colonization of CTCs and the development of metastatic foci.
A pathway of matrix stiffness-tuned exosomal let-7d-5p/HMGA2/E2F1 acetylation/GLUT1, PFKP, and HK2 in lung fibroblasts participates in modulating glucose enrichment
To elucidate the molecular mechanism underlying let-7d-5p-regulated glucose metabolism in lung fibroblasts, we screened the common candidate target proteins of let-7d-5p using three publicly available bioinformatic tools including TargetScan, miRWalk, and miRTarBase, and obtained 154 common candidate proteins (Fig. 5a). Among these candidate proteins, we determined HMGA2 as the target protein of let-7d-5p based on the following reasons: (1) HMGA2 had the highest score in the prediction analysis of let-7d-5p target proteins. (2) TCGA database analysis indicated a positive correlation between HMGA2 and glycolysis pathway in lung and liver cancer tissues (Supplementary Fig. 5a). Given that exosomal let-7d-5p entering the recipient cell (lung fibroblasts) is equivalent to the upregulation of let-7d-5p in the recipient cells, we subsequently infected lung fibroblasts with lentivirus to determine the effect of let-7d-5p upregulation or downregulation on HMGA2 expression. The results demonstrated that overexpression of let-7d-5p significantly suppressed the protein expression of HMGA2 while knockdown of let-7d-5p increased the protein expression of HMGA2 (Fig. 5b), suggesting a negative regulation between let-7d-5p and HMGA2. Additionally, using data from the TargetScan Human version 8.0, we observed that there were seven putative binding regions of let-7d-5p in the 3’UTR of HMGA2 mRNA, and all binding regions contained the common sequence (Fig. 5c). Afterwards, we performed a luciferase reporter gene assay to clarify whether let-7d-5p specifically bound to the 3’UTR of HMGA2 mRNA. The results in Fig. 5c revealed that compared with that of the control group, the luciferase activity of the co-transfection group with let-7d-5p and HMGA2 3’UTR-WT was evidently decreased, but the luciferase activity of the HMGA2 3’UTR-mut group remained unchanged (Fig. 5c), implying that let-7d-5p specifically binds to the 3’UTR of HMGA2 mRNA and regulating its protein expression. These data verified that HMGA2 was the target protein of let-7d-5p. We transfected HMGA2-OE plasmid into lung fibroblasts with let-7d-5p overexpression to further validate whether let-7d-5p modulated glucose metabolism of lung fibroblasts via HMGA2. As expected, HMGA2 overexpression obviously increased GLUT1, PFKP, and HK2 expression in the control cells, also rescued the levels of these proteins that had been depleted by let-7d-5p overexpression (Fig. 5d).
a Predicted target proteins of let-7d-5p using three publicly available bioinformatic tools. b Western blot analysis of HMGA2 in lung fibroblasts with let-7d-5p overexpression or knockdown. c Schematic illustration of luciferase reporter plasmids for HMGA2 3’UTR (left panel) and relative luciferase activity determined after co-transfection of miRNA mimic and plasmids (right panel) (n = 3 biological replicates). d Effects of HMGA2 overexpression (HMGA2-OE) on GLUT1, PFKP, and HK2 expressions in lung fibroblasts with let-7d-5p overexpression. The samples derive from the same experiment but different gels for GLUT1 and PFKP, and another for HK2, HMGA2, and β-actin were processed in parallel. e Immunoprecipitation assay of interaction between HMGA2 and Rb in the nuclear protein of lung fibroblasts. f Immunoprecipitation assay of interaction between HMGA2 or HDAC1 and E2F1-Rb complex in the nuclear protein of lung fibroblasts with let-7d-5p overexpression or downregulation. g E2F1 acetylation level (Pan Ac-Lys levels of E2F1-captured proteins) and HDAC1 interacted with E2F1 in the nuclear protein of lung fibroblasts with let-7d-5p overexpression or downregulation determined by immunoprecipitation assay. h ChIP-qPCR analysis of E2F1 occupancy on SLC2A1, PFKP, and HK2 promotors in lung fibroblasts (relative to input) (n = 3 biological replicates). i Effects of TSA intervention on E2F1 acetylation levels in the nuclear protein (left panel) and GLUT1, PFKP, and HK2 expression in the total protein (right panel) of lung fibroblasts with let-7d-5p overexpression. The samples derive from the same experiment but different gels for GLUT1 and HK2, and another for PFKP and β-actin were processed in parallel. j, k Effects of E2F1 mutation (K117/120/125 R) on E2F1 acetylation levels in the nuclear protein (j) and GLUT1, PFKP, and HK2 expressions in the total protein (k) of lung fibroblasts with let-7d-5p downregulation. The samples derive from the same experiment but different gels for GLUT1 and PFKP, and another for HK2 and β-actin were processed in parallel. Representative blot was shown from 3 biologically independent experiments (b, d–g, i–k). Protein loading was 20 μg in each group and β-actin was used to normalize total protein quantification (b, d, i, k), and Histone H3 was used as a loading control in the nuclear protein (f, g, i, j). Data are presented as mean ± SD, and P-values were calculated using one-way ANOVA (b–d, i, k) or two-tailed unpaired Student’s t-test (h). Ac-Lys acetylated-Lysine, WT wild type, mut mutant, WCL whole cell lysate. Source data are provided as a Source Data file.
HMGA2 functions as the architectural transcriptional regulator to modulate the activities of some transcription factors including E2F151, and HMGA2 interacting with Rb enhances the acetylation and activity of E2F1 in pituitary adenomas52. Thus, we guessed that exosomal let-7d-5p might modulate the acetylation of E2F1 in lung fibroblasts by affecting HDAC1 dissociation from the Rb-E2F1 complex. We confirmed the interaction of HMGA2 and Rb in the nuclear protein of lung fibroblasts using an immunoprecipitation assay (Fig. 5e). Then, we extracted nuclear proteins from lung fibroblasts with let-7d-5p overexpression or downregulation (Supplementary Fig. 5b), and took Rb as a bait protein to capture HMGA2, HDAC1, and E2F1. The results in Fig. 5f showed a more interaction between HDAC1 and Rb in lung fibroblasts with let-7d-5p overexpression and less interaction in the cells with let-7d-5p downregulation. Simultaneously, HMGA2 was able to compete with HDAC1 to bind Rb, but had little effect on the binding of E2F1 to Rb (Fig. 5f). Except that, we performed a quantitative experiment (ELISA) to specifically detect HDAC1 protein levels in the captured proteins after the nuclear proteins were immunoprecipitated with Rb antibody. We found an obvious increase in the level of HDAC1 interacted with Rb in lung fibroblasts with let-7d-5p-OE and a significant decrease in the level of HDAC1 interacted with Rb in lung fibroblasts with anti-let-7d-5p (Supplementary Fig. 5c), suggesting that HMGA2 upregulation increases HDAC1 dissociation from the Rb-E2F1 complex. To further testify whether let-7d-5p could regulate the acetylation of E2F1 via HMGA2, we applied an immunoprecipitation assay to capture E2F1 in the nuclear protein of lung fibroblasts with let-7d-5p overexpression or downregulation for examining the acetylation levels of E2F1. It is observed that there was more HDAC1 that interacted with E2F1 and a low level of E2F1 acetylation in lung fibroblasts with let-7d-5p overexpression (Fig. 5g). On the contrary, in lung fibroblasts with let-7d-5p downregulation, there was less HDAC1 that interacted with E2F1 and an increase in E2F1 acetylation (Fig. 5g). We further applied immunofluorescence co-localization analysis to confirm the interaction between HDAC1 and E2F1 in the nucleus of lung fibroblasts. The results showed more HDAC1 colocalized with E2F1 in lung fibroblasts with let-7d-5p overexpression and less HDAC1 colocalized with E2F1 in cells with let-7d-5p downregulation (Supplementary Fig. 5d). These data suggest that let-7d-5p upregulation indeed increases the binding of HDAC1 to E2F1 and thereby attenuates the level of E2F1 acetylation. Transcription factors often regulate the expression of target genes by binding to their promotor sequences53. Through JASPAR database analysis, we predicted E2F1 as a potential transcription factor that bound to the promotor regions of three glycolysis-related genes including SLC2A1, PFKP, and HK2. By performing ChIP-qPCR, we confirmed increased E2F1 occupancy on the promotors of SLC2A1, PFKP, and HK2 in lung fibroblasts (Fig. 5h and Supplementary Fig. 5e).
To further clarify the role of E2F1 acetylation in modulating the expression of GLUT1, PFKP, and HK2, we used TSA, an inhibitor of HDAC1, to treat lung fibroblasts with let-7d-5p overexpression. TSA intervention obviously rescued the decrease in E2F1 acetylation induced by let-7d-5p overexpression (Fig. 5i), also restored the expression of target proteins GLUT1, PFKP, and HK2 (Fig. 5i). Next, using the online acetylation site prediction tool GPS-PAIL (http://pail.biocuckoo.org/), three putative lysine (K) residue sites including K117, K120, and K125 were identified to be the major acetylated sites of E2F1 (Supplementary Fig. 5f). We respectively transfected Flag-E2F1 (WT)-OE-plasmid or Flag-E2F1 (K117/120/125 R)-OE-plasmid into lung fibroblasts with let-7d-5p downregulation or the control cells and confirmed E2F1 protein overexpression in cells (Supplementary Fig. 5g). Subsequent immunoprecipitation analysis revealed that the acetylation level of E2F1 in lung fibroblasts with Flag-E2F1 (K117/120/125 R) OE-plasmid was significantly lower than that in lung fibroblasts with Flag-E2F1 (WT)-OE-plasmid, regardless of in the let-7d-5p downregulation group or the control group (Fig. 5j). More importantly, K117/120/125 R mutation obviously reversed let-7d-5p knockdown-caused target protein (GLUT1, PFKP, and HK2) upregulation (Fig. 5k). Thus, K117, K120, and K125 are indeed the major acetylation sites of E2F1, and its acetylation level influenced the expressions of the target proteins. Together, all the above evidence suggests that a pathway of matrix stiffness-tuned exosomal let-7d-5p/HMGA2/E2F1 acetylation/GLUT1, PFKP, and HK2 in lung fibroblasts participates in modulating glucose enrichment.
Matrix stiffness-tuned exosomal miR-365a-5p effectively regulates angiogenesis and vascular permeability to influence glucose enrichment in the lung pre-metastatic niche
In addition to the contribution of reduced glucose consumption by lung fibroblasts to glucose enrichment, angiogenesis and vascular permeability, as the key characteristics of the pre-metastatic niche, also influence glucose enrichment by increasing glucose supply. Based on the identified differential miRNAs described above, we continued to determine what miRNA in the matrix stiffness-tuned exosomes effectively controlled angiogenesis and vascular permeability during lung pre-metastatic niche formation. We respectively transfected 9 upregulated miRNA mimics into HUVECs and analyzed the contribution of different miRNAs to angiogenesis and vascular permeability. Only miR-365a-5p obviously inhibited the expression of TJP1 (gene name for ZO-1) and CDH5 (gene name for VE-cadherin) in HUVECs (Fig. 6a). Furthermore, miR-365a-5p mimic noticeably decreased the protein levels of ZO-1 and VE-cadherin but elevated the protein level of VEGFR2, whereas miR-365a-5p inhibitor exhibited the opposite effects (Fig. 6b and Supplementary Fig. 6a). We further analyzed whether miR-365a-5p impaired endothelial barrier integrity and facilitated cancer cell extravasation (Fig. 6c), and found that HUVEC monolayer with ectopic expression of miR-365a-5p was more permeable to FITC-dextran and MHCC97H-GFP cells (Fig. 6d, e). Conversely, knockdown of miR-365-5p in HUVECs caused an obvious decrease in endothelial leakiness (Fig. 6d, e). Besides, we performed an in vivo chorioallantoic membrane (CAM) assay and an in vitro tube formation assay to clarify the role of miR-365a-5p in angiogenesis. miR-365-1-5p (the sequence of mouse miR-365-1-5p is identical to human miR-365a-5p) was stably overexpressed in Hepa1-6 cells (Supplementary Fig. 6b) and their conditioned media were collected for CAM assay. Compared with the control treated with Hepa1-6-Mock/CM, CAM treated with Hepa1-6-miR-365-1-5p-OE/CM exhibited a significant increase in the number of blood vessels (Fig. 6f). In an in vitro tube formation assay, ectopic expression of miR-365a-5p in HUVECs also enhanced the number of tubes, whereas knockdown of miR-365a-5p reduced the number of tubes (Fig. 6g). These results validate that miR-365a-5p (miR-365-1-5p) derived from HCC cells is sufficient to educate vascular endothelial cells and induce vascular permeability and angiogenesis.
a qRT-PCR analysis of tight junction-related genes in HUVECs transfected with indicated mimics or mimic NC (n = 3 biological replicates). Data were normalized to β-actin. b Western blot analysis of ZO-1, VE-cadherin, and VEGFR2 in vascular endothelial cells transfected with miR-365a-5p mimic or inhibitor (n = 3 biological replicates). The samples derive from the same experiment but different gels for ZO-1 and β-actin, and another for VE-cadherin and VEGFR2 were processed in parallel. c Schematic illustration of FITC-dextran leakiness assay (left panel) and trans-endothelial invasion assay (right panel), created in BioRender. Zhao, Y. (2025) https://BioRender.com/d12t168. d Permeability of the HUVEC monolayer (transfected with miR-365a-5p mimic or inhibitor) to FITC-dextran (n = 3 biological replicates). e Invaded HCC cells that passed through the HUVEC monolayer transfected with miR-365a-5p mimic or inhibitor (n = 6 biological replicates). Scale bars: 200 μm. f Chicken embryo chorioallantoic membrane (CAM) incubated with Hepa1-6-Mock/CM or Hepa1-6-miR-365-1-5p-OE/CM (n = 3 biological replicates). g Tube formation assay of HUVECs transfected with miR-365a-5p mimic or inhibitor (n = 6 biological replicates). Scale bars: 200 μm. h, i Immunofluorescence images for concanavalin A co-localization with FITC-dextran (h) and quantification of extravasated dextran (i) in lung tissues induced by PBS, Hepa1-6-Mock/Exo, or Hepa1-6-miR-365-1-5p-OE/Exo (n = 5 mice per group). Scale bars: white, 100 μm; yellow, 20 μm. j, k IHC staining and quantification of CD31 in lung tissues induced by PBS, Hepa1-6-Mock/Exo, or Hepa1-6-miR-365-1-5p-OE/Exo (n = 5 mice per group). Scale bars: black, 200 μm; red, 50 μm. Representative images are presented from indicated biologically independent experiments (e–h, j). Representative blot (20 μg protein per group) was shown from 3 biologically independent experiments (b), and β-actin was used to normalize protein quantification. Data are presented as mean ± SD, and P values were calculated using two-tailed unpaired Student’s t-test (a, f) or one-way ANOVA (b, d, e, g, i, k). WT wild type, OD498, optical density (OD) measured at 498 nm, CM conditioned medium; Exo, exosome. Source data are provided as a Source Data file.
Subsequently, an in vivo FITC-dextran permeability assay was carried out to evaluate the effect of exosomal miR-365a-5p on vascular permeability. We isolated and purified exosomes from the CM of Hepa1-6-Mock and Hepa1-6-miR-365-1-5p-OE cells, and then confirmed miR-365-1-5p upregulation in the purified exosomes (Supplementary Fig. 6b) and the quality of the purified exosomes (Supplementary Fig. 6c, d). The exosomes were injected into mice through the tail vein every other day for 26 days (Supplementary Fig. 6e). Compared with that in PBS or Mock/Exo group, FITC‐dextran in the miR-365-1-5p-OE/Exo group was more easily extravasated from pulmonary vessels (Fig. 6h, i), meaning that exosomal miR-365-1-5p can impair lung vascular integrity by educating vascular endothelial cells. Simultaneously, there was enhanced CD31 expressions in lung tissues in miR-365-1-5p-OE/Exo group (Fig. 6j, k). The above results indicate that exosomal miR-365a-5p (miR-365-1-5p) contributes to angiogenesis and vascular permeability in the lung pre-metastatic niche.
Exosomal miR-365a-5p increases angiogenesis and vascular permeability via inactivating TRPC4AP/Ca2+/CaMKII/ERK5/KLF2/4 pathway
Using three publicly available bioinformatic tools (TargetScan, miRWalk, and miRTarBase), we obtained 97 common target proteins of miR-365a-5p (Fig. 7a). Among these candidate target proteins, TRPC4AP was selected as the target protein of miR-365a-5p due to the great impacts of calcium influx on vascular function54 and a positive correlation of TRPC4AP with TJP1 (gene name for ZO-1) or CDH5 (gene name for VE-cadherin) in TCGA analysis (Supplementary Fig. 7a). As shown in Fig. 7b, miR-365a-5p mimic prominently downregulated the expression of TRPC4AP, while miR-365a-5p inhibitor noticeably upregulated the expression of TRPC4AP in HUVECs, revealing that there is a negative regulation between miR-365a-5p and its target protein TRPC4AP. Furthermore, the luciferase reporter gene assay showed that the luciferase activity was significantly decreased in cells co-transfected with TRPC4AP 3’UTR-WT and miR-365a-5p mimic, but the luciferase activity in the cells co-transfected with TRPC4AP 3’UTR-mut and miR-365a-5p mimic remined unchanged (Fig. 7c), further verifying a specific regulatory role of miR-365a-5p in TRPC4AP expression. TRPC4AP, a calcium ion channel associated protein, can interact with TRPC4 to enhance store-operated Ca2+ entry and increase intracellular calcium55. To unravel whether miR-365a-5p regulates TRPC4AP-induced Ca2+ influx, we used the Ca2+ sensitive dye Fluo-4 AM to examine Ca2+ influx in HUVECs transfected with miR-365a-5p mimic or inhibitor. We found that miR-365a-5p mimic remarkably reduced the fluorescence intensity of intracellular Ca2+ in HUVECs, whereas miR-365a-5p inhibitor obviously elevated the fluorescence intensity (Fig. 7d and Supplementary Fig. 7b), suggesting that miR-365a-5p can inhibit the expression of TRPC4AP to reduce calcium influx in HUVECs.
a Predicted target proteins of miR-365a-5p using three publicly available bioinformatic tools. b Western blot analysis of TRPC4AP in vascular endothelial cells transfected with miR-365a-5p mimic or inhibitor. c Schematic illustration of luciferase reporter plasmids for TRPC4AP 3’UTR (upper panel) and relative luciferase activity determined after co-transfection of miRNA mimic and plasmids (lower panel) (n = 3 biological replicates). d Intracellular Ca2+ fluorescence signals in vascular endothelial cells transfected with miR-365a-5p mimic or inhibitor (n = 5 biological replicates). Scale bars: 100 μm. e Effects of miR-365a-5p mimic or inhibitor on the activation state of CaMKII/ERK5/KLF2/4 pathway in vascular endothelial cells detected by western blot. The samples derive from the same experiment but different gels for p-CaMKII, another for CaMKII and KLF2, another for p-ERK5 and β-actin, and another for ERK5 and KLF4 were processed in parallel. f Effects of TRPC4AP overexpression on the activation state of CaMKII/ERK5/KLF2/4 pathway and the expression levels of ZO-1, VE-cadherin, and VEGFR2 in vascular endothelial cells transfected with miR-365a-5p mimic detected by western blot. The samples derive from the same experiment but different gels for p-CaMKII and p-ERK5, another for CaMKII, ERK5, and β-actin (left), another for TRPC4AP and VEGFR2, another for ZO-1, VE-cadherin and KLF2, and another for KLF4 and β-actin (right) were processed in parallel. g Effects of KLF4 silence on ZO-1 and VE-cadherin expressions in vascular endothelial cells transfected with miR-365a-5p inhibitor detected by western blot. The samples derive from the same experiment but different gels for ZO-1 and KLF4, and another for VE-cadherin and β-actin were processed in parallel. h Effects of KLF2 silence on VEGFR2 expression in vascular endothelial cells transfected with miR-365a-5p inhibitor detected by western blot. The samples derive from the same experiment but different gels for VEGFR2 and β-actin, and another for KLF2 were processed in parallel. Representative images are presented from indicated biologically independent experiments (d). Representative blot (20 μg protein per group) was shown from 3 biologically independent experiments (b, e–h), and β-actin was used to normalize protein quantification. Data are presented as mean ± SD, and P values were calculated using one-way ANOVA (b–c, e–h). WT, wild type; mut, mutant; Ca2+, calcium ions. Source data are provided as a Source Data file.
As the intracellular calcium ion concentration increases, CaMKII, a multifunctional serine/threonine kinase, is often activated to phosphorate downstream molecules such as MAPK, AMPK, AKT, and Src54,56,57,58. In a report, inhibition of CaMKII can reverse Piezo1-induced KLF2/4 expression via MEKK3/MEK5/ERK5 signaling pathway in primary HUVECs56. KLF2 generally negatively regulates angiogenesis by inhibiting the expression of VEGFR2, while KLF4 is involved in maintaining vascular integrity by activating ZO-1 and VE-cadherin expressions59. Thereby, we speculated that miR-365a-5p reversed TRPC4AP-activated KLF2/4 expression via inhibition of CaMKII/ERK5 pathway. We examined the activation state of CaMKII/ERK5/KLF2/4 in HUVECs with miR-365a-5p mimic or inhibitor and discovered that miR-365a-5p mimic significantly suppressed the phosphorylation levels of CaMKII and ERK5 as well as the expression levels of KLF2 and KLF4 (Fig. 7e). Conversely, miR-365a-5p inhibitor obviously reversed the above changes (Fig. 7e), indicating that miR-365a-5p indeed disrupts CaMKII/ERK5/KLF2/4 activation by suppression of TRPC4AP. Next, we transfected TRPC4AP-OE plasmids into HUVECs with miR-365a-5p mimic, and further determined whether miR-365a-5p increased angiogenesis and vascular permeability via inactivating TRPC4AP/Ca2+/CaMKII/ERK5/KLF2/4 pathway. As shown in Fig. 7f, miR-365a-5p mimic significantly increased the expression of VEGFR2 and decreased the expressions of ZO-1 and VE-cadherin, while TRPC4AP upregulation prevented the inactivating role of miR-365a-5p in CaMKII/ERK5/KLF2/4 pathway (Fig. 7f). Notably, KLF4 silence attenuated the promoting effects of miR-365a-5p inhibitor on ZO-1 and VE-cadherin expression (Fig. 7g and Supplementary Fig. 7c), and KLF2 silence reversed the suppressive effect of miR-365a-5p inhibitor on VEGFR2 expression (Fig. 7h and Supplementary Fig. 7c). Collectively, exosomal miR-365a-5p suppresses TRPC4AP expression to inactivate CaMKII/ERK5/KLF2/4 signaling and thereby promotes angiogenesis and vascular permeability.
Association of COL1high/LOXhigh with let-7d-5p and miR-365a-5p in HCC tissues and their clinical significance
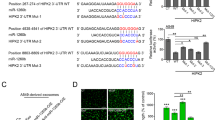
Matrix stiffening is mainly attributed to excessive deposition and crosslinking of extracellular matrix protein such as collagen I (COL1)20. Lysyloxidase (LOX) can effectively enhance the crosslinking level of extracellular matrix proteins20. So, the expression levels of COL1 and LOX are often used to indicate the grade of liver matrix stiffness31. Taking the median expressions of COL1 and LOX in tumor tissues as the threshold, HCC patients were classified into two groups: the COL1low/LOXlow group (low-stiffness group, 24 cases) and the COL1high/LOXhigh group (high-stiffness group, 24 cases). We detected let-7d-5p and miR-365a-5p expressions in HCC tissues using a FISH assay, and discovered that the expression levels of let-7d-5p and miR-365a-5p in the COL1high/LOXhigh group were all obviously higher than those in the COL1low/LOXlow group (Fig. 8a, b), suggesting a positive association between matrix stiffness and let-7d-5p or miR-365a-5p expression (Fig. 8a, b). Next, we analyzed the association of COL1/LOX expression with clinicopathological indexes such as tumor size, microvascular invasion, tumor differentiation, and tumor recurrence (Table 1), and found that HCC tissues in the COL1high/LOXhigh group exhibited higher levels of let-7d-5p and miR-365a-5p and worse tumor differentiation, and HCC patients in the COL1high/LOXhigh group had a higher tumor recurrence. Survival analysis revealed that HCC patients in the COL1high/LOXhigh group had worse overall survival (OS) and poor disease-free survival (DFS) (Fig. 8c). Based on the median expression of let-7d-5p and miR-365a-5p, we further divided HCC patients into two groups including the let-7d-5plow/miR-365a-5plow group (16 cases) and the let-7d-5phigh/miR-365a-5phigh group (16 cases). We found that HCC patients in the let-7d-5phigh/miR-365a-5phigh group had unfavorable OS and DFS in survival analysis (Fig. 8d). In a cohort of TCGA liver cancer patients, we also discovered that let-7dhigh/miR-365ahigh indicated unfavorable OS and DFS (Supplementary Fig. 8a, b). Consequently, these data suggest a close association between high matrix stiffness and increased let-7d-5p or miR-365a-5p expression in HCC tissues, in line with the findings in cell experiments. High matrix stiffness and high expression of let-7d-5p/ miR-365a-5p can better predict poor outcome and tumor recurrence of HCC patients.
a FISH images of let-7d-5p and miR-365a-5p expressions in human HCC tissues with COL1low/LOXlow (low-stiffness group, n = 24 patients) and COL1high/LOXhigh (high-stiffness group, n = 24 patients). Scale bars: white, 200 μm; red, 50 μm. b Quantification of let-7d-5p or miR-365a-5p expression in (a). c Survival curve analysis of HCC patients in the COL1low/LOXlow group (n = 24 patients) and COL1high/LOXhigh group (n = 24 patients). d Survival curve analysis of HCC patients in the let-7d-5plow/miR-365a-5plow group (n = 16 patients) and let-7d-5phigh/miR-365a-5phigh group (n = 16 patients). e Mechanism by which matrix stiffness-tuned exosomal miRNAs modulate glucose enrichment during the formation of the lung pre-metastatic niche in HCC through inhibiting glucose uptake and consumption of lung fibroblasts and enhancing angiogenesis and vascular permeability. Schematic illustration was created in BioRender. Zhao, Y. (2025) https://BioRender.com/s25i400. Specifically, two pathways including matrix stiffness-tuned exosomal let-7d-5p/HMGA2/E2F1 acetylation/GLUT1, PFKP, and HK2 in lung fibroblasts and matrix stiffness-tuned exosomal miR-365a-5p/TRPC4AP/Ca2+/CaMKII/ERK5/KLF2/4 in vascular endothelial cells synergistically promote glucose enrichment during lung pre-metastatic niche formation. Representative images are presented from indicated biologically independent experiments (a). Data are presented as mean ± SD (b), and P values were calculated using two-tailed unpaired Student’s t-test (b) or log-rank test (c, d). COL1 collagen I, LOX lysyloxidase, BMDCs bone marrow-derived cells, CTCs circulating tumor cells. Source data are provided as a Source Data file.
Discussion
With the research progress of tumor pre-metastatic niches, the new characteristics of the pre-metastatic niche are constantly being updated and proposed. Identifying new features of the pre-metastatic niche has become a priority exploration direction in the field of tumor metastasis research. To date, BMDCs recruitment, matrix remodeling, inflammation/immunosuppression, angiogenesis, and vascular permeability have been recognized as features of the pre-metastatic niche to define its formation2. Nevertheless, little is known about whether there are other new unrecognized features in pre-metastatic niches. Based on the roles of glycogen accumulation in mediating liver tumor initiation13 and glucose particularly required for HCC occurrence and development13,60, we speculate that there exists glucose metabolic reprogramming or glucose accumulation in the lung pre-metastatic niche, which is crucial for supporting HCC cell colonization and survival, as well as metastatic foci formation. Increased matrix stiffness has become a significant biomechanical characteristic of HCC, which can be used to indicate and reflect the development and prognosis of HCC26,27. Many studies have suggested that increased matrix stiffness can substantially strengthen the malignant features of HCC cells and promote their metastasis28,29,30,31,32,33,34,35,36. However, the effect of increased matrix stiffness on the pre-metastatic niche and its underlying mechanism remain largely unexplored. We previously reported that increased matrix stiffness facilitated lung pre-metastatic niche formation in HCC via increasing LOXL2 expression and secretion37,38,39, highlighting a linkage between increased matrix stiffness and lung pre-metastatic niche formation in HCC. Regrettably, it has not yet been elucidated whether there exists glucose metabolic reprogramming or glucose enrichment in the lung pre-metastatic niche of HCC, especially triggered by matrix stiffness. Even though a literature on breast cancer indicates that glucose metabolic reprogramming occurs in the lung pre-metastatic niche16, this work is still apparently different from ours due to these metabolic changes not initiated by biomechanical signals. Besides, the contribution of angiogenesis and vascular permeability in the lung pre-metastatic niche to glucose accumulation and its mechanism have not been mentioned.
In the study, we applied H-CM and L-CM to induce tumor-free mouse models with lung pre-metastatic niches for evaluating their contributions to lung pre-metastatic niche formation. Based on the significant changes in BMDCs recruitment, matrix remodeling, immunosuppression, angiogenesis and vascular permeability, and inflammation, we reasonably concluded that H-CM prominently facilitated the formation of the lung pre-metastatic niche. Consistently, the results of cell experiments also supported that H-CM was superior to L-CM in promoting matrix remodeling, angiogenesis, and vascular permeability through educating lung fibroblasts and vascular endothelial cells. These evidences sufficiently validate that conditioned medium from HCC cells under high-stiffness stimulation can obviously accelerate lung pre-metastatic niche formation. We subsequently observed the effects of H-CM and L-CM on glucose metabolism in lung fibroblasts, which were highly abundant in the lung pre-metastatic niche, and discovered that H-CM intervention remarkably suppressed the expressions of GLUT1, PFKP, PKM2, and HK2 in lung fibroblasts, also effectively attenuated their glucose uptake and consumption. Besides, GLUT1 protein expression and glycogen content at the lung tissue level also showed a prominent decrease in glucose uptake and consumption in the H-CM group. Similarly, a noticeable improvement in vascular permeability and angiogenesis caused by H-CM-educated vascular endothelial cells also meant an increase in glucose supply and aggregation in the lung pre-metastatic niche. These findings sufficiently confirm that glucose enrichment is created during H-CM-induced lung pre-metastatic niche. To evaluate whether glucose enrichment serves as a new feature of the lung pre-metastatic niche, we further clarified its pathological significance in favoring the ectopic colonization and survival of HCC cells. Our results demonstrated that glucose enrichment in the pre-metastatic niche not only significantly enhanced adhesion molecule expression to promote HCC cell adhesion, but also increased MDSCs differentiation to strengthen immunosuppression. So, glucose enrichment, like other features of the pre-metastatic niche, is also beneficial for successful realization of subsequent metastasis, illustrating that glucose enrichment can serve as a new feature of the lung pre-metastatic niche in HCC. Considering that conditioned medium from HCC cells contains both extracellular vesicles and soluble factors, we further determined which components played the dominant regulatory role in glucose metabolism. Since H-CM-Exo-free intervention effectively reversed the inhibition effect of H-CM on glucose metabolism, exosomes were determined as the most important contributor for glucose-enriched lung pre-metastatic niche formation. Subsequently, the results from animal models and cell assays all validated that H-Exo intervention same as H-CM intervention prominently facilitated lung pre-metastatic niche formation, attenuated glucose uptake and consumption, and increased glucose supply and aggregation. More importantly, H-Exo-induced glucose-enriched lung pre-metastatic niche significantly improved HCC cell colonization and lung metastasis in vivo. These findings supported that exosomes served as the major contributor to cause glucose enrichment during H-CM-induced lung pre-metastatic niche.
In view of the high abundance of miRNAs in exosomes and their highly regulatory efficiency in intercellular communication9,61, we screened differentially expressed miRNAs between H-Exo and L-Exo and identified let-7d-5p as a key miRNA to participate in modulating glucose enrichment by inhibiting GLUT1, PFKP, and HK2 expressions and glucose uptake in lung fibroblasts. We intravenously injected let-7d-5p-OE/Exo to develop tumor-free mouse models with lung pre-metastatic niches for confirming the contribution of exosomal let-7d-5p to glucose enrichment. Our results revealed that in addition to increasing BMDCs recruitment and immunosuppressive microenvironment, exosomal let-7d-5p also effectively suppressed 2-NBDG uptake by CD45-CD31-CD140a+ lung fibroblasts and elevated the glycogen content in lung tissues of animal models, validating a positive contribution of exosomal let-7d-5p to glucose enrichment during lung pre-metastatic niche formation. Additionally, the glucose-enriched lung pre-metastatic niche induced by let-7d-5p-OE/Exo also showed to be more beneficial for the colonization of CTCs and the development of metastatic foci. miRNAs generally exert their biologic effects by inhibiting the translation of target mRNAs or inducing degradation of mRNAs within the RISC50. Therefore, identifying the target protein of let-7d-5p is a key step to explore its functions. By the bioinformatic analysis, luciferase assay, and negative regulatory analysis, HMGA2 was determined as the target protein of let-7d-5p. Based on the results that HMGA2 overexpression effectively rescued the expressions of GLUT1, PFKP, and HK2 that had been depleted by let-7d-5p overexpression, let-7d-5p was further proved to link with glucose metabolic enzyme expression through targeting HMGA2. HMGA2 as an architectural transcriptional regulator can modulate the activity of some transcription factors including E2F151. Our data supported that E2F1 bound to the promotor regions of SLC2A1, PFKP, and HK2. Furthermore, let-7d-5p-induced HMGA2 downregulation inhibited the interaction of HMGA2 and Rb-E2F1 complex but promoted the binding of HDAC1 to Rb-E2F1 complex. So, E2F1 acetylation level might control the expressions of GLUT1, PFKP, and HK2. TSA intervention obviously rescued the low acetylation level of E2F1 induced by let-7d-5p overexpression and the expressions of target proteins GLUT1, PFKP, and HK2. Consistently, the mutation of E2F1 acetylation sites (K117/120/125 R) obviously reversed let-7d-5p knockdown-caused target protein upregulation (GLUT1, PFKP, and HK2) through decreasing the acetylation level of E2F1. These evidences suggest that exosomal let-7d-5p can modulate HMGA2/E2F1 acetylation axis to suppress GLUT1, PFKP, and HK2 expressions in lung fibroblasts, which results in glucose enrichment in the lung pre-metastatic niche.
Angiogenesis and vascular permeability in the lung pre-metastatic niche are not only beneficial for the extravasation, colonization and metastasis of CTCs59, but also exert great impact on glucose enrichment by increasing glucose supply. Based on differential miRNAs expression and function analysis, we determined miR-365a-5p in matrix stiffness-tuned exosomes as the key miRNA to regulate angiogenesis and vascular permeability. miR-365a-5p noticeably decreased the protein levels of ZO-1 and VE-cadherin, two markers for endothelial barrier integrity62, and in the HUVEC monolayer group with miR-365a-5p overexpression, FITC-dextran or HCC cells were more prone to leakage. Besides, exosomal miR-365-1-5p also enhanced pulmonary vessels permeability in mouse models, further confirming the important role of miR-365a-5p in promoting vascular permeability. The activation of VEGFR2 by VEGF is the most critical driver of tumor angiogenesis63. Our data revealed that VEGFR2 protein level was positively associated with miR-365a-5p level. Moreover, miR-365a-5p (miR-365-1-5p) could effectively induce angiogenesis in vitro and in CAM models. These findings suggest that miR-365a-5p contributes to enhanced vascular permeability and angiogenesis. Subsequently, TRPC4AP was selected as the target protein of miR-365a-5p due to the great impacts of calcium influx on vascular function54,55. After validating the binding between miR-365a-5p and the 3’-UTR regions of TRPC4AP mRNA and a negative correlation between miR-365a-5p and TRPC4AP, we further observed that miR-365a-5p mimic indeed decreased TRPC4AP-induced Ca2+ influx to inactivate CaMKII/ERK5 pathway, and therefore reduced KLF2/4 expressions. Notably, these changes could be rescued by TRPC4AP overexpression. Simultaneously, KLF4 and KLF2 silence also partially reversed miR-365a-5p inhibitor-induced vascular tight junctions and less angiogenesis. Accordingly, we concluded that exosomal miR-365a-5p enhanced angiogenesis and vascular permeability by inactivating TRPC4AP/Ca2+/CaMKII/ERK5/KLF2/4 pathway.
Our data suggested that there existed glucose enrichment during the formation of the lung pre-metastatic niche, and glucose enrichment was a new characteristic of the lung pre-metastatic niche triggered by matrix stiffness-tuned exosomal miRNAs. We explained its underlying mechanism from two different perspectives. One of which is to decrease glucose utilization by attenuating glucose uptake and consumption of lung fibroblasts, and the other is to increase glucose supply by enhancing angiogenesis and vascular permeability. Specifically, two pathways including matrix stiffness-tuned exosomal let-7d-5p/HMGA2/E2F1 acetylation/GLUT1, PFKP, and HK2 in lung fibroblasts and matrix stiffness-tuned exosomal miR-365a-5p/TRPC4AP/Ca2+/CaMKII/ERK5/KLF2/4 in vascular endothelial cells synergistically promoted glucose enrichment during lung pre-metastatic niche formation (Fig. 8e).
Although exosomal let-7d-5p and miR-365a-5p as the major regulators were validated to participate in glucose-enriched lung pre-metastatic niche formation, the effects of other exosomal components such as soluble proteins on glucose enrichment still cannot be excluded. Additionally, restricted by clinical guidelines and ethical, it is almost impossible to obtain lung tissue samples of HCC patients for validating the formation of lung pre-metastatic niche. On the other hand, the quality of serum exosomes was unable to meet clinical validation requirements due to the long follow-up period. These challenges cause great difficulties in verifying new features of the lung pre-metastatic niche using clinical samples.
In summary, this study proposes glucose enrichment, a new characteristic of the lung pre-metastatic niche triggered by matrix stiffness-tuned exosomal miRNAs, is essential for HCC cell colonization and survival as well as metastatic foci formation. To the best of our knowledge, this is the first report to explore the effect of matrix stiffness on glucose-enriched lung pre-metastatic niche formation and its relevant mechanism in HCC. The findings in the study will enrich and update the theory of tumor pre-metastatic niches and provide a new direction for the intervention of tumor pre-metastasis niches in the future.
Methods
Our research strictly complied with all relevant ethical regulations of Zhongshan Hospital, Fudan University and the Affiliated Taizhou People’s Hospital of Nanjing Medical University. All animal experiments were approved by the Animal Care Ethical Committee of Zhongshan Hospital, Fudan University (Approval No. 2022-080). The collection and use of clinical materials was approved by the Taizhou People’s Hospital Research Ethics Committee (Approval No. KY2023-161-01).
Cells and cell culture
Human Hep3B (catalog number: SCSP-5045), 293 T (catalog number: SCSP-502) and mouse Hepa1-6 (catalog number: SCSP-512) cell lines were obtained from the cell bank of Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Human umbilical vein endothelial cells (HUVECs, catalog number: 8000) were acquired from ScienCell Biotechnology Company (ScienCell, San Diego, CA, USA). Human MHCC97H and human embryonic lung fibroblasts (HELFs) cells were from our lab reserved. MHCC97H cells, Hepa1-6 cells, 293 T cells, and HELFs were cultured in DMEM (GenomBio, Hangzhou, China) supplemented with 10% FBS (Biosera, Pays de la Loire, France) and 1% penicillin-streptomycin (GenomBio, Hangzhou, China). Hep3B cells were cultured in MEM medium (GenomBio, Hangzhou, China) supplemented with 12.5% FBS and 1% penicillin-streptomycin. HUVECs were cultured in endothelial cell medium (ScienCell, San Diego, CA, USA) with 5% FBS (ScienCell), 1% penicillin/streptomycin (ScienCell), and 1% endothelial cell growth supplement (ScienCell). When recipient cells were treated with exosomes, 10% exosome-depleted FBS (SBI, USA), not 10% FBS, was supplemented in their culture media.
Animal experiment
Four-to-six-week-old male C57BL/6 mice were purchased from Weitong Lihua Experimental Animal Center (Zhejiang, China). All mice were maintained in a specific pathogen-free (SPF) facility under a 12/12 h dark/light cycle and appropriate temperature (22–24 °C) and humidity (30–70%). They had unrestricted access to food and water, and their diet was commercial feed (growing and reproductive feed for rats and mice, catalog number: P1100) with crude protein ≥ 20.5% and crude fat ≥ 4%, supplied by SLACOM experimental animal company (Shanghai, China). All the animal experiments were performed in accordance with the protocols approved by the Animal Care Ethical Committee of Zhongshan Hospital, Fudan University (Shanghai, China). The maximum diameter of tumor size authorized by the Committees on Animal Research and Ethics was 20 mm, and the size of tumors did not exceed this criterial at any time during all animal experiments.
COL1-coated polyacrylamide gel substrates with the stiffness of 6 kPa and 16 kPa
COL1-coated polyacrylamide gel substrates with stiffness of 6 kPa (low-stiffness substrate, corresponding to stiffness of normal liver) and 16 kPa (high-stiffness substrate, corresponding to stiffness of cirrhotic liver) were prepared by mixing different amounts of 30% acrylamide (Acr), 2% bis-acrylamide (Bis), 10% ammonium persulfate (APS), TEMED, and ddH2O (formula were listed in Supplementary Table 1). Briefly, the polyacrylamide gel substrates were cut into circular gel substrates with a diameter of 6 cm and then subjected to UV irradiation for 30 min. Subsequently, the gel substrates were functionalized with dopamine hydrochloride solution (0.8 mg/mL in 50 mM HEPES, pH 6.8–8.2). After washed with PBS three times, the gel substrates were further covered with 500 µL of COL1 solution (0.1 mg/mL, Corning, USA) for crosslink reaction for 1 h at room temperature (RT). Next, excess COL1 on the gel substrate was washed off by PBS. Thus, the COL1-coated gel substrates were successfully prepared and preserved in basal medium at 4 °C. Approximately 1 × 106 HCC cells in 1 mL culture medium were seeded onto a COL1-coated gel substrate and cultured for 3 h. Then, 12 mL of culture medium were supplemented into the dish, and cells were cultured for another 48 h for the following experiments.
Conditioned medium (CM) preparation
When HCC cells cultured on low- and high-stiffness substrates reached 70% confluence, their culture media were replaced with fresh serum-free medium for another 48 h culture. When cell culture finished, their culture supernatants were collected and centrifuged at 300 × g for 10 min to remove dead cells, subsequently recentrifuged at 20,000 × g for 20 min to remove cellular debris. After that, they were further concentrated using ultrafiltration tubes (3 kD, Millipore, USA) to obtain the concentrated conditioned medium (namely as L-CM and H-CM, respectively). L-CM and H-CM were filtered by 0.22 µm filters and then stored at −80 °C for subsequent cell and animal assays. The protein concentrations of L-CM and H-CM were determined by BCA protein assay (Beyotime Biotechnology, Shanghai, China).
Tumor-free mouse models with lung pre-metastatic niches induced by L-CM and H-CM
Conditioned medium from Hepa1-6 cells grown on low- and high-stiffness substrates (L-CM and H-CM) were prepared as the protocol described above, tumor-free mouse models with lung pre-metastatic niches were developed by tail vein injection of L-CM and H-CM, respectively. Briefly, 300 µL of L-CM and H-CM (1 µg/µL) from Hepa1-6 cells were intravenously injected into the bodies of four-to-six-week-old male C57BL/6 mice via tail vein every other day for 26 days (12 mice in each group). At different time points (days 6, 14, 18, and 22), the lung tissues of two induced mice in each group were collected for flow cytometry analysis. On the 26th day of induction, lung tissues of the remaining four induced mice in each group were collected for subsequent flow cytometry and other analysis to determine the formation of lung pre-metastatic niches.
Lung tissue dissociation and single-cell suspension isolation
Fresh lung tissues from animal models were collected after transcardiac perfusion, and then they were cut into small pieces in normal saline and digested in RPMI-1640 medium with 2 mg/mL collagenase type IV (Sigma, USA) and 0.1 mg/mL DNase I (Sigma, USA) for 1 h at 37 °C under a gentle shaking condition. The suspension was filtered through a 70 μm strainer to remove small fragments of undigested tissues and centrifuged at 300 × g for 5 min. The collected cells were treated with 1 × RBC Lysis Buffer (eBioscience, USA) for 5 min at RT and resuspended in the FACS staining buffer (Invitrogen, USA) for flow cytometry analysis.
Flow cytometry analysis
The collected single cell suspensions were stained by Zombie Aqua™ dye (BioLegend, 423101, 1:300) for 15 min at RT to detect cell viability. After being washed with PBS, the cells were incubated with the following antibodies for 15 min at RT: For tumor-free mouse models with lung pre-metastatic niches induced by L-CM and H-CM (or induced by L-Exo and H-Exo), APC anti-mouse CD45 (BioLegend, 103111, 1:100), FITC anti-mouse CD11b (BioLegend, 101205, 1:200), and BV421 anti-mouse Gr-1 (BioLegend, 108433, 1:100) were used to detect BMDCs and MDSCs. APC anti-mouse CD45 (BioLegend, 103111, 1:100), FITC anti-mouse CD3 (BioLegend, 100203, 1:100), and PE anti-mouse CD8 (BioLegend, 162303, 1:50) were applied to detect CD8+ T cells. For tumor-free mouse models with lung pre-metastatic niches induced by exosomal let-7d-5p, APC anti-mouse CD45 (BioLegend, 103111, 1:100), PerCP/Cyanine5.5 anti-mouse CD31 (BioLegend, 102419, 1:80), and PE anti-mouse CD140a (BioLegend, 135905, 1:80) were applied to identify lung fibroblasts. APC anti-mouse CD45 (BioLegend, 103111, 1:100), PerCP/Cyanine5.5 anti-mouse CD11b (BioLegend, 101227, 1:100), and BV421 anti-mouse Gr-1 (BioLegend, 108433, 1:100) were used to detect BMDCs and MDSCs. APC anti-mouse CD45 (BioLegend, 103111, 1:100), APC/Cyanine7 anti-mouse CD3 (BioLegend, 100221, 1:80), and PE anti-mouse CD8 (BioLegend, 162303, 1:50) were applied to detect CD8+ T cells. For bone marrow cell differentiation analysis, FITC anti-mouse CD11b (BioLegend, 101205, 1:200) and BV421 anti-mouse Gr-1 (BioLegend, 108433, 1:100) were applied to determine MDSCs. To detect the activity of mTOR in MDSCs, PE anti-mouse phospho-mTOR (Ser2448) (Invitrogen, 12-9718-41, 1:50) was added after cell fixation and permeabilization. Subsequently, the incubated cells were washed with PBS and then resuspended in 300 μL of FACS staining buffer for flow cytometry analysis (BD FACSAria™ III machine, BD Bioscience, US). Data were analyzed using FlowJo software (version 10.4), and the representative flow cytometry gate strategy images are shown in Supplementary Fig. 9.
Exosome-free-H-CM (H-CM-Exo-free) preparation
For the preparation of H-CM-Exo-free, ultracentrifugation was employed to deplete exosomes in the collected H-CM. Briefly, the conditioned medium from HCC cells grown on a high-stiffness substrate (H-CM) was subjected to ultracentrifugation at 110,000 × g for 12 h at 4 °C. After that, the exosome-free supernatant was obtained and further concentrated by ultrafiltration as above described. Lastly, the concentrated H-CM-Exo-free was filtered by 0.22 µm filters for subsequent cell assays.
Exosome isolation, identification, and tracking
Conditioned medium from HCC cells grown on low- and high-stiffness substrates were collected, and their exosomes were extracted using a differential ultracentrifugation method. Briefly, the collected CM were subjected to ultracentrifugation at 110,000 × g for 75 min at 4 °C (Beckman Coulter, USA), and then the centrifugal precipitates were collected and washed in PBS. Subsequently, they were was further ultracentrifuged at 110,000 × g for 75 min at 4 °C to obtain the exosomes. The extracted exosomes were then resuspended in 200 µL of PBS. The protein concentration of exosomes was measured by BCA assay. The morphology of exosomes was observed by a Philips CM120 BioTwin transmission electron microscope (FEI Company, USA), and the size of exosomes was analyzed using a Nanoparticle Tracking Analyzer (ZetaView PMX 110, Particle Metrix, Meerbusch, Germany). The molecule markers of exosomes were detected by Western blot. The exosomes used in animal experiments were extracted from the CM of Hepa1-6 cells using the exosome isolation kit (Umibio, Shanghai, China) according to the manufacturer’s instructions.
For exosome labeling and tracking, the exosomes were incubated with 10 μM DIO membrane dye (Beyotime Biotechnology, Shanghai, China) for 20 min in the dark, and then the labeled exosome precipitate washed in PBS was ultracentrifuged at 110,000 × g for 75 min at 4 °C to remove the excess dye in the supernatant. Next, the labeled exosomes in 200 µL PBS (2 µg) were added into the culture medium of recipient cells (2 × 104) for 48 h of culture. Finally, the recipient cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, USA). Internalization of exosomes in the recipient cells was observed using a microscope (High Content Imaging System, Perkin Elmer).
Preparation of lung stiffness substrate
Conditioned medium (CM) from MHCC97H-LOXL2 cells were prepared as the protocol described above, and then lung stiffness substrate (926.18 Pa, mirroring the stiffness of lung tissue in a pathological state) was constructed by a mixture of COL1 (Corning, 3.58 mg/mL), DMEM basal medium, and MHCC97H-LOXL2-CM (310 ng/mL) in a volume ratio of 1:10:5. Briefly, the mixture was added to a 6-well plate at a volume of 2 mL per well and incubated at 37 °C for 4 h to form a gel, and then the gel was stored at 4 °C for another 12 h to form a gel substrate with stiffness of 926.18 Pa37. The resident cells (lung fibroblasts) grown on a lung stiffness substrate were treated with CM or exosomes from HCC cells, simulating a lung pre-metastatic niche environment of HCC in vitro. Specifically, 2 × 105 of lung fibroblasts were seeded on lung stiffness substrate overnight and then incubated with 200 µg CM or 20 µg exosomes for 48 h. After that, the incubated cells were harvested for further analysis.
Intervention of glucose, inhibitors, agonists, and function-blocking antibodies
Different amounts of D-glucose (Beyotime Biotechnology, Shanghai, China) were added to glucose-free DMEM/RPMI-1640 medium (GenomBio, Hangzhou, China) to obtain DMEM/RPMI-1640 medium containing 5.5 mM (NG, normal glucose concentration) and 25 mM (HG, high glucose concentration). 2-DG (a glycolysis inhibitor, 2 mM, MCE, USA) and rapamycin (a mTOR inhibitor, 100 µM, MCE, USA) were utilized in the experiment on MDSCs differentiation for 48 h. In E2F1 acetylation analysis, lung fibroblasts were treated with TSA (0.5 µM, MCE, USA), an HDAC1 inhibitor, for 12 h. To activate AMPK, HCC cells were treated with A-769662 (100 µM, MCE, USA), an AMPK agonist, for 12 h. To block integrin β1, HCC cells were incubated with a specific function-blocking antibody of integrin β1, anti-integrin β1 (50 µg/mL, BioLegend, 921304, USA), for 2 h.
Bone marrow cells (BMCs) isolation and differentiation
BMCs were harvested from C57BL/6 mice by flushing the bone marrow cavities of femurs and tibias with RPMI-1640 medium containing 2% FBS. The harvested BMCs were pre-treated with 1 × RBC Lysis Buffer (eBioscience, USA), and then they were cultured in RPMI-1640 medium containing 40 ng/mL GM-CSF (PeproTech, USA) and 40 ng/mL IL-6 (PeproTech, USA) for 96 h to differentiate into MDSCs. During the process of MDSCs differentiation, HG or HG + 2-DG was added to the above culture system for 48 h to analyze the effects of high glucose on MDSCs differentiation. The morphology of MDSCs was observed and recorded by a light microscope (Olympus, Japan) and the proportion of MDSCs in BMCs was assessed by flow cytometry analysis.
A co-culture system in vitro simulating the pre-metastatic niche environment
A co-culture system in vitro for simulating pre-metastatic niche environments was developed to clarify the effect of exosomes on the formation of the lung pre-metastatic niche. A 6-well Millicell hanging cell culture insert (Millipore, USA) was used to achieve cell co-culture in vitro. Specifically, 1 × 106 BMCs were cultured in the lower chamber in the presence of IL-6 (40 ng/mL) and GM-CSF (40 ng/mL) for 48 h, and then they were co-cultured with 20 µg L-Exo or H-Exo-treated lung fibroblasts (2 × 105) grown on lung stiffness substrates in the upper chamber for another 48 h. Additionally, lung fibroblasts grown on lung stiffness substrates, not co-cultured with BMCs, were taken as the control. After the experiment, the cells in the upper chamber and in the lower chamber were collected for western blot and flow cytometry analysis, respectively.
Cell adhesion assay
Approximately 2 × 105 of lung fibroblasts were cultured on the lung stiffness substrate overnight and then incubated with 200 µg CM or 20 µg exosomes from HCC cells for 48 h. When the treated lung fibroblasts were close to 100% confluence, MHCC97H-GFP cells (2 × 105) in DMEM medium were placed upon the treated lung fibroblast monolayer, simultaneously, NG or HG was added for intervention. After 24 h, the nonadherent HCC cells were removed, and the adherent HCC cells on the surface of lung fibroblasts were photographed using a fluorescence microscope (Olympus, Japan) and counted by Image J software (version 1.8.0).
Glucose uptake and glucose consumption assay
The glucose uptake of lung fibroblasts was measured using the fluorescent glucose analog 2-NBDG (Invitrogen, USA). For the in vitro assay, the indicated lung fibroblasts were incubated in a glucose-free medium at 37 °C for 1 h and then treated with 50 μM 2-NBDG for 45 min. After the cells were washed twice with PBS, their mean fluorescence intensity (MFI) was analyzed using flow cytometry analysis. For the in vivo assay, 4 hours before they were executed, the mouse models were injected with 15 mg/kg of 2-NBDG through the tail vein, and a transcardiac perfusion was performed to remove the excess dye. Their lung tissues were collected and digested into single-cell suspensions for flow cytometry analysis. Glucose consumption in lung fibroblasts was detected using the glucose oxidase assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The culture supernatants of the indicated cells were collected after 48 h culture and glucose consumption (change of extracellular glucose in the culture supernatants) of lung fibroblasts were normalized to total protein amount in each group.
Immunohistochemistry (IHC) and immunofluorescence (IF)
For immunohistochemistry (IHC) staining, tissues were fixed in 4% paraformaldehyde, embedded with paraffin, and sectioned into 4 μm sections. After dewaxing and hydration, the sections were subjected to antigen repair to reveal antigen epitopes by using sodium citrate antigen retrieval solution (pH 6.0, Beyotime Biotechnology, Shanghai, China). Sections were treated in 3% H2O2 for 20 min and blocked with blocking buffer (3% BSA) for 30 min. Subsequently, the sections were incubated with diluted primary antibodies overnight at 4 °C and HRP-labeled polymer anti-rabbit or anti-mouse secondary antibodies (Gene Tech, Shanghai, China) for 30 min at RT. Staining was visualized with DAB (Gene Tech, Shanghai, China), and a haematoxylin counterstain (Gene Tech, Shanghai, China) was performed before dehydration. Stained tissue slides were photographed under an optical microscope (Olympus, Japan), and the average optical density (AOD) was quantified using Image J software (version 1.8.0). The diluted primary antibodies were as follows: Fibronectin (Proteintech, 15613-1-AP, 1:500), CD31 (Abcam, ab182981, 1:2000), GLUT1 (Proteintech, 66290-1-Ig, 1:500), PFKP (CST, 8164, 1:50), HK2 (CST, 2867, 1:400), COL1 (Affinity, AF7001, 1:100), and LOX (Abcam, ab31238, 1:500).
The procedures of immunofluorescence (IF) staining in paraffin-embedded tissues were similar to those of IHC staining. Multiplex IF staining in paraffin-embedded tissues was conducted using Fluorescent Multiplex Reagent Kit (RecordBio, Shanghai, China) according to the instructions. The diluted primary antibodies were as follows: TSG101 (Proteintech, 14497-1-AP, 1:200), CD31 (Abcam, ab182981, 1:2000), VE-cadherin (Abcam, ab33168, 1:1000), S100A4 (CST, 13018, 1:1000), and GLUT1 (Proteintech, 66290-1-Ig, 1:500). For IF staining in OCT-embedded tissues, fresh lung tissues were embedded in Tissue-TEK OCT and cut into 8-μm-thick frozen sections. Since FITC-dextran and Alexa Fluor 594-concanavalin A had been injected into the mouse body, frozen sections were directly counterstained with DAPI solution. For co-localization IF staining in lung fibroblasts, the indicated cells were fixed in 4% paraformaldehyde for 15 min at RT and blocked in 3% BSA for 1 h. Subsequently, the indicated cells were incubated with E2F1 (Invitrogen, 32-1400, 1:200) and HDAC1 antibody (Proteintch, 66085-1-Ig, 1:1000) overnight at 4 °C, and then reacted with the corresponding fluorescent secondary antibodies and DAPI. For IF staining of integrin β1 in MHCC97H cells, integrin β1 (CST, 34971 T, 1:100) antibody was used. For IF staining of VE-cadherin in HUVECs, VE-cadherin (Abcam, ab33168, 1:1000) antibody was used. The fluorescent images were captured using an Olympus FV 1200 scanning laser confocal microscope (Olympus, Japan), and MFI was quantified using Image J software (version 1.8.0).
Hematoxylin-eosin (HE) and periodic acid-Schiff (PAS) staining
Lung tissue sections were dewaxed and rehydrated, and then stained with hematoxylin-eosin (HE) following the instructions of the Hematoxylin and Eosin Staining Kit (Beyotime Biotechnology, Shanghai, China).
Periodic acid-Schiff (PAS) staining in lung tissue sections was done using the Periodic Acid Schiff Diastase (D-PAS) Stain Kit (Solarbio Biotechnology, Beijing, China). Briefly, after deparaffinization and hydration, tissue sections were oxidized with periodate acid solution for 10 min, stained with Schiff reagent for 10 min, and then counterstained with hematoxylin staining solution for 1 min. The PAS staining had been further confirmed using diastase digestion approach. If the PAS positive staining became negative in the parallel tissue slide after diastase digestion, indicating the stained material was glycogen. Stained tissue sections were observed and photographed under an optical microscope (Olympus, Japan).
Western blot
Total proteins were extracted from cells or exosomes using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China). Cytoplasmic protein extraction and nuclear protein extraction were performed using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, USA) in accordance with the manufacturer’s instructions. Membrane protein was extracted using the MinuteTM Plasma Membrane Protein Isolation Kit (Invent Biotechnologies, USA) following the manufacturer’s protocol. Protein concentrations were measured using a BCA Protein Assay Kit. Equal amounts of protein samples were loaded on SDS-PAGE, followed by blotting onto PVDF membrane. After blocked in TBST buffer containing 5% skim milk, the PVDF membrane was incubated with diluted primary antibodies overnight at 4 °C and HRP-conjugated secondary antibodies for 1 h at RT. Protein bands were visualized with the ECL detection kit (Yeasen, Shanghai, China) and photographed using the Bio-Rad ChemiDOC™ XRS Imaging system (Bio-Rad, USA). The intensity of protein bands was analyzed by Image J software (version 1.8.0). Relative expression levels of proteins in experimental groups were normalized to those of the reference β-actin protein level, and then the obtained value was compared with that of the control group depending on the experimental design. Diluted primary antibodies were as follows: β-actin (Proteintech, 20536-1-AP, 1:20000), Fibronectin (Proteintech, 15613-1-AP, 1:1000), MMP9 (Boster, PB9668, 1:1000), GLUT1 (Proteintech, 66290-1-Ig, 1:500), SGLT2 (Proteintech, 24654-1-AP, 1:500), PFKP (CST, 8164, 1:1000), and HK2 (CST, 2867, 1:1000), PKM2 (Proteintech, 60268-1-Ig, 1:5000), ZO-1 (CST, 5406, 1:1000), VE-cadherin (Abcam, ab33168, 1:1000), VEGFR2 (CST, 2479, 1:1000), TSG101 (Proteintech, 14497-1-AP, 1:1000), CD63 (Proteintech, 25682-1-AP, 1:500), Hsp70 (Abmart, T55496, 1:1000), ALIX (Abcam, ab275377, 1:1000), Albumin (Abcam, ab207327, 1:2000), Cytochrome C (Proteintech, 66264-1-Ig, 1:5000), HMGA2 (CST, 5269, 1:1000), Rb (Abcam, ab181616, 1:1000), E2F1 (Invitrogen, 32-1400, 1:500), HDAC1 (Proteintch, 66085-1-Ig, 1:5000), Acetylated-Lysine (CST, 9441, 1:1000), Histone H3 (Proteintech, 17168-1-AP, 1:2000), α-tublin (Proteintech, 11224-1-AP, 1:5000), Flag (Proteintech, 66008-3-Ig, 1:5000), TRPC4AP (Proteintech, 66138-1-Ig, 1:500), Phospho-CaMKII (Thr286) (CST, 12716, 1:1000), CaMKII (Proteintech, 13730-1-AP, 1:1000), Phospho-Erk5 (Thr218/Tyr220) (CST, 3371, 1:1000), ERK5 (Abcam, ab40809, 1:5000), KLF2 (Bioss, bs-2772R, 1:250), KLF4 (Proteintech, 11880-1-AP, 1:1000), integrin β1 (CST, 34971 T, 1:1000), SNX17 (Proteintech, 68256-1-Ig, 1:5000), AMPKα (CST, 5832 T, 1:1000), Phospho-AMPKα (Thr172) (CST, 2535 T, 1:1000), and Na + /K + -ATPase (CST, 3010, 1:1000). Diluted secondary antibodies were as follows: HRP-conjugated goat anti-mouse IgG (Proteintech, SA00001-1, 1:5000) and HRP-conjugated goat anti-rabbit IgG (Proteintech, SA00001-2, 1:5000).
Tumor-free mouse models with lung pre-metastatic niches induced by L-Exo and H-Exo
Exosomes were isolated and purified from Hepa1-6-L-CM or H-CM (named as L-Exo or H-Exo, respectively) to induce lung pre-metastatic niche models for determining the roles of H-Exo in lung pre-metastatic niche formation and subsequent lung metastasis. 50 µg of exosomes in 100 μL PBS were injected into the bodies of four-to-six-week-old male C57BL/6 mice through the tail vein every other day until the 26th day (10 mice in each group). On the 26th day of induction, five induced mice were randomly executed to evaluate lung pre-metastatic niche formation and glucose metabolism of lung tissues. BMDCs, MDSCs, and CD8+ T cells were examined by flow cytometry analysis. Simultaneously, the interstitial fraction fluid was isolated and collected from dissociated lung tissues, and the levels of glucose in the cell-free interstitial fraction fluid was detected using the glucose oxidase assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Subsequently, 2 × 106 Hepa1-6-Luc cells suspended in 100 µL PBS were intravenously injected into the bodies of the remaining five induced mice. Five weeks later, metastatic lesions in the lungs of the mice were observed and assessed by bioluminescent imaging, and their lung tissues were collected for pathological analysis.
qRT-PCR and miRNA qRT-PCR
Total RNA was extracted from frozen tissues or cells or exosomes using TRIzol™ Reagent (Sigma, USA). For mRNA, the cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) according to the manufacturer’s protocols. PCR products were amplified and obtained using Hieff® qPCR SYBR Green Master Mix (Yeasen, Shanghai, China). For miRNA, cDNA template synthesis and PCR amplification were performed using All-in OneTM miRNA qRT-PCR Detection Kit 2.0 (GeneCopoeia, Rockville, MD, USA) according to the manufacturer’s protocol. Real-time quantitative PCR was performed using QuantStudio5 instrument (Thermo Fisher Scientific, USA) and gene expression was quantitated using the comparative CT method (ΔΔCT method). β-actin (ACTB) and U6 (RNU6-1) were applied as internal references for mRNAs and miRNAs, respectively. The primer sequences are provided in Supplementary Table 2.
Metabolomics
Lung fibroblasts (1 × 107 cells, 6 biological replicates per group) treated with MHCC97H-L-CM or MHCC97H-H-CM were collected for the untargeted metabolomics experiment, which was carried out in collaboration with MajorBio company (Shanghai, China). The cells were resuspended in 400 μL of prechilled suspension buffer containing 80% methanol, 20% water, and 0.02 mg/mL L-2-chlorophenylalanin. After grinding and ultrasonic treatment, the samples were incubated at −20 °C for 30 min and centrifugated (13,000 g) at 4 °C for 15 min. Their supernatants were transferred into the LC-MS injection vial for subsequent analysis. Meanwhile, equal volumes of all sample metabolites were mixed to prepare quality control (QC) samples, which were inserted during the instrument analysis process to examine the repeatability of the entire analysis process. A total of 12 experimental samples and 3 QC samples were subjected to LC-MS/MS analysis using an ultra-high-performance liquid chromatography system (UHPLC-Q Exactive HF-X, Thermo Fisher Scientific, USA). In the liquid chromatography (LC), 3 μL of sample was separated on an HSS T3 column (100 mm × 2.1 mm i.d., 1.8 μm), the column temperature was set to 40 °C and flow rate was 0.4 ml/min. The sample mass spectrum (MS) signal was acquired using positive and negative ion scanning mode with a range scanned set at 70–1050 m/z. MS acquisition was performed in data-dependent acquisition (DDA) mode and full MS scans at resolution of 60,000.
Raw data of the mass spectrum signal was analyzed by Progenesis QI software (version 3.0). The metabolites were annotated using the KEGG database (http://www.genome.jp/kegg/), HMDB database (http://www.hmdb.ca/) and Metlin database (http://metlin.scripps.edu/). Subsequently, the data were analyzed through online platform of majorbio choud platform (cloud.majorbio.com). Briefly, total peaks were preprocessed and normalized using the sum normalization method. At the same time, the variables with relative standard deviation of QC samples ≥ 30% were deleted, and then the data were performed log10 transformation to obtain the data matrix. Principal component analysis (PCA) and orthogonal partial least squares discrimination analysis (OPLS-DA) were performed using ropls package (version 1.6.2) in the software R (version 4.1.0). Significant metabolic differences were determined based on the variable importance in projection (VIP) values obtained from the OPLS-DA model and P-values determined by Student’s t-test. Metabolites with VIP > 1 and P < 0.05 were considered as significantly differential metabolites. Metabolic pathways were annotated using KEGG database (http://www.genome.jp/kegg/), pathway enrichment analysis was performed in Python (version 3.7) using the packages scipy.stats (version 1.0.0) and statistical enrichment was calculated using Fisher exact test. Representative secondary mass spectrometry data of differential glycolytic metabolites identified by LC-MS are shown in Supplementary Fig. 10.
Small RNA libraries preparation and sequencing
Small RNA libraries preparation and sequencing were carried out in collaboration with MajorBio company (Shanghai, China). For the preparation and sequencing of small RNA libraries, exosomes were isolated and purified from CM of MHCC97H cells grown on low and high-stiffness substrates (MHCC97H-L-Exo and MHCC97H-H-Exo), and then their total RNAs were extracted using TRIzol™ Reagent (Sigma, USA). The quality and concentration of the RNAs were checked using an Agilent 2100 (Agilent, USA). Small RNA libraries were constructed by Illumina TruSeq Small RNA kit (Illumina, USA). Briefly, small RNAs of 18–32 nt were enriched from total RNAs, ligated with 5′- and 3′-adaptors, amplified by RT-PCR, and purified by 6% Novex TBE PAGE gel to obtain small RNA libraries. The libraries were further subjected to sequencing via the Illumina NovaSeq 6000 platform (Illumina, USA). Differential miRNA expression analysis of the dataset was conducted through the DESeq2 package (version 1.24.0) in the software R (version 4.1.0). The data were analyzed through online platform of majorbio choud platform (cloud.majorbio.com).
Lentivirus, plasmids, miRNA mimics and inhibitors, and RNA interference
Lentivirus vectors expressing hsa-let-7d-5p, mmu-let-7d-5p, and mmu-miR-365-1-5p, lentivirus vectors repressing hsa-let-7d-5p (Anti-hsa-let-7d-5p), and lentivirus vectors expressing luciferase (Luc) were designed, constructed, and packaged by Genechem Co. Ltd. (Shanghai, China). Lung fibroblasts were infected with LV-hsa-let-7d-5p or LV-anti-hsa-let-7d-5p at a MOI of 20, Hepa1-6 cells were infected with LV-mmu-let-7d-5p or LV-mmu-miR-365-1-5p at a MOI of 10. The overexpressing plasmids for human HMGA2, human E2F1 (WT), human E2F1 (K117/120/125 R) (a mutant plasmid of E2F1), and human TRPC4AP were designed by Genechem Co. Ltd. (Shanghai, China). Plasmids were transfected into lung fibroblasts or HUVECs using Lipofectamine 3000 (Invitrogen, USA) following the manufacturer’s protocol. miRNA mimics, including hsa-let-7d-5p, hsa-miR-365a-5p, hsa-miR-193b-5p, hsa-miR-4443, hsa-miR-5100, hsa-miR-23b-3p, hsa-miR-194-5p, hsa-miR-4454, hsa-miR-125a-5p, and mimic NC, as well as hsa-miR-365a-5p inhibitor and inhibitor NC, were purchased from RiboBio (Guangzhou, China). The siRNA oligonucleotides for human KLF2 and KLF4 and the siRNA control were also purchased from RiboBio. Lung fibroblasts and HUVECs were transfected with miRNA mimic, miRNA inhibitor, and siRNA using a Lipofectamine RNAiMax Kit (Invitrogen, USA) following the manufacturer’s protocol. All sequences of siRNAs and overexpression/knockdown fragments are provided in Supplementary Table 3. Sequences of miRNA mimics and inhibitors were listed in Supplementary Table 4.
Tumor-free mouse models with lung pre-metastatic niches induced by exosomal let-7d-5p
Exosomes were isolated and purified from the CM of Hepa1-6-let-7d-5p-OE cells to induce lung pre-metastatic niche models for determining the roles of exosomal let-7d-5p in lung pre-metastatic niche formation and subsequent lung metastasis. 50 µg of exosomes in 100 μL PBS were injected into the bodies of four-to-six-week-old male C57BL/6 mice through the tail vein every other day until the 26th day (9 mice in each group). On the 26th day of induction, four induced mice were randomly executed to evaluate the glucose uptake abilities of lung fibroblasts by an in vivo 2-NBDG uptake assay. BMDCs, MDSCs, and CD8+ T cells were also examined by flow cytometry analysis. Subsequently, 2 × 106 Hepa1-6-Luc cells suspended in 100 µL PBS were intravenously injected into the bodies of the remaining five induced mice. Five weeks later, metastatic lesions in the lungs of the mice were observed and assessed by bioluminescent imaging, and their lung tissues were collected for pathological analysis.
Dual-luciferase reporter assay
The binding sites of let-7d-5p on the 3’-untranslated region (UTR) of HMGA2 mRNA and the binding sites of miR-365a-5p on the 3’-UTR of TRPC4AP mRNA were predicted by the TargetScanHuman 8.0 website. The luciferase reporter plasmids for wild-type HMGA2 3’-UTR and mutant HMGA2 3’-UTR, as well as wild-type TRPC4AP 3’-UTR and mutant TRPC4AP 3’-UTR were synthesized by Genechem Co. Ltd. (Shanghai, China). The sequences of 3’UTR fragments inserted in vectors are listed in Supplementary Table 5. For let-7d-5p and HMGA2, 293 T cells were co-transfected and divided into four groups, including mimic NC + HMGA2 3’UTR-WT, let-7d-5p mimic + HMGA2 3’UTR-WT, mimic NC + HMGA2 3’UTR-mut, and let-7d-5p mimic + HMGA2 3’UTR-mut. For miR-365a-5p and TRPC4AP, 293 T cells were co-transfected and divided into four groups, including mimic NC + TRPC4AP 3’UTR-WT, miR-365a-5p mimic + TRPC4AP 3’UTR-WT, mimic NC + TRPC4AP 3’UTR-mut, and miR-365a-5p mimic + TRPC4AP 3’UTR-mut. Renilla luciferase reporter plasmids served as an internal control. Cell lysates were harvested 48 h after transfection, and their luciferase activities and renilla luciferase activities were measured by Dual-luciferase Reporter Assay Kit (Yeasen, Shanghai, China).
Co-immunoprecipitation (Co-IP)
Nuclear protein from lung fibroblasts was used for immunoprecipitation with the indicated capture antibodies. The extracted nuclear protein (400 µg) was incubated with a captured antibody such as Rb (Abcam, ab181616, 1:100), HMGA2 (CST, 5269, 1:100), E2F1 (Invitrogen, 32-1400, 1:100), Flag (Proteintech, 66008-3-Ig, 1:100), or control antibody IgG (CST, 1:100, 2729 or 61656) overnight at 4 °C under continuous rotation and mixing. On the next day, 30 μL of protein A/G Mix Magnetic Beads (Millipore) were added to the mixture solution, and they were fully incubated for 30 min at RT under continuous rotation and mixing. The beads that captured the antigen-antibody complex were precipitated by a magnetic separation rack (Invitrogen). After being washed three times with PBST, the beads were denatured in 1 × SDS loading buffer containing dithiothreitol and boiled at 95 °C for 10 minutes. Captured proteins in the supernatant were analyzed by western blot.
Chromatin immunoprecipitation (ChIP) assay
Chromatin immunoprecipitation (ChIP) was performed with a ChIP assay kit (Bersinbio, Guangzhou, China) according to the manufacturer’s protocol. In brief, lung fibroblasts were fixed in 1% formaldehyde and terminated by glycine solution, and then they were lysed in ChIP lysis buffer. Subsequently, the chromatin in the cell lysates was sheared into 200–1000 bp fragments using the sonicated method. After being precleared with protein A/G beads, cell lysates were incubated with E2F1 antibody (CST, 3742, 1:100) or control antibody IgG (CST, 2729, 1:100) overnight at 4 °C under continuous rotation and mixing. On the next day, the protein A/G beads were added to the mixture and incubated for 1 h at RT under continuous rotation and mixing. Next, beads were precipitated by a magnetic separation rack and washed three times in low-salt buffer, high-salt buffer, LiCl buffer, and TE buffer, respectively. Bound chromatin was eluted in the ChIP elution buffer and decrosslinked by heating at 65 °C overnight. Subsequently, chromatin DNA was purified using the AFTSpin Multifunction DNA Purification Kit (ABclonal Technology, Wuhan, China), and the target DNA was detected by qRT-PCR. The primers were designed for the ChIP assay based on the potential E2F1 binding sites in the promotor region of SLC2A1/PFKP/HK2 (Supplementary Table 2).
Enzyme-linked immunosorbent assay (ELISA)
The expression levels of HDAC1 in Rb-captured nuclear extracts were determined by an HDAC1 ELISA kit (Enzyme-linked Biotechnology, Shanghai, China). Briefly, the equal amounts of nuclear proteins in each group were immunoprecipitated by Rb antibody and then they were captured by protein A/G beads. The captured proteins were eluted using 100 μL elution buffer (Beyotime Biotechnology, Shanghai, China). Subsequently, each group of elutes and standard products were loaded to analyze their HDAC1 level according to the instructions. HDAC1 content was calculated based on the standard curve. Similarly, the concentration of fibronectin, MMP9, GLUT1, PFKP, PKM2, and HK2 in total proteins of lung fibroblasts were also detected by their corresponding ELISA kits (Enzyme-linked Biotechnology, Shanghai, China).
Chicken embryo chorioallantoic membrane (CAM) assay
The chicken embryo chorioallantoic membrane (CAM) assay was performed to assess the role of miR-365a-5p (miR-365-1-5p) in angiogenesis in vivo. The 2-day-old specific pathogen-free fertilized chicken eggs were cleaned with 75% ethanol and maintained at 38 °C in 60% humidity. After 7 days, a window with a size of 1.0 cm2 was formed in the shell using a sterile tweezer, and the chorioallantoic membrane (CAM) was exposed. CAM that grew well was selected to perform experiments. 1 mL of CM from Hepa1-6-Mock or Hepa1-6-miR-365-1-5p-OE cells were collected and deposited onto the CAM for 3 days of incubation. The results were captured with a photographic document, and the effects of CM on angiogenesis were assessed through assessing the number of second- and third-order vessels according to reported methods64.
Tube formation assay
Matrigel (Corning, USA) solution (mixed with ECM basal medium at 1:1 in volume) was pipetted into the wells of a 12-well plate and solidified at 37 °C for 30 minutes. The treated HUVECs (1 × 105/well) with miR-365a-5p mimic or inhibitor were seeded on the solidified Matrigel and cultured at 37 °C for 8 h. The formed tubes were photographed under a light microscope (Olympus, Japan), and the number of branch points was counted and quantified by Image J software (version 1.8.0).
To evaluate the effect of exosomal miR-365-1-5p on vascular permeability in vivo
Exosomes were extracted from the CM of Hepa1-6-Mock and Hepa1-6-miR-365-1-5p-OE cells to evaluate their roles in vascular permeability. 50 µg of exosomes in 100 μL PBS were injected into the bodies of four-to-six-week-old male C57BL/6 mice through the tail vein every other day until the 26th day (5 mice in each group). On the 26th day of induction, FITC-dextran with a dose of 100 mg/kg (70,000 MW, Sigma, USA) was injected into the bodies of the educated mice through the tail vein. 2.5 h later, Alexa Fluor 594-concanavalin A with a dose of 10 mg/kg (Invitrogen, USA) was also injected into the bodies of the educated mice through the tail vein. 0.5 h later, the excess dye in the educated mice was removed by transcardiac perfusion with 1% PFA in PBS. Their lung tissues were collected and embedded in Tissue-TEK OCT for frozen sections, and subjected to subsequent immunofluorescence (IF) staining.
FITC-dextran leakiness assay
To determine vascular leakiness in vitro, the treated HUVECs (3.5 × 104 in 100 μL ECM medium) with CM or exosomes were seeded on the bottom membrane of the upper chamber in a Boyden chamber (0.4 μm pore size; Corning, USA), and 600 μL of ECM medium was added into the lower chamber. At the same time, the “no monolayer cells” group (there were no monolayer cells on the upper chamber) served as a control. When HUVECs grew to be a monolayer, a mixed solution of FITC-dextran (70,000 MW, 10 mg/mL; Sigma, USA) and ECM medium (volume ratio 3:7) was added into the upper chamber. The optical density (OD) of liquid in the lower chamber was measured at 498 nm by a microplate reader at different time points of dextran leakiness. For the in vivo Dextran leakiness assay, FITC-dextran was injected into the educated mouse models as above described.
Trans-endothelial invasion assay
The trans-endothelial invasion assay was employed to evaluate vascular permeability in vitro. The treated HUVECs (3.5 × 104 in 100 μL ECM medium) with miR-365a-5p mimic or inhibitor were seeded on the bottom membrane of the upper chamber in a Boyden chamber (8 μm pore size; Corning, USA) for 12 h to form a monolayer. Subsequently, MHCC97H-GFP cells (1 × 104) were placed on the HUVEC monolayer. After 24 h of culture, the non-invasive cells on the front side of the bottom membrane were gently scraped and washed with PBS. The invaded HCC cells on the back side of the bottom membrane were observed and photographed by a light microscope (Olympus, Japan), as well as counted by Image J software (version 1.8.0).
Intracellular Ca2+ measurement
The level of intracellular Ca2+ was measured by the Fluo-4 AM (Beyotime Biotechnology, Shanghai, China) according to the instructions. Briefly, HUVECs (1 × 105/well), seeded on a 12-well plate, were transfected with miR-365a-5p mimic, mimic NC, miR-365a-5p inhibitor, or inhibitor NC and cultured for 48 h. Subsequently, the cells were incubated with 1 μM of Fluo-4 AM diluted in the basal ECM medium for 30 min at 37 °C. After being washed with PBS three times, levels of intracellular Ca2+ in HUVECs in each group were observed and photographed by a fluorescence microscope (Olympus), and their mean fluorescence intensities (MFI) were analyzed by Image J software (version 1.8.0).
Clinical human HCC specimens
Clinical data of 52 HCC patients who underwent curative hepatectomy in Taizhou People’s Hospital from March 2015 to December 2020 were collected. All participants provided written informed consent. All the HCC patients did not receive neoadjuvant therapy, and their tumor tissue sections were obtained from the Department of Pathology, Taizhou People’s Hospital. Taking the median expressions of LOX and COL1 in tumor tissues as the threshold, HCC patients were classified into COL1low/LOXlow group (24 cases), COL1high/LOXhigh group (24 cases), COL1low/LOXhigh group (2 cases) and COL1high/LOXlow group (2 cases). COL1high/LOXhigh group was defined as the high-stiffness group, while COL1low/LOXlow group were defined as the low-stiffness group. Two groups of COL1low/LOXhigh and COL1high/LOXlow were excluded for further analysis since their stiffness level could not be accurately determined. Of the remaining 48 HCC patients, 9 were female and 39 were male, with 30 patients over the age of 59. HCC patients were followed up until November 2023 and their clinical data were retrospectively analyzed. Tumor differentiation was graded by the Edmondson grading system. Microvascular invasion was determined based on the presence of tumor emboli within either the central hepatic vein, the portal, or the large capsular vessels31. The study was approved by the Taizhou People’s Hospital Research Ethics Committee (Approval No. KY2023-161-01).
Fluorescence in situ hybridization (FISH)
The FISH assay was performed to examine the expressions of let-7d-5p or miR-365a-5p in tissue sections using the fluorescence in situ hybridization (FISH) kit (Servicebio, Wuhan, China) and the let-7d-5p or miR-365a-5p detection probe (Servicebio, Wuhan, China). Specifically, tissue sections were dewaxed, rehydrated, and repaired, and then digested with protease K for 25 min at 37 °C. After pre-hybridization with hybridization solution at 40 °C for 1 h, tissue sections were incubated with the hsa-let-7d-5p probe or the hsa-miR-365a-5p probe overnight at 40 °C for hybridization. Subsequently, tissue sections were washed with SSC washing buffer at 40 °C, and their fluorescence signals were amplified by a Cy3-conjugated label probe (for hsa-let-7d-5p detection) or an Alexa Fluor 488-conjugated label probe (for hsa-miR-365a-5p). Lastly, tissue sections were stained with DAPI. The fluorescent images of tissue sections were observed and captured using a fluorescence microscope (Olympus, Japan), and MFI were analyzed by Image J software (version 1.8.0). Sequences of miRNA probes were listed in Supplementary Table 6.
The cancer genome atlas (TCGA) database
Clinical data of 372 HCC patients and the results of mRNA and miRNA expressions in HCC tissues were retrieved and downloaded from the TCGA database (https://www.cancer.gov/tcga, Data Release 32.0). Kaplan-Meier (K-M) method was performed to generate survival curves.
Statistics and reproducibility
The in vitro assays were derived from at least three biological samples. The cells or conditions were assigned randomly to each experimental group. The in vivo experiments were performed with at least four animals in each animal model. Mice were randomly grouped before different treatments. For in vitro and in vivo experiments, the investigators were blinded from the experimental conditions/groups. For experiments of clinical samples, the investigators were blinded for the clinical information of each sample prior to the analysis. All quantitative data are presented as mean ± standard deviation (SD), and GraphPad Prism software (version 9.0.0) was used for statistical analysis and graphs. Statistical comparisons between two groups were carried out by two-tailed unpaired Student’s t-test. When comparing more than two groups with one independent factor, one-way ANOVA was used to calculate the statistical significance. Categorical variables were analyzed by Pearson’s chi-squared test. For survival analysis, the Kaplan-Meier method and log-rank test were used. The Pearson correlation test was used for correlation analysis. P values were indicated in figures and tables, and statistical significance was determined at P < 0.05. The schematic illustration images were created with BioRender.com with publication licenses.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA-sequencing expression profiles and clinical information for TCGA-HCC patients are publicly available and downloaded from the TCGA database (https://www.cancer.gov/tcga). Raw sequencing data of small RNA in this study have been deposited in the GSA database in National Genomics Data Center, China National Center for Bioinformation, under the accession number HRA006931 (https://ngdc.cncb.ac.cn/gsa-human/browse/HRA006931). Mass spectrometry metabolomics data in this study have been deposited in the OMIX database in National Genomics Data Center, China National Center for Bioinformation, under the accession number OMIX008314 (https://ngdc.cncb.ac.cn/omix/release/OMIX008314). The raw data is available for non-commercial purposes under controlled access because of data privacy laws, and access can be obtained by request to the corresponding authors. The corresponding authors will check the requests with caution and make the decision whether to permit or not in one month. The data will be available for one month once access has been granted. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided as a Source Data file. Source data are provided with this paper.
References
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Liu, Y. & Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 30, 668–681 (2016).
Massague, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
Zhang, S. et al. RNF219/alpha-Catenin/LGALS3 Axis Promotes Hepatocellular Carcinoma Bone Metastasis and Associated Skeletal Complications. Adv. Sci. (Weinh.) 8, 2001961 (2021).
Wang, D., Sun, H., Wei, J., Cen, B. & DuBois, R. N. CXCL1 Is Critical for Premetastatic Niche Formation and Metastasis in Colorectal Cancer. Cancer Res. 77, 3655–3665 (2017).
Ye, L. et al. Influence of Exosomes on Astrocytes in the Pre-Metastatic Niche of Lung Cancer Brain Metastases. Biol. Proceed. Online 25, 5 (2023).
Zhu, G. et al. LOXL2-enriched small extracellular vesicles mediate hypoxia-induced premetastatic niche and indicates poor outcome of head and neck cancer. Theranostics 11, 9198–9216 (2021).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Guo, Y. et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer 18, 39 (2019).
Fang, T. et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 9, 191 (2018).
Yuan, X. et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 11, 1429–1445 (2021).
Zhao, S. et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 13, 156 (2020).
Liu, Q. et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell 184, 5559–5576.e5519 (2021).
Zanotelli, M. R., Zhang, J. & Reinhart-King, C. A. Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab. 33, 1307–1321 (2021).
Bergers, G. & Fendt, S. M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21, 162–180 (2021).
Fong, M. Y. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015).
Gong, Z. et al. Lipid-laden lung mesenchymal cells foster breast cancer metastasis via metabolic reprogramming of tumor cells and natural killer cells. Cell Metab. 34, 1960–1976.e1969 (2022).
Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 12, 31–46 (2022).
Mao, X. et al. Nidogen 1-Enriched Extracellular Vesicles Facilitate Extrahepatic Metastasis of Liver Cancer by Activating Pulmonary Fibroblasts to Secrete Tumor Necrosis Factor Receptor 1. Adv. Sci. (Weinh.) 7, 2002157 (2020).
Jiang, Y. et al. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 15, 34 (2022).
Kalli, M., Poskus, M. D., Stylianopoulos, T. & Zervantonakis, I. K. Beyond matrix stiffness: targeting force-induced cancer drug resistance. Trends Cancer 9, 937–954 (2023).
Wei, S. C. et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 17, 678–688 (2015).
Wang, W. et al. Matrix stiffness regulates tumor cell intravasation through expression and ESRP1-mediated alternative splicing of MENA. Cell Rep. 42, 112338 (2023).
Papalazarou, V. et al. The creatine-phosphagen system is mechanoresponsive in pancreatic adenocarcinoma and fuels invasion and metastasis. Nat. Metab. 2, 62–80 (2020).
Woo, H. Y. et al. Lung and lymph node metastases from hepatocellular carcinoma: Comparison of pathological aspects. Liver Int 42, 199–209 (2022).
Marasco, G. et al. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J. Hepatol. 70, 440–448 (2019).
Serra-Burriel, M. et al. Development, validation, and prognostic evaluation of a risk score for long-term liver-related outcomes in the general population: a multicohort study. Lancet 402, 988–996 (2023).
Li, M. et al. Activation of Piezo1 contributes to matrix stiffness-induced angiogenesis in hepatocellular carcinoma. Cancer Commun. (Lond.) 42, 1162–1184 (2022).
Wang, Y. et al. Integrin alphaVbeta5/Akt/Sp1 pathway participates in matrix stiffness-mediated effects on VEGFR2 upregulation in vascular endothelial cells. Am. J. Cancer Res 10, 2635–2648 (2020).
Dong, Y. et al. Increasing matrix stiffness upregulates vascular endothelial growth factor expression in hepatocellular carcinoma cells mediated by integrin beta1. Biochem Biophys. Res Commun. 444, 427–432 (2014).
Dong, Y. et al. Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J. Hematol. Oncol. 12, 112 (2019).
Zhang, X. et al. A synergistic regulation works in matrix stiffness-driven invadopodia formation in HCC. Cancer Lett. 582, 216597 (2024).
You, Y. et al. Matrix stiffness-mediated effects on stemness characteristics occurring in HCC cells. Oncotarget 7, 32221–32231 (2016).
Liu, H. H. et al. An SCD1-dependent mechanoresponsive pathway promotes HCC invasion and metastasis through lipid metabolic reprogramming. Mol. Ther. 30, 2554–2567 (2022).
Gao, X. et al. Matrix Stiffness-Upregulated MicroRNA-17-5p Attenuates the Intervention Effects of Metformin on HCC Invasion and Metastasis by Targeting the PTEN/PI3K/Akt Pathway. Front Oncol. 10, 1563 (2020).
Wu, B. et al. Stiff matrix induces exosome secretion to promote tumour growth. Nat. Cell Biol. 25, 415–424 (2023).
Wu, S. et al. The pathological significance of LOXL2 in pre-metastatic niche formation of HCC and its related molecular mechanism. Eur. J. Cancer 147, 63–73 (2021).
Wu, S. et al. Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation. J. Exp. Clin. Cancer Res 37, 99 (2018).
Xing, X. et al. Matrix stiffness-mediated effects on macrophages polarization and their LOXL2 expression. FEBS J. 288, 3465–3477 (2021).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Lee, H. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234 (2020).
Jiang, P. et al. Negative regulation of AMPK signaling by high glucose via E3 ubiquitin ligase MG53. Mol. Cell 81, 629–637.e625 (2021).
Fan, Y. et al. Sirt6 Suppresses High Glucose-Induced Mitochondrial Dysfunction and Apoptosis in Podocytes through AMPK Activation. Int J. Biol. Sci. 15, 701–713 (2019).
Schaffer, B. E. et al. Identification of AMPK Phosphorylation Sites Reveals a Network of Proteins Involved in Cell Invasion and Facilitates Large-Scale Substrate Prediction. Cell Metab. 22, 907–921 (2015).
McNally, K. E. et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat. Cell Biol. 19, 1214–1225 (2017).
Georgiadou, M. & Ivaska, J. Tensins: Bridging AMP-Activated Protein Kinase with Integrin Activation. Trends Cell Biol. 27, 703–711 (2017).
Giles, A. J. et al. Activation of Hematopoietic Stem/Progenitor Cells Promotes Immunosuppression Within the Pre-metastatic Niche. Cancer Res 76, 1335–1347 (2016).
Li, Y. et al. mTOR inhibitor INK128 promotes wound healing by regulating MDSCs. Stem Cell Res Ther. 12, 170 (2021).
Deng, Y. et al. mTOR-mediated glycolysis contributes to the enhanced suppressive function of murine tumor-infiltrating monocytic myeloid-derived suppressor cells. Cancer Immunol. Immunother. 67, 1355–1364 (2018).
O’Brien, K., Breyne, K., Ughetto, S., Laurent, L. C. & Breakefield, X. O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 21, 585–606 (2020).
Ozturk, N., Singh, I., Mehta, A., Braun, T. & Barreto, G. HMGA proteins as modulators of chromatin structure during transcriptional activation. Front Cell Dev. Biol. 2, 5 (2014).
Fedele, M. et al. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell 9, 459–471 (2006).
Francois, M., Donovan, P. & Fontaine, F. Modulating transcription factor activity: Interfering with protein-protein interaction networks. Semin Cell Dev. Biol. 99, 12–19 (2020).
Filippini, A., D’Amore, A. & D’Alessio, A. Calcium Mobilization in Endothelial Cell Functions. Int. J. Mol. Sci. 20, 4525 (2019).
Mace, K. E. et al. TRUSS, TNF-R1, and TRPC ion channels synergistically reverse endoplasmic reticulum Ca2+ storage reduction in response to m1 muscarinic acetylcholine receptor signaling. J. Cell Physiol. 225, 444–453 (2010).
Zheng, Q. et al. Mechanosensitive Channel PIEZO1 Senses Shear Force to Induce KLF2/4 Expression via CaMKII/MEKK3/ERK5 Axis in Endothelial Cells. Cells 11, 2191 (2022).
Tokumitsu, H. & Sakagami, H. Molecular Mechanisms Underlying Ca(2+)/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction. Int. J. Mol. Sci. 23, 11025 (2022).
Villalobo, A. Ca(2+) Signaling and Src Functions in Tumor Cells. Biomolecules 13, 1739 (2023).
Zeng, Z. et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 9, 5395 (2018).
Plaz Torres, M. C. et al. Diabetes medications and risk of HCC. Hepatology 76, 1880–1897 (2022).
Isaac, R., Reis, F. C. G., Ying, W. & Olefsky, J. M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 33, 1744–1762 (2021).
Giannotta, M., Trani, M. & Dejana, E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell 26, 441–454 (2013).
Kanugula, A. K. et al. Novel noncanonical regulation of soluble VEGF/VEGFR2 signaling by mechanosensitive ion channel TRPV4. FASEB J. 33, 195–203 (2019).
Deng, F. et al. Tumor-secreted dickkopf2 accelerates aerobic glycolysis and promotes angiogenesis in colorectal cancer. Theranostics 9, 1001–1014 (2019).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 82272959 to J.-F.C.).
Author information
Authors and Affiliations
Contributions
J.-F.C., K.-Z.Z., and Z.-M.W. conceived and supervised this project. J.-F.C., Y.-Y.Z., H.-M.Y., and J.-J.L. designed this project. Y.-Y.Z., H.-M.Y., J.-J.L., J.-L.Q., M.L., X.Z., M.-M.W., and Y.-H.W. performed and analyzed all the experiments. Y.-Y.D. and Y.Y. analyzed clinical samples. Q.-W.Z., D.-M.G., and Y.Z. performed the animal experiments. B.-B.L., R.-X.C., and Z.-G.R. provided suggestions for experiments. Y.-Y.Z., H.-M.Y., and J.-J.L. wrote the manuscript, and J.-F.C., K.-Z.Z., and Z.-M.W. reviewed it. All authors discussed and agreed on the presentation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Atsushi Ochiai, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, Y., Yu, H., Li, J. et al. A glucose-enriched lung pre-metastatic niche triggered by matrix stiffness-tuned exosomal miRNAs in hepatocellular carcinoma. Nat Commun 16, 1736 (2025). https://doi.org/10.1038/s41467-025-56878-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-56878-8
This article is cited by
-
Colorectal cancer cells-derived exosomal miR-188-3p promotes liver metastasis by creating a pre-metastatic niche via activation of hepatic stellate cells
Journal of Translational Medicine (2025)
-
Mechanical regulation of extracellular vesicle activity during tumour progression
Nature Biomedical Engineering (2025)