Abstract
Doublecortin is a neuronal microtubule-associated protein that regulates microtubule structure in neurons. Mutations in Doublecortin cause lissencephaly and subcortical band heterotopia by impairing neuronal migration. We use CRISPR/Cas9 to knock-out the Doublecortin gene in induced pluripotent stem cells and differentiate the cells into cortical neurons. DCX-KO neurons show reduced velocities of nuclear movements and an increased number of neurites early in neuronal development, consistent with previous findings. Neurite branching is regulated by a host of microtubule-associated proteins, as well as by microtubule polymerization dynamics. However, EB comet dynamics are unchanged in DCX-KO neurons. Rather, we observe a significant reduction in α-tubulin polyglutamylation in DCX-KO neurons. Polyglutamylation levels and neuronal branching are rescued by expression of Doublecortin or of TTLL11, an α-tubulin glutamylase. Using U2OS cells as an orthogonal model system, we show that DCX and TTLL11 act synergistically to promote polyglutamylation. We propose that Doublecortin acts as a positive regulator of α-tubulin polyglutamylation and restricts neurite branching. Our results indicate an unexpected role for Doublecortin in the homeostasis of the tubulin code.
Similar content being viewed by others
Introduction
During human brain development, ~1010 newborn neurons migrate out of their stem cell niche to form the cerebral cortex1. Migrating neurons generally have two slender processes that extend from the cell body2. Their migration can be divided into two steps: (1) elongation of the leading process and (2) translocation of the cell body in the direction of motion3. Both steps rely heavily on the neuronal microtubule cytoskeleton4. In the leading process, microtubules contribute pushing forces5, activate and reinforce actin polymerization, respond to guidance cues, and steer neuronal migration6. In the cell body, microtubules and motor proteins generate forces that pull the nucleus forward7. In addition, microtubules serve as roadways for the transport of organelles, synaptic vesicle precursors, and neurotrophic factors by motor proteins8.
To enable these diverse functions, the microtubule network is regulated and patterned at multiple levels. First, in neurites, microtubules are dynamic in neurite tips and stable in neurite shafts9. In axons, microtubules have uniform orientation (plus-end out); in dendrites, microtubule orientation is mixed10. Second, microtubules are altered by post-translational modifications (PTMs), known as the “tubulin code”11, to create subpopulations of microtubules with variable chemical and mechanical properties12,13. Third, the tubulin code governs a host of microtubule-associated proteins (MAPs), including structural MAPs14,15, polymerases and depolymerases16, plus-end and minus-end regulators17, and motor proteins18,19. Each MAP has a specific localization, affinity, and function towards microtubules. Together, these regulatory mechanisms create complex microtubule “cartography”20. For example, dendritic microtubules are organized into adjacent bundles that differ in their polarity and their PTMs21. Dysregulation of MAPs or loss of microtubule patterning can lead to brain malformation, neurodegeneration, and/or impaired neural function.
Human brain formation depends critically on the MAP Doublecortin (DCX)22,23. The Dcx gene is located on the X-chromosome and was discovered in 1998 in a cohort of patients with double cortex syndrome and X-linked lissencephaly24,25. These diseases are caused by mutations in DCX that lead to a failure in the radial migration of cortical neurons and result in a disorganized lamination of the cerebral cortex26. How DCX mutations cause migration defects remains only partially understood, in part due to competing hypotheses about the structural role of DCX in microtubule regulation.
Most disease-causing mutations in DCX are missense mutations that cluster in a pair of tandem globular domains, known as the DC domains27,28. DC domains bind to microtubules at the vertex of 4 tubulin dimers29,30, a binding site shared with end-binding (EB) family proteins31 and doublecortin-like kinase 1 (DCLK1)32,33. The location of this binding site, in close proximity to inter- and intra-protofilament interfaces, makes DC domains (and EBs) highly responsive to the structural state of the microtubule lattice. The N-terminal domain, DC1, is tightly folded33 and binds to straight GDP lattices30. The second domain, DC2, is only partly folded33,34 and binds GDP-Pi lattices35, curved lattices36, and other tubulin intermediates37,38. Together, the DC domains enable DCX to bind cooperatively to the microtubule lattice39,40. In addition, the DC domains bind in close proximity to kinesin motor domains on microtubules41 and impact kinesin-1 and dynein motility in vitro42,43. Many of these diverse structural interactions are disrupted by patient mutations in the DC domains36,38,39, suggesting a direct role for DCX in regulating microtubule structure and intracellular trafficking. Yet how mutations in DC domains disrupt function in the full context of the neuronal proteome remains unclear, especially at neurite tips and cell bodies where DCX is enriched44,45,46.
The link between the disease phenotype seen in patients and the mechanistic behavior seen in reconstitution assays has been partially elucidated by several in vitro and in vivo models. Surprisingly, Dcx-/y (hemizygote) mice have very mild phenotypes47, with a cortical lamination indistinguishable from wildtype; only a double knock-out of DCX and DCLK1 gives rise to a disorganized cortex48. Stronger phenotypes were observed with shRNA-mediated knock-down of DCX in rat embryos, which resulted in severe cortical migration defects49. This RNAi approach was shown to have significant off-target effects mediated by the miRNA let7 50, complicating the interpretation of shRNA-mediated knock-down experiments41. However, explants of neurons from Dcx-/y mice allowed for an initial characterization of cortical neuron behavior: DCX knock-out disrupted the movement of the nucleus (nucleokinesis) and increased neurite branching51,52. So far, these phenotypes have not been linked to a clear mechanism of microtubule regulation. Basic cellular data on microtubules remains unmeasured, such as whether microtubule dynamics and intracellular trafficking are perturbed in non-shRNA-mediated neuronal models.
To better understand DCX’s role in neuronal migration, we took advantage of human induced pluripotent stem cells (iPSCs) and CRISPR/Cas9 gene editing. Human iPSC models have the following advantages: (1) human-specific genotypes, which are significant in the context of cortical development53; (2) relatively simple knock-in of patient mutations in an isogenic background; and (3) direct observation of neurons immediately after terminal differentiation as opposed to harvesting and plating of neurons from E18 rodent brains. Thus, we generated a series of DCX-KO and patient mutant iPSC models.
In our iPSC models, DCX-KO neurons showed impaired nucleokinesis and increased neurite branching, consistent with phenotypes in Dcx-/y rodent studies51,52. Using our DCX-KO model, we find that excessive branching is the result of higher rates of branch initiation, a pathway known to involve severing enzymes and structural MAPs. In addition, the processivity of lysosomes is impaired in DCX-KO neurons, linking DCX directly to intracellular trafficking by kinesins and dynein. These diverse phenotypes involve multiple protein networks, suggesting disruption of an upstream regulator of the microtubule network. While searching for a common mechanism, we made the unexpected observation that DCX-KO neurons exhibit a significant reduction in α-tubulin polyglutamylation, one of the central PTMs of the neuronal tubulin code. Expressing exogenous DCX in DCX-KO neurons was sufficient to rescue polyglutamylation levels and to restrict neurite branching back to control. Rescue was also achieved by over-expression of TTLL11, an α-tubulin specific polyglutamylase, or by driving polyglutamylation with paclitaxel. Using U2OS cells as an alternate model, we established that co-expression of DCX and TTLL11 was sufficient to create a significant, synergistic increase in polyglutamylation. Finally, we confirmed that polyglutamylation levels are reduced in 6 patient mutant knock-in neuronal models. Our results on DCX and polyglutamylation demonstrate that MAPs not only “read” the tubulin code but also regulate it.
Results
Nuclear mobility is impaired in DCX-KO cortical neurons
To understand the role of DCX in regulating the neuronal cytoskeleton, we used CRISPR/Cas9 genome editing to knock-out the expression of DCX in a male iPSC line. We designed a gRNA to target a sequence upstream of the first microtubule binding domain DC1, resulting in multiple iPSC clones expressing only N-terminal fragments (Fig. 1A and Fig. S1A, B). We confirmed DCX knock-out by Western blots and immunocytochemistry using antibodies against DCX’s C-terminal domain (Fig. 1B, C and Fig. S1C). The morphology of our DCX-KO cells was indistinguishable from control cells at the iPSC stage as well as after induction to neural progenitor cells (NPCs). Terminal differentiation of DCX-KO NPCs into cortical neurons was confirmed by immunocytochemistry and Western blots against β3-tubulin (Tubb3), a neuron-specific isotype (Fig. 1C and Fig. S1D). At DIV3 (3 days in terminal differentiation medium), iPSC-derived cortical neurons showed a bipolar morphology reminiscent of migrating neurons, with two or more slender processes that extended from the cell body2,3.
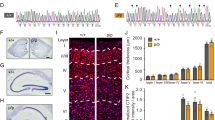
A DCX contains two microtubule binding domains, DC1 and DC2. DCX-KO cells were obtained by indels introduced in Dcx’s gene sequence before DC1. CTRL0 and DCXKO1 amino acid sequences are shown from residue 41–60. The asterisk shows the indel location and the resulting amino acid sequence in DCXKO1 (cyan). B Immunoblots representative of 3 experiments, showing CTRL0 and DCXKO1 neuron lysates at 3 days of differentiation (DIV3), stained with antibodies against β3-Tubulin (Tubb3) and DCX. C CTRL0 and DCXKO1 neurons representative of 4 experiments stained for Tubb3 and DCX at DIV3. D (top panel) Still images of live neurons with a nuclear dye. Nuclei detected by Trackmate are circled (light blue), and their trajectories over the 8 h period of imaging are shown (gray). (Bottom panel) Position plots showing a sample of trajectories extracted from CTRL0 and DCXKO1 nuclei tracking. The first timepoint of all tracks is (0,0). Tracks are color-coded according to the displacement of the nucleus. Distribution of displacement for both genotypes are shown on the right end side of the panel. E Schematics showing the displacement, distance traveled, and directionality index parameters. F Distribution of mean speed achieved by CTRL and DCXKO nuclei during the imaging period. G Distribution of directionality index or efficiency of the trajectory for CTRL and DCXKO nuclei. Higher directionality indexes indicate more unidirectional movements. H Distribution of distance traveled by CTRL and DCXKO nuclei during the imaging period. For (F–H) Violin plots are split between the two control (CTRL0, light gray, and CTRL1, dark gray) and two DCXKO (DCXKO1, cyan, and DCXKO4, dark cyan) lines used in this study. These three plots were cut-off to better show the main population, but the maximum values for distance traveled, mean speed, and directionality index are 497 µm, 2.7 µm/min and 1 respectively. n = 21826 tracks for CTRL0, 24801 tracks for CTRL1, 6461 tracks for DCXKO1 and 14,837 tracks for DCXKO4. Each line was used in 3 experiments, tracks with less than 30 min of imaging time were excluded from the dataset. Statistical analysis was performed using the Kruskal-Wallis non parametric H test: ***p < 0.001. p-values are detailed in Supplementary Table 1.
To determine if DCX-KO cortical neurons show phenotypes consistent with lissencephaly, we first measured the translocation of nuclei in control and DCX-KO cells using automated tracking algorithms (Fig. 1D–H, Fig. S2, and Supplementary Movies 1–2). Representative images of nuclei and their tracks are shown in Fig. 1D (top panel), above 2-dimensional plots of nuclear position (n ≅ 2800 tracks) over an 8 h observation window. These tracks showed a broad distribution of nuclear displacements (distance between first and last coordinates, Fig. 1E) in all directions, as some nuclei move significantly more than others of the same genotype. Despite this heterogeneity, DCX-KO neurons have a greater proportion of small displacements (<10 µm) while control neurons have more large displacements (>10 µm) (Fig. 1D, right panel and Fig. S2A, right panel). To mitigate the effect of false-positive tracks from our algorithm, only tracks that stayed visible for ≥6 frames (30 min) through the whole imaging period were analyzed (Fig. 1F–H and Fig. S2B, C). The nuclear movements were also slower. In control neurons, the nuclei had a mean speed of 0.27 ± 0.01 µm/min on average over the observation window (Fig. 1F). In contrast, in DCX-KO neurons, the nuclei had a significantly reduced speed of 0.17 ± 0.01 µm/min on average over the same window (p < 0.0001, Fig. 1F). Moreover, we measured the maximum speed, or the fastest segment of each nucleus track (Fig. S2B). DCX-KO nuclei had reduced maximum speeds compared to control nuclei, suggesting a generalized decrease in speed as opposed to increased pausing. In parallel, we measured a “directionality index”, which is the ratio between displacement and distance traveled, or the efficiency of the movement (Fig. 1E, G). A perfectly straight trajectory would result in a directionality index of 1. The directionality index for both genotypes are relatively low as the direction of movement is random and not chemotactic or durotactic. However, DCX-KO neurons have a reduced directionality index compared to control (DCX-KO 0.20 ± 0.01 vs control 0.26 ± 0.01, p < 0.0001), implying that they are 20% less efficient in their displacement relative to control neurons (Fig. 1G). This inefficiency causes DCX-KO nuclei to travel shorter distances (Fig. 1H, control 61.5 ± 0.2 µm vs DCX-KO 48.1 ± 0.3 µm, p < 0.0001). We conclude that DCX-KO nuclei are slower, cover less distance, and are less directional, consistent with the nucleokinesis defects observed in Dcx-/y explant neurons51,52,54.
DCX-KO neurons establish more branches through increased rates of initiation
Nucleokinesis is one of two steps in the process of neuronal migration; the other step being neurite extension55,56,57. As expected, DCX is enriched at neurite tips in our cell model (Fig. S4E), so we wondered if a defect in this compartment could also contribute to defects in neuronal migration. To test this idea, we imaged individual neurites using either phase contrast microscopy or fluorescence microscopy with live cell fluorescent labels for microtubules and actin filaments (SiR-Tubulin and CellMask Actin, respectively). As shown in Fig. 2A–C (Supplementary Movies 3-6), we observed the canonical sequence of events58,59: (A) first, neurites were initiated when a transient burst of actin polymerization was stabilized by microtubules; (B) the initiated neurite elongated at a relatively consistent velocity; (C) in most cases, the neurite later retracted. The balance of initiation and retraction events determines the net increase in neurite number per cell. We measured no significant change between control and DCX-KO cells in the rates of elongation or shrinkage (Fig. 2D, E); rather, only initiation and retraction events were affected. In DCX-KO neurons, the number of initiation events increased (Fig. 2G), as did the number of retraction events, albeit to a lesser extent (Fig. 2F), resulting in an imbalance relative to controls. We measured a 2-fold increase in neurite formation (number of neurite initiations minus number of neurite retractions) per cell in DCX-KO neurons (Fig. 2H). We confirmed that DCX-KO increases neurite number using both types of live-cell imaging as well as direct counting of neurites in immunocytochemistry of fixed neurons (Fig. 2H, J). Figure 2D–H report the results from phase contrast microscopy of unlabeled cells to avoid any confounding effects of the fluorescent reagents. To account for variations in cell developmental stage, we also measured the neurite density, or the number of neurites divided by the total length of the cell’s neurites (Fig. 2I and Fig. S3) and found again a significant increase in DCX-KO neurons. We conclude that DCX plays a significant role in restricting neurite formation51,60,61. Cortical neurons with an excessive number of neurites may struggle to migrate, because each branch pulls the cell body in a different direction62,63,64. These results are therefore consistent with previously-published hypotheses that excessive branching may contribute to the neuronal migration defects observed in patients with X-linked lissencephaly and double cortex syndrome52.
Images representative of 3 experiments, showing a neurite initiation (A), growth/shrinkage (B) and retraction (C) events in a CTRL0 neuron recorded after incubation with tubulin and actin binding dyes: SiR-tubulin (green) and Cellmask actin (magenta). Yellow arrowheads show actin protrusions where a new neurite is initiated. cyan arrowheads show the remains of a neurite retracting completely. Yellow arrows show growth events and cyan arrows show retraction events. These events were used for calculation of growth and retraction speed (D, E), by measuring neurite length and lifetime on kymographs. An example kymograph is shown in the bottom panel in (B) with brightfield and fluorescence imaging. D Distribution of neurite growth rate in CTRL0 and DCXKO1 neurons. E Distribution of neurite retraction rate in CTRL0 and DCXKO1 neurons. For (D, E), n = 26 CTRL0 and 38 DCXKO1 events, from 2 independent experiments. Statistical analysis was performed using a Mann-Whitney U test: ns, nonsignificant. F Distribution of the number of neurite retraction events/min seen in CTRL0 and DCXKO1 neurons. Cells showing no retraction events were included in the analysis, but the points were not represented on the plot. G Distribution of the number of neurite initiation events/minute per neuron in CTRL0 and DCXKO1 neurons. Cells showing no initiation events were included in the analysis, but the points were not represented on the plot. H Number of neurites found per neuron in CTRL0 and DCXKO1 neurons. For (F–H), n = 68 CTRL0 and 93 DCXKO1 neurons from 2 independent experiments. Statistical analysis was performed using a Mann-Whitney U test: ns nonsignificant, *p < 0.05, ***p < 0.001. I Distribution of neurite density per neuron for CTRL0 and DCXKO1 neurons in fixed samples. J Distribution of neurite number per neuron for CTRL0 and DCXKO1 neurons in fixed samples. For (I, J), n = 323 CTRL0 and 351 DCXKO1 neurons from 3 independent experiments. Statistical analysis was performed using a Mann-Whitney U test: ***p < 0.001. p-values are detailed in Supplementary Table 1.
Microtubule dynamics are unaffected in DCX-KO neurons
What could cause the increase in neurite branching in DCX-KO neurons? Microtubule dynamics play a major role in branching via the polymerization of microtubules into nascent branches65,66,67. Hence, one plausible explanation for the branching phenotype is altered microtubule dynamics, whereby increased microtubule lifetimes/numbers in DCX-KO neurons stabilize a greater percentage of the transient bursts of actin polymerization. In reconstitution assays, DCX promotes microtubule nucleation29,39, reduces the microtubule catastrophe frequency38, and slows their post-catastrophe shrinkage37. But DCX appears to be excluded from microtubule plus ends by end-binding proteins in cell lines68, so the potential impact of DCX-KO on microtubule dynamics in neurons is difficult to predict. As a first approach to look for changes in the microtubule cytoskeleton in DCX-KO neurons, we stained for α-tubulin (DM1A) and the neuronal specific Tubb3, as markers of the total microtubule population (Fig. 3A and S4A, B). We found that DCX-KO neurons have equivalent levels of total tubulin, albeit with a slight reduction in the intensity of Tubb3.
A (Top) Immunostaining representative of 3 experiments showing CTRL0 and DCXKO1 neurons at DIV3 with an α-Tubulin (DM1A) specific antibody, and (Bottom) mean intensity profile found in the distal tip of neurons from both genotypes, SEM are shown in lighter gray (CTRL0) and lighter cyan (DCXKO1). Images included in the analysis are from n = 135 CTRL0 neurites and n = 134 DCXKO1 neurites from 3 experiments. B Schematics of the experimental procedure and analysis method for measuring microtubule dynamics. NPCs are electroporated with the plus-tip marker EB3-mCherry, and neurons are imaged at DIV3. Each comet is assigned to a domain (Shaft or growth cone (GC)), and its characteristics are measured: length, lifetime, growth rate, and direction. The EB3 snapshot and kymograph image are representative of 4 similar experiments, (C) Distribution of growth rates for plus end out comets in shaft or growth cone of CTRL0 and DCXKO1 neurons. D Distribution of lifetimes for plus end out comets in shaft or growth cone of CTRL0 and DCXKO1 neurons. E Distribution of the proportion (%) of minus end out (retrograde) comets in the shaft and growth cone of CTRL0 and DCXKO1 neurons. For clarity reasons, datapoints at 0 are not shown, but are included in the analysis. For (C–E), n = 63 CTRL0 and n = 63 DCXKO1 cells from 4 experiments. Statistical analysis was performed using the Mann-Whitney non parametric U test: ns nonsignificant, *p < 0.05. p-values are detailed in Supplementary Table 1.
We then looked specifically for changes in microtubule dynamics. We observed dynamic microtubules by transient expression of the end-binding protein EB3-mCherry in DCX-KO neurons (Fig. 3B and Supplementary Movies 7–8). Since DCX is enriched in neurite tips (Fig. S4E), we measured neurite tips and shafts separately. However, regardless of region, we found no significant differences in EB3 comet dynamics between control and DCX-KO (Fig. 3C, D and Fig. S5), including in the densities of EB3 comets (0.40 ± 0.04 µm−2 control vs 0.44 ± 0.04 µm−2 DCXKO1, p = 0.43), their velocities (10.5 ± 0.3 µm/min control vs 10.0 ± 0.4 µm/min DCXKO1, p = 0.24), and lifetimes (GC: 13.1 ± 0.5 s control vs 12.5 ± 0.6 s DCXKO1, p = 0.18). The characteristics of EB3 comet dynamics in our iPSC-derived neurons were equivalent to what has been reported in other studies69,70. We did observe a slight increase in the proportion of retrograde comets, suggesting a minor role for DCX in the orientation of growing microtubules (Fig. 3E and Fig. S5E), consistent with recent cryo-electron tomography of microtubules in Dcx-/y mouse neurons71. We also noticed that the area covered by EB3-decorated microtubules was reduced in DCX-KO neurites, indicating that DCX may contribute to growth cone morphology (Fig. S5C). Taken together, we conclude that DCX does not regulate EB3 comet dynamics in our model. Rather, other factors determine the growth rates, lifetimes, and nucleation rates in neurons. DCX-KO does appear to increase the rate of post-catastrophe shrinkage46, which is consistent with reconstitution results37. However, faster shrinkage rates in DCX-KO may not be sufficient to explain the increase in branch initiation events reported here (Fig. 2G).
DCX-KO neurons show altered DCLK1 localization and lysosome motility
DCX has also been proposed to play a role in regulating intracellular trafficking, and dysfunctional cargo transport could be responsible for the observed excessive neurite branching. However, there is some ambiguity between observations made in vitro and in cells. For example, in vitro, DCX reduces the landing rate and run length of kinesin-1 motors but not kinesin-3 motors42, while in neurons, DCX shRNA caused reduced run lengths of kinesin-3 cargos41, a result that may be confounded by off-target RNAi effects50. In addition, DCX binds directly to dynein complexes and negatively regulates retrograde transport43. The absence of DCX in our DCX-KO neurons might also modify the localization or behavior of other MAPs involved in the regulation of trafficking, such as Tau72,73, MAP274, DCLK175, and others14,15,76. As an example of this competition, DCX and Tau were shown to bind the microtubule lattice in a mutually exclusive manner in non-neuronal cell lines68 and in rat neurons45,68.
To determine how intracellular trafficking may be directly or indirectly impacted by DCX-KO, we measured the intensity profile of several molecular motors and MAPs in control and DCX-KO neurons by immunocytochemistry (Fig. 4A, B and Fig. S6). To maximize signal, we did not use detergent to extract the cytoplasmic pool of proteins (See “Methods”; bound proteins only, Fig. S6A) and detect both microtubule-bound and unbound proteins. The intensity of polarity markers MAP2 and Tau did not change in DCX-KO neurons, perhaps because they are not strongly expressed in neurons at this early pre-polarization stage69. We found an increased signal by 1.4-fold for DCLK1 in DCX-KO neurons (Fig. 4A), which was not associated with higher expression levels (Fig. S6C). DCLK1 is enriched in the distal tip of neurites in our cell model (Fig. S6B), suggesting increased occupancy of the DCX binding site by its homolog48,77. Kinesin-1, kinesin-2, kinesin-3, and dynein intensities were not significantly modified in DCX-KO neurons (Fig. 4A and Fig. S6A). However, the lack of change in motor localization (Fig. S6B) does not exclude a defect in cargo trafficking.
A Compared mean intensity for MAP2, Tau, DCLK1, Kif1A, Kif3A, Kif5B, and Dynein in CTRL0 or DCXKO1 DIV3 immuno-stained neurons. The analysis pipeline is shown above the plot: a mask of the microtubules (MTs) was made, and the intensity of the marker was normalized to the intensity of the mask and to the control condition. Each transparent point represents a single image, while the opaque points show mean values, error bars represent the confidence intervals. Analyses are from n = 5, 5, 10, 5, 6,13, 6 CTRL0 images or 5, 5, 10, 5, 5, 13, 5 DCXKO1 images (MAP2, Tau, DCLK1, Kif1A, Kif3A, Kif5B, and Dynein respectively) from 2–4 experiments. Statistical analysis was performed using the Mann-Whitney non parametric U test: *p < 0.05. B Images representative of 3 experiments showing CTRL0 and DCXKO1 DIV3 neurons stained for DCLK1, Kif5B and Tubb3. C (Top) Representative still images chosen from 3 experiments showing CTRL0 and DCXKO1 straight neurite sections after incubation with the Lysotracker dye, and 2 min kymographs of those same sections. A = anterograde, and R = retrograde. (Bottom) Position plots for lysosomes detected using Trackmate during the 241 frames for CTRL0 and DCXKO1 neurons. The distribution of these positions was also plot and is represented on the right end side of the position plots. D Mean squared displacement (MSD) of lysosomes inside CTRL0 and DCXKO1 neurites. Data are presented as mean values ± SEM. E Proportion of tracked cargos that are either stationary (Stat.), Diffusive (Diff.) or Processive (Proc.). Data are presented as mean values ± SEM. Each point represents a single neuron. F Directional bias of lysosome cargos in CTRL0 and DCXKO1 neurons, where 0 corresponds to retrograde only and 1 corresponds to anterograde only. For D-F, n = 41 control and 30 DCXKO1 neurons from 3 independent experiments. Statistical analysis was performed using the Student’s t-test: ns nonsignificant, *p < 0.05. G Violin plots of the average velocity for anterograde and retrograde displacement of lysotracker cargos in CTRL0 and DCXKO1 DIV3 neurons. n = 1828 tracks for control and 1131 for DCXKO1 from 3 independent experiments. Statistical analysis was performed using the Student’s t-test: ns nonsignificant. p-values are detailed in Supplementary Table 2.
To test the role of DCX in regulating cargo trafficking, we measured the trajectories of lysosomes using the pH-sensitive Lysotracker dye (Supplementary Movies 9–10). Lysosomes move in a bidirectional manner using kinesin-1, kinesin-2, kinesin-3, and dynein motors, making them well-suited to assess broad trends in intracellular trafficking78,79. Figure 4C shows Lysotracker kymographs and extracted trajectories for control and DCX-KO neurons. In both cases, we observed bidirectional transport with similar distributions of trajectories (right panel). We characterized this transport further by measuring the mean-squared displacement (MSD) of lysosome position, radius of gyration (Rg), directional bias, and velocities as a function of time (Fig. 4D–G and S7). The Rg, MSD, and extracted α value (See “Methods”: slope of the MSD plot), which assess general motility of cargos, are significantly decreased in DCX-KO neurons (Fig. 4D and Fig. S7A, B), confirming the impact of the KO on intracellular trafficking. All the trajectories used for MSD assessment can be sorted as stationary, diffusive, or processive depending on their characteristics (Fig. 4E, see “Methods”). Interestingly, the proportion of processive motility is significantly reduced in DCX-KO neurons (control 31.0 ± 1.3 % vs DCX-KO 26.2 ± 1.3 %, p = 0.012, *) and correspondingly the fraction of diffusive (control 57.0 ± 1.0 % vs DCX-KO 59,8 ± 0.9 %, p = 0.053, ns) and stationary motility increased (control 11,9 ± 0.5 % vs DCX-KO 14,0 ± 0.6 %, p = 0.010, *). We noticed that lysosomes in DCX-KO neurons are more unidirectional than in control cells, as indicated by a bimodal directional bias (Fig. 4F). However, lysosome velocity was not affected in either the minus- or plus-end direction (Fig. 4G). Altogether, in DCX-KO neurons, lysosomes are less processive. These results support the principle that DCX-KO impacts intracellular trafficking, and a comprehensive analysis of neuronal cargos will be an important future direction of research. However, the defect in lysosome trafficking is mild and possibly insufficient to explain the excessive branching phenotype found in DCX-KO neurons (Fig. 2).
Thus far, our analysis of DCX-KO neurons has revealed complex and pleiotropic phenotypes, namely a slowed nucleokinesis, increased rate of branch initiation, increased proportion of misoriented microtubules, and reduction in lysosome processivity. These disparate phenotypes point to an upstream regulator of microtubule physiology: something which, if dysregulated, disrupts microtubule homeostasis at many levels. We hypothesized that this upstream regulator could be the tubulin code.
DCX-KO disrupts microtubule polyglutamylation
The tubulin code is an information system for microtubule specialization that contributes to the processivity of motor proteins21,80, binding affinity of MAPs81,82, and microtubule spacing and geometry83. For example, acetylated microtubules (Acetyl) are more flexible and predominantly bundled21,84,85. Recently, acetylation has been linked to neurite branching in a model of an α-tubulinopathy86. Tyrosinated microtubules (Tyr) act as preferred tracks for kinesin-3, kinesin-580,87, and dynein21,88; Polyglutamylation (polyE) and detyrosination (detyr) regulate MCAK and severing enzymes binding, that are responsible for modified branching patterns when depleted89,90 (reviewed in refs. 91,92). Our results pointed to many of these proteins and pathways. Therefore, we wondered if the tubulin code could be altered in DCX-KO neurons.
To test for changes in the tubulin code, we performed immunocytochemistry and Western blots in control and DCX-KO neurons, with antibodies against acetylation (611B1), tyrosination (YL1/2), detyrosination (detyr), initial glutamylation (GT335) and polyglutamylation (polyE) where glutamate side chains are reversibly added to the C-terminal tails of α- and β-tubulin93,94. (Figs. 5A, B and S8). GT335 recognizes all glutamate chains through the initial residue, while polyE detects glutamate chains of 4 residues or more95,96. Remarkably, we observed a dramatic reduction specifically in tubulin polyglutamylation by both Western blot and immuno-cytochemistry (polyE – Fig. 5A, B and Fig. S8). The lower polyglutamylation levels in DCX-KO neurons were maintained at later differentiation stages (up to DIV14, see Fig. S9). In addition, detection of all glutamate chains with GT335 in Western blots is mildly increased in DCX-KO neurons. We analyzed the subcellular distribution of staining in neurites and cell bodies (CB) and confirmed an increase in initial glutamylation specific to cell bodies and a drastic reduction in polyglutamylation only in neurites (Fig. 5C, D). In parallel, we observed that acetylation and detyrosination levels, which label long-lived microtubules, were unchanged in DCX-KO neurons compared to control, while tyrosination is mildly increased in cell bodies. These results indicate that DCX expression regulates the tubulin code, especially polyglutamylation levels in neurites. The reduced levels of polyglutamylation in DCX-KO will impact a broad spectrum of MAPs and motors.
A Compared mean intensity for polyglutamylation (polyE), glutamylation (GT335), tyrosination (Tyr), detyrosination (detyr), and acetylation (Acetyl) in CTRL0 or DCXKO1 DIV3 immunostained neurons. The analysis pipeline is shown above the plot: a mask of the microtubules (MTs) was made, and the intensity of the marker was normalized to the intensity of the mask and to the control condition. Each transparent point represents a single image, while the opaque points show mean values, error bars represent the confidence intervals. Plots are from n = 31, 9, 44, 12, 10 CTRL0 images or 21, 9, 39, 12, 10 DCXKO1 images (polyE, GT335, Tyr, detyr, Acetyl respectively) from at least 3 experiments. Statistical analysis was performed using the Mann-Whitney non parametric U test: *p < 0.05, ***p < 0.001. B Immunoblots representative of 3 experiments showing the relative post-translational modifications levels in CTRL1 and DCXKO4 DIV3 neurons. The same samples were loaded on different gels for each antibody. C Images representative of at least 3 experiments showing CTRL0 and DCXKO1 neurons stained for PTMs with the following antibodies: polyE, GT335, YL1/2, detyr, 611B1. D Intensity profiles for the PTMs levels in distal neurites and cell bodies (CB) of CTRL0 and DCXKO1 DIV3 neurons. Data are presented as mean values with error bars representing the SEM for neurites, and the confidence interval for CB. Plots are from n = 106, 48, 109, 96, 67 CTRL0 and 116, 105, 196, 122, 106 DCXKO1 neurites, and n = 66, 34, 90, 42, 23 CTRL0 and 45, 41, 69, 36, 33 DCXKO1 cell bodies (polyE, GT335, Tyr, detyr, and Acetyl respectively) from at least 3 experiments. Statistical analysis was performed using the Mann-Whitney non parametric U test: ns nonsignificant, **p < 0.01, ***p < 0.001. p-values are detailed in Supplementary Table 2.
DCX reduces neurite density by increasing tubulin polyglutamylation levels
We next asked if the regulation of PTM levels by DCX could result in the branching phenotype we have seen in DCX-KO neurons. To mechanistically link DCX and polyglutamylation, we tested whether the expression of DCX could rescue polyglutamylation levels and the branching phenotype in DCX-KO neurons. We electroporated a DCX-GFP construct in control and DCX-KO neurons and measured both the polyglutamylation (polyE) levels and neurite density (Fig. 6A–C and Fig. S10A). In DCX-KO neurons re-expressing DCX-GFP, we found that polyglutamylation levels were increased and neurite density was accordingly reduced to match control neurons. Surprisingly, the overexpression of DCX-GFP in control neurons reduced polyglutamylation levels and increased neurite density, similar to DCX-KO neurons (Fig. 6B, C). This result is consisent with overexpression experiments done in mouse models, which also led to an increase in neurite numbers97,98. Moreover, our data indicates that the effects of DCX protein concentrations are biphasic, where an intermediate protein concentration achieves healthy polyglutamylation levels and neurite densities, while reduced or elevated levels of DCX lead to dysregulation.
A Images representative of 3 experiments showing CTRL1 and DCXKO4 DIV3 neurons electroporated with DCX-GFP and stained for polyglutamylation. The arrowheads are showing the same cell in both channels (GFP, and polyE). B Violin plots showing the polyglutamylation (polyE) intensity normalized by Tubb3 intensity in CTRL and DCXKO DIV3 neurons with (+ or blue background) or without (− or white background) DCX-GFP electroporation. C Violin plot showing the neurite density in CTRL and DCXKO DIV3 neurons with or without DCX-GFP electroporation. For (B, C), the analysis included n = 97 CTRL0 (light gray), 178 CTRL1 (dark gray), 225 DCXKO1 (cyan), 171 DCXKO4 (dark cyan), 11 CTRL0 + DCX-GFP, 34 CTRL1 + DCX-GFP, 38 DCXKO1 + DCX-GFP, and 45 DCXKO4 + DCX-GFP neurons from 3 experiments. These two plots were cut-off to better show the main population, but the maximum values for polyE intensity, and neurite density are 9.4 (arb. units), 0.156 neurite/µm respectively. Statistical analysis was performed using the Mann-Whitney non parametric U test: ns nonsignificant, **p < 0.01, ***p < 0.001. p-values are detailed in Supplementary Table 3. D Images representative of 2 experiments showing CTRL0 and DCXKO1 DIV3 neurons stained for polyglutamylation (polyE) after 48 h in regular media or 48 h in media supplemented with 100 nM paclitaxel. E Violin plots showing the polyglutamylation (polyE) intensity in CTRL0 and DCXKO1 DIV3 neurons with (+ or green background) or without (− or white background) treatment with paclitaxel. F Violin plots showing the neurite density in CTRL0 and DCXKO1 DIV3 neurons with or without treatment with paclitaxel. For (E, F), images included in the analysis are from n = 84 CTRL0, 172 DCXKO1, 120 CTRL0+paclitaxel and 160 DCXKO1 + paclitaxel neurons from 2 experiments. Statistical analysis was performed using the Mann-Whitney non parametric U test: *p < 0.05, ***p < 0.001. p-values are detailed in Supplementary Table 3.
Chemical induction of polyglutamylation by paclitaxel restricts neurite density
As our next attempt to rescue polyglutamylation levels and neurite density, we treated cells with paclitaxel, a well-known chemotherapy drug, that stabilizes microtubules99. Low doses of paclitaxel were previously shown to increase PTM levels by promoting detyrosination, acetylation, and polyglutamylation100. We therefore applied paclitaxel (100 nM) for 48 h to neurons and measured their polyglutamylation levels and neurite density (Fig. 6D–F and Fig. S10B–D). Neurons treated with paclitaxel showed an increase in polyglutamylation levels in their branches independent of their genotype (Fig. 6E). Polyglutamylation was increased by ~1.2-fold in control cells and by 1.6-fold in DCX-KO cells. In parallel to these changes in polyglutamylation levels, neurite density decreased by about 20% in paclitaxel-treated cells (Fig. 6F), indicating a correlation between polyglutamylation levels and neurite density. However, because paclitaxel affects multiple PTMs and causes lattice expansion101,102, we sought a more targeted approach to rescue polyglutamylation in our DCX-KO system.
Expression of the α-tubulin elongase TTLL11 in DCX-KO neurons rescues neurite density
Polyglutamylation levels are determined by modifying enzymes, and we hypothesized that the transient expression of a glutamylating enzyme could also rescue polyglutamylation levels together with neurite density in our model. A family of 9 glutamylases (Tubulin Tyrosine Ligase Like, or TTLLs)103,104 competes against a family of 6 deglutamylases (Cytosolic CarboxyPeptidase, or CCPs)105 to create glutamate chains of variable length and structure92. The members of each family have specialized roles, such as branch initiation (e.g., TTLL4, TTLL7) versus elongation (e.g., TTLL6, TTLL11)106, or shortening (e.g., CCP1, CCP6) versus branch removal (CCP5)105. Additionally, α-tubulin and β-tubulin polyglutamylation are distinct107,108,109, with elongase TTLL’s showing preference for α-tubulin. We confirmed that our neurons show exclusively α-tubulin polyglutamylation (Fig. S11A), consistent with previous studies of early differentiated neurons110. Thus, we choose to express an α-tubulin specific elongase, namely TTLL11. As one of the main autonomous enzyme expressed in neurons111, TTLL11 is able to elongate pre-existing α-tubulin glutamate chains104.
To enhance our measurements of TTLL11-mRuby expression in neurons after fixation and staining, we modified our electroporation protocol to include a sorting step (See “Methods” and Fig. S11B). Figure 7A shows representative images of polyglutamylation levels in control and DCX-KO neurons with (mRuby +) or without (mRuby −) TTLL11-mRuby expression. In both control and DCX-KO neurons expressing TTLL11-mRuby, polyglutamylation levels increased about 5-fold (Fig. 7B and Fig. S11E, F). In parallel, we found that the expression of TTLL11 in DCX-KO neurons rescued neurite density back to control levels (Fig. 7C and Fig. S11C, D). These results match DCX expression and paclitaxel treatments in neurons (Fig. 6), where DCX is sufficient but not required to control polyglutamylation levels. We can, therefore, confirm that DCX restricts neurite branching through regulation of polyglutamylation levels.
A Images representative of 6 experiments showing CTRL1 and DCXKO4 DIV3 neurons control (mRuby-) or electroporated with TTLL11-mRuby (mRuby +) and stained for polyglutamylation (polyE). B Violin plots showing the polyglutamylation (polyE) intensity in CTRL1 and DCXKO DIV3 neurons with (+ or purple background) or without (− or white background) TTLL11-mRuby electroporation. DCXKO1 and DCXKO4 are shown as split violins, with DCXKO1 in cyan (top) and DCXKO4 in dark cyan (bottom). C Violin plot showing the neurite density in CTRL1 and DCXKO DIV3 neurons with or without TTLL11-mRuby electroporation. For B-C, images included in the analysis are from n = 372 CTRL1 (dark gray), 380 DCXKO1 (cyan), 654 DCXKO4 (dark cyan), 199 CTRL1 + TTLL11-mRuby, 186 DCXKO1 + TTLL11-mRuby and 408 DCXKO4 + TTLL11-mRuby neurons from 6 independent experiments. These two plots were cut-off to better show the main population, but the maximum values for polyE intensity, and neurite density are 91.6 (arb. units), 0.143 neurite/µm respectively. Statistical analysis was performed using the Mann-Whitney non parametric U test: ns nonsignificant, ***p < 0.001. p-values are detailed in Supplementary Table 4. D Images representative of 3 experiments showing U2OS cells transfected respectively with DCX-GFP (green), TTLL11-mRuby (magenta) or DCX-GFP and TTLL11-mRuby together. All conditions were fixed and stained for polyglutamylation (polyE). E Violin plots showing the quantification of polyglutamylation (polyE) levels in U2OS transfected cells as in (D). Images included in the analysis are from n = 27 control (untransfected), 58 DCX-GFP, 46 TTLL11-mRuby, and 95 DCX + TTLL11 cells from 3 experiments. This plot was cut-off to better show the main population, but the maximum values for polyE intensity is 2.46 (arb. units). Statistical analysis was performed using the Mann-Whitney non parametric U test: *p < 0.05, ***p < 0.001. p-values are detailed in Supplementary Table 4. F Immunoblot representative of 3 experiments showing U2OS cells untransfected or transfected respectively with DCX-GFP, TTLL11-mRuby or DCX + TTLL11, and stained for polyglutamylation (polyE) and tubulin (DM1A). DCX and TTLL11 transfections were visually checked before lysis of the samples. The same samples were loaded on multiple gels for each antibody.
DCX and TTLL11 synergize to regulate polyglutamylation levels
The rescue of DCX-KO phenotypes by TTLL11 expression caused us to wonder if DCX is able to regulate polyglutamylation by direct activation of TTLL11. To better understand the relationship between these two proteins, we used U2OS cells as a simplified model system. As with other cancer lines, U2OS cells have low TTLL expression and thus minimal polyglutamylation levels at interphase95,104,112. In addition, U2OS cells do not express DCX, which is specific to developing neurons. The use of this model allowed us to isolate the effect of DCX and TTLL11 expression on polyglutamylation levels. As illustrated in Fig. 7D, U2OS cells were transfected either with DCX-GFP (green), TTLL11-mRuby (magenta) or both constructs together, and their polyglutamylation levels were measured by immunocytochemistry and Western blots (Fig. 7D–F and Fig. S12). Both methods revealed equivalent results: untransfected cells had no detectable polyE signal, while cells expressing DCX, TTLL11, or both showed different degrees of polyglutamylation (Fig. 7E, F and Fig. S12C), with signal clearly localizing to microtubules (Fig. 7D). DCX expressing cells had 12 times more polyglutamylation than control: DCX is not a glutamylase, so its expression alone likely affects the modifying enzymes naturally present in the cells to trigger polyglutamylation. TTLL11 expressing cells had 36 times more polyglutamylation than control: the expression of TTLL11 alone is sufficient to shift the balance of modifying enzymes, as in our neuronal model. In coexpressing cells, only lower levels of DCX and TTLL11 expression were achieved (35% less DCX and 30% less TTLL11, Fig. S12A). However, the polyglutamylation produced by co-expression was 53 times more than control (Fig. 7E). Thus, polyglutamylation levels are 60% higher than expected from the added activity of DCX and TTLL11 (Fig. S12B). These results show that DCX and TTLL11 work synergistically to control polyglutamylation in U2OS cells. We found similar results in neurons, as polyglutamylation levels were 40% higher than expected from the addition of DCX-induced and TTLL11-induced glutamylase activity (Fig. 6B). Altogether, these experiments demonstrate DCX as a regulator of neurite density through control of tubulin polyglutamylation, and more specifically activation of glutamylases like TTLL11.
DCX patient-mutant neurons have reduced polyglutamylation levels
To test whether polyglutamylation is perturbed by mutations in DCX, rather than gene knockout, we used our CRISPR/Cas9 approach to introduce point mutations into DCX found in patients with X-linked lissencephaly. We used previously characterized missense mutations located in both domains of DCX: S47R, Y64N, D86H in DC1 and R178L, P191R, and R192W in DC236,113,114. We assessed polyglutamylation levels in neurons derived from these isogenic mutated iPSC lines by Western blot and immunocytochemistry (Fig. 8A, B). Remarkably, all patient mutations tested resulted in reduced polyglutamylation levels independent of the mutation locus. Overall, these findings demonstrate a central role for DCX in regulating polyglutamylation levels and thus MAP affinity and microtubule organization.
A Violin plots showing the polyglutamylation (polyE) intensity in stained DIV3 neurons carrying the following DCX patient mutations: S47R, Y64N, D86H, R178L, P191R, R192W, and compared to CTRL0 and DCXKO1. Images included in the analysis are from n = 384 CTRL0, 314 D86H, 177 DCXKO1, 323 P191R, 260 R178L, 287 R192W, 215 S47R, 289 Y64N neurons from 3 experiments. Statistical analysis was performed using the Mann-Whitney non-parametric U test: ***p < 0.001. p-values are detailed in Supplementary Table 4. B Immunoblots representative of 3 experiments, showing polyglutamylation levels in CTRL0, DCXKO1 and mutant DIV3 neurons (DC1: S47R, Y64N, D86H, DC2: R178L, P191R, R192W). The same samples were loaded on different gels for both antibodies. C Control neurons have high polyglutamylation levels. In this context, DCX activates TTLLs leading to two molecular phenotypes in DCX-KO neurons: a decrease in polyglutamylation of the microtubule lattice and an impeded cargo motility. The addition or association of these two mechanisms results in an excessive production of neurite and a decrease in migration speed and efficacy in DCX-KO and mutated neurons.
Discussion
In summary, using a DCX knockout iPSC model, an orthogonal U2OS system, and iPSC models with disease mutations, we show that DCX regulates α-tubulin polyglutamylation to restrict neurite branching. We believe these results provide insight into the cellular basis of double-cortex syndrome and X-linked lissencephaly. DCX-KO neurons showed pleiotropic defects, including meandering nucleokinesis (Fig. 1), excessive branch initiation (Fig. 2), and impaired lysosome trafficking (Fig. 4), but without changes in EB3 comet dynamics (Fig. 3). Rather, our results indicated that these pleiotropic defects originate from a strong reduction in α-tubulin polyglutamylation levels (Fig. 5), an unexpected finding not predicted in the literature on DCX (Fig. 8). Both polyglutamylation levels and neurite density were rescued by DCX expression, by paclitaxel treatment (Fig. 6), and by TTLL11 expression (Fig. 7), indicating a robust connection between DCX and the tubulin code. These results contribute to a growing body of evidence on the importance of polyglutamylation for morphology in many types of neurons115,116,117.
How could DCX help maintain a healthy level of polyglutamylation in developing neurites? Polyglutamylation is a complex, reversible, and heterogenous feature of neuronal microtubules. The patterns of polyglutamylation are generated by the competition between glutamylases (TTLLs) and deglutamylases (CCPs), proteins whose regulation is essential for neuronal health. Excessive polyglutamylation upon loss of CCP activity causes neurodegeneration12,118, while our results link reduced polyglutamylation to a neuronal migration disease. DCX could contribute to a healthy level between these extremes by stimulating the activity of TTLL’s (more specifically, α-tubulin elongases) or by reducing the activity of CCP’s. At present, our data provides evidence for a model based on TTLL activation, as DCX and TTLL11 co-expression caused a synergistic increase in polyglutamylation levels in U2OS cells (Fig. 7D–F). However, it is important to note that we cannot yet rule out inhibition of CCP’s, which could occur concurrently. Because mono-glutamylation is preserved in our model (Fig. 5D), the relevant CCP’s would be chain-shortening enzymes (CCP1, CCP2, CCP3, CCP4, CCP6), not de-branching enzymes (CCP5). In either case, regulation of long α-tubulin polyglutamate chains (as detected by polyE antibodies) explains how DCX restricts the number of neurite branches (Fig. 8C). Interestingly, it has been shown previously that TTLLs are present at low levels at this stage of maturation69 have low microtubule affinity104, and are enriched mainly in the cell bodies of neurons119 rather than the neurites. Thus, neurites appear to establish healthy polyglutamylation levels using a relatively small number of polyglutamylases. In this context, DCX may play a key role as a positive activator of TTLL-microtubule interactions.
We can imagine two mechanisms by which DCX could regulate TTLLs and cause, e.g., the synergistic effect observed in U2OS cells. First, DCX could directly bind the TTLL enzymes to bring them to the microtubule lattice, e.g., by direct recruitment of the conserved cationic microtubule-binding domain of TTLLs120. The cationic domain of the TTLL6 bound to the α-tubulin tail shows a helix extending into the interprotofilament groove121, which the authors note is near the binding site of DCX, EB proteins, as well as kinetochore components. Second, DCX could indirectly affect the TTLL enzymes by changing the structural feature(s) of αβ-tubulins in the lattice, in other words by altering the enzymes’ substrate in a way that accelerates the enzyme’s kinetics. Along these lines, recent work has linked other PTMs to the structural feature of “lattice spacing”, or the measured dimer rise of the microtubule lattice, which varies between 81–84 Å in cryo-electron microscopy122,123. More specifically, the detyrosination complex VASH/SVBP is more active on expanded, GTP-like microtubules lattice124, as is the acetyltransferase αTAT1125. A cognate model based on “altered substrates” makes sense for DCX, given DCX’s sensitivity to structural features such as lattice curvature36, protofilament number29,39, and lattice spacing35. However, an altered substrate model is not incompatible with direct recruitment, and detailed biophysical analysis will be needed to define these models and distinguish between them.
Our observation that DCX regulates polyglutamylation to restrict neurite branching (Figs. 6–7) harmonizes with many recently-discovered links between MAPs involved in branching pathways and the tubulin code. Consider the severing enzymes, spastin and katanin126. The severing rate of these enzymes is increased by long polyglutamate chains96 and sensitive to both α-tail and β-tail modifications127. Reconstitution experiments indicated that the severing rate first increases as glutamate chains get longer but then decreases beyond some optimal chain length in vitro90,96. Severing enzymes were originally proposed to fragment the microtubule network at branch points128, such that the proliferation of short microtubules would stabilize transient actin protrusions129. But fragmentation is not the only outcome of severing enzyme activity in the presence of soluble tubulin130; rather, severing enzymes create holes and defects in the lattice that are subsequently repaired by soluble GTP-tubulin. This process of microtubule “self-repair”131 may refresh the lattice with respect to nucleotide state, PTMs, etc. Whether via fragmentation or damage, severing enzymes will be impacted by the reduction in polyglutamylation in DCX-KO neurons. A link between microtubule damage and DCX is hinted at by cryo-electron tomography of microtubules in Dcx-/y mouse neurons, in which the number of large lattice defects increased relative to control71. To further complicate the picture, severing enzymes are inhibited by structural MAPs such as tau76,132, and tau’s envelope formation on microtubules132 is increased by polyglutamylation127. The dynamics of this complex multi-protein system will be exciting to measure and to model.
Healthy polyglutamylation levels are important for motor proteins80,100, but DCX also regulates motor proteins directly, potentially having complex effects. In reconstitution assays, kinesin-1 has a reduced run length on polyglutamylated microtubules127, and DCX-coated microtubules reduce kinesin-1’s landing rate42. Similarly, the run length of kinesin-3 is increased on polyglutamylated microtubules87, while DCX-coated microtubules do not affect kinesin-3 motility in vitro42. Both kinesin-1 and kinesin-3 are present on lysosomes133, and our results indicate that DCX-KO primarly affects the fraction of lysosomes which are stationary versus processive. Other cargos regulated by DCX-family proteins are synaptophysin48, Vamp241, dense-core vesicles75, and TrkB43. The phenotypes of disrupted intracellular trafficking will need to be explained in light of both direct interactions between DCX and motor proteins41,43 as well as changes in polyglutamylation, and polyglutamylation’s effects on affinity of the other MAPs like tau127 (Fig. 8).
Our study did not address a potential role for DCX in the regulation of the actin cytoskeleton. The data on DCX and actin are mixed. DCX has been proposed to modulate actin through interactions with spinophilin, an actin-binding protein134. Dcx-/y neurons showed reduced actin staining in some experiments135, but this result was not reproduced in recent cryo-ET71; if anything, our DCX-KO neurons show increased actin staining (Fig. S4C, D). In any case, our results on polyglutamylation do not exclude a link between DCX and actin. Indeed, given the complexity of MT-actin crosstalk55, it’s likely that the tubulin code impacts the actin cytoskeleton as well. We look forward to exploring these links in the future.
Sadly for us, our results argue against a role for DCX in the regulation of microtubule dynamics, which we and others had proposed based on DCX’s potent nucleation and anti-catastrophe activity in reconstitution assays29,39. Rather, we only detected a minor misorientation of EB comets in our DCX-KO neurons (Fig. 3). The lack of change in EB comet behavior suggests that EB proteins outcompete DCX for their shared plus-end binding sites, e.g., via faster association kinetics136, differences in binding site preferences68, or exclusion of DCX from EB condensates137.
DCX also shares its binding site with its close relative, DCLK1. Both proteins regulate neuronal migration and morphology138,139, and double-KO mice have severe defects in brain development48. Hence, DCLK1 might compensate for DCX-KO or interact differently with patient-mutant DCX. The kinase domain of DCLK1 is auto-regulatory140, effectively tuning the affinity of DCLK1 for microtubules, which may impact DCX’s affinity as well. Understanding the overlapping and independent roles of these two proteins is an important area of future research, including but not limited to their roles in the regulation of the tubulin code.
The PTMs of the tubulin code are frequently correlated; e.g., multiple PTMs are increased simultaneously by paclitaxel100, perhaps because stabilized microtubules have more time to accumulate PTMs. However, polyglutamylated microtubules specifically enhance detyrosination141, indicating a more specific correlation, perhaps depending on the “lattice spacing” of the microtubule lattice124. Our work demonstrates that one PTM can be significantly reduced (polyglutamylation) while others remain constant, indicating that PTMs can be individually regulated.
The identification of tubulin modifying enzymes has enabled significant progress in our understanding of the “tubulin code”, but we do not fully understand how these enzymes are regulated. Our work has identified DCX as a key regulator of polyglutamylation, and more generally implicates MAPs as potential regulators of tubulin PTMs. MAPs that regulate the tubulin code may share DCX’s sensitivity to structural features of the microtubule lattice. If the tubulin code has “writers” (like TTLLs) and “readers” (like spastin and tau), then DCX acts as a positive activator of a “writer”, somewhat like a good editor.
Methods
iPSCs origin, maintenance, and differentiation
Our use of stem cells was approved by McGill’s Institutional Review Board under permit number A12-M38-17A. hiPSCs initial stocks were obtained from the C-BIGR repository at the Montreal Neurological Institute142. The AIW-002-02 line was generated from the PBMCs of an unaffected male donor with its informed consent. The cells were grown on Matrigel coated surfaces in mTeSR Plus media in feeder-free conditions, they were passaged using Gentle Cell dissociation reagent (GCDR) and supplemented with 10 μM Y-27362 for 24 h when required after thawing, gene-editing or low-density plating. hiPSCs were induced into Neuronal progenitor cells using Stemdiff Smadi induction media provided by StemCell Technologies. Briefly, the hiPSCs were seeded in a monolayer at high density and feeded with fresh induction media every other day for 6–12 days. Then the cells were dissociated using accutase and amplified on PO-laminin coated plates for an additional period of 10–15 days before cryopreservation or differentiation. Terminal differentiation into cortical neurons was performed using commercially available BrainPhys and supplemented as required by manufacturer’s guidelines. NPCs were seeded on PO-laminin coated dishes or coverslips at variable densities depending on the experiment and were differentiated for 3 days. Cell fixation was performed following143 methods: after 1,5 min incubation in extraction buffer when specified (PIPES 80 mM, EGTA 1 mM, MgCl2 7 mM, NaCl 150 mM, D-Glucose 5 mM, Triton X-100 0.3%, Glutaraldehyde 0.25%), PFA 4% warmed at 37 °C was applied to the cells for 15 min before rinsing and storage in PBS until further use.
CRISPR gene-edition
AIW-002-02 hiPSCs line was gene edited using CRISPR/Cas9 techniques. gRNAs for DCX Knock-In were designed using the CRISPRdirect online tool (https://crispr.dbcls.jp/). All the gRNA sequences used in this study are detailed in the Excel file Supplementary Data 1. They were ordered as ssDNA oligos (IDT) and cloned into the pX458 plasmid generously gifted by Feng Zhang (#48138), using the BBsI restriction site. The newly generated plasmids were transformed into Stbl3 strains and amplified according to nucleobond Maxiprep kit supplier’s recommendations. The actual gene edition was performed using electroporation, as follows: the hiPSCs were detached from the substrate using accutase, counted and about 5millions cells were resuspended in 100 μl of cold Internal Neuronal Buffer (KCl 135 mM, CaCl2 200 µM, MgCl2 2 mM, HEPES 10 mM, EGTA 5 mM). The cell suspension was added to 5 µg of gRNA/Cas9 plasmid with 30 µg of the corresponding single stranded template before application to a 100 μl BTX cuvette. The BTX Gemini X2 twinwave electroporator was used to deliver a single 10 ms pulse at 60 V. Warmed up mTeSR was then added on top of the electroporated cells before transfer of the cell suspension to a new matrigel coated dish with rock inhibitor supplemented mTeSR. Three days post-electroporation, the cells were again dissociated from the substrate using accutase and were passed through a 70 μm strainer before FACs-sorting for GFP positive cells. The cell sorting was performed in the Flow Cytometry Core Facility for flow cytometry and single cell analysis of the Life Science Complex and supported by funding from the Canadian Foundation for Innovation. The positive cells were seeded in individual wells of 96 well plates and left to grow for about 2 weeks. For proper recovery and growth of the cells, rock inhibitor or CloneR were kept in the mTeSR media for 24 to 72 h post-sorting. After sufficient growth of individual hiPSCs clones in the 96 well plates, the clones were collected and split in two for amplification and screening for indels in extracted genomic DNA. For screening, each clone’s genomic DNA was extracted in Quick Extract buffer, amplified by PCR (see Excel file Supplementary Data 1 for primer sequences) and depending on the profile of the PCR product and response to T7 assay, the clones were subjected to Sanger sequencing performed at Genome Québec. The gene edited iPSCs were tested for presence of pluripotency markers (OCT4, SSEA3), amplified, and cryopreserved in FBS-20%DMSO until further use for differentiation.
Neuronal cell preparation
Following fixation, the coverslips were quenched in NaBH4 at 1 mg/ml for 10 min, then they were subjected to permeabilization in PBS-Triton 0.5% for 10 min. Unspecific bindings were blocked by bathing the coverslips in PBS-Triton0.1%-BSA3% for 30 min before incubation with the primary antibodies (see Excel file Supplementary Data 1) overnight at 4 °C. Incubation with the secondary antibodies were done at room temperature for 1 h, before a DAPI stain and mounting of the coverslips with Fluorsave.
Transient expression of EB3-mCherry, DCX-GFP, or TTLL11-mRuby in neurons were performed using electroporation. Briefly, the NPCs were dissociated and about 0.5-1 million cells were mixed with 5 µg of plasmid in 200 μl of INB. The electroporation was performed with a 1 ms pulse at 350 V in 620 BTX cuvettes. The cells were then seeded on glass bottom dishes or coverslips and imaged in BrainPhys or fixed as previously described. When dyes were used to look at specific structures in live neurons, they were all diluted in warmed up BrainPhys and incubated for specified timing with the cells before removal and imaging: SiR-tubulin 100 nM 30 min, CellMask Green Actin 0.5X 30 min, Lysotracker 50 nM 10 min, Syto Nucleus Deep red 250 nM 30 min. For sorting of TTLL11 electroporated neurons, electroporation was performed similarly as described above, with 10 million cells and 12 µg of plasmid. After electroporation, the cells were seeded on regular plastic dishes and cultured in terminal differentiation media (BrainPhys) for 2 days. On the second day, the cells were collected using accutase, sorted at the FACs sorting facility based on their level of mRuby fluorescence, and subsequently plated again at ~15,000 cells/cm2 in BrainPhys media for one additional day. The cells were then fixed and stained for polyE and Tubb3 as described above.
TTLL11 plasmid generation
The human TTLL11 primary transcript (NCBI: NM_001139442) in frame with restriction sites at the N and C-terminal ends of the sequence, was synthesized by IDT after manual codon optimization (see Excel file Supplementary Data 1 for sequence). The gene was digested by HindIII and AgeI enzymes alongside a pCMV-mRuby plasmid generously gifted by Pr. Arnold Hayer. The digested TTLL11 gene was then ligated into the linearized mRuby plasmid. The validity of this construct was assessed by sequencing and expression in model cells. Moreover, polyglutamylation levels increased significantly upon expression, confirming the polyglutamylase activity of the expressed TTLL11.
Cell line culture, transfection
Human osteosarcoma cells (U2OS) were used as a secondary non-neuronal model system. The cells were grown at 37 °C and 5% CO2 in high glucose DMEM supplemented with 10% serum and 1% antibiotic. Once seeded at 40,000 cells/cm2 on coverslips, the cells were transfected with an equivalent amount of DNA: either 1.5 µg of TTLL11-mRuby, 1.5 µg of DCX-GFP, or 1.5 µg total of both plasmids, using the calcium phosphate method144. The cells were left to express 24 h before extraction (PIPES 80 mM, EGTA 1 mM, MgCl2 7 mM, NaCl 150 mM, D-Glucose 5 mM, Triton X-100 0.3%, Glutaraldehyde 0.25%) for 1 min 30 s and fixation for 10 min using cold methanol, or lysis (2X SDS loading buffer: 125 mM Tris-HCl pH 6.8, 20% glycerol, 0.04% Bromophenol blue, 4% SDS, 100 mM DTT). The coverslips were then stained for polyE as described above.
Western blots
The cells were lysed by incubation in cold RIPA or 2X SDS loading buffer (125 mM Tris-HCl pH 6.8, 20% glycerol, 0.04% Bromophenol blue, 4% SDS, 100 mM DTT) and were collected by scraping the plates. Lysates in RIPA buffer were supplemented with complete-T for long term storage. Before loading onto a 12% acrylamide gel (precasted, Genscript), the lysates in RIPA were mixed with 2X SDS loading buffer and all samples were sonicated, spun down, and boiled for 10 min at 95 °C. Electrophoresis was done in MOPS buffer at 160 constant voltage, then proteins were transferred to nitrocellulose membranes and blocked in 5% milk. Primary antibodies were diluted according to specific dilutions (see Excel file Supplementary Data 1) and incubated with the membrane at 4 °C overnight. The membranes were rinsed with TBST and incubated for 2 h with anti-HRP secondary antibodies. The proteins were revealed using ECL pico PLUS, and 2 s-30 min membrane exposure to Diamed films. Since DCX, native tubulin, and modified tubulin have similar molecular weight, all films are from different loadings-gels of the same samples. For the separation of α and β-tubulin SDS-PAGE gels were prepared as described in Banerjee et al. 145 (7.5% gel with dirty SDS: purity <95%), sample loadings were done on the same gel, and migration were performed in Glycine/Tris buffer at 120 V.
Imaging and analysis
Image collection for this manuscript was performed in the McGill University Advanced BioImaging Facility (ABIF), RRID:SCR_017697. All images were analyzed primarily in Fiji and processed using Microsoft office Excel, and Python. The Zeiss LSM710 inverted confocal and X-Cite 120 LED with Zen 2.0 software were used to screen and acquire images of fixed samples. The objectives 10X EC plan neofluar NA 0.3, 20X plan apochromat NA 0.8 and 63X plan apochromat NA 1.4 oil were used. Intensity profiles in neuronal fixed samples were obtained from thick ROI lines drawn on neurites from the tip to the cell body, or by creation of a dilated mask of the DAPI signal for cell bodies. The intensity per pixel was interpolated from pixels along the width of the line, the generated profiles were then further compiled with other images using Excel and Python. For intensity quantification in entire field of views, all images were acquired in the same laser power and gain conditions, the Tubb3 or tyrosinated (YL1/2) microtubule signal was used as a mask for cells and intensity with the other channels were measured inside the mask and normalized to the mask intensity. The number of branches per cell were manually counted and their length measured with fiji line tools. For DCX and TTLL11 expression in U2OS cells, ROIs were drawn around the expressing cells and signal intensity for DCX-GFP, TTLL11-mRuby, and polyE (Alexa-647) were measured and normalized to the cell area.
The Quorum WaveFX-X1 spinning disk confocal system on a Leica DMI6000B inverted microscope equipped with a Photometrics prime BSI Cmos camera was used for short term live-cell imaging. We used the 63X plan apochromat NA 1.4 oil objective and the Metamorph software to record EB3mCherry and lysotracker dynamics. All timelapse movies recorded were 2 min total, and live cells were never imaged for more than 1 h. For EB3, images were acquired at 2 s intervals, in neurons with low-medium expression levels. Kymographs were generated from wide multipoint ROI lines drawn on processes from the tip to the cell body. Then straight ROI lines were drawn on each comet and a homemade Python script extracted the comet direction, lifetime, length and growth rate. For each cell, the growth cone and process areas were measured by thresholding a maximum projection of the timelapse. All further analyses were made in Python and used a mean value per cell.
For lysotracker, 241 frames were acquired at 500 ms intervals. Straight sections of the processes excluding cell bodies were selected using the polygon ROI tool and cargo trajectories were analyzed inside, using the Trackmate plugin146,147. Trackmate was set up to use the LoG detector to identify 1 µm diameter spots, with sub-pixel localization. Then the simple LAP tracker was used to define and filter trajectories that were exported as xml files. To analyze the TrackMate data, we had previously written custom MATLAB codes148, available on Github: https://github.com/hendricks-lab/Lysosome_Tracking_DCX. For each trajectory, we converted coordinate x and y position values to a single position vector with respect to the location of the soma. This position vector had a positive value when the tracked cargo moved anterogradely, and a negative value when moving retrogradely, as shown in Fig. 4. Using the position vector, we reduced the noise using a Savitzky-Golay smoothing filter of power 2 and span of 9 and segmented the trajectory into runs at each inflection point. These runs were used in the run length and velocity analysis. Between each inflection point, we determined the absolute value of the cargo’s displacement. If the displacement within the run was larger than 2 μm, the run was considered processive, while if it was between 0.03 μm and 2 μm, the run was categorized as diffusive, and finally if the displacement was 0.03 μm or smaller, the run was considered stationary. The threshold for a run to be considered processive was determined by plotting the α value of processive runs compared to that of diffusive runs, as well as the directional bias of diffusive and processive runs. We determined that 2 μm was optimal because the processive runs had an α value near 2, while the diffusive runs had an α near 1, as expected. We also observed that there was no directional bias for diffusive runs at the set minimum run length threshold, indicating the runs are purely diffusive. We then determined the run length and velocity per trajectory by taking the mean of the processive runs or processive runs over time, respectively, for each trajectory. We calculated the α by calculating the slope of the mean squared displacement plot for each trajectory and subsequently taking the mean for each cell. We calculated mean squared displacement using a time average for time intervals from the exposure time of 0.5 s up to 20 s. We used (Eq. 1) to calculate the mean squared displacement (MSD), where t is the frame, τ is the time interval, and x is the position vector value at the specified time.
The first 5 s of log-log plot of the mean squared displacement is used to calculate the α value for each trajectory, to ensure only the linear portion is fitted. We calculated the radius of gyration (Rg) using (Eq. 2). Here, r represents the sum of the squared x and y components, and N represents the number of position values in the entire trajectory.
To determine the directional bias, we determined the fraction of time the processive runs spent moving anterogradely compared to retrogradely, using the identified processive runs from the previously described function analyzing run length and velocity. To calculate the fraction of processive, stationary, and diffusive runs, we calculated the fraction of time for each run within each trajectory spent in each of these states, as defined by the previously described thresholds.
The perkin-elmer high content screening microscopes operaphenix in confocal mode with a 20X NA 1.0 water objective and the Harmony 9.0 software were used for long time recording of neuronal movements and branch dynamics. Images were taken at 5–10 min interval for 8–15 h. For each imaging session, multiple FOV from the same well were taken and analyzed individually. Nuclei tracking was done using trackmate within an automated Python-based pipeline149 available on github: https://github.com/brouhardlab/Sebastien-2024/. The LoG detector and LAP simple tracker were used with subpixel detection of 10 µm spots, and 40 µm maximum linking distance. Trajectories and spot data were exported as xml files, visually checked, and compiled within Python. Only the trajectories that lasted more than 30 min in total were included in the analysis.
Statistical analysis
The violin plots in this work show all the values from the minimum to the maximum (unless otherwise specified in the legend), the median values as white dots, mean values are written next to the associated violin, while the thick black lines represent the quartiles. All statistical tests used are indicated in the figure legends: Kruskal-Wallis non parametric H test, two-sided Mann-Whitney non parametric U test, or two-sided Student’s t test. P-values are detailed in Supplementary Tables 1–4 and were considered nonsignificant when above 0.05.
For the lysotracker dataset: For each per trajectory analysis, we took 1000 bootstrapped means with replacement and averaged them the same number of times as the minimum number of trajectories from either dataset. We then compared these means by subtracting the DCX-KO data from the control data and comparing the fraction of the difference distribution overlapping with 0. If this fraction was less than 0.05%, then we considered it a statistically significant difference, with a p value equal to the fraction that overlaps with the 0 line. Each value calculated per cell was analyzed using Student’s two-tailed t test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Accession codes and unique identifiers for all reagents are provided in the Excel file Supplementary Data 1. Due to size considerations, the raw data generated in this study are available only upon request, when a transfer media is provided (i.e., hard drive, server). Processed data for all figures are provided as Source Data file. Source data are provided with this paper.
Code availability
Custom code for lysosome and nuclear tracks analysis are available for download on Zenodo [https://doi.org/10.5281/zenodo.14735751] and Github [https://github.com/brouhardlab/Sebastien-2024/], respectively. Custom scripts for comet analysis and intensity profiles are available for download at http://sites.imagej.net/Hadim/.
References
Herculano-Houzel, S. The human brain in numbers: a linearly scaled-up primate brain. Front. Hum. Neurosci. 3, 31 (2009).
Rakic, P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J. Comp. Neurol. 145, 61–83 (1972).
Nadarajah, B. & Parnavelas, J. G. Modes of neuronal migration in the developing cerebral cortex. Nat. Rev. Neurosci. 3, 423–432 (2002).
Kapitein, L. C. & Hoogenraad, C. C. Building the neuronal microtubule cytoskeleton. Neuron 87, 492–506 (2015).
Dogterom, M., Kerssemakers, J. W., Romet-Lemonne, G. & Janson, M. E. Force generation by dynamic microtubules. Curr. Opin. Cell Biol. 17, 67–74 (2005).
Menon, S. & Gupton, S. L. Building blocks of functioning brain: cytoskeletal dynamics in neuronal development. Int. Rev. Cell Mol. Biol. 322, 183–245 (2016).
Umeshima, H., Hirano, T. & Kengaku, M. Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc. Natl Acad. Sci. USA 104, 16182–16187 (2007).
Maday, S., Twelvetrees, A. E., Moughamian, A. J. & Holzbaur, E. L. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron 84, 292–309 (2014).
Baas, P. W. & Black, M. M. Individual microtubules in the axon consist of domains that differ in both composition and stability. J. Cell Biol. 111, 495–509 (1990).
Baas, P. W., Deitch, J. S., Black, M. M. & Banker, G. A. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl Acad. Sci. USA 85, 8335–8339 (1988).
Verhey, K. J. & Gaertig, J. The tubulin code. Cell Cycle 6, 2152–2160 (2007).
Magiera, M. M., Singh, P., Gadadhar, S. & Janke, C. Tubulin posttranslational modifications and emerging links to human disease. Cell 173, 1323–1327 (2018).
Roll-Mecak, A. The tubulin code in microtubule dynamics and information encoding. Dev. Cell 54, 7–20 (2020).
Ramkumar, A., Jong, B. Y. & Ori-McKenney, K. M. ReMAPping the microtubule landscape: How phosphorylation dictates the activities of microtubule-associated proteins. Dev. Dyn. 247, 138–155 (2018).
Bodakuntla, S., Jijumon, A. S., Villablanca, C., Gonzalez-Billault, C. & Janke, C. Microtubule-associated proteins: structuring the cytoskeleton. Trends Cell Biol. 29, 804–819 (2019).
Howard, J. & Hyman, A. A. Microtubule polymerases and depolymerases. Curr. Opin. Cell Biol. 19, 31–35 (2007).
Akhmanova, A. & Steinmetz, M. O. Control of microtubule organization and dynamics: two ends in the limelight. Nat. Rev. Mol. Cell Biol. 16, 711–726 (2015).
Hirokawa, N., Noda, Y., Tanaka, Y. & Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 10, 682–696 (2009).
Verhey, K. J. & Ohi, R. Causes, costs and consequences of kinesin motors communicating through the microtubule lattice. J. Cell Sci. 136, jcs260735 (2023).
Iwanski, M. K. & Kapitein, L. C. Cellular cartography: towards an atlas of the neuronal microtubule cytoskeleton. Front. Cell Dev. Biol. 11, 1052245 (2023).
Tas, R. P. et al. Differentiation between oppositely oriented microtubules controls polarized neuronal transport. Neuron 96, 1264–1271.e1265 (2017).
Francis, F. et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23, 247–256 (1999).
Gleeson, J. G., Lin, P. T., Flanagan, L. A. & Walsh, C. A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23, 257–271 (1999).
Gleeson, J. G. et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 92, 63–72 (1998).
Des Portes, V. et al. doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH). Hum. Mol. Genet. 7, 1063–1070 (1998).
Feng, Y. & Walsh, C. A. Protein-protein interactions, cytoskeletal regulation and neuronal migration. Nat. Rev. Neurosci. 2, 408–416 (2001).
Sapir, T. et al. Doublecortin mutations cluster in evolutionarily conserved functional domains. Hum. Mol. Genet. 9, 703–712 (2000).
Taylor, K. R., Holzer, A. K., Bazan, J. F., Walsh, C. A. & Gleeson, J. G. Patient mutations in doublecortin define a repeated tubulin-binding domain. J. Biol. Chem. 275, 34442–34450 (2000).
Moores, C. A. et al. Mechanism of microtubule stabilization by doublecortin. Mol. Cell 14, 833–839 (2004).
Fourniol, F. J. et al. Template-free 13-protofilament microtubule-MAP assembly visualized at 8 A resolution. J. Cell Biol. 191, 463–470 (2010).
Maurer, S. P., Fourniol, F. J., Bohner, G., Moores, C. A. & Surrey, T. EBs recognize a nucleotide-dependent structural cap at growing microtubule ends. Cell 149, 371–382 (2012).
Omori, Y. et al. Expression and chromosomal localization of KIAA0369, a putative kinase structurally related to Doublecortin. J. Hum. Genet. 43, 169–177 (1998).
Kim, M. H. et al. The DCX-domain tandems of doublecortin and doublecortin-like kinase. Nat. Struct. Biol. 10, 324–333 (2003).
Burger, D. et al. Crystal structures of the human doublecortin C- and N-terminal domains in complex with specific antibodies. J. Biol. Chem. 291, 16292–16306 (2016).
Manka, S. W. & Moores, C. A. The role of tubulin-tubulin lattice contacts in the mechanism of microtubule dynamic instability. Nat. Struct. Mol. Biol. 25, 607–615 (2018).
Bechstedt, S., Lu, K. & Brouhard, G. J. Doublecortin recognizes the longitudinal curvature of the microtubule end and lattice. Curr. Biol. 24, 2366–2375 (2014).
Moores, C. A. et al. Distinct roles of doublecortin modulating the microtubule cytoskeleton. EMBO J. 25, 4448–4457 (2006).
Manka, S. W. & Moores, C. A. Pseudo-repeats in doublecortin make distinct mechanistic contributions to microtubule regulation. EMBO Rep. 21, e51534 (2020).
Bechstedt, S. & Brouhard, G. J. Doublecortin recognizes the 13-protofilament microtubule cooperatively and tracks microtubule ends. Dev. Cell 23, 181–192 (2012).
Rafiei, A. et al. Doublecortin engages the microtubule lattice through a cooperative binding mode involving its C-terminal domain. Elife 11, e66975 (2022).
Liu, J. S. et al. Molecular basis for specific regulation of neuronal kinesin-3 motors by doublecortin family proteins. Mol. Cell 47, 707–721 (2012).
Monroy, B. Y. et al. A combinatorial MAP code dictates polarized microtubule transport. Dev. Cell 53, 60–72.e64 (2020).
Fu, X. et al. Doublecortin and JIP3 are neural-specific counteracting regulators of dynein-mediated retrograde trafficking. Elife 11, e82218 (2022).
Friocourt, G. et al. Doublecortin functions at the extremities of growing neuronal processes. Cereb. Cortex 13, 620–626 (2003).
Tint, I., Jean, D., Baas, P. W. & Black, M. M. Doublecortin associates with microtubules preferentially in regions of the axon displaying actin-rich protrusive structures. J. Neurosci. 29, 10995–11010 (2009).
Dema, A. et al. Doublecortin reinforces microtubules to promote growth cone advance in soft environments. Curr. Biol. 34, 5822–5832.e5825 (2024).
Corbo, J. C. et al. Doublecortin is required in mice for lamination of the hippocampus but not the neocortex. J. Neurosci. 22, 7548–7557 (2002).
Deuel, T. A. et al. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron 49, 41–53 (2006).
Bai, J. et al. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat. Neurosci. 6, 1277–1283 (2003).
Baek, S. T. et al. Off-target effect of doublecortin family shRNA on neuronal migration associated with endogenous microRNA dysregulation. Neuron 82, 1255–1262 (2014).
Kappeler, C. et al. Branching and nucleokinesis defects in migrating interneurons derived from doublecortin knockout mice. Hum. Mol. Genet 15, 1387–1400 (2006).
Koizumi, H. et al. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nat. Neurosci. 9, 779–786 (2006).
Buchsbaum, I. Y. & Cappello, S. Neuronal migration in the CNS during development and disease: insights from in vivo and in vitro models. Development 146, dev163766 (2019).
Belvindrah, R. et al. Mutation of the alpha-tubulin Tuba1a leads to straighter microtubules and perturbs neuronal migration. J. Cell Biol. 216, 2443–2461 (2017).
Dogterom, M. & Koenderink, G. H. Actin-microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 20, 38–54 (2019).
Tsai, L. H. & Gleeson, J. G. Nucleokinesis in neuronal migration. Neuron 46, 383–388 (2005).
Coles, C. H. & Bradke, F. Coordinating neuronal actin-microtubule dynamics. Curr. Biol. 25, R677–R691 (2015).
Kalil, K. & Dent, E. W. Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nat. Rev. Neurosci. 15, 7–18 (2014).
Pacheco, A. & Gallo, G. Actin filament-microtubule interactions in axon initiation and branching. Brain Res Bull. 126, 300–310 (2016).
Bilimoria, P. M. et al. A JIP3-regulated GSK3beta/DCX signaling pathway restricts axon branching. J. Neurosci. 30, 16766–16776 (2010).
Li, H. et al. MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons. Protein Cell 5, 160–169 (2014).
Martini, F. J. et al. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development 136, 41–50 (2009).
Chai, X. et al. Reelin Induces branching of neurons and radial glial cells during corticogenesis. Cereb. Cortex 25, 3640–3653 (2015).
Gupta, A. et al. Layering defect in p35 deficiency is linked to improper neuronal-glial interaction in radial migration. Nat. Neurosci. 6, 1284–1291 (2003).
Dehmelt, L. & Halpain, S. Actin and microtubules in neurite initiation: are MAPs the missing link? J. Neurobiol. 58, 18–33 (2004).
Kawauchi, T. & Hoshino, M. Molecular pathways regulating cytoskeletal organization and morphological changes in migrating neurons. Dev. Neurosci. 30, 36–46 (2008).
Cooper, J. A. Cell biology in neuroscience: mechanisms of cell migration in the nervous system. J. Cell Biol. 202, 725–734 (2013).
Ettinger, A., van Haren, J., Ribeiro, S. A. & Wittmann, T. Doublecortin is excluded from growing microtubule ends and recognizes the GDP-microtubule lattice. Curr. Biol. 26, 1549–1555 (2016).
Lindhout, F. W. et al. Quantitative mapping of transcriptome and proteome dynamics during polarization of human iPSC-derived neurons. Elife 9, e58124 (2020).
Dema, A., Charafeddine, R., Rahgozar, S., van Haren, J. & Wittmann, T. Growth cone advance requires EB1 as revealed by genomic replacement with a light-sensitive variant. Elife 12, e84143 (2023).
Atherton, J., Stouffer, M., Francis, F. & Moores, C. A. Visualising the cytoskeletal machinery in neuronal growth cones using cryo-electron tomography. J. Cell Sci. 135, jcs259234 (2022).
Chaudhary, A. R., Berger, F., Berger, C. L. & Hendricks, A. G. Tau directs intracellular trafficking by regulating the forces exerted by kinesin and dynein teams. Traffic 19, 111–121 (2018).
Monroy, B. Y. et al. Competition between microtubule-associated proteins directs motor transport. Nat. Commun. 9, 1487 (2018).
Gumy, L. F. et al. MAP2 defines a pre-axonal filtering zone to regulate KIF1- versus KIF5-dependent cargo transport in sensory neurons. Neuron 94, 347–362.e347 (2017).
Lipka, J., Kapitein, L. C., Jaworski, J. & Hoogenraad, C. C. Microtubule-binding protein doublecortin-like kinase 1 (DCLK1) guides kinesin-3-mediated cargo transport to dendrites. EMBO J. 35, 302–318 (2016).
Siahaan, V. et al. Microtubule lattice spacing governs cohesive envelope formation of tau family proteins. Nat. Chem. Biol. 18, 1224–1235 (2022).
Tanaka, T., Koizumi, H. & Gleeson, J. G. The doublecortin and doublecortin-like kinase 1 genes cooperate in murine hippocampal development. Cereb. Cortex 16, i69–i73 (2006).
Pu, J., Guardia, C. M., Keren-Kaplan, T. & Bonifacino, J. S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 129, 4329–4339 (2016).
Bentley, M., Decker, H., Luisi, J. & Banker, G. A novel assay reveals preferential binding between Rabs, kinesins, and specific endosomal subpopulations. J. Cell Biol. 208, 273–281 (2015).
Sirajuddin, M., Rice, L. M. & Vale, R. D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 16, 335–344 (2014).
Boucher, D., Larcher, J. C., Gros, F. & Denoulet, P. Polyglutamylation of tubulin as a progressive regulator of in vitro interactions between the microtubule-associated protein Tau and tubulin. Biochemistry 33, 12471–12477 (1994).
Bonnet, C. et al. Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J. Biol. Chem. 276, 12839–12848 (2001).
Cueva, J. G., Hsin, J., Huang, K. C. & Goodman, M. B. Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Curr. Biol. 22, 1066–1074 (2012).
Portran, D., Schaedel, L., Xu, Z., Thery, M. & Nachury, M. V. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 19, 391–398 (2017).
Balabanian, L., Berger, C. L. & Hendricks, A. G. Acetylated microtubules are preferentially bundled leading to enhanced kinesin-1 Motility. Biophys. J. 113, 1551–1560 (2017).
Hoff, K. J., Aiken, J. E., Gutierrez, M. A., Franco, S. J. & Moore, J. K. TUBA1A tubulinopathy mutants disrupt neuron morphogenesis and override XMAP215/Stu2 regulation of microtubule dynamics. Elife 11, e76189 (2022).
Lessard, D. V. et al. Polyglutamylation of tubulin’s C-terminal tail controls pausing and motility of kinesin-3 family member KIF1A. J. Biol. Chem. 294, 6353–6363 (2019).
McKenney, R. J., Huynh, W., Vale, R. D. & Sirajuddin, M. Tyrosination of alpha-tubulin controls the initiation of processive dynein-dynactin motility. EMBO J. 35, 1175–1185 (2016).
Homma, N. et al. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell 114, 229–239 (2003).
Valenstein, M. L. & Roll-Mecak, A. Graded control of microtubule severing by tubulin glutamylation. Cell 164, 911–921 (2016).
Moutin, M. J., Bosc, C., Peris, L. & Andrieux, A. Tubulin post-translational modifications control neuronal development and functions. Dev. Neurobiol. 81, 253–272 (2021).
Janke, C. & Magiera, M. M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 21, 307–326 (2020).
Audebert, S. et al. Reversible polyglutamylation of alpha-tubulin and beta-tubulin and microtubule dynamics in mouse-brain neurons. Mol. Biol. Cell 4, 615–626 (1993).
Redeker, V., Melki, R., Prome, D., Le Caer, J. P. & Rossier, J. Structure of tubulin C-terminal domain obtained by subtilisin treatment. The major alpha and beta tubulin isotypes from pig brain are glutamylated. FEBS Lett. 313, 185–192 (1992).
Wolff, A. et al. Distribution of glutamylated alpha and beta-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur. J. Cell Biol. 59, 425–432 (1992).
Lacroix, B. et al. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J. Cell Biol. 189, 945–954 (2010).
Cohen, D., Segal, M. & Reiner, O. Doublecortin supports the development of dendritic arbors in primary hippocampal neurons. Dev. Neurosci. 30, 187–199 (2008).
Yap, C. C. et al. Different Doublecortin (DCX) Patient Alleles Show Distinct Phenotypes in Cultured Neurons: EVIDENCE FOR DIVERGENT LOSS-OF-FUNCTION AND “OFF-PATHWAY” CELLULAR MECHANISMS. J. Biol. Chem. 291, 26613–26626 (2016).
Yang, C. H. & Horwitz, S. B. Taxol((R)): the first microtubule stabilizing agent. Int. J. Mol. Sci. 18, 1733 (2017).
Hammond, J. W. et al. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol. Biol. Cell 21, 572–583 (2010).
Arnal, I. & Wade, R. H. How does taxol stabilize microtubules? Curr. Biol. 5, 900–908 (1995).
Alushin, G. M. et al. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell 157, 1117–1129 (2014).
Janke, C. et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 308, 1758–1762 (2005).
Van Dijk, J. et al. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell 26, 437–448 (2007).
Rogowski, K. et al. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell 143, 564–578 (2010).
Mahalingan, K. K. et al. Structural basis for polyglutamate chain initiation and elongation by TTLL family enzymes. Nat. Struct. Mol. Biol. 27, 802–813 (2020).
Edde, B. et al. Posttranslational glutamylation of alpha-tubulin. Science 247, 83–85 (1990).
Alexander, J. E. et al. Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc. Natl Acad. Sci. USA 88, 4685–4689 (1991).
Bodakuntla, S. et al. Distinct roles of alpha- and beta-tubulin polyglutamylation in controlling axonal transport and in neurodegeneration. EMBO J. 40, e108498 (2021).
Magiera, M. M. & Janke, C. Investigating tubulin posttranslational modifications with specific antibodies. Methods Cell Biol. 115, 247–267 (2013).
McKenna, E. D., Sarbanes, S. L., Cummings, S. W. & Roll-Mecak, A. The tubulin code, from molecules to health and disease. Annu. Rev. Cell Dev. Biol. 39, 331–361 (2023).
Zadra, I. et al. Chromosome segregation fidelity requires microtubule polyglutamylation by the cancer downregulated enzyme TTLL11. Nat. Commun. 13, 7147 (2022).
Bahi-Buisson, N. et al. New insights into genotype-phenotype correlations for the doublecortin-related lissencephaly spectrum. Brain 136, 223–244 (2013).
Leger, P. L. et al. The location of DCX mutations predicts malformation severity in X-linked lissencephaly. Neurogenetics 9, 277–285 (2008).
Gavoci, A. et al. Polyglutamylation of microtubules drives neuronal remodeling. bioRxiv, 2024.2003.2011.584412 (2024).
Lopes, A. T. et al. Spastin depletion increases tubulin polyglutamylation and impairs kinesin-mediated neuronal transport, leading to working and associative memory deficits. PLoS Biol. 18, e3000820 (2020).
Ziak, J. et al. Microtubule-binding protein MAP1B regulates interstitial axon branching of cortical neurons via the tubulin tyrosination cycle. EMBO J. 43, 1214–1243 (2024).
Shashi, V. et al. Loss of tubulin deglutamylase CCP1 causes infantile-onset neurodegeneration. EMBO J. 37, e100540 (2018).
O’Hagan, R. et al. Glutamylation regulates transport, specializes function, and sculpts the structure of cilia. Curr. Biol. 27, 3430–3441.e3436 (2017).
Garnham, C. P. et al. Multivalent microtubule recognition by tubulin tyrosine ligase-like family glutamylases. Cell 161, 1112–1123 (2015).
Mahalingan, K. K. et al. Structural basis for alpha-tubulin-specific and modification state-dependent glutamylation. Nat. Chem. Biol. 20, 1493–1504 (2024).
LaFrance, B. J. et al. Structural transitions in the GTP cap visualized by cryo-electron microscopy of catalytically inactive microtubules. Proc. Natl Acad. Sci. USA 119, e2114994119 (2022).
Zhang, R., LaFrance, B. & Nogales, E. Separating the effects of nucleotide and EB binding on microtubule structure. Proc. Natl Acad. Sci. USA 115, E6191–E6200 (2018).
Yue, Y., Hotta, T., Higaki, T., Verhey, K. J. & Ohi, R. Microtubule detyrosination by VASH1/SVBP is regulated by the conformational state of tubulin in the lattice. Curr. Biol. 33, 4111–4123.e4117 (2023).
Shen, Y. & Ori-McKenney, K. M. Microtubule-associated protein MAP7 promotes tubulin posttranslational modifications and cargo transport to enable osmotic adaptation. Dev. Cell 59, 1553–1570.e7 (2024).
Costa, A. C. & Sousa, M. M. The role of spastin in axon biology. Front. Cell Dev. Biol. 10, 934522 (2022).
Genova, M. et al. Tubulin polyglutamylation differentially regulates microtubule-interacting proteins. EMBO J. 42, e112101 (2023).
Roll-Mecak, A. & Vale, R. D. Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays? J. Cell Biol. 175, 849–851 (2006).
Yu, W. et al. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell 19, 1485–1498 (2008).
Vemu, A. et al. Severing enzymes amplify microtubule arrays through lattice GTP-tubulin incorporation. Science 361, eaau1504 (2018).
Schaedel, L. et al. Microtubules self-repair in response to mechanical stress. Nat. Mater. 14, 1156–1163 (2015).
Tan, R. et al. Microtubules gate tau condensation to spatially regulate microtubule functions. Nat. Cell Biol. 21, 1078–1085 (2019).
Guardia, C. M., Farias, G. G., Jia, R., Pu, J. & Bonifacino, J. S. BORC functions upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep. 17, 1950–1961 (2016).
Bielas, S. L. et al. Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist. Cell 129, 579–591 (2007).
Fu, X. et al. Doublecortin (Dcx) family proteins regulate filamentous actin structure in developing neurons. J. Neurosci. 33, 709–721 (2013).
Reid, T. A. et al. Structural state recognition facilitates tip tracking of EB1 at growing microtubule ends. Elife 8, e48117 (2019).
Song, X. et al. Phase separation of EB1 guides microtubule plus-end dynamics. Nat. Cell Biol. 25, 79–91 (2023).
Friocourt, G. et al. Both doublecortin and doublecortin-like kinase play a role in cortical interneuron migration. J. Neurosci. 27, 3875–3883 (2007).
Shin, E. et al. Doublecortin-like kinase enhances dendritic remodelling and negatively regulates synapse maturation. Nat. Commun. 4, 1440 (2013).
Agulto, R. L. et al. Autoregulatory control of microtubule binding in doublecortin-like kinase 1. Elife 10, e60126 (2021).
Ebberink, E. et al. Tubulin engineering by semi-synthesis reveals that polyglutamylation directs detyrosination. Nat. Chem. 15, 1179–1187 (2023).
Das, S. et al. The C-BIG repository: an institution-level open science platform. Neuroinformatics 20, 139–153 (2022).
Chazeau, A., Katrukha, E. A., Hoogenraad, C. C. & Kapitein, L. C. Studying neuronal microtubule organization and microtubule-associated proteins using single molecule localization microscopy. Methods Cell Biol. 131, 127–149 (2016).
Kingston, R. E., Chen, C. A. & Okayama, H. Calcium phosphate transfection. Curr. Protoc. Cell Biol. 20, 20–23 (2003).
Banerjee, A., Bovenzi, F. A. & Bane, S. L. High-resolution separation of tubulin monomers on polyacrylamide minigels. Anal. Biochem. 402, 194–196 (2010).
Ershov, D. et al. TrackMate 7: integrating state-of-the-art segmentation algorithms into tracking pipelines. Nat. Methods 19, 829–832 (2022).
Tinevez, J. Y. et al. TrackMate: an open and extensible platform for single-particle tracking. Methods 115, 80–90 (2017).
Prowse, E. N. P., Chaudhary, A. R., Sharon, D. & Hendricks, A. G. Huntingtin S421 phosphorylation increases kinesin and dynein engagement on early endosomes and lysosomes. Biophys. J. 122, 1168–1184 (2023).
Sébastien, M., Paquette, A. L., Ferotin, L., Hendricks, A. G. & Brouhard, G. J. Measurements of neurite extension and nucleokinesis in an iPSC-derived model system following microtubule perturbation. Mol. Biol. Cell 36, mr1 (2025).
Acknowledgements
We would like to thank current members of the Brouhard, Hendricks, and Bechstedt labs, for fruitful discussions during all phases of this project, and more particularly past members Hadrien Mary and Piper Stevens for their help in code writing. Many thanks to Geneviève Dorval and Thomas Durcan for thoughtful support with the use of iPSCs and CRISPR methods. We would also like to acknowledge the McGill University Advanced BioImaging Facility (ABIF), RRID: SCR_017697, the Flow cytometry core facility of the Life Science Complex, the Genome Québec sequencing platform, and their personal for providing services and helpful advice for data collection and analysis. G.J.B. acknowledges support from Natural Sciences and Engineering Research Council of Canada (RGPIN-2014-03791), the Canadian Institutes of Health Research (PJT-148702), and McGill University. A.G.H. is supported by the Canadian Institutes of Health Research (PJT-185997).
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design: G.J.B., M.S., and A.G.H. Experimental procedures: M.S. and A.L.P. Code writing: E.N.N.P., A.G.H. Data analysis: M.S., E.N.P.P., and A.L.P. Figures and schematics design: M.S. Manuscript writing: M.S., G.J.B., and A.G.H. All authors participated in editing the manuscript. Funding acquisition: G.J.B. and A.G.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sébastien, M., Paquette, A.L., Prowse, E.N.P. et al. Doublecortin restricts neuronal branching by regulating tubulin polyglutamylation. Nat Commun 16, 1749 (2025). https://doi.org/10.1038/s41467-025-56951-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56951-2