Abstract
Metabolic disturbances are hallmarks of vascular smooth muscle cell (VSMC) phenotypic transitions, which play a critical role in the pathogenesis of aortic dissection (AD). In this study, we identify and characterize glucose metabolism regulatory protein (GMRSP), a protein encoded by lncRNA H19. Using VSMC-specific GMRSP induction in knock-in mice, adeno-associated virus-mediated GMRSP overexpression, and exosomal GMRSP delivery, we demonstrate significant improvements in AD and mitochondrial dysfunction. Mechanistically, GMRSP inhibits heterogeneous nuclear ribonucleoprotein (hnRNP) A2B1-mediated alternative splicing of pyruvate kinase M (PKM) pre-mRNA, leading to reduced PKM2 production and glycolysis. This reprogramming preserves the contractile phenotype of VSMCs and prevents their transition to a proliferative state. Importantly, pharmacological activation of PKM2 via TEPP-46 abrogates the protective effects of GMRSP in vivo and in vitro. Clinical relevance is shown by elevated plasma PKM2 levels in AD patients, which correlate with poor prognosis. Collectively, these findings indicate GMRSP as a key regulator of VSMC metabolism and phenotypic stability, highlighting its potential as a therapeutic target for AD.
Similar content being viewed by others
Introduction
Aortic dissection (AD) is a severe vascular emergency that is associated with a high risk of morbidity and mortality1. While degeneration of the extracellular matrix and phenotypic transition of vascular smooth muscle cells (VSMCs) have been identified as crucial mechanisms in AD2,3, effective pharmacological treatments for the prevention and management of AD remain limited. Therefore, gaining a deep understanding of the pathophysiology of AD is essential for the identification of potential therapeutic targets for this disease.
Metabolic reprogramming, particularly the shift from oxidative phosphorylation (OXPHOS) to aerobic glycolysis, plays a role in the phenotypic transition of VSMCs4. This process is partially regulated by the relative abundance of the pyruvate kinases M1 (PKM1) and M2 (PKM2) through alternative splicing (AS) of PKM pre-mRNA. An elevated PKM2/PKM1 ratio has been observed during the transition process as VSMCs move from a contractile to a synthetic phenotype and has been implicated in atherosclerosis5, vascular injury6, and pulmonary arterial hypertension7. Targeting PKM2 has shown some promise in alleviating these pathological processes8. However, the role of PKM2 in AD remains unexplored, particularly in terms of the factors regulating its expression and activity in VSMCs.
Long noncoding RNAs (lncRNAs), which are RNAs >200 nucleotides in length that lack protein-coding capacity, have been implicated in various biological activities, including chromatin modification9, DNA repair10, and translation11. Recent studies have revealed that a small subset of lncRNAs contain an open reading frame (ORF) with translational capacity, leading to biologically active peptide production. These peptides have been shown to influence muscle regeneration12, cancer progression13, and angiogenesis14. However, the functional role of lncRNA-derived peptides and proteins in AD remains largely unexplored.
LncRNA H19 (H19) is an imprinting gene with only maternal expression that is involved in multiple biological processes15,16. H19 is markedly upregulated in abdominal aortic aneurysms17 and regulates VSMC survival, although its precise role in AD pathology remains to be fully elucidated. Here, we show that H19 was highly downregulated in patients with AD and in a β-aminopropionitrile (BAPN)-induced mouse model. Furthermore, we discovered that the previously annotated lncRNA H19 encodes a small, conserved 131-amino acid protein named “GMRSP”. The GMRSP protein, encoded by lncRNA H19, was found to play a crucial role in suppressing the phenotypic transition of VSMCs by affecting heterogeneous nuclear ribonucleoprotein (hnRNP) A2B1-mediated pre-PKM splicing, which inhibits PKM2 production and subsequent metabolic reprogramming in AD. Furthermore, we demonstrated that VSMC-specific GMRSP overexpression significantly reduces the incidence and severity of BAPN monofumarate-induced AD. Our findings suggest that GMRSP could serve as a potential therapeutic target for slowing AD progression.
Results
LncRNA H19 is downregulated in AD tissues and encodes a small protein
RNA sequencing was conducted to identify potential candidate lncRNAs associated with AD, and a heatmap was generated to visualize differential lncRNA expression. Among these candidates, lncRNA H19 (ENSG00000130600) is highly expressed in normal human aortic tissue. Furthermore, H19 was significantly downregulated in AD tissue compared with normal aortic tissue (Fig. 1A). Moreover, analysis of differentially expressed genes from the GSE153434 dataset revealed a significant downregulation of lncRNA H19 in AD tissues compared with normal human aortic tissues (Fig. S1A)18. To validate H19 expression, reverse transcriptase (RT)-PCR and Q-PCR were performed, and the experimental results were consistent with the high-throughput sequencing data. H19 expression levels were found to be lower in aortic tissues from patients with AD than in those from controls without AD (Figs. 1B and S1B). Originally annotated as a lncRNA gene in Homo sapiens (NR_131224.1), H19 exhibited intriguing properties according to the ribosome profiling sequencing (Ribo-seq) data, which indicated the presence of a small protein encoded by a small ORF within H19 (Fig. S1C). We also searched the GWIPS-viz database, which contained data that were consistent with our ribosome data (Fig. S1D). Bioinformatics analysis identified ORF1 as the most likely translated ORF, as this ORF consists of 396 nucleotides that encode a 131-amino acid microprotein with classical start (ATG) and stop (TGA) codons (Figs. 1C and S1E). This microprotein was named GMRSP. GMRSP is highly conserved across primates, whereas mouse GMRSP (132 aa) shares moderate homology with human GMRSP at the C-terminus (Fig. S1F). We thus generated a rabbit polyclonal antibody against GMRSP and verified its antibody specificity through siRNAs (Fig. S1G and S1H). Like H19, GMRSP expression levels were also lower in aortic tissues from AD patients than in those from controls without AD (Figs. 1D and S1I). Subsequently, immunofluorescence staining was performed to examine the expression and localization of GMRSP, which was predominantly expressed in arterial VSMCs rather than in arterial macrophages, endothelial cells or fibroblasts (Fig. S2A-S2C and 1E). Additionally, GMRSP expression was lower in human AD VSMCs than in arterial VSMCs from control individuals (Fig. 1E).
A Heatmap of the top 15 differentially expressed lncRNAs in normal human aortic tissues and AD tissues (q < 0.05, |Log2FC | >1). B Q-PCR analysis of H19 levels in the aorta of controls without AD (n = 7 biologically independent samples) and patients with AD (n = 9 biologically independent samples). C The 1253-nucleotide lncRNA H19 contains a potential small protein-encoding ORF, which may encode a 131-amino acid small protein, GMRSP. D Western blot analysis of the levels of GMRSP in the aorta of controls without AD (n = 7 biologically independent samples) and patients with AD (n = 9 biologically independent samples). E Representative images of GMRSP (red) co-localized with α-SMA (green)-positive vascular smooth muscle cells in aortic tissues from controls without AD (n = 5 biologically independent samples) and patients with AD (n = 5 biologically independent samples), Scale bar: 100 μm. After transfecting the indicated constructs into primary human VSMCs cells for 48 h, GFP fluorescence was detected by fluorescence microscope, Scale bar: 50 μm (F), and GMRSP-GFP fusion protein expression was detected by western blotting using anti-GFP and GMRSP antibodies (G). After transfecting the indicated constructs into primary human VSMCs for 48 h, GMRSP-Flag fusion protein levels were immunostained using anti-Flag, Scale bar: 20 μm (H) and GMRSP antibodies, Scale bar: 20 μm (I). J GMRSP-Flag was overexpressed in VSMCs, followed by detection with anti-Flag and GMRSP antibodies. Data are presented as the mean ± SD. The p values were calculated by a two-tailed unpaired Student’s t-test in (B, D). The data presented in (F–J) are representative of three independent experiments. Source data are provided as a Source Data file. AD, aortic dissection; RT-PCR; reverse transcriptase-polymerase chain reaction; VSMCs, vascular smooth muscle cells; GMRSP; glucose metabolism regulatory small protein; SMA, smooth muscle actin; GFP, green fluorescent protein; IP, immunoprecipitation.
To investigate the functionality of the in-frame ATG codon within H19, the start codon ATG in the predicted ORF1 was mutated to ATT. Similarly, the start codon ATGGTG of the GFP plasmid was mutated to ATTGTT, resulting in GFPmut. We then generated a construct called GMRSP-GFPmut by fusing GFPmut to the C-terminus of GMRSP (Fig. S2D). These constructs were transfected into human primary VSMCs and HEK293T cells. Approximately 48 hours of post-transfection, GMRSP expression-GFPmut fluorescence was detected in both the VSMCs and the HEK293T cells (Figs. 1F and S2E). Additionally, western blotting was performed using anti-GFP and anti-GMRSP antibodies to confirm the presence of GMRSP-GFPmut fusion proteins with the predicted relative molecular masses (Figs. 1G and S2F). However, the GMRSP-GFPmut protein was not detected when the ATG codon in GMRSP was mutated (ORFmut-GFPmut) (Figs. 1G and S2F). To rule out potential interference from the size of the GFP tag on the fusion protein, we generated a series of GMRSP-Flag constructs. Immunofluorescence staining and western blotting with anti-Flag and anti-GMRSP antibodies confirmed the presence of GMRSP-Flag fusion proteins in human primary VSMCs and HEK293T cells (Figs. 1H–J and S2G-S2I). These proteins were consistent with GMRSP-GFPmut fusion proteins. Mutation of the GMRSP start codon from ATG to ATT (ORFmut-Flag) abolished GMRSP-Flag fusion protein expression (Figs. 1H–J and S2G-S2I). Finally, the results of mass spectrometry (MS) further demonstrated the endogenous production of GMRSP in human VSMCs (Fig. S2J). Collectively, these data provide evidence that the ORF1 of H19 encodes a small protein called GMRSP in human VSMCs, which is decreased in patients with AD.
GMRSP overexpression inhibits contractile-to-synthetic phenotypic transition in VSMCs and alleviates AD Formation in vivo
Immunofluorescence analysis revealed a decrease in the protein levels of GMRSP, along with contractile markers of VSMCs (α-smooth muscle actin [α-SMA] and SM22α), upon stimulation with platelet-derived growth factor (PDGF)-BB (Fig. S3A–S3B). To investigate the potential role of GMRSP in the phenotypic transition of VSMCs, we initially examined its effects on human primary VSMCs. GMRSP overexpression was induced in VSMCs using GMRSP-Flag fusion expression constructs (Fig. S3C). In vitro, GMRSP overexpression via H19 ORF constructs inhibited VSMC migration and MMP2 secretion following PDGF-BB treatment (Fig. S3D–S3F). In contrast, ORFmut-Flag, which contains a mutated start codon and does not encode GMRSP, did not affect VSMC migration or MMP2 secretion (Fig. S3D–S3F). Furthermore, GMRSP significantly promoted α-SMA protein expression in response to PDGF-BB treatment in vitro (Fig. S3G). However, the ORFmut-Flag construct did not alter the expression of markers associated with the contractile-to-synthetic phenotype transition (Fig. S3G). Next, to explore which specific phenotype of PDGF-BB-induced VSMCs was reversed by GMRSP, we detected the expression of four synthetic VSMC markers by Q‒PCR. We used FN1, BGN, and COL1A1 for the fibroblast phenotype, CD45 and CD68 for the macrophage phenotype, SP-7, SOX9, and RUNX2 for the osteogenic phenotype, and UCP1, ELOVL3, and PRDM16 for the adipocyte phenotype according to previous reports19. GMRSP significantly reversed the PDGF-BB-induced fibroblast and macrophage phenotypes, but had no significant effect on the osteoblast or adipocyte phenotypes (Fig. S3H). To demonstrate that the observed effects are due to the GMRSP protein rather than the RNA, we silenced endogenous H19 and simultaneously restored GMRSP expression through exogenous introduction (Fig. S3I). To further investigate and differentiate the functions of GMRSP and its H19 lncRNA in VSMC phenotypic conversion, we restored its expression with H19 ORF-Flag or ORFmut-Flag (MUT) vectors in H19-knockdown cells. We found that H19 KD-induced alterations were restored to the control level after re-expression with H19 ORF-Flag, which can encode GMRSP, but not after re-expression of H19 ORFmut-Flag, as this does not encode GMRSP (Fig. S3J-S3K).
To assess the relevance of GMRSP in AD progression in vivo, we established an overexpression model by injecting adeno-associated virus (AAV) vectors, namely, AAV-ORF or AAV-ORFmut, into AD mice induced with BAPN monofumarate dissolved in drinking water (Fig. S4A-S4B). After BAPN monofumarate administration, 100% (n = 15) of the C57BL/6 J mice injected with the AAV-vector, 46.7% (n = 7) of those injected with AAV-ORF, and 93.3% (n = 14) of those injected with AAV-ORFmut developed AD (Fig. S4C). After four weeks of BAPN monofumarate administration, 60% (n = 9) of the C57BL/6 J mice injected with the AAV-vector, 20% (n = 3) of those injected with AAV-ORF, and 53.3% (n = 8) of those injected with AAV-ORFmut died from AD-related rupture (Figs. S4D and S4E). The measurement of the maximal aortic diameter on day 28 indicated that, compared with the AAV-vector control, GMRSP mitigated BAPN monofumarate-induced aortic dilation, whereas AAV-ORFmut did not alleviate BAPN monofumarate-induced aortic dilation (Fig. S4F). H&E staining revealed that GMRSP significantly reduced BAPN monofumarate-induced aortic medial thickness, whereas AAV-ORFmut did not have the same effect as did AAV-vector controls (Figs. S4G and S4H). Moreover, elastic-van Gieson (EVG) staining showed that GMRSP significantly reduced BAPN monofumarate-induced fragmentation of elastic fibers, indicating improved elastic tissue integrity compared with the AAV-vector control (Fig. S4G and S4I). The transfection efficiency was confirmed, as shown in Fig. S4G. As expected, AAV-ORF markedly increased the expression of GMRSP in vivo, whereas ORFmut-Flag did not (Fig. S4J). GMRSP significantly promoted SM22α and α-SMA expression and inhibited BAPN monofumarate-induced MMP2 expression in VSMCs from AD mice (Fig. S4J and S4K).
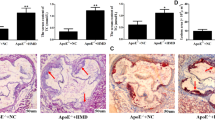
To further investigate the role of GMRSP in AD, we conducted in vivo experiments using a conditional GMRSP overexpression model driven by the CAG promoter (GMRSP OE-Flox; Fig. S5A). Smooth muscle cell-specific expression was achieved through tamoxifen-induced Tagln-Cre activation (Fig. S5B and S5C). Both GMRSPOE-flox and SM22α+GMRSPOE-flox mice were subjected to AD induction by administration of 0.25% BAPN monofumarate for four weeks (Fig. 2A). No significant differences were observed in the maximal aortic diameter between GMRSPOE-flox and SM22α+GMRSPOE-flox mice after vehicle treatment (Fig. 2B and E). Notably, compared with GMRSPOE-flox mice, SM22α+GMRSPOE-flox mice exhibited significantly better survival rates following BAPN-induced AD (Fig. 2C). After four weeks of BAPN monofumarate administration, 70% (n = 7) of the GMRSPOE-flox and 20% (n = 2) of the SM22α+GMRSPOE-flox mice died due to AD and rupture (Fig. 2D). In addition, compared with GMRSPOE-flox mice, SM22α+GMRSPOE-flox mice presented a significantly reduced maximal aortic diameter following BAPN monofumarate administration (Fig. 2B and Fig. 2E). Consistent with previous findings, H&E and EVG staining revealed that GMRSP expression significantly reduced vessel wall dissection and elastin disorganization, highlighting its protective role in AD pathology (Fig. 2F–H). Besides, GMRSP significantly promoted SM22α and α-SMA expression and inhibited BAPN monofumarate-induced MMP2 expression in VSMCs from AD mice (Fig. 2I, J). Additionally, we also detected GMRSP function in female murine AD models (Fig. S5D). We found the GMRSP could also reduce the incidence and severity of AD in female mice (Fig. S5E–S5G). Collectively, these data demonstrate that GMRSP maintains the contractile phenotype of VSMCs and attenuates AD formation both in vitro and in vivo.
A Male mice were induced with tamoxifen for 5 days at two weeks. One week after the last tamoxifen treatment, GMRSPOE-flox and SM22α+GMRSPOE-flox mice were treated with 0.25% BAPN monofumarate for four weeks (n = 10 biological replicates per group). B Representative images of the aorta of the mice are shown. C The survival rate was estimated by the Kaplan-Meier method and compared using the Breslow test (n = 10 biological replicates per group). D The incidence of AD was statistically analyzed. E The maximum aortic diameter was measured (n = 10 biological replicates per group). F Representative macroscopic images of aorta sections stained with H&E and EVG, Scale bar: 200 μm and 50 μm. The wall thickness (G) and elastin integrity (H) of each groups were analyzed (n = 10 biological replicates per group). I Immunofluorescence staining of α-SMA (green) and GMRSP (red) in the aorta of the mice is shown. Nuclei were counterstained with DAPI. J Immunofluorescence staining of SM22α (green) and MMP2 (red) in the aorta of the mice is shown. Nuclei were counterstained with DAPI,Scale bar: 200 μm. Data are presented as the mean ± SD.The p values were calculated by a two-tailed unpaired Student’s t-test in (E,G,H). The p values were calculated by Kaplan–Meier analysis and two-sided Breslow Test (C).The data presented in (F,I, J) are representative of three independent experiments. Source data are provided as a Source Data file. GMRSP; glucose metabolism regulatory small protein; SMC; smooth muscle cell; AD, aortic dissection; BAPN, β-aminopropionitrile; H&E, hematoxylin and eosin; EVG, elastic–Van Gieson; SMA, smooth muscle actin; DAPI, 4’,6-diamidino-2-phenylindole; SM22α, smooth muscle protein 22-alpha; MMP2, matrix mettaloproteinase2.
GMRSP inhibits aerobic glycolysis by regulating Pre-PKM mRNA splicing
To characterize the mechanism by which GMRSP functions in VSMCs, we analyzed a publicly available single-cell sequencing dataset of patients with AD20. The analysis revealed seven major cell clusters identified via the Uniform Manifold Approximation and Projection (UMAP) method (Fig. S6A). We subsequently performed a pathway analysis on the differentially expressed metabolic-related genes in the smooth muscle cell populations using the scMetabolism algorithm21. By comparing the landscape of VSMCs in aortic tissue from patients with and without AD, we observed activation of the glycolysis and pyruvate metabolism pathways, along with suppression of OXPHOS in AD samples (Fig. 3A). Additionally, we conducted gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses on H19-positive VSMC populations from patients with AD and compared the results with those of H19-negative VSMC populations. GO analysis revealed that H19 is associated with mRNA splicing (P = 4.93E-14), adenosine triphosphate (ATP) metabolism (P = 2.20E-11), and muscle cell differentiation (P = 4.53E-08) (Fig. 3B). The KEGG analysis indicated that H19 is associated with OXPHOS (P = 1.28E-15), the spliceosome (P = 1.66E-07), and VSMC contraction (P = 1.25E-06) (Fig. 3C). Glycolytic stress testing revealed a significant increase in the extracellular acidification rate (ECAR), which reflects glycolytic activity, and glycolytic capacity in PDGF-BB-treated VSMCs (Fig. S6B). The mitochondrial membrane potential plays a crucial role in mitochondrial ATP synthesis22. To assess the mitochondrial membrane potential, we utilized tetramethylrhodamine ethyl ester (TMRE), a cell-permeant dye that accumulates in mitochondria in proportion to membrane potential23. Our findings revealed significant mitochondrial polarization in PDGF-BB-treated VSMCs compared with control VSMCs (Fig. S6C). Additionally, we observed a significant increase in mitochondrial membrane potential and downregulation of F-actin expression in PDGF-BB-treated VSMCs (Fig. S6D). These findings suggest that metabolic reprogramming occurs in the aortas of patients with AD, whose VSMCs exhibit a preference for glycolysis and inhibition of OXPHOS.
A Dotplot showing metabolic characteristics of vascular smooth muscle cells from patients with/without aortic dissection using scMetabolism algorithm based on the GSE213740. The circle size and color darkness both represent the scaled metabolic score. B, C GO analysis (D) and KEGG (E) analysis between H19-positive and H19-negative vascular smooth muscle cells in GSE213740. D After transfection with the indicated constructs for 36 hours, followed by PDGF-BB (20 ng/mL) treatment for 24 h, RNA sequencing was performed in human primary VSMCs. GMRSP regulates PKM pre-mRNA splicing, promoting PKM1 isoform formation and inhibiting PKM2 isoform formation. E Representative immunofluorescent images of F-actin (red), PKM1 (yellow), and PKM2 (green) in human primary VSMCs were determined, Scale bar: 50 μm. The seahorse glycolysis stress test (n = 8 biologically independent samples) (F) and representative images of TMRE staining, Scale bar: 50 μm (G), F-actin and MitoTracker staining, Scale bar: 5 μm (H) were obtained in human primary VSMCs transfected with the indicated constructs for 36 h, followed by PDGF-BB (20 ng/mL) treatment for 24 hours. Data are presented as the mean ± SD. The p values were calculated by a two-tailed unpaired Student’s t-test in (F). The data presented in (E–G) are representative of three independent experiments. Source data are provided as a Source Data file. GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PDGF, platelet-derived growth factor; VSMCs, vascular smooth muscle cells; GMRSP; glucose metabolism regulatory small protein; PKM, pyruvate kinase M; TMRE, tetra-methyl-rhodamine ester.
On the basis of the above single-cell data analysis, we speculated that GMRSP inhibits metabolic reprogramming and maintains the contractile phenotype of VSMCs by regulating mRNA splicing. To investigate the mechanism by which GMRSP maintains the contractile phenotype, RNA sequencing and AS analyses were conducted to identify changes in gene expression in human primary VSMCs overexpressing GMRSP compared with control plasmid-treated cells upon stimulation with PDGF-BB (Fig. S6E and S6F). Using a ∆PSI cutoff of 0.1 and an FDR < 0.05, we identified 2245 GMRSP-regulated AS events (Fig. S6E and S6F). We observed the impact of GMRSP on PKM pre-mRNA splicing, which resulted in the expression of the PKM1 isoform and the inhibition of PKM2 isoform expression (Fig. 3D). To validate the effect of GMRSP on PKM pre-mRNA splicing, we performed RT‒PCR and restriction digestion assays to assess the levels of PKM1 and PKM2 mRNA (Fig. S6G). GMRSP overexpression resulted in increased levels of PKM1 mRNA and decreased levels of PKM2 mRNA, whereas mutation of the H19 ORF start codon had no effect on PKM splicing regulation (Fig. S6G). Immunofluorescence analysis yielded consistent results and demonstrated changes in protein expression levels (Fig. 3E). GMRSP overexpression counteracted the PDGF-BB-induced increase in the ECAR and the increase in glycolytic capacity (Fig. 3F). Similarly, GMRSP also attenuated the PDGF-BB-induced lactate production and glucose uptake (Fig. S6H and S6I). GMRSP overexpression reversed the PDGF-BB-induced mitochondrial damage and phenotypic transition of VSMCs (Fig. 3G, H). Collectively, these findings indicate that GMRSP regulates the pre-mRNA expression of PKM, promoting the expression of PKM1 isoform and inhibiting the PKM2 isoform. This regulation reduces aerobic glycolysis and helps maintain the contractile phenotype of VSMCs, providing insight into the mechanisms underlying VSMC metabolic reprogramming in AD.
Activation of PKM2 Reverses the Protective Effect of GMRSP in BAPN Monofumarate-Induced AD both in vitro and in vivo
To investigate the role of GMRSP in regulating the AS of PKM and its impact on PKM1 and PKM2 mRNA and protein expression, we employed TEPP-46 to disrupt the protective function of GMRSP. TEPP-46 is a PKM2-specific activator that promotes the conversion of PKM2 dimers to tetramers, enhancing their enzymatic activity and promoting a more active glycolytic state24. VSMCs were transfected with the indicated constructs for 36 h. After transfection, cells were treated with TEPP-46 (20 µmol/L) and PDGF-BB (20 ng/mL) for 24 h. The GMRSP protein levels were detected (Fig. S7A). TEPP-46 significantly increased PKM2 enzyme activity according to the results of the pyruvate kinase assay (Fig. S7B). GMRSP inhibited the migration of and MMP2 secretion in PDGF-BB-treated human primary VSMCs (Fig. S7C–S7E). However, these inhibitory effects were disrupted after TEPP-46 treatment (Fig. S7C–S7E). Additionally, a Seahorse assay showed that TEPP-46 aggravated the metabolic dysfunctions, which were initially ameliorated by GMRSP (Fig. 4A). Finally, TEPP-46 reversed the inhibitory effect of GMRSP on lactate production and glucose uptake in PDGF-BB-treated VSMCs (Fig. S7F and S7G). Despite the positive effect of GMRSP on the mitochondrial membrane potential, TEPP-46 significantly reduced its protective effect in PDGF-BB-treated VSMCs (Fig. 4B). Furthermore, TEPP-46 reversed the GMRSP-induced increase in mitochondrial size and disrupted the maintenance of the contractile phenotype (Fig. 4C). To examine whether TEPP-46 could also reverse metabolic and phenotypic changes in SM22α+GMRSPOE-flox mice, we administered TEPP-46 by oral gavage for seven days following tamoxifen induction (Fig. 4D). In the in vivo study, compared with those without TEPP-46 treatment, the vascular injury of the TEPP-46-treated SM22α + GMRSPOE-flox mice was aggravated after BAPN monofumarate induction (Fig. 4E). Similarly, TEPP-46-treated SM22α+GMRSPOE-flox mice exhibited a higher incidence of dissection and increased mortality compared to untreated controls (Fig. 4F and G). GMRSP effectively reduced aortic wall thickness and alleviated the degradation of elastic fibers, which the therapeutic effect of GMRSP was reversed by TEPP-46 treatment (Fig. 4H-J). In addition, GMRSP significantly inhibited the BAPN monofumarate-induced increase in the plasma expression levels of PKM2 and lactate (Fig. S7H and S7I). However, TEPP-46 reversed the GMRSP overexpression-induced changes in lactate levels (Fig. S7H and S7I). These findings indicate that PKM2 activation could reverse the protective effect of GMRSP on metablic changes and contractile phenotype of VSMCs both in vitro and in vivo.
Seahorse glycolysis stress test (n = 8 biologically independent samples) (A), representative images of TMRE staining, Scale bar: 50 μm (B), F-actin and MitoTracker staining, Scale bar: 50 μm (C) were performed in human primary VSMCs transfected with the indicated constructs for 36 h, followed by TEPP-46 (20 µmol/L) and PDGF-BB (20 ng/mL) treatment for 24 h. D SM22α+GMRSPOE-flox mice were induced with tamoxifen for 5 days at two weeks. One week after the last tamoxifen treatment, mice were treated with 0.25% BAPN monofumarate and TEPP-46 (10 mg/kg) for four weeks (n = 10 biological replicates per group). E Representative images of the mouse aorta are shown. F Maximum aortic diameter measurement (n = 10 biological replicates per group). G Survival rate estimation using the Kaplan-Meier method and comparison using the Breslow test (n = 10 biological replicates per group). H Representative macroscopic images of aorta sections were stained with H&E and EVG, Scale bar: 200 μm and 50 μm. The wall thickness (I) and elastin integrity (J) of each groups were analyzed(n = 10 biological replicates per group). Data are presented as the mean ± SD. The p values were calculated by a two-tailed unpaired Student’s t-test in (A, F, I, J). The p values were calculated by Kaplan–Meier analysis and two-sided Breslow test (G). The data presented in (A, B, C, H) are representative of three independent experiments. Source data are provided as a Source Data file. BAPN, β-aminopropionitrile.
The expression level of lactate and PKM2 in the plasma could increase the diagnostic performance and predict the prognosis of patients with AD
Glycolysis is regulated by three rate-limiting enzymes: hexokinase (HK), phosphofructokinase (PFK), and PKM. We observed higher PKM2/PKM1 expression at both the mRNA and protein levels in aortic tissues from AD patients compared to normal aortic tissues (Fig. 5A and S8A), suggesting enhanced glycolysis in AD. Furthermore, we detected negative correlations between PKM2 mRNA levels and H19 levels (R = − 0.8491, P < 0.001) and positive correlations between PKM1 mRNA levels (R = 0.5434, P = 0.0011) and H19 levels in tissue samples (Fig. 5B). Additionally, the protein levels of PKM2 and PKM1 were negatively correlated (R = − 0.5249, P = 0.0015) and positively correlated (R = 0.4207, P = 0.0066), respectively, with the levels of GMRSP in tissue samples (Figure S8B). Multiplex immunofluorescence assays yielded the same results (Fig. 5C).
A The PKM splicing assay was performed in the aorta of controls without AD (n = 7 biologically independent samples) and patients with AD (n = 9 biologically independent samples). B The levels of PKM1 and PKM2 mRNA were positively and negatively correlated with the levels of GMRSP mRNA in the aorta of controls without AD (n = 7 biologically independent samples) and patients with AD (n = 9 biologically independent samples), respectively. C Immunofluorescence staining of PKM1, GMRSP, PKM2 and SM22α in the aorta from controls without AD (n = 5 biologically independent samples) and patients with AD (n = 5 biologically independent samples) were shown. Nuclei were counterstained with DAPI, Scale bar: 100 μm. D Distribution of plasma levels of PKM2 in healthy controls (n = 60 biologically independent samples) and patients with type B AD (n = 89 biologically independent samples)or type A AD (n = 56 biologically independent samples). E Receiver operating characteristic curves: patients with AD vs. healthy controls for PKM2. F The discrepancy of the maximum aortic diameter in patients with high (>2.40 ng/mL,n = 68 biologically independent samples) or low levels of PKM2 ( ≤ 2.40 ng/mL,n = 77 biologically independent samples). G Cumulative Kaplan-Meier estimates of MACE during follow-up stratified by the levels of PKM2 (2.40 ng/mL). Data are presented as the mean ± SD. The p values was calculated using two-sided Spearman’s correlation test in B. The p values were calculated by a two-tailed unpaired Student’s t-test in (D, F). Area under the Receiver operating characteristic curve (AUC) and the 95% confidence interval (CI) were used to evaluate the predictive accuracy of PKM2(E). The p values were calculated by Kaplan–Meier analysis and two-sided Breslow Test (G). Source data are provided as a Source Data file. MACE, major adverse events.
In our prospective dataset, we enrolled 205 individuals, including 60 controls, 89 patients with type B AD, and 56 patients with type A AD. The baseline characteristics and clinical parameters of the participants are presented in Supplementary Table S3. We observed a moderatepositive correlation between PKM2 levels and lactate (R = 0.29, P < 0.001; Fig. S8C). The expression levels of PKM2 were increased across the groups, with a median of 1.47 (interquartile range [IQR]: 1.27–1.87) ng/mL in controls, 2.26 (1.64–2.90) ng/mL in patients with type B AD, and 2.42 (1.98–3.34) ng/mL in patients with type A AD (P < 0.001; Fig. 5D). To assess the utility of plasma PKM2 concentrations as a potential biomarker of AD, we evaluated the predictive ability of PKM2 using the area under the receiver operating characteristic curve, which yielded a value of 0.78 (95% CI, 0.71–0.84; Fig. 5E). Furthermore, plasma PKM2 concentrations remained an independent predictor (OR, 5.95; 95% CI, 2.30–15.40) for the occurrence of AD even after adjusting for common risk factors, including age, sex, smoking status, history of hypertension, coronary artery disease, diabetes, hyperlipemia, chronic kidney disease, and anemia. To enhance clinical applicability, we categorized the PKM2 levels into two groups according to the median value of PKM2: high risk (PKM2 > 2.4 ng/mL) and low risk (PKM2 ≤ 2.4 ng/mL). Although no significant difference in survival rate was observed between the groups (Fig. S8D), patients with PKM2 > 2.4 ng/mL exhibited a larger aortic diameter (median [IQR]: 44.50 [38.25–46.75] vs. 40.0 [36.75–44.25] mm, respectively, P = 0.024; Fig. 5F) and a higher incidence of major adverse events (MAE), including dissection related death, rupture, retrograde type A aortic dissection [RTAD], stent graft induced new entry tear [SINE], secondary endoleaks, and follow up re-intervention (P = 0.015; Fig. 5G). CT angiography (CTA) results revealed that patients with high PKM2 expression had a larger aortic diameter (Fig. S8E). Additionally, the PKM2 levels in the supernatant of cultured VSMCs from AD patients were significantly higher than those in control VSMC supernatants (Fig. S8F). The level of PKM2 in the supernatant of normal smooth muscle cells treated with PDGF-BB was also upregulated (Fig. S8G).
GMRSP interacts with the glycine-rich domain of hnRNPA2B1 and Inhibits hnRNPA2B1-mediated PKM Pre-mRNA splicing
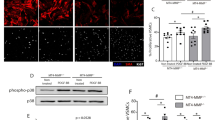
To further investigate the mechanism inhibiting the transition of VSMCs from the contractile to synthetic phenotype, we performed (co-immunoprecipitation) co-IP combined with MS to identify proteins interacting with GMRSP (Fig. 6A). In all, 165 proteins that interact with GMRSP were identified and are listed in Table S2. Among these proteins, the splicing factor hnRNPA2B1 was particularly interesting, and its unique peptide was detected (Fig. S9A). hnRNPA2B1 is a key regulator of the phenotypic transition in pulmonary arterial hypertension (PAH)-PASMCs25 and binds repressively to sequences flanking exon 9 of PKM pre-mRNA, promoting exon 10 inclusion. This mechanism ensures a high PKM2/PKM1 ratio, facilitating cellular metabolic reprogramming26. To confirm the interactions between GMRSP and hnRNPA2B1, we performed co-IP experiments with or without RNase A treatment (Fig. 6B, C) and conducted fluorescence colocalization studies (Fig. 6D). HnRNPA2B1 consists of two N-terminal RNA recognition motifs (RRM1 and RRM2), a glycine-rich (Gly-rich) motif, and a nuclear targeting sequence (M9)27. To determine the hnRNPA2B1 motif required for its interaction with GMRSP, we constructed truncated fusion constructs of hnRNPA2B1 with a C-terminal HA tag and cotransfected them with the GMRSP-Flag vector in HEK293T cells (Fig. 6E). Our results revealed that only hnRNPA2B1 constructs containing the Gly-rich motif bound to GMRSP, which indicates the essential role of the Gly-rich motif in GMRSP binding (Fig. 6F, G). Furthermore, molecular docking simulations revealed that residues L12, C16, and T94 in GMRSP interact with residues G217, G218, G221, and Y222 in hnRNPA2B1, forming salt bridges and hydrogen bond (Fig. 6H). In vitro co-IP experiments further supported the direct binding between GMRSP and hnRNPA2B1, and mutations in critical amino acid residues disrupted this interaction (Fig. 6I–L). To provide additional evidence of this interaction, we then performed microscale thermophoresis (MST) analysis using purified GMRSP, GMRSPL/C/T, and hnRNPA2B1. The fluorescence-labeled recombinant full-length hnRNPA2B1 bound to recombinant GMRSP and GMRSPL/C/T, with KD values of 3.1885E-7 and 2.0333E-5, respectively (Fig. 6M). Collectively, these findings suggest that the L12, C16, and T94 residues in GMRSP interact with the Gly-rich domain (G217, G218, G221, and Y222) of hnRNPA2B1.
A Silver staining identifies the specific bands for GMRSP-co-IP. B The GMRSP-Flag plasmid was transfected into HEK293T cells, cellular lysates were treated with 10 mg/mL RNase A for one hour or left untreated, GMRSP-Flag complexes were co-IPed by anti-Flag antibody, and hnRNPA2B1 was determined. C The hnRNPA2B1-HA plasmid was transfected into HEK293T cells, cellular lysates were treated with 10 mg/mL RNase A for one hour or left untreated, hnRNPA2B1-HA complexes were co-IPed by anti-HA antibody, and GMRSP was determined. D Immunofluorescence of GMRSP (red) co-localized with hnRNPA2B1 (green) in VSMCs. Nuclei were stained with DAPI (blue), Scale bar: 20 μm. E An illustration of the truncated hnRNPA2B1 domains is shown. F, G The WT and the indicated mutants of hnRNPA2B1-HA, together with the GMRSP-Flag vector, were co-transfected into HEK293T cells. hnRNPA2B1-HA (F) and GMRSP-Flag (G) complexes were co-IPed with anti-HA and anti-Flag antibodies, respectively, to detect GMRSP-Flag and hnRNPA2B1-HA, respectively. H Molecular docking analysis of the GMRSP and hnRNPA2B1 Gly-rich domains. I, L The indicated hnRNPA2B1-HA mutant (hnRNPA2B13G/Y: G217A, G218A, G221A, and Y222A) together with the GMRSP-Flag vector were co-transfected into HEK293T cells. The indicated GMRSP-Flag mutants (GMRSPL/C/T: L12A, C16A, and T94A), together with the hnRNPA2B1-HA vector, were co-transfected into HEK293T cells. The interaction of the hnRNPA2B1 with GMRSP was determined using anti-Flag antibodies (I and J) and anti-HA antibodies (K and L). M MST detection of interactions between GMRSP and GMRSPL/C/T with hnRNPA2B1, respectively(i = 3 biologically independent samples). Data are presented as the mean ± SD. The data presented in (A-M) are representative of three independent experiments. Source data are provided as a Source Data file. Co-IP, co-immunoprecipitation; hnRNP, heterogeneous nuclear ribonucleoprotein; MST, microScale thermophoresis.
To investigate whether GMRSP regulates the AS of PKM pre-mRNA by binding to hnRNPA2B1, we performed RNA pull-down assays using 5’ biotin-labeled PKM RNA EI9 (50–68) containing the UAGGGC sequence and a mutant version, EI9 (50–68) G3C (where the G3 nucleotide was mutated to C in UAGGGC), as previously described28. Our results showed that hnRNPA2B1 was directly bound to the EI9 (50–68) sequence of PKM, whereas GMRSP did not bind directly to the EI9 sequence (Fig. S9B). GMRSP expression significantly reduced hnRNPA2B1’s ability to bind to the EI9 sequences of PKM in a dose-dependent manner (Fig. S9C), suggesting a modulatory role in the regulation of PKM splicing. Furthermore, we observed that GMRSP expression inhibited the binding of the hnRNPA2B1 Gly-rich domain to the EI9 sequence of PKM (Fig. S9D). Additionally, GMRSP failed to inhibit the binding of a mutant hnRNPA2B1 (hnRNPA2B13G/Y) to the EI9 sequence of PKM (Fig. S9E). Moreover, when the L12, C16, and T94 residues in GMRSP were mutated, GMRSP lost its ability to regulate hnRNPA2B1 binding to the EI9 sequence of PKM (Fig. S9F). To further investigate the inhibitory effect of GMRSP on hnRNPA2B1 binding, we performed UV crosslink IP (CLIP) experiments. Our findings showed that hnRNPA2B1 strongly bound to exon 9 of the PKM pre-mRNA (Fig. S9G). GMRSP markedly reduced this binding, an effect that was abolished when the L12, C16, and T94 residues in GMRSP were mutated (Fig. S9G). Overall, our data suggest that GMRSP interacts with hnRNPA2B1 and inhibits hnRNPA2B1-mediated AS of pre-mRNA PKM.
The GMRSP-HnRNPA2B1 interaction is critical for maintaining the contractile phenotype and oxidative metabolism of VSMCs
To examine the effects of GMRSP on the functions of hnRNPA2B1, we coexpressed H19 ORF-Flag, a construct expressing GMRSP, or ORFmut-Flag (mutant control), along with hnRNPA2B1 in PDGF-BB-treated VSMCs. Our findings revealed that GMRSP had no effect on the protein level of hnRNPA2B1 (Figs. 7A, S10A, and S10B). However, GMRSP effectively blocked the hnRNPA2B1 overexpression-induced increase in PKM2 mRNA and protein levels, the decrease in PKM1 mRNA and protein levels, and the phenotypic transition of the VSMCs (Figs. 7A–C, S10A, and S10B). Moreover, GMRSP antagonized the hnRNPA2B1 overexpression-induced increase in cell migration and MMP2 secretion in PDGF-BB-treated VSMCs (Fig. S10C–E). GMRSP also counteracted the promoting effect of hnRNPA2B1 on metabolic dysfunction, as detected by the seahorse assay (Fig. 7D). Additionally, GMRSP reversed the suppression of the membrane potential, mitochondrial size, and phenotypic transition induced by hnRNPA2B1 overexpression (Fig. 7E, F). Notably, GMRSP attenuated hnRNPA2B1 overexpression-induced lactate production and glucose uptake in PDGF-BB-treated VSMCs (Fig. S10F and S10G). In summary, our results demonstrate that GMRSP suppresses hnRNPA2B1-dependent PKM splicing, inhibits PKM2 isoform formation, and preserves the contractile phenotype and oxidative metabolism of VSMCs.
A–F The GMRSP-Flag vector, together with the hnRNPA2B1-HA vector, was co-transfected into human primary VSMCs for 36 h, followed by PDGF-BB (20 ng/mL) treatment for 24 h (n = 5 per group). The indicated protein levels were detected (A). The PKM splicing assay was performed (B). Representative immunofluorescent images of F-actin (red), PKM1 (yellow), and PKM2 (green) were determined, Scale bar: 50 μm (C). The seahorse glycolysis stress test (n = 8 biologically independent samples) (D) representative images of TMRE staining, Scale bar: 50 μm (E), and MitoTracker staining, Scale bar: 50 μm (F) were performed. Data are presented as the mean ± SD. The p values were calculated by a two-tailed unpaired Student’s t-test in D. The data presented in (A–F) are representative of three independent experiments. Source data are provided as a Source Data file.
EVs-GMRSP inhibit contractile-to-synthetic phenotype transition and attenuateAD formation In Vitro and In Vivo
To investigate the therapeutic potential of GMRSP in the prevention and treatment of AD, we isolated and cultured VSMCs from patients with AD and observed significant downregulation of contractile markers (SM22α and SMA) and GMRSP in VSMCs from patients with AD compared with normal VSMCs obtained from donors (Fig. S11A and S11B). Extracellular vesicles (EVs), a component of cell paracrine signaling, offer several advantages over cell therapy, as they overcome the drawbacks associated with cell transplantation, evade systemic immune rejection, and possess targeting capabilities29. Therefore, we engineered MOVAS-derived EVs to overexpress GMRSP- or GMRSPL/C/T using genetic engineering techniques. This strategy endows EVs with the ability to target VSMCs. Transmission electron microscopy (TEM) images revealed the presence of heterogeneous round or elliptical membranous vesicles in all the EV groups (Fig. 8A). NTA revealed average particle sizes that ranged from 100 to 150 nm (Fig. 8B). Furthermore, purified EVs were confirmed via the use of specific EV biomarkers, including CD9, CD63, TSG101, Alix, and GM130 (Fig. S11C). Importantly, the presence of the Flag tag was detected in the engineered EV-GMRSP and EV-GMRSPL/C/T groups, whereas the blank EV group did not exhibit this signal (Fig. S11C). Fluorescence confocal microscopy revealed significant enrichment of EVs from all the groups within the VSMC population (Fig. 8C). Furthermore, we detected that MOVAS-derived EV-GMRSP specifically targeted VSMCs in vivo (Fig. S11D).
A Representative images of EVs were visualized using TEM. Scale bars: left, 50 nm; right, 100 nm. B The particle size of EVs was measured by NTA. C Representative images of the internalization of mCherry (red)-labeled EVs were visualized, Scale bar: 50 μm. D A schematic diagram of the experimental therapy: mice were intravenously injected with 500 ug/kg/day EVs for 7 days. Meanwhile, EV-treated mice were also treated with 0.25% BAPN monofumarate for four weeks (n = 15 biological replicates per group). E Representative images of the aorta of the mice are shown. F The survival rate was estimated using the Kaplan-Meier method(n = 15 biological replicates per group). G, H AD incidence and maximum aortic diameter were detected (n = 15 biological replicates per group). I Representative macroscopic images of aorta sections were performed with H&E staining and EVG staining, Scale bar: 200 μm and 50 μm. J Schematic representation of signaling events mediated by GMRSP signaling during the pathogenesis of AD.Data are presented as the mean ± SD. The p values were calculated by a two-tailed unpaired Student’s t-test in H. The p values were calculated by Kaplan–Meier analysis and two-sided Breslow test (F). The data presented in (A, B, C, I) are representative of three independent experiments.Source data are provided as a Source Data file. EV, extracellular vesicles; TEM, transmission electron microscope; NTA, nanoparticle tracking analysis.
We subsequently examined the therapeutic effects of the EVs-GMRSP and EVs-GMRSPL/C/T in vitro. Our results revealed that, compared with EVs-GMRSPL/C/T, EVs-GMRSP significantly inhibited the migration of primary VSMCs from patients with AD and reduced MMP2 secretion by these cells (Fig. S11E–S11G). Additionally, EVs-GMRSP attenuated the increase in PKM2 mRNA and the decrease in PKM1 mRNA in VSMCs from patients with AD, whereas EVs-GMRSPL/C/T did not elicit the same effect (Fig. S11H). Moreover, the EVs-GMRSP effectively suppressed lactate production and glucose uptake in VSMCs from patients with AD (Fig. S11I and S11J). Furthermore, we evaluated the potential of EVs-GMRSP as a therapeutic agent for the prevention of AD in vivo (Fig. 8D). Remarkably, the EVs-GMRSP resulted in significant reductions in vascular injury, the incidence of AD, and the mortality rate compared with EVs-GMRSPL/C/T and blank EVs (Fig. 8E–H). Moreover, the EVs-GMRSP significantly reduced aortic wall thickness and alleviated the degradation of elastic fibers (Fig. 8I and Fig. S12A-12B). EVs-GMRSP significantly promoted SM22α, α-SMA and PKM1 expression and inhibited BAPN monofumarate-induced MMP2 and PKM2 expression in VSMCs from AD mice (Fig. S12C–S12E). These findings suggest that GMRSP is a promising target for the prevention and treatment of AD.
Discussion
In this study, we identified and characterized a functional small protein, GMRSP, encoded by the putative lncRNA H19. Our study focused on the role of this protein in the regulation of the phenotypic transition of VSMCs and in vascular remodeling. Mechanistically, GMRSP directly interacts with hnRNPA2B1, inhibiting hnRNPA2B1-mediated AS of PKM and the consequent reduction of PKM2 production. This, in turn, results in the reprogramming of vascular metabolic profiles. Additionally, we observed a significant increase in the plasma levels of PKM2 in patients with AD, which strongly correlates with a poor prognosis and highlights the potential of PKM2 as a diagnostic and therapeutic target for AD.
Numerous studies have discussed the dual effects of lncRNA H19 in cardiovascular diseases. Some studies have reported a protective role of lncRNA H19 in conditions such as diabetic retinopathy, cardiac hypertrophy, endothelial cell aging, and cardiac ischemia‒reperfusion injury30,31,32,33. Conversely, other studies have reported adverse consequences associated with lncRNA H19 in atherosclerotic calcification, ischemic stroke, and abdominal aortic aneurysm (AAA)17,34,35. Although elevated lncRNA H19 expression has been reported in human AAA/AD tissues as well as in AngII-stimulated and porcine pancreatic elastase-induced animal models17,36,37, our sequencing data revealed significant downregulation in tissues from patients with AD. This finding was further confirmed in a publicly available GEO dataset (GSE153434; Fig. S1A), which compared the transcriptome characteristics of patients with type A AD with those of healthy controls. This discrepancy might be partly attributed to large interindividual variability, different stimulus durations, and different animal models.
Thus, lncRNA H19 expression not only varies between AAA and AD but also across different experimental models of vascular diseases38. Intriguingly, using MS, we identified a protein (GMRSP) encoded by the putative nonprotein-coding lncRNA H19 and validated its low expression in tissues from patients with AD, mice with BAPN monofumarate-induced AD, and PDGF-BB-stimulated human primary VSMCs via a custom-made GMRSP-specific antibody. Furthermore, we demonstrated that the protein, rather than the lncRNA, prevented the phenotypic transition of VSMCs and MMP2 secretion, thereby suppressing BAPN monofumarate-induced AD occurrence and progression.
Emerging evidence suggests that metabolic disorders, including disturbances in amino acid metabolism, glycometabolism, and lipid metabolism, are involved in the pathogenesis of aortic aneurysm and dissection by affecting multiple functional aortic cells39. Our retrospective clinical data revealed that deceased patients presented higher lactate values upon hospital admission. Additionally, we observed increased glycolysis and lactate production in PDGF-BB-stimulated VSMCs. These findings suggest that glucose metabolic distress may contribute to the pathophysiological deterioration of AD. Further supporting this notion, a human aortic single-cell RNA sequencing dataset revealed reduced OXPHOS and elevated glycolysis in H19-positive VSMCs20. These findings indicate that the protective effect of GMRSP may be mediated through the regulation of glucose metabolism-related targets. Previous studies have proposed that energy metabolism reprogramming can facilitate the phenotypic transition of VSMCs6,7,40,41. The inhibition of PKM2 and lactate dehydrogenase A, two rate-limiting enzymes involved in the transformation of pyruvate into lactate, has been shown to suppress VSMC proliferation, migration, and secretion6,41. However, the role of metabolic distress in VSMCs during AD, as well as its upstream regulation, remains unclear. Our findings further support the role of the suppression of glycolysis in maintaining the contractile properties of VSMCs5 and reveal the mechanism by which metabolic homeostasis is regulated through the H19-encoded protein, GMRSP.
To elucidate the mechanism by which GMRSP regulates glucose metabolism, we conducted transcriptome sequencing after GMRSP overexpression and reported disrupted PKM2/PKM1 AS events. Although a few transcripts feature skips from exon 8 to exon 11, PKM1 and PKM2 are the two major isoforms of PKM (Fig. 3D), which result from the inclusion of either exon 9 (PKM1) or 10 (PKM2). Exon skipping is closely related to the occurrence and development of many diseases. Studies have shown that exon 47 skipping in FBN1 preferentially leads to cardiovascular defects and is closely related to TAAD42. In addition, polypyrimidine variants in ABCA1 cause exon skipping and contribute to HDL cholesterol deficiency in familial early-onset coronary heart disease43. PKM1 is expressed primarily in differentiated adult tissues, where it promotes OXPHOS, whereas PKM2 is expressed mainly in embryonic cells and highly proliferative cells, including proliferating VSMCs, where it facilitates aerobic glycolysis6,13,44,45. Imbalances in the levels of PKM2/PKM1 can lead to chromosome segregation issues, mitochondrial dysfunction, and altered biosynthesis, thereby contributing to cell proliferation, cell cycle progression, and tumorigenesis46,47,48,49. Our clinical data, which were used for prognostic analyses, indicate that patients with AD with high levels of PKM2 had poor outcomes (Fig. 5H). Previous studies have reported that hnRNP family proteins, particularly the polypyrimidine tract-binding proteins hnRNPA1 and hnRNPA2, can regulate the AS of pre-PKM mRNA26. Through co-IP followed by LC‒MS and MST, our research revealed that GMRSP can directly bind to hnRNPA2B1. Furthermore, GMRSP inhibits hnRNPA2B1-mediated PKM2 production, thereby attenuating aerobic glycolysis. Additionally, we found that PKM2 activation by TEPP-46 could counteract the protective effect of GMRSP in BAPN monofumarate-induced SM22α+GMRSPOE-flox AD mice, which further confirms the pivotal role of the GMRSP-hnRNPA2B1-PKM axis in AD.
Despite the promising results of GMRSP-AAV2 overexpression in vivo, its clinical application is greatly restricted due to poor bioavailability and immunogenicity50. To overcome these limitations, we employed MOVAS-derived exosomes as a delivery system for the GMRSP protein. Treatment with these exosomes resulted in a significant improvement in the incidence and severity of AD. Cellular experiments further confirmed that exosome-loaded GMRSP could penetrate the nucleus and suppress the phenotypic transition of VSMCs. Our future work will focus more efforts on improving the natural targeting ability of GMRSP. In summary, we have demonstrated the vital role of the lncRNA H19-encoded protein in maintaining metabolic homeostasis and the contractile properties of VSMCs by targeting hnRNPA2B1-mediated PKM2 production. These findings provide alternative targets for the intervention and treatment of AD.
Methods
The study was approved by the Institutional Review Board of Guangdong Provincial People’s Hospital in Guangzhou, China, and adhered to the principles outlined in the Declaration of Helsinki. The software, primers, and reagents used in this study are listed in the Supplementary Table S1.
Study population and data collection
Written informed consent was obtained from all participants involved in the study. From 2019 to 2022, a total of 89 type B AD, 56 type A AD, and 60 controls were recruited. The inclusion criteria were as follows: (1) patients with AD > 18 years of age; (2) obvious clinical manifestations of AD, such as sudden onset of severe chest, back, or abdominal pain; and (3) false lumen or free intima detected using computed tomography angiography (CTA). Patients with connective tissue diseases, cancer, aortic trauma, pseudoaneurysm, or infectious diseases were excluded. Detailed patient information is summarized in Supplementary Table S3. The control individuals, matched for sex and age, were recruited from those suspected of AD but excluded by CTA in our hospital.
Blood samples were collected from all participants before endovascular or surgical treatments within 12 h of admission, and plasma was separated by immediate centrifugation and stored at −80 °C until further analysis. Besides, human aortic tissues were collected from type A aortic dissection patients who underwent cardiothoracic surgery in accordance with the Helsinki Declaration. Aortic tissue samples from individuals without AD were obtained from heart donors during explantation of Guangdong Provincial People’s Hospital. This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (approval number: 2019KY094).
Animal studies
All animal experiments conducted in this study followed the Guidelines for the Care and Use of Laboratory Animals, jointly formulated by the National Institutes of Health and the National Academy of Sciences. The animal program was approved by the Ethics Committee of Guangdong Provincial People’s Hospital, ensuring humane care and avoiding cruelty to animals. GMRSP OE-Flox mice (OE) were generated by Cyagen Biosciences, Inc. The sequences of genotyping primers are listed in Table S1. Tagln-CreERT2 mice (Cat. NO. NM-KI-225006) were purchased from Shanghai Model Organisms Center, Inc. All wild-type C57BL/6 J mice used in this study were obtained from GemPharmatech Co., Ltd. Two-week-old mice were intraperitoneally injected once per day with either vehicle (oil) or tamoxifen (75 mg/kg) for 5 days to induce Cre-Tagln activity. One week after the last tamoxifen treatment, mice were treated TEPP-46 (10 mg/kg) for four weeks via oral gavage once a day. Three-week-old wild-type mice were injected with the respective virus (1 × 1011 plaque-forming units per mouse) via the tail vein. Additionally, 3-week-old wild-type mice received the indicated EVs (500 µg/kg/day) via the tail vein for 7 days. Wild-type C57BL/6 J, Tagln-GMRSP-OE-Flox, and AAV-GMRSP-OE mice aged 3.5 weeks were administered BAPN monofumarate in their drinking water for four weeks to induce AD. Adult male and female mice were used for verifying the function of GMRSP in AD. The underlying mechanisms were analyzed in male mice. All mice were maintained in a sterile barrier facility with light (reverse 12 h light/12 h dark cycle), humidity (55 ± 10%), and temperature (25 °C) control, provided with normal chow diets (#GDMLAC-224) and sterile water. Intraperitoneal anesthesia with 1% pentobarbital sodium was administered to euthanize the mice, and samples were collected. No animals were excluded from any of the animal studies, and all animal experiments and data analysis were conducted by two independent researchers using a blind method.
RNA sequencing and data analysis
Tissue or cell samples were used for RNA isolation with the Trizol Reagent (10296010, Invitrogen, CA, USA). Sequencing libraries were generated using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, USA). All samples underwent Illumina Novaseq6000 sequencing (Novogene Company, Guangzhou, China) with a paired-end 150 bp read length. The following data filtering schemes were applied: (1) removal of reads with adapter sequences; (2) removal of reads with a percentage of unknown bases (N) > 10%; and (3) removal of reads with >50% of bases having a mass value of ≤5 in a single reading. The default parameter (fastq) was used for the quality assessment of RNA-sequencing readings and the removal of residual adapter sequences or low-quality reads2. The first 18 bases of each read were trimmed due to the GC deviation at the read start. After quality control, the clean reads were mapped to the GRCh38 human reference sequence (accessible at ftp://ftp.ncbi.nlm.nih.gov/genomes/all/GCA/000/001/405/GCA_000001.405.15_GRCh38/seqs_for_alignment_pipelines.ucsc_ids/GCA_000001405.15_GRCh38_no_alt_analysis_set.fna.gz) using hisat2 (hisat2 -p 10 --dta -xGCA_000001405.15_GRCh38_no_alt_analysis_set -1 sample.1.fastq.gz -2 sample.2.fastq.gz | samtools sort-@ 10 -o sample.bam)3,4. Alternative splicing (AS) events were detected using the rMATS program on the hisat2 output bam file5.
Single cell RNA (scRNA) sequencing data analysis
Human aortic dissection scRNA sequencing dataset was downloaded from GEO with the accession number GSE21374020. R software (Seurat package [V4.4.0]) were used for the downstream analysis. Low-quality cells were excluded based on the following criteria: <600 or >5000 genes; >30% mitochondrial genes. The Seurat manual instructions were followed, and the actions were as follows: NormalizeData, ScaleData, and FindClusters with resolution of 0.3. Each cell cluster was visualized using uniform manifold approximation and projection (UMAP), and variable genes were found using FindVariableGenes. Cells were annotated according to the marker genes as follow: T cells (CD3D, CD3E, GZMA, NKG7), endothelial cells (VWF, CLDN5, ICAM2, CDH5), fibroblasts (FGF7, MME, DCN, LUM, FBN1, COL1A1, COL3A1), neutrophil (CST3, LYZ, FCGR3B, CSF3R), monocytes (CD68, CD163, S100A8, S100A9), VSMCs (TAGLN, MYH11, CNN1, ACTG2, ACTA2). The sub-cluster of VSMCs was extracted for further analysis. FindAllMarkers in the Seurat package was used to search differentially expressed genes between normal control and AD patients in VSMCs. GO and KEGG analysis were performed based on the ClusterProfile packages (V3.14.3)51. scMetabolism package was used to analyze the metabolic activity in single cell level21.
Human aorta VSMCs Isolation and cell culture
Primary human aorta VSMCs were isolated from the aorta and cultured in a complete smooth muscle cell medium (SMCM; ScienCell, CA, USA). The medium was supplemented with 10% (v/v) fetal bovine serum (FBS; Cat. No. 0010, ScienCell, CA, USA), smooth muscle cell growth factor (SMCGS; 1152, ScienCell, CA, USA), and 1% penicillin and streptomycin (P/S; 0503, ScienCell, CA, USA). Mouse vascular smooth muscle cells (MOVAS; American Type Culture Collection [ATCC]) and human HEK293T cells (ATCC) were cultured in Dulbecco’s Modified Eagle Medium (DMEM, 11995065; Gibco, CA, USA) supplemented with 10% FBS (FSP500; ExCell, Suzhou, China). Both HASMC, MOVAS and HEK293T cells were cultured at 37 °C with 5% CO2.
Plasmid construction and transfection
The GMRSP-Flag or hnRNPA2B1-HA fusion protein constructs were cloned into the pcDNA3.1(+) vector using the Clon Express MultiS One Step Cloning Kit (C113-01; Vazyme) following the provided instructions. Mutations in the GMRSP-Flag (GMRSPL/C/T) and hnRNPA2B1-HA (hnRNPA2B1 mut1 ~ 4-HA and hnRNPA2B13G/Y) constructs were generated using the Mut Express II Fast Mutagenesis Kit V2 (C214; Vazyme, Nanjing, China). The primers used for plasmid construction are shown in Table S1. The plasmid constructs were transfected into HASMC using an advanced transfection reagent (AD600150; Zeta Life, CA, USA).
Immunofluorescence staining
We used 0.3% Triton X-100 to permeabilize 4% formaldehyde-fixed cells or tissue sections and then blocked them with 3% bovine serum albumin (BSA). The cells or tissue sections were incubated overnight at 4 °C with the specific primary antibodies shown in Table S1. Subsequently, the fluorescent secondary antibodies shown in Table S1 were applied to the cells or tissue sections and incubated for two hours at room temperature. To visualize the nuclei, 4’,6-diamidino-2-phenylindole (DAPI; C1006, Beyotime, Shanghai, China) was added and incubated with the cells or tissue sections for five minutes. Immunofluorescence was detected using a confocal microscope (LSM 900, Zeiss, Oberkochen, Germany) for subsequent observation.
Western blotting
Cell samples or tissue samples were lysed using sodium dodecyl sulfate (SDS) lysis buffer (P0013G; Beyotime, Shanghai, China). The proteins from all samples were separated by 10–15% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. After incubating with specific primary antibodies, the blots were further incubated with a horseradish peroxidase-conjugated (HRP) secondary antibody and detected using Supersignal West Pico substrate (34080; Thermofisher, CA, USA). The specific primary and secondary antibodies used in this study are listed in Table S1.
Migration assays
Cell migration activity was assessed using both the wound healing and transwell migration assays. For the wound healing assay, treated human primary VSMCs were seeded in a 12-well plate and manually wounded by scraping the cells with a 200 µl pipette tip. The medium was then replaced with an FBS-free F12 medium. The migration areas were observed and captured under a microscope at 0 and 12 h after wounding. The migration area was quantified using Image-Pro Plus 6.0 software. In the transwell migration assay, 5 × 104 treated human primary VSMCs were seeded in the top chambers of the 8 µm transwell plates (353097; BD Biosciences, NJ, USA) in an FBS-free F-12 medium with a membrane inserted. In the well of the plate, 0.75 mL of F12 medium supplemented with 10% FBS was added. After 24 h, the cells that migrated to the lower surface of the membrane were fixed with 4% formaldehyde for 20 minutes. The cells were then stained with 1% crystal violet for 20 min, captured, and counted under a 10x microscope.
Gelatin zymography
To analyze the activity of matrix metalloproteinase 2 (MMP2), the culture supernatant of treated human primary VSMCs was loaded onto gelatin gels and subjected to electrophoresis. The Gelatin-Zymography Kit (RTD6143; Real-Jimer, Beijing, China) was used to detect MMP2 activity following the manufacturer’s protocol.
Preparation and characterization of extracellular vesicles
Extracellular vesicles (EVs) were isolated from gene-engineered MOVAS cells using a Beckman floor-standing ultracentrifuge (Optima XPN-100; Beckman, CA, USA). Briefly, MOVAS cells were cultured for four days in media containing exosome-depleted FBS to promote EV production. The media were then centrifuged at 3500 g at 4 °C for 10 min to remove any dead cells present. Subsequently, the EVs in the media were purified by high-speed centrifugation at 20,000 g at 4 °C for 30 min. The filtered media, using a 0.22 µm membrane, were further centrifuged at 2,00,000 g at 4 °C for two hours. Following a wash with phosphate-buffered saline (PBS), the EVs were re-suspended in Trehalose PBS and stored at −80 °C for future use. The size distribution and concentration of the isolated EVs were assessed using nanoparticle tracking analysis (NTA).
Hematoxylin and eosin and elastic fiber staining
Aortic tissue samples were fixed using 4% paraformaldehyde (PFA) and subsequently embedded in paraffin. Aortic sections were stained with hematoxylin and eosin (H&E, C0105; Beyotime, Shanghai, China) and Verhoeff–Van Gieson (G1598; Solarbio, Beijing, China) stains following the manufacturer’s instructions. Elastin degradation was assessed using van Gieson staining and assigned an elastin score based on established criteria. Grade 1 represented a complete and well-organized elastic sheet; Grade 2 represented an elastic sheet with some interruptions and fractures; Grade 3 represented severe elastin fragmentation or loss; and Grade 4 indicated severe elastin degradation with visible rupture sites6. Microscopic images were captured for analysis.
Immunoprecipitation and liquid chromatography-tandem mass spectrometry analysis
Treated cells were lysed using immunoprecipitation (IP) lysis buffer (P0013; Beyotime, Shanghai, China) supplemented with a 1x phosphatase inhibitor cocktail (4693132001; Roche, Basel, Switzerland). After incubation on ice for 30 min, the lysates were centrifuged at 12,000 rpm at 4 °C for 15 min. The protein concentrations in the lysates were measured using the Pierce™ BCA protein assay kit (23227; Thermofisher, CA, USA). Equal amounts of proteins were immunoprecipitated with specific primary antibodies overnight at 4 °C. The lysates were incubated with Protein A/G PLUS-Agarose beads (SC-2003; Santa Cruz, CA, USA) for four hours at 4 °C. The samples were then separated by a 10–15% SDS-PAGE gel. Following the manufacturer’s protocol, the gel was stained using the fast silver stain kit (P0017S; Beyotime, Shanghai, China). The differential protein bands were subjected to nano-liquid chromatography-tandem mass spectrometry (LC-MS)/MS (ABSCIEX TripleTOF 5600, USA). The MS/MS data were analyzed for protein identification and quantification using PEAKS Studio 8.5. The local false discovery rate at PSM was 1.0% after searching against Human database with a maximum of two missed cleavages. The following settings were selected: Oxidation (M), Acetylation (Protein N-term), Deamidation (NQ), Pyro-glu from E, Pyro-glu from Q for variable modifications as well as fixed Carbamidomethylation of cysteine. Precursor and fragment mass tolerance were set to 10 ppm and 0.05 Da, respectively. The differential proteins were analyzed and identified using the Mascot program and the UniProt human protein database.
Molecular docking
The protein was pretreated using Discovery Studio software, which involved removing water molecules, hydrogenation, and charge adjustments. The original ligand in the protein structure was extracted, and the protein was visualized using PyMOL. The protein-protein docking study was conducted using the Zdock module within Discovery Studio software. The receptor protein hnRNPA2B1 was docked with the ligand-protein GMRSP. For the docking sampling, an angular step size of 15 was set, and the most accurate prediction results were obtained with a sampling angle of 15 out of 54,000 combined configurations. When setting the root mean square deviation (RMSD) cutoff to a cluster radius of 10.0 Å and the interface cutoff to 10.0 Å, the maximum number of clusters was 100, and the clustering result was considered the best. The poses with Zdockscore >16 were selected as the baseline and filtered for Rdock docking analysis. Rdock is an energy optimization process based on CHARMm that optimizes the binding configurations of protein-protein complexes identified by ZDOCK. It utilizes an energy-scoring function to evaluate and score these binding configurations.
Protein purification and micro-scale thermophoresis
The His-GMRSP or His-hnRNPA2B1 recombinant plasmids were transformed into Escherichia coli BL21 (DE3)-competent cells. The transformed bacteria were cultured in a shaker at 37 °C and 150 rpm for four hours until the optical density (OD) value reached a value of 0.6–0.8. Isopropyl β-D-thiogalactoside (IPTG) was then added to induce the target protein expression at 18 °C and 180 rpm. The following day, the bacteria were lysed and centrifuged at 13,000 rpm for 30 minutes at 4 °C. The resulting supernatant was collected and mixed with Ni-NTA resin (Qiagen Inc., Valencia, CA), followed by incubation at 4 °C for one hour. The target proteins were purified using AKTA systems. The fusion protein of His-hnRNPA2B1 was labeled with the MonolithTM RED NHS protein labeling kit (MO-L011, AmineReactive) and then mixed with His-GMRSP or His-GMRSPL/C/T. Finally, the sample was extracted using a capillary (MO-K022, NanoTemper, Germany) and measured on the micro thermophoresis meter (Monolith NT.115, NanoTemper, Germany).
RNA affinity purification
The 5’ biotin-labeled PKM EI9 RNAs were synthesized by Genscript (Nanjing, China). RNA affinity purification was performed following previously described methods7. To prepare RNA-immobilized beads, 50 μl of Streptomyces avidin agarose beads (GE17-5113-01; Sigma, MO, USA) were used to bind to 1 nmol biotin-labeled RNA. Treated cells were lysed using IP lysis buffer (P0013; Beyotime, Shanghai, China) supplemented with 1x phosphatase inhibitor cocktail (4693132001; Roche, Basel, Switzerland). Nuclear particles from the cell lysates were collected using a nuclear and cytoplasmic protein extraction kit (78833; Thermofisher, CA, USA). The extracted nuclear lysate was then incubated with the RNA-immobilized beads at room temperature for 30 min. After the protein and RNA were combined, the samples were lysed using SDS lysis buffer (P0013G; Beyotime, Shanghai, China) and subjected to western blot analysis.
UV-crosslinking IP
The UV-crosslinking IP was performed following established protocols8. After UV irradiation on ice (100 mJ/cm2), treated cells were lysed using lysis buffer containing 50 mM Tris (pH 8.0), 1 mM MgCl2, 100 mM NaCl, 0.5 mM Na3VO4, 1% NP-40, 1 mM dithiothreitol (DTT), 0.1 mM CaCl2, an RNase inhibitor, and a protease inhibitor cocktail. Following a brief ultrasound treatment on ice, the cell lysate was incubated with DNase (AM2222; Ambion, CA, USA) at 37 °C for 20 min and then centrifuged at 12,000 g at 4 °C for 10 min. After diluting the supernatant to 1 mL with lysis buffer, 1 mg of the supernatant was incubated with anti-hnRNPA2B1 or immunoglobulin G (IgG; negative control) for IP in the presence of protein A/G plus agarose beads (SC-2003; Santa Cruz, CA, USA). Additionally, 1 mg of the supernatant was incubated with 1000 IU of RNase I (AM2294; Ambion, CA, USA) at 4 °C for two hours with rotation. After three thorough washes, 10% of the samples were used as IP controls, while the remaining 90% were incubated with proteinase K (AM2546; Invitrogen, CA, USA) at 55 °C for 30 min. The RNA in the samples was extracted using the TRIzol method, reverse transcribed, and detected by quantitative polymerase chain reaction (qPCR).
Reverse transcriptase-PCR and PKM splicing assays
Total RNA from tissue and cell samples was isolated using the Trizol Reagent (10296010; Invitrogen, CA, USA). cDNA synthesis from the RNA samples was performed using the PrimeScript TM RT reagent kit with gDNA Eraser (RR037A; TaKaRa, Beijing, China). The PCR products were analyzed by monitoring 1% agarose gels. The specific primers used in this study are listed in Table S1. PKM splicing assays were conducted following established protocols9. The PCR products of PKM were digested with PstI endonuclease (D6566; Beyotime, Shanghai, China) and separated by 8% non-denaturing PAGE.
Extracellular acidification rate assay
The extracellular acidification rate (ECAR) assay was conducted using a Seahorse Bioscience XF-96 extracellular analyzer (Agilent, CA, USA) following the manufacturer’s instructions. Briefly, 20,000 treated HASMC were seeded on Seahorse XF-96 polystyrene tissue culture plates in an XF base medium. ECAR measurements were obtained by adding three reagents at specified time intervals with the following concentrations: glucose (10 mM), oligomycin (1 μM), and 2-deoxy-D-glucose (2-DG; 50 mM). The results were presented as a graph with time on the X-axis and mpH/min/well for ECAR on the Y-axis. Each experiment was repeated at least six times.
Measurement of glucose and lactate production
The glucose concentration in the treated cell supernatant was measured using a glucose colorimetric assay kit (K606-100; BioVision, SF, USA) following the manufacturer’s instructions. Similarly, the lactate production in the treated cell supernatant was detected using the lactate colorimetric assay kit II (K627-100; BioVision, SF, USA) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
The levels of PKM2 in human serum were measured using the enzyme-linked immunosorbent assay (ELISA) obtained from Mbbiology (MB-4462, Jiangsu, China). The samples were diluted with a dilution buffer, and 100 µL of each diluted sample was added to the wells according to the manufacturer’s protocol. The absorbance was measured at 450 nm using an ELISA reader (Multiskan, ThermoFisher, CA, USA). Each experiment was independently repeated five times.
Statistical analysis
The data are presented as the means ± standard error of the mean (SEM). Shapiro-Wilk normality test was used to test the normality. Statistical comparisons between 2 groups were evaluated by using the 2-sample t-test (normal data; 2 groups), Mann–Whitney U test (nonnormal data; 2 groups), or χ2 test. One-way ANOVA or 2-way ANOVA ( > 2 groups) was conducted followed by Bonferroni post hoc test for comparisons among multiple groups. GraphPad Prism 8.0 and IBM SPSS Statistics (version 25, IBM Corp., Armonk, NY, USA) were used to analyze and plot the data. Survival curves were generated using the Kaplan-Meier method, and the difference between groups was assessed using the Breslow Test. Logistic regression analysis was performed to evaluate the association between plasma levels of PKM2 and risk for AD, calculating odds ratios (OR) and 95% confidence intervals (CIs). A P-value of <0.05 was considered statistically significant, denoted as *P < 0.05, **P < 0.01, and ***P < 0.001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All sequencing data generated in this study have been deposited in the Gene Expression Omnibus (GEO) database under accession code GSE241265 and GSE252721. Human aortic dissection scRNA sequencing dataset was downloaded from GEO with the accession number GSE21374020. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium with the dataset identifier PXD059430. The raw numbers for charts and graphs are available in the Source Data file. Source data are provided with this paper.
References
Evangelista, A. et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 137, 1846–1860 (2018).
Chakraborty, A. et al. Epigenetic Induction of Smooth Muscle Cell Phenotypic Alterations in Aortic Aneurysms and Dissections. Circulation 148, 959–977 (2023).
Gao, J. et al. The mechanism and therapy of aortic aneurysms. Signal Transduct. Target Ther. 8, 55 (2023).
Ali, L., Schnitzler, J. G. & Kroon, J. Metabolism: The road to inflammation and atherosclerosis. Curr. Opin. Lipido. 29, 474–480 (2018).
Caruso, P. et al. Identification of MicroRNA-124 as a Major Regulator of Enhanced Endothelial Cell Glycolysis in Pulmonary Arterial Hypertension via PTBP1 (Polypyrimidine Tract Binding Protein) and Pyruvate Kinase M2. Circulation 136, 2451–2467 (2017).
Jia, Y. et al. PHB2 Maintains the Contractile Phenotype of VSMCs by Counteracting PKM2 Splicing. Circ. Res. 131, 807–824 (2022).
Zhou, Q. et al. Warburg effect is involved in apelin-13-induced human aortic vascular smooth muscle cells proliferation. J. Cell Physiol. 234, 14413–14421 (2019).
Zhao, X. et al. PKM2-dependent glycolysis promotes the proliferation and migration of vascular smooth muscle cells during atherosclerosis. Acta Biochim. Biophys. Sin. (Shanghai) 52, 9–17 (2020).
Tsai, M.-C. et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 (2010).
Wang, X. et al. Long noncoding RNA HCP5 participates in premature ovarian insufficiency by transcriptionally regulating MSH5 and DNA damage repair via YB1. Nucleic Acids Res. 48, 4480–4491 (2020).
Statello L., Guo C.-J., Chen L.-L. & Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118 (2021).
Matsumoto, A. et al. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 541, 228–232 (2017).
Huang, J.-Z. et al. A Peptide Encoded by a Putative lncRNA HOXB-AS3 Suppresses Colon Cancer Growth. Mol. Cell 68, 171–184 (2017).
Spencer, H. L. et al. The LINC00961 transcript and its encoded micropeptide, small regulatory polypeptide of amino acid response, regulate endothelial cell function. Cardiovasc Res. 116, 1981–1994 (2020).
Gabory, A., Jammes, H. & Dandolo, L. The H19 locus: role of an imprinted non-coding RNA in growth and development. Bioessays 32, 473–480 (2010).
Thorvaldsen, J. L., Duran, K. L. & Bartolomei, M. S. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 12, 3693–3702 (1998).
Li, D. Y. et al. H19 Induces Abdominal Aortic Aneurysm Development and Progression. Circulation 138, 1551–1568 (2018).
Zhou, Z. et al. Exaggerated Autophagy in Stanford Type A Aortic Dissection: A Transcriptome Pilot Analysis of Human Ascending Aortic Tissues. Genes (Basel) 11, 1110–1187 (2020).
Yap, C., Mieremet, A., de Vries, C. J. M., Micha, D. & de Waard, V. Six Shades of Vascular Smooth Muscle Cells Illuminated by KLF4 (Kruppel-Like Factor 4). Arterioscler Thromb. Vasc. Biol. 41, 2693–2707 (2021).
Zhang, B. et al. Single-Cell RNA-Seq Analysis Reveals Macrophages Are Involved in the Pathogenesis of Human Sporadic Acute Type A Aortic Dissection. Biomolecules 13, 390–399 (2023).
Wu, Y. et al. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov. 12, 134–153 (2022).
Wang, T. et al. C9orf72 regulates energy homeostasis by stabilizing mitochondrial complex I assembly. Cell Metab. 33, 531–546 (2021).
Cerrato, C. P., Pirisinu, M., Vlachos, E. N. & Langel, Ü. Novel cell-penetrating peptide targeting mitochondria. FASEB J. 29, 4589–4599 (2015).
Tang, Y. et al. Jmjd4 Facilitates Pkm2 Degradation in Cardiomyocytes and Is Protective Against Dilated Cardiomyopathy. Circulation 147, 1684–1704 (2023).
Ruffenach, G., Medzikovic, L., Aryan, L., Li, M. & Eghbali, M. HNRNPA2B1: RNA-Binding Protein That Orchestrates Smooth Muscle Cell Phenotype in Pulmonary Arterial Hypertension. Circulation 146, 1243–1258 (2022).
David, C. J., Chen, M., Assanah, M., Canoll, P. & Manley, J. L. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463, 364–368 (2010).
Kim, H. J. et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473 (2013).
Chen, M., David, C. J. & Manley, J. L. Concentration-dependent control of pyruvate kinase M mutually exclusive splicing by hnRNP proteins. Nat. Struct. Mol. Biol. 19, 346–354 (2012).
de Abreu, R. C. et al. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 17, 685–697 (2020).
Hofmann, P. et al. Long non-coding RNA H19 regulates endothelial cell aging via inhibition of STAT3 signalling. Cardiovasc Res. 115, 230–242 (2019).
Li, X. et al. lncRNA H19 Alleviated Myocardial I/RI via Suppressing miR-877-3p/Bcl-2-Mediated Mitochondrial Apoptosis. Mol. Ther. Nucleic Acids 17, 297–309 (2019).
Thomas, A. A. et al. lncRNA H19 prevents endothelial-mesenchymal transition in diabetic retinopathy. Diabetologia 62, 517–530 (2019).
Viereck, J. et al. Targeting muscle-enriched long non-coding RNA H19 reverses pathological cardiac hypertrophy. Eur. Heart J. 41, 3462–3474 (2020).
Dai, X. et al. Epigenetic Upregulation of H19 and AMPK Inhibition Concurrently Contribute to S-Adenosylhomocysteine Hydrolase Deficiency-Promoted Atherosclerotic Calcification. Circ. Res. 130, 1565–1582 (2022).
Wang, J. et al. Long Noncoding RNA H19 Promotes Neuroinflammation in Ischemic Stroke by Driving Histone Deacetylase 1-Dependent M1 Microglial Polarization. Stroke 48, 2211–2221 (2017).
Sun, Y. et al. LncRNA H19 promotes vascular inflammation and abdominal aortic aneurysm formation by functioning as a competing endogenous RNA. J. Mol. Cell Cardiol. 131, 66–81 (2019).
Ren, M. et al. LncRNA H19 regulates smooth muscle cell functions and participates in the development of aortic dissection through sponging miR-193b-3p. Biosci. Rep. 41, (2021).
Sénémaud, J. et al. Translational Relevance and Recent Advances of Animal Models of Abdominal Aortic Aneurysm. Arteriosclerosis, Thrombosis, Vasc. Biol. 37, 401–410 (2017).
Zhou, Y. et al. Research Progress on the Pathogenesis of Aortic Aneurysm and Dissection in Metabolism. Curr. Probl. Cardiol. 49, 102040 (2024).
Lambert, C. M., Roy, M., Robitaille, G. A., Richard, D. E. & Bonnet, S. HIF-1 inhibition decreases systemic vascular remodelling diseases by promoting apoptosis through a hexokinase 2-dependent mechanism. Cardiovasc Res. 88, 196–204 (2010).
Wu, X. et al. LDHA mediated degradation of extracellular matrix is a potential target for the treatment of aortic dissection. Pharm. Res 176, 106051 (2022).
Wang, W. J. et al. Exon 47 skipping of fibrillin-1 leads preferentially to cardiovascular defects in patients with thoracic aortic aneurysms and dissections. J. Mol. Med. (Berl.) 91, 37–47 (2013).
Hong, S. H., Rhyne, J. & Miller, M. Novel polypyrimidine variation (IVS46: del T -39…-46) in ABCA1 causes exon skipping and contributes to HDL cholesterol deficiency in a family with premature coronary disease. Circ. Res 93, 1006–1012 (2003).
Lv, L. et al. Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol. Cell 42, 719–730 (2011).
Morita, M. et al. PKM1 Confers Metabolic Advantages and Promotes Cell-Autonomous Tumor Cell Growth. Cancer Cell 33, 355–367 (2018).
Jiang, Y. et al. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol. Cell 53, 75–87 (2014).
Li, X. et al. LncRNA GACAT2 binds with protein PKM1/2 to regulate cell mitochondrial function and cementogenesis in an inflammatory environment. Bone Res. 10, 29 (2022).
Ye, J. et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl. Acad. Sci. USA 109, 6904–6909 (2012).
Lunt, S. Y. et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol. Cell 57, 95–107 (2015).
Srivastava, A. AAV Vectors: Are They Safe? Hum. Gene Ther. 31, 697–699 (2020).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Acknowledgements
This study is supported by the National Natural Science Foundation of China (grant 82070478 and 82100382), Guangdong Provincial Clinical Research Center for Cardiovascular disease (grant 2020B1111170011), Multi-channel minimally invasive vascular interventional surgery robot project (ZTZB-23-990-011).
Author information
Authors and Affiliations
Contributions
L.J.F., L.J., and L.S.Y. conceived and supervised the whole project; W.J.Z. and L.J.T. performed the animal and the in vitro cellular functional experiments; W.J.Z. and Y.F. performed the model building, prepared the samples for the mass spectrometry; C.J.H., S.Y.H. and L.J. performed bioinformatics analysis. S.T.C., P.F. and F.R.X. performed the structural analysis; W.J.Z. and L.J.T. wrote the manuscript with inputs from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wei Kong, Fulvio Morello and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, J., Liu, J., Yang, F. et al. GMRSP encoded by lncRNA H19 regulates metabolic reprogramming and alleviates aortic dissection. Nat Commun 16, 1719 (2025). https://doi.org/10.1038/s41467-025-57011-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-57011-5
This article is cited by
-
Machine-learning–guided transcriptomic integration identifies GFM1 as a lactylation-related candidate biomarker in aortic dissection
Scientific Reports (2026)
-
Lactylation associated biomarkers and immune infiltration in aortic dissection
Scientific Reports (2025)